Abstract
Cocaine use disorder is a major health crisis that is associated with increased oxidative stress and neuroinflammation. While the role of NLRP3 inflammasome in mediating neuroinflammation is well-recognized, whether cocaine induces this response remains unexplored. Based on the premise that cocaine induces both reactive oxygen species (ROS) as well as microglial activation, we hypothesized that cocaine-mediated microglial activation involves both ROS and NLRP3 signaling pathways. We examined activation of the NLRP3 pathway in microglia exposed to cocaine, followed by validation in mice administered either cocaine or saline for 7 days, with or without pretreatment with the NLRP3 inhibitor, MCC950 and in post-mortem cortical brain tissues of chronic cocaine-dependent humans. We found that microglia exposed to cocaine exhibited significant induction of NLRP3 and mature IL-1β expression. Intriguingly, blockade of ROS (Tempol) attenuated cocaine-mediated priming of NLRP3 and microglial activation (CD11b). Blockade of NLRP3 by both pharmacological (MCC950) as well as gene silencing (siNLRP3) approaches underpinned the critical role of NLRP3 in cocaine-mediated activation of inflammasome and microglial activation. Pretreatment of mice with MCC950 followed by cocaine administration for 7 days mitigated cocaine-mediated upregulation of mature IL-1β and CD11b, in both the striatum and the cortical regions. Furthermore, cortical brain tissues of chronic cocaine-dependent humans also exhibited upregulated expression of the NLRP3 pathway mediators compared with non-cocaine dependent controls. Collectively, these findings suggest that cocaine activates microglia involving the NLRP3 inflammasome pathway, thereby contributing to neuroinflammation. NLRP3 can thus be considered as a potential therapeutic target for alleviating cocaine-mediated neuroinflammation.
Keywords: cocaine, NLRP3, IL-1β, reactive oxygen species, tempol, MCC950
Background
Cocaine use disorder (CUD) is estimated to affect up to 22.5 million people worldwide resulting in marked changes in behavior and lifestyle emanating from its psychoactive and addictive effects [1]. In the US, cocaine use is increasing among young adults suggesting that cocaine abuse is an emerging issue among young adults [2]. Accumulating evidence suggests a close link between drug abuse and neuroinflammation [3,4]. In fact, CUD is associated with enhanced immune activation, inflammation and neuronal injury which, in turn, further contributes to behavioral alterations [5–9]. Several mechanism(s) such as cocaine-induced production of reactive oxygen species (ROS) and inflammasome activation have been suggested to underlie cocaine-associated behavioral deficits [10,11]. Cocaine is a well-known inducer of ROS [6], which has been implicated as an upstream mediator in the activation of NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome [12,13].
Several studies have demonstrated the contribution of ROS in cocaine-mediated physiological alterations and addictive behavior [6,14–18]. Increased ROS production is shown to be associated with oxidized metabolites of cocaine (e.g., norcocaine derivatives) which, subsequently activate the redox cycling pathways and subsequent electron transfer [19,10]. Increased ROS has also been well-documented to elicit inflammatory responses within the CNS via activation of glial cells such as the microglia - the primary immunocompetent cells of the brain [20,21,15,22]. Under normal homeostasis, microglia play essential roles involving surveillance of the brain environment, phagocytosis, synaptic pruning and neuronal plasticity [23–25]. Several psychostimulant drugs, such as cocaine and methamphetamine, have been shown to activate microglia, resulting in the production of pro-inflammatory cytokines, ultimately contributing to a neuroinflammatory milieu that underlies neurodegeneration and behavioral changes [26–29]. The interrelationship of cocaine-induced ROS and inflammasome activation, however, remains poorly understood. In this study we rationalized that inhibition of cocaine-mediated ROS generation could result in abrogation of cellular activation by dampening activation of the NLRP3 inflammasome, which has been linked to ROS [13,12].
Activation of the NLRP3 inflammasome is a two-step process comprising of signals 1 and 2; with the first signal priming the pathway by enhancing levels of NLRP3, followed by the second signal that triggers caspase-1 dependent cleavage, maturation and release of the pro-inflammatory cytokines IL-1β and IL-18 [30–34]. Although a previous report has shown an increase in the expression of NLRP3 mRNA in macrophages exposed to cocaine and HIV-1 [35], the causal association between NLRP3 activation and ROS in microglia and its contribution to cocaine-induced behavioral changes, however, remains poorly understood. Herein, we investigated the molecular mechanism(s) underlying cocaine-mediated NLRP3 inflammasome activation in microglia and the in vivo effects of NLRP3 blockade in reducing microglial activation and inflammation, in mice administered cocaine.
Using both primary microglia and cocaine administration of mice for 7 days, our findings suggested that cocaine activates microglia via the NLRP3 inflammasome. Moreover, our findings also identified ROS-dependent priming of NLRP3 as a potential mechanism underlying cocaine-induced activation of microglia and neuroinflammation. Intriguingly, pharmacological inhibition of NLRP3 was shown to dampen cocaine-mediated microglial activation in mice. Overall, these findings thus demonstrate a critical association between ROS, NLRP3 inflammasome and microglial activation as contributors of neuroinflammation.
Methods
Reagents
Antibodies and reagents used in this work were purchased from the indicated sources: NLRP3 (AdipoGen; AG-20B-0014); ASC (Novus; NB1–78978 for western blots and Adipogen; AG-25B-0006 for IHC), caspase-1 p20 (Santa Cruz; sc-398715), Iba-1 (Novus NB1001028 or Wako; 19–19741), CD11b (Novus; NB11089474), goat anti-mouse-HRP (Santa Cruz Biotechnology; sc-2005) and goat anti-rabbit-HRP (Santa Cruz Biotechnology; sc-2004); IL-1 beta (Abcam; ab9722); actin (Sigma-Aldrich; A1978). Cryopyrin/NLRP3 siRNA (sc-45470) was from Santa Cruz Biotechnology. MCC950/CP-456773 (Sigma; pz0280) was from Sigma-Aldrich. Cocaine hydrochloride (C5776) and LPS (L2880) was from Sigma-Aldrich. Sterile water was used to dissolve cocaine and LPS. ATP (tlrl-atpl) was from Invivogen. FAM-FLICA caspase assay kit (#97) was from ImmunoChemistry
Animals
All animal procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Centre and the National Institutes of Health. The C57BL/6N mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed under standard vivarium conditions. Food and water were available ad libitum [34]. C57BL/6 wild type mice were divided into groups; receiving cocaine or saline injections for seven consecutive days in the presence of absence of MCC950 pretreatment. Mice were injected with NLRP3 inhibitor MCC950 (50 mg/kg or vehicle, i.p.) daily followed by cocaine administration (20 mg/kg, i.p.) an hour later. Mice were sacrificed 1 h following the last injection. Brain tissues were dissected, and homogenates of the cortex and the striatum were used to assess the protein levels of NLRP3 and microglial activation markers.
Human tissues
Frozen frontal cortex tissues from cocaine abusers or non-cocaine abusing controls or were obtained from the Douglas-Bell Canada Brain Bank (DBCBB; Douglas Mental Health University Institute, Montreal, Quebec, Canada). The DBCBB samples were collected post-mortem following consent according to tissue banking practices regulated under IUSMD-04–21 Banque de cerveaux suicides du Québec. Patient characteristics are shown in Table S1. Protein was isolated and the expression of NLRP3 pathway was analyzed by western blotting.
Isolation of primary mice microglial cells
Primary microglial cells were isolated from C57BL/6N newborn mice pup brains as previously described [36,22,34]. The purity of isolated microglia was confirmed by immunohistochemical staining with antibodies specific for Iba-1 and was routinely found to be >95% pure [34,36].
BV-2 cells
Mouse BV-2 microglial cell line was generously provided by Dr. Sanjay Maggirwar (George Washington University, Department of Microbiology, Immunology, and Tropical Medicine, USA). These cells were grown and routinely maintained at 37°C, and 5% CO2 in DMEM supplemented with 10 % heat-inactivated Fetal Calf Serum (# S11150H, Atlanta Biologicals, GA, USA), 100 IU/ml Penicillin and 100μg/ml streptomycin as previously described [36].
Western blotting
Mouse brain tissue homogenates or microglia exposed to cocaine were lysed with RIPA buffer supplemented with a protease inhibitor cocktail (#78430, ThermoFisher Scientific, MA) followed by ultrasonication for 15 sec at 80% amplitude. Western blotting was performed as previously described [36,22,34,37]. Densitometric analyses was performed using NIH ImageJ software (ImageJ v1.44, NIH) as previously described [37]. Protein amounts for bands of interest were normalized to β-actin.
Small interfering RNA (siRNA) transfection
siRNA transfections were performed using Lipofectamine 2000 (11668027, Life Technologies, CA) according to the manufacturer’s instructions. Briefly, cells were transfected with targeted siRNA or scrambled siRNA (20 pM) mixed with 6 μl of Lipofectamine 2000 diluted in 150 μl Opti-MEM Reduced Serum Medium (#31985062, Life Technologies, CA). The resulting siRNA-lipid complexes were added onto cells, incubated for 6 h and the medium was refreshed and maintained up to 24 h. Cells were subsequently treated with cocaine (10 μM) or left untreated and harvested after an additional 24 h as previously described [22,36,34]. Knockdown efficiencies were determined by western blotting.
FAM-FLICA caspase-1 assay
Microglia were seeded into 24-well plates overnight, followed by exposure to medium with cocaine, LPS or left untreated for 24 hr. Cells were treated with ATP (1 mM) for 1h followed by FAM-FLICA assay (#97) according to the manufacturer’s instructions.
IL-1β Cytokine assay
Microglia were seeded onto 96-well plates (8000 cells/well) overnight, and subsequently exposed to medium with cocaine or left untreated. Supernatant fluids were collected at 24 h post-cocaine treatment, followed by quantification of IL-1β by ELISA (MLB00C, R&D, Minneapolis, USA) according to the manufacturer’s instructions.
Immunohistochemistry
Double immunofluorescence staining for NLRP3 and Iba1 or ASC was performed in the whole brain sections from mice injected with either cocaine (20 mg/kg body weight, i.p.) or saline for 7 consecutive days or on microglia cultured as previously described [22]. Briefly, for brain slides, formalin-fixed, paraffin-embedded brain slides were baked overnight at 55°C. The sections were deparaffinized using xylene and rehydrated by incubating the slide in graded series (100, 95 and 70%; each 5 min) of alcohol. Next, the slides were subjected to antigen retrieval by boiling them in Tris/EDTA buffer (pH 9) for about 20 min. Microglia cells on coverslips were fixed with 4% formaldehyde in PBS for 10 min at room temperature, washed three times with PBS, permeabilized with 0.3% Triton X-100 in PBS with 10 % goat serum for 1 h as previously described [22]. The tissues were then blocked with 10% goat serum (#S-1000–20, Vector Laboratories, CA, USA) in PBS for 2hr followed by overnight co-incubation with primary antibodies (NLRP3, ASC or Iba-1) at 4°C. Next day, the slides were washed with PBS for three times, followed by incubation with corresponding secondary Alexa Fluor 488 goat anti-mouse IgG (#A-11008, Invitrogen, CA) or Alexa Fluor 594 goat anti-rabbit (#A-11032, Invitrogen, CA) for 2 h. Finally, the slides were washed with PBS for three times and mounting with ProLong Gold Antifade Reagent with DAPI. Fluorescent images were taken on a Zeiss Observer using a Z1 inverted microscope (Carl Zeiss, Thornwood, NY, USA). The acquired images were analyzed for the fluorescence intensity and co-localization using the Axio Vs 40 Version 4.8.0.0 software (Carl Zeiss MicroImaging GmbH). To calculate microglial process length, images were converted to a binary format and skeletonized for analysis using Image J software. All experiments were repeated at least three times.
Statistical analysis
Graphs and statistical analyses were performed using GraphPad software V5.0 (GraphPad Prism Software). Student’s t test was used to compare results between test and controls. One-way ANOVA was used for multiple comparisons. P values less than 0.05 were considered statistically significant.
Results
Cocaine exposure upregulated the expression of NLRP3 in microglia
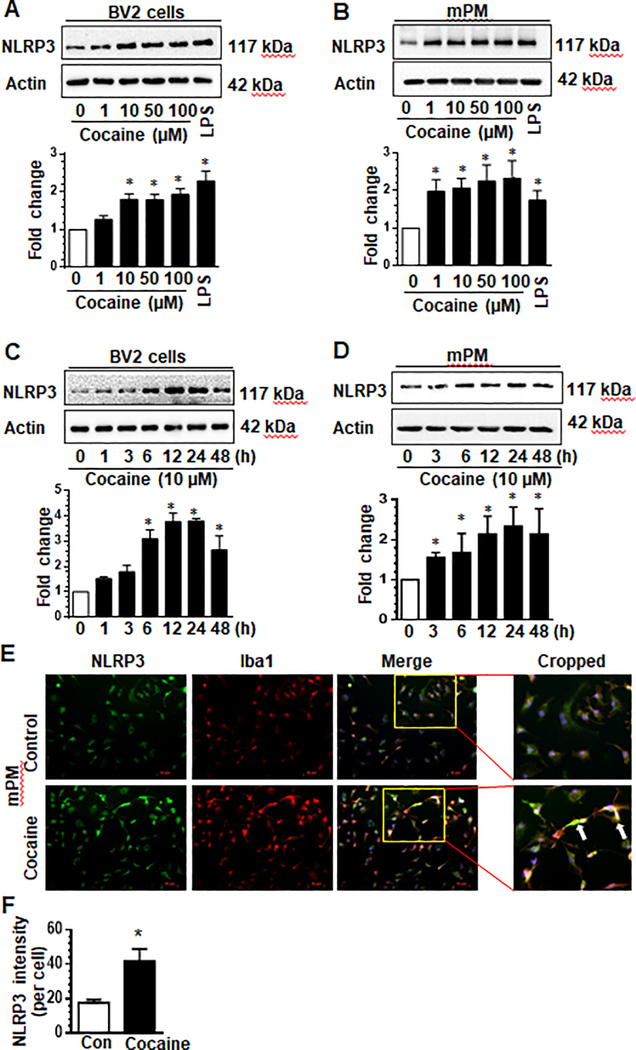
We first sought to examine whether exposure of microglia to cocaine could activate these cells involving the NLRP3 inflammasome. Mouse primary microglia (mPM) or BV-2 microglial cells were exposed to various concentrations of cocaine (0, 1, 10, 50, 100 μM) for 24 hours and analyzed for the expression of NLRP3. As shown in Fig. 1a & b, cocaine dose-dependently increased the expression of NLRP3, with significant increases at 10 – 100 μM in both mPM and BV-2 cells. As anticipated, exposure of cells to bacterial lipopolysaccharide (LPS) (50 ng/ml), also caused a significant increase in NLRP3 expression compared with control cells (Fig. 1a & b). Based on these findings, we chose the lowest dose of cocaine (10 μM) that upregulated expression of NLRP3 in BV-2 cells, for all the ensuing experiments.
Fig. 1. Cocaine upregulates the expression of NLRP3 in microglia cells in a dose- and time-dependent manner.
BV-2 cells and mouse primary microglia (mPMs) were seeded into 6-well plates and exposed to the indicated doses of cocaine for 24 h. Cocaine (10 μM) concentration increased the expression of NLRP3 in both cell types (a, b). BV-2 cells and mPMs were seeded into 6-well plates and exposed to cocaine (10 μM) for the indicated time periods. Exposure of these cells to cocaine increase the expression of NLRP3 time dependently in both cell types (c, d). mPMs were seeded into 24 well plates and exposed to cocaine for 24 h. The expression of NLRP3 and Iba-1 was analyzed at 24 h post-exposure (e). Quantification of Iba-1 fluorescence Intensity (f). All experiments were done at least three independent times, and representative figures are shown. LPS and actin served as positive or loading controls respectively. Quantification of western blots is shown under each blot. Data are shown as mean±SEM and *p< 0.05 vs control.
The next step then was to assess the time-course of cocaine-mediated upregulation of NLRP3. As shown in Fig. 1c, we found significant upregulation of NLRP3 protein at 6 h post-cocaine exposure (10 μM) that was sustained up to 48 h (p < 0.05) in BV-2 cells. These findings were also confirmed in mPMs exposed to cocaine which revealed a sustained increase in the expression of NLRP3 up to 48 h (Fig. 1d). To further validate cocaine-induced upregulation of NLRP3, immunofluorescence imaging of mPMs exposed to cocaine was performed. As shown in Fig. 1e, cocaine increased the expression of both NLRP3 and Iba-1 compared with control cells. Quantification of NLRP3 fluorescence intensity is shown in Fig. 1f. Collectively, these data demonstrated that cocaine upregulated the expression of microglial NLRP3.
Effect of cocaine on NLRP3 inflammasome formation and activation of caspase-1 and IL-1β in microglial cells
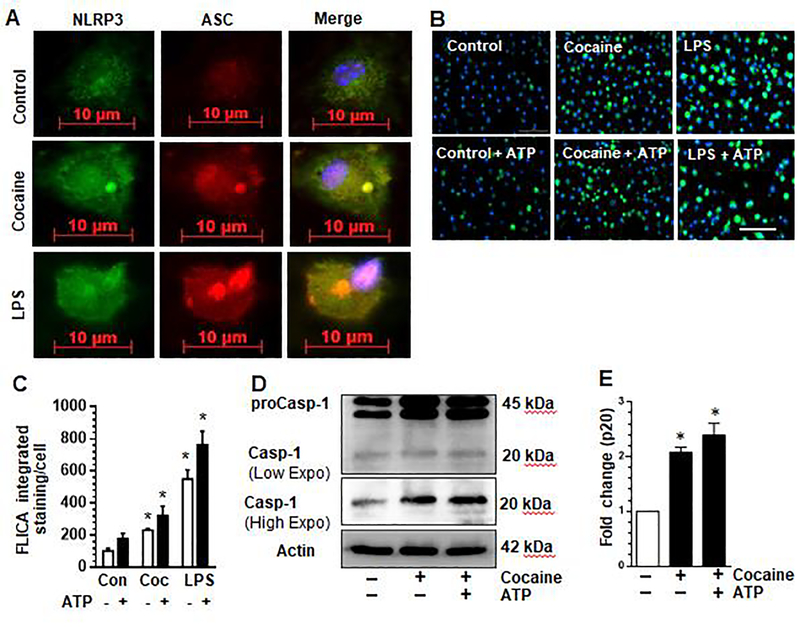
Next, we examined whether cocaine exposure could increase generation of the NLRP3 inflammasome complex by immunostaining microglia for both NLRP3 and the adaptor molecule, Apoptosis-associated speck-like protein containing a CARD (ASC) by immunofluorescence. As shown in Fig. 2a ASC speck formation was observed in the cocaine exposed or LPS positive control cells compared with untreated control cells. We next sought to assess whether exposure of microglia to cocaine could activate pro-caspase-1 which undergoes proteolytic maturation leading to detection of active caspase-1 using the FAM-FLICA assay. The FLICA probe is a non-cytotoxic Fluorescent Labeled Inhibitor of Caspase-1 that covalently bind to active caspase-1 enzyme. Mouse primary microglia were seeded in 24 well plates and exposed to cocaine for 24 hours followed by subjecting cells to the FAM-FLICA assay according to manufacturer’s instructions. As shown in Fig. 2b, exposure of microglia to cocaine (10 μM) or LPS (50 ng/ml) resulted in increased FLICA fluorescence signal. FLICA intensity was higher in cells exposed to cocaine or LPS in the presence of ATP. The effect of LPS in increasing FLICA intensity was at least twice as much as that of cocaine suggesting that cocaine-induced caspase-1 activation is milder than that induced by LPS at the concentrations assayed. Quantification of FLICA fluorescence intensity is shown in Fig. 2c. To validate caspase-1 cleavage we analyzed expression of both total and cleaved caspase-1 p20 by western blot. As shown in Fig. 2d and quantified in Fig. 2e, exposure of cells to cocaine increased the expression of cleaved caspase-1.
Fig. 2. Cocaine induced NLRP3 inflammasome formation and activation caspase-1 in microglia.
Mouse primary microglia (mPMs) were seeded into 24-well plates and exposed to cocaine for 24 h. The expression and presence of ASC specks was analyzed at 24 h post-exposure (a). Cells were seeded in 24-well black plates with glass bottom and exposed to cocaine (10 μM) or LPS (50 ng/ml) for 24 h followed by addition of ATP (2.5 mM) or left untreated for 1h. The FLICA probe was added for 1 h, followed by washing and fluorescence imaging (b). Densitometric quantification of FLICA fluorescence (c). Expression of procaspase-1 and cleaved caspase-1, p20. Cleaved caspase-1 p20 is shown at both low- and high exposure (d). The high exposure images of caspase-1 p20 were quantified (e). All experiments were done at least three independent times. Data are shown as mean±SEM and *p< 0.05 vs control.
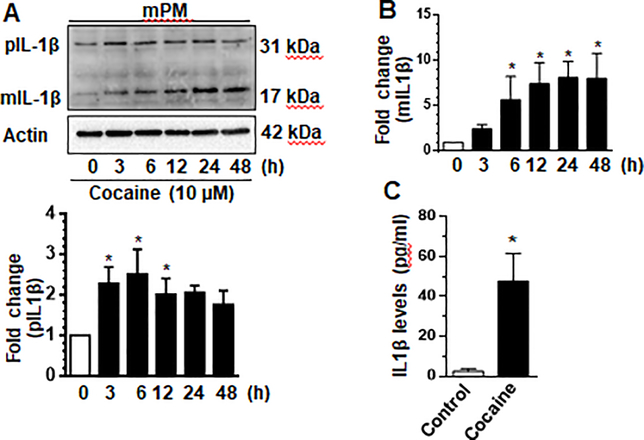
To further validate cocaine-mediated activation of NLRP3 inflammasome, we determined the expression levels of IL-1β in microglia exposed to cocaine over a 48-h time-course. As shown in Fig. 3a & b, mPMs exposed to cocaine demonstrated a time-dependent increase of the mature IL-1β, (p17) at 6–48 h. We also sought to examine cocaine-mediated release of IL-1β into the supernatant fluids. As shown in Fig. 3c, there was a significantly (p<0.05) increased release of IL-1β 24 h in mPMs exposed to cocaine compared with cells not exposed to cocaine.
Fig. 3. Cocaine mediated the maturation of IL-1β in microglia.
Mouse primary microglia (mPMs) were seeded into 6-well plates and exposed to cocaine (10 μM) for the indicated time periods. Exposure to cocaine induced the expression of mature IL-1β (mIL-1β) time-dependently (a, b). Cocaine exposure resulted in increased release of IL-1β into supernatants from mPMs as quantified by ELISA (c). ELISA experiments were done with 6 replicates per each condition. All experiments were done at least three independent times. Actin served as a loading control. Quantification of pro IL-1β (pIL-1β) and mIL-1β is shown in (a) and (b) respectively. Data are shown as mean±SEM and *p< 0.05 vs control.
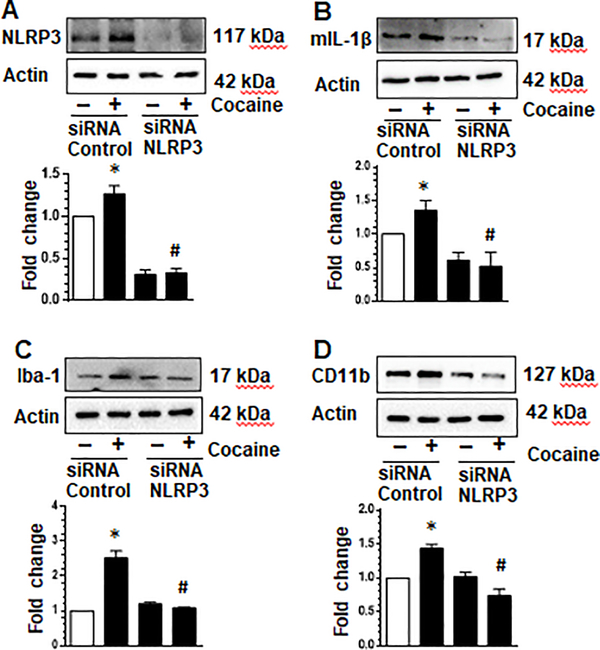
Genetic silencing of NLRP3 attenuated cocaine-mediated activation of NLRP3 inflammasome and cellular activation in microglia
Next, we used a gene silencing approach to validate the role of NLRP3 in cocaine-induced activation of the NLRP3 inflammasome. Herein, mPMs were transfected with either scrambled or NLRP3 siRNA (24 h) followed by exposure of transfected cells to cocaine for an additional 24 hr. As shown in Fig. 4a, in cells transfected with NLPR3 siRNA cocaine failed to upregulate the expression of NLRP3 compared with cells transfected with the scrambled siRNA. Similarly, there was abrogation of cocaine-mediated maturation of IL-1β as evidenced by reduced expression of mature IL-1β in cells knocked down for NLRP3 (Fig. 4b). In addition, cells knocked out for NLRP3 failed to demonstrate cocaine-mediated activation of cellular activation markers Iba-1 and CD11b (Fig. 4c & d). These findings thus underscore the role of NLRP3 in cocaine-mediated activation of both microglial inflammasome as well as cellular activation.
Fig. 4. Cocaine-mediated NLPR3 inflammasome and cellular activation was attenuated by NLRP3 siRNA.
Mouse primary microglia were seeded into 6-well plates and treated with either siNLRP3 or control siRNA. Cells were subsequently exposed to cocaine (10 μM) or left untreated for 24 h and expression of NLRP3, IL-1β, Iba-1 and CD11b analyzed by western blotting. NLRP3 expression was reduced by siNLRP3 knockdown (a). Exposure of cells treated with siNLRP3 to cocaine did not increase NLRP3 levels compared with control cells (a); or mature IL-1b, Iba-1 and CD11b (b-d respectively). All experiments were done at least three independent times, and representative figures are shown. Actin served as a loading control and quantification of western blots are shown under each blot. Data are shown as mean±SEM and *p< 0.05 vs control or # vs cocaine/control siRNA group.
Cocaine upregulated NLRP3 inflammasome pathway via the sigma receptor in microglia
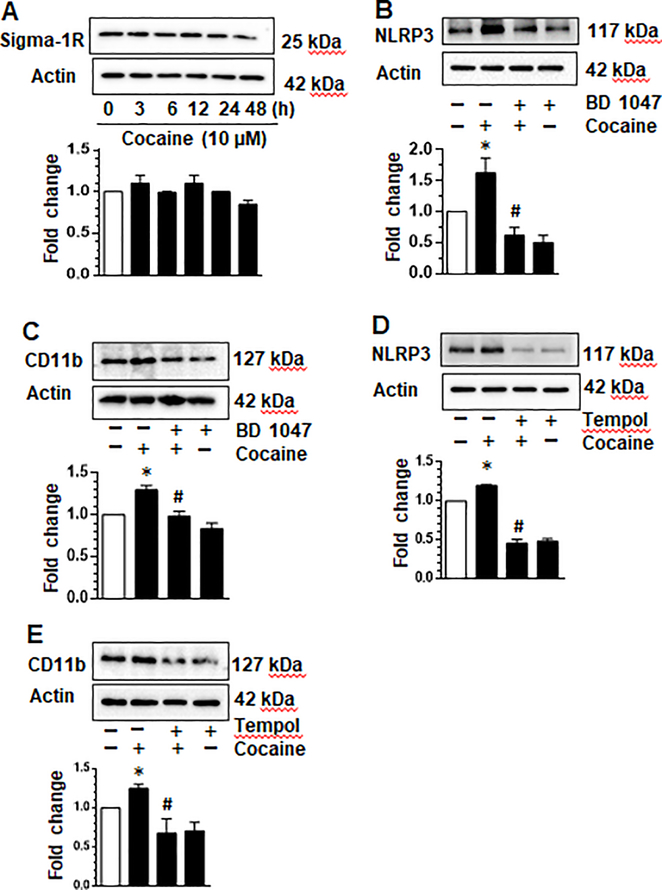
Having demonstrated that cocaine activated microglia via the NLRP3 pathway, we next explored the role of cognate cocaine receptor - sigma-1 receptor (σ−1R), in this process. The σ−1R is shown to be expressed in most cells of the CNS and has been shown to be activated following binding of cocaine [38–40]. Its role in the priming of NLRP3 however, remains to be investigated. To address this, we assessed the expression of σ−1R in mPMs exposed to cocaine. As shown in figure Fig. 5a, the expression of σ−1R remained constant to the last time point analyzed (48 hrs). Next, mPMs were pretreated with the σ−1R inhibitor - BD1047 (10 μM), followed by exposure of cells to cocaine for 24 h and analyzed for the expression of NLRP3 and the activation marker CD11b, by western blotting.
Fig. 5. Cocaine-induced ROS and the sigma receptor are involved in the upregulation of NLRP3 and cellular activation in microglia.
Mouse primary microglia (mPMs) exposed to cocaine express the sigma-1 receptor (a). mPMs were pretreated with BD1047 (10 μM) for 1 h followed by exposure to cocaine for 24 h. Exposure of cells to cocaine resulted in increased expression of NLRP3. Pre-treatment of cells with BD1047 attenuated cocaine-mediated upregulation of NLRP3 expression (b). Similarly, BD1047 attenuated cocaine-mediated upregulation of CD11b (c). mPMs were pretreated with tempol (50 μM) for 1 h followed by exposure to cocaine for 24 h. Exposure of cells to cocaine resulted in increased expression of NLRP3. Pre-treatment of cells with tempol attenuated cocaine-mediated upregulation of NLRP3 expression (d). Similarly, tempol attenuated cocaine-mediated upregulation of CD11b (e). All experiments were done at least three independent times, and representative figures are shown. Actin served as a loading control and quantification of western blots are shown under each blot. Data are shown as mean±SEM and *p< 0.05 vs control or # vs cocaine group.
As presented in Fig. 5b, cocaine exposure alone increased the expression of NLRP3 compared with untreated control cells. Pretreatment of mPMs with BD1047, on the other hand, resulted in significant abrogation of cocaine-mediated upregulation of NLRP3 compared with cells exposed to cocaine alone. In keeping with these findings, there was also inhibition of CD11b expression (Fig. 5c) in cells exposed to the pharmacological inhibitor of σ−1R, followed by exposure to cocaine. Overall, these results underscore the involvement of σ−1R in cocaine-mediated upregulation of NLRP3 inflammasome and subsequent activation of microglia.
Cocaine-induced ROS is involved in the upregulation of NLRP3 inflammasome pathway and activation of microglia
Previous reports have demonstrated cocaine-mediated induction of ROS in microglia [22]. Herein we sought to explore whether inhibiting ROS could lead to inhibition of both NLRP3 priming and microglial activation. To test this hypothesis, mPMs were pre-treated with tempol- a brain penetrant ROS inhibitor (1 h), followed by exposure of cells to cocaine (10 μM, 24 h) and assessed for the expression of NLRP3 and CD11b. As shown in Fig. 5d, and as expected, exposure of mPMs to cocaine resulted in an increase in the expression of NLRP3 compared with the control cells. Interestingly, pretreatment of mPMs with tempol attenuated cocaine-mediated upregulation of NLRP3 compared with cells exposed to cocaine alone. As expected, pretreatment of mPMs with tempol alone did not upregulate the expression of NLRP3. In keeping with the findings on NLRP3 expression, tempol pretreatment also abrogated cocaine-mediated upregulation of the microglial activation marker, CD11b (Fig. 5e), thereby underscoring the role of ROS in cocaine-mediated activation of microglia. Together, these data suggest that ROS lies upstream of cocaine-mediated induction of the NLRP3 inflammasome and cellular activation, thereby underscoring the potential role of ROS inhibitor(s) in ameliorating cocaine-mediated activation of the NLRP3 inflammasome.
In vivo administration of cocaine upregulated the expression of NLRP3 inflammasome mediators and microglial activation
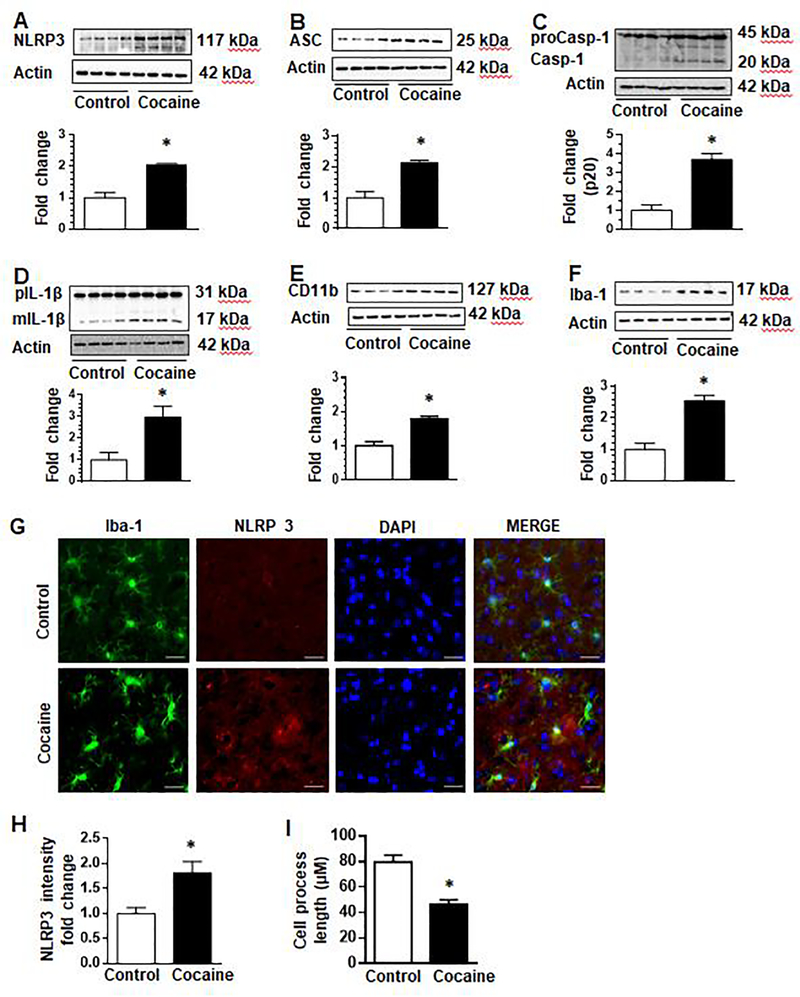
Having demonstrated the effects of cocaine on the NLRP3 inflammasome and microglial activation in vitro, we next sought to validate these findings in vivo in mice administered cocaine for 7 days. Mice were randomly assigned into two groups (n=4/group) and administered either saline or cocaine (20 mg/kg, i.p.) once a day for 7 consecutive days. One hour following the last cocaine injection, mice were sacrificed, brains removed, and striatal tissue homogenates assessed for expression of NLRP3 as well as markers of microglial activation. Our findings demonstrated that cocaine administration resulted in significant upregulation of NLRP3 in the striatal brain tissues compared with the striatum from saline-injected controls (Fig. 6a). Additionally, to further validate these findings, we also assessed the expression of mediators such as caspase-1, ASC and IL-1β in the striatum of cocaine-administered mice. As shown in Fig. 6b–d, expression of pro- and cleaved forms of caspase-1, ASC and mature IL-1β was significantly upregulated in cocaine-administered mice compared with the saline-injected controls. To further ascertain the role of microglia in this process, we also monitored the expression of microglial activation markers CD11b and Iba-1 in the brains of these mice. As expected, and similar to our in vitro studies, cocaine administration upregulated the expression of CD11b and Iba-1 proteins (Fig. 6e & f) compared with the saline administered controls.
Fig. 6. Cocaine upregulated the expression of NLRP3 pathway and microglial activation in the striatum.
Wild-type mice (C57BL/6) were administered cocaine (i.p. 20 mg/kg) or saline for 7 consecutive days followed by euthanasia within 1 h of the last injection and removal of brains for analysis of NLRP3 pathway mediators. The expression of NLRP3, ASC, Caspase-1 and IL-1β were significantly upregulated in the striatum (a-d). The expression of microglial markers CD11b and Iba-1 was upregulated in cocaine treated mice (e & f). Immunofluorescence analysis similarly showed increased NLRP3 and Iba-1 staining in presence of cocaine (g) and the fold change in NLRP3 fluorescence intensity analyzed (h). Cocaine administration in these mice decreased microglial cell process length (i). N =4/ group. Quantification of western blots is shown under each blot. Data are shown as mean±SEM and *p< 0.05.
To further validate cocaine-mediated activation of the microglial NLRP3 inflammasome, we also performed immunofluorescence staining for NLRP3 and Iba-1 or ASC in striatal brain sections obtained from mice administered either cocaine or saline for 7 consecutive days. Our results demonstrated increased expression of NLRP3 in Iba-1 positive microglial cells in the striatum of cocaine administered mice versus control animals (Fig. 6g). As shown in Fig. 6h, the fluorescence intensity of NLRP3 in cocaine-administered mice was upregulated (1.82 folds, p<0.05) compared with control mice. Furthermore, microglial process length was significantly reduced in cocaine-administered mice compared to control mice (Fig. 6i). As shown in supplementary Fig. 1, formation of ASC specks was increased in Iba-1 positive cells in cocaine administered mice compared with control animals. Increased expression of ASC is also seen in non-Iba-1 positive cells. Together these data suggest that cocaine exposure modulates the NLRP3 inflammasome in vivo in the brains of mice.
Pharmacological inhibition of NLRP3 attenuated cocaine-induced upregulation of inflammasome markers and microglial activation
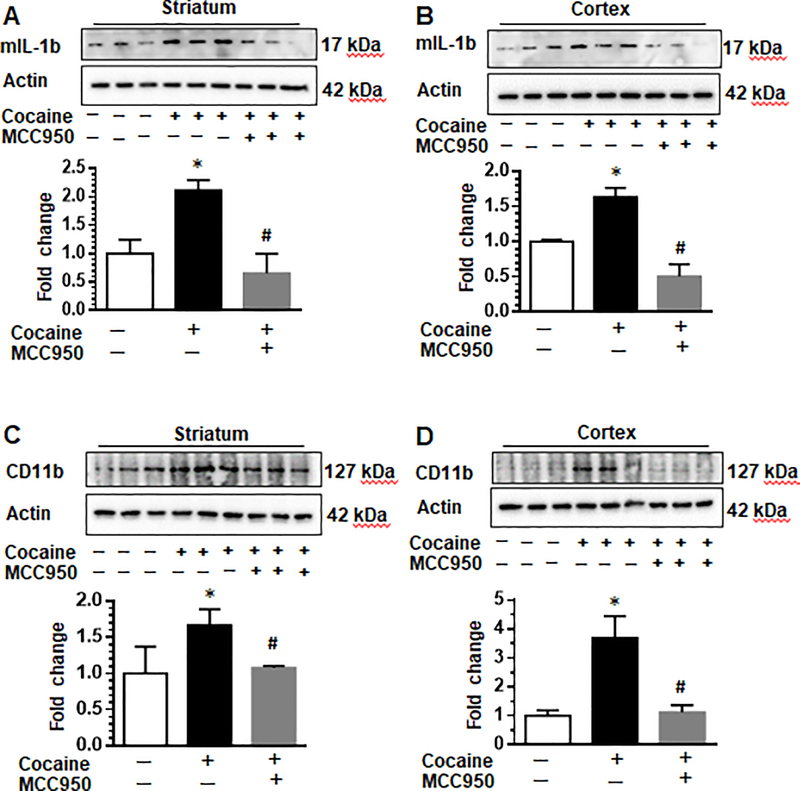
Next, we investigated in vivo the effects of pharmacological inhibition of NLRP3 by the small molecule inhibitor, MCC950. C57BL/C mice were randomly divided into four groups; receiving cocaine or saline injections for seven consecutive days in the presence or absence of MCC950 pretreatment. Mice were injected with NLRP3 inhibitor MCC950 (50 mg/kg or vehicle, i.p.) followed by cocaine administration (20 mg/kg, i.p.) an hour later, daily. On the seventh day, mice were euthanized and brains removed for expression of proteins by western blotting. As shown in Fig. 7a & b and as expected, cocaine increased the expression of mature IL-1β in both the striatum and cortex compared with control animals. Importantly, pre-treatment with MCC950 suppressed the expression of mature IL-1β in both the brain regions (Fig. 7a & b), thereby suggesting MCC950 mediated inhibition of cocaine-induced NLRP3 inflammasome activation. We also monitored the expression of the microglial activation marker CD11b in the striatum and cortex regions of these mice and found that MCC950 also suppressed cocaine-induced expression of CD11b (Fig. 7c - d).
Fig. 7. Pharmacological inhibition of NLRP3 attenuated cocaine-induced upregulation of inflammasome markers and microglial activation.
C57BL/6 wild type mice were divided into groups; receiving cocaine or saline injections for seven consecutive days in the presence of absence of MCC950 pretreatment. Mice were injected with NLRP3 inhibitor MCC950 (50 mg/kg or vehicle, i.p.) daily followed by cocaine administration (20 mg/kg, i.p.) an hour later. Mice were euthanasia within 1 h of the last injection followed by removal of brains for analysis of IL-1β and microglial activation. Cocaine increased the expression of mature IL-1β in both the striatum and cortex compared with control animals. Pre-treatment with MCC950 suppressed the expression of mature IL-1β in both the brain regions (a & b). The expression of microglial activation markers Iba-1 and CD11b in the striatum and cortex regions was attenuated by MCC950 (c-d). N = 3 / group. Quantification of western blots is shown under each blot. Data are shown as mean±SEM and *p< 0.05
Chronic cocaine dependence upregulated the expression of NLRP3 pathway mediators and microglial activation in human brains of cocaine addicts
We next sought to validate the effects of cocaine on the NLRP3 inflammasome in archival human brain sections with or without cocaine abuse. To address this, we assessed the expression of NLRP3 pathway mediators in frontal cortices of postmortem brain tissues from cocaine abusers and non-cocaine using controls. We found increased expression of NLRP3, ASC, Caspase-1 and IL-1β in the brains of cocaine abusers compared with cocaine naïve controls (Fig. 8a – d). As shown in Fig. 8e & f, expression of the microglial markers Iba-1 and CD11b was also increased in the brains of cocaine addicts compared with cocaine naive controls. Together, these results suggest the involvement of NLRP3 inflammasome and microglial activation in cocaine-induced inflammation in humans with chronic cocaine use.
Fig. 8. Chronic cocaine dependence upregulated the expression of NLRP3 pathway mediators and microglial activation in human brains of cocaine addicts.
Post mortem cortex brain tissues from chronic cocaine dependent addicts and non-cocaine using controls were analyzed for the expression of NLRP3 pathway mediators. The expression of NLRP3, ASC, Caspase-1 and IL-1β was increased in the brains of cocaine addicts compared with no-cocaine using controls (a – d). As shown in e & f, expression of the microglial markers Iba-1 and CD11b was also increased in the brains of cocaine abusers compared with no-cocaine using controls. N = 4 / group. Quantification of western blots is shown under each blot. Data are shown as mean±SEM and *p< 0.05
Discussion
CUD remains a significant public health concern globally that affects up to 22 million people worldwide and is closely associated with severe dysregulation of the immune system in both periphery and the CNS [7–9,1,2]. Previous studies have demonstrated the involvement of reactive oxygen species, activation of NF-kB and induction of proinflammatory cytokines such as IL-1β and TNFα, in functional neurocognitive decline associated with cocaine [6,15,11,7,8,41,42]. Interestingly, while activation of the NLRP3 inflammasome has been implicated in cocaine-induced activation of peripheral macrophages [35], the causal association between NLRP3 activation, ROS, and cocaine-induced behavioral changes remains poorly understood. The current study provides insights into the mechanism(s) of cocaine-induced priming and activation of the NLRP3 inflammasome. Our findings suggest that cocaine administration activates the NLRP3 inflammasome resulting in increased levels of proinflammatory IL-1β.
NLRP3 is highly expressed in microglia and is activated by various external and internal stimuli including urea crystals, asbestos, viral and bacterial proteins [30–32,43]. Activation of the NLRP3 inflammasome leads to increased levels of IL-1β, which, in turn, modulates neuronal excitability of the neighboring cells [44–46]. NLRP3 inflammasome has been demonstrated to play critical roles in microglial over-activation, which is an underlying mechanism critical for promoting the pathogenesis of multiple neurodegenerative diseases including Parkinson’s diseases (PD), Alzheimer’s disease, & multiple sclerosis [47–52]. Additionally, chronic unpredictable mild stress has also been reported to accelerate LPS-induced NLRP3 inflammasome activation in the PD model of rats, resulting in the death of dopaminergic neurons in this rat model, thereby signifying the importance of microglial activation in NLRP3 signaling [53]. Cocaine is well-known to activate glial cells in both in vitro and in vivo model systems [22,54,36]. Whether NLRP3 is also involved in cocaine-mediated activation of microglia and subsequent behavioral changes has not been explored. In this study, we demonstrated that exposure of mPMs to cocaine resulted in the upregulation of NLRP3, followed by recruitment of ASC and release of IL-1β from microglial cells. Our findings suggested that a physiologically relevant dose of cocaine (10 μM) resulted in activation of the NLRP3 inflammasome as demonstrated by increased expression of mature IL-1β in microglia. It has been shown that the plasma levels of cocaine in humans who administered cocaine intranasally range between 0.4–1.6 μM,[55] while the plasma cocaine levels in tolerant abusers was found to be 13 μM [56]. Further, the levels of cocaine in postmortem brains of chronic cocaine users with acute intoxications has been found to be greater than 100 μM [57]. Cocaine concentrations used in this study are in keeping with the physiological levels observed in humans abusing cocaine. Our in vivo validation study also demonstrated that the expression of multiple NLRP3 pathway mediators (NLRP3, ASC, caspase-1 and IL-1β) as well as microglial activation (Iba-1 and CD11b) was upregulated in the striatum of mice administered cocaine repeatedly (20 mg/kg i.p., daily for 7 days) as well as in humans with reported chronic cocaine abuse compared to the levels in the control brains, thereby underscoring the involvement of NLRP3 in cocaine-induced microglial activation and neuroinflammation. Furthermore, our results demonstrating increased expression of Iba-1 in microglia exposed to cocaine are consistent with previous studies that have also shown upregulation of Iba-1 following cocaine exposure [58–60].
To examine the molecular changes involved in cocaine-mediated activation of microglia, we employed pharmacological approaches. Our findings demonstrated that pharmacological inhibition of ROS using tempol, reduced cocaine-mediated priming of NLRP3 inflammasome. These data thus implicate the role of ROS in cocaine-induced priming of NLRP3, a finding that is consistent with previous studies showing ROS-mediated priming of the NLRP3 inflammasome [12,13,15]. Tempol has been previously shown to reduce oxidative damage and the development of behavioral sensitization in rodents [17]. Recently, in an in vitro model of diabetic nephropathy, exposure of HK-2 cells (an immortalized proximal tubule epithelial cell line from normal adult human kidney) to high glucose demonstrated increased expression of ROS-mediated NLRP3 activation, which was notably inhibited in cells pretreated with Tempol [61]. Our findings also suggest that ROS elevation lies upstream of NLRP3 activation.
Our in vivo findings demonstrated that expression of mature IL-1β was upregulated in the striatum of cocaine-administered mice compared with control mice, thus suggesting cocaine-induced cleavage of pro-IL-1β. In keeping with these findings, it has also been reported that circulating levels of IL-1β are increased in humans with crack-cocaine disorders and could be associated with an activation of the reward, immune and inflammatory systems [8]. Activation of the NLRP3 inflammasome by mechanism(s) involving ROS, lysosome rapture or potassium efflux has been well documented [12,62,63,31,32,30]. The effects of cocaine on the cleavage and release of mature IL-1β, however, has not yet been clearly elucidated. In this study we provide data that cocaine-exposed microglia activate NLRP3 inflammasome signaling with the release of IL-1β involving the upstream activation of ROS. Studies are underway to ascertain the detailed molecular mechanism(s) underlying cocaine-mediated activation of the NLRP3 inflammasome.
The critical role of NLRP3 in cocaine-mediated microglial activation was further explored by both pharmacological and gene silencing (siRNA) approaches. Our results demonstrated that both MCC950 (NLRP3 inhibitor) as well as the NLRP3 siRNA attenuated cocaine-mediated activation of the NLRP3 inflammasome, as evidenced by the reduced expression of mature IL-1β. There are numerous studies reporting the use of MCC950 as a potential pharmacological inhibitor of NLRP3 to alleviate the NLRP3-mediated immune activation in various disease settings [64–75,52,76–78]. Our findings also demonstrated that both pharmacological inhibition and gene silencing of NLRP3 resulted in significant inhibition of cocaine-mediated expression of IL-1β and activation of microglia, as evidenced by decreased expression of both Iba-1 and CD11b. Our results thus suggest that targeting the NLRP3 inflammasome could be developed as an adjunctive therapeutic approach to ameliorate the deleterious effects of cocaine.
Conclusion
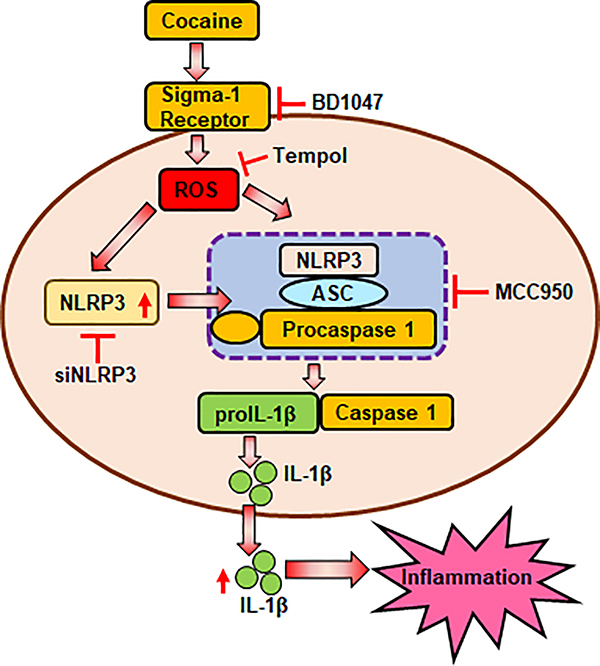
In summary, herein we report that the NLPR3 inflammasome signaling involving generation of reactive oxygen species plays a role in cocaine-mediated activation of microglia. Blockade of NLRP3 reduced cocaine-mediated activation of NLRP3 inflammasome and ensuing microglial activation and inflammation is depicted in Fig. 9. Taken together, inhibiting the NLRP3 inflammasome could thus be developed as a potential therapeutic strategy for dampening cocaine-mediated neuroinflammation.
Fig. 9. Schematic description of cocaine-mediated activation of the NLRP3 inflammasome in microglia.
Cocaine upregulates the expression of NLRP3 and increases generation of ROS. The NLRP3 inflammasome is subsequently activated resulting in the maturation of IL-β that contributes to inflammation. Blockade of ROS (Tempol) attenuates cocaine-mediated induction of NLRP3. Blockade of NLRP3 by either pharmacological (MCC950) or gene silencing (siNLRP3) approaches reduced cocaine-mediated activation of the inflammasome
Supplementary Material
Acknowledgements
We thank the Douglas-Bell Canada Brain Bank (DBCBB) for providing the postmortem brain samples of cocaine abusers and controls. The DBCBB is supported by the Quebec Suicide Research Network of the Fonds de Recherche du Quebec - Sante (FRQS) and by the Douglas Institute Foundation.
Funding
This work was supported by NIH grant R01DA050545 (SB & MG), R01DA050545-02S1 (PI: SB & ETC as Research Supplement recipient), R21DA046831 (ETC), R01DA047156 (MG & SB) and the Nebraska Centre for Substance Abuse Research (NCSAR).
Abbreviations
- ROS
Reactive oxygen species
- NLRP3
NLR Family Pyrin Domain Containing 3
- ASC
Apoptosis-associated speck-like protein containing a CARD
- CUD
Cocaine use disorder
- LPS
Lipopolysaccharide
Footnotes
Conflicts of interest/Competing interests
The authors declare that they have no competing interests.
DECLARATIONS
Ethics approval consent to participate
All animal procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Centre and the National Institutes of Health.
Consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not Applicable
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.Pomara C, Cassano T, D’Errico S, Bello S, Romano AD, Riezzo I, Serviddio G (2012) Data available on the extent of cocaine use and dependence: biochemistry, pharmacologic effects and global burden of disease of cocaine abusers. Curr Med Chem 19 (33):5647–5657. doi: 10.2174/092986712803988811 [DOI] [PubMed] [Google Scholar]
- 2.Hughes A, Williams MR, Lipari RN, Van Horn S (2013) State Estimates of Past Year Cocaine Use among Young Adults: 2014 and 2015. In: The CBHSQ Report. Rockville (MD), pp 1–9 [PubMed] [Google Scholar]
- 3.Crews FT, Zou J, Qin L (2011) Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 25 Suppl 1:S4–S12. doi: 10.1016/j.bbi.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark KH, Wiley CA, Bradberry CW (2013) Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res 23 (2):174–188. doi: 10.1007/s12640-012-9334-7 [DOI] [PubMed] [Google Scholar]
- 5.Periyasamy P, Liao K, Kook YH, Niu F, Callen SE, Guo ML, Buch S (2018) Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4 Signaling. Mol Neurobiol 55 (4):3196–3210. doi: 10.1007/s12035-017-0584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich JB, Mangeol A, Revel MO, Burgun C, Aunis D, Zwiller J (2005) Acute or repeated cocaine administration generates reactive oxygen species and induces antioxidant enzyme activity in dopaminergic rat brain structures. Neuropharmacology 48 (7):965–974. doi: 10.1016/j.neuropharm.2005.01.018 [DOI] [PubMed] [Google Scholar]
- 7.Moreira FP, Medeiros JR, Lhullier AC, Souza LD, Jansen K, Portela LV, Lara DR, da Silva RA, Wiener CD, Oses JP (2016) Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug Alcohol Depend 158:181–185. doi: 10.1016/j.drugalcdep.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 8.Narvaez JC, Magalhaes PV, Fries GR, Colpo GD, Czepielewski LS, Vianna P, Chies JA, Rosa AR, Von Diemen L, Vieta E, Pechansky F, Kapczinski F (2013) Peripheral toxicity in crack cocaine use disorders. Neuroscience letters 544:80–84. doi: 10.1016/j.neulet.2013.03.045 [DOI] [PubMed] [Google Scholar]
- 9.Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, Sinha R (2012) Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol 27 (2):156–166. doi: 10.1002/hup.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valente MJ, Carvalho F, Bastos M, de Pinho PG, Carvalho M (2012) Contribution of oxidative metabolism to cocaine-induced liver and kidney damage. Curr Med Chem 19 (33):5601–5606. doi: 10.2174/092986712803988938 [DOI] [PubMed] [Google Scholar]
- 11.Kovacic P (2005) Role of oxidative metabolites of cocaine in toxicity and addiction: oxidative stress and electron transfer. Med Hypotheses 64 (2):350–356. doi: 10.1016/j.mehy.2004.06.028 [DOI] [PubMed] [Google Scholar]
- 12.Lupfer CR, Anand PK, Liu Z, Stokes KL, Vogel P, Lamkanfi M, Kanneganti TD (2014) Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog 10 (9):e1004410. doi: 10.1371/journal.ppat.1004410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Na R, Boldt E, Ran Q (2015) NLRP3 inflammasome activation by mitochondrial reactive oxygen species plays a key role in long-term cognitive impairment induced by paraquat exposure. Neurobiology of aging 36 (9):2533–2543. doi: 10.1016/j.neurobiolaging.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Chen X, Simet SM, Hu G, Cai Y, Niu F, Kook Y, Buch SJ (2016) Reactive Oxygen Species/Hypoxia-Inducible Factor-1alpha/Platelet-Derived Growth Factor-BB Autocrine Loop Contributes to Cocaine-Mediated Alveolar Epithelial Barrier Damage. Am J Respir Cell Mol Biol 55 (5):736–748. doi: 10.1165/rcmb.2016-0096OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang EY, Ryu YH, Lee BH, Chang SC, Yeo MJ, Kim SH, Folsom RJ, Schilaty ND, Kim KJ, Yang CH, Steffensen SC, Kim HY (2015) Involvement of reactive oxygen species in cocaine-taking behaviors in rats. Addict Biol 20 (4):663–675. doi: 10.1111/adb.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu W, Wang H, Wei J, Sartor GC, Bao MM, Pierce CT, Wahlestedt CR, Dykxhoorn DM, Dong C (2018) Cocaine Exposure Increases Blood Pressure and Aortic Stiffness via the miR-30c-5p-Malic Enzyme 1-Reactive Oxygen Species Pathway. Hypertension 71 (4):752–760. doi: 10.1161/HYPERTENSIONAHA.117.10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Numa R, Kohen R, Poltyrev T, Yaka R (2008) Tempol diminishes cocaine-induced oxidative damage and attenuates the development and expression of behavioral sensitization. Neuroscience 155 (3):649–658. doi: 10.1016/j.neuroscience.2008.05.058 [DOI] [PubMed] [Google Scholar]
- 18.Lattanzio FA Jr., Tiangco D, Osgood C, Beebe S, Kerry J, Hargrave BY (2005) Cocaine increases intracellular calcium and reactive oxygen species, depolarizes mitochondria, and activates genes associated with heart failure and remodeling. Cardiovasc Toxicol 5 (4):377–390 [DOI] [PubMed] [Google Scholar]
- 19.Kloss MW, Rosen GM, Rauckman EJ (1984) Biotransformation of norcocaine to norcocaine nitroxide by rat brain microsomes. Psychopharmacology (Berl) 84 (2):221–224. doi: 10.1007/bf00427449 [DOI] [PubMed] [Google Scholar]
- 20.Muriach M, Lopez-Pedrajas R, Barcia JM, Sanchez-Villarejo MV, Almansa I, Romero FJ (2010) Cocaine causes memory and learning impairments in rats: involvement of nuclear factor kappa B and oxidative stress, and prevention by topiramate. J Neurochem 114 (3):675–684. doi: 10.1111/j.1471-4159.2010.06794.x [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Pedrajas R, Ramirez-Lamelas DT, Muriach B, Sanchez-Villarejo MV, Almansa I, Vidal-Gil L, Romero FJ, Barcia JM, Muriach M (2015) Cocaine promotes oxidative stress and microglial-macrophage activation in rat cerebellum. Front Cell Neurosci 9:279. doi: 10.3389/fncel.2015.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S (2016) Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation 13:33. doi: 10.1186/s12974-016-0501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima K, Kohsaka S (1993) Functional roles of microglia in the brain. Neuroscience research 17 (3):187–203. doi: 10.1016/0168-0102(93)90047-t [DOI] [PubMed] [Google Scholar]
- 24.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29 (13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Bernhardi R, Heredia F, Salgado N, Munoz P (2016) Microglia Function in the Normal Brain. Advances in experimental medicine and biology 949:67–92. doi: 10.1007/978-3-319-40764-7_4 [DOI] [PubMed] [Google Scholar]
- 26.Perry VH, Holmes C (2014) Microglial priming in neurodegenerative disease. Nat Rev Neurol 10 (4):217–224. doi: 10.1038/nrneurol.2014.38 [DOI] [PubMed] [Google Scholar]
- 27.Smith JA, Das A, Ray SK, Banik NL (2012) Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain research bulletin 87 (1):10–20. doi: 10.1016/j.brainresbull.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang WY, Tan MS, Yu JT, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 3 (10):136. doi: 10.3978/j.issn.2305-5839.2015.03.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinhard L, d’Errico P, Leng Tay T (2018) Headmasters: Microglial regulation of learning and memory in health and disease. AIMS Molecular Science 5 (1):63–89. doi: 10.3934/molsci.2018.1.63 [DOI] [Google Scholar]
- 30.Gross O, Thomas CJ, Guarda G, Tschopp J (2011) The inflammasome: an integrated view. Immunol Rev 243 (1):136–151. doi: 10.1111/j.1600-065X.2011.01046.x [DOI] [PubMed] [Google Scholar]
- 31.Rathinam VA, Vanaja SK, Fitzgerald KA (2012) Regulation of inflammasome signaling. Nat Immunol 13 (4):333–342. doi: 10.1038/ni.2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutterwala FS, Haasken S, Cassel SL (2014) Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci 1319:82–95. doi: 10.1111/nyas.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon JS, Lee S, Park MA, Siempos II, Haslip M, Lee PJ, Yun M, Kim CK, Howrylak J, Ryter SW, Nakahira K, Choi AM (2015) UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J Clin Invest 125 (2):665–680. doi: 10.1172/JCI78253 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Chivero ET, Guo ML, Periyasamy P, Liao K, Callen SE, Buch S (2017) HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J Neurosci 37 (13):3599–3609. doi: 10.1523/JNEUROSCI.3045-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atluri VS, Pilakka-Kanthikeel S, Garcia G, Jayant RD, Sagar V, Samikkannu T, Yndart A, Nair M (2016) Effect of Cocaine on HIV Infection and Inflammasome Gene Expression Profile in HIV Infected Macrophages. Sci Rep 6:27864. doi: 10.1038/srep27864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo ML, Liao K, Periyasamy P, Yang L, Cai Y, Callen SE, Buch S (2015) Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 11 (7):995–1009. doi: 10.1080/15548627.2015.1052205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chivero ET, Ahmad R, Thangaraj A, Periyasamy P, Kumar B, Kroeger E, Feng D, Guo ML, Roy S, Dhawan P, Singh AB, Buch S (2019) Cocaine Induces Inflammatory Gut Milieu by Compromising the Mucosal Barrier Integrity and Altering the Gut Microbiota Colonization. Sci Rep 9 (1):12187. doi: 10.1038/s41598-019-48428-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, Prat A, Wang J, Su TP, Buch S (2011) Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci 31 (16):5942–5955. doi: 10.1523/JNEUROSCI.5618-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ (2003) Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol 469 (1–3):1–12. doi: 10.1016/s0014-2999(03)01723-0 [DOI] [PubMed] [Google Scholar]
- 40.Tsai SY, Chuang JY, Tsai MS, Wang XF, Xi ZX, Hung JJ, Chang WC, Bonci A, Su TP (2015) Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc Natl Acad Sci U S A 112 (47):E6562–6570. doi: 10.1073/pnas.1518894112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B, Lesselyong A, Perrotti LI, Bolanos CA, Kumar A, Clark MS, Neumaier JF, Neve RL, Bhakar AL, Barker PA, Nestler EJ (2009) Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci 29 (11):3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahu G, Farley K, El-Hage N, Aiamkitsumrit B, Fassnacht R, Kashanchi F, Ochem A, Simon GL, Karn J, Hauser KF, Tyagi M (2015) Cocaine promotes both initiation and elongation phase of HIV-1 transcription by activating NF-kappaB and MSK1 and inducing selective epigenetic modifications at HIV-1 LTR. Virology 483:185–202. doi: 10.1016/j.virol.2015.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassel SL, Joly S, Sutterwala FS (2009) The NLRP3 inflammasome: a sensor of immune danger signals. Semin Immunol 21 (4):194–198. doi: 10.1016/j.smim.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stemkowski PL, Noh MC, Chen Y, Smith PA (2015) Increased excitability of medium-sized dorsal root ganglion neurons by prolonged interleukin-1beta exposure is K(+) channel dependent and reversible. J Physiol 593 (16):3739–3755. doi: 10.1113/JP270905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang P, Bi RY, Gan YH (2018) Glial interleukin-1beta upregulates neuronal sodium channel 1.7 in trigeminal ganglion contributing to temporomandibular joint inflammatory hypernociception in rats. J Neuroinflammation 15 (1):117. doi: 10.1186/s12974-018-1154-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vezzani A, Viviani B (2015) Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96 (Pt A):70–82. doi: 10.1016/j.neuropharm.2014.10.027 [DOI] [PubMed] [Google Scholar]
- 47.Rossi S, Furlan R, De Chiara V, Motta C, Studer V, Mori F, Musella A, Bergami A, Muzio L, Bernardi G, Battistini L, Martino G, Centonze D (2012) Interleukin-1beta causes synaptic hyperexcitability in multiple sclerosis. Ann Neurol 71 (1):76–83. doi: 10.1002/ana.22512 [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, Hu G (2016) MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Molecular neurodegeneration 11:28. doi: 10.1186/s13024-016-0094-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, Yuan YH, Chen NH, Wang HB (2019) The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int Immunopharmacol 67:458–464. doi: 10.1016/j.intimp.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 50.Mao Z, Liu C, Ji S, Yang Q, Ye H, Han H, Xue Z (2017) The NLRP3 Inflammasome is Involved in the Pathogenesis of Parkinson’s Disease in Rats. Neurochem Res 42 (4):1104–1115. doi: 10.1007/s11064-017-2185-0 [DOI] [PubMed] [Google Scholar]
- 51.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493 (7434):674–678. doi: 10.1038/nature11729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O’Neill LAJ, Lynch MA (2017) Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun 61:306–316. doi: 10.1016/j.bbi.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 53.Kong H, Yang L, He C, Zhou JW, Li WZ, Wu WN, Chen HQ, Yin YY (2019) Chronic unpredictable mild stress accelerates lipopolysaccharide- induced microglia activation and damage of dopaminergic neurons in rats. Pharmacol Biochem Behav 179:142–149. doi: 10.1016/j.pbb.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 54.Periyasamy P, Guo ML, Buch S (2016) Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy 12 (8):1310–1329. doi: 10.1080/15548627.2016.1183844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Dyke C, Barash PG, Jatlow P, Byck R (1976) Cocaine: plasma concentrations after intranasal application in man. Science 191 (4229):859–861. doi: 10.1126/science.56036 [DOI] [PubMed] [Google Scholar]
- 56.Stephens BG, Jentzen JM, Karch S, Mash DC, Wetli CV (2004) Criteria for the interpretation of cocaine levels in human biological samples and their relation to the cause of death. Am J Forensic Med Pathol 25 (1):1–10. doi: 10.1097/01.paf.0000118960.58334.a9 [DOI] [PubMed] [Google Scholar]
- 57.Kalasinsky KS, Bosy TZ, Schmunk GA, Ang L, Adams V, Gore SB, Smialek J, Furukawa Y, Guttman M, Kish SJ (2000) Regional distribution of cocaine in postmortem brain of chronic human cocaine users. J Forensic Sci 45 (5):1041–1048 [PubMed] [Google Scholar]
- 58.Burkovetskaya ME, Small R, Guo L, Buch S, Guo ML (2020) Cocaine self-administration differentially activates microglia in the mouse brain. Neuroscience letters 728:134951. doi: 10.1016/j.neulet.2020.134951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jarvis R, Tamashiro-Orrego A, Promes V, Tu L, Shi J, Yang Y (2019) Cocaine Self-administration and Extinction Inversely Alter Neuron to Glia Exosomal Dynamics in the Nucleus Accumbens. Front Cell Neurosci 13:581. doi: 10.3389/fncel.2019.00581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang ZJ, Martin JA, Gancarz AM, Adank DN, Sim FJ, Dietz DM (2017) Activin A is increased in the nucleus accumbens following a cocaine binge. Sci Rep 7:43658. doi: 10.1038/srep43658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu M, Han W, Song S, Du Y, Liu C, Chen N, Wu H, Shi Y, Duan H (2018) NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol Cell Endocrinol 478:115–125. doi: 10.1016/j.mce.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 62.Okada M, Matsuzawa A, Yoshimura A, Ichijo H (2014) The lysosome rupture-activated TAK1-JNK pathway regulates NLRP3 inflammasome activation. J Biol Chem 289 (47):32926–32936. doi: 10.1074/jbc.M114.579961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katsnelson MA, Rucker LG, Russo HM, Dubyak GR (2015) K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol 194 (8):3937–3952. doi: 10.4049/jimmunol.1402658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ward R, Li W, Abdul Y, Jackson L, Dong G, Jamil S, Filosa J, Fagan SC, Ergul A (2019) NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol Res 142:237–250. doi: 10.1016/j.phrs.2019.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qu J, Yuan Z, Wang G, Wang X, Li K (2019) The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int Immunopharmacol 70:147–155. doi: 10.1016/j.intimp.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 66.Luo Y, Lu J, Ruan W, Guo X, Chen S (2019) MCC950 attenuated early brain injury by suppressing NLRP3 inflammasome after experimental SAH in rats. Brain research bulletin 146:320–326. doi: 10.1016/j.brainresbull.2019.01.027 [DOI] [PubMed] [Google Scholar]
- 67.Umiker B, Lee HH, Cope J, Ajami NJ, Laine JP, Fregeau C, Ferguson H, Alves SE, Sciammetta N, Kleinschek M, Salmon M (2019) The NLRP3 inflammasome mediates DSS-induced intestinal inflammation in Nod2 knockout mice. Innate Immun 25 (2):132–143. doi: 10.1177/1753425919826367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan Y, Du L, Fu Q, Zhou Z, Zhang J, Li G, Wu J (2018) Inhibiting the NLRP3 Inflammasome With MCC950 Ameliorates Isoflurane-Induced Pyroptosis and Cognitive Impairment in Aged Mice. Front Cell Neurosci 12:426. doi: 10.3389/fncel.2018.00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathur A, Feng S, Hayward JA, Ngo C, Fox D, Atmosukarto II, Price JD, Schauer K, Martlbauer E, Robertson AAB, Burgio G, Fox EM, Leppla SH, Kaakoush NO, Man SM (2019) A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nat Microbiol 4 (2):362–374. doi: 10.1038/s41564-018-0318-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, Robertson AAB, Butler MS, Rowe DB, O’Neill LA, Kanthasamy AG, Schroder K, Cooper MA, Woodruff TM (2018) Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med 10 (465). doi: 10.1126/scitranslmed.aah4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu L, Zhang C, Jiang N, He D, Bai Y, Xin Y (2019) Rapamycin combined with MCC950 to treat multiple sclerosis in experimental autoimmune encephalomyelitis. J Cell Biochem 120 (4):5160–5168. doi: 10.1002/jcb.27792 [DOI] [PubMed] [Google Scholar]
- 72.Xu KY, Wu CY, Tong S, Xiong P, Wang SH (2018) The selective Nlrp3 inflammasome inhibitor Mcc950 attenuates lung ischemia-reperfusion injury. Biochem Biophys Res Commun 503 (4):3031–3037. doi: 10.1016/j.bbrc.2018.08.089 [DOI] [PubMed] [Google Scholar]
- 73.Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson AAB, Schroder K, Kunde D, Eri R (2018) MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep 8 (1):8618. doi: 10.1038/s41598-018-26775-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhai Y, Meng X, Ye T, Xie W, Sun G, Sun X (2018) Inhibiting the NLRP3 Inflammasome Activation with MCC950 Ameliorates Diabetic Encephalopathy in db/db Mice. Molecules 23 (3). doi: 10.3390/molecules23030522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen W, Foo SS, Zaid A, Teng TS, Herrero LJ, Wolf S, Tharmarajah K, Vu LD, van Vreden C, Taylor A, Freitas JR, Li RW, Woodruff TM, Gordon R, Ojcius DM, Nakaya HI, Kanneganti TD, O’Neill LAJ, Robertson AAB, King NJ, Suhrbier A, Cooper MA, Ng LFP, Mahalingam S (2017) Specific inhibition of NLRP3 in chikungunya disease reveals a role for inflammasomes in alphavirus-induced inflammation. Nat Microbiol 2 (10):1435–1445. doi: 10.1038/s41564-017-0015-4 [DOI] [PubMed] [Google Scholar]
- 76.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21 (3):248–255. doi: 10.1038/nm.3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC (2017) NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 66 (5):1037–1046. doi: 10.1016/j.jhep.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ismael S, Zhao L, Nasoohi S, Ishrat T (2018) Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep 8 (1):5971. doi: 10.1038/s41598-018-24350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.