Abstract
BACKGROUND:
The prevalence of abdominal aortic aneurysm is high in chronic obstructive pulmonary disease (COPD) population. Emphysema involves proteolytic destruction of elastic fibers. Therefore, emphysema may also contribute to thoracic aorta dilatation. This study assessed aorta dilation in smokers stratified by presence of COPD, emphysema and airway thickening.
METHODS:
Aorta diameters were measured on 3D magnetic resonance angiography in smokers recruited from the Multi-Ethnic Study of Atherosclerosis (MESA), the Emphysema and Cancer Action Project (EMCAP), and the local community. COPD was defined by standard spirometric criteria; emphysema was measured quantitatively on computed tomography and bronchitis was determined from medical history.
RESULTS:
Participants (n=315, age 58–79) included 150 with COPD and 165 without COPD, of whom 56% and 19%, respectively, had emphysema. Subjects in the most severe quartile of emphysematous change showed the largest diameter at all four aorta locations compared to those in the least severe quartiles (all p<0.001). Comparing subjects with and without COPD, aorta diameters were larger in participants with severe COPD in ascending and arch (both p < 0.001), and abdominal aorta (p = 0.001). Chronic bronchitis and bronchial wall thickness did not correlate with aorta diameter. In subjects with emphysema, subjects with coexistence of COPD showed larger aorta than those without COPD in ascending (p = 0.003), arch (p = 0.002), and abdominal aorta (p = 0.04).
CONCLUSIONS:
This study showed larger aorta diameter in subjects with COPD and severe emphysema compared to COPD related to chronic bronchitis or bronchial wall thickening.
Keywords: aortopathy, aneurysm, MRI, MRA, emphysema, COPD
INTRODUCTION
Advanced age and smoking are risk factors for aortic aneurysms as they are for other cardiovascular and pulmonary diseases [1–7]. Other known risk factors include increased pressure [8], i.e. hypertension or bicuspid aortic valve jets [1] and diseases that weaken the aortic wall including atherosclerosis [8], Marfan’s and other connective tissue diseases [10].
High prevalence of aortic aneurysm is also reported in chronic obstructive pulmonary disease (COPD) [11] which is commonly observed in smokers. COPD is defined by airflow limitation on spirometry that does not fully reverse [12,13]. Smoking may also be associated with pulmonary emphysema, chronic bronchitis and airway thickening which can co-exist with COPD making it difficult to determine which features are more likely to be associated with aortic aneurysms[14]. This gap in knowledge is particularly relevant since emphysema and airway thickening can be measured on chest computed tomography (CT). Understanding which features are associated with aortic aneurysms could help potentially guide future screening and prevention strategies.
A striking histological feature of aortic aneurysms is destruction of the media and elastic tissue from excessive proteolytic enzyme activity in the aortic wall [2]. Emphysema also involves proteolytic destruction of elastic fibers. Thus, we hypothesize that both COPD and emphysema are at increased risk of aneurysm along the entire aorta. To test this hypothesis, we assessed aorta diameters in smokers stratified by presence of COPD, emphysema, chronic bronchitis and measures of airway thickening.
METHODS
Study participants:
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study that recruited 6814 participants in 2000 to 2002 from six U.S. communities, who were white, African-American, Hispanic, or Chinese-American, aged 45–84 years and free of clinical cardiovascular disease. Between 2010 and 2012, 4716 participants returned for follow-up.
The MESA Lung Study enrolled 3965 MESA participants who all underwent full-lung CT and spirometry in 2010–2012.
The protocols of MESA and all studies described herein were approved by the Institutional Review Boards of all collaborating institutions (Columbia University, New York, NY; Johns Hopkins University, Baltimore, MD; Northwestern University, Chicago, IL; University of California, Los Angeles, LA; University of Minnesota, Twin Cities, MN; University of Iowa, Iowa City, IA) [15].
The population of this study was created from three cohorts: 1) all the subjects with COPD or emphysema from the MESA Lung Study (n=190) [16], 2) all participants from the Emphysema and Cancer Action Project (EMCAP), a nonoverlapping lung cancer screening study (n= 89) [17], and 3) outpatient community at Columbia University Medical Center (n=36), as described in previous studies [18,19]. Inclusion criteria were 50–79 years of age with ≥ 10 pack-year smoking history. Exclusion criteria were clinical cardiovascular disease (myocardial infarction, angina, heart failure, valve disease, atrial fibrillation or stroke), stage IIIb-V chronic kidney disease, asthma prior to age 45 years, prior lung resection, cancer, allergy to gadolinium, claustrophobia, metal in the body, pregnancy, and weight > 300lbs. The present report includes these 315 participants who all underwent spirometry, CT scanning and contrast-enhanced cardiopulmonary magnetic resonance imaging (MRI) including three dimensional (3D) time-resolved magnetic resonance angiography (MRA, TRICKS or TWIST).
Study Oversight:
Study procedures were approved by institutional review boards of the participating institutions, Columbia Medical Center (AAAD6395), Johns Hopkins University (NA_00030361/CR00017844), North Western University (STU00021057-MOD0031), University of California, Los Angeles (11–002392-AM-00051), University of Washington (STUDY00001485), and by the National Heart, Lung, and Blood institute. Written informed consent was obtained from all participants.
Magnetic Resonance Imaging:
The MRI protocol included time-resolved 3D MRA to assess pulmonary vasculature [18]. Images were obtained using a 1.5 Tesla whole-body MRI system (Signa LX, GE Healthcare or Avanto, Siemens). TRICKS or TWIST was performed at 1.5 second temporal resolution following injection of 0.1 mMol/kg of Gd (Gd:DTPA, Bayer, Wayne, NY), and coronal 3D image of the aorta was obtained from cranial to aortic arch and caudal to aortic bifurcation.
Aorta assessment:
MRA images were evaluated for the diameter of ascending aorta at the level of pulmonary artery, aortic arch at the level of brachiocephalic artery, intra thoracic descending aorta at the level of pulmonary artery, the abdominal aorta at the level of the mesenteric artery, and the infrarenal aorta immediately distal to the renal arteries. Aorta diameters were measured independently by a radiologist and a cardiologist blinded to all clinical information about the subjects using electronic calipers on a computer workstation with 3D visualization software (volume Viewer Plus Suite 15.10.4 on Advantage Windows Workstation (GE Medical Systems, Waukesha, WI). Reliability and inter observer agreement between the two readers were assessed by intraclass correlation coefficient (ICC). Discrepancies between the observers were resolved with a secondary consensus reading.
COPD case status:
We used the standard definition of COPD to define case status: a post-bronchodilator ratio of the forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC) ratio < 0.70 [13,20] (Supplemental Figure 1). Post-bronchodilator spirometry was measured in all participants based on American Thoracic Society/European Respiratory Society guidelines. Rolling barrel spirometer was used as previously described in the MESA Lung protocol [21]. COPD severity was classified as mild, FEV1 ≥80% predicted; moderate, FEV1 50% to 79% predicted; and severe, FEV1 <50% predicted [22].
Computed tomography (CT), assessment of emphysema and airway anatomy:
All participants underwent full-lung CT scans with breath holding at maximum inspiration without intravenous contrast and reconstructed using a high-spatial-contrast algorithm with 0.625-mm-slice thickness on Siemens and GE 64-slice scanners (GE Healthcare, Waukesha, WI). All scans were acquired following the SPIROMICS protocol [16,17,23].31
Image attenuation was assessed using a modified version of the Pulmonary Analysis Software Suite (VIDA Diagnostics, Coralville, IA) at a single reading center by trained readers without knowledge of other participants’ information. Emphysema-like lung (also known as percent low attenuation area and hereafter referred to as percent emphysema) was defined as the number of lung voxels with outside-air corrected attenuation less than −950 Hounsfield units on chest CT [24] (Supplemental Table 1).
Stratified analyses defined patients with emphysema as those with percent emphysema voxels above the median value in this study. Sensitivity analyses used alternate thresholds of 25th percentile, as well as median of emphysema [25].
The central airway tree was identified using Apollo Software (VIDA Diagnostics, Coralville, IA). Segmentation and labelling of the airways were visually verified by an image analyst and all labelled airways were assigned a generation number based upon the number of branch points from the trachea. Cross-sectional airway wall thickness and lumen area were measured at the 5th generation perpendicular to the local airway segment’s long axis and measurements were averaged along the middle third of each labelled airway segment [26].
Chronic bronchitis was defined as a chronic productive cough for 3 or more months in two or more years [21].
Anthropometry, smoking status and other co-varieties:
Age, gender, race/ethnicity, educational attainment, smoking status, pack-years, and medical history were self-reported. Medication use was assessed by medication inventory. Height and weight were measured following the MESA protocol [3]. Resting blood pressure was measured 3 times in the seated position. Glucose, total cholesterol and high-density lipoprotein (HDL) cholesterol levels, and complete blood counts were measured from blood samples after at least twelve-hour of fasting. Low-density lipoprotein (LDL) cholesterol level was calculated using the Friedewald equation [27]. Smoking history was assessed using standard questionnaire items and was confirmed with plasma cotinine levels [28]. Study participants reporting at least 1 cigarette in the 30 days prior to assessment were classified as current smokers. Information on medication use was obtained by medication inventory. Diabetes was defined as a fasting plasma glucose ≥ 126 mg/dL or self-report of physician diagnosis. Hypertension was defined as systolic blood pressure (Psys) ≥ 140 mmHg, diastolic blood pressure (Pdiast) ≥ 90 mmHg or physician diagnosis. The Mean Arterial Pressure (MAP) was calculated using the systolic and diastolic pressure values in the equation (Pdiast + 1/3 (Psys - Pdias)), and the Pulse Pressure (PP) as the difference between the systolic and diastolic pressure readings. All the blood pressure associated values were measured in mmHg [29].
Statistical analysis:
Dichotomous variables are presented as proportions and continuous variables as means with standard deviation unless otherwise indicated. Fisher’s exact test was used to assess the significance of differences in the number of aortic aneurysms based upon in percent emphysema and COPD severity. Multiple comparisons across levels of COPD severity were addressed by Holm’s step-down procedure. Associations of aorta diameter with percent emphysema, COPD status and severity, chronic bronchitis status, and airway anatomy (wall thickness and inner area) were tested with linear regression.
Potential confounders were selected based upon biological plausibility and examination of correlations with covariates. Unadjusted and adjusted linear regression models were performed, controlling for age, gender, race/ethnicity, height, weight, body surface area (BSA), cohort, smoking status, blood pressure, LDL-Cholesterol, HDL-Cholesterol and triglycerides, diabetes, smoking status, cotinine levels, and pack-years smoked [30]. Given that study participants were recruited based upon COPD status rather than as a cohort study, analyses of percent emphysema and other continuous variables were weighted proportionate to the inverse ratio of the sample prevalence to the source study prevalence, COPD cases recruited from the community were assigned the same weights as cases from EMCAP [31]. A two-tailed P value < 0.05 was considered statistically significant. Analyses were performed using SAS 9.4 (Cary, NC) statistical software.
RESULTS
The 315 participants had a mean age of 68 ± 7 years old, and 188 (60%) were male. Race/ethnic distribution was 169 (54%) White, 83 (26%) African-American, 44 (14%) Hispanic, and 19 (6%) Asian. All participants were either current (n=88) or former (n=227) smokers. Additional demographic data are presented in Table 1.
Table 1.
Characteristics of the Study Participants by Quartile of Percent Emphysema.
| Percent emphysema (−950 HU) | ||||
|---|---|---|---|---|
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | |
| n=79 | n=79 | n=79 | n=78 | |
| Demographics | ||||
| Age, mean ± SD, years | 66 ± 7 | 69 ± 6 | 68 ± 7 | 69 ± 7 |
| Male, N (%) | 36 (46) | 44 (56) | 53 (67) | 55 (70) |
| Race/Ethnicity, N (%) | ||||
| White | 34 (43) | 39 (49) | 46 (58) | 50 (64) |
| Chinese | 7 (9) | 3 (4) | 6 (8) | 3 (4) |
| African-American | 24 (30) | 22 (28) | 17 (21) | 20 (26) |
| Hispanic | 14 (18) | 15 (19) | 10 (13) | 5 (6) |
| Educational attainment, N (%) | ||||
| ≤ High school degree | 24 (30) | 12 (24) | 16 (20) | 17 (22) |
| Some college/Assoc. degree/Vocational school | 25 (32) | 20 (25) | 27 (34) | 20 (26) |
| ≥ College degree | 30 (38) | 40 (51) | 36 (45) | 41 (52) |
| Hypertension, N (%) | 36 (46) | 33 (42) | 40 (51) | 36 (46) |
| Diabetes Mellitus, N (%) | 12 (15) | 12 (15) | 11 (14) | 10 (13) |
| Smoking status, N (%) | ||||
| Current | 29 (37) | 19 (24) | 19 (24) | 21 (27) |
| Former | 50 (63) | 60 (76) | 60 (76) | 57 (73) |
| Pack-years of smoking, median [IQR] | 32 [20–46] | 32 [22–48] | 32 [19–51] | 33 [20–52] |
| Characteristics | ||||
| Height, mean ± SD, cm | 165 ± 10 | 168 ± 10 | 170 ± 8 | 171 ± 9 |
| Weight, mean ± SD, kg | 78 ± 17 | 83 ± 18 | 80 ± 16 | 78 ± 18 |
| Body mass index, mean ± SD, kg/m2 | 28 ± 5 | 29 ± 5 | 28 ± 5 | 27 ± 5 |
| Body surface area, mean ± SD, m2 | 1.8 ± 0.22 | 1.9 ± 0.23 | 1.9 ± 0.20 | 1.9 ± 0.23 |
| Systolic blood pressure, mean ± SD, mmHg | 122 ± 17 | 121 ± 18 | 121 ± 20 | 122 ± 15 |
| Diastolic blood pressure, mean ± SD, mmHg | 70 ± 10 | 69 ± 9 | 69 ± 10 | 72 ± 8 |
| MAP, mean ± SD, mmHg | 87 ± 11 | 86 ± 10 | 87 ± 12 | 89 ± 9 |
| PP, mean ± SD, mmHg | 51 ± 15 | 52 ± 15 | 52 ± 15 | 49 ± 13 |
| Labs | ||||
| Cotinine levels, mean ± SD, mg/dL | 133 ± 211 | 360 ± 2245 | 88 ± 180 | 139 ± 647 |
| LDL, mean ± SD, mg/dL | 106 ± 31 | 109 ± 32 | 110 ± 32 | 97 ± 31 |
| HDL, mean ± SD, mg/dL | 58 ± 20 | 54 ± 16 | 58 ± 19 | 56 ± 18 |
| Triglycerides, mean ± SD, mg/dL | 101 ± 44 | 112 ± 50 | 109 ± 43 | 120 ± 84 |
| Cholesterol, median [IQR] | 178 [160–205] | 182 [159–210] | 187 [160–222] | 178 [147–196] |
| Pulmonary function test | ||||
| FEV1 percent of predicted, mean ± SD | 91 ± 17 | 95 ± 20 | 92 ± 17 | 75 ± 25 |
| FVC percent of predicted, mean ± SD | 92 ± 13 | 97 ± 14 | 101 ± 16 | 97 ± 18 |
| FEV1/FVC ratio, mean ± SD | 0.75 ± 0.09 | 0.73 ± 0.08 | 0.69 ± 0.1 | 0.56 ± 0.14 |
Definition of abbreviations: FVC = forced vital capacity; FEV1= forced expiratory volume in 1 second; IQR = interquartile range; MAP = Mean Arterial Pressure; Percent emphysema (−950 HU) = percentage of emphysema-like lung at −950 Hounsfield units; PP = Pulse Pressure.
Intra- and inter-observer agreements of the aorta diameter measurements are as follows: ascending aorta (ICC= 0.71, 0.89, respectively), arch (ICC= 0.72, 0.74, respectively), descending aorta (ICC= 0.75, 0.79, respectively), abdominal aorta (ICC=0.77, 0.86, respectively), infra-renal aorta (ICC=0.76, 0.83, respectively).
Aorta Diameter and Emphysema:
Ascending, arch, descending, abdominal and infra-renal aorta diameters were monotonically related to percent emphysema in unadjusted analyses (Table 2A; all P < 0.001). This positive association of aortic diameter with percent emphysema was statistically significant in the fully adjusted multivariable model (Table 2B, Figure 1A).
Table 2.
Aorta diameters (mm) according to emphysema quartiles.
| (A) Distributions of aorta diameters, by quartile of percent of emphysema-like lung. | |||||
|---|---|---|---|---|---|
| Percent of emphysema-like lung |
|||||
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
| (n=79) | (n=79) | (n=79) | (n=78) | ||
| Normal | Borderline | Mild | Severe | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p-value | |
| Ascending Aorta | 30 ± 3 | 31 ± 4 | 34 ± 3 | 35 ± 4 | <0.001 |
| Arch | 26 ± 2 | 27 ± 2 | 28 ± 3 | 29 ± 3 | <0.001 |
| Descending Aorta | 23 ± 3 | 24 ± 3 | 27 ± 3 | 27 ± 3 | <0.001 |
| Abdominal Aorta | 20 ± 3 | 21 ± 3 | 23 ± 3 | 24 ± 3 | <0.001 |
| Infrarenal Aorta | 14 ± 3 | 14 ± 3 | 16 ± 2 | 17 ± 3 | <0.001 |
| (B) Adjusted model | |||||
| Percent of emphysema-like lung |
|||||
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
| (n=79) | (n=79) | (n=79) | (n=77) | ||
| Normal | Borderline | Mild | Severe | ||
| β (95% CI) | β (95% CI) | β (95% CI) | p-value | ||
| Ascending Aorta | Ref | 0.71 (−0.29, 1.71) | 3.25 (2.26, 4.24) | 4.19 (2.98, 5.41) | <0.001 |
| Arch | Ref | 0.59 (−0.18, 1.35) | 1.84 (1.05, 2.64) | 2.32 (1.37, 3.26) | <0.001 |
| Descending Aorta | Ref | 0.74 (−0.05, 1.53) | 2.80 (2.05, 3.54) | 2.86 (2.07, 3.65) | <0.001 |
| Abdominal Aorta | Ref | 0.06 (−0.74, 0.87) | 2.82 (2.00, 3.64) | 2.69 (1.84, 3.54) | <0.001 |
| Infrarenal Aorta | Ref | −0.47 (−1.40, 0.46) | 1.77 (0.86, 2.68) | 2.57 (1.58, 3.57) | <0.001 |
Linear regression model adjusted for age, race, gender, cohort, height, weight, BSA, current smoker, pack-years smoked, cardiovascular risk factors (hypertension, cholesterol, triglycerides, LDL and diabetes).
Figure 1.
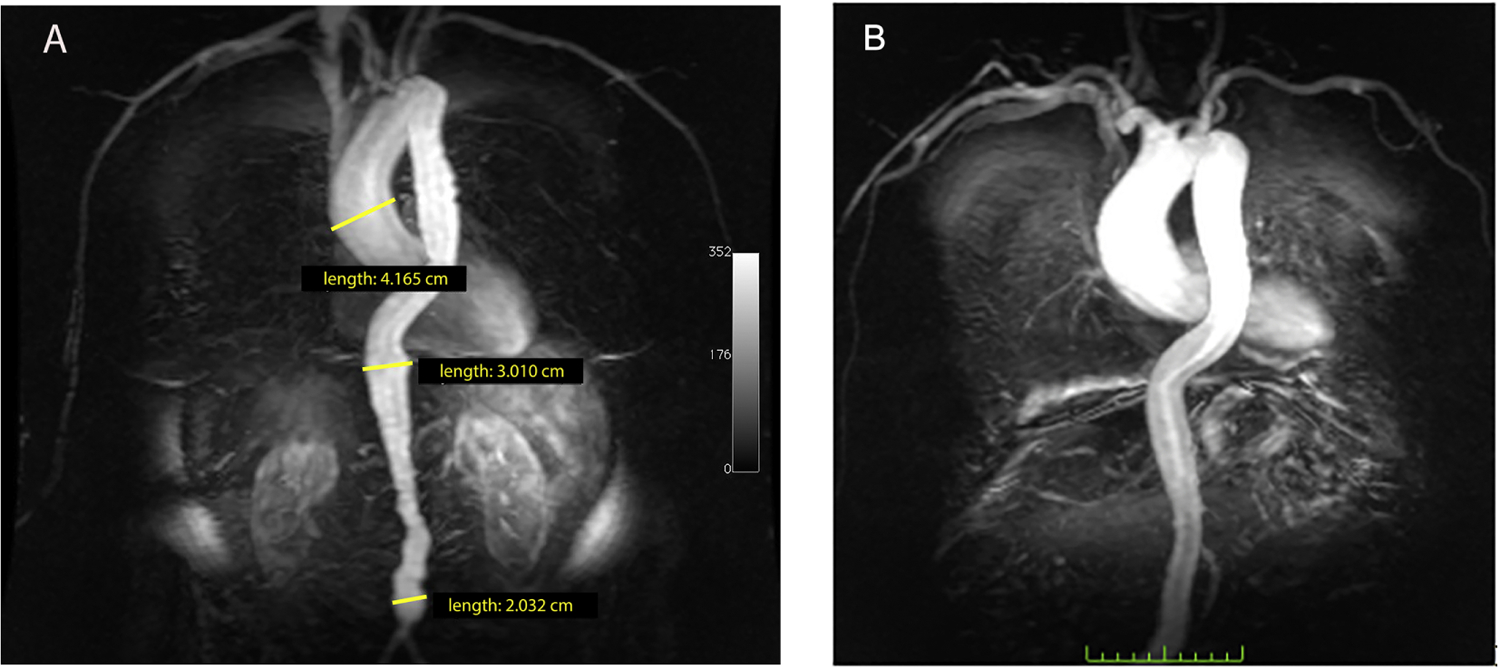
Patient with emphysema MRA post gadolinium TRICKS coronal image. (A) Ecta-sia of the aorta. The ascending aorta measured 4.2 cm, diaphragmatic aorta was 3 cm, and infrarenal aorta 2 cm ectasia. (B) Aneurysmal ascending aorta.
Using ≥ 40mm as the threshold for diagnosing ascending aortic aneurysm, and ≥ 30mm for diagnosing abdominal aneurysm, 30 of the 315 participants had aneurysm, with a maximum diameter = 48 mm. There were 11 aneurysms in the 4th quartile of emphysema severity, 5 in the 3rd quartile, and 11 in the 2nd quartile compared to 3 in the 1st quartile participants. Therefore, in subjects with emphysema, the number of aneurysms increased across quartiles (p=0.04) of emphysema severity (Figure 1B).
Aorta Diameter and COPD severity:
Greater severity of COPD was associated with greater diameter of the ascending aorta, aortic arch, and abdominal aorta (all p-values for trends across COPD severities categories <0.001), with little changes after multivariable adjustment (Table 3). Diameters of the aortic arch and abdominal aorta were significantly greater in severe, moderate and mild COPD compared to participants with no COPD; the ascending aorta diameter was significantly greater in severe and moderate COPD compared to no COPD; and the descending thoracic aorta was significantly in greater in diameter in severe COPD only. Although aortic diameter was greater with COPD, this did not translate into more aortic aneurysms. Among the 30 total aneurysms, 11 were among the 154 participants without COPD, 8 were among the 53 participants with mild COPD, 8 were among the 62 subjects with moderate COPD, and 3 were among the 13 individuals with severe COPD, showing no linear relationship (p=0.2).
Table 3.
Aorta diameters (mm) across COPD severity groups.
| (A) Distributions of aorta diameters, by COPD severity | |||||
|---|---|---|---|---|---|
| COPD on Spirometry |
|||||
| Normal | Mild | Moderate | Severe | ||
| (n=165) | (n=61) | (n=70) | (n=19) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | p-value for trend | |
| Ascending Aorta | 32 ± 4 | 33 ± 4 | 33 ± 5 | 34 ± 4 | <0.001 |
| Arch | 27 ± 3 | 28 ± 3 | 28 ± 3 | 29 ± 3 | <.0.001 |
| Descending Aorta | 24 ± 3 | 26 ± 3 | 25 ± 4 | 26 ± 4 | 0.11 |
| Abdominal Aorta | 21 ± 3 | 22 ± 3 | 22 ± 4 | 24 ± 3 | <0.001 |
| Infrarenal Aorta | 15 ± 3 | 16 ± 2 | 16 ± 3 | 17 ± 3 | <0.001 |
| (B) Adjusted model | |||||
| COPD on Spirometry |
|||||
| Normal | Mild | Moderate | Severe | ||
| (n=164) | (n=61) | (n=70) | (n=19) | ||
| β (95% CI) | β (95% CI) | β (95% CI) | p-value for trend | ||
| Ascending Aorta, mm | Ref | 0.98 (−0.16, 2.12) | 1.60 (0.50, 2.71)* | 2.80 (0.74, 4.86)* | <0.001 |
| Arch, mm | Ref | 1.06 (0.25, 1.87)* | 1.09 (0.29, 1.89)* | 2.32 (0.85, 3.78)* | <0.001 |
| Descending Aorta, mm | Ref | 0.74 (−0.13, 1.61) | 0.81 (−0.04, 1.65) | 1.80 (0.23, 3.37)* | 0.02 |
| Abdominal Aorta, mm | Ref | 1.11 (0.22, 1.99)* | 1.14 (0.24, 2.03)* | 2.32 (0.72, 3.91)* | 0.001 |
| Infrarenal Aorta, mm | Ref | 1.12 (0.26, 1.98)* | 1.15 (0.27, 2.04)* | 1.11 (−0.42, 2.65) | 0.01 |
P-value <0.05, indicating statistical significance compared to Normal
Linear regression model adjusted for age, race, gender, cohort, height, weight, BSA, current smoker, pack-years smoked, cardiovascular risk factors (hypertension, cholesterol, triglycerides, LDL and diabetes).
Aorta Diameter and coexistence of Emphysema and COPD:
In subjects with emphysema, those with coexistence of COPD showed greater aorta diameter than those without COPD in ascending (p = 0.003), arch (p = 0.002), and abdominal aorta (p = 0.04) (Supplemental Table 1).
Aorta Diameter and Chronic Bronchitis:
Both the unadjusted and adjusted models showed no evidence for a continuous association of chronic bronchitis with greater aortic diameter (Supplemental Table 2).
Aorta Diameter and Airway Anatomy:
Bronchial wall thickness was not associated with any difference in aorta diameter with the fully adjusted model (Supplemental Table 3). Furthermore, there was no evidence for a continuous association of bronchial lumen area and aorta diameter (Supplemental Table 4).
DISCUSSION
This large and ethnically diverse population allowed for multiple stratified analyses of aortic aneurysm risk in COPD according to the presence of emphysema, chronic bronchitis and bronchial wall thickening. Among 315 current and former smokers, subjects with COPD and especially emphysema, diagnosed on CT, showed greater thoracic and abdominal aorta cross-sectional diameters, compared to controls with no COPD. A greater number of subjects with severe emphysema showed thoracic and abdominal aortic aneurysms compared to participants without emphysema. Increased aorta diameter was not correlated with chronic bronchitis or airway dimensions.
Aortic aneurysm is the result of multifactorial processes, including inflammation, genetic abnormalities, biomechanical wall stress, apoptosis, and proteolytic degradation of connective tissue including elastin and collagen [32,33]. Risk factors for development of aortic aneurysms include factors that increase stress on the aortic wall (e.g. hypertension and elevated diastolic blood pressure), factors that promote degradation of the elastin and collagen fibers within the aorta wall (e.g. aging, smoking, atherosclerosis), and genetic factors that predispose the aortic wall to degeneration (e.g. Marfan syndrome, Ehler-Danlos, Cutis Laxa).
Since emphysema also results from destruction of elastin and collagen [34,35], it is not surprising that emphysema is associated with increasing aortic diameters and aortic aneurysms [36]. Tobacco smoke inhibits the anti-elastase activity of alpha1-antitrypsin (alpha1 - protease inhibitor), causing an imbalance between elastolytic and antielastolytic factors, which leads to emphysema [6]. Furthermore, autoimmune T-cell activation has been shown to be associated with smoking in patients with thoracic aortic aneurysm or dissection without COPD [37]. Destruction of elastin in aortic aneurysms may be via the same mechanism.
The relative risk of abdominal aortic aneurysms (AAA) in individuals who have ever smoked is 2.5 times greater than the relative risk for coronary heart disease [4]. Lederle et al [5] described AAA more closely associated with cigarette smoking than any other tobacco-related disease except lung cancer. MacSweeney et al [7] assessed active smoking (serum cotinine levels), blood pressure, cholesterol, and triglycerides in patients with small (< 40 mm) AAAs. Active smoking was the only parameter associated with a significant increase in growth rate, and double the rate of thoraco-abdominal aneurysm expansion [9]. In Marfan syndrome [10], subjects are highly predisposed to thoracic aortic aneurysm and/or Type A dissection, with virtually every patient developing aortic disease at some point during their lifetime [38]. In addition, giant bullous and emphysema have been reported in Marfan patients [39]. Cystic medial aortic degeneration also occurs in Ehlers-Danlos syndrome [40] and in particular the vascular type IV showed the presence of bullous emphysema and aortic aneurysm [41]. Individuals with Cutis Laxa (generalized elastolysis), characterized by abnormal elastic fibers and loose skin [43], also showed aortic aneurysm and emphysema [44].
Bicuspid aortic valve is often seen in patients with connective tissue diseases. The ACC/AHA Valvular Heart Disease Guidelines specifically address bicuspid aortic valve as a cause of thoraco-abdominal aneurysm [1], and there are reports of bicuspid aortic valve patients with emphysema [44]. There are also reports of aortic dissection in patients with autosomal dominant polycystic kidney disease [42]. Previous studies showed a coexistence of polycystic kidney disease and alpha-1 antitrypsin deficiency associated emphysema [46].
The continuous association between greater aortic diameters and emphysema may also relate to endothelial damage; endothelium is present in both airways and vessels [47]. Several proinflammatory cytokines, including interleukin-6, interleukin-1β, tumor necrosis factor-α, and interferon-γ, have been identified in AAA walls. These cytokines activate lymphocytes and macrophages [32] and promote matrix metalloproteinases MMP-2 and MMP-9 [48] production in the smooth muscle of aortic wall that degrade elastin and collagen and leads to the cystic medial necrosis found in aortic aneurysms [37]. These inflammatory processes are seen in both aneurysms and emphysema [33]. A recent study in animal models, has showed how treatment with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), inhibit transforming growth factor- β in the endothelium, improve endothelial function and restore airspace architecture. ARB/ACE inhibitor therapy was associated with slower progression of percent emphysema over ten years, particularly among former smokers. ACE and ARB agents are currently being developed for the prevention and treatment of emphysema [49]. ACE inhibitors are also used in preventing aortic aneurysm rupture [50].
We used validated instruments including MRA, Spirometry and CT with data collected in a blinded manner. MRA has an advantage that it does not risk patients with radiation. Especially for patients requiring series of imaging tests to follow-up disease processes, cumulative radiation dose via CT may become somewhat problematic. TRICKS or TWIST has reasonable temporal and spatial resolution for aortic diameter measurements and allows the visualization of whole aorta with optimal contrast-enhancement.
There are some limitations in this study. First, our study was observational and cross-sectional, thus neither causality nor temporality can be inferred. Secondly, there may be residual confounding factors that were not included in our models. The study includes a limited age range, 50–79 years. Aorta diameter had to be obtained from MRA instead of the higher resolution CT images since the CT was non-contrast and low dose optimized for assessing lung parenchyma in volunteers not aorta diamter.
CONCLUSIONS
In this large, multi-ethnic sample of individuals who are former/current smokers free of clinical cardiovascular disease recruited from the community, we have found that COPD with emphysema is associated with increased thoracic and abdominal aortic diameters but this is not observed in the chronic bronchitis and thickened airway subtypes of COPD. There may be similar elastolytic mechanisms destroying lung parenchyma to cause emphysema and weakening the aorta wall, however it requires further investigation.
Supplementary Material
Supplemental Figure 1. Correlation between COPD, emphysema, and chronic bronchitis. COPD was defined as the forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC) ratio < 0.70. COPD severity was classified as mild, FEV1 ≥80% predicted; moderate, FEV1 50% to 79% predicted; and severe, FEV1 <50% predicted. Emphysema was evaluated on chest CT and was defined as percentage of voxels with outside-air corrected attenuation < −950 HU above the median in this study. Chronic bronchitis was defined as a chronic productive cough for 3 or more months in two or more years.
Highlights.
High prevalence of aortic aneurysm in COPD population
COPD with emphysema associates with increased thoracic and abdominal aortic diameters
Aorta dilatation is not associated with bronchitis and bronchial wall thickness.
Acknowledgments
Authors would like to acknowledge Benjamin M. Smith1, MD, MS and Chia Liu2, PhD for their contribution to the conception and study design and in the acquisition, analysis and interpretation of the data.
Acknowledgement of grant support
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). This publication was developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. KF’s work on this paper was funded in part by the Division of Intramural Research, NHLBI, NIH, United States Department of Health and Human Services (DHHS).
Funding
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). This publication was developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. This work was partially supported by the Division of Intramural Research, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
LIST OF ABBREVIATIONS:
- AAA
abdominal aortic aneurysm
- ACC/AHA
American College of Cardiology/American Heart Association
- ACE
Angiotensin-converting enzyme
- ARB
Angiotensin II receptor blocker
- BSA
body surface area
- COPD
chronic obstructive pulmonary disease
- CT
Computed Tomography
- EMCAP
Emphysema and Cancer Action Project
- FEV1
forced expiratory volume in 1 second
- FRC
functional residual capacity
- FVC
forced vital capacity
- Gd
gadolinium
- HDL
high-density lipoprotein
- ICC
Infraclass Correlation Coefficient
- IQR
Interquartile range
- LDL
low-density lipoprotein
- MAP
Mean Arterial Pressure
- MMP
Metalloproteninases
- MRI
magnetic resonance imaging
- MRA
magnetic resonance angiography
- MESA
Multi-Ethnic Study of Atherosclerosis
- PP
Pulse Pressure
- TRICKS
time-resolved imaging of contrast kinetics (3D time-resolved MRA)
- TWIST
time-resolved angiography with interleaved stochastic trajectories (3D time-resolved MRA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Any potential conflicts of interest
None
Author Agreement Form – International Journal of Cardiology
This statement is to certify that all authors have seen and approved the manuscript being submitted, have contributed significantly to the work, attest to the validity and legitimacy of the data and its interpretation, and agree to its submission to the International Journal of Cardiology.
On behalf of all Co-Authors, the first and corresponding Authors shall bear full responsibility for the submission. Any changes to the list of authors, including changes in order, additions or removals will require the submission of a new author agreement form approved and signed by all the original and added submitting authors.
Martin R. Prince reports patent agreements with GE, Siemens, and Bayer whose products were utilized in this study. None of the other authors report relationships that could be construed as a conflict of interest.
This author takes responsibility for all aspects of the reliability and freedom, from bias of the data presented and their discussed interpretation.
REFERENCES
- [1].Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Practice Guidelines. Circulation 2020. (online ahead of print). [DOI] [PubMed]
- [2].Davies MJ. Aortic aneurysm formation: lessons from human studies and experimental models. Circulation 1998;98:193–195. [DOI] [PubMed] [Google Scholar]
- [3].Powell JT, Worrell P, MacSweeney ST, Franks PJ, Greenhalgh RM. Smoking as a risk factor for abdominal aortic aneurysm. Ann N Y Acad Sci 1996;800:246–248. [DOI] [PubMed] [Google Scholar]
- [4].Lederle FA, Nelson DB, Joseph AM. Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg 2003;38:329–334. [DOI] [PubMed] [Google Scholar]
- [5].Muley T, Wiebel M, Schulz V, Ebert W. Elastinolytic activity of alveolar macrophages in smoking-associated pulmonary emphysema. Clin investig 1994;72:269–276. [DOI] [PubMed] [Google Scholar]
- [6].MacSweeney ST, Ellis M, Worrell PC, Greenhalgh RM, Powell JT. Smoking and growth rate of small abdominal aortic aneurysms. Lancet 1994;344:651–652. [DOI] [PubMed] [Google Scholar]
- [7].Vizzardi E, Maffessanti F, Lorusso R, et al. Ascending Aortic Dimensions in Hypertensive Subjects: Reference Values for Two-Dimensional Echocardiography. J Am Soc Echocardiog 2016;29:827–837. [DOI] [PubMed] [Google Scholar]
- [8].Dapunt OE, Galla JD, Sadeghi AM, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994;107:1323–1332. [PubMed] [Google Scholar]
- [9].Jeremy RW, Huang H, Hwa J, McCarron H, Hughes CF, Richards JG. Relation between age, arterial distensibility, and aortic dilatation in the Marfan syndrome. Am J Cardiol 1994;74:369–373. [DOI] [PubMed] [Google Scholar]
- [10].Kubota Y, Folsom AR, Matsushita K, Couper D, Tang W. Prospective study of lung function and abdominal aortic aneurysm risk: The Atherosclerosis Risk in Communities study. Atherosclerosis 2018;268:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557–582. [DOI] [PubMed] [Google Scholar]
- [12].Marsh SE, Travers J, Weatherall M, et al. Proportional classifications of COPD phenotypes. Thorax 2008;63:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].2021 Gold Reports, Global initiative for chronic obstructive lung disease. https://goldcopd.org/2021-gold-reports/ (accessed 16 December 2020).
- [14].Aaron CP, Hoffman EA, Lima JAC, et al. Pulmonary vascular volume, impaired left ventricular filling and dyspnea: The MESA Lung Study. PloS one 2017;12:e0176180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- [16].Hueper K, Vogel-Claussen J, Parikh MA, et al. Pulmonary Microvascular Blood Flow in Mild Chronic Obstructive Pulmonary Disease and Emphysema. The MESA COPD Study. Am J Respir Crit Care Med 2015;192:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barr RG, Mesia-Vela S, Austin JH, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med 2007;176:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kawut SM, Poor HD, Parikh MA, et al. Cor pulmonale parvus in chronic obstructive pulmonary disease and emphysema: the MESA COPD study. J Am Coll Cardiol 2014;64:2000–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hankinson JL, Odencrantz JR and Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- [20].Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest 2010;137:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sieren JP, Newell JD Jr, Barr RG, et al. SPIROMICS Protocol for Multicenter Quantitative Computed Tomography to Phenotype the Lungs. Am J Respir Crit Care Med 2016;194:794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. [DOI] [PubMed] [Google Scholar]
- [23].Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med 2010;362:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Oelsner EC, Smith BM, Hoffman EA, et al. Associations between emphysema-like lung on CT and incident airflow limitation: a general population-based cohort study. Thorax 2018;73:486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smith BM, Hoffman EA, Rabinowitz D, et al. Comparison of spatially matched airways reveals thinner airway walls in COPD. The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax 2014;69:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Burkart KM, Manichaikul A, Wilk JB, et al. APOM and high-density lipoprotein cholesterol are associated with lung function and per cent emphysema. Eur Respir J 2014;43:1003–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med 2010;152:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- [29].Podolanczuk AJ, Oelsner EC, Barr RG, et al. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J 2016;48:1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Smith BM, Kawut SM, Bluemke DA, et al. Pulmonary hyperinflation and left ventricular mass: the Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation 2013;127:1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Reilly JM, Brophy CM, Tilson MD. Characterization of an elastase from aneurysmal aorta which degrades intact aortic elastin. Ann Vasc Surg 1992;6:499–502. [DOI] [PubMed] [Google Scholar]
- [32].Maclay JD, McAllister DA, Rabinovich R, et al. Systemic elastin degradation in chronic obstructive pulmonary disease. Thorax 2012;67:606–612. [DOI] [PubMed] [Google Scholar]
- [33].Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 2000;117:684–694. [DOI] [PubMed] [Google Scholar]
- [34].Kasahara Y, Tuder RM, Cool CD, Voelkel NF. Expression of 15-lipoxygenase and evidence for apoptosis in the lungs from patients with COPD. Chest 2000;117:260s. [DOI] [PubMed] [Google Scholar]
- [35].Lindholt JS, Heickendorff L, Antonsen S, Fasting H, Henneberg EW. Natural history of abdominal aortic aneurysm with and without coexisting chronic obstructive pulmonary disease. J Vasc Surg 1998;28:226–233. [DOI] [PubMed] [Google Scholar]
- [36].Gu BH, Choi JC, Shen YH, et al. Elastin-Specific Autoimmunity in Smokers With Thoracic Aortic Aneurysm and Dissection is Independent of Chronic Obstructive Pulmonary Disease. J Am Heart Assoc 2019;8:e011671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Meffert P, Tscheuschler A, Beyersdorf F, et al. Characterization of serum matrix metalloproteinase 2/9 levels in patients with ascending aortic aneurysms. Interact Cardiovasc Thorac Surg 2017;24:20–26. [DOI] [PubMed] [Google Scholar]
- [38].Corsico AG, Grosso A, Tripon B, et al. Pulmonary involvement in patients with Marfan Syndrome. Panminerva Med 2014;56:177–182. [PubMed] [Google Scholar]
- [39].Shannon VR, Nanda AS, Faiz SA. Marfan Syndrome Presenting as Giant Bullous Emphysema. Am J Respir Crit Care Med 2017;195:827–828. [DOI] [PubMed] [Google Scholar]
- [40].Ascione R, Gomes WJ, Bates M, Shannon JL, Pope FM, Angelini GD. Emergency repair of type A aortic dissection in type IV Ehlers-Danlos syndrome. Cardiovasc Surg. 2000;8:75–78. [DOI] [PubMed] [Google Scholar]
- [41].Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 2000;342:673–680. [DOI] [PubMed] [Google Scholar]
- [42].Berk DR, Bentley DD, Bayliss SJ, Lind A, Urban Z. Cutis laxa: a review. J Am Acad Dermatol 2012;66:842.e1–17. [DOI] [PubMed] [Google Scholar]
- [43].Champion P, Ryan F. A case of congenital cutis laxa (generalized elastolysis). Can Respir J 2005;12:151–152. [DOI] [PubMed] [Google Scholar]
- [44].Carrel T, Berdat P, Pavlovic M, Sukhanov S, Englberger L, Pfammatter JP. Surgery of the dilated aortic root and ascending aorta in pediatric patients: techniques and results. Eur J Cardiothorac Surg 2003;24:249–254. [DOI] [PubMed] [Google Scholar]
- [45].Adeola T, Adeleye O, Potts JL, Faulkner M, Oso A. Thoracic aortic dissection in a patient with autosomal dominant polycystic kidney disease. J Natl Med Assoc 2001;93:282–287. [PMC free article] [PubMed] [Google Scholar]
- [46].Pintacuda S, Di Blasi S, Morici G, Amato S. The polycystic kidney and serum alpha-1-antitrypsin deficiency. Observations on 2 family groups. Minerva medica. 1981;72:1697–1701. [PubMed] [Google Scholar]
- [47].Bersi MR, Khosravi R, Wujciak AJ, Harrison DG, Humphrey JD. Differential cell-matrix mechanoadaptations and inflammation drive regional propensities to aortic fibrosis, aneurysm or dissection in hypertension. J R Soc Interface 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tscheuschler A, Meffert P, Beyersdorf F, et al. MMP-2 Isoforms in Aortic Tissue and Serum of Patients with Ascending Aortic Aneurysms and Aortic Root Aneurysms. PloS One 2016;11:e0164308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Parikh MA, Aaron CP, Hoffman EA, et al. Angiotensin-Converting Inhibitors and Angiotensin II Receptor Blockers and Longitudinal Change in Percent Emphysema on Computed Tomography. The Multi-Ethnic Study of Atherosclerosis Lung Study. Ann Am Thorac Soc 2017;14:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kristensen KE, Torp-Pedersen C, Gislason GH, Egfjord M, Rasmussen HB, Hansen PR. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with abdominal aortic aneurysms: nation-wide cohort study. Arterioscler Thromb Vasc Biol 2015;35:733–740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Correlation between COPD, emphysema, and chronic bronchitis. COPD was defined as the forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC) ratio < 0.70. COPD severity was classified as mild, FEV1 ≥80% predicted; moderate, FEV1 50% to 79% predicted; and severe, FEV1 <50% predicted. Emphysema was evaluated on chest CT and was defined as percentage of voxels with outside-air corrected attenuation < −950 HU above the median in this study. Chronic bronchitis was defined as a chronic productive cough for 3 or more months in two or more years.


