Abstract
Elimination of both rapidly dividing epithelial mammary cancer cells as well as breast cancer stem-like cells (bCSC) is essential for maximizing antitumor response. Withaferin A (WA), a small molecule derived from a medicinal plant (Withania somnifera), is highly effective in reducing burden and/or incidence of breast cancer in vivo in various preclinical models. We have shown previously that suppression of breast cancer incidence by WA administration in a rat model is associated with a decrease in self-renewal of bCSC but the underlying mechanism is still elusive. This study investigated the role of forkhead box Q1 (FoxQ1) transcription factor in antitumor responses to WA. Exposure of MDA-MB-231 and SUM159 cells to WA resulted in downregulation of protein and mRNA levels of FoxQ1 as well as inhibition of its transcriptional activity. FoxQ1 overexpression in SUM159 and MCF-7 cells resulted in a marked protection against WA-mediated inhibition of bCSC as judged by flow cytometric analysis of CD49fhigh population and mammosphere assay. RNA-seq analysis revealed upregulation of many bCSC-associated genes by FoxQ1 overexpression in SUM159 cells, including interleukin-8 (IL-8) whose expression was decreased by WA treatment in SUM159 and MCF-7 cells. FoxQ1 was recruited to the promoter of IL-8 that was inhibited significantly by WA treatment. On the other hand, WA-mediated inhibition of cell proliferation or migration was not affected by FoxQ1 overexpression. The FoxQ1 overexpression partially attenuated WA-mediated G2/M phase cell cycle in SUM159 cells only. These results indicate that FoxQ1 is a target of WA for inhibition of bCSC fraction.
Keywords: withaferin A, FoxQ1, breast cancer stem-like cells
Introduction
Medicinal plants including Withania somnifera continue to be used in various formulations of Ayurvedic medicine that is still very popular in South Asian countries including India (1). Clinical studies have been conducted to determine the safety and pharmacological effects of Withania somnifera extract (2–6). Clinical effects of Withania somnifera extract have been studied on memory and cognitive function, reproductive system, hormonal effects in aging and overweight men, etc. (2–6). The extract of this medicinal plant also seems beneficial against many human illnesses including epilepsy, depression, arthritis, and diabetes (7). While clinical trials to determine potential antitumor effects of Withania somnifera extract are yet to be undertaken, this plant extract is highly effective in suppressing tumor growth in preclinical models (8–11). One such study reported a 55% complete response rate in Balb/c mice intradermally injected with sarcoma 180 cells and treated daily intraperitoneally with an alcoholic extract of the root of Withania somnifera (1000 mg/kg body weight dose) (9). In a preclinical murine model of fibrosarcoma induced by 20-methylcholanthrene, administration of the root extract of Withania somnifera (20 mg/mouse by intraperitoneal route) not only decreased disease development but also increased survival (10). Widodo et al. (11) reported selective killing of cancer cells using the leaf extract of Withania somnifera. Oral administration of the alcoholic root extract of Withania somnifera (400 mg/kg body weight) inhibited 7,12-dimethylbenz[a]anthracene-induced skin cancer development in Swiss albino mice (12). These preclinical observations are promising and justify future clinical trials to determine antitumor effect of this medicinal plant.
More recent studies have focused on one or more small molecule constituents of Withania somnifera for the in vivo preclinical efficacy determinations as well as cellular mechanistic studies (reviewed in 13). Structurally diverse small molecules, including withanolides, alkaloids, and sitoindosides have been isolated from the extract of Withania somnifera but withanolides have been most extensively investigated for anticancer properties. Among different withanolides [e.g., withaferin A (WA), withanone, withanolide A], the WA seems most effective in reducing the growth of tumor cells. In one such study, the antitumor effect of WA, withanone, and withanolide A were compared using human breast cancer cell lines (14). This study reported mitotic growth arrest in breast cancer cells belonging to different subtypes following WA treatment, but no such effect was observed with withanone or withanolide A (14). In another study, a combination of withanolides was highly effective in selective killing of cancer cells (15).
While antitumor effects of WA have been studied using cellular and animal models of different solid tumors, this phytochemical is relatively more extensively characterized in breast cancer (13, 16). The WA was shown to inhibit growth of human breast cancer cell lines by causing mitotic arrest and apoptosis induction (14, 17). Interestingly, normal mammary epithelial cells (MCF-10A and HMEC) were resistant to mitotic arrest and apoptosis induction resulting from WA treatment (14, 17). The WA administration was shown to inhibit in vivo growth of MDA-MB-231 human breast cancer cell line orthotopically implanted in female nude mice (18). A decrease in mammary cancer incidence (primary prevention) and/or wet tumor weight was observed following WA administration in MMTV-neu transgenic mice and in a rat model of breast cancer induced by N-methyl-N-nitrosourea (MNU) (19, 20).
In recent years, experimental evidence has accumulated to implicate breast cancer stem-like cells (bCSC) in cancer development as well as in therapy resistance (21). We have reported previously that WA administration inhibits self-renewal of bCSC in vitro and in vivo (20, 22). However, the mechanism underlying bCSC inhibition by WA is still not fully understood. In our previously published study, we also found that inhibition of bCSC population by WA administration in the MNU-rat model was accompanied by a marked decrease in protein level of forkhead box Q1 (FoxQ1) transcription factor, which has emerged as a key regulator of bCSC maintenance (23, 24). Therefore, the present study was logically undertaken to determine the potential role of FoxQ1 in WA-mediated inhibition of self-renewal of bCSC.
Materials and Methods
Ethics statement
Plasma and mammary tumors from our previously published study (20), which was approved by the University of Pittsburgh Animal Care and Use Committee, were used to determine the effect of WA administration on levels of interleukin-8 (IL-8).
Reagents and cell lines
The WA (purity > 95%) was purchased from ChromaDex (Irvine, CA) and dissolved in dimethyl sulfoxide (DMSO). Reagents for cell culture, including fetal bovine serum, cell culture media, and antibiotic mixture were purchased from Life Technologies-Thermo Fisher Scientific (Waltham, MA). An antibody against FoxQ1 was purchased from Proteintech (Rosemont, IL) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was from Genetex (Irvine, CA). An antibody against β-Actin was from Sigma-Aldrich (St. Louis, MO). The MDA-MB-231 and MCF-7 cell lines were purchased from the American Type Culture Collection (Manassas, VA), whereas SUM159 cell line was from Asterand Bioscience (Detroit, MI). Each cell line was last authenticated by us in 2017 by short tandem repeat profiling. Each cell line was cultured according to the supplier’s recommendations. Details of stable transfection of MCF-7 and SUM159 cells with pCMV6 empty vector and the same vector encoding FoxQ1 have been described by us previously (24).
Western blotting
Cell lysate from DMSO-treated control cells or WA-treated cells were prepared as described by us previously (25). Western blotting was performed as described by us previously (19, 25). The blots were stripped and re-probed with anti-GAPDH or anti-β-Actin antibody for protein normalization. The change in protein level was determined by densitometric quantitation using UN-SCAN-IT software (Silk Scientific, Orem, UT).
Quantitative real-time polymerase chain reaction (qRT-PCR) assay
Total RNA from control and WA-treated cells was extracted using RNeasy kit from Qiagen (Germantown, MD) and cDNA was synthesized using Superscript Reverse Transcriptase (Invitrogen-Life Technologies) with oligo (dT)20 primer. The qRT-PCR was performed using 2× SYBR green qPCR kit (Thermo Fisher Scientific) with 95°C (15 seconds), 60°C (30 seconds), and 72°C (20 seconds) for 40 cycles. Primer for FoxQ1 was purchased from GeneCopoeia (Rockville, MD). GAPDH was used as a normalization control with the following primers: Forward: 5’- GGACCTGACCTGCCGTCTAGAA-3’; Reverse; 5’-GGTGTCGCTGTTGAAGTCAGAG-3’.
Relative gene expression was calculated by the method of Livak and Schmittgen (26).
Luciferase reporter assay
Cells were transfected with FoxQ1-luciferase reporter (GeneCopoeia) plasmid using FuGENE HD (Promega, Madison, WI) as a transfection reagent. After 24 hours of transfection, the cells were treated with either DMSO or different concentrations of WA for 24 hours. Luciferase activity was determine using Secrete-Pair Gaussia Luciferase assay kit (Genecopoeia) as recommended by the supplier.
Flow cytometric analysis of CD49fhigh population
The control (DMSO-treated) and WA-treated cells were incubated with anti-CD49f (PE-conjugated) antibody in the dark for 1 hour at room temperature. The cells were washed with phosphate-buffered saline (PBS), and then analyzed using a BD Accuri ™ C6 flow cytometer (BD Bioscience, San Jose, CA).
Mammosphere formation assay
Mammosphere assay was done as described by us previously (22). Briefly, one thousand cells were plated in 24-well ultralow attachment plates in medium containing B27, insulin, hydrocortisone, epidermal growth factor, basic fibroblast growth factor, 2-mercaptoethanol, and methylcellulose. Desired concentration of WA was then added to the plates. After a 5-day (MCF-7) or 7-day (SUM159) incubation, the mammospheres were imaged and scored under an inverted microscope.
Measurement of IL-8 levels
Secreted level of IL-8 in the conditioned media of cells was determined using a kit from R&D systems (Minneapolis, MN) according to the manufacturer’s instructions. In brief, cells (SUM159– 4 × 105 cells/6-cm dish and MCF-7– 1 × 106 cells/10-cm dish) were plated in triplicate, incubated overnight, and then treated with DMSO or WA (0.1 and 0.2 μmol/L) in serum-free media. After 24 hours, the medium was collected for IL-8 measurement. Rat plasma and tumor IL-8 levels were measured using a kit from MyBioSource (San Diego, CA) and by following the supplier’s instructions.
Chromatin immunoprecipitation assay
The chromatin immunoprecipitation (ChIP) assay was performed as described by us previously (24) by using Magnetic ChIP kit (Pierce) and by following the manufacturer’s protocol. Normal mouse IgG and FoxQ1 antibodies were used for chromatin immunoprecipitation. The FoxQ1 binding sites in the IL-8 promoter were amplified (60°C, 1 minute, 40 cycles) with the following region-specific primers: Site 1 (forward) 5′-ATGCACTGTGTTCCGTATGC-3′ and (reverse) 5′-GCTTTGCTAGTACAGGACAGG −3′, and site 3 (forward) 5′-TGCTTTCTTCTTCTGATAGACCA −3′ and (reverse) 5′-TGTTAACAGAGTGAAGGGGCA-3′. Fold enrichment was normalized to the input.
Determination of cell cycle distribution and mitotic fraction
The cells were treated with DMSO or WA for 24 hours, washed with PBS, harvested by trypsinization followed by fixation in 70% ethanol overnight at 4°C. Fixed cells were washed with PBS, stained with propidium iodide (PI, 50 μg/mL) and RNase A (80 μg/mL) for 30 minutes, and then analyzed using a BD Accuri™ C6 flow cytometer. Mitotic fraction was determined by flow cytometric analysis as described by us previously (14). Briefly, control and WA-treated cells were collected and fixed in 70% ethanol at 4°C for 2 hours and then permeabilized with 0.25% Triton X-100 for 15 minutes, followed by incubation with Alexa Fluor 488-conjugated phospho-(Ser10) histone H3 antibody. After 1 hour of incubation, the cells were stained with PI (50 μg/mL) and RNase A (80 μg/mL) for 30 minutes at room temperature. Stained cells were analyzed using BD Accuri™ C6 flow cytometer.
Cell proliferation assay
Cell proliferation was performed using CellTiter 96® AQueous Non-Radioactive Cell Proliferation assay kit from Promega according to the manufacturer’s instructions.
Cell migration assay
Transwell Boyden chambers (Corning, New York, NY) containing 8 μm polycarbonate filter were used to determine cell migration as described by us previously (27).
Statistical analysis
Statistical tests were performed using GraphPad Prism (version 7.02). Student’s t test was performed for statistical comparisons between two groups. One-way analysis of variance (ANOVA) followed by Dunnett’s test or Bonferroni’s correction were used for dose-response or multiple group comparisons, respectively. A P value of <0.05 was considered statistically significant.
Results
WA treatment inhibited expression and transcriptional activity of FoxQ1
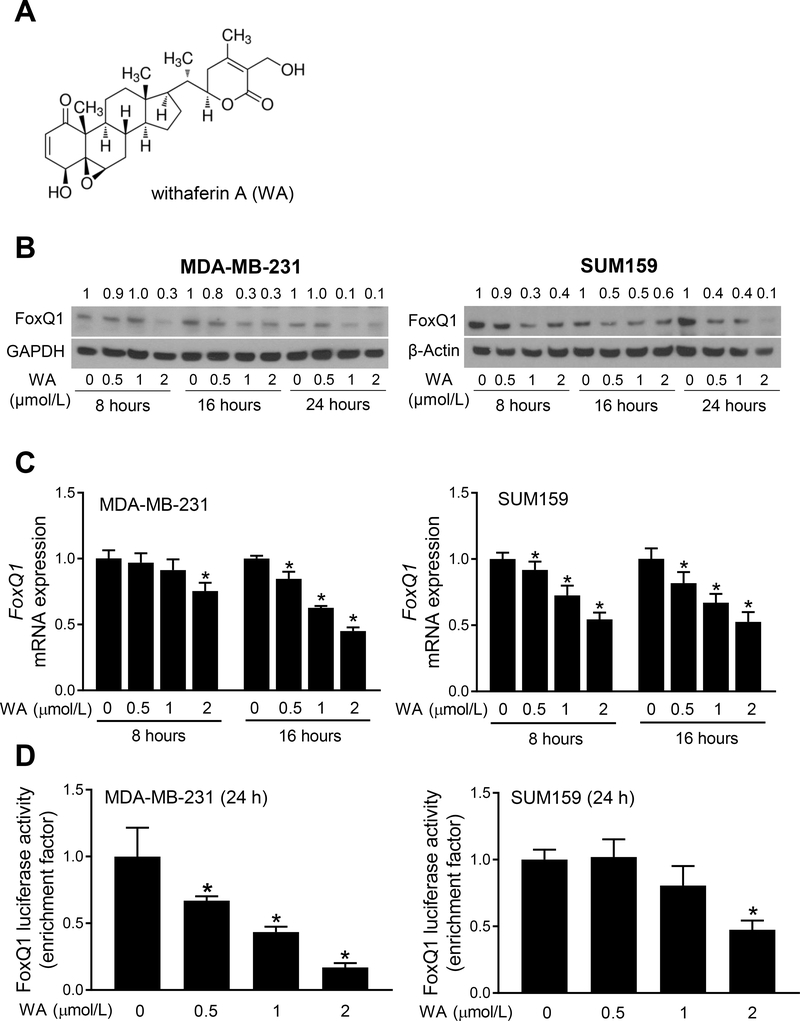
Pharmacokinetic studies using mice and rats have revealed peak plasma concentrations of 1.8–6.5 μmol/L (28, 29). Therefore, WA (chemical structure is shown in Fig. 1A) concentrations < 2 μmol/L were used in this study. Exposure of MDA-MB-231 and SUM159 cells to WA resulted in a decrease in protein level of FoxQ1 as judged by western blotting (Fig. 1B). The WA-mediated decrease in protein level of FoxQ1 was evident as early as 16 hours of treatment in both MDA-MB-231 and SUM159 cells (Fig. 1B). The WA treatment also caused a decrease in mRNA levels of FoxQ1 in both cell lines (Fig. 1C). The WA-mediated downregulation of FoxQ1 was accompanied by a decrease in its transcriptional activity as judged by luciferase reporter assay (Fig. 1D). These results indicated inhibition of expression and transcriptional activity of FoxQ1 upon exposure to WA in human breast cancer cells.
Figure 1.
Withaferin A (WA) treatment suppresses expression and activity of FoxQ1 in breast cancer cells. A, Chemical structure of WA. B, Western blots showing effect of WA treatment on FoxQ1 protein expression in MDA-MB-231 and SUM159 cells. Numbers above bands are changes in FoxQ1 protein level relative to corresponding DMSO-treated control cells. C, Quantification of FoxQ1 mRNA expression by qRT-PCR after treatment with DMSO or WA in breast cancer cells (mean ± SD, n=3). *Significantly different (P<0.05) compared with DMSO-treated control as determined by one-way ANOVA followed by Dunnett’s test. D, FoxQ1 luciferase activity after 24-hour treatment with DMSO or the indicated concentrations of WA. Results shown are mean ± SD (n=3). *Significantly different (P<0.05) compared with DMSO-treated control by one-way ANOVA followed by Dunnett’s test. Each experiment was repeated at least twice, and the results were comparable.
FoxQ1 overexpression attenuated WA-mediated decrease in bCSC
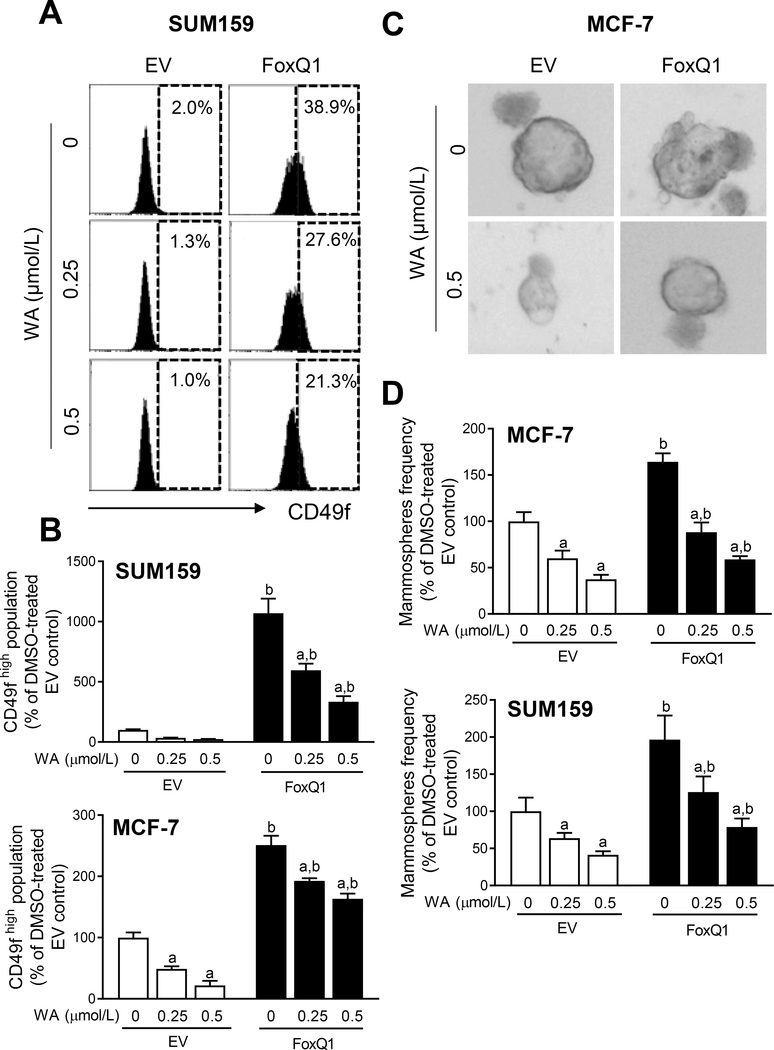
We have shown previously that WA administration inhibits bCSC fraction in vivo in both MMTV-neu transgenic mice and MNU-rat model (20, 22). We also found a remarkable decrease in protein level of FoxQ1 in mammary tumors of WA-treated rats than that of control rats (20). Therefore, we raised the question of whether suppression of FoxQ1 expression and activity accounted for WA-mediated inhibition of bCSC. The MCF-7 and SUM159 cells with stable overexpression of FoxQ1 and corresponding empty vector transfected control cells (hereafter abbreviated as EV) were used to address the above question. Figure 2A shows flow histograms for CD49fhigh fraction in EV and FoxQ1 overexpressing SUM159 cells. Overexpression of FoxQ1 resulted in a significant increase in CD49fhigh population in both cell lines than in corresponding EV cells (Fig. 2B). Exposure of EV and FoxQ1 overexpressing cells to WA resulted in a decrease in CD49f high population (Fig. 2B). The WA-mediated decrease in CD49fhigh population was partly attenuated by overexpression of FoxQ1 in both cell lines (Fig. 2B). These results were confirmed by mammosphere formation assay. The size of mammospheres was slightly bigger in FoxQ1 overexpressing cells in comparison with EV cells (Fig. 2C). The frequency of mammospheres was decreased in WA-treated EV cells and FoxQ1 overexpression partly attenuated this effect in MCF-7 and SUM159 cells (Fig. 2D).
Figure 2.
FoxQ1 overexpression confers partial protection against inhibition of CD49fhigh population by withaferin A (WA) treatment. A, Representative flow histograms showing CD49fhigh population in empty vector transfected control cells (EV) or FoxQ1 overexpressing SUM159 cells after 48-hour of treatment with DMSO or the indicated doses of WA. B, Bar graphs showing quantitation of the CD49f high population in EV or FoxQ1 overexpressing SUM159 and MCF-7 cells (mean ± SD; n=3). Statistically significant (P<0.05) compared with a corresponding DMSO-treated control or b between EV and FoxQ1 cells at the same dose of WA by one-way ANOVA followed by Bonferroni’s multiple comparisons test. C, Representative images of mammospheres from EV or FoxQ1 overexpressing MCF-7 cells following treatment with DMSO or WA. D, Bar graphs showing the quantitation of mammosphere frequency. Combined results from two independent experiments are shown as mean ± SD (n=6). Statistically significant (P<0.05) compared with a corresponding DMSO-treated control or b between EV and FoxQ1 cells at the same dose of WA by one-way ANOVA followed by Bonferroni’s multiple comparisons test.
The bCSC-associated genes downregulated by WA treatment in breast cancer cells
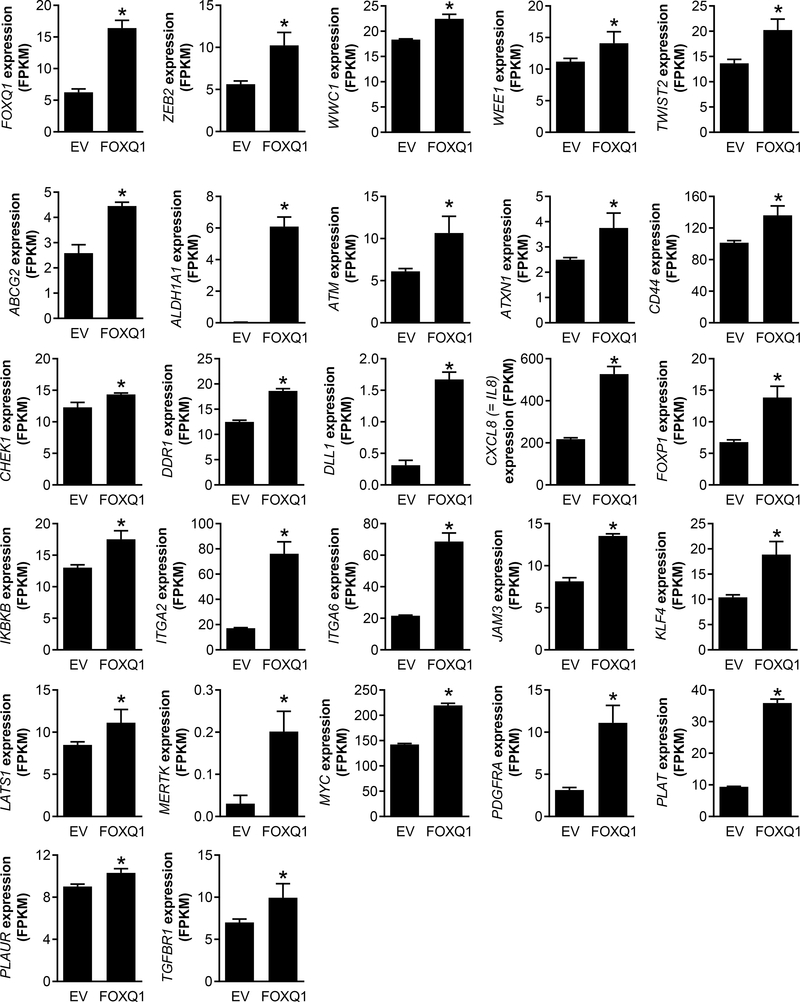
We recently performed RNA-seq analysis using EV and FoxQ1 overexpressing SUM159 cells (30). Analysis of the 86 bCSC-associated genes from this RNA-seq data revealed upregulation of 26 genes by FoxQ1 overexpression (Fig. 3). Recently, we also completed RNA-seq analysis using MCF-7 and MDA-MB-231 cells following 16 hours of treatment with DMSO (control) or 2 μmol/L WA (31). Only three genes (ABCG2, CHEK1, and IL-8) were commonly downregulated by WA treatment in both cell lines with a P value of < 0.05 (Supplementary Fig. S1A). On the other hand, some genes were significantly downregulated in MCF-7 or MDA-MB-231 cells only (Supplementary Fig. S1B).
Figure 3.
RNA-seq analysis identifies breast cancer stemness-related genes upregulated by FoxQ1 overexpression in SUM159 cells (30). Bar graphs showing upregulation of 26 stemness genes in FoxQ1 overexpressing SUM159 cells in comparison with EV cells. Data shown are mean ± SD (n=3). *Significantly different (P<0.05) compared with control by Student’s t test.
WA treatment inhibited IL-8 level in breast cancer cells
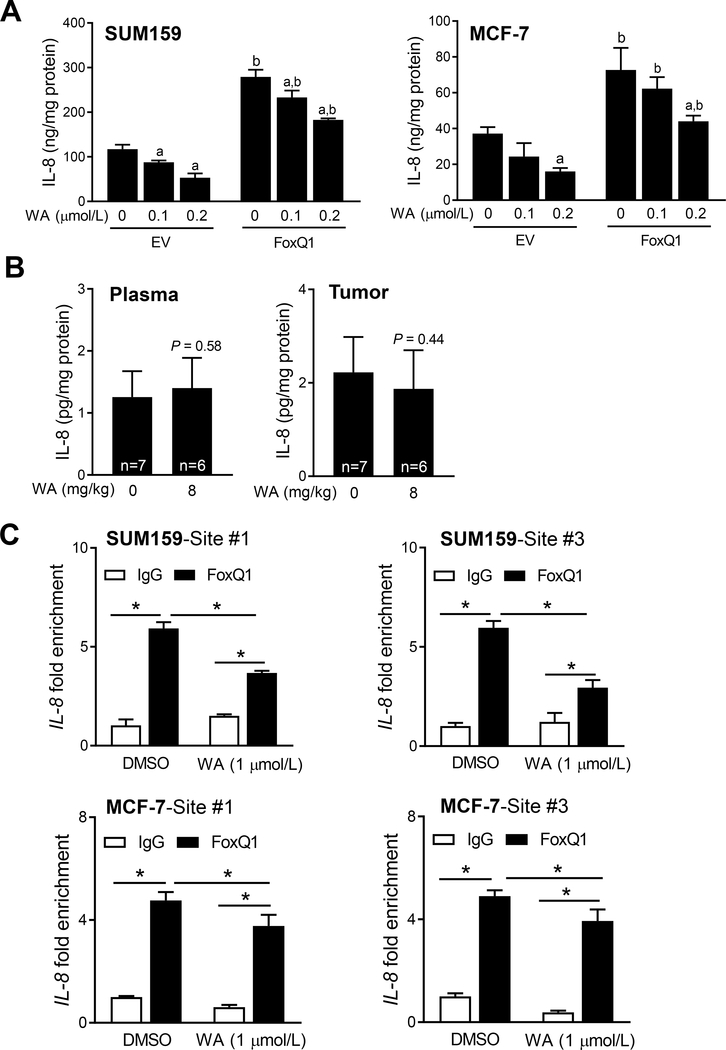
From the list of genes that were commonly downregulated by WA treatment in both MCF-7 and MDA-MB-231 cells, the IL-8 appeared interesting to study further based on following considerations: (a) IL-8 signaling is implicated in regulation of bCSC activity (32); (b) FoxQ1 expression is positively and statistically significantly associated with that of IL-8 in the breast cancer based on analysis of The Cancer Genome Atlas dataset (30); (c) stable overexpression of FoxQ1 significantly increases expression of IL-8 mRNA in SUM159 cells (30); (d) secretion of IL-8 was significantly higher in the conditioned media of FoxQ1 overexpressing SUM159 cells than that in EV cells (30); and finally (e) the promoter of IL-8 contains three FoxQ1 consensus binding sites and FoxQ1 occupancy was observed at two sites (30). The WA treatment caused a decrease in levels of IL-8 in the conditioned media of EV and FoxQ1 overexpressing SUM159 and MCF-7 cells (Fig. 4A). On the other hand, WA administration failed to decrease plasma or mammary tumor levels of IL-8 in rats in vivo (Fig. 4B). However, FoxQ1 occupancy at the promoter of IL-8 was decreased significantly by WA treatment in both SUM159 and MCF-7 cells (Fig. 4C). These results indicated inhibitory effect of WA on FoxQ1-regulated IL-8 expression.
Figure 4.
Withaferin A (WA) treatment decreases secretion of interleukin-8 (IL-8) in breast cancer cells. A, Quantification of IL-8 secretion in the media of EV and FoxQ1 overexpressing SUM159 and MCF-7 cells after 24 hours of treatment with DMSO or the indicated doses of WA. Results are shown as mean ± SD (n=3). Significantly different (P<0.05) compared with a respective DMSO-treated control or b between EV and FoxQ1 overexpressing cells at the same dose of WA by one-way ANOVA followed by Bonferroni’s multiple comparisons test. B, Rat plasma and tumor levels of IL-8. Statistical significance was analyzed by unpaired Student’s t test. C, Effect of WA treatment on FoxQ1 binding to the IL-8 promoter region (mean ± SD; n=3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparisons test. Experiments (A and C) were repeated at least twice with comparable results.
FoxQ1 was dispensable for WA-mediated mitotic arrest and its inhibitory effects on cell proliferation and migration
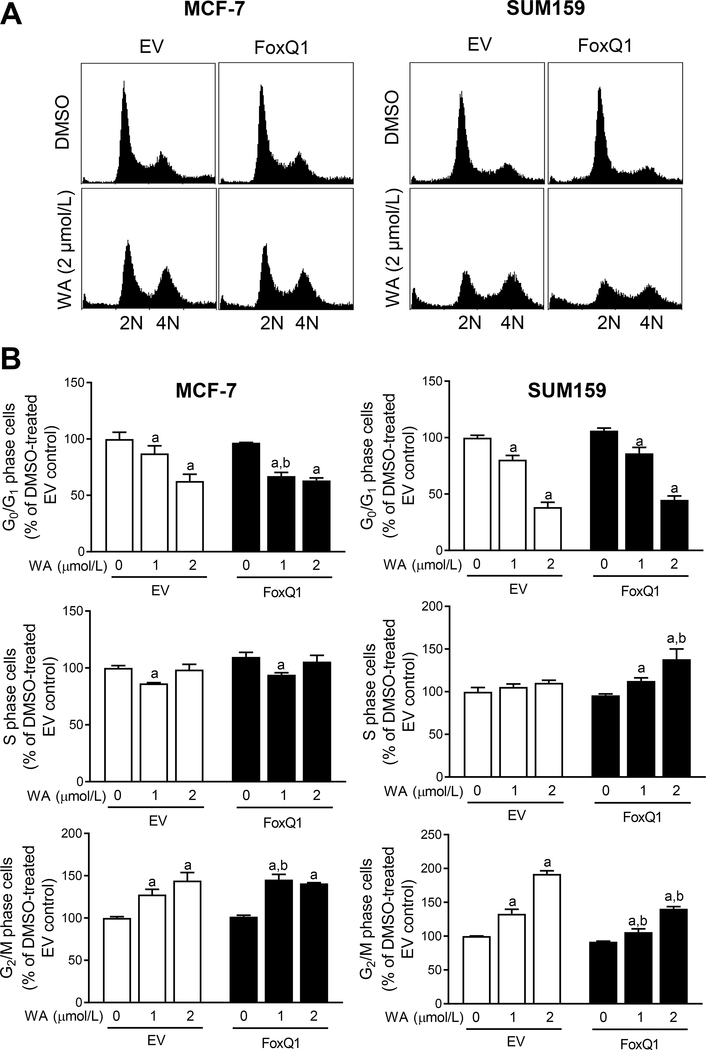
We have reported previously that WA treatment causes G2/M phase cell cycle arrest in breast cancer cells (33). Therefore, we determined the effect of WA treatment on cell cycle distribution using EV and FoxQ1 overexpressing MCF-7 and SUM159 cells. Flow histograms for cell cycle distribution in MCF-7 and SUM159 cells are depicted in Figure 5A. Consistent with published reports (14, 33), WA treatment caused G2/M phase cell cycle arrest in both EV and FoxQ1 overexpressing MCF-7 and SUM159 cells (Fig. 5B). The G2/M phase cell cycle arrest resulting from WA treatment was significantly attenuated by FoxQ1 overexpression in SUM159 cells but not in the MCF-7 cell line (Fig. 5B). The WA-mediated mitotic arrest was not affected by FoxQ1 overexpression in SUM159 cells (Fig. 6A), and this assay was not done in the MCF-7 cells.
Figure 5.
Effect of FoxQ1 overexpression on withaferin A (WA)-mediated G2/M phase cell cycle arrest. A, Representative flow histograms depicting cell cycle distribution in FoxQ1 overexpressing MCF-7 and SUM159 cells or corresponding EV cells after 24 hours of treatment with DMSO or WA. B, Bar graphs showing effect of FoxQ1 overexpression and WA treatment on cell cycle distribution. Results shown are mean ± SD (n=3). Statistically significant (P<0.05) compared with a corresponding DMSO-treated control or b between EV and FoxQ1 overexpressing cells at the same dose of WA by one-way ANOVA followed by Bonferroni’s multiple comparisons test. Comparable results were observed in replicate experiments.
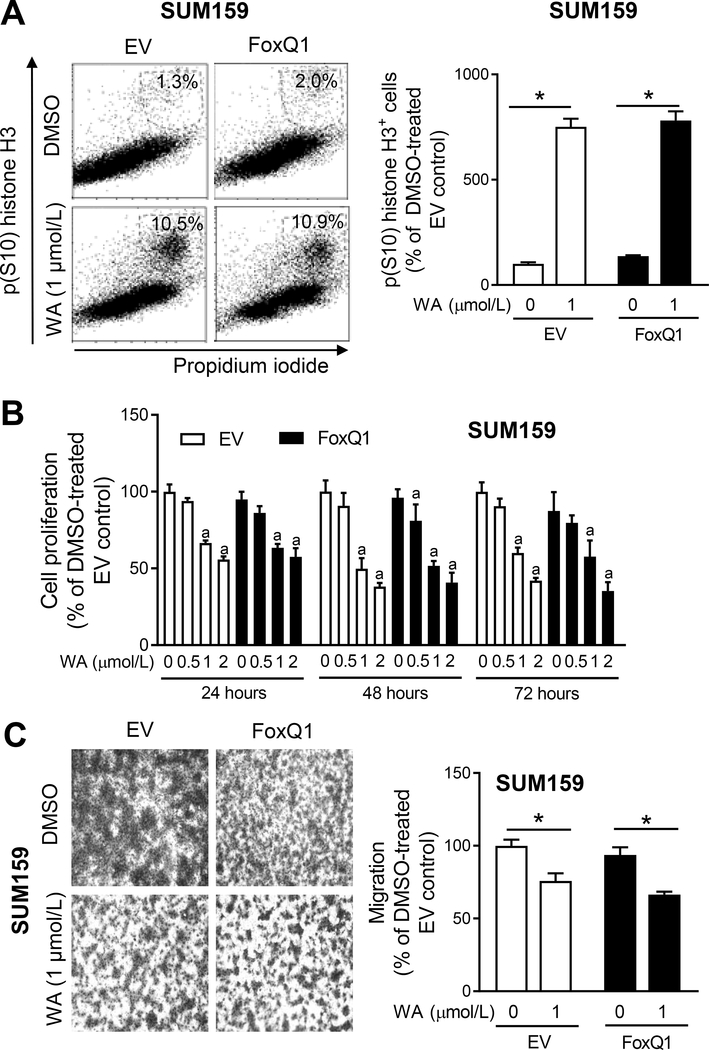
Figure 6.
FoxQ1 overexpression fails to confer protection against withaferin A (WA)-mediated mitotic arrest or inhibition of cell proliferation or cell migration. A, Representative flow histograms showing mitotic fraction in EV cells or FoxQ1 overexpressing SUM159 cells after 8 hours of treatment with DMSO or WA. Bar graph shows quantitation of mitotic fraction. Data shown are mean ± SD (n=3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparisons test. B, Effect of WA treatment on cell proliferation in EV and FoxQ1 overexpressing SUM159 cells. Results shown are mean ± SD (n=4). a Significantly different (P<0.05) compared with DMSO-treated EV by one-way ANOVA followed by Bonferroni’s multiple comparisons test. C, Representative images (Boyden chamber assay) depicting migration by EV or FoxQ1 overexpressing SUM159 cells after 24 hours of treatment with DMSO or WA (100× magnification). The bar graph shows quantitation of cell migration. Results shown are mean ± SD (n=3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparisons test. Each experiment was done twice with comparable results.
Proliferation of EV and FoxQ1 overexpressing SUM159 cells was dose dependently inhibited by WA treatment (Fig. 6B). The cell proliferation inhibition resulting from WA treatment was not affected by FoxQ1 overexpression (Fig. 6B). We also determined the effect of WA on cell migration because FoxQ1 overexpression is known to promote metastasis in vivo (34). Interestingly, FoxQ1 overexpression did not increase SUM159 cell migration. Like cell proliferation data, the WA-mediated inhibition of cell migration was not affected by FoxQ1 overexpression in the SUM159 cell line (Fig. 6C). These results indicated that FoxQ1 was dispensable for inhibitory effects of WA on cell proliferation and migration at least in the SUM159 cell line.
Discussion
We have shown previously that level of FoxQ1 protein is significantly higher in basal-like and luminal-type human breast cancer tissues when compared to normal mammary tissues (24, 30). Previous studies have also documented oncogenic functions of FoxQ1 in breast cancer (34–36). For example, FoxQ1 overexpression was shown to promote epithelial-mesenchymal transition in breast cancer cells (34). Overexpression of FoxQ1 in a human mammary epithelial cell line (HMLE) resulted in increased cell migration, whereas this effect was reversed by knockdown of this protein in the 4T1 mouse mammary carcinoma cells (34). FoxQ1 expression correlated with high-grade basal-like breast cancer and poor clinical outcomes (35). The bCSC fraction was shown to be increased by overexpression of FoxQ1 (35). The results of the present study indicate that FoxQ1 is a target of WA for inhibition of bCSC. However, FoxQ1 may not be the sole participant in WA-mediated inhibition of bCSC fraction as this effect is only partly attenuated by overexpression of FoxQ1. Nevertheless, FoxQ1 protein level may be a useful pharmacodynamic biomarker of WA in future clinical trials because its level is decreased by WA treatment in vitro (present study) and in vivo in a rat mammary tumor model (20).
The present study reveals that the IL-8 is a target of WA downstream of FoxQ1 for inhibition of bCSC. There is a positive correlation between serum levels of IL-8 and survival in patients with early and metastatic breast cancer (37). The IL-8 was shown to promote growth and aggressiveness of estrogen receptor negative breast cancer cells (38). Treatment with recombinant IL-8 resulted in increased mammosphere formation as well as promotion of self-renewal of bCSC as judged by Aldefluor assay (39). The present study reveals that FoxQ1 directly regulates expression of IL-8 and WA treatment decreases IL-8 level in breast cancer cells in vitro. It is interesting to note, however, that the plasma or tumor levels of IL-8 are not decreased in vivo at least in the rat model despite marked suppression of the FoxQ1 protein expression. The reasons for this discrepancy are not clear and require further investigation.
The FoxQ1 is known to promote cell migration in vitro and in vivo growth and metastasis of breast cancer cells (34–36). It is surprising to note that the proliferation and migration was not affected by FoxQ1 overexpression at least in the SUM159 cell line (present study). The FoxQ1 also seems dispensable for WA-mediated inhibition of cell proliferation and migration in SUM159 cells. The FoxQ1 also appears to have a cell line-specific role in WA-mediated G2/M phase cell cycle arrest. Enrichment of G2/M population, but not the mitotic fraction, resulting from WA exposure is significantly attenuated by overexpression of FoxQ1 in SUM159 cell line but not in the MCF-7 cell line. This difference may be attributable to genetic differences in these cell lines. For example, the MCF-7 cell line expresses estrogen receptor that is lacking in the SUM159 cell line. The ligands for estrogen receptor are known to regulate cell cycle progression (40). Similarly, the MCF-7 cell line expresses wild-type p53 but a functional copy of this tumor suppressor is lacking in the SUM159 cell line.
In conclusion, the present study reveals that WA treatment decreases expression and activity of FoxQ1 in breast cancer cells translating to inhibition of bCSC self-renewal. However, FoxQ1 is dispensable for WA-mediated inhibition of breast cancer cell proliferation and cell migration.
Supplementary Material
Acknowledgement
This study was supported by the National Cancer Institute at the National Institutes of Health grant R01 CA142604 (to SVS). This study used the UPMC Hillman Cancer Center Flow Cytometry Facility supported by the National Cancer Institute at the National Institutes of Health grant P30 CA047904. The funders had no role in the design of the study, data collection, analysis or interpretation of the data, manuscript preparation or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: None
References
- 1.Tandon N, Yadav SS. Safety and clinical effectiveness of Withania Somnifera (Linn.) Dunal root in human ailments. J Ethnopharmacol 2020;255:112768. [DOI] [PubMed] [Google Scholar]
- 2.Nasimi Doost Azgomi R, Zomorrodi A, Nazemyieh H, Fazljou SMB, Sadeghi Bazargani H, Nejatbakhsh F, et al. Effects of Withania somnifera on reproductive system: A systematic review of the available evidence. Biomed Res Int 2018;4076430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarris J Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother Res 2018;32:1147–62. [DOI] [PubMed] [Google Scholar]
- 4.Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of Ashwagandha (Withania somnifera L. Dunal) root extract in improving memory and cognitive functions. J Diet Suppl 2017;14:599–612. [DOI] [PubMed] [Google Scholar]
- 5.Lopresti AL, Drummond PD, Smith S. A randomized, double-blind, placebo-controlled, crossover study examining the hormonal and vitality effects of Ashwagandha (Withania somnifera) in aging, overweight males. J.Am J Mens Health 2019;13:1557988319835985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegenfuss TN, Kedia AW, Sandrock JE, Raub BJ, Kerksick CM, Lopez HL. Effects of an aqueous extract of Withania somnifera on strength training adaptations and recovery: The STAR trial. Nutrients 2018;10:1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dar NJ, Hamid A, Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci 2015;72:4445–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta R, Khalil R, Green R, Mohapatra SS, Mohapatra S. Withania somnifera (Ashwagandha) and withaferin A: Potential in integrative oncology. Int J Mol Sci 2019;20:5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devi PU, Sharada AC, Solomon FE, Kamath MS. In vivo growth inhibitory effect of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, Sarcoma 180. Indian J Exp Biol 1992;30:169–72. [PubMed] [Google Scholar]
- 10.Davis L, Kuttan G. Effect of Withania somnifera on 20-methylcholanthrene induced fibrosarcoma. J Exp Clin Cancer Res 2000;19:165–7. [PubMed] [Google Scholar]
- 11.Widodo N, Priyandoko D, Shah N, Wadhwa R, Kaul SC. Selective killing of cancer cells by Ashwagandha leaf extract and its component Withanone involves ROS signaling. PLoS One 2010;5:e13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash J, Gupta SK, Dinda AK. Withania somnifera root extract prevents DMBA-induced squamous cell carcinoma of skin in Swiss albino mice. Nutr Cancer. 2002;42:91–7. [DOI] [PubMed] [Google Scholar]
- 13.Hahm ER, Kim SH, Singh KB, Singh K, Singh SV. A comprehensive review and perspective on anticancer mechanisms of withaferin A in breast cancer. Cancer Prev Res (Phila) 2020;13:721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antony ML, Lee J, Hahm ER, Kim SH, Marcus AI, Kumari V, et al. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of β-tubulin. J Biol Chem 2014;289:1852–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao R, Shah N, Lee JS, Katiyar SP, Li L, Oh E, et al. Withanone-rich combination of Ashwagandha withanolides restricts metastasis and angiogenesis through hnRNP-K. Mol Cancer Ther 2014;13:2930–40. [DOI] [PubMed] [Google Scholar]
- 16.Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin A, a naturally occurring steroidal lactone. AAPS J 2014;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One 2011;6:e23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res 2008;68:7661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahm ER, Lee J, Kim SH, Sehrawat A, Arlotti JA, Shiva SS, et al. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst 2013;105:1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samanta SK, Sehrawat A, Kim SH, Hahm ER, Shuai Y, Roy R, et al. Disease subtype-independent biomarkers of breast cancer chemoprevention by the Ayurvedic medicine phytochemical Withaferin A. J Natl Cancer Ins 2016;109(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien CS, Farnie G, Howell SJ, Clarke RB. Breast cancer stem cells and their role in resistance to endocrine therapy. Horm Cancer 2011;2:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Singh SV. Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prev Res (Phila) 2014;7:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng F, Speyer CL, Zhang B, Zhao Y, Chen W, Gorski DH, et al. PDGFRα and β play critical roles in mediating Foxq1-driven breast cancer stemness and chemoresistance. Cancer Res 2015;75:584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SH, Kaschula CH, Priedigkeit N, Lee AV, Singh SV. Forkhead box Q1 is a novel target of breast cancer stem cell inhibition by diallyl trisulfide. J Biol Chem 2016;291:13495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 2003;24:891–7. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Hahm ER, Singh KB, Singh SV. Diallyl trisulfide inhibits leptin-induced oncogenic signaling in human breast cancer cells but fails to prevent chemically-induced luminal-type cancer in rats. J Cancer Prev 2020;25:1–12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129:2744–55. [DOI] [PubMed] [Google Scholar]
- 29.Dai T, Jiang W, Guo Z, Wang Z, Huang M, Zhong G, et al. Studies on oral bioavailability and first-pass metabolism of withaferin A in rats using LC-MS/MS and Q-TRAP. Biomed Chromatogr 2019;33:e4573. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Hahm ER, Singh KB, Singh SV. Novel mechanistic targets of forkhead box Q1 transcription factor in human breast cancer cells. Mol Carcinog. 2020;59:1116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahm ER, Kim SH, Singh KB, Singh SV. RNA-seq reveals novel cancer-selective and disease subtype-independent mechanistic targets of withaferin A in human breast cancer cells. Mol Carcinog. 2020;2021;60:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh JK, Simoes BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res 2013;15:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer 2008;60 (Suppl 1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Meng F, Liu G, Zhang B, Zhu J, Wu F, Ethier SP, Miller F, Wu G. Forkhead transcription factor foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res 2011;71:1292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao Y, Jiang X, Lee ST, Karuturi RK, Hooi SC, Yu Q. FOXQ1 regulates epithelial-mesenchymal transition in human cancers. Cancer Res 2011;71:3076–86. [DOI] [PubMed] [Google Scholar]
- 36.Meng F, Speyer CL, Zhang B, Zhao Y, Chen W, Gorski DH, et al. PDGFRα and β play critical roles in mediating Foxq1-driven breast cancer stemness and chemoresistance. Cancer Res 2015;75:584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpé S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res 2004;10:7157–62. [DOI] [PubMed] [Google Scholar]
- 38.Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, et al. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer 2007;121:1949–57. [DOI] [PubMed] [Google Scholar]
- 39.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 2009;69:1302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doisneau-Sixou SF, Sergio CM, Carroll JS, Hui R, Musgrove EA, Sutherland RL. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr Relat Cancer 2003;10:179–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.