Abstract
Whether stomach influences the progression of nonalcoholic steatohepatitis (NASH) remains largely unknown. Ghrelin, a 28-amino acid gastric hormone, is critical for the regulation of energy metabolism and inflammation. We investigated whether ghrelin affects the progression of NASH.
NASH were induced with LPS (240μg/kg/day) in male C57BL/6J mice fed HFD. Ghrelin (11nmol/kg/day) was administrated by a subcutaneous mini-pump. Liver steatosis, inflammation and fibrosis were assessed. Kupffer cells and hepatocytes isolated from wild type, GHSR1a−/− or PPARγ+/− mice were co-cultured to determine the cellular and molecular mechanism by which ghrelin ameliorates NASH
Low concentration of LPS activates the Kuppfer cells, leading to the development of NASH in mice fed HFD. Ghrelin blocked the progression of NASH induced by LPS via GHSR1a-mediated attenuation of Kupffer cells M1 polarization. GHSR1a was detected in Kupffer cells isolated from wildtype mice but not in GHSR1a deficient animals. Upon binding with ghrelin, internalization of GHSR1a occurred. Ghrelin reduced levels of TNFα and iNOS, while increasing Arg1 in Kupffer cells treated with LPS. Ghrelin markedly attenuated the up-regulation of lipid accumulation induced by the supernatant of Kupffer cells under both basal and LPS-treated conditions. Deficiency of PPARγ significantly reduced the effect of LPS on the hepatic steatosis in mice and in cultured hepatocytes.
Our studies indicate that the stomach may improve the development of NASH via ghrelin. Ghrelin may serve as a marker and therapeutic target for NASH.
Keywords: NAFLD, Gut-liver interaction, X/A like cells, Ghrelin, PPARγ
Abbreviated abstract
Upper gut may alter the progression of NASH.
In addition to the neuronal mechanism, the stomach improves metabolic inflammation in liver via a chemical signal: ghrelin.
PPARγ mediates the effects of LPS and ghrelin on hepatic steatosis.
Introduction
Activation of Kupffer cells, the macrophages resided in hepatic sinusoids, is critical for the pathogenesis of nonalcoholic steatohepatitis (NASH). Depletion of Kupffer cells using clodronate liposomes significantly ameliorates NASH evidenced by reduction in hepatic steatosis, inflammation and necrosis(Rivera, Adegboyega et al. 2007). Up-regulation of CD14 positive Kupffer cells exacerbates the progression of nonalcoholic fatty liver disease (NAFLD) from simple steatosis to steatohepatitis(Imajo, Fujita et al. 2012). Further studies have shown that M1 polarization of Kupffer cells, the classical pro-inflammatory state of macrophages, contributes to the occurrence of NASH by producing tumor necrosis factor a (TNF-α), interleukin 1 (IL-1), IL-12, and reactive oxygen species (ROS) (Wan, Benkdane et al. 2014). The phenotype switch from the classical pro-inflammatory M1 to the alternative anti-inflammatory M2 state may thus provide an alternative strategy for the intervention of NASH and its associated metabolic dysfunction.
Obesity is the culprit of NASH. The mechanism by which Kupffer cells are activated to the M1 state during the progression of obesity remains incompletely understood. According to the “multiple hits” hypothesis, a variety of factors ranging from ROS to cytokines such as TNF-α and interleukins concur in the activation of hepatic immune cells including macrophages(Buzzetti, Pinzani et al. 2016). Recent studies have suggested that dysbiosis of gut microflora may also contribute to the activation of Kupffer cells and subsequent development of NASH, although the molecules mediating this effect remain to be determined. Lipopolysaccharides (LPS), an endotoxin derived from intestinal gram-negative bacteria, may contribute to the M1 polarization of residual Kupffer cells in patients with obesity. Dysbiosis of intestinal microbiota increases the production of endotoxin, as well as impairs the gut permeability, leading to the subsequent endotoxemia. Despite of this general agreement, the influence of endotoxemia on the development and progression of NASH remains under dispute. First, whether levels of serum LPS are significantly elevated in patients with NASH than those with simple steatosis remains controversial. Although studies by Harte et al(Harte, da Silva et al. 2010) showed a higher levels of serum LPS in patients with NAFLD relative to control subjects, no endotoxemia was detected in patients with NAFLD in the study by Loguercio et al(Loguercio, De Simone et al. 2004). Second, it is currently unclear at what threshold that endotoxemia triggers the progression from simple steatosis to NASH. Moderate elevation of portal LPS levels was reported in healthy subject, suggesting that mild portal endotoxemia is insufficient to cause liver dysfunction(Jacob, Goldberg et al. 1977). Instead, Imajo et al(Imajo, Fujita et al. 2012) proposed that chronic exposure to low dose of LPS (250μg/kg body weight) in the obese mice is required for the progression to NASH. However, LPS was administrated by intraperitoneal injection daily in their study. Such administration of LPS led to transient robust increase in serum LPS concentration, which does not replicate the natural condition of NASH. Reasons accounting for these controversial observations remain unknown but may be complicated by the alteration in endogenous responses such as secretion of gut hormones. Indeed, LPS has been reported to alter the secretion of gut hormones: ghrelin and nesfatin-1(Stengel, Goebel et al. 2010, Stengel, Goebel-Stengel et al. 2011), suggesting an integration between gut-derived inflammatory factors and endocrine molecules. Currently, little is known about the impact of these interactions in the development and progression of NAFLD.
In addition to its functioning as a signal to convert negative energy balance, ghrelin, a 28 amino acid peptide hormone from the gastric X/A like endocrine cells, has also been recognized as a critical modulator in inflammatory response. Ghrelin receptor has been detected in immune organs such as lymph nodes, spleen and thymus(Gnanapavan, Kola et al. 2002), as well as in immune cells including T lymphocytes and monocytes(Cohen, Chandra et al. 2010). Dependent on the pathophysiological conditions, ghrelin may function as an enhancer(Koo, Huang et al. 2001) or suppressor of inflammatory response(Dixit, Schaffer et al. 2004, Gonzalez-Rey, Chorny et al. 2006, Chorny, Anderson et al. 2008). Further, obesity impairs the acylation of ghrelin and its signaling, leading to the exacerbation of inflammatory response(Harvey, Howard et al. 2017), Because low grade chronic inflammation is often associated with obesity and circulating ghrelin is significantly lower in obese animals and human beings(Shiiya, Nakazato et al. 2002, Xu, Li et al. 2009), ghrelin may alter the inflammatory states and thus impact the progression of obesity and its associated metabolic diseases like NAFLD. To test this concept, we examined how ghrelin affects the activation of Kupffer cells and its subsequent influence on the progression of NAFLD. Our studies demonstrate that continuous infusion of gut-derived endotoxin LPS at a low dose relative to the level of high fat diet (HFD)-induced obese mice led to the M1 polarization of Kupffer cells/macrophages and the progression from simple steatosis to NASH. Further, ghrelin significantly attenuated the progression to NASH by suppressing the M1 polarization of Kupffer cells/macrophages.
Materials and Methods
Animals
Four-week-old male C57BL/6J mice weighing about 20g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd, and fed either normal chow diet (10% kcal fat) or high-fat diet (60% kcal fat, Research Diets, Inc., USA) for 12 weeks prior to experiments. PPARγ+/− mice were from Jackson laboratory. All animals were housed in the SPF-level environment with temperature of 24°C and 12h light-12h dark cycle. Mice were fed ad libitum except for fasting before oral glucose tolerance test (OGTT), insulin tolerance test (ITT) and intestinal permeability test.
Implantation of micro-osmotic pump
The micro-osmotic pumps were purchased from ALZET (Cupertino, CA) were soaked in normal saline solution immediately after unpacking for at least 6h before drug filling. Mice were anesthetized with intraperitoneal injection of 1% sodium pentobarbital sodium (50mg/kg body weight). A 0.5 cm incision was made in the middle of the posterior cervical scapula. Micro-osmotic pump (model 1002) filled with test agent or vehicle control was implanted subcutaneously. Incision was closed by suture. The pump was gently moved daily to avoid adhesion. Through the implanted micro-osmotic pump, LPS at a dose of 240μg/kg/day and ghrelin 11nmol/kg/day were infused for 14 days. Control experiment used normal saline solution.
Preparation of samples
Mice were anesthetized with 1% sodium pentobarbital. Blood was collected through the right ventricule of heart. Subcutaneous and epididymal fat tissues, liver, gastric and intestinal tissues were harvested and treated with RNAtrip, tissue lysing buffer or 4% paraformaldehyde respectively. After fixation with 4% paraformaldehyde, tissues were embeded with paraffin or OCT, section at the thickness of 6 μm, mounted on the polylysine-treated slides, then stained with hematoxylin and eosin or oil-red O.
Isolation of hepatic primary cells
Hepatic primary cells were isolated as described before(Li, Xu et al. 2014, Yin, Li et al. 2015). Briefly, mice were anesthetized with 1% sodium pentobarbital and abdominal cavity was exposed through a laparotomy. The portal vein was exposed and cannulated for perfusion with 20ml of 37°C preheated Dhanks (about 20ml), followed by 0.02% collagenase (Sigma Aldrich, CO. St. Louis. MO) at a flow rate of 2 ml/min. Heparin was used for anticoagulation. After perfusion, liver tissues were removed and the capsule of the liver was removed and incubated in 20 ml of 0.02% collagenase at 37°C for 15 mins. Cell suspension was then filtered through two layers of 60–80 μm nylon mesh, centrifuged at 500 rpm and washed twice with DMEM medium. Dispersed hepatocytes were then seeded at a concentration of 2*106 cells/100 mm dish containing 10 ml high glucose DMEM medium supplemented with 10% FBS. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2. Culture medium was changed daily.
Isolation of Kupffer cells/macrophages
Hepatic primary cells were isolated as described above and enriched for non-parenchymal cells by centrifugation at 800g for 10min in 4°C. The sediment cells were re-suspended in PBS containing 10%FBS at a concentration of 4 ml per mouse. Cell suspension was applied over two layers of percoll gradient, 60% lower layer and 30% upper layer, and centrifuged at 400g in 4°C for 20min. The cell suspension as well as the upper layer percoll were removed and disposed. The lower layer percoll was transferred to a new centrifuge tube, diluted with PBS containing 10% FBS, then centrifuged at 800g, 4°C for 10min. Precipitated cells were re-suspended in PBS containing FBS and centrifuged again. This procedure was repeated twice to wash off the percoll. The enriched Kupffer cells/macrophages were cultured in 1640 medium containing 10% FBS. Cultured medium was gently replaced after 2h.
Detection of LPS
Concentration of LPS was measured using the Tachypleus Amebocyte Lysate (TAL) assay. Plasma samples were diluted 10-fold using the formula: 5μl plasma + 45μl purified water, heated in 70°C water bath for 10min, then cooled on ice. LPS standards were prepared as follows: LPS standard powder was dissolved into 1ml purified water at the concentration of 10EU/ml, then gradually diluted to 1, 0.5, 0.25, 0.1, 0.05, 0.025, 0.0125 and 0EU/ml. TAL reagent was dissolved in purified water. Reaction was performed in the presence of Azo reagent 1 and Limulus reagent at 37°C for 50min. Chromogenic reaction was performed at 37°C for 6min. Absorbance values at 545nm wavelength was detected using the plate reader iMark from Bio-Rad.
Rhodamine labeled ghrelin binding experiments
Kupffer cells/macrophages cultured on the slide were washed three times with PBS and incubated with Rhodamine-labeled ghrelin (#FR-G-031–31) purchased from GENTAUR U.S.A Phoenix peptide (San Jose, CA) for 0, 5, 15, 45 min. Cells were then washed three times with PBS, fixed with pre-cooled 4% paraformaldehyde for 30 min, stained with Hoechst for 15min before detection.
Measurement of triglyceride
Triglyceride was extracted from liver tissues or cultured hepatocytes, and measured by Triglyceride Colorimetric Assay Kit from Cayman Chemical Company according to the manufacturer’s instructions. Values were normalized to protein concentrations using the Pierce BCA protein quantitative assay kit (Thermo-Fisher Scientific).
Detection of cytokines
Proteins were extracted from 100mg liver tissues using 500μl of pre-cooled 1x AimPlex Tissue Lysis Buffer at 4°C. Supernatant was collected and stored at −80°C before usage. Cytokines were measured using the Aimplex® Mouse custom 7-plex kit from QuantoBio (Beijing, China).
Western blot analysis
Liver tissues or cultured cells were harvested and homogenized in lysis buffer. Proteins were subjected to SDS-PAGE with a 10% running gel, then transferred to a polyvinylidene fluoride membrane. Membranes were incubated for 1 hr at room temperature with 5% fat-free milk in Tris buffered saline containing Tween 20, followed by incubation overnight at 4°C with primary antibodies. Specific reaction was detected using IRDye-conjugated second antibody and visualized using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Real-time RT-PCR
RNA was isolated from liver tissue or cultured cells using Trizol (Invitrogen) and reverse-transcribed into cDNAs using the First-Strand Synthesis System for RT-PCR kit (Invitrogen). SYBR Green-based quantitative RT-PCR was performed using the Mx3000 multiplex quantitative PCR system (Stratagene, La Jolla, CA). Triplicate samples were collected for each experimental condition to determine relative expression levels. Sequences for the primer pairs used in this study were listed below:
| Gene name | Sequence of the sense strand | Antisense strand sequence |
|---|---|---|
| IL-1β | CCCTAAACAGATGAAGTGCTCCTT | GGTGGTCGGAGATTCGTAGCT |
| Col IV | CACCATAGAGAGAAGCGAGATGTTC | GGCTGACGTGTGTTCGC |
| TNFα | CCAGACCCTCACACTCAGATC | CACTTGGTGGTTTGCTACGAC |
| GHSR | CTATCCAGCATGGCCTTCTC | AAGACGCTCGACACCCATAC |
| Arg-1 | CTCCAAGCCAAAGTCCTTAGAG | AGGAGCTGTCATTAGGGACATC |
| GAPDH | ATGACATCAAGAAGGTGGTG | CATACCAGGAAATGAGCTTG |
| PPARγ2 | CTCTGGGAGATTCTCCTGTTGA | GGTGGGCCAGAATGGCATCT |
| PPARγ | TCAGCTCTGTGGACCTCTCC | ACCCTTGCATCCTTCACAAG |
| CD36 | TGGTCAAGCCAGCTAGAAA | CCCAGTCTCATTTAGCCAC |
Data analysis
Results were expressed as Mean±SEM. Differences between groups were analyzed by two-way ANOVA and Newman-Student-Keuls test. Comparisons between two groups involved use of the Student’s t test. Significant difference was denoted by p<0.05.
Results
1. Persistent elevation of low concentration of LPS is critical for the development of NASH
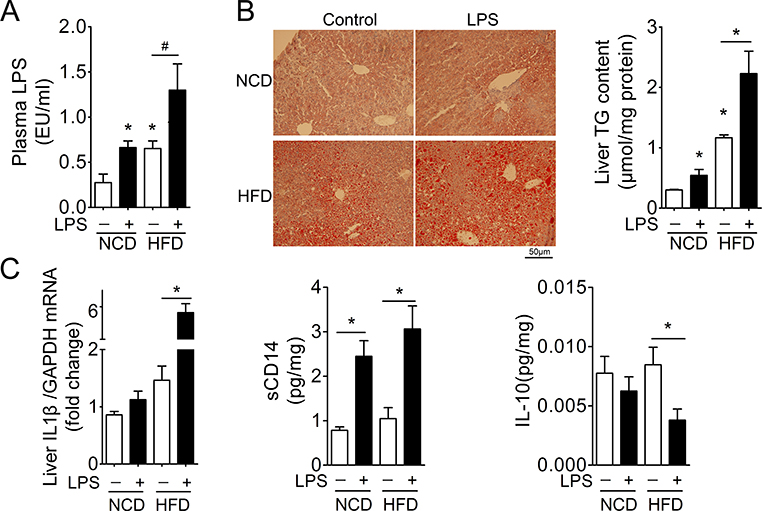
To investigate whether persistent elevation of low concentration of LPS plays a pivotal role in the development of NASH, mice were continuously infused 240μg/kg/day of LPS by a subcutaneous micro-osmotic pump. This dose increased plasma LPS in mice fed NCD to the levels comparative to those in animals fed HFD (Fig 1A). This low level of circulating LPS was not sufficient to induce NASH evidenced by steatosis and cytokine levels such as IL1β and IL10 (Fig 1B, C), despite of its effect on the inflammatory cells in liver measured by sCD14 (Fig 1C). However, this low dose of LPS increased the circulating LPS to levels sufficient to induce the development of NASH from simple steatosis in mice fed HFD. Both hepatic steatosis and levels of IL1β and sCD14 increased significantly, whereas IL10 levels reduced (Fig. 1C).
Figure 1. Induction of NASH by continuous infusion of low dose of LPS.
Mice fed NCD or HFD for 12 weeks were continuously infused with LPS at a dose of 240μg/kg/day for 14 days via the implanted micro-osmotic pump. Plasma and liver tissues were then harvested for analysis of NASH. n=6, * denotes P<0.05 vs control.
A. Levels of plasma LPS.
B. H&E staining and hepatic triglyceride (TG) levels.
C. Levels of hepatic cytokines.
Ghrelin ameliorates NASH induced by LPS
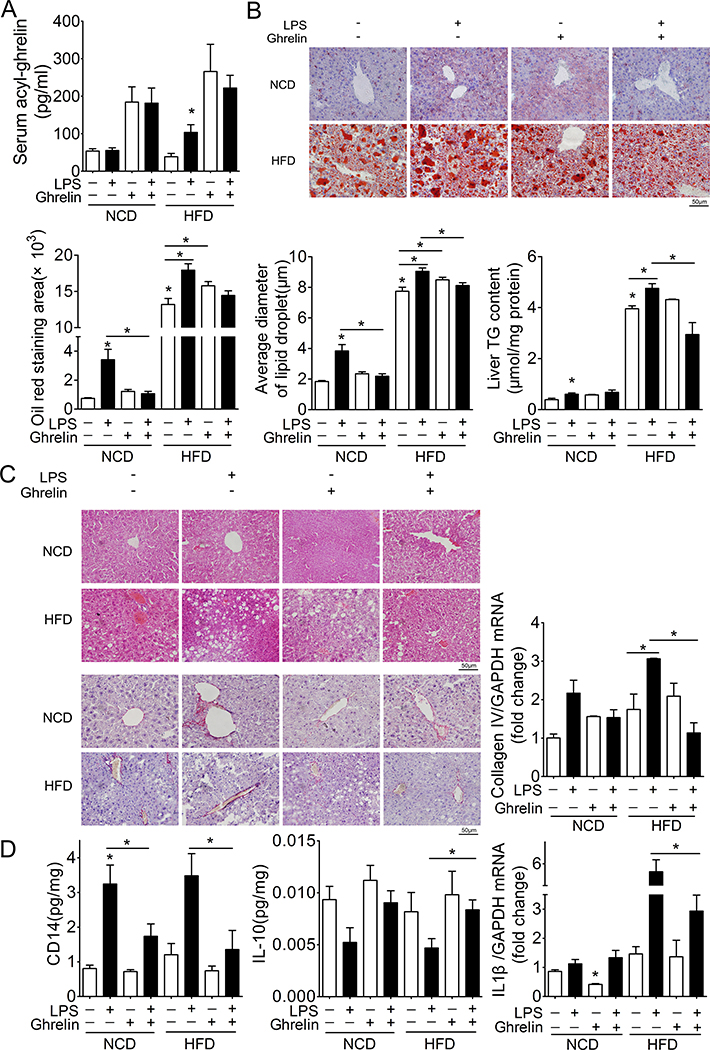
To determine whether ghrelin contributes to the progression of simple steatosis to NASH, we administrated exogenous ghrelin at a dose of 11nmol/kg/day via a subcutaneous micro-osmotic pump. As shown in Fig 2A, administration of exogenous ghrelin reversed the reduction of circulating ghrelin in mice fed HFD. Supplementation of exogenous ghrelin significantly ameliorated the development of NASH induced by low dose of LPS in mice fed HFD. Hepatic steatosis was significantly attenuated as evidenced by oil-red staining and levels of triglycerides (Fig 2B). Hepatic ballooning was markedly reduced (Fig. 2C). The increase in hepatic fibrosis was also reversed by ghrelin as evidenced by the reduction in collagen IV mRNA and Sirius red staining (Fig. 2C). The increment in proinflammatory cytokines such as IL1b and sCD14 was significantly attenuated by ghrelin in HFD mice receiving low dose of LPS (Fig. 2D). On the other hand, ghrelin reversed the reduction of anti-inflammatory cytokine IL10 in these animals (Fig. 2D). Further, plasma ghrelin in HFD mice was significantly reduced (Supplemental Fig 1). These observations demonstrate that reversal of circulating ghrelin to its physiological level is sufficient to block the hepatic inflammation and the development of NASH induced by low dose of LPS in mice fed HFD.
Figure 2. Ghrelin ameliorates NASH induced by low dose LPS.
Ghrelin (11nmol/kg/day) was administrated by subcutaneous micro-osmotic pump implantation into NCD or HFD mice receiving continuous infusion of LPS. Plasma and liver tissues were then harvested for analysis of NASH. n=12, * denotes P<0.05 vs control.
A. Levels of plasma ghrelin.
B. Oil-red staining and hepatic triglyceride (TG) levels.
C. H&E staining and sirius red staining and mRNA levels of collagen IV.
D. Levels of hepatic cytokines.
2. Kupffer cells/macrophages-dependent mechanism
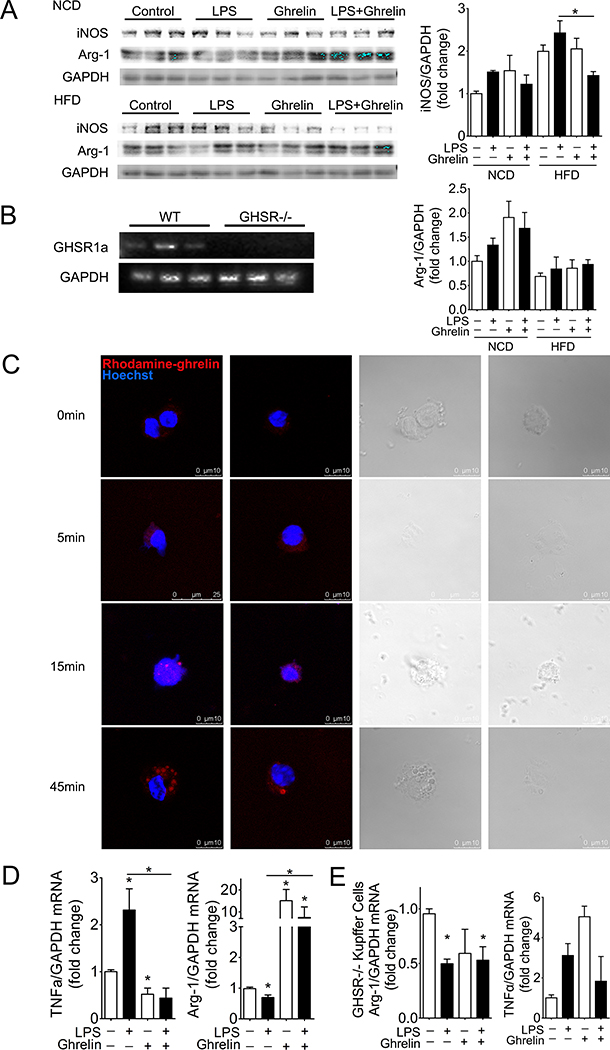
Since Kupffer cells/macrophages are critical for the progression of NASH, we next examined whether exogenous ghrelin alters the M1 polarization of these inflammatory cells. Ghrelin significantly suppressed the expression of iNOS, the M1 marker, while increasing levels of M2 marker Arg-1 in HFD mice receiving LPS (Fig 3A). This observation indicates that ghrelin may function through the Kupffer cells/macrophages to block the progression of NASH. We thus examined the presence of ghrelin receptor, the growth hormone secretagogue receptor 1a, (GHSR1a) in these cells. As shown in Fig. 3B, GHSR1a mRNA was detected in Kupffer cells/macrophages isolated from wild type mice but not from GHSR1a deficient transgenes. Incubation of isolated Kupffer cells/macrophages with rhodamine-labelled ghrelin demonstrated an internalization of GHSR1a upon binding with ghrelin (Fig. 3C). To further examine the effects of ghrelin on LPS-induced M1 polarization of isolated Kupffer cells/macrophages, we first analyzed the dose-dependent effects of LPS on TNFα and Arg-1. As shown in supplemental Fig 2, LPS at the dose of 4ng/ml demonstrated an efficient effect on the M1 polarization of isolated Kupffer cells/macrophages. This dose was used for the rest of experiments in this study. Ghrelin at the concentration of 10−8mol/L significantly reversed the up-regulation of TNFα and the suppression of Arg1 induced by LPS (Fig. 3D). This effect is GHSR1a-dependent because ghrelin demonstrated no effect on Kupffer cells/macrophages with deficiency of GHSR1a, the ghrelin receptor (Fig. 3E).
Figure 3. Ghrelin attenuates LPS-induced M1 polarization of Kupffer cells/macrophages.
Cultured Kupffer cells/macrophages were treated with LPS at the dose of 4ng/ml to induce M1 polarization. The effects of ghrelin on the M1 polarization of Kupffer cells/macrophages were examined by measurement of iNOS, TNFα and Arg-1. n=12, * denotes P<0.05 vs control.
A. Effects on hepatic levels of iNOS and Arg-1.
B. Presence of GHSR1a mRNA in isolated Kupffer cells/macrophages.
C. GHSR1a internalization. Kupffer cells/macrophages were treated with rhodamine-labelled ghrelin for indicated times and fluorescent signal detected by Leica microscope.
D. Effects on mRNA levels of TNFα and Arg-1 in cultured Kupffer cells/macrophages.
E. Ghrelin demonstrates no effect on TNFα and Arg-1 in Kupffer cells/macrophages with GHSR1a deficiency.
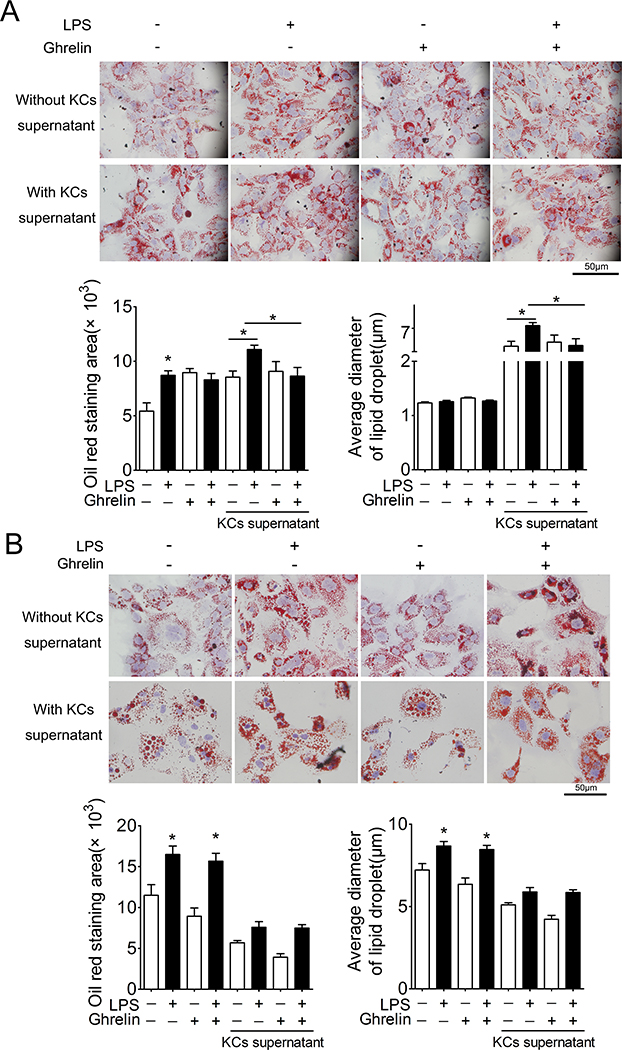
To determine whether ghrelin functions through Kupffer cells/macrophages to alter the lipid accumulation in hepatocytes, we used the supernatant of Kupffer cells/macrophages to treat cultured hepatocytes. As shown in Fig 4A, supernatant of Kupffer cells/macrophages treated with LPS demonstrated a more profound effect on lipid accumulation relative to the direct action of LPS on hepatocytes. Consistent with previous report(Li, Xu et al. 2014, Li, Yu et al. 2019), ghrelin significantly increased the lipid content in cultured hepatocytes treated with or without supernatants of Kupffer cells/macrophages. Further, supernatants from Kupffer cells/macrophages treated with ghrelin or vehicle demonstrate identical effects. However, ghrelin markedly blocked the stimulation of lipid accumulation in hepatocytes induced by supernatant of Kupffer cells/macrophages treated with LPS. Deletion of GHSR1a in Kupffer cells/macrophages eliminated the effect of ghrelin, indicating a GHSR1a-dependent action (Fig. 4B lower panel). In addition, deficiency of GHSR1a in hepatocytes significantly attenuated the effect of ghrelin on lipid accumulation in cultured hepatocytes treated with or without LPS (Fig 4B upper panel).
Figure 4. Ghrelin acts via GHSR1a on Kupffer cells/macrophages to attenuates LPS-induced lipid accumulation in hepatocytes.
Cultured Kupffer cells/macrophages were treated with LPS (4ng/ml) in the presence of ghrelin (10−8mol/L) or control vehicle for 6 h. Cultured medium was replaced with fresh medium to withdraw LPS and ghrelin. Cells were then cultured for another 12h. Supernatants were harvested and centrifuged, then added to cultured hepatocytes pretreated with 62.5μmol/L oleic acid for 24 h. Cultured hepatocytes were stained with Oil-red O and positive signal measured by area and average diameter. n=5, * denotes P<0.05 vs control.
A. Effects of ghrelin on Kupffer cells/macrophages. Both the hepatocytes and Kupffer cells/macrophages used were isolated from wild-type mice.
B. No effects of ghrelin on Kupffer cells/macrophages isolated from GHSR1a−/− mice. Upper panel: ghrelin could not block the effect of LPS on hepatocytes isolated from GHSR1a−/− mice.
Lower panel: hepatocytes were isolated from wild-type mice, while Kupffer cells/macrophages were from GHSR1a−/− mice.
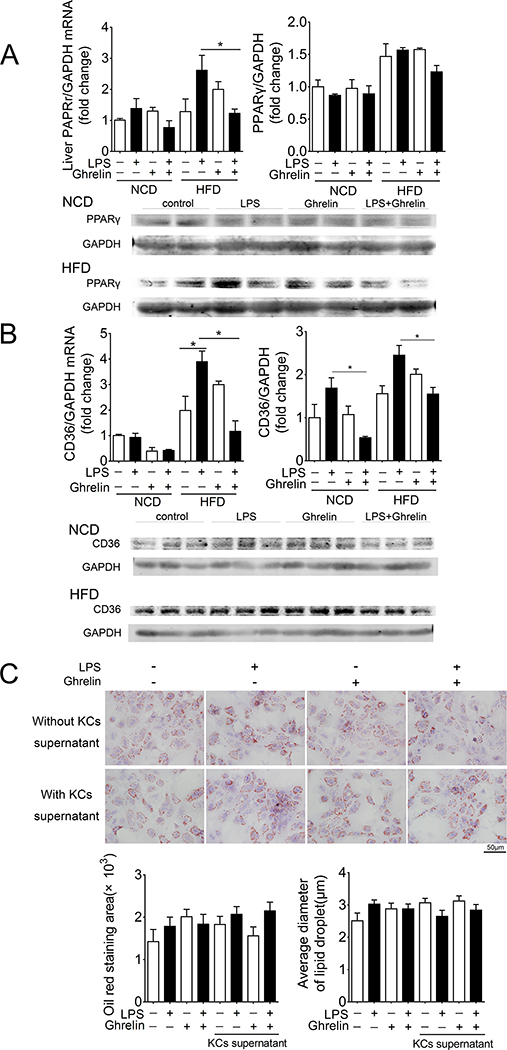
3. PPARγ-CD36-dependent mechanism
PPARγ-CD36 pathway plays an essential role in the hepatic lipid accumulation(Zhou, Febbraio et al. 2008). We thus examined this signaling pathway using the PPARγ+/− heterozygous mice. LPS increased the mRNA and protein levels of hepatic PPARγ (Fig. 5A) as well as CD36 in mice fed HFD (Fig. 5B). This effect was significantly attenuated by exogenous ghrelin. Further, deficiency of PPARγ significantly reduced the lipid accumulation under LPS-stimulated condition in mice fed HFD (Supplemental Fig. 3). PPARγ deficiency also blocked the effects of LPS and ghrelin on lipid accumulation in hepatocytes treated with or without supernatant of Kupffer cells/macrophages (Fig. 5C).
Figure 5. PPAR-γ dependent effects.
A. Ghrelin attenuates the up-regulation of hepatic PPAR-γ induced by low dose of LPS (240μg/kg/day) in mice fed NCD or HFD.
B. Ghrelin attenuates the up-regulation of hepatic CD36 induced by low dose of LPS (240μg/kg/day) in mice fed NCD or HFD.
C. No effects of ghrelin and LPS on lipid accumulation in hepatocytes treated with or without Kupffer cells/macrophages supernatants in the condition of PPAR-γ deficiency. n=5. Both Kupffer cells/macrophages and hepatocytes were isolated from PPAR-γ+/− mice.
n=5–6 each unless indicated otherwise. * denotes P<0.05 vs control.
Discussion
The switch from the M2 to M1 status of Kupffer cells in the liver is the key event for the pathogenesis of NAFLD and insulin resistance. While this switch is subject to the induction of intestinal bacteria byproducts, the role of LPS in the development and progression of NASH remains enigmatic. In this study, we found that low grade endotoxemia alone is insufficient to trigger hepatic inflammation if animals remain lean and healthy. This concept is supported by the following observations. First, continuous infusion of low dose LPS to increase its circulating concentration to the levels of those in HFD-induced obese mice did not alter hepatic levels of pro-inflammatory and anti-inflammatory cytokines IL1β and IL10 in lean mice fed NCD. Together with previous study(Jacob, Goldberg et al. 1977) detecting mild portal endotoxemia, this observation suggests that low grade endotoxemia alone is not sufficient to induce hepatic inflammation. Second, animals fed HFD for 8 weeks demonstrated moderate liver steatosis without significant inflammation even circulating LPS is mildly elevated. Third, this low dose of LPS resulted in a profound inflammatory response in the liver only in HFD-fed animals in which both lipid accumulation and circulating LPS are increased. Pro-inflammatory cytokine IL1β was significantly elevated, while anti-inflammatory cytokine IL10 down-regulated. Accompanied with these changes, there was a significant increase in hepatic ballooning and steatosis. All these changes suggest the progression to NASH.
Whether low grade endotoxiemia exacerbates the progression to NASH through its direct action on hepatocytes or indirect activation of Kupffer cells remains controversial. Previous studies have shown that gut-derived LPS can activate Kupffer cells either through its direct action on toll like receptor 4 (TLR4) or indirectly via the complement system: C3a/C3aR and C5a/C5aR(Baffy 2009). On the other hand, studies by Imajo et al(Imajo, Fujita et al. 2012) have indicated a direct mechanism: obesity renders hepatocytes hypersensitive to low dose of LPS, leading to the acceleration of NASH progression characterized by inflammation and fibrosis in the liver. This concept was further supported by the finding that deficiency of TLR4 in hepatocytes protects hepatic steatosis and insulin resistance induced by high fat diets(Jia, Vianna et al. 2014). Our study indicates that M1 activation induced by LPS is critical for the progression to NASH. First, low dose LPS increased CD14 positive Kupffer cells. This effect occured even in the absence of HFD-induced steatosis. Second, LPS increased levels of iNOS while decreased Arg1 in the liver and cultured Kupffer cells, suggesting a M1 polarization. Third, supernatant of cultured Kupffer cells treated with LPS was able to induce lipid accumulation in hepatocytes. This effect is likely mediated by PPAR-γ because deficiency in PPAR-γ significantly attenuated the capability of LPS to stimulate lipid accumulation in hepatocytes both in vivo and in vitro.
If M1 polarization of Kupffer cells is critical for the progression from simple steatosis to NASH induced by low grade endotoxemia, blockage of M1 polarization would be expected to improve or even reverse the NASH. Our studies provide further support for this concept. First, we demonstrated that both acute and chronic administration of exogenous ghrelin significantly ameliorated the NASH induced by low dose of LPS in mice fed HFD. Upon treatment with ghrelin, lipid accumulation, hepatic inflammation and fibrosis were significantly reduced. Second, ghrelin blocked the M1 activation of Kupffer cells induced by LPS while stimulated the M2 alternative activation of these immune cells. Third, supernatant of Kupffer cells pretreated with ghrelin was able to reduce LPS-stimulated lipid accumulation in hepatocytes. All these observations suggest that ghrelin, an endogenous anti-inflammatory factor, may counter the effects of low grade endotoxemia and thus attenuate the development and progression to NASH. Consistent with this concept, previous studies have shown that levels of ghrelin are significantly reduced in both obese animals and patients. Further, LPS has been reported to significantly inhibit circulating levels of ghrelin. Taken together, these studies indicate that the interaction between low-grade endotoxemia and gastrointestinal endocrine hormones may ultimately determine the progression of NAFLD. Only patients with low circulating ghrelin and subsequent impairment in anti-inflammatory response will deteriorate from simple steatosis to NASH. This concept may provide a reasonable explanation for the clinical observation that progression from simple steatosis to NASH occurs in a long-lasting course and in only a proportion of patients.
Majority of studies supports the concept that the anti-inflammatory effect of ghrelin occurs through the direct inhibition on immune cells: monocytes and Th1 lymphocytes. Ghrelin treatment suppresses T cell differentiation, inhibits the Th1-mediated inflammatory response, decreases the percentage of macrophage and the secretion of proinflammatory cytokines including IL-6, TNF-a, IL-1b, IL-12, IL-15, IL-17, IL-18 as well as chemokines responsible in recruitment of immune cells such as macrophage inflammatory protein (MIP) 1, MIP-2, interferon-gamma-inducible protein. Further, deletion of ghrelin-O-acytransferase (GOAT) significantly increases splenic macrophage in mice fed high fat diet(Harvey, Howard et al. 2017). The anti-inflammatory action of ghrelin appears to occur through the activation of its receptor: growth hormone secretagogue receptor 1a (GHSR1a). First, GHSR1a was detected in T lymphocytes and monocytes. Second, genetic and pharmacological intervention of GHSR1a significantly increased macrophage infiltration and activity(Dixit, Schaffer et al. 2004, Lin, Lee et al. 2016). Consistent with these observations, our studies extend the anti-inflammatory action of ghrelin to the hepatic Kupffer cells. Our finding demonstrated that ghrelin is a potent inhibitor for the M1 polarization of Kupffer cells and its action is dependent on the GHSR1a. mRNA specific for GHSR1a was detected in Kupffer cells by RT-PCR. Fluorescently-labelled ghrelin was demonstrated by confocal microscopy and ligand internalization detected. Deficiency in GHSR1a blocked the effect of ghrelin on the M1 activation of Kupffer cells and its subsequent up-regulation on hepatic lipid accumulation induced by LPS. Although some researches have shown the pro-inflammatory action of ghrelin-GHSR1a signaling(Zhao, Zhan et al. 2006, Liu, Wang et al. 2015), our findings do not support the pro-inflammatory effect on Kupffer cells for this signaling pathway. It is worth of noting that the effect of reduced lipid accumulation is much profound in GHSR1a−/− mice than ghrelin treated KCs supernatant. Whether this observation indicates that ghrelin and GHSR1a exercise independent effects as proposed by other investigators (Hedegaard and Holst B 2020, Mende, Hundahl et al. 2018) in Kupffer cells/macrophages and hepatocytes requires further exploration.
Several intracellular mechanisms have been proposed to mediate the anti-inflammatory effect of ghrelin. Ghrelin has been shown to activate p38mitogen-activated protein kinases (MAPK), leading to the up-regulation in the production of IL-10(Waseem, Duxbury et al. 2008). Dependent on the cell types, ghrelin has been reported to either stimulate NF-κB binding activity and NF-κB p65 subunit phosphorylation, or suppress the nuclear translocation of this nuclear factor, leading to increase in IL-8 production or protection of intestinal barrier(Cheng, Zhang et al. 2015). Our previous study has established mTOR/STAT3 pathway as a critical mechanism for the action of ghrelin on Th17 cell differentiation. In the present study, we have found that the effect of ghrelin on LPS-elicited M1 polarization of Kupffer cells may occur through a mechanism dependent on PPAR-γ. Deficiency of peroxisome proliferator-activated receptor-γ (PPARγ) in Kupffer cells significantly attenuated the efficacy of ghrelin to block the up-regulation of lipid accumulation in hepatocytes treated with supernatant of activated Kupffer cells. PPARγ is a key factor in the regulation of lipid metabolism in macrophages and has long been recognized as a suppressor for pro-inflammatory gene expression(Lawrence and Natoli 2011). In the condition of PPARγ deficiency, the ability of ghrelin to alter the lipid metabolism in hepatocytes may be complicated. This PPAR-γ dependent effect of ghrelin may occur via two pathways: the direct action on hepatocytes(Li, Xu et al. 2014) in the physiological condition and the indirect action mediated by Kupffer cells/macrophages evidenced by present study. In the condition of low grade inflammation, the indirect effect via Kupffer cells/macrophages out-performs the direct effect.
According to the concept of lipotoxicity, lipids such as fatty acids are the noxious molecules responsible for organ injury(Listenberger, Han et al. 2003, Kusunoki, Kanatani et al. 2006, McClain, Barve et al. 2007). On the other hand, triglycerides are protective. Our previous studies have demonstrated that ghrelin stimulates the de novo synthesis of triglycerides in hepatocytes. Our present study also proves that in the absence of inflammation, ghrelin enhances the synthesis of triglyceride in liver. Under the condition of low grade inflammation induced by low dose LPS, ghrelin functions as a potent anti-inflammatory factor to suppress the M1 polarization of Kupffer cells/macrophages, leading to the subsequent inhibition of NASH. Our study thus provide evidence supporting the dual action of ghrelin (Fig. 6). Under physiological condition, ghrelin functions to clean the extra lipid by increasing lipid accumulation in hepatocytes. Consistent with this concept, ghrelin demonstrates negligible effect on HFD-induced steatohepatitis in our current study. In case of low grade inflammation, this peptide acts to block the development of NASH via suppression of Kupffer cells/macrophages M1 polarization.
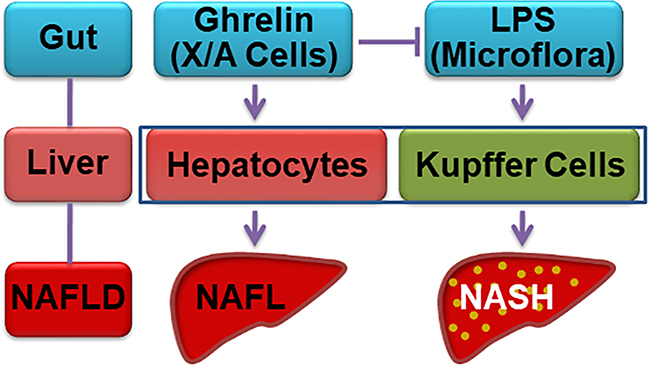
Figure 6. Stomach-liver crosstalk in NASH.
Under physiological condition, ghrelin acts directly on hepatocytes to stimulate lipogenesis. In the condition of chronic inflammation induced by low concentration of LPS derived from intestine microflora, ghrelin suppresses the inflammation by acting on Kupffer cells, leading to the amelioration of NASH.
In summary, our studies reveal a chemical signal-dependent mechanism underlying the gut-liver crosstalk. Interaction between the intestine-derived LPS and gastric endocrine hormone ghrelin is critical for the development and progression from simple steatosis to NASH. Ghrelin may serve as a marker in predicting the progression to NASH, and also provide an attractive strategy for the intervention of NASH.
Supplementary Material
Acknowledgements:
This work was supported by funds from the National Key R&D Program of China (2017YFC0908900), the National Natural Science Foundation of China (81730020, 81700516, 81930015) and National Institutes of Health Grant R01 DK112755 and 1R01DK110273.
Abbreviations
- NASH
nonalcoholic steatohepatitis
- GHSR1a
growth hormone secretagogue receptor 1a
- LPS
lipopolysaccharides
- PPARγ
peroxisome proliferator-activated receptor γ
- HFD
High fat diet
- NCD
normal chow diet
Footnotes
Declaration of interests:
All authors declare no conflict of interest.
Data sharing:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Baffy G (2009). “Kupffer cells in non-alcoholic fatty liver disease: the emerging view.” J Hepatol 51(1): 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzetti E, Pinzani M and Tsochatzis EA (2016). “The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD).” Metabolism 65(8): 1038–1048. [DOI] [PubMed] [Google Scholar]
- Cheng J, Zhang L, Dai W, Mao Y, Li S, Wang J, Li H, Guo C and Fan X (2015). “Ghrelin ameliorates intestinal barrier dysfunction in experimental colitis by inhibiting the activation of nuclear factor-kappa B.” Biochem Biophys Res Commun 458(1): 140–147. [DOI] [PubMed] [Google Scholar]
- Chorny A, Anderson P, Gonzalez-Rey E and Delgado M (2008). “Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria.” J Immunol 180(12): 8369–8377. [DOI] [PubMed] [Google Scholar]
- Cohen RI, Chandra S, Koenig S, Tsang D, Wilson D and McCloskey T (2010). “Ghrelin receptor expression in lymphocytes isolated from adult cystic fibrosis patients.” Respiration 79(2): 141–146. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW Jr. and Taub DD (2004). “Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells.” J Clin Invest 114(1): 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB and Korbonits M (2002). “The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans.” J Clin Endocrinol Metab 87(6): 2988. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A and Delgado M (2006). “Therapeutic action of ghrelin in a mouse model of colitis.” Gastroenterology 130(6): 1707–1720. [DOI] [PubMed] [Google Scholar]
- Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM, Amin AI, Burt AD, Kumar S, Day CP and McTernan PG (2010). “Elevated endotoxin levels in non-alcoholic fatty liver disease.” J Inflamm (Lond) 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RE, Howard VG, Lemus MB, Jois T, Andrews ZB and Sleeman MW (2017). “The Ghrelin/GOAT System Regulates Obesity-Induced Inflammation in Male Mice.” Endocrinology 158(7): 2179–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard MA and Holst B (2020). “The Complex Signaling Pathways of the Ghrelin Receptor.” Endocrinology 161(4):bqaa020. [DOI] [PubMed] [Google Scholar]
- Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, Kato S, Mawatari H, Shibata W, Kitani H, Ikejima K, Kirikoshi H, Nakajima N, Saito S, Maeyama S, Watanabe S, Wada K and Nakajima A (2012). “Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling.” Cell Metab 16(1): 44–54. [DOI] [PubMed] [Google Scholar]
- Jacob AI, Goldberg PK, Bloom N, Degenshein GA and Kozinn PJ (1977). “Endotoxin and bacteria in portal blood.” Gastroenterology 72(6): 1268–1270. [PubMed] [Google Scholar]
- Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C, Sun K, Liu T, Harper MJ, Lee CE, Lee S, Scherer PE and Elmquist JK (2014). “Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance.” Nat Commun 5: 3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo GC, Huang C, Camacho R, Trainor C, Blake JT, Sirotina-Meisher A, Schleim KD, Wu TJ, Cheng K, Nargund R and McKissick G (2001). “Immune enhancing effect of a growth hormone secretagogue.” J Immunol 166(6): 4195–4201. [DOI] [PubMed] [Google Scholar]
- Kusunoki J, Kanatani A and Moller DE (2006). “Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome.” Endocrine 29(1): 91–100. [DOI] [PubMed] [Google Scholar]
- Lawrence T and Natoli G (2011). “Transcriptional regulation of macrophage polarization: enabling diversity with identity.” Nat Rev Immunol 11(11): 750–761. [DOI] [PubMed] [Google Scholar]
- Li Z, Xu G, Qin Y, Zhang C, Tang H, Yin Y, Xiang X, Li Y, Zhao J, Mulholland M and Zhang W (2014). “Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARgamma signaling pathway.” Proc Natl Acad Sci U S A 111(36): 13163–13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yu R, Yin W, Qin Y, Ma L, Mulholland M and Zhang W (2019). “mTOR Signaling in X/A-Like Cells Contributes to Lipid Homeostasis in Mice.” Hepatology 69(2): 860–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Lee JH, Buras ED, Yu K, Wang R, Smith CW, Wu H, Sheikh-Hamad D and Sun Y (2016). “Ghrelin receptor regulates adipose tissue inflammation in aging.” Aging (Albany NY) 8(1): 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr., Ory DS and Schaffer JE (2003). “Triglyceride accumulation protects against fatty acid-induced lipotoxicity.” Proc Natl Acad Sci U S A 100(6): 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZZ, Wang WG, Li Q, Tang M, Li J, Wu WT, Wan YH, Wang ZG, Bao SS and Fei J (2015). “Growth hormone secretagogue receptor is important in the development of experimental colitis.” Cell Biosci 5: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loguercio C, De Simone T, D’Auria MV, de Sio I, Federico A, Tuccillo C, Abbatecola AM, Del Vecchio Blanco C and Italian ACG (2004). “Non-alcoholic fatty liver disease: a multicentre clinical study by the Italian Association for the Study of the Liver.” Dig Liver Dis 36(6): 398–405. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Barve S and Deaciuc I (2007). “Good fat/bad fat.” Hepatology 45(6): 1343–1346. [DOI] [PubMed] [Google Scholar]
- Mende F, Hundahl C, Plouffe B, Skov LJ, Sivertsen B, Madsen AN, Lückmann M, Diep TA, Offermanns S, Frimurer TM, Bouvier M and Holst B (2018). “Translating biased signaling in the ghrelin receptor system into differential in vivo functions.” Proc Natl Acad Sci U S A 115(43):E10255–E10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M and Wallace M (2007). “Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis.” J Hepatol 47(4): 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K and Matsukura S (2002). “Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion.” J Clin Endocrinol Metab 87(1): 240–244. [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel-Stengel M, Jawien J, Kobelt P, Tache Y and Lambrecht NW (2011). “Lipopolysaccharide increases gastric and circulating NUCB2/nesfatin-1 concentrations in rats.” Peptides 32(9): 1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Reeve JR Jr., Tache Y and Lambrecht NW (2010). “Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: potential role of the circulating ghrelin-acylating enzyme GOAT.” Peptides 31(9): 1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A, Lotersztajn S and Pavoine C (2014). “M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease.” Hepatology 59(1): 130–142. [DOI] [PubMed] [Google Scholar]
- Waseem T, Duxbury M, Ito H, Ashley SW and Robinson MK (2008). “Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways.” Surgery 143(3): 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Li Y, An W, Li S, Guan Y, Wang N, Tang C, Wang X, Zhu Y, Li X, Mulholland MW and Zhang W (2009). “Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake.” Endocrinology 150(8): 3637–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Li Z, Gao L, Li Y, Zhao J and Zhang W (2015). “AMPK-dependent modulation of hepatic lipid metabolism by nesfatin-1.” Mol Cell Endocrinol 417: 20–26. [DOI] [PubMed] [Google Scholar]
- Zhao D, Zhan Y, Zeng H, Moyer MP, Mantzoros CS and Pothoulakis C (2006). “Ghrelin stimulates interleukin-8 gene expression through protein kinase C-mediated NF-kappaB pathway in human colonic epithelial cells.” J Cell Biochem 97(6): 1317–1327. [DOI] [PubMed] [Google Scholar]
- Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li S, Silverstein RL and Xie W (2008). “Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis.” Gastroenterology 134(2): 556–567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.