Abstract
Background:
Aerosolized Azacitidine has been shown to inhibit orthotopic lung cancer growth and induce re-expression of methylated tumor suppressor genes in murine models. We hypothesized that inhaled Azacitidine is safe and effective in reversing epigenetic changes in the bronchial epithelium secondary to chronic smoking.
Patients and Methods:
We report the first in human study of inhaled Azacitidine. Azacitidine in aqueous solution was used to generate an aerosol suspension of 0.25 to 5μm particle size. Main inclusion criteria: Stage IV or recurrent NSCLC with predominantly lung involvement, ≥1 prior systemic therapy, ECOG PS 0-1, and adequate pulmonary function. Patients received inhaled Azacitidine daily on days 1-5 and 15-19 of 28-day cycles, at 3 escalating doses (15, 30 and 45mg/m2 daily). The primary objective was to determine the feasibility and tolerability of this new therapeutic modality. The key secondary objectives included pharmacokinetics, methylation profiles and efficacy.
Results:
From 3/2015 to 2/2018, eight patients received a median number of 2 (IQR =1) cycles of inhaled Azacitidine. No clinically significant adverse events were observed, except one patient treated at the highest dose developed an asymptomatic grade 2 decreased DLCO which resolved spontaneously. One patient receiving 12 cycles of therapy had an objective and durable partial response, and two patients had stable disease.
Plasma Azacitidine was only briefly detectable in patients treated at the higher doses. Moreover, in 2 of 3 participants who agreed and underwent pre- and post-treatment bronchoscopy, the global DNA methylation in the bronchial epithelium decreased by 24% and 79% post-therapy, respectively. The interval between last inhaled treatment and bronchoscopy was 3 days.
Conclusions:
Inhaled Azacitidine resulted in negligible plasma levels compared to the previously reported subcutaneous administration and was well-tolerated. The results justify the continued development of inhaled Azacitidine at non-cytotoxic doses for patients with lung-confined malignant and/or premalignant lesions.
Keywords: Lung cancer, inhaled Azacitidine
1. Introduction
Despite advances in screening, diagnosis, and treatment, lung cancer remains the leading cause of cancer-related death. Although smoking identifies the population at risk that could benefit from a preventive strategy, multiple oral chemoprevention strategies have unfortunately failed1. An ideal chemoprevention strategy would require a non-toxic agent that is able to reverse genetic lesions central to the carcinogenesis process, administered in a selective manner to the at-risk bronchial epithelium.
Epigenetic alterations that result in loss of tumor suppressor function are among the earliest genetic alterations in the tobacco carcinogenesis process 1-9. Azacitidine functions as both demethylating and cytotoxic agent exerting a DNA demethylating effect in cultured lung cancer cells at 3 logs lower than cytotoxic concentrations10. Several demethylating agents demonstrated minimal antitumor activity against lung cancer when used at cytotoxic doses by systemic administration11-13.
Aerosol administration is an ideal strategy to deliver drugs selectively to the bronchial epithelium with reduced systemic exposure14. We and others have demonstrated in orthotopic lung tumor models that inhaled demethylating therapy shortly after tumor inoculation can significantly reduce tumor growth and lead to the re-expression of tumor suppressors via demethylating effects10, 15-17. As compared to the systemic route inhaled demethylating therapy for lung-confined limited malignancy and premalignancy has a much stronger rationale but has not been explored in patients before.
Continuous direct delivery of non-cytotoxic demethylating doses of Azacitidine via aerosol delivery to tobacco-damaged bronchial epithelium is a rational strategy to reverse the tobacco-induced carcinogenesis process. To explore this novel preventive strategy, we developed and characterized an aerosol formulation of Azacitidine, performed all preclinical toxicology, and conducted a pilot study under an Institutional IND in patients with advanced NSCLC to get a preliminary assessment of its feasibility and tolerability. In preclinical models, a 2.5 mg/m2 intrapulmonary deposited dose of aerosolized Azacitidine led to DNA demethylation and re-expression of tumor suppressors without observed pulmonary toxicity16, 17. As efficiency of drug delivery to pulmonary tissue by clinically used nebulizer systems is about 15%18, we selected Azacitidine 15mg/m2 as the starting dose for this pilot study. The primary focus was to monitor pulmonary toxicity, determine systemic absorption and examine changes in global methylation in the bronchial epithelium. The main objective was to select a dose for subsequent studies using a chronic administration schedule to study its efficacy in chronic smokers with bronchial premalignancy.
2. Patients and Methods
2.1. Study population
The study was conducted under IND 121399, reviewed and approved by the institutional review boards, and in accordance with the Declaration of Helsinki and Good Clinical Practice. Informed consent was obtained for all subjects. Sex was considered as a biological variable, with both male and female patients being enrolled. Main inclusion criteria were: Histologic or cytological diagnosis of Stage IV or recurrent NSCLC with predominantly lung involvement, at least one prior standard systemic therapy, age ≥ 18, ECOG performance status (PS) 0-1, adequate bone marrow reserve and organ function, including adequate pulmonary function as determined by a measured Forced Expiratory Volume (FEV1) greater than 50% of predicted value and FEV1/Forced Vital Capacity (FVC) ratio of 65% or greater.
2.2. Study design
Azicitidine (Vidaza®, Celgene), as a lyophilized powder was dissolved in sterile water to generate a clear solution (10-15 mg/ml) and aerosolized using a clinical standard aerosol system (PARI nebulizer®). The final volume of aerosolate was 7 ml (6 ml of drug dose and 1 ml representing residual volume retained by the nebulizer). The majority of aerosolized droplets were between 0.25~5 μm in diameter10, 16, suitable for reaching the distal airways in humans17. The drug was administered at a rate of 0.3ml/minute which could be adjusted in the subsequent nebulization depending on the residual volume left after the first session. The reconstituted solution could be stored at 25°C, but administration had to be completed within 1 hour of reconstitution.
Patients were treated with inhaled Azacitidine daily for 5 days on days 1-5 and days 15-19 of a 28-day cycle. There were three escalating dose levels: 15, 30 and 45 mg/m2 (Table 1). Escalation to a higher dose level proceeded after 3 patients enrolled at a given dose level completed 1 cycle (28 days) of treatment without drug related toxicity of grade 2 or more. Intra-patient dose escalation was not permitted. Each aerosolization session generally lasted for 20 minutes, but occasionally up to 40 minutes at higher doses. Patients were treated inside a separate procedure room adjacent to the chemotherapy infusion facility under a plastic tent to prevent environmental contamination, and wearing a gown, gloves, goggles, and hat as previously described for a phase I study of aerosolized SLIT cisplatin conducted by us14. They were continuously visually monitored during treatment through a glass window. CT chest/abdomen/pelvis was done every two cycles and RECIST 1.1 was used for tumor measurements. To closely monitor the impact of inhaled therapy in the lung, pulmonary function testing (PFT) was assessed every two cycles.
Table 1:
Patient Characteristics, dose levels and clinical efficacy.
| Patients (n=8) N (%) |
|
|---|---|
| Median age in years (IQR) | 70 (11.5 ) |
| Gender | |
| Male | 3 (37.5%) |
| Female | 5 (62.5%) |
| Race | |
| African American (AA) | 5 (62.5%) |
| non-AA | 3 (37.5%) |
| ECOG PS=1 | 8 (100%) |
| Smoking status | |
| Never | 3 (37.5%) |
| Active/Former | 5 (62.5%) |
| Histology | |
| Adenocarcinoma | 7 (87.5%) |
| SQCLC | 1 (12.5%) |
| Type of mutations (all with adenocarcinoma) | |
| EGFR | 1 (12.5%) |
| KRAS | 2 (25%) |
| Median lines of prior Treatment (IQR) | 2.5 (2.5) |
| Median follow-up in months (IQR) | 15.4 (10.5) |
| Escalating doses | |
| 15 mg/m2 | 3 (37.5%) |
| 30 mg/m2 | 3 (37.5%) |
| 45 mg/m2 | 2 (25%) |
| Median Treatment cycles (IQR) | 2 (1) |
| Clinical efficacy | |
| Partial Response (PR) | 1 (12.5%) |
| Stable disease (Stable) | 2 (25%) |
| Progression (PD) | 5 (62.5%) |
| Clinical efficacy based on dose levels | |
| 15 mg/m2 | 3 (100%) PD |
| 30 mg/m2 | 1 (33%) PR, 2 (67%) PD |
| 45 mg/m2 | 2 (100%) Stable |
Toxicity was graded using the NCI Common Toxicity Criteria (CTCAE) version 4 except for decreased DLCO and decreased FEV1 since most lung cancer patients have reduced DLCO (due to anemia) and/or FEV1 already at baseline. For likely treatment-related decreased DLCO or decreased FEV1, grade 1, 2, 3, and 4 was defined as 80-89%, 70-79%, 60-69% and ≤ 60% of baseline, respectively. Dose limiting toxicity (DLT) was defined as any of the following: Grade 2 or higher defined pulmonary toxicity (FEV1, DLCO, pneumonitis/ pulmonary infiltrate); Grade 4 neutropenia, Grade 4 thrombocytopenia or Grade 3 thrombocytopenia associated with bleeding; Any grade 3 or higher non-hematological toxicity related to study treatment.
2.3. Pharmacokinetic (PK) study
Blood samples for pharmacokinetic studies were drawn at different time points (Table 2B), including cycle 1 day 1 pre-dose; cycle 1 day 15 pre-dose, 0 hour/2 hour/4 hour post-dose; and cycle 1 day 19 pre-dose, 0 hour and 2 hours post-dose. The plasma Azacitadine was measured by Bioanalytic Services, PPD labs (USA). A 50-μL matrix aliquot was fortified with 20 μL of 200 ng/mL internal standard, Azacitidine-15N4, working solution and extracted on ice. The final extract was analyzed via HPLC with column switching and MS/MS detection using positive ion electrospray. A dilution series of known Azacitidine-15N4 standards was established and clinical samples were then quantitated to this standard curve.
Table 2.
Effects of inhaled Azacitidine on 2A: toxicity, response, pulmonary function (FEV1 and DLCO) and 2B: pharmacokinetics (PK, plasma Azacitidine levels).
| 2A. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pt | Dose Level mg/m2 |
Cycles (n) |
TRAE | DLT | Response | FEV1 L (% predicted) |
DLCO mL/min/mmHg (% predicted) |
||
| Baseline | End of study |
Baseline | End of study |
||||||
| #1 | 15 | 2 | G1 FEV1 decreased | No | PD | 1.8 (76) | 1.5 (62) | 14.9 (69) | 14.8 (68) |
| #2 | 15 | 2 | None | No | PD | 2.1(109) | 2.2(116) | 13.2 (55) | 15.1 (64) |
| #3 | 15 | 2 | None | No | PD | 1.1 (64) | 1.0 (61) | 6.5 (40) | 6.8 (42) |
| #4 | 30 | 12 | None | No | PR | 2.2 (72) | 2.1 (72) | 17.2 (57) | 22.3 (78) |
| #5 | 30 | 1 | None | No | PD | 1.8 (85) | 1.7 (81) | 19.9 (67) | 18.6 (62) |
| #6 | 30 | 2 | None | No | PD | 1.4 (87) | 1.3 (78) | 9.2 (45) | 8.3 (41) |
| #7 | 45 | 4 | None | No | Stable | 1.6 (54) | 1.7 (61) | 11.2 (33) | 10.9 (33) |
| #8 | 45 | 2 | G2 DLCO decreased (no symptoms) | Yes | Stable | 1.6 (73) | 1.7 (80) | 16.3 (75) | 12.6 (58) |
| TRAE: Treatment-related adverse events. DLT: Dose-limiting toxicity. Pt: patient. Tx: treatment. PD: Progression of Disease. PR: Partial Response. | |||||||||
| 2B. | ||||||||
|---|---|---|---|---|---|---|---|---|
| PK* | Cycle 1 day 1 |
Cycle 1 day 15 | Cycle 1 day 19 | |||||
| Pre- dose |
Pre- dose |
0 h post dose |
2 h post dose |
4h post dose |
Pre- dose |
0 h post dose |
2 h post dose |
|
| Mean plasma (ng/ml) | 0 | 0 | 6.66 | 0.76 | 0 | 0 | 17.87 | 2.48 |
| SEM | 0 | 0 | 9.72 | 0.88 | 0 | 0 | 18.10 | 3.50 |
PK summary for 5 patients who received inhaled Azacitidine at 30 and 45mg/m2. (Plasma Azacitidine was undetectable in any of the 3 patients who were treated at the lowest dose level of 15mg/m2).
2.4. Global DNA methylation study
As per manufacturer’s instruction [MethylFlash™ Global DNA Methylation (5-mC) ELISA kit, EpiGentek, USA), the global DNA methylation status was determined colorimetrically by specifically quantifying levels of 5-methylcytosine (5-mC) in an ELISA-like reaction. Methylation studies were performed in paired pre-Azacitidine and post-Azacitidine bronchial epithelium specimens from 3 participants who agreed to pre and post-treatment bronchoscopy. Interval between last inhaled treatment and post-treatment bronchoscopy was 3 days.
2.5. Statistical methods and outcomes
Demographic variables and clinical outcomes were summarized using median and interquartile range (IQR) for continuous variables, and proportions for categorical variables. To evaluate response rate (RR), the proportion of patients who achieved complete response (CR) or partial response (PR) according to RECIST 1.1 was computed. Median overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier method. Since this was a pilot feasibility and toxicity study, formal statistical hypothesis testing was not performed.
3. Results
3.1. Patient characteristics and dose escalation
Eight eligible patients were enrolled and treated at our institution from March 2015 to February 2018 (Figure 1; Table 1). The median age was 70 years (IQR=11.5). Of 8 participants, 5 (62.5%) were female, 5 (62.5%) were African American, and 5 (62.5%) were active or former smokers. All participants had ECOG performance status of 1. All had histologically or cytologically proven NSCLC, including 7 (87.5%) adenocarcinoma with one participant harboring an EGFR and two harboring KRAS mutations. All had lung-predominant disease at the time of registration. The median number of prior lines of therapies was 2.5 (IQR=2.5), mostly chemotherapy, and PD-1/PD-L1 inhibitors in 4 (50%) patients. Most patients without prior immunotherapy were enrolled before immunotherapy became FDA approved.
Figure 1.
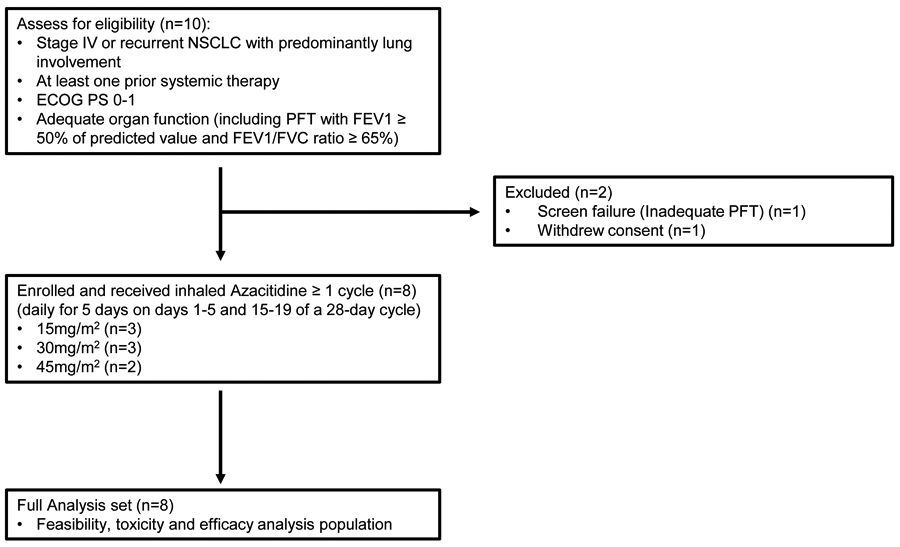
Patient disposition (PFT, pulmonary function test).
The participants were treated with 3 escalating doses of inhaled Azacitidine (Table 1 and Table 2A). The starting dose was 15mg/m2 which was derived from our preclinical studies that showed it to be a safe, non-cytotoxic and DNA demethylating dose16, 17, followed by 30 and 45 mg/m2. Most patients received two cycles of treatment, except one participant who withdrew from the study after one cycle (not able to comply with frequency of treatment visits), one at 30 mg/m2 received 12 cycles, and one at 45 mg/m2 received 4 cycles of treatment. The median number of study treatment cycles was 2 (IQR = 1) and ranged from 1 to 12 cycles. The main reason for the termination of study treatment in participants was due to progression of disease. The median follow-up time was 15.4 months (IQR = 20.5).
3.2. Toxicities and Pulmonary functions
No clinically significant adverse events including pulmonary toxicity (such as new or worsening dyspnea, cough or hoarseness) were observed (Table 2). Pulmonary function tests (PFT) were monitored to investigate the effects of inhaled Azacitidine on the respiratory tract and gas exchange (Table 2). Grade 1 decreased FEV1 (83% of baseline) was noticed in the first participant without any symptoms. Moreover, in our second patient receiving the highest dose 45 mg/m2, there was a grade 2 decreased DLCO (77% of baseline) after 2 cycles of treatment, which was also defined as DLT based on study protocol. The patient did not develop any new pulmonary symptoms and her DLCO spontaneously recovered to baseline without any intervention. She did not receive any additional treatments and no further participants were enrolled after the patient developed the DLT.
3.3. Pharmacokinetics
Over the 5-day consecutive treatment course (cycle 1 day 1 to 5 and day 15 to 19), measurable plasma Azacitidine was not detected at any time point in all 3 patients who received inhaled Azacitidine at the lowest dose level, 15 mg/m2. In patients who received higher doses (30 or 45 mg/m2), the plasma Azacitidine concentration was measurable at the completion of day 1 treatment [6.66 (SD = 9.72) ng/ml, 0 hour post-dose], and increased after day 5 treatment [17.87 (SD = 18.10ng/ml), 0 hour post-dose] (Table 2B). Previous study reported that the peak plasma Azicitadine concentration following a single subcutaneous dose (75mg/m2) occurred in 0.5 hour, and was approximately 750 ng/ml19. Thus, the mean maximum plasma concentration following inhaled Azacitidine is much lower compared to the historical subcutaneous administration. The plasma level quickly decreased by 80%-90% at 2 hours post inhalation and became undetectable 4 hours afterwards in all patients.
3.4. Efficacy assessment
Among 8 treated patients, 1 (12.5%) had a partial response (PR), 2 (25%) had stable disease (SD), and 5 (62.5%) had progression of disease (PD) (Table 1). The PR and SD events occurred in patients who received inhaled Azacitidine at 30 or 45 mg/m2 (Table 1 and Table 2A). Median PFS and OS were 1.8 and 15.4 months, respectively.
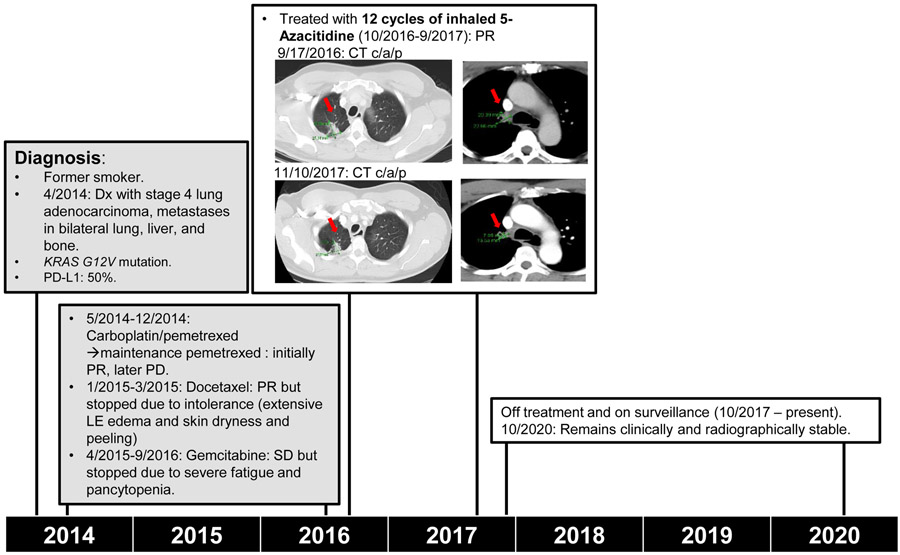
Patient #4 is a 57 years old African American male, former smoker (10 pack years, quit in 2014), with medical history of hypertension. He was initially diagnosed with stage IV lung adenocarcinoma in 4/2014, with metastases in bilateral lung, liver and bone. Molecular profiling revealed KRAS G12V mutation and PD-L1 TPS was 50%. He was treated with carboplatin and pemetrexed followed by maintenance pemetrexed (5/2014-12/2014, initially PR, later progressed), next docetaxel (1/2015-3/2015, PR with liver metastases mostly resolved, but discontinued due to intolerance with extensive lower extremity edema, skin dryness and peeling), then gemcitabine (4/2015-9/2016, SD but stopped due to severe fatigue and pancytopenia). He subsequently declined any further intravenous treatment and was enrolled in our study, received 12 cycles of inhaled Azacitidine treatment at 30mg/m2 (10/2016-9/2017), and had a PR. He tolerated the treatment well with no AEs. He has since been on surveillance and off cancer treatment. He has remained clinically and radiographically stable for more than three years since completing the inhalation treatments (Table 2A and Figure 2).
Figure 2.
Representative case. A 57 year-old African American male with heavily pre-treated stage IV lung adenocarcinoma received 12 cycles of inhaled Azacitidine treatment. (Dx: Diagnosed; PR: partial response; PD: progression of disease; LE: lower extremity; SD: stable disease)
Patient #8 is a 70 years old former smoker (30 pack years, quit in 2011). She was diagnosed with stage IIIA squamous cell lung cancer in 8/2016 and was treated with concurrent chemoradiotherapy. She was found to have loco-regional recurrence in 3/2017 and received nivolumab (3/2017-10/2017) complicated by grade 3 colitis and arthralgia. She was then enrolled in our study and received 2 cycles of inhaled Azacitidine treatment at 45mg/m2 (10/30/2017-12/15/2017) complicated by grade 2 decreased DLCO as aforementioned. She has remained stable for approximately three years since completing the inhalation treatments.
3.5. Methylation study
To test our main hypothesis that inhaled Azacitidine will cause hypomethylation in the bronchial epithelium the global methylation profile was investigated by quantifying levels of 5-methylcytosine in 3 participants who underwent pre- and post-treatment bronchoscopies yielding paired pre- and post-treatment bronchial epithelium tissue. The interval between the last inhaled treatment and bronchoscopy was 3 days. A significant demethylating effect was observed in 2 out of 3 (66%) patients in the post-therapy bronchial epithelium sample (24% and 79%), treated at 15mg/m2 and 45mg/m2, respectively (Figure 3). No significant methylation change was detected in the third patient receiving 45mg/m2.
Figure 3.
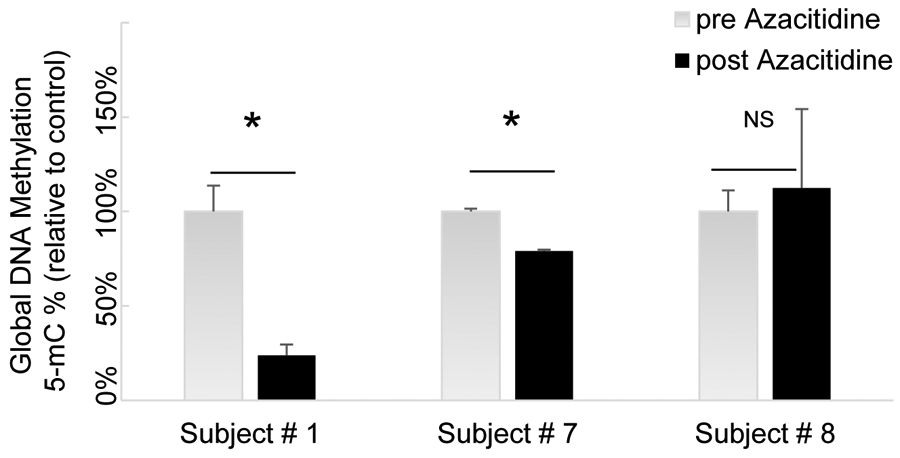
Global methylation profile in 3 participants who underwent pre- and post-treatment bronchoscopy with paired pre- and post-treatment bronchial epithelium specimens. The Interval between last inhaled treatment and bronchoscopy was approximately 3 days. *=P<0.05; NS, not significant.
4. Discussion
We report the first in-human pilot study of inhaled Azacitidine. Aerosol drug delivery has the advantage of direct deposition of the drug on the airways epithelial lining, resulting in a preferential accumulation of the drug into the lungs compared with intravenous administration. However, delayed systemic drug absorption from the lungs occurs and as a result inhalation has also been explored for the systemic delivery of drugs like insulin20. A formulation of aerosolized Azacitidine using the drug in powder form showed markedly enhanced systemic absorption, probably reflecting the preservation of drug stability when given in powder form15. In designing an inhaled formulation of Azacitidine for the purpose of targeting the airways epithelium and minimizing systemic absorption of biologically active Azacitidine, we adjusted the aerosol formulation parameters to obtain a particle size of 0.25 to 5 um and used the aqueous solution of Azacitidine to take advantage of its short stability, thus mostly restricting the delivery of active drug to the lungs and decreasing the systemic absorption of intact drug that could lead to systemic toxicity.
The results of this pilot study show that inhaled Azacitidine administered daily was very well tolerated, with no significant detectable systemic absorption of intact drug, thus explaining the lack of systemic toxicity. There were no symptoms of lung toxicity but one patient had a transient and modest asymptomatic decrease in DLCO at 45 mg/m2. Such dose may be still below the maximum tolerated dose (MTD) but requires inhalation sessions of 40 minutes in some cases, the significant time commitment required of patients becoming a practical limiting factor. Because of this and the fact that a demethylating effect had been observed at lower doses, we decided that there was no need to continue the study to establish a MTD as the intent of this pilot study was not to use Azacitidine as a cytotoxic agent, but to prove technical feasibility and find a chronic dose for future studies that is feasible, non-toxic, and able to exert a demethylating effect in the target tissue, the bronchial epithelium. We concluded that inhaled Azacitidine administered daily at 30 mg/m2 is feasible, well-tolerated and the logical dose of choice for a subsequent study using a continuous daily dose schedule in patients with tobacco-related stage I lung cancer, as an adjuvant treatment post resection as these patients have extensive genetic damage in their bronchial epithelium and are at high risk of developing second primary or recurrent lung cancers. Moreover, we are in the process of developing a more concentrated formulation of inhaled Azacitidine to decrease the length of treatment and establish a toxicity-defined MTD in future studies. Our study also paves the way for further clinical development of this promising epigenetic approach either alone or in combination to treat and prevent cancers with predominant lung involvement.
Highlights.
In heavily-pretreated lung cancer patients with predominantly lung involvement, inhaled Azacitidine was feasible and well-tolerated.
Disease control was observed in 3 out of 8 patients and demethylating effects were noted in 2 out of 3 of patients whose bronchial epithelium was sampled pre- and post-treatment.
Inhaled Azacitidine may exemplify a new approach for managing patients with lung-confined malignant lesions and related premalignant conditions.
Acknowledgments
This work was supported by NIH CA154755 (to HC, YZ, SS, and RPS). Clinical trial information: NCT02009436. The authors wish to thank all patients and their families for their invaluable support. The authors also wish to thank our supporting staff and nurses at cancer center (especially Elizabeth Ravera, Cheryl Baker, Yoko Eng, Betty Silchenstedt, Norman Williams and Akash Shah) for their important assistance in the trial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
None of below are relevant to this work: HC has received research funding from Genentech, Spectrum, and Array; and has received compensation as a scientific advisor to AstraZeneca, Bayer, and Takeda. MK has received compensation as a scientific advisor to Celgene and Eli Lilly. AV has received research funding from GlaxoSmithKline, BMS, Jannsen, Incyte, MedPacto, Celgene, Novartis, Curis and Eli Lilly and Company, has received compensation as a scientific advisor to Novartis, Stelexis Therapeutics, Acceleron Pharma, and Celgene, and has equity ownership in Throws Exception and Stelexis Therapeutics. BP is an employee of Merck. BH has received research funding from Astra Zeneca, Merck, Boehringer Ingelheim, BMS, Pfizer, Takeda, Guardant Health, Mirati, AbbVie, Novartis, and GlaxoSmithKline; and has received compensation as a scientific advisor to Foundation Medicine, Guardant Health, Astra Zeneca, Pfizer, Novartis, Merck, BMS, Boehringer-Ingelheim, Genentech, Spectrum, Ignyta. RPS has received compensation as a scientific advisor to Stelexis Therapeutics, Acceleron Pharma, and has ownership in Stelexis Therapeutics.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Ashraf-Uz-Zaman M, Bhalerao A, Mikelis CM, Cucullo L and German NA. Assessing the Current State of Lung Cancer Chemoprevention: A Comprehensive Overview. Cancers (Basel). 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H, Ji Y, Zhang J, Su Z, Liu M, Tong J, Ge C, Chen T and Li J. Aberrant DNA methylation in radon and/or cigarette smoke-induced malignant transformation in BEAS-2B human lung cell line. J Toxicol Environ Health A. 2017;80:1321–1330. [DOI] [PubMed] [Google Scholar]
- 3.Lyn-Cook L, Word B, George N, Lyn-Cook B and Hammons G. Effect of cigarette smoke condensate on gene promoter methylation in human lung cells. Tob Induc Dis. 2014;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato T, Arai E, Kohno T, Takahashi Y, Miyata S, Tsuta K, Watanabe S, Soejima K, Betsuyaku T and Kanai Y. Epigenetic clustering of lung adenocarcinomas based on DNA methylation profiles in adjacent lung tissue: Its correlation with smoking history and chronic obstructive pulmonary disease. Int J Cancer. 2014;135:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deutschmeyer V, Breuer J, Walesch SK, Sokol AM, Graumann J, Bartkuhn M, Boettger T, Rossbach O and Richter AM. Epigenetic therapy of novel tumour suppressor ZAR1 and its cancer biomarker function. Clin Epigenetics. 2019;11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stueve TR, Li WQ, Shi J, Marconett CN, Zhang T, Yang C, Mullen D, Yan C, Wheeler W, Hua X, Zhou B, Borok Z, Caporaso NE, Pesatori AC, Duan J, Laird-Offringa IA and Landi MT. Epigenome-wide analysis of DNA methylation in lung tissue shows concordance with blood studies and identifies tobacco smoke-inducible enhancers. Hum Mol Genet. 2017;26:3014–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tellez CS, Juri DE, Do K, Picchi MA, Wang T, Liu G, Spira A and Belinsky SA. miR-196b Is Epigenetically Silenced during the Premalignant Stage of Lung Carcinogenesis. Cancer Res. 2016;76:4741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh DK, Bose S and Kumar S. Regulation of expression of microRNAs by DNA methylation in lung cancer. Biomarkers. 2016;21:589–99. [DOI] [PubMed] [Google Scholar]
- 9.Brzezianska E, Dutkowska A and Antczak A. The significance of epigenetic alterations in lung carcinogenesis. Mol Biol Rep. 2013;40:309–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahesh S, Saxena A, Qiu X, Perez-Soler R and Zou Y. Intratracheally administered 5-azacytidine is effective against orthotopic human lung cancer xenograft models and devoid of important systemic toxicity. Clin Lung Cancer. 2010;11:405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrump DS, Fischette MR, Nguyen DM, Zhao M, Li X, Kunst TF, Hancox A, Hong JA, Chen GA, Pishchik V, Figg WD, Murgo AJ and Steinberg SM. Phase I study of decitabine-mediated gene expression in patients with cancers involving the lungs, esophagus, or pleura. Clin Cancer Res. 2006;12:5777–85. [DOI] [PubMed] [Google Scholar]
- 12.Levy BP, Giaccone G, Besse B, Felip E, Garassino MC, Domine Gomez M, Garrido P, Piperdi B, Ponce-Aix S, Menezes D, MacBeth KJ, Risueno A, Slepetis R, Wu X, Fandi A and Paz-Ares L. Randomised phase 2 study of pembrolizumab plus CC-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. European journal of cancer. 2019;108:120–128. [DOI] [PubMed] [Google Scholar]
- 13.Momparler RL. Epigenetic therapy of non-small cell lung cancer using decitabine (5-aza-2'-deoxycytidine). Front Oncol. 2013;3:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittgen BP, Kunst PW, van der Born K, van Wijk AW, Perkins W, Pilkiewicz FG, Perez-Soler R, Nicholson S, Peters GJ and Postmus PE. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 2007;13:2414–21. [DOI] [PubMed] [Google Scholar]
- 15.Kuehl PJ, Tellez CS, Grimes MJ, March TH, Tessema M, Revelli DA, Mallis LM, Dye WW, Sniegowski T, Badenoch A, Burke M, Dubose D, Vodak DT, Picchi MA and Belinsky SA. 5-Azacytidine inhaled dry powder formulation profoundly improves pharmacokinetics and efficacy for lung cancer therapy through genome reprogramming. Br J Cancer. 2020;122:1194–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu X, Liang Y, Sellers RS, Perez-Soler R and Zou Y. Aerosol azacytidine inhibits orthotopic lung cancers in mice through Its DNA demethylation and gene reactivation effects. PloS one. 2014;9:e109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu X, Liang Y, Sellers RS, Perez-Soler R and Zou Y. Toxicity and Pharmacokinetic Studies of Aerosolized Clinical Grade Azacitidine. Clin Lung Cancer. 2016;17:214–222 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labiris NR and Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Manero G, Stoltz ML, Ward MR, Kantarjian H and Sharma S. A pilot pharmacokinetic study of oral azacitidine. Leukemia. 2008;22:1680–4. [DOI] [PubMed] [Google Scholar]
- 20.Pittas AG, Westcott GP and Balk EM. Efficacy, safety, and patient acceptability of Technosphere inhaled insulin for people with diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:886–94. [DOI] [PubMed] [Google Scholar]