Abstract
Background:
Central airway stenosis (CAS) is a severe airway complication after lung transplantation associated with bronchial ischemia and necrosis. We sought to determine if hyperbaric oxygen therapy (HBOT), an established treatment for tissue ischemia, attenuates post-transplant bronchial injury.
Methods:
We performed a randomized, controlled trial of usual care vs. HBOT (2 atmospheres absolute for 2 hours × 20 sessions) in subjects with extensive airway necrosis 4 weeks post-transplant. Endobronchial biopsies were collected at 4, 7, and 10 weeks post-transplant for quantitative PCR. Co-primary outcomes were incidence of airway stenting and acute cellular rejection (ACR) at one year.
Results:
The trial was stopped after enrolling 20 subjects (n=10 per group) after a pre-planned interim analysis showed no difference between usual care and HBOT groups in stenting (both 40%), ACR (70% and 40%, respectively), or CAS (40% and 60%, respectively). Time-to-first-stent placement (median [IQR]) was significantly shorter in the HBOT group (150 [73–150] vs. 186 [167–206] days, P<0.05). Hypoxia-inducible gene expression was significantly increased in donor tissues at 4, 7, and 10 weeks post-transplant, but was not altered by HBOT. Subjects that developed CAS or required stenting had significantly higher HMOX1 and VEGFA expression at 4 weeks (both P<0.05). Subjects that developed ACR had significantly FLT1, TIE2, and KDR expression at 4 weeks (all P<0.05).
Conclusions:
Incidence of CAS is high after severe, established airway necrosis post-transplant. HBO therapy does not reduce CAS severity or stenting. Elevated HMOX1 and VEGFA expression appears to associate with airway complications.
Keywords: lung transplantation, hyperbaric oxygenation, cell hypoxia/genetics, gene expression, postoperative complications
INTRODUCTION
Central airway stenosis (CAS) is a debilitating complication of lung transplantation that leads to loss of lung function, respiratory infections, hospitalizations, and possibly death1–3. While the pathophysiology is incompletely understood, accumulating evidence points to bronchial ischemia acquired intra-operatively due to sacrifice of the bronchial arteries4–8. Soon after transplant, donor bronchial tissue hypoxia is evident, with reduced tissue oxygen saturations and up-regulation of hypoxia-inducible factor-dependent (HIF) genes6. Severe bronchial ischemia is associated with post-transplant respiratory failure and prolonged hospitalization, and ultimately leads to mucosal necrosis, fibrosis, and central airway stenosis6.
Strategies proposed to mitigate post-transplant bronchial ischemia include bronchial artery anastomosis8,9, which prolongs operative time and may be technically challenging, topical application of HIF stabilizers7, and hyperbaric oxygen therapy (HBOT)10. HBOT involves breathing pure oxygen in a chamber pressured to 1.4 atmospheres absolute (ATA) or higher11, and is an established therapy for ischemic flaps and grafts12,13. In small case series HBOT has been reported to improve anastomotic healing after native tracheobronchial reconstruction14–17. Furthermore, HBOT can be safely administered to patients after lung transplantation10,18 and may reduce the need for airway stent placement for CAS10. On this basis, we performed a randomized, controlled, phase 2 clinical trial evaluating the efficacy of HBOT to reduce airway complications following lung transplantation in patients with significant airway necrosis. We also serially measured bronchial mucosal gene expression of HIF-dependent genes to develop a timeline for host response, and validate our prior biomarker studies6.
METHODS
Subject Selection
The study protocol was approved by the Duke Institutional Review Board (Pro00055849) and posted on www.clintrials.gov (NCT02363959). All subjects provided written informed consent prior to study procedures. Subjects were eligible for the study that had developed extensive (stage 3–4) (Table S1) post-transplantation airway necrosis10 after lung transplantation that did not spontaneously resolve after 2–3 weeks. Subjects were excluded that required mechanical ventilation with fraction of inspired oxygen (FiO2) > 0.4, required extracorporeal membrane oxygenation at the time of screening, used inhaled nitric oxide at the time of screening, had a pneumothorax at the time of screening, were pregnant, or were unable to provide informed consent. Subjects that agreed to participate were randomized 1:1 to usual care or HBOT (total target enrollment of 40 subjects) using pre-prepared envelopes containing the group assignment.
Surgical Technique
Single or bilateral orthotopic lung transplantation (SOLT/BOLT) was performed as described10,19. Briefly, the membranous portions of the bronchi were sutured end-to-end using a running, 4–0 absorbable monofilament suture (e.g. PDS) with the smaller cartilaginous portion partially intussuscepted into the larger airway. When technically feasible, the donor bronchus is preferentially intussuscepted into the recipient bronchus. No tissue flap or additional coverage of the airway is performed. There were five surgeons during the time of the study that performed the airway anastomosis in a similar fashion. None of the enrolled subjects received lungs from donors after cardiac death (DCD) or after ex vivo lung perfusion (EVLP).
Bronchoscopy Protocol
Informed consent was obtained prior to each procedure. Bronchoscopies were performed at approximately 4 weeks, 7 weeks, and 10 weeks post-transplantation. Airway balloon dilation was performed for airway stenosis, defined as inability to traverse an airway that would otherwise permit passage of a 6.4 mm outer-diameter bronchoscope. Balloon dilation procedures were performed up to three times, in 2–3 week intervals. For subjects with stenosis refractory to three separate balloon dilation procedures, or who were at risk for complete airway obstruction, endobronchial stents were placed. Subjects with excessive granulation tissue underwent mechanical debridement as needed to open the airway lumen. Endobronchial biopsies (1–2 mm) of the airway epithelium were performed in triplicate at the main carina and the first subcarina of each donor bronchus. Subjects with prior SOLTs underwent biopsy of the main carina and the donor bronchus subcarina only. The endobronchial biopsy specimens were labeled numerically for blinding, placed immediately into RNAlater RNA stabilization reagent (Qiagen), and stored at −80°C.
Hyperbaric Oxygen Therapy
Subjects randomized to HBOT breathed >99% medical-grade oxygen via head tent inside a multi-place hyperbaric chamber (Duke Center for Hyperbaric Medicine and Environmental Physiology, Durham, NC) pressured to 2 ATA for two hours daily for up to 20 sessions total. Subjects were evaluated after each treatment for signs or symptoms of barotrauma or oxygen toxicity.
Blinding
While the study was designed so the proceduralists were blinded to randomization, unblinding inevitably occurred during the study due to the clinical reporting of the HBOT administration in the subjects’ electronic medical record. Nevertheless, the proceduralists were blinded for as long as possible, until it was discoverable in the chart.
Crossover
Subjects randomized to usual care were eligible to crossover to HBOT between 8 – 12 weeks after initial study bronchoscopy if they: 1) developed CAS or another airway complication (e.g. dehiscence, malacia); or 2) failed to improve pseudomembrane severity based on a semi-quantitative scoring system (Table S1). For data analysis, subjects that crossed-over remained in their original group assignments per our intention-to-treat protocol.
Primary and Secondary Outcomes
The co-primary outcomes were need for airway stenting and development of acute cellular rejection (ACR) at one year. We chose ACR as a co-primary outcome for safety monitoring. The secondary outcomes were development of clinically significant central airway stenosis (CAS) due to fibrotic strictures, need for dilation/balloon bronchoplasty, development of clinically significant lung infection requiring antibiotics, development of airway dehiscence, chronic lung allograft dysfunction (CLAD)20, development of other airway complications such as granulomatous strictures or bronchomalacia, and bronchial epithelial gene expression. Clinical outcomes were assessed at 12 months. An interim analysis was pre-planned after enrolling 20 subjects with the option to terminate the trial early for futility.
Polymerase Chain Reaction
Total RNA was extracted from endobronchial biopsy specimens using the RNeasy Midi Kit (Qiagen). RNA purity was confirmed on a 1.2% agarose gel and RNA was reverse transcribed into cDNA using the ImProm-II reverse transcription system (Promega). Quantitative real-time RT-PCR was performed on an ABI StepOnePlus using gene expression assays (Applied Biosystems). Gene expression assay primers were used to amplify HMOX1, VEGFA, FLT1 (VEGFR1), KDR (VEGFR2), TIE2 (TEK), and TGFB1. 18S rRNA was used as an endogenous control. Quantification of gene expression was determined by the comparative threshold cycle and relative quantification method. Each sample was assayed in triplicate. All mRNA work was performed in a blinded fashion.
Statistical Analysis
Assuming a CAS incidence of 60%, a reduction by HBOT to 10%10, and alpha of 0.05, we calculated 14 subjects/group would achieve 80% power to detect statistical significance. We targeted 20 subjects/group (40 total) to compensate for subject withdrawal or drop-out.
Grouped data are shown as median (interquartile range) unless otherwise specified. Primary and secondary outcomes were analyzed by intention-to-treat analysis. Categorical data were analyzed using Fisher’s exact test. PCR data were analyzed by one sample Wilcoxon signed-rank test against a hypothetical value of 1 with Bonferroni correction (Figure 2), or by 2-way ANOVA with Benjamin-Hochberg post-hoc test (to correct for multiple comparisons) for group differences within each time point (Figures 3–6) (Prism v8.1.1, GraphPad Software, San Diego, CA). P<0.05 was accepted as significant.
Figure 2: Donor/native tissue gene expression ratios.
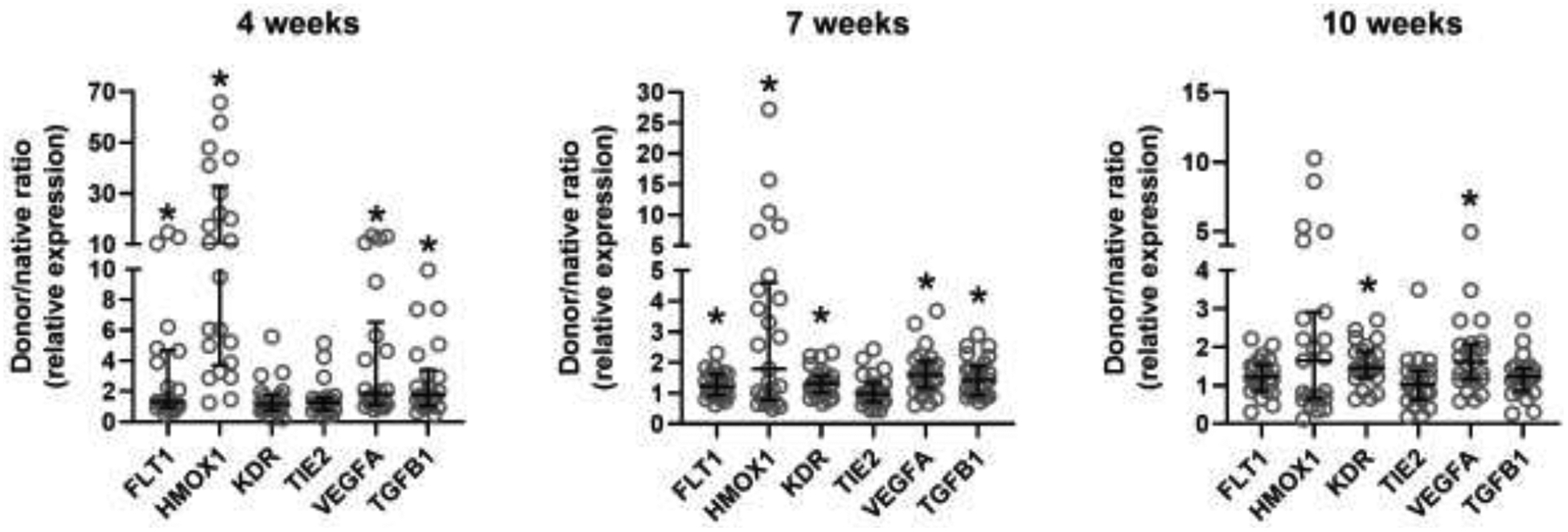
Bronchial mucosal gene expression shown as donor/native ratio (relative to 18S mRNA) at 4, 7, and 10 weeks post-transplant. *P<0.05 by one sample Wilcoxon signed-rank test compared to hypothetical value of 1.
Figure 3: Gene expression of subjects by group assignment (Usual Care vs. HBOT).
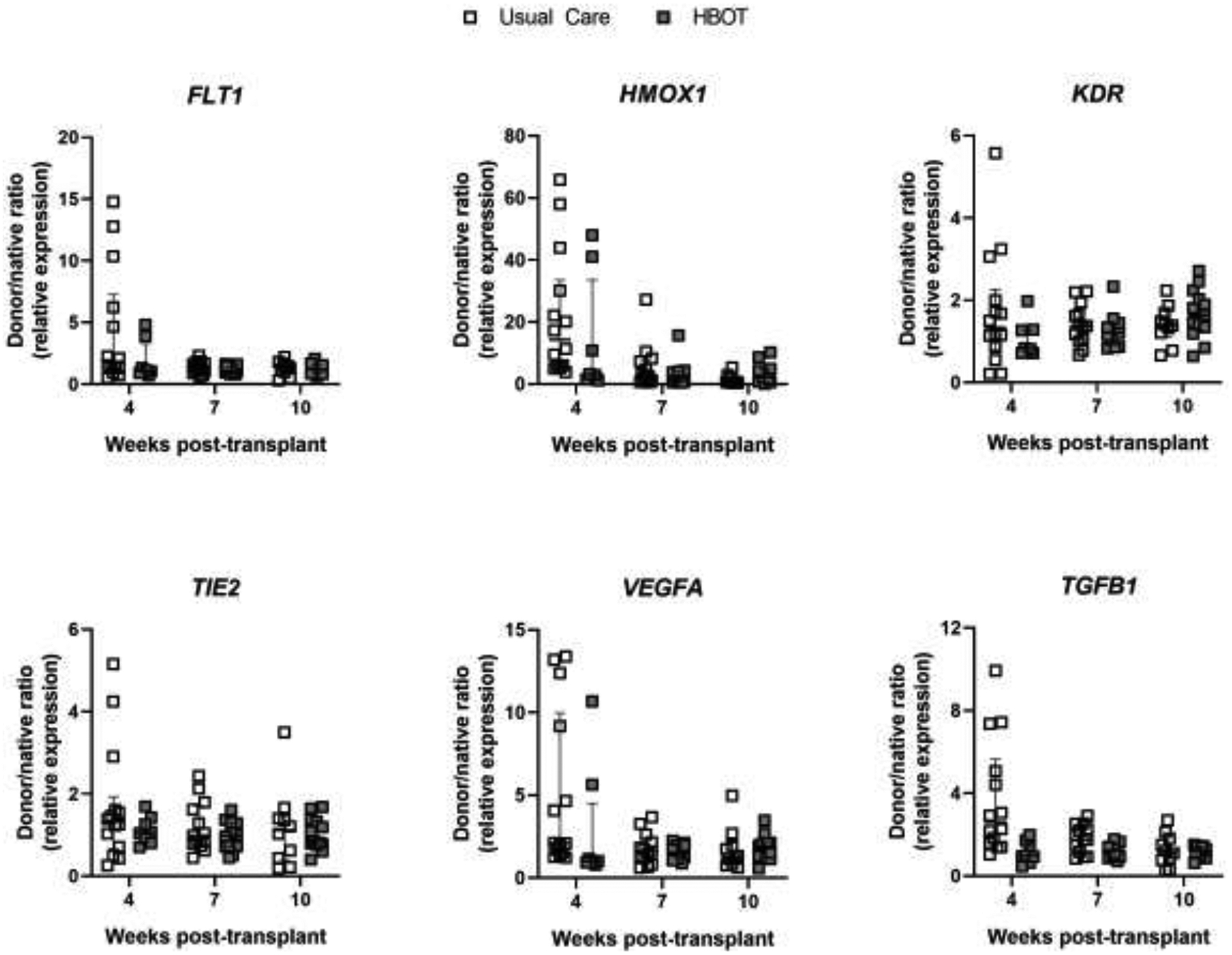
Bronchial mucosal gene expression shown as donor/native ratio (relative to 18S mRNA) in Usual Care (open boxes) and HBOT (grey boxes) groups for FLT1, HMOX1, KDR, TIE2, VEGFA, and TGFB1 at 4, 7, and 10 weeks post-transplant. Error bars are median with IQR.
Figure 6: Gene expression of subjects by Acute Cellular Rejection status.
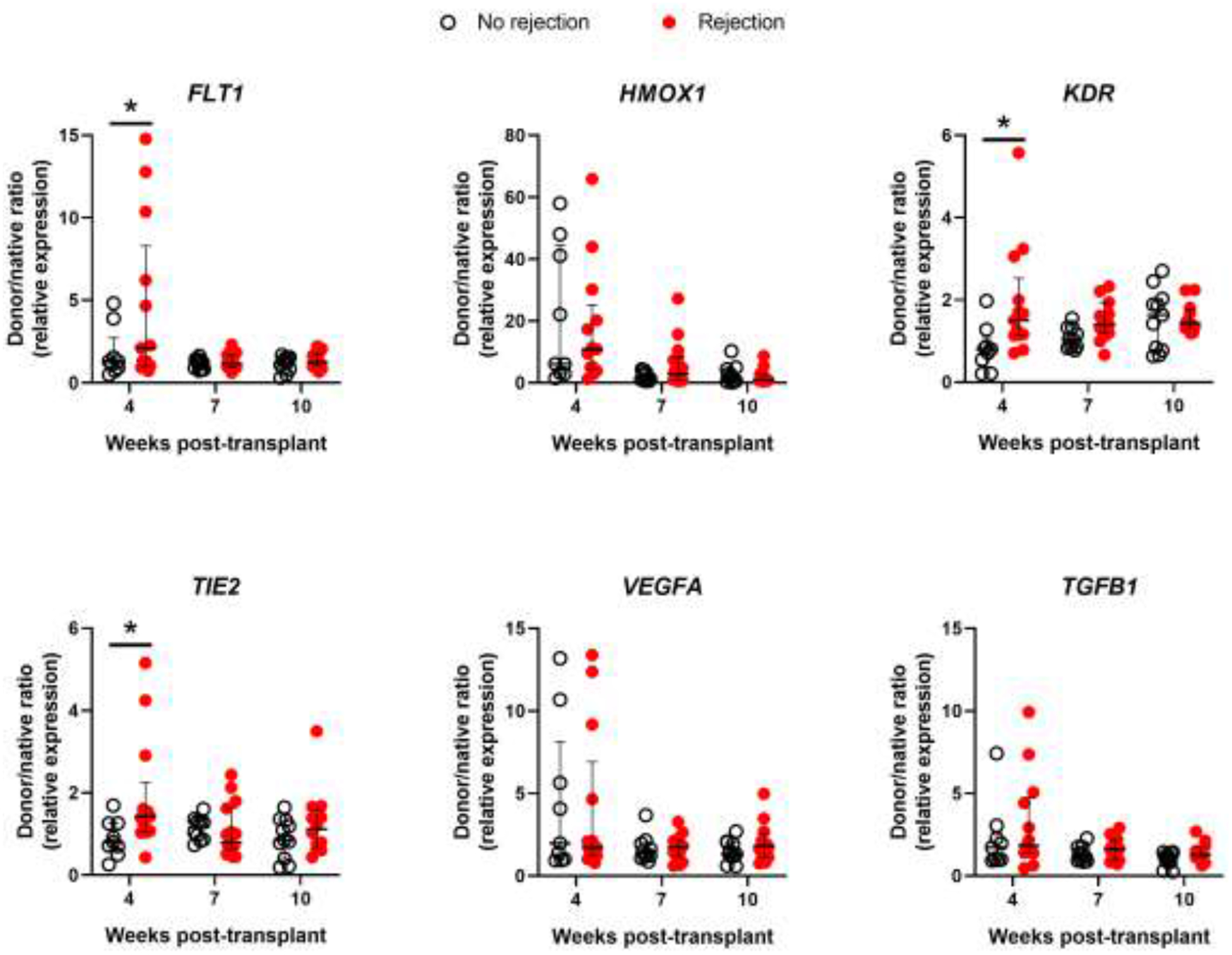
Bronchial mucosal gene expression shown as donor/native ratio (relative to 18S mRNA) in subjects without ACR (open circles) and with ACR (red circles) for FLT1, HMOX1, KDR, TIE2, VEGFA, and TGFB1 at 4, 7, and 10 weeks post-transplant. Error bars are median with IQR. *P<0.05 for group differences within each time point by 2-way ANOVA with the Benjamin-Hochberg post-hoc test.
RESULTS
Twenty subjects (11 males, 9 females) that were 4.6 (4 – 5.6) weeks post-transplant were enrolled and randomized to either usual care (n=10) or hyperbaric oxygen therapy (n=10). Age and sex distributions were comparable between the groups: 54.5 (36 – 65) vs. 59.7 (41 – 62) years, and 50% vs. 40% female, respectively. Individual subject characteristics are shown in Table 1. Two subjects were screened but not randomized due to transplant-related complications developed prior to study activities (seizures, n=1; anastomotic dehiscence, n=1). The most common indications for transplant were interstitial lung disease (n=8) and cystic fibrosis (n=7). All but three subjects underwent BOLT (left SOLT, n=1; right SOLT, n=2). All patients had undergone evaluation for gastroesophageal reflux and esophageal dysmotility as part of their routine transplant evaluation. The groups were comparable with respect to gastroesophageal disorders (Table S2).
Table 1.
Patient Characteristics
| Subject ID | Age | Sex | Lung Disease | Donor Lung | Weeks post-transplant | Group Assignment | Necrosis Score (Right/Left) |
|---|---|---|---|---|---|---|---|
| 001 | 58 | M | IPF | Bilateral | 3.9 | Usual care | 3/2 |
| 002 | 67 | M | IPF | Right | 4.6 | Hyperbaric oxygen | 3/0 |
| 003 | 78 | M | IPF | Right | 2.6 | Usual care | 4/0 |
| 004 | 50 | F | CF | Bilateral | 4.0 | Usual care | 3/3 |
| 005 | 20 | F | CF | Bilateral | 3.3 | Screened, not enrolled | 2/1 |
| 006 | 31 | M | CF | Bilateral | 4.1 | Usual care | 2/1 |
| 007 | 19 | M | CF | Bilateral | 2.3 | Usual care | 3/3 |
| 008 | 60 | M | A1AT deficiency | Bilateral | 8.3 | Hyperbaric oxygen | 3/1 |
| 009 | 63 | F | COPD | Bilateral | 7.7 | Usual care | 3/3 |
| 010 | 66 | F | IPF | Left | 3.6 | Usual care | 0/4 |
| 011 | 37 | F | CF | Bilateral | 6.9 | Hyperbaric oxygen | 3/1 |
| 012 | 29 | F | CF | Bilateral | 5.1 | Usual care | 3/3 |
| 013 | 58 | M | Chronic HP | Bilateral | 4.7 | Hyperbaric oxygen | 4/3 |
| 014 | 73 | M | IPF | Bilateral | 4.3 | Usual care | 3/3 |
| 015 | 51 | F | IPF | Bilateral | 5.6 | Hyperbaric oxygen | 1/3 |
| 016 | 31 | F | CF | Bilateral | 7.3 | Hyperbaric oxygen | 3/1 |
| 017 | 49 | F | Kartagener’s syndrome | Bilateral | 4.9 | Usual care | 2/3 |
| 018 | 62 | F | COPD | Bilateral | 3.9 | Hyperbaric oxygen | 4/3 |
| 019 | 76 | M | COPD | Bilateral | 5.6 | Hyperbaric oxygen | 3/3 |
| 020 | 30 | M | CF | Bilateral | 4.6 | Hyperbaric oxygen | 4/3 |
| 021 | 30 | M | GVHD/drug toxicity | Bilateral | 3.9 | Screened, not enrolled | 4/4 |
| 022 | 60 | M | IPF | Bilateral | 4.1 | Hyperbaric oxygen | 3/3 |
Abbreviations: A1AT, Alpha-1-antitrypsin; COPD, chronic obstructive pulmonary disease; CF, cystic fibrosis; F, female; GVHD, graft-versus-host-disease; HP, hypersensitivity pneumonitis; IPF, idiopathic pulmonary fibrosis; M, male.
Additional post-transplant clinical factors are shown in Table S3. There were no significant differences between groups in ischemic times, primary graft dysfunction (PGD) severity21, vasopressor-free days, usage of ECMO post-transplant, ventilator-free days, tracheostomy-free days, or time to readmission (all p>0.05). However, despite randomization, the number of oxygen-free days was significantly lower in the Usual Care group compared with the HBOT group (p<0.05).
For subjects randomized to HBOT, time to treatment was 8.5 (6 – 13) days after randomization. All subjects completed twenty HBOT sessions, except for one subject who discontinued HBOT after five sessions due to claustrophobia. Due to poorly resolving bronchial mucosal necrosis, two subjects that had randomized to usual care were offered to crossover to HBOT, one of which was agreeable and completed twenty HBO treatments.
Patient outcomes are shown in Table 2. After enrolling twenty patients, the trial was stopped after a pre-planned interim analysis showed no difference between groups in incidence of airway stenting, acute cellular rejection, CAS, airway balloon dilation, anastomotic dehiscence, airway infections, or CLAD (Table 2 and Table S4). Plaque scores were identical between both groups over time (Figure 1A). While the time to first dilation was also similar between usual care and HBOT groups (98 [93 – 128] vs. 97 [87 – 126] days, respectively) (Figure 1B), the time to stent placement was significantly shorter in the HBOT group (150 [73 – 150] vs. 186 [167 – 206] days, P<0.05) (Figure 1C). One subject (# 001) in the usual care group underwent stent placement for right mainstem bronchomalacia but did not require balloon dilation. A secondary analysis showed that oxygen-free days did not correlate with the need for stent placement, development of CAS, plaque score severity, or plaque score change over time.
Table 2.
Primary and Secondary Endpoints
| Co-Primary Endpoints | Secondary Endpoints | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group Assignment | Stent | ACR | GS | Dehiscencea | Infectionsb | BM | CAS | Dilation | CLAD |
| Usual care, n (%) | 4 (40) | 7 (70) | 1 (10) | 1 (10) | 3 (30%) | 2 (20) | 4 (40) | 3 (30) | 1 (10) |
| Hyperbaric oxygen, n (%) | 4 (40) | 4 (40) | 0 (0) | 1 (10) | 5 (50%) | 1 (10) | 6 (60) | 6 (60) | 0 (0) |
Abbreviations: ACR, acute cellular rejection; CAS, central airway stenosis due to fibrotic strictures; CLAD, chronic lung allograft dysfunction; GS, granulomatous strictures; BM, bronchomalacia.
Includes full thickness mucosal breakdown.
Airway infections at 10 weeks based on bronchoalveolar lavage fluid culture. See Table S3 for additional details on microbiology at all time points.
Figure 1: Clinical outcomes.
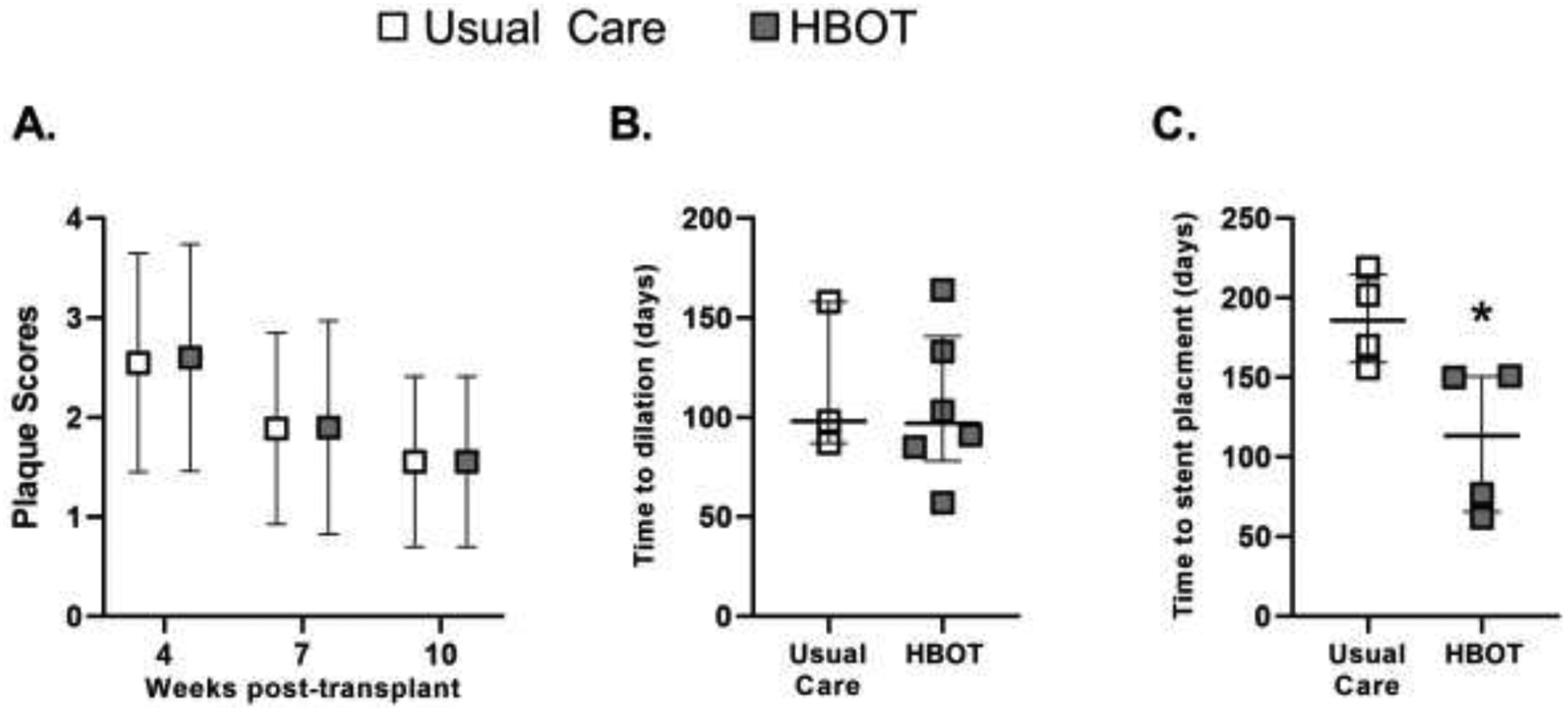
(A) Plaque scores (mean ± SD) over time for Usual care (open boxes) and HBOT (grey boxes) groups. (B) Time to first balloon airway dilation (median and IQR). (C) Time to first stent placement (median and IQR). *P<0.05 by Mann Whitney test.
Based on previous work that identified associations between hypoxia-inducible gene expression and post-transplant airway complications6, we performed quantitative PCR on bronchial mucosal biopsies for select hypoxia-inducible genes. Donor/native tissue expression ratios were significantly >1 for FLT1, HMOX1, and TGBF1 at 4 and 7 weeks, and for VEGFA at 4, 7, and 10 weeks (all P<0.05) (Figure 2). We also found elevated ratios for KDR at 7 and 10 weeks (P<0.05).
We next compared gene expression between the HBOT and usual care groups to determine if HBOT exposure altered hypoxic gene expression (Figure 3). Despite the randomization procedure, we initially found baseline differences in gene expression of FLT1 and TGFB1 at 4 weeks, prior to the initiation of HBOT. However, when accounting for oxygen-free days, these differences were no longer apparent. No other differences in mRNA levels of the measured genes (FLT1, HMOX1, KDR, TIE2, VEGFA, or TGFB1) were observed between the usual care and HBOT groups.
We then analyzed gene expression as a function of development of CAS and need for stent placement. We found significantly higher expression of HMOX1 and VEGFA (P<0.05) at 4 weeks post-transplantation in subjects that would go on to develop CAS (Figure 4) and in subjects that required airway stenting (Figure 5). Furthermore, expression of KDR, the VEGFA receptor 2, was significantly lower at 10 weeks post-transplant in subjects that required airway stenting (P<0.05). An HMOX1 donor/native gene expression ratio of > 40 was poorly sensitive for the development of CAS or need for stenting (36% and 38%, respectively) but 100% specific with a positive predictive value of 100%. Similarly, VEGFA donor/native gene expression ratio > 5 was poorly sensitive for development of CAS or need for stenting (43% and 46%, respectively), but 100% specific with a positive predictive value of 100%. Finally, we analyzed gene expression as a function of development of acute cellular rejection. There were significantly higher mRNA levels of FLT1, KDR, and TEK (all P<0.05) at 4 weeks post-transplant in subjects that would develop rejection compared with subjects that would not (Figure 6). However, there were no differences noted at 7 or 10 weeks post-transplant.
Figure 4: Gene expression of subjects by Central Airway Stenosis status.
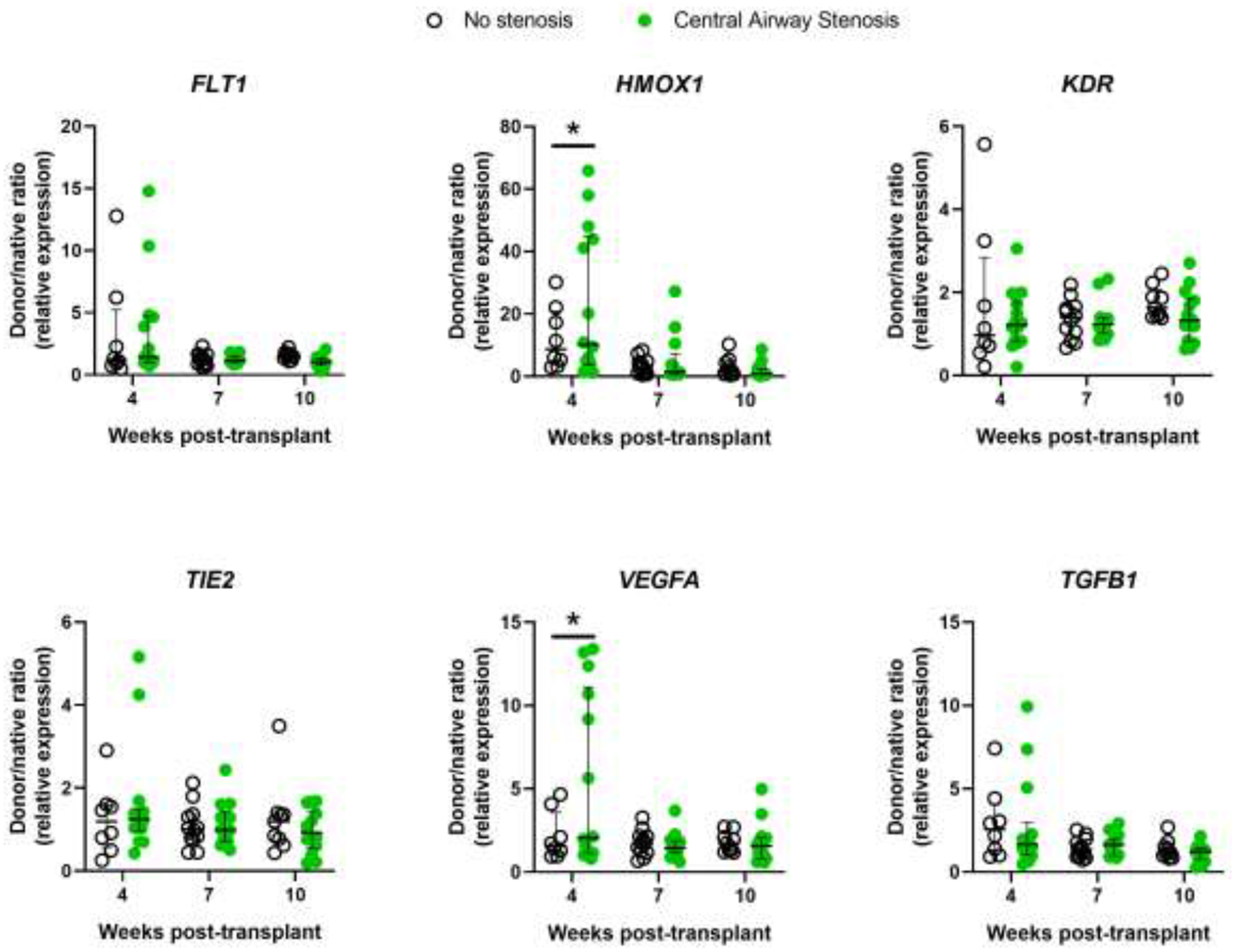
Bronchial mucosal gene expression shown as donor/native ratio (relative to 18S mRNA) in subjects without central airway stenosis (open circles) and with central airway stenosis (green circles) for FLT1, HMOX1, KDR, TIE2, VEGFA, and TGFB1 at 4, 7, and 10 weeks post-transplant. Error bars are median with IQR. *P<0.05 for group differences within each time point by 2-way ANOVA with the Benjamin-Hochberg post-hoc test.
Figure 5: Gene expression of subjects by Airway Stent status.
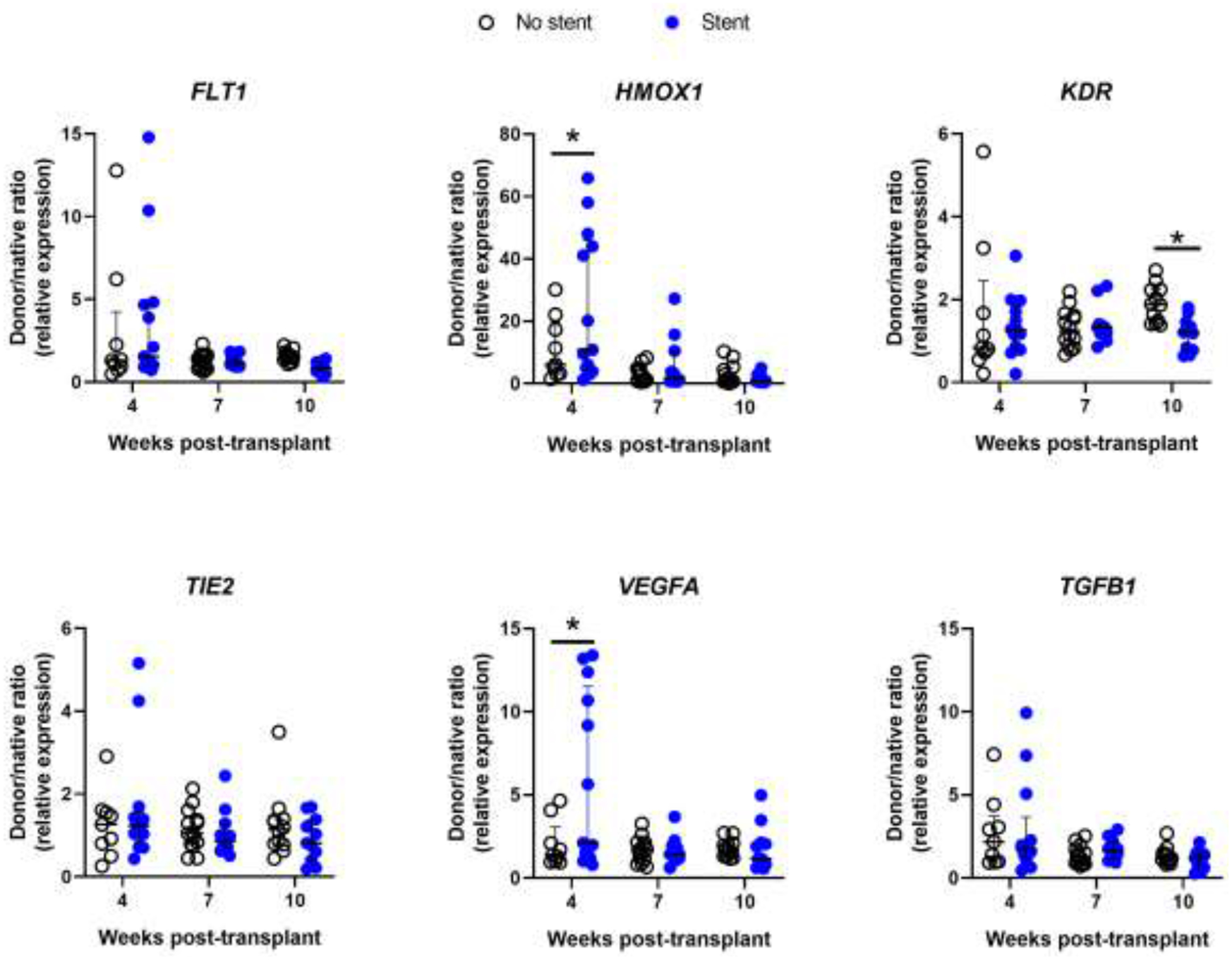
Bronchial mucosal gene expression shown as donor/native ratio (relative to 18S mRNA) in subjects without airway stents (open circles) and with airway stents (blue circles) for FLT1, HMOX1, KDR, TIE2, VEGFA, and TGFB1 at 4, 7, and 10 weeks post-transplant. Error bars are median with IQR. *P<0.05 for group differences within each time point by 2-way ANOVA with the Benjamin-Hochberg post-hoc test.
DISCUSSION
We report the first randomized, controlled trial of hyperbaric oxygen therapy compared with usual care to treat established airway necrosis after lung transplantation. While many clinical factors are known to contribute to ischemic airway complications, such as hypotension, prolonged mechanical ventilation, infection, PGD, and ACR1,2,6,22–24, our hypothesis was that HBOT might improve donor bronchial mucosal healing and reduce the incidence of CAS and need for stenting. However, the study was terminated early for futility, as there was no difference in incidence of airway complications or acute cellular rejection. However, we did find a significantly shorter time to stent placement in the HBOT group. We also validated two mRNA biomarkers (HMOX1 and VEGFA) associated with development of airway complications, and discovered three novel RNA biomarkers (FLT1, KDR, and TIE2) associated with development of acute cellular rejection.
Our study rationale was that HBOT is safe10, has been reported to promote healing in native airways following tracheobronchial resections14–17, and therefore may be useful in post-transplant donor airway ischemia. Lung transplantation is unlike other solid organ transplant procedures as there is no anastomosis of the arterial (bronchial) blood supply. This renders the transplanted lung dependent on low flow, retrograde perfusion from the poorly oxygenated pulmonary circulation8, which predisposes to donor airway ischemia, a leading risk factor for airway complications4–8. This system mimics ischemic grafts and flaps, where HBOT has been shown to improve oxygen delivery, recruit endothelial stem cells, promote neovascularization and fibroblast proliferation, and reduce inflammation12,13,25,26. Therefore, we performed a randomized, controlled trial of HBOT vs. usual care in lung transplant patients with established airway necrosis. However, we found no difference between groups in incidence of airway complications, such as CAS, need for balloon dilation, need for airway stenting, infection, or dehiscence, and no difference in development of ACR or CLAD. We chose ACR as a co-primary outcome to monitor for any effect of HBOT on ACR in either direction: HBOT has immunosuppressive effects and has been studied as a treatment for ACR in other solid organ allografts27; however, T-cell metabolism is largely oxidative28 and we could not rule out that lung T-cells could become activated after hyperoxic exposure. Because we only enrolled subjects with severe, established airway necrosis that persisted at 4 weeks post-transplant, it is possible that the donor airway injury was too advanced for HBOT to have had any effects. Future studies could administer HBOT as soon as post-transplant airway necrosis is recognized, or even preemptively to all patients post-operatively16.
Our study did identify several notable clinical findings. First, the incidence of CAS in our patient population that was enriched for airway necrosis was 40–60%, much higher than the generally reported incidence of 10–15%2,3, further strengthening the relationship between airway ischemia, necrosis, and stenosis. Second, the incidence of airway stenting was the same between groups, but occurred significantly sooner in the HBOT group. The reason for this is not entirely clear, but was seen in our earlier case-control study as well10, and is suggestive of an accelerated fibrotic healing response in ischemic airway tissues by HBOT similar to that seen in other tissues12,26,29.
We have previously shown that mRNA levels of HIF-dependent genes, such as VEGFA, FLT1, HMOX1, were upregulated in ischemic donor airways at 4 weeks and associated with post-transplant respiratory complications6. In this study, we again found mRNA levels of these genes were higher at 4 week. We also found they remained elevated through 10 weeks post-transplant, longer than was previously known. We further hypothesized that by augmenting oxygen delivery, HBOT might down-regulate donor bronchial mucosal HIF-dependent gene expression, although we found no such effect on the measured genes. However, we did find that HMOX1 and VEGFA mRNA levels were significantly associated with the development of CAS and need for stent placement. Taken together, these findings validate our earlier observations6, and suggest the HIF pathway may be a viable target for pharmacologic intervention7.
Prior studies have shown chronic rejection (CLAD) is associated with small airway ischemia30–33, and activation of the HIF-1α-VEGFA-VEGFR2 (KDR) pathway34. Moreover, experimental CLAD is attenuated by HIF-1α transfection33, suggesting this pathway is not merely a biomarker for disease but is involved in pathogenesis. While we did not show a significant difference between the HBOT and usual care groups in development of CLAD (0% vs. 10%, respectively), our study only followed subjects for one year, where the incidence of CLAD is still low. Therefore, we are limited in assessing whether HBOT affected CLAD incidence.
Prior studies have correlated gene expression in various lung tissues (e.g. bronchoalveolar lavage cells, transbronchial biopsy, lung explant) with ACR35 and CLAD36–38. This work has focused primarily on pro-fibrotic and immunologic genes. Our study focused on hypoxic gene expression and is the first to show associations between ACR and angiogenic markers, such as the VEGFA pathway (KDR and FLT1), and TIE2 expression. TIE2 is an angiogenic cell surface marker previously associated with chronic renal allograft rejection39 and CLAD33,40, but is a novel finding for ACR. Our study is limited due to measuring gene expression in large airways rather than small ones where acute cellular rejection occurs. However, we hypothesize that these small airways display hypoxic gene expression too, especially 1) those with wall thickness > 1 mm, which is the maximum distance that oxygen diffuses41; and 2) because they are perfused (down to respiratory bronchioles) by the bronchial arteries42 which are sacrificed at transplant. However, these findings are hypothesis generating and require confirmation in future lung ACR studies.
Our study has several limitations. First, it was not possible to use a blinded study design, which may have biased the results, particularly the use of stents. However, the randomization procedure itself was blinded and our stent insertion followed a strict protocol (see Methods) which would limit this. Second, despite the randomization step, there were still imbalances between the groups with respect to oxygen-free days. However, as the Usual Care group had fewer oxygen-free days, this would have biased the study in favor of the HBOT intervention, which we did not see.
In conclusion, the study provided no support for the use of HBOT to reduce airway complications in lung transplant patients with established airways ischemia. Higher levels of HMOX1 and VEGFA RNA expression at 4 weeks appeared to be associated with increasing airways complications, such as CAS and need for stenting, irrespective of whether receiving HBOT or not, and may be pharmacologic targets for airway-directed therapy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the chamber operators, nurses, and physicians at the Duke Center for Hyperbaric Medicine and Environmental Physiology for assistance with this study, and thank the Duke Lung Transplant clinical coordinators and patient care providers.
Funding: Duke Pulmonary and Duke Hyperbaric Center Divisional funds, and NHLBI (K08HL130557, PI: Kraft).
FINANCIAL CONFLICTS OF INTEREST:
The study was funded by Duke Pulmonary Divisional funds and Duke Hyperbaric Center funds. Dr. Kraft reports additional funding from the NIH/NHLBI (K08HL130557). None of the funding sources had any role in data collection, analysis, or interpretation, or in approving or disapproving of the manuscript. The authors have no other conflicts of interest pertinent to the submitted work.
GLOSSARY
- A1AT
Alpha-1-anti-trypsin
- ACR
Acute cellular rejection
- ANOVA
Analysis of Variance
- ATA
Atmospheres absolute
- BM
Bronchomalacia
- BOLT
Bilateral orthotopic lung transplant
- CAS
Central Airway Stenosis
- cDNA
Complimentary DNA
- CF
Cystic fibrosis
- CLAD
Chronic lung allograft dysfunction
- COPD
Chronic obstructive pulmonary disease
- DCD
Donors after cardiac death
- ECMO
Extracorporeal membrane oxygenation
- EVLP
Ex vivo lung perfusion
- FiO2
Fraction of inspired oxygen
- FLT1
Vascular Endothelial Growth Factor A Receptor 1
- GS
Granulomatous stricture
- GVHD
Graft-versus-host disease
- HBOT
Hyperbaric oxygen therapy
- HIF
Hypoxia-inducible factor
- HMOX1
Heme oxygenase-1
- HP
Hypersensitivity pneumonitis
- IPF
Idiopathic pulmonary fibrosis
- IQR
Interquartile range
- KDR
Vascular Endothelial Growth Factor A Receptor 2
- mRNA
Messenger ribonucleic acid
- PCR
Polymerase chain reaction
- PDS
Polydioxanone suture
- PGD
Primary graft dysfunction
- RNA
Ribonucleic acid
- rRNA
Ribosomal ribonucleic acid
- SOLT
Single orthotopic lung transplant
- TGFB1
Transforming growth factor beta-1
- TIE2
Angiopoietin Receptor 1
- VEGFA
Vascular Endothelial Growth Factor A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Castleberry AW, Worni M, Kuchibhatla M, et al. A comparative analysis of bronchial stricture after lung transplantation in recipients with and without early acute rejection. The Annals of thoracic surgery. 2013;96(3):1008–1017; discussion 1017–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shofer SL, Wahidi MM, Davis WA, et al. Significance of and risk factors for the development of central airway stenosis after lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(2):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzetta A, Porzio M, Riou M, et al. Patients Treated for Central Airway Stenosis After Lung Transplantation Have Persistent Airflow Limitation. Ann Transplant. 2019;24:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhillon GS, Zamora MR, Roos JE, et al. Lung transplant airway hypoxia: a diathesis to fibrosis? American journal of respiratory and critical care medicine. 2010;182(2):230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkes DS. Airway hypoxia, bronchiolar artery revascularization, and obliterative bronchiolitis/bronchiolitis obliterans syndrome: are we there yet? American journal of respiratory and critical care medicine. 2010;182(2):136–137. [DOI] [PubMed] [Google Scholar]
- 6.Kraft BD, Suliman HB, Colman EC, et al. Hypoxic Gene Expression of Donor Bronchi Linked to Airway Complications After Lung Transplantation. American journal of respiratory and critical care medicine. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasnupneti S, Nicolls MR. Airway hypoxia in lung transplantation. Curr Opin Physiol. 2019;7:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crespo MM, McCarthy DP, Hopkins PM, et al. ISHLT Consensus Statement on adult and pediatric airway complications after lung transplantation: Definitions, grading system, and therapeutics. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2018;37(5):548–563. [DOI] [PubMed] [Google Scholar]
- 9.Pettersson GB, Karam K, Thuita L, et al. Comparative study of bronchial artery revascularization in lung transplantation. The Journal of thoracic and cardiovascular surgery. 2013;146(4):894–900 e893. [DOI] [PubMed] [Google Scholar]
- 10.Mahmood K, Kraft BD, Glisinski K, et al. Safety of hyperbaric oxygen therapy for management of central airway stenosis after lung transplant. Clin Transplant. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon RE, Camporesi EM. Hyperbaric oxygen therapy: from the nineteenth to the twenty-first century. Respir Care Clin N Am. 1999;5(1):1–5. [PubMed] [Google Scholar]
- 12.Baynosa RC, Zamboni WA. The effect of hyperbaric oxygen on compromised grafts and flaps. Undersea Hyperb Med. 2012;39(4):857–865. [PubMed] [Google Scholar]
- 13.Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127 Suppl 1:131S–141S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickhoff C, Daniels JM, van den Brink A, Paul MA, Verhagen AF. Does hyperbaric oxygen therapy prevent airway anastomosis from breakdown? The Annals of thoracic surgery. 2015;99(2):682–685. [DOI] [PubMed] [Google Scholar]
- 15.Stock C, Gukasyan N, Muniappan A, Wright C, Mathisen D. Hyperbaric oxygen therapy for the treatment of anastomotic complications after tracheal resection and reconstruction. The Journal of thoracic and cardiovascular surgery. 2014;147(3):1030–1035. [DOI] [PubMed] [Google Scholar]
- 16.Endoh M, Oizumi H, Kato H, et al. Hyperbaric oxygen therapy for postoperative ischemic bronchitis after resection of lung cancer. J Thorac Dis. 2018;10(11):6176–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benhamed L, Bellier J, Fournier C, et al. Postoperative ischemic bronchitis after lymph node dissection and primary lung cancer resection. The Annals of thoracic surgery. 2011;91(2):355–359. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi T, Oto T, Millar IL, Levvey BJ, Williams TJ, Snell GI. Preliminary report of the safety and efficacy of hyperbaric oxygen therapy for specific complications of lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2006;25(11):1302–1309. [DOI] [PubMed] [Google Scholar]
- 19.Hartwig MG, Snyder LD, Finlen-Copeland A, et al. Lung transplantation at Duke University. Clinical transplants. 2009:197–210. [PubMed] [Google Scholar]
- 20.Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2019;38(5):493–503. [DOI] [PubMed] [Google Scholar]
- 21.Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(10):1454–1459. [DOI] [PubMed] [Google Scholar]
- 22.Olland A, Reeb J, Puyraveau M, et al. Bronchial complications after lung transplantation are associated with primary lung graft dysfunction and surgical technique. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2017;36(2):157–165. [DOI] [PubMed] [Google Scholar]
- 23.Dark JH. Pathophysiology and Predictors of Bronchial Complications After Lung Transplantation. Thorac Surg Clin. 2018;28(3):357–363. [DOI] [PubMed] [Google Scholar]
- 24.Yserbyt J, Dooms C, Vos R, Dupont LJ, Van Raemdonck DE, Verleden GM. Anastomotic airway complications after lung transplantation: risk factors, treatment modalities and outcome-a single-centre experience. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2016;49(1):e1–8. [DOI] [PubMed] [Google Scholar]
- 25.Hopf HW, Gibson JJ, Angeles AP, et al. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13(6):558–564. [DOI] [PubMed] [Google Scholar]
- 26.Sander AL, Henrich D, Muth CM, Marzi I, Barker JH, Frank JM. In vivo effect of hyperbaric oxygen on wound angiogenesis and epithelialization. Wound Repair Regen. 2009;17(2):179–184. [DOI] [PubMed] [Google Scholar]
- 27.Muralidharan V, Christophi C. Hyperbaric oxygen therapy and liver transplantation. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2007;9(3):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brismar K, Lind F, Kratz G. Dose-dependent hyperbaric oxygen stimulation of human fibroblast proliferation. Wound Repair Regen. 1997;5(2):147–150. [DOI] [PubMed] [Google Scholar]
- 30.Luckraz H, Goddard M, McNeil K, Atkinson C, Sharples LD, Wallwork J. Is obliterative bronchiolitis in lung transplantation associated with microvascular damage to small airways? The Annals of thoracic surgery. 2006;82(4):1212–1218. [DOI] [PubMed] [Google Scholar]
- 31.Luckraz H, Goddard M, McNeil K, et al. Microvascular changes in small airways predispose to obliterative bronchiolitis after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2004;23(5):527–531. [DOI] [PubMed] [Google Scholar]
- 32.Babu AN, Murakawa T, Thurman JM, et al. Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis. The Journal of clinical investigation. 2007;117(12):3774–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X, Khan MA, Tian W, et al. Adenovirus-mediated HIF-1alpha gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. The Journal of clinical investigation. 2011;121(6):2336–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Abuduwufuer A, Lv W, et al. The role of HIF-1alpha-VEGF pathway in bronchiolitis obliterans after lung transplantation. J Cardiothorac Surg. 2019;14(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patil J, Lande JD, Li N, Berryman TR, King RA, Hertz MI. Bronchoalveolar lavage cell gene expression in acute lung rejection: development of a diagnostic classifier. Transplantation. 2008;85(2):224–231. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee B, Ling KM, Sutanto EN, et al. The airway epithelium is a direct source of matrix degrading enzymes in bronchiolitis obliterans syndrome. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30(10):1175–1185. [DOI] [PubMed] [Google Scholar]
- 37.Sacreas A, Yang JYC, Vanaudenaerde BM, et al. The common rejection module in chronic rejection post lung transplantation. PloS one. 2018;13(10):e0205107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonigk D, Izykowski N, Rische J, et al. Molecular Profiling in Lung Biopsies of Human Pulmonary Allografts to Predict Chronic Lung Allograft Dysfunction. The American journal of pathology. 2015;185(12):3178–3188. [DOI] [PubMed] [Google Scholar]
- 39.Ma X, Lu YP, Yang L, et al. Expressions of Angiopoietin-1, Angiopoietin-2, and Tie2 and their roles in rat renal allografts with chronic allograft nephropathy. Transplantation proceedings. 2008;40(8):2795–2799. [DOI] [PubMed] [Google Scholar]
- 40.Jiang X, Hsu JL, Tian W, et al. Tie2-dependent VHL knockdown promotes airway microvascular regeneration and attenuates invasive growth of Aspergillus fumigatus. Journal of molecular medicine. 2013;91(9):1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neuman TS, Thom SR. Physiology and medicine of hyperbaric oxygen therapy. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 42.Pump KK. The bronchial arteries and their anastomoses in the human lung. Dis Chest. 1963;43:245–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


