1. Introduction
Neuroendocrine tumors (NETs) are a rare group of neoplasms that can arise at sites throughout the body, with the most common sites being the lung and gastrointestinal (GI) tract [1]. Most data on the management of lung NETs stems from the study of GI NETs, although specific studies dedicated to lung NETs are emerging [2]. Well-differentiated NETs of the lung, also known as typical and atypical carcinoids (referred to as lung carcinoids hereafter), are relatively well-behaved biologically with a decreased incidence of lymph node and distant metastases compared to poorly differentiated lung NETs (i.e. small cell carcinoma and large cell neuroendocrine carcinoma) [3].
Prior to 2020, the most current lung neuroendocrine tumor expert consensus guidelines were from the European Neuroendocrine Tumor Society (ENETS) in 2015 [4]. However, an endorsement and update of these guidelines has recently been published from a North American Neuroendocrine Tumor Society (NANETS) and Commonwealth Neuroendocrine Tumour (CommNETS) research collaborative [5]. For patients with lung carcinoids, when feasible, complete anatomic surgical resection (i.e. lobectomy) and systematic lymph node dissection is recommended, particularly if the tumor is peripheral. Sublobar resection is now considered an acceptable alternative for peripheral < 2 cm lung typical carcinoids if complete resection is achievable.
There are no clinical trial data or consensus on adjuvant therapy following a complete resection for lung carcinoids, as official cancer guidelines (i.e. ENETS, NANETS, National Comprehensive Cancer Network (NCCN), and European Society for Medical Oncology) either do not provide recommendations or have contradictory recommendations [4–8]. Generally, for completely resected stage I-IIIA typical lung carcinoids using the American Joint Committee on Cancer (AJCC) AJCC/Internal Union for Cancer Control (UICC) 8th TNM classification, observation alone is performed [8]. In the 2015 ENETS guidelines, adjuvant therapy is considered for patients with completely resected atypical carcinoids and lymph node metastases in the context of a high proliferative rate and only after discussion in a multidisciplinary tumor board setting [4]. However, in the NANETS/CommNETs endorsement of the 2015 ENETS guidelines, adjuvant therapy is not recommended given the lack of data [5].
Based on the relatively limited data in the literature and the lack of consensus for postoperative management for lung carcinoids with lymph node metastases, we sought to elucidate the following in our single institution retrospective cohort study: (i) the incidence of lymph node metastases for typical and atypical lung carcinoid tumors treated with surgical resection at Stanford University, (ii) the clinical, radiographic, surgical and pathologic factors associated with lymph node metastases, and (iii) the post-surgical management of patients with lymph node metastases.
2. Methods
2.1. Definitions
This retrospective analysis was performed under an institutional review board approved protocol. There were 220 patients identified with a pathologic diagnosis of typical or atypical lung carcinoid tumor who were seen at Stanford University between October 1998 and September 2017. These patients were identified from the Stanford Cancer Institute Research Database (SCIRDB)1, the Surgical Thoracic Database, and the Stanford Neuroendocrine Tumor Program using the search terms “neuroendocrine”, “well differentiated neuroendocrine”, “carcinoid”, “typical carcinoid”, and “atypical carcinoid”. Of these patients, 101 who underwent a surgical resection performed at our institution were identified. Demographic, radiographic, tumor, and surgical variables (listed in Table 1) were abstracted from the electronic medical record.
Table 1.
Clinical characteristics and clinicopathological features associated with lymph node disease
| Characteristic | All Patients | Without Lymph Node disease (N0) N= 81 |
With Lymph Node disease (N1 or N2) N= 17 |
Univariable Analysis (p value) |
|---|---|---|---|---|
| Age, years | 0.65 | |||
| Median-years (range) | 58(19–84) | 58 (19–84) | 56 (36–78) | |
| Race | 0.57 | |||
| White | 67 (68.4%) | 54 (66.7%) | 13 (76.5%) | |
| Non-White | 31 (31.6%) | 27 (33.3%) | 4 (23.5%) | |
| Sex | 1.00 | |||
| Male | 24 (24.5%) | 20 (24.7%) | 4 (23.5%) | |
| Female | 74 (75.5%) | 61 (75.3%) | 13 (76.5%) | |
| Prior Smoking History | 1.00 | |||
| Yes | 32 (32.7%) | 27 (33.3%) | 5 (29.4%) | |
| No | 66 (67.3%) | 54 (66.7%) | 12 (70.6%) | |
| Prior Malignancy | 0.43 | |||
| Yes | 12 (12.2%) | 9 (11.1%) | 3 (17.6%) | |
| No | 86 (87.8%) | 72 (88.9%) | 14 (82.4%) | |
| Second Primary Cancer (non- lung carcinoid) | 0.20 | |||
| Yes | 10 (10.2%) | 10 (12.3%) | 0 (0%) | |
| No | 88 (89.8%) | 71 (87.7%) | 17 (100%) | |
| Functional Syndrome1 | 0.58 | |||
| Yes | 10 (10.2%) | 8 (9.9%) | 2 (11.8%) | |
| No | 37 (37.8%) | 29 (35.8%) | 8 (47.1%) | |
| Not specified | 51 (52.0%) | 44 (54.3%) | 7 (41.2%) | |
| Preoperative Systemic Imaging Performed2 | ||||
| CT | 85 (86.7%) | 70 (86.4%) | 15 (88.2%) | 1.00 |
| 18FDG PET | 58 (59.2%) | 50 (61.7%) | 8 (47.1%) | 0.29 |
| SSTR Imaging | 18 (18.4%) | 12 (14.8%) | 6 (35.3%) | 0.08 |
| Suspected Lymph Node Involvement on Preoperative Imaging | 0.13 | |||
| Yes | 15 (15.3%) | 10 (12.3%) | 5 (29.4%) | |
| No | 83 (84.7%) | 71 (87.7%) | 12 (70.6%) | |
| Location of Tumor | 0.42 | |||
| Central | 57 (58.2%) | 49 (60.5%) | 8 (47.1%) | |
| Peripheral | 41 (41.8%) | 32 (39.5%) | 9 (52.9%) | |
| Tumor Laterality | 0.40 | |||
| Right | 35 (35.7%) | 27 (33.3%) | 8 (47.1%) | |
| Left | 63 (64.3%) | 54 (66.7%) | 9 (52.9%) | |
| Surgical Approach | 0.83 | |||
| Thoracotomy | 73 (74.5%) | 59 (72.8%) | 14 (82.4%) | |
| VATS | 23 (23.5%) | 20 (24.7%) | 3 (17.6%) | |
| Robotic | 2 (2.0%) | 2 (2.5%) | 0 (0%) | |
| Extent of Surgery | 1.00 | |||
| Lobectomy | 89 (90.8%) | 73 (90.1%) | 16 (94.1%) | |
| Sublobar resection | 9 (9.2%) | 8 (9.9%) | 1 (5.9%) | |
| Number of Lymph Nodes Sampled, median [range] | 9[1–32] | 9 (1–30) | 9 (1–32) | 1.00 |
| Tumor Size (pathologic), median [range] | 2.1 [0.7–9.0] | 2.1 (0.7–9.0) | 2.5 (0.7–4.5) | 0.91 |
| Number of Lymph Node Stations Sampled median [range] | 4 [1–10] | 4[1–10] | 4 [1–8] | 0.62 |
| Primary Tumor | 0.57 | |||
| T1a | 50 (51.0%) | 42 (51.9%) | 8 (47.1%) | |
| T1b | 24 (24.5%) | 21 (25.9%) | 3 (17.6%) | |
| T2a | 20 (20.4%) | 14 (17.3%) | 6 (35.3%) | |
| T2b | 2 (2.0%) | 2 (2.5%) | 0 | |
| T3 | 2 (2.0%) | 2 (2.5%) | 0 | |
| Regional Lymph Nodes3 | ||||
| N0 | 81 (83.7%) | ---- | ---- | ---- |
| N1 | 11 (10.2%) | ---- | ---- | ---- |
| N2 | 6 (6.1%) | ---- | ---- | ---- |
| Distant Metastases | ||||
| M0 | 98 (100%) | ---- | ---- | ---- |
| M1a | 0 | ---- | ---- | ---- |
| M1b | 0 | ---- | ---- | ---- |
| Overall Stage (n=98)4 | ||||
| IA | 63 (64.3%) | ---- | ---- | ---- |
| IB | 14 (14.3%) | ---- | ---- | ---- |
| IIA | 11 (11.2%) | ---- | ---- | ---- |
| IIB | 4 (4.1%) | ---- | ---- | ---- |
| IIIA | 6 (6.1%) | ---- | ---- | ---- |
| IIIB | 0 | ---- | ---- | ---- |
| IV | 0 | ---- | ---- | ---- |
| Histologic Diagnosis | 0.40 | |||
| Typical Carcinoid | 87 (88.8%) | 73 (90.1%) | 14 (82.4%) | |
| Atypical Carcinoid | 11 (11.2%) | 8 (4.9%) | 3 (17.6%) | |
| Resection Status | ||||
| Complete | 94 (95.9%) | 79 (97.5%) | 15 (88.2%) | 0.14 |
| Incomplete | 4 (4.1%) | 2 (2.5%) | 2 (11.8%) | |
| Mitotic Index (mitosis/2 mm2) | 0.10 | |||
| <2 | 91 (92.9%) | 77 (95.1%) | 14 (82.4%) | |
| 2–10 | 7 (7.1%) | 4 (4.9%) | 3 (17.6%) | |
| Necrosis | 0.65 | |||
| Yes | 9 (9.2%) | 7 (8.6%) | 2 (11.8%) | |
| No | 89 (90.8%) | 74 (91.4%) | 15 (88.2%) | |
| Recurrence | ||||
| Yes | 10 (10.2%) | 5 (6.2%) | 5 (29.4%) | 0.013 |
| No | 88 (89.8%) | 76 (93.8%) | 12 (70.6%) |
Abbreviations: 18FDGPET =Fludeoxyglucose Positron Emission Tomography; CT = Computed Tomography; SSTR imaging = Somatostatin Receptor imaging includes OctreoScan = Octreotide Scan and 68Ga-DOTATATE PET = 68 Gallium-DOTATATE Positron Emission Tomography; VATS = video-assisted thoracic surgery.
Carcinoid Syndrome was detected in 8 patients and Cushing’s Syndrome in 2 patients.
9 had OctreoScan, 11 had 68Ga-DOTATATE PET scans, and 2 had both.
2.1.1. Pathologic Diagnosis
The pathologic diagnosis was classified according to 2015 WHO Classification and 93 (92%) of cases with available specimens underwent re-review by a single thoracic pathologist (G.J.B.) [3]. Classification of typical versus atypical carcinoid was based on morphology, mitotic count, and the presence of necrosis. Typical carcinoid was defined as having no necrosis and mitotic rates of <2 division figures per 2 mm2 [2], while atypical carcinoids were defined as focal or punctate necrosis and/or increased mitotic activity of 2–10 division figures per 2 mm2 [3]. A minimum of 1 slide per case was reviewed. For tumors near the mitoses count cutoffs, 2–3 slides were reviewed, multiple microscopic fields were enumerated, and the mean was used for determining the mitotic rate in line with the recommendations from the 2015 WHO Classification [3]. Ki-67 as assessed by immunohistochemistry is not routinely performed on lung neuroendocrine tumors and was not examined in this study.
2.1.2. Staging
Lymph node stations and staging were notated according to AJCC version 7, including re-classification of cases before 2010 [9]. Lymph node summary categories included N1, defined as metastasis in ipsilateral hilar or intrapulmonary lymph nodes, including involvement by direct extension; N2, defined as metastasis in ipsilateral mediastinal lymph nodes; and N3, defined as metastasis in contralateral or supraclavicular lymph nodes [9].
For our study, lymph node positive disease was defined as having at least one lymph node involved with tumor at any lymph node station irrespective of number or levels of lymph node stations sampled. Patients without lymph nodes sampled or reported in the pathologic specimen were excluded from the analysis.
2.1.3. Tumor, Radiographic, and Surgical Variables
In accordance with previously reported definitions, central tumors were defined as involving proximal bronchi, and peripheral tumors were defined as involving sub-segmental or more distal bronchi [11]. Size of the tumor was determined pathologically and if there were multiple lesions present, the size of the largest lesion was recorded. Suspected lymph node disease was examined on patient’s radiology reports and defined as increased nodal uptake on 18F-fluorodeoxyglucos (FDG) PET-CT or somatostatin receptor imaging (i.e. Octreoscan or 68 Gallium DOTATATE PET), or enlarged lymph nodes (defined as greater than 1cm on the short axis) noted on anatomical imaging with computed tomography (CT) scan. A detailed analysis of somatostatin receptor PET imaging was performed in a subset of 11 patients by a nuclear medicine board certified physician (T.K.Y), and the characteristics of both the primary tumor and any associated lymph nodes were reported. Type of lung resection was categorized as lobar (lobectomy, bilobectomy, sleeve resection, pneumonectomy) or sub-lobar (wedge resection or segmentectomy).
2.2. Statistical Analysis
Cross-sectional analyses were conducted to examine the association between of a set of selected factors and lymph node metastases. Descriptive statistics were used to summarize the baseline and tumor characteristics, with continuous variables reported as medians and ranges and categorical variables reported as frequencies and relative percentages. The Fisher’s exact test and Wilcoxon-test were used to compare categorical variables and continuous variables, respectively, between patients with and without lymph node disease. This was followed by a multivariate logistic regression analysis to determine whether lung carcinoid histologic type, number of lymph node stations sampled, mitotic index, presence of necrosis, and performance of preoperative SSTR imaging could predict lymph node metastases in patients with lung carcinoids. These five independent variables were selected a priori. The 95% confidence interval of the odds ratio associated with each independent variable was reported. Significance was determined at p < 0.05 for all statistical tests. In addition, a p-value between 0.05 and 0.10 is considered evidence of a trend warranting further research. Lastly, we describe in detail the management of the 17 cases with lymph node disease. All analyses were performed with R version 3.1.2 (R Foundation for Statistical Computing).
3. Results
3.1. Cohort Characteristics
From the 101 patients identified with lung carcinoid who underwent surgical resection at Stanford University, the final cohort for analysis included 98 patients: 87 patients (89%) with typical carcinoid and 11 patients (11%) with atypical carcinoid (Figure 1). This final cohort reflects a total of 5 cases that had their original diagnosis changed on re-review, including 4 cases of atypical carcinoid changed to typical carcinoid and 1 case changed from atypical carcinoid to large cell neuroendocrine carcinoma, with the latter case being excluded from the analysis. This final cohort also reflects the exclusion of 3 cases without lymph nodes sampled or reported in the pathologic specimen.
Figure 1.
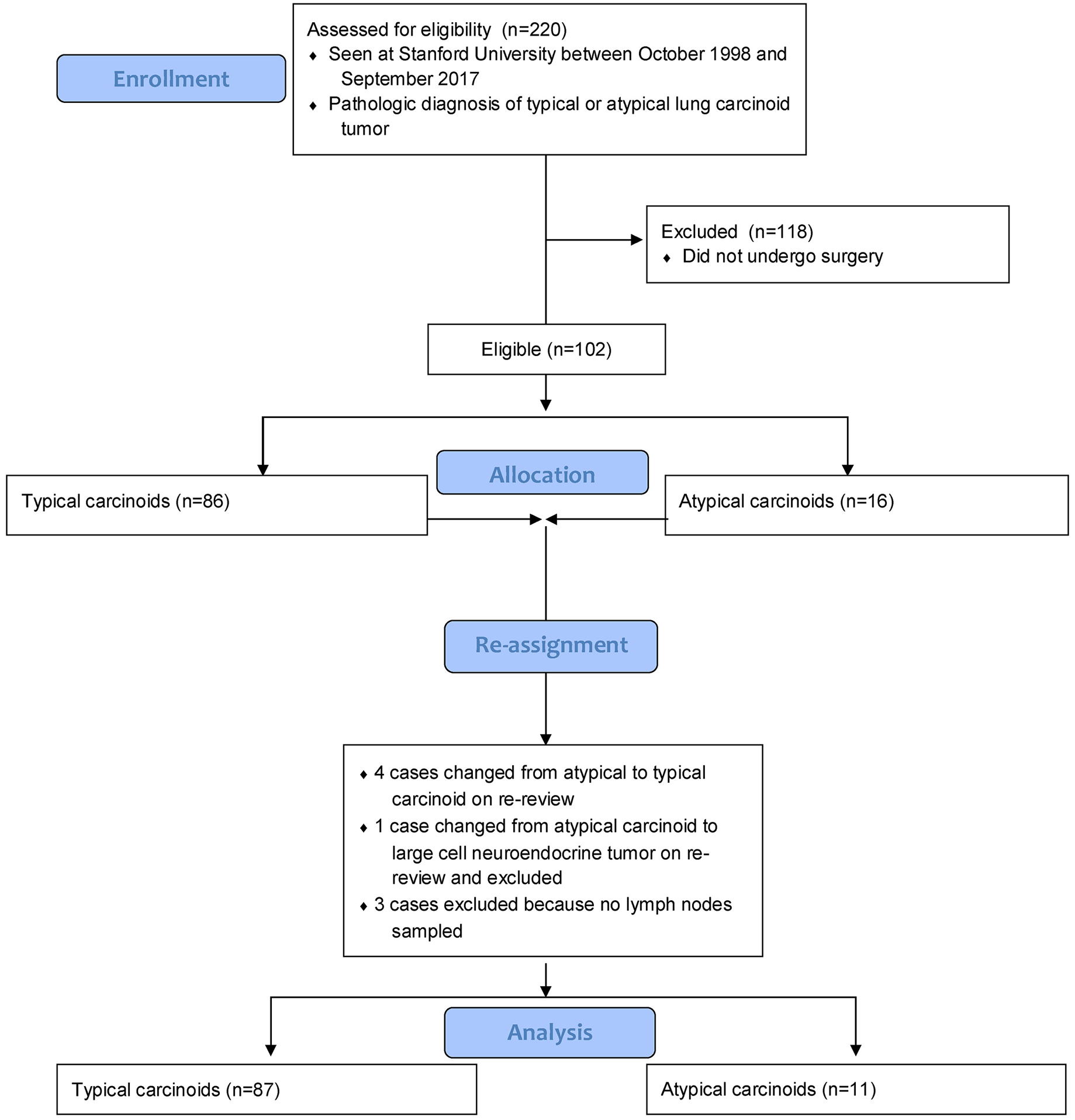
Cohort Selection
Most patients were white (68%), female (76%), and the median age of the patients was 58 years (Table 1). Almost one-third of the patients with typical or atypical carcinoid tumors had a smoking history (33%). Almost one-fourth of the patients had a prior malignancy or second primary malignancy (12% and 10%, respectively). The most common preoperative imaging performed was computed tomography (87%) either with or without contrast and 18FDG PET (59%), although 9 patients and 11 patients underwent OctreoScan and 68 Gallium (68Ga) DOTATATE PET, respectively. The majority of patients underwent lobectomy (91%) and a thoracotomy approach (75%). No patients had a preoperative mediastinoscopy. All patients had at least one lymph node sampled, with a median of 4 lymph node stations sampled and a median of 9 total lymph nodes sampled.
These variables were examined in association with presence of lymph node metastases. In the univariable analysis, patients with lymph node disease were associated with having a higher rate of recurrence of lung carcinoid compared to those without lymph node disease (29% vs. 6%, respectively; p= 0.01). Recurrence, however, was not analyzed as a time-to-event category and some recurrences may not have been documented due to loss of follow-up. There was also a trend towards association of type of preoperative imaging performed and presence of lymph node metastases, with a higher proportion of patients with lymph node disease having completed preoperative SSTR imaging (35% vs. 15%, respectively; p=0.08).
3.2. Lymph Node Metastases in Cohort
In our cohort, 17 patients were found to have at least one positive lymph node, with 11 having N1 disease and 6 having N2 disease. Of the lymph node positive cases, 14 were typical carcinoids (8 N1, 6 N2) and 3 were atypical carcinoids (3 N1). This subgroup of patients had a median age of 56 years and the majority were white (77%). Interestingly, the median tumor size was only 2.5cm with a range between 0.7–4.5cm. The T-stages of these tumors were the following: 8 (47%) were T1a, 3 (18%) were T1b, and 6 (35%) were T2a. Of the lymph node positive cases, 12 (71%) patients did not have suspected lymph node disease on pre-operative imaging whereas 5 (29%) did. None of the patients received adjuvant therapy after surgery.
3.3. Multivariate Model to Predict Lymph Node Metastases
Given the limited sample size of patients with lymph node involvement, five variables of clinical interest were chosen a priori for the multivariable regression model (Table 3). None of the a priori selected factors were significant in a multivariable logistic regression for association with lymph node involvement, including lung carcinoid histologic type, number of lymph node stations sampled, mitotic index, presence of necrosis, and preoperative SSTR imaging. However, there was a trend towards performance of preoperative SSTR imaging and lymph node involvement (OR=3.06, p=0.07).
Table 3.
Clinicopathological features associated with lymph node involvement (Multivariable Logistic Regression)
| Variables | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Lung Carcinoid Histologic type (Atypical versus Typical Carcinoid) | 0.46 | 0.02 – 11.7 | 0.63 |
| Number of Stations Sampled (each additional lymph node station sampled) | 0.96 | 0.73 – 1.26 | 0.77 |
| Performance of SSTR Imaging (Octreoscan or 68Ga-DOTATATE performed versus not performed) | 3.06 | 0.93 – 10.1 | 0.07 |
| Mitotic Index (2–10 versus <2 mitoses/2 mm2) | 6.16 | 0.41 – 92.1 | 0.19 |
| Presence of Necrosis (present versus absent) | 1.56 | 0.12 – 19.4 | 0.73 |
Abbreviations: SSTR imaging = Somatostatin Receptor imaging includes OctreoScan = Octreotide Scan and 68Ga-DOTATATE PET = 68 Gallium-DOTATATE Positron Emission Tomography.
3.4. Somatostatin Receptor Imaging
Given the trend in association of preoperative SSTR imaging and lymph node positive disease in on our study, we examined this patient subcohort in more detail. Of the 18 patients who underwent SSTR imaging, 9 patients received an OctreoScan, 11 patients 68Ga-DOTATATE PET, and 2 patients both OctreoScan and 68Ga-DOTATATE PET.
Of the 9 patients who received an OctreoScan, 7 were available to be reviewed by a nuclear medicine radiologist (T.K.Y.). Of the 7 patients who were reviewed, 3 were noted to have ‘positive’ scans, defined as uptake in any of the lobes of the lung or lymph nodes. Of these 3 patients, only two were found to have pathologically proven lymph node positive disease. In addition, one patient who did not have a ‘positive’ OctreoScan was found to have pathologically proven lymph node positive disease.
Of the 11 patients who underwent 68Ga-DOTATATE PET, 3 were found to have suspicion of lymph node involvement as evaluated by nuclear medicine radiologist (T.K.Y). Of these 3 patients, only one had pathologically proven lymph node involvement. Two other patients who had pathologic involvement of lymph nodes did not have suspicious lymph node involvement on preoperative 68Ga-DOTATATE PET.
In summary, the imaging findings of this subcohort included an average SUV max for primary tumor of 29.2 and an average SUV max of the spleen of 29.7. Several characteristics found in the literature to be independent predictors for tumor progression and prognosis were also examined, including tumor-to-spleen ratio along with 68Ga-DOTATATE-avid tumor volume [12–13]. The imaging findings of these patients and the association with pathologic findings are noted in appendix table A.1. Given the small proportion (11%) of preoperative 68Ga-DOTATATE PET performed in this cohort and highly censored outcomes, clinical correlation was not possible.
4. Discussion
There is relatively limited data to direct lung carcinoid tumor management and data on the natural history of these tumors has been limited. Studies have shown that the 8th TNM staging system provides reliable prognostic discrimination of outcomes, but subcategories of this classification do not provide adequate separation from their neighbors, highlighting the need for more information on the factors related to prognosis in these tumors [14]. When early stage and resectable, surgery is the treatment of choice for lung carcinoids and provides the optimum chance of offering cure. The type of resection and assessment of the lymph nodes, particularly in the mediastinum, are noted to be important across several cancer guidelines [4–6]. Here, we described a retrospective single-institution cohort of 98 patients with resected lung carcinoids and examined clinicopathologic features associated with lymph node involvement.
The cohort consisted of 89% lung typical carcinoids and 11% lung atypical carcinoids, a similar proportion of each type observed in other studies [15]. As is consistent with the the CommNETS/NANETS 2020 endorsement and update of the ENETS 2015 guidelines, the majority of patients in our cohort underwent an anatomic resection (i.e. lobectomy or greater) [5]. A thoracotomy approach was utilized for the majority of our patients (75%), however, minimally invasive strategies with VATs and robotic surgery are now acceptable modalities and account for more than a quarter of patients in our cohort. Another important part of surgical resection is lymph node sampling. Currently, consensus for the completeness or adequacy of lymph node staging is lacking. The International Association for the Study of Lung Cancer (IASLC) defines adequate lymph node staging to include stations 2R, 4R, 7, 10R, 11R for right sided tumors and stations 5, 6, 7, 10L, 11L for left sided tumors. In addition, it is recommended to sample mediastinal lymph node station 9 for lower lobe tumors, and examine stations 12–14 contained within the surgical specimen if lobectomy or greater is performed and to sample these stations separately if segmentectomy is performed [10]. The NCCN defines adequate mediastinal lymph node staging as three N2 lymph node stations sampled or dissected, with dissection performed if the patient has known N2 disease.
In our study, we found a rate of lymph node metastases of 17% among 98 patients (11 N1 and 6 N2). The relatively high rate of lymph node metastases in our cohort along with the imperfect sensitivity or specificity of detection of lymph node metastases on preoperative imaging suggests that lymph node sampling should be completed at the time of surgery for lung carcinoids. It is possible that the rate of lymph node metastases could have been even higher in our cohort had all of the patients received a complete mediastinal lymph node dissection. The definition of lymph node sampling was liberal in our study, defined as at least one lymph node being sampled irrespective of lymph node station sampled.
There are conflicting studies on the prognostic impact of lymph node metastases after complete surgical resection for patients with lung carcinoids, although the majority do show worse prognosis [13, 16–18]. Given the lack of available long term outcome data for this cohort, we were unable to examine the impact of any lymph node variables on clinical outcomes, including number of nodes sampled and the number of stations sampled. More research also needs to be done to elucidate the independent significance of histologic type (i.e. typical vs. atypical carcinoid) and nodal status on overall survival.
With mounting evidence tying the association between lymph node involvement and prognosis, it is important to understand the clinical, radiographic, surgical, and pathologic factors associated with lymph node involvement. We evaluated greater than 20 factors in our lung carcinoid cohort in a univariable analysis. Among these factors, none were significantly associated with lymph node involvement and there was only a trend noted for performance of preoperative SSTR imaging. Part of the reason we may not have found more significant associations was due to the relatively limited sample size of only 17 patients with lymph node involvement versus 81 without lymph node involvement. The decision by surgeons on the extent of the performance of lymph node sampling could represent biases from the surgeon based on preoperative knowledge of tumor biology and other information not ascertained in this analysis. Tumor recurrence was also significantly associated with lymph node involvement (p < 0.01) and is not surprising; however, the weakness of this analysis was that recurrence was not examined as a time-to-event analysis, as there were limited events and 9 patients were lost to follow-up.
There were also interesting findings in terms of lack of significant associations with lymph node involvement, including size of the primary tumor. First, the size of the tumor was not significantly associated with lymph node involvement. In the 17 cases with lymph node involvement, there were tumors as small as 0.7cm that were found to have lymph node involvement and 7 of 17 had tumors <2cm. This is in contrast to a study by Kneuertz et al that analyzed 3335 patients with typical or atypical carcinoid tumors and found that large tumor size was a predictor of nodal disease [19], as well as a study by Wurtz et al showing that tumor size >3cm was associated with lymphatic spread [20]. It was also interesting that histologic pattern (typical carcinoid vs. atypical carcinoid) was not associated with lymph node metastases given multiple studies have shown a higher rate of lymph node metastases in atypical carcinoids [16–18]. In our cohort, of the 17 cases of lymph node involvement, 14 had typical carcinoid (82%) and 3 (18%) had atypical carcinoid. In addition, there was no independent association of mitotic count and/or presence of necrosis with lymph node positive disease in multivariable analysis.
Since we could not include all factors in a multivariable logistic regression due to the sample size, a priori factors of clinical interest were selected. However, none had a significant association with lymph node involvement, including lung carcinoid type, number of lymph node stations sampled, mitotic index, presence of necrosis, and preoperative SSTR imaging.
While it did not reach significance, performance of preoperative somatostatin receptor (SSTR) imaging (i.e., OctreoScan and 68-Gallium DOTATATE PET) showed a trend towards association with lymph node metastases in both univariable (p=0.08) and multivariable (p=0.07) analyses. SSTR imaging, particularly DOTA-PET, has demonstrated a significant improvement in the management of patients with NETs and may improve staging at diagnosis, including preoperative lymph node staging for lung carcinoids. In 2017, Hope and colleagues proposed criteria for SSTR-PET, including 68Ga-DOTATATE PET, which was validated in patients with well-differentiated gastroentero-pancreatic (GEP)-NETs [21]. These included initial staging following the histologic diagnosis of NET and staging of NET prior to planned surgery. However, there are limited studies of SSTR PET imaging dedicated to lung carcinoids and further studies are needed, as there may be differences in its utility for typical carcinoids versus atypical carcinoids [19–20]. In a study comparing 68Ga-DOTATATE PET with contrast enhanced CT in patients with histologically confirmed NET (including pancreas, gastroenteric, lung, endometrium, paraganglioma), the sensitivity, specificity, and positive predictive value for lymph node detection with 68Ga-DOTATATE PET versus contrast enhanced CT was 92% versus 64% (p = 0.0004), 83% versus 59% (p=0.0386), and 82% versus 57%, respectively, for lymph node detection [22]. Despite a small sample of just 11 68Ga DOTATATE PETs performed in our study, both false positives (n=2) and negatives (n=2) were observed, indicating SSTR PET sensitivity and specificity for lymph node involvement in lung carcinoids needs further study. While SSTR-PET can be valuable for staging of a NET prior to planned surgery, it is important to recognize the limitation of these imaging modalities. Although SSTR PET may be able to identify occult lymph node metastases, it should not be used to influence breadth of lymph node sampling at the time of surgery until further studies are completed.
None of the 17 patients with lung carcinoid tumors with lymph node involvement received adjuvant therapy due in part to the lack of data to support use of adjuvant therapy for resected lung carcinoids. In population-based studies and other retrospective cohort studies, there was no survival advantage of adjuvant therapy observed for patients with lymph node metastases in typical carcinoid or atypical carcinoid [15, 23–26]. Filosso et al. found lymph node metastases were a predictor for the development of distant metastases, however, adjuvant therapy did not reduce to risk of developing this [15]. Future prospective studies are needed to elucidate the utility of adjuvant therapy for lung carcinoids.
5. Conclusions
There is a small but growing body of evidence on the prognostic implications of lymph node metastases in lung carcinoid tumors. Our study’s findings were limited by the small sample size of patients with positive lymph node metastases (n=17; 17%). That said, the study benefited from extensive characterization of the variables associated with lymph node involvement. We found a trend for the performance of SSTR imaging and association of lymph node metastases in both univariable and multivariable analysis. This suggests the potential importance of incorporating SSTR imaging in our routine practice for pre-operative staging of lung carcinoids, although we acknowledge that both false positives and negatives were observed with SSTR imaging in this cohort. It was also notable that a large proportion (41%) of patients with lymph node positive disease had < 2 cm tumors, suggesting that we should not restrict using this staging modality in patients with small tumors. It additionally highlights the importance of mediastinal lymph node sampling during surgery. Future studies for lung carcinoids should evaluate the role of preoperative SSTR imaging for lymph node staging, additional factors associated with lymph node metastases given its poor prognostic implication across several studies and importantly, establish the role of adjuvant therapy for lymph node positive disease.
Table 2.
17 cases of Lymph Node Metastases
| Histologic Diagnosis | Functional Syndrome | Types of Preoperative Imaging performed | Location of Tumor | 18FDG PET LN Uptake | SSTR LN Positive | Surgical Approach | Extent of Resection1 | N status2 | Number of positive Lymph Nodes per station3 | Method of lymph nodes sampled | Primary Tumor Size (cm) | Stage (AJC C 7) | Adjuvant Therapy | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Typical | NonFunctional | PET/CT FDG plus Octreoscan | Peripher al LUL | No | No | VATs | Left Upper Lobectomy | N2 | Station 5: 1/1 Station 11L: 2/2 | Surgery | 0.7 | IIIA | None |
| 2 | Typical | Not specified | PET/CT FDG plus CT with contrast | Central LLL | No | N/A | Thoracotomy | Left Lower Lobectomy | N1 | Station 5: 0/3 Station 6: 0/1 Station 7: 0/2 Station 10: 1/2 Station 11L: 0/1 Station 12: 0/4 | Surgery | 1 | IIA | None |
| 3 | Typical | Not specified | CT with contrast | Central LUL | N/A | N/A | Thoracotomy | Left Upper Lobectomy | N1 | Station 5: 0/3 Station 7: 0/1 Station 9: 0/1 Station 10L: 0/1 Station 12: 1/3 | Surgery | 3.6 | IIA | None |
| 4 | Typical | Not specified | PET/CT FDG plus CT with contrast | Peripher al RLL | No | N/A | VATs | Right Lower Lobectomy | N2 | Station 2R: 0/2 Station 4R: 0/2 Station 7: 2/6 Station 9: 0/1 Station 11R: 1/2 Station 12: 0/1 | Surgery | 1.3 | IIIA | None |
| 5 | Typical | Not specified | PET/CT FDG plus CT with contrast plus PET/CT DOTA | Peripher al LUL | Yes | Yes | Thoracotomy | Left Upper Lobe Wedge Resection | N1 | Station 2L: 0/1 Station 4L: 0/6 Station 7: 0/1 Station 9: 0/1 Station 10L: 0/1 Station 11L: 0/1 Station 12: 5/5 | Surgery and Mediastinoscopy | 4.5 | IIA | None |
| 6 | Typical | Not specified | PET/CT FDG plus CT with contrast plus Octreoscan | Central Right Hilum | No | Yes | Thoracotomy | Right Pneumonectomy | N2 | Station 7: 1/1 Station 8: 1/1 Station 10: 1/1 Station 12–14 (peribronchial): 4/8 | Surgery | 4.1 | IIIA | None |
| 7 | Typical | Not specified | PET/CT FDG | Central RML | Yes | N/A | Thoracotomy | Right Middle Lobectomy | N2 | Station 2R: 0/6 Station 4R: 1/12 Station 7: 0/3 Station 9: 0/1 Station 10R: 0/2 Station 12: 2/8 | Surgery | 2.7 | IIIA | None |
| 8 | Typical | Cushing’s | PET/CT FDG plus CT with contrast plus PET/CT DOTA | Peripher al RML | No | No | VATs | Right Middle Lobectomy | N1 | Station 12: 1/1 | Surgery | 1.5 | IIA | None |
| 9 | Atypical | Not specified | PET/CT FDG plus CT without contrast plus PET/CT DOTA | Peripher al LLL | No | No | Thoracotomy | Left Lower Lobectomy | N1 | Station 7: 0/5 Station 9: 0/2 Station 10L: 0/1 Station 12: 2/2 | Surgery | 2.9 | IIA | None |
| 10 | Typical | Carcinoid | Unknown | Central Left Endobronchial | N/A | N/A | Thoracotomy | Left Pneumonectomy | N1 | Station 6: 0/1 Station 7: 0/1 Station 9: 0/1 Station 10L: 1/1 Station 12: 0/7 | Surgery | 2.5 | IIA | None |
| 11 | Typical | Nonfunctional | Unknown | Central RML | N/A | N/A | Thoracotomy | Right Middle Lobectomy | N2 | Station 7: 2/4 Station 12: 1/1 | Surgery | 3.5 | IIIA | None |
| 12 | Atypical | Nonfunctional | CT (unknown with or without contrast) | Peripher al LUL | N/A | N/A | Thoracotomy | Left Upper Lobectomy | N1 | Station 4L: 0/1 Station 9: 0/1 Station 10: 1/3 | Surgery | 2.9 | IIA | None |
| 13 | Typical | Non- functional | CT with contrast | Peripher al LUL | N/A | N/A | Thoracotomy | Left Upper Lobectomy | N1 | Station 12–14 (peribronchial): 1/4 | Surgery | 1.5 | IIA | None |
| 14 | Typical | Nonfunctional | CT with contrast | Peripher al RLL | N/A | N/A | Thoracotomy | Right Lower Lobectomy | N2 | Station 4R: 0/1 Station 7: 1/1 Station 10R: 0/2 Station 11: 0/1 Station 12: 0/2 | Surgery | 4.0 | IIIA | None |
| 15 | Typical | Nonfunctional | CT (unknown with or without contrast) | Central RUL | N/A | N/A | Thoracotomy | Right Upper Lobectomy | N1 | Station 4: 0/2 Station 7: 0/2 Station 10R: 1/3 | Surgery | 1.2 | IIA | None |
| 16 | Typical | Nonfunctional | CT (unknown with or without contrast) plus Octreoscan | Central Left Hilum | N/A | Yes | Thoracotomy | Left Pneumonectomy | N1 | Station 12: 2/6 | Surgery | 3.5 | IIA | None |
| 17 | Atypical | Nonfunctional | Unknown | Peripher al RUL | N/A | N/A | Thoracotomy | Right Upper Lobectomy | N1 | Station 12: 1/7 | Surgery | 1.6 | IIA | None |
Abbreviations: 18FDGPET =Fludeoxyglucose Positron Emission Tomography; CT = Computed Tomography; SSTR imaging = Somatostatin Receptor imaging includes OctreoScan = Octreotide Scan and 68Ga-DOTATATE PET = 68 Gallium-DOTATATE Positron Emission Tomography; VATS = video-assisted thoracic surgery; LUL = Left Upper Lobe; LLL = Left Lower Lobe; RUL = Right Upper Lobe; RML = Right Middle Lobe; RLL = Right Lower Lobe.
Extent of resection included pneumonectomy, lobectomy or sub-lobar resection (i.e. wedge resection).
N1 disease was defined as metastasis in ipsilateral hilar nodes and intrapulmonary lymph nodes, including metastases by direct extension; N2 disease was defined as metastasis in ipsilateral mediastinal lymph nodes.
Lymph node stations and staging were notated according to AJCC version 7.
Highlights.
There is a high rate of lymph node metastases for lung carcinoid tumors
Somatostatin-receptor imaging directionally associated with lymph node metastases
Sampling of ≥ 1 lymph node and tumor recurrence are associated with node involvement
Lymph node positive disease was not associated with the size of the tumor
7. Acknowledgements
This work was supported by Stanford Cancer Institute Research Database (SCIRDB), NCI Cancer Center Support Grant 5P30CA124435 and Stanford NIH/NCRR CTSA Award Number UL1 RR025744.
1 This work was supported by a National Cancer Institute Cancer Center Support Grant (P30CA124435). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
6. Appendices
Table A.1.
68Ga-DOTATATE PET Imaging Findings
| SUV Max Primary Tumor | Location | SUV Max spleen | SUV Tumor-to-Spleen ratio | Tumor Volume (mL) | Suspected Lymp Node Metastases on Imaging | Level of Suspected Lymph node Metastases on Imaging | Lymph Nodes Sampled During Surgery | Pathologic Positive Lymph Nodes | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.7– 3.3 | RUL, LUL | 33.4 | 0.10 | 10.8 | Yes | 4R, 6, 7 | 2R, 4R, 7, 9, 12 | Yes: 4R, 12 |
| 2 | 52.9 | L mainstem bronchus | 24.8 | 2.13 | 1.9 | No | N/A | 5, 6, 7, 9, 10 | None |
| 3 | 75.2 | R bronchus intermedius | 28.1 | 2.67 | 4.6 | Yes | 10R, 4R, 4L, 7 | 4R, 7 | None |
| 4 | 0.7 | RML | 19.3 | 0.04 | 0.1 | No | N/A | 7, 9, 11R, 12 | None |
| 5 | 7.7 | RML | 34.5 | 0.22 | 1.1 | No | N/A | 12 | Yes: 12 |
| 6 | 119.2 | RUL | 35.5 | 3.36 | 5.9 | Yes | 10R, 4R, 2R | 4R, 7, 10 | None |
| 7 | 15.5 | LLL, multifocal | 40.5 | 0.38 | 7.2 | No | N/A | 7, 9, 10, 12 | Yes: 12 |
| 8 | 17 | RML | 25.9 | 0.66 | 8.7 | No | N/A | 4R, 7, 11 | None |
| 9 | 24.9 | LUL | 37.7 | 0.66 | 6.6 | No | N/A | 6, 7 | None |
| 10 | 1.3 | RUL | 22.1 | 0.06 | 0.9 | No | N/A | 2R, 4R, 7, 10, 11 | None |
| 11 | 30.1 | LLL | 25.0 | 1.20 | 3.2 | No | N/A | 7, 9, 10 | None |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None
References
- 1.Dasari Arvind, et al. “Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States.” JAMA oncology 3.10 (2017): 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao James C., et al. “Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study.” The Lancet 387.10022 (2016): 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis William D., et al. “The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification.” Journal of thoracic oncology 10.9 (2015): 1243–1260. [DOI] [PubMed] [Google Scholar]
- 4.Caplin Martyn E., et al. “Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids.” Annals of Oncology 26.8 (2015): 1604–1620. [DOI] [PubMed] [Google Scholar]
- 5.Singh Simron, et al. “Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients With Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines.” Journal of Thoracic Oncology 15.10 (2020): 1577–1598.. [DOI] [PubMed] [Google Scholar]
- 6.Gosain Rohit, et al. “Management of typical and atypical pulmonary carcinoids based on different established guidelines.” Cancers 10.12 (2018): 510. oncology26.8 (2015): 1604–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah Manisha H., et al. “NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018.” Journal of the National Comprehensive Cancer Network 16.6 (2018): 693–702. [DOI] [PubMed] [Google Scholar]
- 8.Öberg Kjell, et al. “Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.” Annals of oncology 23.suppl_7 (2012): vii120–vii123. [DOI] [PubMed] [Google Scholar]
- 9.Edge Stephen B., and Compton Carolyn C.. “The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM.” Annals of surgical oncology 17.6 (2010): 1471–1474. [DOI] [PubMed] [Google Scholar]
- 10.Rusch Valerie W., et al. “The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer.” Journal of thoracic oncology 4.5 (2009): 568–577. [DOI] [PubMed] [Google Scholar]
- 11.Miyauchi Eisaku, et al. “Distinct characteristics of small cell lung cancer correlate with central or peripheral origin: subtyping based on location and expression of transcription factor TTF-1.” Medicine 94.51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huag Alexander, et al. “68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin-receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors.” Journal of nuclear medicine 51.9 (2010): 1349–1356 [DOI] [PubMed] [Google Scholar]
- 13.Tirosh Amit, et al. “Prognostic Utility of Total 68Ga-DOTATATE-Avid Tumor Volume in Patients with Neuroendocrine Tumors.” Gastroenterology (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon Ji Yoon, et al. “Evaluation of the prognostic significance of TNM staging guidelines in lung carcinoid tumors.” Journal of Thoracic Oncology 14.2 (2019): 184–192. [DOI] [PubMed] [Google Scholar]
- 15.Filosso Pier Luigi, et al. “Multidisciplinary management of advanced lung neuroendocrine tumors.” Journal of thoracic disease 7.Suppl 2 (2015): S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raz Dan J., et al. “Natural History of Typical Pulmonary Carcinoid Tumors: A Comparison of Non surgical and Surgical Treatment.” Chest 147.4 (2015): 1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Yuste Mariano, et al. “Typical and atypical carcinoid tumours: analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung.” European Journal of Cardio-Thoracic Surgery 31.2 (2007): 192–197 [DOI] [PubMed] [Google Scholar]
- 18.Steuer Conor E., et al. “Atypical carcinoid tumor of the lung: a surveillance, epidemiology, and end results database analysis.” Journal of Thoracic Oncology 10.3 (2015): 479–485. [DOI] [PubMed] [Google Scholar]
- 19.Kneuertz Peter J., et al. “Incidence and Prognostic Significance of Carcinoid Lymph Node Metastases.” The Annals of thoracic surgery 106.4 (2018): 981–988. [DOI] [PubMed] [Google Scholar]
- 20.Wurtz Alain, et al. “Results of systematic nodal dissection in typical and atypical carcinoid tumors of the lung.” Journal of Thoracic Oncology 4.3 (2009): 388–394. [DOI] [PubMed] [Google Scholar]
- 21.Hope Thomas A., et al. “Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors.” Journal of nuclear medicine: official publication, Society of Nuclear Medicine 59.1 (2018): 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albanus Dirk Robert, et al. “Clinical value of 68Ga-DOTATATE-PET/CT compared to stand-alone contrast enhanced CT for the detection of extra-hepatic metastases in patients with neuroendocrine tumours (NET).” European journal of radiology 84.10 (2015): 1866–1872. [DOI] [PubMed] [Google Scholar]
- 23.Anderson Kevin L. Jr, et al. “Adjuvant chemotherapy does not confer superior survival in patients with atypical carcinoid tumors.” The Annals of thoracic surgery 104.4 (2017): 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nussbaum Daniel P., et al. “Defining the role of adjuvant chemotherapy after lobectomy for typical bronchopulmonary carcinoid tumors.” The Annals of thoracic surgery 99.2 (2015): 428–434. [DOI] [PubMed] [Google Scholar]
- 25.Fink Gershon, et al. “Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature.” Chest 119.6 (2001): 1647–1651. [DOI] [PubMed] [Google Scholar]
- 26.Rea Federico, et al. “Outcome and surgical strategy in bronchial carcinoid tumors: single institution experience with 252 patients.” European journal of cardio-thoracic surgery 31.2 (2007): 186–191. [DOI] [PubMed] [Google Scholar]


