Abstract
Eating fish is often recommended as part of a healthful diet. However fish, particularly large predatory fish, can contain significant levels of the highly toxic methylmercury (MeHg). Ocean fish in general also contain high levels of selenium (Se), which is reported to confer protection against toxicity of various metals including mercury (Hg). Se and Hg have a high mutual binding affinity, and each can reduce the toxicity of the other. This is an evolving area of extensive research and controversy with variable results in the animal and epidemiologic literature. MeHg is toxic to many organ systems through high affinity for –SH (thiol) ligands on enzymes and microtubules. Hg toxicity also causes oxidative damage particularly to neurons in the brain. Hg is a potent and apparently irreversible inhibitor of the selenoenzymes, glutathione peroxidases (GPX) and thioredoxin reductases (TXNRD) thar are important antioxidants, each with a selenocysteine (SeCys) at the active site. Hg binding to the SeCys inhibits these enzymes, accounting in part for the oxidative damage that is an important manifestation of Hg toxicity, particularly if there is not a pool of excess Se to synthesize new enzymes. A molar excess of Se reflected in an Se/Hg molar ratio >1 is often invoked as evidence that the Hg content can be discounted. Some recent papers now suggest that if the Se:Hg molar ratio exceeds 1:1, the fish is safe and the mercury concentration can be ignored. Such papers suggested that the molar ratio rather than the Hg concentration, should be emphasized in fish advisories. This paper examines some of the limitations of current understanding of the Se:Hg molar ratio in guiding fish consumption advice, Se is certainly an important part of the Hg toxicity story, but it is not the whole story. We examine how Hg toxicity relates also to thiol binding. We suggest that a 1:1 molar ratio cannot be relied on because not all of the Se in fish or in the fish-eater is available to interact with Hg. Moreover, in some fish Se levels are sufficiently high to warrant concern about Se toxicity.
Keywords: Methylmercury, selenium, fish consumption, tubulin, microtubules, selenocysteine, oxidative stress, enzyme inhibition, selenoproteins, thioredoxin reductase, glutathione peroxidase, Se:Hg molar ratio
Introduction
Fish are often recommended as part of a healthful diet. However, some fish have high levels of methylmercury (MeHg). This requires careful consideration of risks and benefits (Budtz-Jorgensen et al. 2007). All forms of mercury (Hg) are toxic to all forms of life. No organism needs or benefits from Hg, yet humans have exploited its physical and toxic properties in various ways including its use as an antiseptics, pesticide, fungicide, diuretic, and vaccine stabilizer. (IARC 1993). Selenium (Se) in the form of the amino acid selenocysteine (SeCys) is a critical component of selenoenzymes such as glutathione peroxidases (GPX) and thioredoxin reductases (TXNRD). These enzymes defend cells against oxidative damage (Lubos et al. 2011; Benhar 2018). Se compounds are also toxic (Vinceti et al. 2001; ATSDR 2003; Hamilton 2004), with only a narrow margin between essentiality and toxicity (Spallholz 1993; Surai 2006). Hg and Se have a high mutual affinity and binding constant (Sugiura et al 1978; Dyrssen and Wedborg 1991), and each can confer protection against the toxicity of the other (El-Begearmi et al. 1977; Farina et al. 2003, Raymond and Ralston 2004; Dauplais et al. 2013). That Se can mitigate mercury toxicity in some situations has been known since the 1960s (Parizek and Ostádalová 1967; Ganther et al. 1972; Ganther and Sunde 1974) and has been subject to periodic reviews at approximately decade intervals (Skerving 1978; Magos and Webb 1980; Cuvin-Aralar and Furness 1991; Watanabe 2002; Yang et al. 2008; Ralston and Raymond (2018). However, protection is not universal and synergism (Penglase et al 2014) or enhancement of Hg toxicity has been reported (Brandão 2011; Polevoy et al. 2020). Experiments on the interactions of He and Se using different compounds, protocols, administration sequence or endpoints may yield different results (Penglase et al 2014) and this is still an active research area (e.g., Baldisetta et al 2020; Carvalho et al. 2019).
The toxicokinetics of MeHg is complex with various individual factors influencing the biological half-life (Rand and Caito 2019). Most MeHg in fish is complexed with Cys, but some is already bound to Se, mainly selenomethionine (Cabañero et al. 2005). A fraction of the ingested MeHg may be demethylated by gut microflora (Liu et al. 2019), but most, about 90–95% of the MeHg ingested is absorbed from the gastrointestinal tract (Gad 2014). Much of the absorbed Hg enters red blood cells where it binds to hemoglobin (Gad 2014). Other Hg remains in the blood transported partly bound to the SeCys of selenoprotein P (Liu et al 2018) or to the Cys of albumin, metallothionein or other proteins.
Hg enters cells exploiting various transporters, for example Hg-Cys via an amino acid transporter (Bridges and Zalups 2010). In the cell the Hg may encounter and bind to a SeCys perhaps inactivating an antioxidant enzyme or may bind to GSH, or interact with Cys on enzymes or tubulin molecules. Some MeHg may enter the brain or kidney where demethylation and oxidative damage may occur (Gad 2014). MeHg in the liver may be secreted in the bile, enter the intestine where most of it is demethylated (Liu et al 2019), and then excreted. A large portion of each ingested dose will eventually be excreted mainly in the feces amounting to 95% of elimination for organomercurials (Caito et al 2018). Published half-lives for MeHg in the body vary greatly with most estimates in the 40–70 day range, but up to 130 days (Rand and Caito 2019). A carefully controlled human exposure study showed that the broad range represents true individual variability in toxicokinetics, including demethylation rate and is not a methodologic artefact (Caito et al 2018).
Individuals who experience a long half-life for MeHg and eat fish frequently are at risk of bioaccumulating Hg in tissues, taking in more than they excrete, meal after meal (Caito et al. 2018). In this paper we explore the benefits and risks of eating fish with respect to the Hg and Se content of fish tissue. We focus on the reliability of the Se:Hg molar ratio that is one metric of the risk of eating a lot of high mercury fish, and on whether reference to a “1:1” molar ratio or “>1:1” ratio is reliable and protective in risk communication. The benefits of fish consumption are widely publicized, tempered with warnings focused on “contaminants” including methylmercury (MeHg) and polychlorinated biphenyls (EPA 2020). In the best case, the benefits of eating fish would be maximized at a consumption level before a toxic threshold is reached (Gochfeld and Burger 2005). Most at risk are pregnant women and children as well as “people who eat a lot of fish” (EPA 2020). We refer to the latter as “high end fish consumers”, people who eat more than 2–3 fish meals per week (Gochfeld and Burger 2005).
How much Se in fish is needed to protect against Hg toxicity is uncertain (Ralston and Raymond 2018; Spiller 2018; Gerson et al. 2020). Much of the Se-protection discussion has focused on the safety of consuming fish, including large quantities of predatory marine fish (Ralston et al. 2016). Most of the mercury in fish, about 80–95%, is methylmercury (MeHg) (Cappon and Smith 1982; Bloom 1992) and fish is the main source of exposure to MeHg for most fish-eaters (Schartup et al. 2019), although rice grown in mercury-contaminated mining areas can be a major source of Hg exposure (Feng et al. 2007).
Fish Consumption Advisories
The health benefits of eating fish are due in part to beneficial nutrients such as polyunsaturated fatty acids and Se. The benefits may be offset by the high intake of MeHg (Roman et al. 2012) in people who eat high-mercury fish frequently. Many states and countries have fish advisories based on the Hg or polychlorinated biphenyl (PCB) content (EPA 2011). Mercury warnings typically target pregnant women and children, or people who eat a lot of fish such as recreational and subsistence anglers. Health effects are mainly neurodevelopmental, but MeHg is also neurotoxic in adults and also increases cardiovascular disease in adults (Roman et al. 2012). Most advisories also provide guidance on the health benefits of eating fish. In the past 15 years, as our understanding of Se biochemistry and protectiveness against Hg has evolved, there has been a tendency for official advisories to discount the Hg concentration, and emphasize the benefits (EPA 2020), as long as fish contain a molar excess of Se (Spiller 2018). Most fish species do have Se:Hg >1, although there is great inter- and intra- specific variability (Burger and Gochfeld 2013; Cusack et al. 2017). The Se “protectiveness” against Hg toxicity has engendered numerous recent studies (see Spiller 2018), and contributed greatly to our understanding of Hg toxicity. It has become common to mention that as long as Se is present in at least a 1:1 molar ratio with Hg, it will protect against Hg toxicity (e.g., Azad et al 2019). There is little empirical evidence, and limited population based validation of what ratio would connote safety but see Lemire et al. (2011).
The question of how much molar excess of Se is protective remains unresolved. In our view, confidence in a 1:1 ratio ignores the following: 1) there are abundant endogenous metals such as Cu and Zn as well as other xenobiotic metals that bind Se, and 2) there are far more –SH (thiol) ligands that bind Hg and also Se. Hence, at any point in time not all the Se would be available to react with or sequester Hg. By this reasoning a 1:1 molar ratio in fish is not sufficiently protective, but the relationship between ratio and risk has not been worked out. The molar ratio has come to play a controversial role in risk communication about eating fish. Ralston et al (2016) emphasize the strong supporting evidence for relying on the molar ratio, while Gerson et al (2020) have emphasized that the supporting evidence is “limited and ambiguous”.
Ralston et al. (2008) explained that Hg poisoning occurs by creating a deficiency of Se needed for activity and synthesis of critical selenoenzymes. Hg causes essentially irreversible inhibition of selenoenzymes, especially those required to prevent and reverse oxidative damage in the brain (GPX, TXNDR), and this is considered as primarily responsible for the characteristic oxidative damage of Hg neurotoxicity (Spiller 2018; Ralston and Raymond 2018). Their depiction of this mechanism for mercury toxicity is compelling. Se is clearly a major player in Hg toxicity. Their important work has stimulated many subsequent studies including our own (Burger 2009, Burger and Gochfeld 2011, 2012a,b, 2013, 2020) to examine the molar relationship of Se and Hg in a variety of organisms. It is an important story, but we point out here that it is not the whole story, and that sulphur (S) and sulfhydryl groups (thiols, -SH) are an abundant target for Hg with toxic consequences (Ajsuvakova et al. 2020)..
Mercury Poisoning is Not the Same as Selenium Deficiency
Some authors (Ralston and Raymond 2018) have suggested that mercury toxicity occurs mainly through creating a selenium deficiency, jeopardizing its antioxidant role by inhibiting GPX and TXNDR. We compare the features of MeHg poisoning and Se deficiency to show they are not congruent. Serious MeHg poisoning events have occurred from both contaminated fish (e.g. Japan) and grain (e.g. Iraq), with a syndrome progressing from paresthesias to ataxia, dysarthria, tunnel vision, hearing loss, coma and death (Grandjean et al. 2010). Based on our clinical experience at the Environmental and Occupational Health Sciences Institute (Rutgers University, New Jersey, USA), a typical story of mild MeHg poisoning is a patient with paresthesias and visual changes who reports “I ate a can of tuna every day for years” (Gochfeld 2003; Grandjean et al. 2010). Even ingesting only “light tuna” averaging 0.1 μg/g at 140 g/day would exceed the EPA Reference Dose of 0.1 μg/kg-day, leading to the toxicity our patients have described.
Se deficiency is widespread where crops are grown on Se-poor soil (Shreenath et al. 2020). Mild to moderate Se deficiency is associated with infertility and neurologic effects including fatigue, muscle weakness and brain fog. Severe deficiency syndromes include Kashin-Beck disease (deformities of bones and joints), and Keshan disease (muscle pain and weakness) (Rayman 2012 Shreenath et al. 2020). These are not prominent features of mercury toxicity. We conclude that the syndromes of Hg poisoning and Se deficiency are not congruent. Antunes dos Santos et al. (2016) said it well: “No single process can explain the multitude of effects observed in MeHg-induced neurotoxicity” The roles of Se and S or selenols and thiols cannot be completely separated in a dynamic system.
The high affinity of Hg for Se has suggested that co-sequestration occurs as one of the major interactions. This is a suicidal relationship whereby Se binds Hg irreversibly but at the expense of inactivating crucial enzymes and tying up SeCys needed for synthesis of new enzyme as proposed by Ralston and Raymond (2018). In support of sequestration, Korbas et al (2010) were able to identify nanoparticle HgSe precipitate in the brain.
The brain, and also the kidney (Zalups and Lash 1996) are major sites for demethylation of MeHg. After chronic oral dosing with MeHg, monkeys accumulated Hg2+ in most brain regions (Vahter et al 1995). Selenite and glutathione enhanced the demethylation of MeHg (Komsta-Szumska et al.1983). Liu et al (2019) reported that MeHg altered gut flora while Se protected gut flora that enhanced the decomposition of MeHg in the GI tract.
Hg compounds have a high affinity for thiols (Clarkson et al 1972; Ajsuyakova et al 2020) and an even higher affinity for selenols (Sugiura et al. 1978). Therefore, selenols and thiols play important roles in the toxicokinetics and toxicodynamics of Hg (Spiller 2018; Ajsuvakova et al. 2020). The relationship and protectiveness between Hg and Se are complicated by the several forms of Hg (elemental, inorganic, organic) and of Se (selenite, selenate, selenide, organic) that have been used in the laboratory or found in fish (Skerfving 1978; Dang and Wang 2011). For Se, the essentiality or toxicity differs among the compounds (García-Barrera et al. 2019). Ralston et al. (2007) pointed out the importance of Se sequestering Hg as an insoluble Hg-selenide, but subsequently (Ralston and Raymond 2018) emphasized sequestering of SeCys by Hg as the critical mechanism.
Kaneko and Ralston (2007) is often cited as the authority for using molar ratios in risk communication about fish consumption, however they carefully do not identify 1:1 as a critical value for the ratio. Since then, the molar ratio has gained much attention as a way of emphasizing the benefits of eating fish. Many authors have mentioned or emphasized the 1:1 molar ratio as if it were a “bright line” (our term) between Hg toxicity and safety (Polak-Juszczak 2015; Cusack et al. 2017; Azad et al. 2018; Reyes-Avila et al. 2019; Ulusoy et al 2019 to name a few). This assumes that most of the ingested Hg molecule have encountered Se, either in the fish or in the consumer, before exerting a toxic effect. We concur that a molar excess of Se is highly beneficial, but how much of an excess is needed remains uncertain and controversial (Gerson et al 2020). Does a 2:1 ratio provide adequate protection or is a 5:1 or even 10:1 ratio assurance of safety. A definitive answer is elusive given our current state of knowledge.
Reliance on the Se:Hg molar ratio measured in fish treats the numbers as static for a given species. However, the ratios vary greatly within as well as among fish species (Burger and Gochfeld 2013; Cusack et al. 2017) and they vary by geography and ecology (Azad et al. 2019). The interactions of Hg and Se are very complex and research has demonstrated that for each, the behavior in the environment, the toxicokinetics (uptake, distribution, elimination), and toxicodynamics differ among the chemical species. This accounts, in part, for the variety of results and interpretations. As Ralston and Raymond (2018) assert, “selenium toxicology is a rapidly evolving field which continually disproves dogma”.
Mercury and Selenium
Experiments showing that under some conditions Se compounds protected animals from Hg toxicity began in the 1960s, even before the amino acid SeCys was identified. Mercury poisoning (primarily from elemental Hg0) was recognized in antiquity (Hunter 1978), and knowledge of inorganic Hg poisoning grew with the industrial and pharmacologic introduction of mercurials in the 19th century. Although organic mercurials came into use as pesticides and diuretics in the early 1900s, we generally point to 1960 as a turning point when MeHg was identified as the cause of the mysterious epidemic of poisoning from fish around Minamata Bay, Japan (Kurland et al. 1960, Hachiya 2006). This was a landmark event for environmental toxicology. Ironically, prior to 1960, Se was a suspected cause of the Minamata epidemic due to its high concentration in Minamata Bay fish (McAlpine and Araki 1958). In the late 1960s, ecotoxicologists demonstrated the biomethylation of Hg by bacteria in sediment (Jensen and Jernelöv 1969), which led to appreciation of the bioamplification of MeHg in the food chain (New Jersey Mercury Task Force 2001).
By contrast, our understanding of Se and the function of selenoenzymes is much newer with important questions about metabolism (Burk and Hill 2015), health effects (Rayman 2012), and interactions (Ralston and Raymond 2018, Gerson et al. 2020) to be clarified. Surai (2006:pp xix) opens his monumental (974 pp) monograph “Among many minerals Se has a special place being the most controversial trace element.” Until the mid-1900s, Se was known only as a toxic metalloid (Hill 1975), and research on fish deformities caused by Se continues today (Johnson et al. 2020). Se was recognized as essential in mammals in 1958 and glutathione peroxidase (GPX) was identified as a selenoprotein in 1973 (Surai 2006). It is a cytoplasmic enzyme that reduces the reactive H2O2 to water. In the 1980s selenocysteine (SeCys) was recognized as the 21st amino acid.
The active site of the GPX enzymes includes a SeCys identified as primary targets of Hg, accounting for the oxidative stress attributed to it (Ralston and Raymond 2018, Spiller 2018). However, these proteins also contain Cys sites which can also be targets of Hg (Picaud and Desbois 2006, Carvahlo et al. 2008). Hg blocks the active site of the selenoenzymes and also creates deficits of Se preventing synthesis of new SeCys necessary to replenish the inactivated enzymes, thereby reducing antioxidant defense (reviewed in Surai 2006, ch 2).
Many papers have reviewed the protectiveness of Se against Hg toxicity. Ralston and Raymond (2018) suggest a different approach, that Hg toxicity is due to its affinity for Se, resulting in the inactivation of selenoenzymes, a major antioxidant defense. Oxidative damage does the rest in many systems (Ralston and Raymond 2018). An excess of Se is then necessary to replace the damaged enzymes, which uniquely, in the case of SeCys has to be synthesized anew. The Hg-SeCys cannot be reused (Papp et al. 2007). This has been a valuable observation, changing the way we think of Hg and Se interactions. However, there is more to Hg poisoning than solely tying up Se. Conversely Se supplementation may not block all Hg toxicity (Branco et al. 2012a).
In animals (including humans) mercury toxicity has usually been ascribed to its high affinity for sulfur (S) (Poopal 2013) and thiols (-SH, Ajsuvakoa et al 2020), which in the form of the amino acid cysteine (Cys) is responsible for forming disulfide (-S-S) bridges in protein chains, holding proteins in various folded configurations essential for their function as enzymes, Mercury is capable of blocking thiols, thereby inhibiting the enzymes. Emphasizing the affinity of Hg for –SH, Ajsuvakova et al (2020) provide alternative explanations for the mechanisms of Hg toxicity and oxidative stress.
Mercury and Oxidative Stress
Since the early 1990s, oxidative stress has evolved as a major phenomenon in redox biology, pathogenesis, and toxicology (Sies 2015). Oxidative stress is recognized as playing significant roles in metal toxicology, particularly for mercury. Before oxidative stress was a familiar term, Ganther (1978) had proposed that Hg poisoning involved free radicals, particularly the methyl radical (CH3*). However, oxidative stress did not figure prominently in understanding Hg toxicology until the 1990s, (Miller and Woods 1993). by which time the selenoenzyme role was being recognized. Chang’s compendium on Toxicology of Metals published in 1997 has a chapter on oxidative stress that mentions many metals, but mercury is notably absent (Kaspryzak 1997).
Using PUBMED and the rubric “mercury and oxidative stress”, we found that the first few entries are from 1992, and the literature doesn’t begin to increase rapidly until 2005. Zhang et al. (2020) provide a current review of the selenoenzymes as antioxidants with SeCys at the active site of GPX and TXNRD. These enzymes each with a single SeCys amino acid, are targets for Hg (Branco et al.2012a, Spiller 2018). However, Carvahlo et al (2011) note that the active site of TXNRDC has both a Cys(497) and SeCys(498), and Hg links these with high affinity inhibitng the enzyme. However, in vitro some –SH rich chelating agents can remove the Hg, restoring activity and selenite is even more effective at rescue (Carvalho et al. 2011). But while Hg is inhibiting GPX, it is also tying up other Se molecules that could be used by the cell to replace the depleted enzyme. If the Se content of brain cells, for example, fall below 60% according to Ralston and Raymond (2018), the antioxidant protection fails, and brain cells, including neurons die. Branco et al (2012a) noted that Se has “limited capacity to prevent mercury effects in the brain and kidney”, particularly with regard to GPX. Indeed Se alone at high levels can promote oxidative stress (Hoffman and Heinz 1998).
Inhibition of GPX and particularly TXNRD (Branco et al. 2012a), account in part for oxidative stress caused by Hg, and by other cations, such as Cd (Hurna et al. 1997) that also have a high affinity for Se. Branco et al. (2012b) suggest that Hg inhibition of TXNRD is a potential biomarker of Hg toxicity. GPX and TXNRD are important parts of antioxidant defense, that includes the tripeptide glutathione (GSH)(Nesci et al. 2016; Farina and Aschner 2019). Markers of oxidative stress and lipid peroxidation, including malondialdehyde (MDA) and thiobarbituric acid reactive substances (TBARs), increase in the kidney as MeHg is converted to Hg2+ (Carneiro et al. 2014).
Oxidative stress from mercury is not solely due to Se-affinity. Hg has a high affinity for the thiols of superoxide dismutase (Ajsuvakova et al. 2020) and for the –SH of GSH as demonstrated by Fuhr and Rabenstein (1973). GSH is part of the antioxidant chain interacting with GPX as it undergoes redox cycling from –G-S-S-G to -GSH- (Rubino 2015). Woods and Ellis (1995) showed that Hg2+ increased the synthesis of GSH, which in turn lowered the MDA and TBARs markers consistent with its known role in oxidative defenses. The selenoezymes TXNRD and GPX each have one SeCys, but several Cys which could be targets. Carvalho et al. (2011) reported that the active site of TXNRD involves Hg reacting with both Cys497 and SeCys498.. Picaud and Desbois (2006) identified Hg inactivating GPX at the Cys45 and Cys50 sites. Hg also binds to Cys on Manganese superoxide dismutase (Ajsuvakova et al. 2020) thereby inhibiting another antioxidant defense.
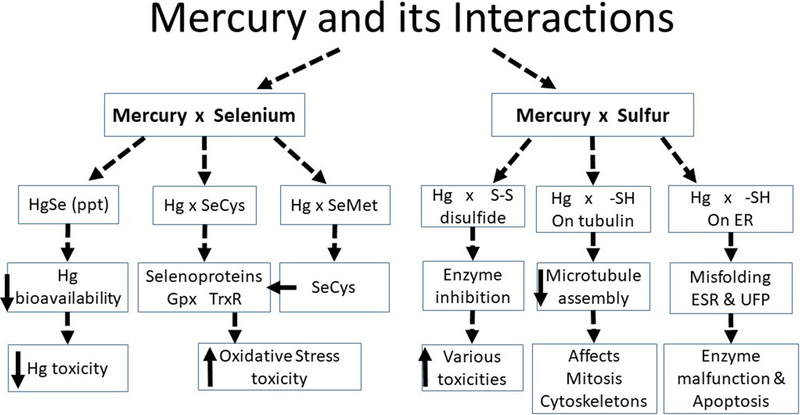
Below we discuss key issues in Hg interactions and toxicity, including interaction with S resulting in enzyme inhibition and microtubule disruption. Figure 1 presents our conceptualization of the interactions and consequences of Hg interacting with Se and S as discussed in this paper.
FIG. 1.
Mercury and its interactions and effects with Se or selenols (left side) and with S or thiols. (right side).
Mercury and sulfur
Sulfur (S) is one of the most common minerals in the body, occurring mostly as amino acids cysteine (Cys) and methionine (Met) in proteins, with smaller quantities present as GSH and as homocysteine and taurine. Ajsuvakova et al (2020) provide an extensive review of sulphur containing ligands for Hg. There is more than a million times more S atoms than Se atoms in the body (IOM 2006) counter balancing the apparently much greater Hg affinity for Se vs S. Raymond and Ralston (2004) emphasized that selenium’s higher affinity (Sugiura et al. 1978) results in Hg binding preferentially to Se or Se disrupting Hg-S bonds. The extent to which, and the circumstances under which, Se can displace Hg from other ligands is not completely known. More importantly, by mass action, an Hg molecule has a much higher probability of encountering a thiol than a selenol (IOM 2006).
Clarkson (1972) writing at a time when the protective role of Se was barely known, emphasized the affinity of Hg for SH groups on proteins, noting that the abundance of this ligand, was sufficient to bind all Hg (inorganic or organic) administered even at lethal doses.. Other proteins involved in Hg kinetics involving S binding are metallothioneins and hemoglobin. Metallothioneins are low molecular weight proteins rich in Cys that serve as reservoirs or transporters for metals, particularly Cu and Zn, but also xenobiotics such as Cd, Pb, and Hg. Hg is an efficient inducer of metallothionein in the liver which is often mentioned as a detoxification or elimination mechanism (Palacios and Capdevila 2013). Sørmo et al. (2011) demonstrated that metallothionein level could be a biomarker of a Se:Hg ratio below 1:1. Hemoglobin is also an important ligand for Hg in red cells due to –SH binding (Weed et al. 1962). A study of dolphins identified that hemoglobin (specifically Cys residues in the beta-chain) is a major ligand for MeHg (Zayas et al. 2014). Less well-defined are sulfur-containing compounds such as the amino acid taurine, which can reduce hepatotoxicity (Jagadeesan and Sankarsami Pillai 2007). Zalups and Lash (1997) describe a Hg-cys conjugate as a vehicle leading to binding of Hg to renal luminal membranes. These exemplify the diverse roles of thiol binding influencing Hg toxicity.
Mercury and enzyme inhibition
Enzyme inhibition by mercury is recognized as an important part of mercury toxicity (Stratton 2017). Mercury inhibition of selenoenzymes has been discussed above, but Hg compounds inhibit many non-selenoenzymes and induce others. The varied manifestations of Hg toxicity result in part from the variety of enzyme reactions or receptors inhibited (Spulber et al 2018). In some cases inhibition is due to binding exposed thiols. This binding by Hg can be reversible or not, or can result in denaturation or protein aggregation (Frasco et al. 2007). Enzymes inhibited by Hg include acetylcholinesterase (Frasco et al. 2007), catalase (Chen et al. 2015), amylase, lipase, lactase and glucose-6-phosphatase (Temel and Taysi 2019), delta-aminolevulinic acid dehydratase (Rocha et al 1995), and Na/K ATPase (Kade 2012). Chymotrypsin lacks available Cys, but Hg inactivates it by binding to histidine residues (Stratton et al. 2017). Of particular interest is inhibition of δ-aminolevulinic acid dehydratase (ALA-D) that catalyzes a rate-limiting step in hemoglobin synthesis, resulting in hypochromic anemia. Both lead and Hg compounds (Rocha et al. 1995), as well as Se compounds (Barbosa et al. 1998) inhibit this enzyme experimentally. Hg2+ and selenite both inhibit the ALA-D with some evidence of synergism, due to binding an –SH group and displacing zinc at the active site (Farina et al. 2003).
Na/K ATPase, a ubiquitous membrane pump enzyme that maintains membrane potential, cell volume, and active transport is inhibited by MeHg blocking thiols, specifically a Cys113 (Wang and Horisberger 1996), but this can be reversed by organoselenium, or by GSH or Cys (Kade 2012). Recently (Baldiserra et al 2020) reported that pre-treatment of fish with diphenyl selenide confers some protection against MeHg inhibition of Na-K ATPase activity. They invoked the Se protection as evidence that the enzyme inhibition was due indirectly to oxidative stress, rather than directly to –SH binding of the enzyme (Poopal et al 2013).
MeHg exposure also results in a complex pattern of protein up and down regulation at the genome level, some changes manifesting toxicity and others apparently as adaptive defenses. (Jenko et al. 2012). That is beyond the scope of the present paper.
Mercury and Microtubules
Microtubules are elongated hollow fibers that play critical roles in the cytoskeleton of cells and in cell cycling, for example in mitosis. They are polymers of the tubulin protein. They are characterized by ‘dynamic instability’ and by the suddenness of ‘catastrophic disassembly’ and of polymerization or ‘rescue’ (Gardner et al. 2013). A significant manifestation of Hg toxicity involves disruption of microtubules (Graff and Reuhl 1996). The microtubules arise from centromeres to form the mitotic spindle that separates sister chromatids during mitosis. In the 1960s studies of onion root and fruit flies exposed to MeHg, revealed a potent disruption of mitosis in metaphase arrest, (Ramel 1969; Ramel and Magnusson 1969), similar to that caused by colchicine (Eigsti et al. 1949). They attributed the disruption to breaking of disulfide bonds (-S-S-), thereby disrupting enzyme functions and halting the function of the mitotic spindle. Subsequent cell culture studies showed the methylmercury disrupted microtubules, depressing the polymerization of tubulin (Imura et al. 1980) essential for mitotic spindle formation.
Microtubules form from the assembly of tubulin subunits. Tubulin, a heterodimer protein, was first discovered in the 1950s, but its structure was not elucidated until 1998 (Yarris 1998). The tubulin protein polymerizes into the filaments that form the tubules. This is a very dynamic, rapidly reversible, phenomenon as microtubule structure has to constantly change, for example, during mitosis, or in the regulation of intracellular transport. Abe et al. (1975) reported that MeHg caused depolymerization of microtubules.
Sager et al. (1983) showed that MeHg disrupted microtubules of cultured fibroblasts in a dose-dependent manner. Stoiber et al. (2004) reported a dose-dependent inhibition of tubulin assembly (NOEL 1 μM) and microtubule motility (NOEL 0.05 μM). Wasteneys et al. (1988) showed that MeHg caused a dose-dependent disassembly of the mitotic spindle in cultured fibroblast cell, which was reversed by washout of the MeHg. Vogel et al. (1989) studied MeHg effects on microtubules in vitro, finding inhibition of polymerization as well as depolymerization. MeHg bound to 15 ‘high affinity sites” (-SH) on the surface of the tubulin dimer, and binding to only 2 of the sites inhibited polymerization (Vogel et al. 1989). Microtubule function is complex requiring both the structure of the tubulin polymer and the ordered motility of the associated kinesin motor (Goshima and Vale 2003). Bonacker et al. (2004) showed that Hg inhibited both the microtubule function as well as assembly in a dose-dependent manner.
Selenium-mercury interaction.
The Parizek and Ostadalova (1967) paper is generally recognized as the first report of Se mitigating Hg toxicity. Na-selenite injected a few hours after an HgCl2 dose in rats reduced toxicity. However, reversal of the sequence resulted in increased toxicity (Parizek et al. 1971). Ganther et al. (1972) brought this into the fish consumption risk domain by feeding Japanese Quail MeHg in a diet containing tuna vs corn-fed controls. The tuna-fed chicks survived, and an experiment using Se equivalent to that found in the tuna diet, “confirmed” the protectiveness of Se against MeHg. Rats fed a diet containing 0.5 ppm of Se survived MeHg in drinking water whereas most of those on a Se-deficient diet died by week 6 (Ganther et al. 1972).
In Amazonian Brazil where indigenous people are exposed to Hg from gold mining directly and through fish consumption, high dietary Se protects motor function (Lemire et al 2011). Many papers have confirmed that Se confers protection, while others found little or no protection or even synergism (Penglase et al. 2014; Heinz et al. 2012). Some authors report that Se sequesters Hg, and others (Ralston and Raymond 2018) suggested that Hg sequesters Se as the primary toxic affect, leading to oxidative damage if Se is inadequate. Se also has a high affinity for –SH groups (Mykkanen and Wasserman 1990) and may inhibit enzymes by binding to Cys. Excess Se has been implicated in lipid peroxidation (Hoffman 2002).
Not all Se and Hg interactions are protective. Se-methionine (Se-met) and MeHg were injected into bird eggs separately and together. Se-met caused more defects and hatching failure than MeHg, and injected together the hatching rate went up but paradoxically the deformity rate also went up (Heinz et al. 2012) and the effects were greater than similar concentrations conveyed to the egg from the female.
Is a 1:1 molar ratio protective?
Now we return to the question of whether a 1:1 or even >1:1 Se:Hg assures protection. There seems to be agreement that where the Se:Hg molar ratio is below 1:1, Hg toxicity is likely to occur. A great excess of Se is likely to confer protection until the Se itself is at a toxic level. Few people address the latter. However, the designation of “1:1” as a bright line (our term), although convenient, is misleading. Ralston et al. (2019) have proposed a new equation called the Selenium Health Benefit Value to incorporate the benefits of Se as well as its Hg- protectiveness. This is beginning to be used in some papers (Vega-Sánchez et al. 2020). Ralston et al. (2019) report “The HBV criterion provides a reliable basis for differentiating seafoods whose intake should be limited during pregnancy from those that should be consumed to obtain health benefits.”
Although Ralston et al. (2007) emphasized the importance of the molar ratio and Se excess in evaluating Hg risk to humans from eating fish, they carefully do not identify a 1:1 molar ratio as a divide between toxicity and safety. However, the 1:1 ratio has taken on a life of its own. It is attractive to visualize a system in which every MeHg molecule meets a Se molecule, binds and co-precipitates as insoluble HgSe. But there are many more sulphur targets in the body, and Hg molecules are much more likely to encounter sulphur, for example, in enzymes and tubulin molecules, where the toxicity may be manifest, before Se comes to the rescue.
Some examples of the emphasis on a 1:1 ratio include the following:
“Since Se is present in molar excess of Hg, Hg exposures from eating these fish is not a public health concern” (Ulusoy et al. 2019 from Turkey)
“The mean Se/Hg ratios were greater than one in all fish species indicating that Se antidotal effect in counteracting Hg occurred.” (Barone et al. 2017 from Italy).
“The mean molar ratios for each species were all above 1:1.” (Cusack et al. 2017, from USA).
“Selenium binds to organic mercury at a molar ratio of 1:1 Se/Hg to inhibit the toxic effects of Hg” (Donald 2016 from Canada).
“When the molar ratios of Se to Hg in fish exceeds 1.0 ingestion of the fish is unlikely to deplete Se reserves” (Reyes-Avila et al. 2019 from USA).
“A tissue Se:Hg molar ratio greater than 1 is suggested as a threshold for the protecting action of Se against Hg toxicity”(Mulder et al. 2012 from Norway).
In addition to thiol-mediated toxicity, another reason to question the protectiveness of Se at a 1:1 ratio or any ratio close to 1:1 for human fish consumption advice is that Se has many other ligands including other cations that are present either naturally or not. Se itself binds extensively to –SH groups on proteins. And there are specific Se-binding proteins such as selenium-binding protein 1 (SBP1) that participates in cellular motility, redox modulation, protein degradation and other important functions including sulfur metabolism (Elhodaky and Diamond 2018). Taken together we conclude that despite high affinity for Hg, not all Se molecules are available to interact with Hg.
In general, as Hg concentration increases the Se:Hg ratio declines. Hg generally increases with fish size, while Se which is homeostatically regulated does not (Burger and Gochfeld 2011). Therefore, it is predictable that Wang et al. (2018) and others (Burger 2009; Gochfeld et al. 2012; Santos and Silva 2017) reported a decrease in Se;Hg with increasing fish size within species and at higher trophic level in the food web (Polak-Juszcak 2015, Squadrone et al. 2015). This is not a universal observation, however. Many studies reported that Hg and Se levels in fish tissue tend to be correlated suggesting they are already complexed in the fish tissue. Although this may reduce the toxicity of the Hg to the first consumer, it is also a mechanism for enhancing the bioamplification of Hg in the food chain (Beijer and Jernelöv 1978).
We examined molar ratios in a variety of fish species and found them too variable within species to be useful in risk communication, (Burger and Gochfeld 2012a,b) and urged that Hg concentration itself not be ignored in developing fish consumption advisories (Burger and Gochfeld 2020; Gochfeld et al. 2012). We acknowledge that a substantial excess of Se over Hg confers protection, but how much of an excess is needed requires more study and whether excess Se can completely protect against high dose of MeHg is uncertain (Cusack et al. 2017, Gerson et al. 2020).
Significance of variations in Se:Hg molar ratios
We assembled data from several of our papers on Hg and Se concentrations and molar ratios in freshwater and marine fish (Burger and Gochfeld 2013). Table 1 shows that among 53 marine fish, 13 had some individuals with total Hg (THg) > 0.75 ppm. Among predators 72% of Swordfish (Xiphias gladius) and 94% of Mako Shark (Isurus oxyrinchus) had Hg > 0.75 ppm. Mako averaged 1.96 ppm THg(n=51). Only Mako Shark had a mean Se:Hg ratio below 1. However, 24 species had mean Se:Hg ratios below 5:1. Among 16 freshwater species 67% of Bowfin (Amia calva)) had Hg> 0.75 ppm. Among fresh water species 11 (68%) had a mean ratio below 5:1. At present we don’t have a good estimate of whether even a 5:1 molar excess is adequate for protection, and there is no reason to believe that the degree of protectiveness bears a linear relationship to the ratio in edible portions of a fish or whether the same ratio is protective across species. Recently, Gerson et al (2020), relying on different information also concluded there is not a “strong scientific basis for modifying current fish consumption advisories on the basis of Se:Hg ratios.”
Table 1.
Comparison of high mercury levels and Se:Hg ratios less than 1.0 among 54 salt water species and 16 fresh water species (from Burger and Gochfeld 2013).
| Species with individuals having Total.Hg > 0.75 ppm | Species with mean Se:Hg ratio less than 1:1 | Species with mean Se:Hg ratio less than 5:1 | Species with mean Se:Hg ratio greater than 5:1 | |
|---|---|---|---|---|
| 58 salt water species | 13 species 22% | 1 species 2% | 23 species 40% | 35 species 60% |
| 16 fresh water species | 7 species 44% | 1 species 6% | 11 species 69% | 5 species 31$ |
As an example from another source, a study of 7 fish species from Louisiana (Reyes-Avila et al. (2019) found two species with Se:Hg ratios <1, and three species with ratios between 1:1 and 3:1, which we consider a gray area of only slight Se excess..
Discussion
Research indicates that MeHg irreversibly inhibits the selenoenzymes that normally prevent or/reverse oxidative damage in the brain and other tissues. Unless excess Se is available or supplemental Se is provided, consequences increase as Se:Hg declines towards unity (Ralston et al. 2016). How much of a Se excess is needed for protection? This would be amenable to laboratory studies, but relevant human studies would be very difficult to conduct. Choi et al. (2008) used cord blood Hg and Se concentrations, to analyze the Faroe Island prospective cohort neurobehavioral outcome data. They reported “no evidence was found that Se was an important protective factor against MeHg neurotoxicity,” despite the 10:1 molar excess of Se in cord blood measured years earlier. Due to the long time lag between exposure and outcome, this should be interpreted with caution.
The relative abundance of thiols vs selenols ests that any Hg molecule in fish, is probably already bound to a Cys in protein by chance alone. Once absorbed, the Hg-Cys may dissociate as other S or Se ligands are encountered, and an Hg-Cys may indeed survive without ever encountering a Se ligand. Important missing information is whether a Se bound for selenoprotein P or already incorporated in SelP will attract or break an HgS bond and form an HgSe bond that renders the Se, irreversibly unavailable. Protection against Hg toxicity comes at a significant cost.
Nothing in this paper should be construed as arguing against eating fish, or even occasional large predatory fish that may have high concentrations of MeHg. Nor do we underestimate the importance of Se. We believe there is abundant evidence that people who eat fish rarely will attain some health benefits from eating fish more frequently (Rimm et al. 2018) up to a maximum of about two meals a week (Gochfeld and Burger 2005). Bernstein et al. (2019) and Domingo (2016) for example, share our cautious recommendations. People who eat fish more frequently (> 2x week) or large meals (>200 g) should pay attention to Hg content, avoiding high Hg fish, and favoring those with Hg < 0.1 ppm. There is no benefit other than preference from choosing high Hg fish, and our experience with clinical cases suggests, not surprisingly, that a predilection for predatory fish is a risk factor for Hg poisoning. Likewise, we are confident that Se does confer some protection when present in molar excess, but probably substantial molar excess. We recommend that the utility of the Selenium Health Benefit Value (Ralston et al. 2019), continue to be evaluated in future studies. We have found (Burger and Gochfeld 2013) that Se:Hg ratios mainly reflect the Hg in the denominator and that Hg concentration is a more direct and reliable indicator of health risk, than the molar ratio..
Conclusions
Hg and Se have a high mutual affinity and each can counteract the toxic potential of the other. A primary manifestation of Hg toxicity is oxidative stress, mainly mediated by Hg irreversibly binding SeCys on GPX and TXNRD. Hg that encounters Se can form an insoluble HgSe precipitate, or a complex, rendering Se unavailable for synthesis of SeCys and replacement of the inactivated enzymes. Hg has other oxidative stress effects through targeting SOD and catalase. Although Hg can cause a relative Se deficiency, the symptoms of Hg poisoning are not the same as the symptoms of Se deficiency. Much of the toxicity of Hg is mediated through its high affinity for sulfur, including its ability to inhibit enzymes by reacting with thiols and to interfere with microtubule assembly by binding the high affinity –SH groups on the tubulin molecule. Although this paper argues that a 1:1 Se:Hg molar ratio is not sufficient to prevent Hg toxicity, we do not attempt to estimate a molar excess that assures safety from MeHg. Indeed, at a very high concentration of Se present in some fish it may be Hg that protects against Se toxicity. Future laboratory and epidemiologic research may give us at least a range for a protective molar ratio. Reuhl (1988) wrote “The multiplicity of MeHg actions upon biochemical pathways and organelles makes it improbable that a single mechanism or biochemical target adequately explains MeHg’s cytotoxic effects.” We agree with Gerson et al (2020) that significantly more information is needed to provide a strong scientific basis for modifying current fish consumption advisories on the basis of Se:Hg ratios
Implications
Considering the many other ligands available for Hg, and the many other metals that avidly bind Se, a substantial molar excess of Se in fish tissue is necessary to mitigate Hg toxicity. The molar ratio may be an important biochemical and toxicological aspect of Hg toxicokinetics and toxicodynamics, but it is confusing for risk communication. The degree of protectiveness or conversely of risk may not be a linear function of the Se:Hg molar ratio. We believe that at the present state of knowledge, Hg concentrations in fish are more informative for risk communication to pregnant women or to people who consume fish very frequently.
Highlights:
Fish have high nutritional value but some also have high levels of methylmercury.
Mercury exerts toxic effects by binding to sulfhydryl (-SH) groups which are its most abundant ligand in the fish and in the human..
Mercury inhibits many enzymes by reacting with thiols and also selenols.
Mercury inhibits microtubule formation essential for cell structure, cellular transport and cell division, by binding to –SH sites on the tubulin protein to prevent assembly
Mercury has high affinity for selenium and causes oxidative damage by inhibiting selenoenzymes (e.g., GPX, TXNRD) that are major components of cells’ antioxidant defenses. Some believe that this is the major mechanism for Hg toxicity.
Exposure to methylmercury from fish poses a public health risk, although a great excess of Se may partially mitigate the risk by assuring an adequate supply of selenocysteine for selenoprotein replacement. However, the risk of Se toxicity should not be ignored.
The Se:Hg molar ratio provides information on the relative excess of Se, but people who eat fish frequently should avoid fish with high mercury content. Fish advisories should take into account the mercury concentration and not rely solely on the molar ratio.
Acknowledgments
We thank our colleagues Michael Gallo, Jeffrey Laskin, Dan Morse, Ken Reuhl, and Helmut Zarbl who have shared their extensive knowledge of biochemistry and toxicology in general and mercury and selenium in particular. Nicholas Ralston provided valuable discussions, questioned our assumptions, and addressed our questions. We have benefitted from decades of discussions and collaborations on issues of fish consumption advice and advisories including Ned Groth, Philippe Grandjean, and Alan Stern. This paper benefitted greatly from two knowledgeable and thoughtful anonymous reviewers who encouraged us to re-examine and substantiate many of our assumptions. And thanks to our patients who came to our clinic with high mercury levels and symptoms of mercury poisoning from eating a lot of fish, usually for “health reasons”. Fortunately most of them have gotten better as their mercury levels declined. Our clinic colleagues Howard Kipen, Iris Udasin, Michael Pratt, Nancy Fiedler, and Rob Laumbach contributed with valuable discussions of differential diagnoses and management options. Christian Jeitner handled searches, manuscript preparation, formatting and submission details in addition to preparing graphics for the oral presentations. Last but not least we thank Carlos Lodeiro Espiño and Jose Luis Capello for the invitation to participate in the 3rd PTIM conferences and present these ideas to an audience of diverse expertise.
Funding: This research was funded by the National Institute of Environmental Health Science Center for Environmental Exposure and Disease (NIH-NIEHS P30ES0050022), the USDA National Institute of Food and Agriculture (Hatch Multistate Project 1008906 through NJAES (Hatch NJ 12233, W4045), and the U.S. DOE (DE-FC 01-06EW 07503 grant to the Consortium for Risk Evaluation with Stakeholer Participation (CRESP). Travel support for J Burger was provided by the PTIM conference organization as a plenary speaker.
Footnotes
Competing Interests: The authors have no competing or financial interests to declare.
Disclosure: We have no conflict of interest related to mercury, selenium or fish consumption. A summary version of this paper was presented at the 3rd International Caparica Conference on Pollutant Toxic Ions and Molecules November 2019, Caparica, Portugal.
Declarations
Ethical Approval: The paper is a conceptualization and analysis of the published literature on the topic. No human subjects or animals were used in this paper.
Consent to Participate: Not applicable.
Consent to Publish: Not applicable.
Availability of data and materials: Data sharing is not applicable to this paper because no data sets were generated or analyzed during the study. All references used are listed in the reference section. All are available through PUBMED or at the URLs provided (updated 10 DECEMBER 2020).
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Abe T, Haga T, Kurokawa M (1975) Blockage of axoplasmic transport and depolymerisation of reassembled microtubules by methyl mercury. Brain Res 86:504–508 [DOI] [PubMed] [Google Scholar]
- Ajsuvakova OP, Tinkov AA, Aschner M, Rocha JBT, Michalke B, Skalnaya MG, Skalny AV, Butnarin M, Dadar M, Sarac I, Aaseth J, Bjorklund G (2020) Sulfhydryl groups as targets of mercury toxicity. Coord Chem Rev 417:213343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes Dos Santos A, Appel Hort M, Culbreth M, López-Granero C, Farina M, Rocha JB, Aschner M (2016) Methylmercury and brain development: A review of recent literature. J Trace Elem Med Biol 38:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. 2003. Toxicological Profile for Selenium. Agency for Toxic Substances and Disease Research, Centers for Disease Control and Prevention, Atlanta, GA: https://www.atsdr.cdc.gov/toxprofiles/tp92.pdf [accessed 10 December 2020] [Google Scholar]
- Azad AM, Frantzen S, Bank MS, Nilsen BM, Duinker A, Madsen L, Maage A (2019). Effects of geography and species variation on selenium and mercury molar ratios in Northeast Atlantic marine fish communities. Science Total Environment 652:1482–1496 [DOI] [PubMed] [Google Scholar]
- Baldissera MD, Souza CF, de Silva AS, Henn AS, Flores EMM, Baldisserotto B (2020) Diphenyl diselenide dietary supplementation alleviates behavior impairment and brain damage in Grass Carp (Ctenopharyngodon idella) exposed to methylmercury chloride. Compar Biochem Physiol C229: 108674. [DOI] [PubMed] [Google Scholar]
- Barbosa NBV, Rocha JBT, Zenia G, Emanuelli T, Beque MC, Braga AL (1998) Effect of organic forms of selenium on δ-aminolevulinate dehydratase from liver, kidney, and brain of adult rats. Toxicol & Appl Pharm 149:243–253 [DOI] [PubMed] [Google Scholar]
- Barone G, Storelli A, Mallamaci R, Storelli MM (2017) Comparative study on trace metal accumulation in liver of Mediterranean deep-sea fish and their selenium/mercury molar ratios. Water Air Soil Pollut 228: Article No. 211 [Google Scholar]
- Beijer K, Jernelöv A (1978) Ecological aspects of mercury-selenium interactions in the marine environment. Environ Health Perspect. 23:43–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhar M (2018) Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic Biol Med 1;127:160–164 [DOI] [PubMed] [Google Scholar]
- Bernstein AS, Oken E, de Ferranti S (2019) Council on Environmental Health; Committee on Nutrition. Fish, Shellfish, and Children’s Health: An Assessment of Benefits, Risks, and Sustainability. Pediatrics. 143(6):e20190999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom N (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49:1010–1017 [Google Scholar]
- Bonacker D Stoiber T, Wang M, Böhm KJ, Prots I, Unger E, Thier R, Bolt HM, Degen GH (2004) Genotoxicity of inorganic mercury salts based on disturbed microtubule function. Arch Toxicol 78:575–583 [DOI] [PubMed] [Google Scholar]
- Branco V, Canario J, Lu J, Holmgren A, Carvalho C (2012a) Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase. Free Radical Biol Med. 52:781–793 [DOI] [PubMed] [Google Scholar]
- Branco V, Ramos P, Canario J, Lu J, Holmgren A, Carvalho C (2012b) Biomarkers of Adverse Response to Mercury: Histopathology versus thioredoxinreductase activity J. Biomed Biotechnol Article 3598879:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão R, Moresco RN, Bellé LP, Leite MR, de Freitas ML, Bianchini A, Nogueira CW (2011) Diphenyl diselenide potentiates nephrotoxicity induced by mercuric chloride in mice.. J Appl Toxicol. 31(8):773–782. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK (2010) Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev 13(5): 385–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Grandjean P, Weihe P (2007). Separation of risks and benefits of seafood intake. Environ Health Perspect 115:323–327, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J (2009) Risk to consumers from mercury in Bluefish (Pomatomus saltatrix) from New Jersey: size, season and geographical effects. Environ Res 1099:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J Gochfeld M (2011) Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size and season. Science Total Envron. 409:1418–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J (2012a) Selenium: mercury molar ratios in fish from the Savannah River: implications for risk management. J. Risk Res 15:627–644 [Google Scholar]
- Burger J, Gochfeld M (2012b). Selenium and mercury molar ratios in saltwater fish from New Jersey: individual and species variability complicate use in human health fish consumption advisories. Environ Res 114:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M (2013) Selenium/mercury molar ratios in freshwater, marine, and commercial fish from the USA: variation, risk and health management. Rev Environ Health 28:129–143 [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M (2020) Importance of biomonitoring levels of selenium, mercury, and selenium:mercury molar ratios in selected species in Northeastern United States estuaries: risks to biota and humans. J Environ Sci Pollution Res, this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk RF, Hill KE (2015) Regulation of Selenium Metabolism and Transport. Annu Rev Nutr. 35:109–134 [DOI] [PubMed] [Google Scholar]
- Cabañero AL, Carvalho C, Madrid Y, Batoreu C, Camara C (2005) Quantification and speciation of mercury and selenium in fish samples of high consumption in Spain and Portugal Biol Trace Elem Res 103:17–35 [DOI] [PubMed] [Google Scholar]
- Caito SW, Jackson BP, Punshon T, Scrimale T, Grier A, Gill SR, Love TM, Watson GE, van Wijngaarden E, Rand MD (2018) Variation in methylmercury metabolism and elimination status in humans following fish consumption. Toxicolog Sciences 161:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappon CJ, Smith JC (1982) Chemical form and distribution of mercury and selenium in edible seafood. J Anal Toxicol 6:10–21 [DOI] [PubMed] [Google Scholar]
- Carneiro MF, Grotto D, Barbosa F Jr (2014) Inorganic and methylmercury levels in plasma are differentially associated with age, gender, and oxidative stress markers in a population exposed to mercury through fish consumption. J Toxicol Environ Health A77:69–79 [DOI] [PubMed] [Google Scholar]
- Carvalho CML, Chew E, Hashemy SI, Lu J, Holmgren A (2008) Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity J Biol Chem 283:11913–11923 [DOI] [PubMed] [Google Scholar]
- Carvalho CM, Lu J, Zhang X, Arnér ES, Holmgren A (2011) Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: implications for treatment of mercury poisoning FASEB J. 25 370–381 [DOI] [PubMed] [Google Scholar]
- Carvalho LVB, Hacon SS, Vega CM, Vieira JA, Larentis AL, Mattos RCO, Valente D, Costa-Amaral IC, Mourão Silva GP, Oliveira BFA. (2019) Oxidative Stress Levels Induced by Mercury Exposure in Amazon Juvenile Populations in Brazil Internatl J Environ Res Public Health 16(15):2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang J, Zhu Y, Zhang Y (2015) Molecular interaction of inorganic mercury(ii) with catalase: a spectroscopic study in combination with molecular docking† RSC Advances No.97. 2015 https://pubs.rsc.org/en/content/articlelanding/2015/ra/c5ra15301h#!divAbstract [accessed 10 December 2020] [Google Scholar]
- Choi AL, Budtz-Jørgensen E, Jørgensen PJ, Steuerwald U, Debes F, Weihe P, Grandjean P (2008) Selenium as a potential protective factor against mercury developmental neurotoxicity. Environ Res 107:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW (1972) The pharmacology of mercury compounds. Annual Rev Pharmacol 12:375–406 [DOI] [PubMed] [Google Scholar]
- Cusack LK, Eagles-Smith C, Hardin AK, Kile M, Stone D (2017) Selenium:mercury molar ratios in freshwater fish in the Columbia River Basin: potential applications for specific fish consumption advisories. Biol Trace Elem Res 18:136–146 [DOI] [PubMed] [Google Scholar]
- Cuvin-Aralar ML, Furness RW (1991) Mercury and selenium interaction: a review Ecotoxicol Environ Saf 21:348–364 [DOI] [PubMed] [Google Scholar]
- Dang F, Wang W-X. (2011) Antagonistic interaction of mercury and selenium in a marine fish is dependent on their chemical species. Environ Sci Tech 45:3116–3122 [DOI] [PubMed] [Google Scholar]
- Dauplais M, Lazard M, Blanquet S, Plateau P (2013) Neutralization by Metal Ions of the Toxicity of Sodium Selenide. PLoS One. 2013; 8(1): e54353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL (2016) Nutrients and Chemical Pollutants in Fish and Shellfish. Balancing Health Benefits and Risks of Regular Fish Consumption. Crit Rev Food Sci Nutr 56:979–988 [DOI] [PubMed] [Google Scholar]
- Donald DB (2016) Relationships for mercury and selenium in muscle and ova of gravid freshwater fish. Environ Monitor Assess 188: Article #582. [DOI] [PubMed] [Google Scholar]
- Dyrssen D, Wedborg M (1991) The Sulphur-mercury (II) system in natural waters. Water Air Soil Pollut 56:507–519 [Google Scholar]
- Eigsti OJ, Dustin P jr, Gay-Winn N (1949) On the discovery of the action of colchicine on mitosis in 1889. Science 110:692. [DOI] [PubMed] [Google Scholar]
- El-Begearmi MM, Sunde ML, Ganther H (1977) A mutual protective effect of mercury and selenium in Japanese quail. Poult Sci 56:313–322 [DOI] [PubMed] [Google Scholar]
- Elhodaky M, Diamond AM (2018) Selenium-binding protein 1 in human health and disease. Int J Mol Sci 19(11): 3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (2011) National listing of fish advisories. U.S. Environmental Protection Agency. https://19january2017snapshot.epa.gov/sites/production/files/2015-06/documents/technical-factsheet-2011.pdf [accessed 10 December 2020] [Google Scholar]
- EPA (2020) Choose Fish and Shellfish Wisely: Fish and Shellfish Advisories and Safe Eating Guidelines. U.S. Environmental Protection Agency; http://www.epa.gov/choose-fish-and-shellfish-wisely/fish-and-shellfish-advisories-and-safe-eating-guidelines#guidelines [accessed 10 December 2020] [Google Scholar]
- Farina M, Aschner M (2019) Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay. Biochim Biophys Acta Gen Sub 1863(12): 129285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Brandão R, Lara FS, Soares FA, Souza DO, Rocha JB (2003) Mechanisms of the inhibitory effects of selenium and mercury on the activity of delta-aminolevulinate dehydratase from mouse liver, kidney and brain. Toxicol Lett 139:55–66 [DOI] [PubMed] [Google Scholar]
- Feng X et al. (2007) Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environ. Sci. Technol. 42: 326–332 [DOI] [PubMed] [Google Scholar]
- Frasco MF, Colletier JP, Weik M, Carvalho F, Guilbermino L, Stojan J, Fournier D (2007). Mechanisms of cholinesterase inhibition by inorganic mercury. FEBS J. 274:1849–1861 [DOI] [PubMed] [Google Scholar]
- Fuhr BJ, and Rabenstein DL (1973) Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX. The binding of cadmium, zinc, lead, and mercury by glutathione. J. Am. Chem. Soc 95(21):6944–6950 [DOI] [PubMed] [Google Scholar]
- Gad SC (2014) Methylmercury, pp 318–320 Wexler P (ed) Encyclopedia of Toxicology Academic Press, New York, 3rd edition [Google Scholar]
- Ganther HE, Sunde ML (1974) Effect of tuna fish and selenium on the toxicity of methylmercury: a progress report. J Food Sci 39:1–5 [Google Scholar]
- Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner P, Oh S-H, Hoekstra WG (1972) Selenium relation to decreased toxicity of methylmercury added to diets containing tuna. Science 175:1122–1124 [DOI] [PubMed] [Google Scholar]
- García-Barrera T, Moro GR, Acosta SR, Borrego AA, Leblic BC, Abril N, Roldan FN, Gómez-Ariz JL (2019) Metabolic impairments caused by “chemical cocktails” in mammals and the protective role of selenium. Keynote Lecture, 3rd International Caparica Conference on Pollutant Toxic Ions & Molecules, Caparica, Portugal [Google Scholar]
- Gardner MK, Zanic M, Howard J (2013) Microtubule catastrophe and rescue. Curr Opin Cell Biol 25:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson JR, Walters DM, Eagles-Smith CA, Bernhardt ES, Brandt JE (2020) Do Two Wrongs Make a Right? Persistent uncertainties tegarding environmental selenium–mercury interactions. Environ. Sci. Technol 54: 9228–9234 [DOI] [PubMed] [Google Scholar]
- Gochfeld M (2003) Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Safety 56:174–179 [DOI] [PubMed] [Google Scholar]
- Gochfeld M, Burger J (2005) Good fish/Bad fish: a composite benefit-risk by dose curve. NeuroToxicology 26:511–520 [DOI] [PubMed] [Google Scholar]
- Gochfeld M, Burger J, Jeitner C, Donio M, Pittfield T (2012) Seasonal, locational and size variations in mercury and selenium levels in Striped Bass (Morone saxatilis) from New Jersey. Environ Res 112: 8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Vale RD (2003) The roles of microtubule-based motor proteins in mitosis comprehensive RNAi analysis in the Drosophila S2 cell line J Cell Biol. 162:1003–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff RD, Reuhl KR (1996) Chapter 38: Cytoskeletal toxicity of heavy metals, pp 639–658 in Toxicology of Metals (Chang LW, ed) CRC-Lewis, Boca Raton FL [Google Scholar]
- Grandjean P, Satoh H, Murata K, Eto K (2010) Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 118:1137–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya N (2006) The history and the present of Minamata---Entering the second half century.JMAJ 49:112–118 [Google Scholar]
- Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Science Total Environ 326:1–31 [DOI] [PubMed] [Google Scholar]
- Heinz GH1, Hoffman DJ, Klimstra JD, Stebbins KR (2012) A comparison of the teratogenicity of methylmercury and selenomethionine injected into bird eggs. Arch Environ Contam Toxicol 62:519–528 [DOI] [PubMed] [Google Scholar]
- Hill CH. (1975) Interrelationships of selenium with other trace elements. Feder. Proceed 14:2096–2100 [PubMed] [Google Scholar]
- Hoffman DJ (2002) Role of selenium toxicity and oxidative stress in aquatic birds Aquat Toxicol 57:11–26 [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Heinz GH (1998) Effects of mercury and selenium on glutathione metabolism and oxidative stress in Mallard Ducks. Environ Toxicol Chem 17:161–165 [Google Scholar]
- Hunter D (1978). Diseases of Occupations. Hodder and Stoughton, London; 6th edition [Google Scholar]
- Hurna E, Siklenka P, Hurna S. (1997) Effect of selenium on cadmium genotoxicity investigated by micronucleus assay. Vet. Med (Praha) 42:339–342. [PubMed] [Google Scholar]
- Imura N, Miura K, Inokawa M, Nakada S (1980) Mechanism of methylmercury cytotoxicity: by biochemical and morphological experiments using cultured cells. Toxicology 17:241–254 [DOI] [PubMed] [Google Scholar]
- IARC (1993). Beryllium, cadmium, mercury and exposures in the glass manufacturing industry. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 58. International Agency for Research on Cancer, Lyon, France. https://www.ncbi.nlm.nih.gov/books/NBK499756/ [Accessed 10 December 2020] [PMC free article] [PubMed] [Google Scholar]
- IOM (2006) Dietary Reference Intakes:. Institute of Medicine, National Academies Press, The Essential Guide to Nutrient Requirements; Washington DC. pp. 313–19, 415–22 10.17226/11537. [Accessed 12 December 2020] [DOI] [Google Scholar]
- Jagadeesan G1, Sankarsami Pillai S (2007). Hepatoprotective effects of taurine against mercury induced toxicity in rats. J Environ Biol 28:753–756 [PubMed] [Google Scholar]
- Jenko K, Karouna-Reier NK, Hoffman DJ (2012).Gene expression, glutathione status, and indicators of hepatic oxidative stress in Laughing Gull (Larus atricilla) hatchlings exposed to methylmercury. Environ Toxicol Chem 31:2588–2596 [DOI] [PubMed] [Google Scholar]
- Jensen S, Jernelöv A (1969) Biological methylation of mercury in aquatic organisms. Nature 223(5207):753–754 [DOI] [PubMed] [Google Scholar]
- Johnson RC, Stewart AF, Limburg KE, Huang R, Cocherell D, Feyrer F (2020) Lifetime chronicles of selenium exposure linked to deformities in an imperiled migratory fish. Environ Sci Technol 54:2892–2901 [DOI] [PubMed] [Google Scholar]
- Kade IJ (2012) Mercury toxicity on sodium pump and organoseleniums intervention: a paradox. J Biomed Biotechnol 2012:924549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko JJ, Ralston NVC (2007) Selenium and mercury in pelagic fish in the central North Pacific near Hawaii. Biol Trace Elem Res 119: 242–254 [DOI] [PubMed] [Google Scholar]
- Kasprzak K(1997) Oxidative DNA damage in metal-induced carcinogenesis pp.299–320 In: Chang LW (ed) Toxicology of Metals. CRC Lewis Publ. Boca Raton FL [Google Scholar]
- Komsta-Szumska E, Reuhl KE, Miller DR. (1983) Effect of selenium on distribution, demethylation and excretion of methylmercury by the guinea pig. J Toxicol Environ Health 12:775–785 [DOI] [PubMed] [Google Scholar]
- Korbas M, O’Donoghue JL, Watson GE, Pickering IJ, Singh SP, Myers GJ, Clarkson TW, George GN (2010) The Chemical Nature of Mercury in Human Brain Following Poisoning or Environmental Exposure. ACS Chem Neurosci. 1: 810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland LT, Faro SN, Siedler H. (1960). Minamata Disease. The outbreak of a neurologic disorder in Minamata, Japan, and its relationship to the ingestion of seafood contaminated by mercuric compounds. World Neurol 1960:370–395 [PubMed] [Google Scholar]
- Lemire M, Fillion M, Frenette B, Passos CJ, Guimarães JR, Barbosa F Jr, Mergler D. (2011). Selenium from dietary sources and motor functions in the Brazilian Amazon. Neurotoxicology. 32:944–953 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang W, Zhao J, Lin X, Liu J, Cui L, Gao U, Zhang TL, Li B, Li YF (2018) Selenoprotein P as the major transporter for mercury in serum from methylmercury-poisoned rats. J Trace Elem Med Biol 50:589–595 [DOI] [PubMed] [Google Scholar]
- Liu Y, Ji J, Zhang W, Suo Y, Zhao J, Lin X, Cui L, Bai L, Hu H, Chen C, Li, Y (2019) Selenium modulated gut flora and promoted decomposition of methylmercury in methylmercury-poisoned rats. Ecotoxicol Environ Saf 185:109720. [DOI] [PubMed] [Google Scholar]
- Lubos E, Loscalzo J, Handy DE (2011) Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal 15: 1957–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine D, Araki S (1958) Minamata Disease: an unusual neurological disorder caused by contaminated fish. Lancet. 1958; 2(7047):629–631 [DOI] [PubMed] [Google Scholar]
- Magos L, Webb M. (1980) The interactions of selenium with cadmium and mercury. Crit Rev Toxicol 8(1):1–42 [DOI] [PubMed] [Google Scholar]
- Miller DM, Woods JS (1993) Urinary porphyrins as biological indicators of oxidative stress in the kidney. Interaction of mercury and cephaloridine. Biochem Pharmacol 46:2235–2241 [DOI] [PubMed] [Google Scholar]
- Mulder PJ, Lie E, Eggen GS, Ciesielski TM, Berg T, Skaare JU, Jenssen BM, Sørmo EG (2012) Mercury in molar excess of selenium interferes with thyroid hormone function in free-ranging freshwater fish. Environ Sci Technol 46:9027–9037 [DOI] [PubMed] [Google Scholar]
- Mykkanen HM, Wasserman RH (1990). Relationship of membrane-bound sulfhydryl groups to vitamin D-stimulated uptake of [75Se]Selenite by the brush border membrane vesicles from chick duodenum J Nutr 120:882–888 [DOI] [PubMed] [Google Scholar]
- Nesci S, Trombetti F, Pirini M, Ventrella V, Pagliarani A (2016) Mercury and protein thiols: stimulation of mitochondrial F1Fo-ATPase and inhibition of respiration. Chemico-Biol Interact 260:42–49 [DOI] [PubMed] [Google Scholar]
- New Jersey Mercury Task Force (2001) Final Report Vol 1 Executive Summary & Recommendations. New Jersey Department of Environmental Protection, Trenton, NJ: https://www.state.nj.us/dep/dsr/nj-mercury-volume1.PDF [accessed 10 December 2020] [Google Scholar]
- Palacios Ò, Capdevila M. (2013) Metallothioneins and Mercury. In: Kretsinger RH, Uversky VN, Permyakov EA (eds) Encyclopedia of Metalloproteins. Springer, New York, NY. 10.1007/978-1-4614-1533-6_309 [accessed 10 December 2020] [DOI] [Google Scholar]
- Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 9:775–806 [DOI] [PubMed] [Google Scholar]
- Parízek J, Ostádalová I (1967) The protective effect of small amounts of selenite in sublimate intoxication. Experientia 23:142–143 [DOI] [PubMed] [Google Scholar]
- Parízek J, Ostádalová I, Kalouskova J Babicky A, Benes J (1971) The detoxifying effects of selenium, Interrelations between compounds of selenium and certain metals, pp. 85–122 In: Metz W, Cornatzer WE (eds) Newer Trace Elements in Nutrition Marcel Dekker, New York [Google Scholar]
- Penglase S, Hamre K, Ellinngsen.(2014) Selenium and mercury have a synergistic negative effect on fish reproduction. Aquatic Toxicol. 149: 16–24, 2014 [DOI] [PubMed] [Google Scholar]
- Picaud T, Desbois A (2006) Interaction of glutathione reductase with heavy metal: the binding of Hg(II) or Cd(II) to the reduced enzyme affects both the redox dithiol pair and the flavin Biochemistry 45:15829–15837 [DOI] [PubMed] [Google Scholar]
- Polak-Juszczak L (2015) Selenium and mercury molar ratios in commercial fish from the Baltic Sea: additional risk assessment criterion for mercury exposure. Food Control 50:881–888 [Google Scholar]
- Polevoy C, Arbuckle TE, Oulhote Y, Lanphear BP, Cockell KA, Muckle G, Saint-Amour D (2020) Prenatal exposure to legacy contaminants and visual acuity in Canadian infants: a maternal-infant research on environmental chemicals study (MIREC-ID). Environmental Health 19(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poopal RK, Ramesh M, Dinesh B (2013) Short-term mercury exposure on Na+/K+-ATPase activity and ionoregulation in gill and brain of an Indian major carp, Cirrhinus mrigala. J Trace Elem Med Biol 27:70–75 [DOI] [PubMed] [Google Scholar]
- Ralston NV, Blackwell JL 3rd, Raymond LJ (2007) Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol Trace Elem Res 119:255–268 [DOI] [PubMed] [Google Scholar]
- Ralston NV, Ralston CR, Blackwell JL 3rd, Raymond LJ (2008) Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology 29:802–811 [DOI] [PubMed] [Google Scholar]
- Ralston NVC, Raymond LJ (2018) Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim Biophys Acta Gen Subj pii: 1862 (2018):2405–2416 [DOI] [PubMed] [Google Scholar]
- Ralston NVC, Ralston CR, Raymond LJ (2016) Selenium Health Benefit Values: Updated Criteria for Mercury Risk Assessments Biol Trace Elem Res. 171:262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston NVC, Kaneko JJ, Raymond LJ. (2019). Selenium health benefit values provide a reliable index of seafood benefits vs. risks. J Trace Elem Med Biol 55:50–57 [DOI] [PubMed] [Google Scholar]
- Ramel C (1969) Genetic effects of organic mercury compounds. I. Cytological investigations on Allium roots. Hereditas.61:208–230 [PubMed] [Google Scholar]
- Ramel C, Magnusson J (1969) Genetic effects of organic mercury compounds. II. Chromosome segregation in Drosophila melanogaster. Hereditas 61:231–254 [PubMed] [Google Scholar]
- Rand MD, Caito SW (2019) Variation in the biological half-life of methylmercury in humans: Methods, measurements and meaning Biochim Biophysica Acta Gen Sub. 1863(12):129301. [DOI] [PubMed] [Google Scholar]
- Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268 [DOI] [PubMed] [Google Scholar]
- Raymond LJ, Ralston NVC (2004) Mercury:selenium interactions and health implications. Seychelles Med & Dent J.(Special Issue) 7:72–77 [DOI] [PubMed] [Google Scholar]
- Reuhl KR (1988) Role of cytoskeletal damage in congenital methylmercury poisoning. Pp 211–224 In: Singer TP, Castanoli N, Wang CC (Eds). Molecular Basis of the Action of Drugs and Toxic Substances. Walter de Gruyter, New York [Google Scholar]
- Reyes-Avila AD, Laws ED, Herrman AD, DeLaune RD, Blanchard TP (2019) Mercury and selenium levels, and Se:Hg molar ratios in freshwater fish from South Louisiana. J Environ Sci Health (Part A) 54:238–245 [DOI] [PubMed] [Google Scholar]
- Rimm EB, Appel LJ, Chiuve SE, Diousse L, Engler MB, Kris-Eterton PM, Mazaffarian D, Siscovick DS, Lichtenstein AH (2018) Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 138:e35–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha JB, Pereira ME, Emanuelli T, Christofari RS, Souza DO (1995) Effect of treatment with mercury chloride and lead acetate during the second stage of rapid postnatal brain growth on delta-aminolevulinic acid dehydratase (ALA-D) activity in brain, liver, kidney and blood of suckling rats. Toxicology 100:27–37 [DOI] [PubMed] [Google Scholar]
- Roman HA, Walsh TL, Couli BA, Dewailly E, Guallar E, Hattis D, Marian K, Schwartz J, Stern AH, Virtanen JK, Rice G (2012) Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose-response function for regulatory benefits analysis. Environ Health Perspect 119:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino FM (2015) Toxicity of Glutathione-Binding Metals: A Review of Targets and Mechanisms. Toxics 26:20–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager PR, Doherty RA, Olmsted JB (1983) Interaction of methylmercury with microtubules in cultured cells and in vitro. Exp Cell Res 146:127–137 [DOI] [PubMed] [Google Scholar]
- Santos AB, Silva WL. New evaluation of selenium mercury ratios in fish and crabs from an impacted tropical estuary in southeastern Brazil. Int J Environ Sci & Natural Resources 2(3):001 [Google Scholar]
- Schartup AT, Thackray CP, Qureshi A, Dassuncao C, Gillespie K, Hanke A, Sunderland EM (2019) Climate change and overfishing increase neurotoxicant in marine predators. Nature 572:648–650 [DOI] [PubMed] [Google Scholar]
- Shreenath AP, Ameer MA, Dooley J (2020). Selenium deficiency. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020, https://pubmed.ncbi.nlm.nih.gov/29489289/ [accessed 10 December 2020] [Google Scholar]
- Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4:180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerfving S (1978) Interaction between selenium and methylmercury. Environ Health Perspect. 25:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørmo EG, Ciesielski TM, Øverjordet IB, Lierhagen S, Eggen GS, Berg T, Jenssen BM (2011) Selenium moderates mercury toxicity in free-ranging freshwater fish. Environ Sci Technol 45:6561–6566 [DOI] [PubMed] [Google Scholar]
- Spallholz JE (1993) On the nature of selenium toxicity and carcinostatic activity. Free Radical Biol Med 17:45–64 [DOI] [PubMed] [Google Scholar]
- Spiller HA (2018) Rethinking mercury: the role of selenium in the pathophysiology of mercury toxicity. Clin Toxicol 56:313–326 [DOI] [PubMed] [Google Scholar]
- Spulber S, Raciti M, Dulko-Smith B, Lupu D, Ruegg J, Nam K, Ceccatelli S (2018) Methylmercury interferes with glucocorticoid receptor: Potential role in the mediation of developmental neurotoxicity. Toxicol Appl Pharm 354:94–100 [DOI] [PubMed] [Google Scholar]
- Squadrone S, Benedetto A, Brizio P, Prearo M, Abete MC (2015) Mercury and selenium in European Catfish (Silurus glanis) from northern Italian Rivers: can molar ratio be a predictive factor for mercury toxicity in a top predator? Chemosphere 119:24–30 [DOI] [PubMed] [Google Scholar]
- Stoiber T, Bonacker D, Böhm KJ, Bolt HM, Thier R, Degen GH, Unger E (2004) Disturbed microtubule function and induction of micronuclei by chelate complexes of mercury(II). Mutat Res 563:97–106 [DOI] [PubMed] [Google Scholar]
- Stratton A, Ericksen M, Harris TV, Symmonds N, Silverstein TP (2017) Mercury(II) binds to both of chymotrypsin’s histidines, causing inhibition followed by irreversible denaturation/aggregation. Protein Science. 26:292–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Tamai Y, and Tanaka H (1978). Selenium protection against mercury toxicity: high binding affinity of methylmercury by selenium-containing ligands in comparison with sulfur-containing ligands. Bioinorg. Chem 9:167–180 [DOI] [PubMed] [Google Scholar]
- Surai PF (2006). Selenium in nutrition and Health. Nottingham Univ Press, Nottingham, UK: 974 pp [Google Scholar]
- Temel Y, Taysi MS (2019) The Effect of Mercury Chloride and Boric Acid on Rat Erythrocyte Enzymes. Biol Trace Element Res 191:177–182 [DOI] [PubMed] [Google Scholar]
- Ulusoy S, Mol S, Karakula FS, Kahraman AE (2019) Selenium-mercury balance in commercial fish species in Turkish waters. Biol Trace Element Res 191:207–213 [DOI] [PubMed] [Google Scholar]
- Vahter ME Mottet NK, Friberg LT, Lind SB, Charleston JS, Burbacher TM (1995) Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol App Pharmacol 143:273–284. [DOI] [PubMed] [Google Scholar]
- Vega-Sánchez B, Ortega-García S, Ruelas-Inzunza J, Frías-Espericueta M, Escobar-Sánchez O, Jara-Marini M. (2020) Selenium and mercury in Dolphinfish (Coryphaena hippurus) from the Gulf of California: inter-annual variations and selenium health benefit value. Environ Sci Pollut Res Int. 27:2311–2318 [DOI] [PubMed] [Google Scholar]
- Vinceti M, Wei ET, Malagoli C, Bergomi M, Vivoli G.(2001) Adverse health effects of selenium in humans. Rev Environ Health. 16:233–251 [DOI] [PubMed] [Google Scholar]
- Vogel DG, Margolis RL, Mottet NK (1989) Analysis of methyl mercury binding sites on tubulin subunits and microtubules. Pharmacol Toxicol 64:196–201 [DOI] [PubMed] [Google Scholar]
- Wang X, Horisberger JD. (1996) Mercury binding site on Na+/K(+)-ATPase: a cysteine in the first transmembrane segment. Mol Pharmacol 50:687–691 [PubMed] [Google Scholar]
- Wang X, Wu L, Sun J, Wei Y, Zhou Y, Rao Z1, Yuan L, Liu X (2018) Mercury Concentrations and Se:Hg Molar Ratios in Flyingfish (Exocoetus volitans) and squid (Uroteuthis chinensis). Bull Environ Contam Toxicol 101:42–48 [DOI] [PubMed] [Google Scholar]
- Wasteneys GO, Cadrin M, Reuhl KR, Brown DL (1988) The effects of methylmercury on the cytoskeleton of murine embryonal carcinoma cells. Cell Biol Toxicol. 4:41–60 [DOI] [PubMed] [Google Scholar]
- Watanabe C (2002) Modification of mercury toxicity by selenium: practical importance? Tohoku J Exp Med 196:71–77 [DOI] [PubMed] [Google Scholar]
- Weed R, Eber J, Rothstein A. (1962) Interaction of mercury with human erythrocytes. J Gen Physiol 45:395–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Ellis ME (1995) Up-regulation of glutathione synthesis in rat kidney by methyl mercury. Relationship to mercury-induced oxidative stress. Biochem Pharmacol 50:1719–1724 [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Y, Gunn JM, Belzile N. (2008) Selenium andmercury in organisms: interactions and mechanisms. Environ Review 16:71–92 [Google Scholar]
- Yarris L(1998) Mystery of vital cell protein solved after 30 Years. Berkeley Lab Research News January 8, 1998 https://www2.lbl.gov/Science-Articles/Archive/3D-tubulin.html [accessed 10 December 2020]
- Zalups RK, Lash LH (1996) Interactions between glutathione and mercury in the kidney, liver, and blood, pp. 145–163 in Toxicology of Metals. (Chang LW, ed). CRC Press-Lewis, Boca Raton, FL [Google Scholar]
- Zalups RK, Lash LH (1997) Binding of mercury in renal brush-border and basolateral membrane-vesicles Biochem Pharmacol 53:1889–1900 [DOI] [PubMed] [Google Scholar]
- Zayas ZP, Ouerdane L, Mounicou S, Lobinski R, Monperrus M, Amouroux D (2014) Hemoglobin as a major binding protein for methylmercury in white-sided dolphin liver. Anal Bioanal Chem 406:1121–1129 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Roh YJ, Han S, Park I, Lee HM, Ok YS, Lee BC, Lee SR (2020) Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review Antioxidants 9(5):383. [DOI] [PMC free article] [PubMed] [Google Scholar]