Abstract
Diabetic kidney disease remains the leading cause of end‐stage kidney disease and a major risk factor for cardiovascular disease. Large cardiovascular outcome trials and dedicated kidney trials have shown that sodium‐glucose cotransporter (SGLT)2 inhibitors reduce cardiovascular morbidity and mortality and attenuate hard renal outcomes in patients with type 2 diabetes (T2D). Underlying mechanisms explaining these renal benefits may be mediated by decreased glomerular hypertension, possibly by vasodilation of the post‐glomerular arteriole. People with T2D often receive several different drugs, some of which could also impact the renal vasculature, and could therefore modify both renal efficacy and safety of SGLT2 inhibition. The most commonly prescribed drugs that could interact with SGLT2 inhibitors on renal haemodynamic function include renin‐angiotensin system inhibitors, calcium channel blockers and diuretics. Herein, we review the effects of these drugs on renal haemodynamic function in people with T2D and focus on studies that measured glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) with gold‐standard techniques. In addition, we posit, based on these observations, potential interactions with SGLT2 inhibitors with an emphasis on efficacy and safety.
Keywords: calcium channel blockers, diabetic kidney disease, diuretics, RAS inhibitors, renal haemodynamic function, SGLT2 inhibition, type 2 diabetes humans
SUMMARY AT A GLANCE
This invited review describes the renal haemodynamic and protective effects of commonly prescribed drugs in people with type 2 diabetes and their interaction with SGLT2 inhibitors.
1. DIABETIC KIDNEY DISEASE: A ROLE FOR IMPAIRED GLOMERULAR FUNCTION
Diabetic kidney disease (DKD), characterized by increased urinary albumin excretion and/or impaired glomerular filtration rate (GFR), is a common complication of type 2 diabetes (T2D). 1 DKD is the leading cause of kidney failure and a major risk factor for cardiovascular disease (CVD). 2 The pathogenesis of DKD is complex and multifactorial, and is associated with risk factors such as hyperglycaemia, obesity, hypertension as well as dyslipidaemia, as reviewed in detail elsewhere. 2 , 3 , 4 Despite optimal treatment with intensive glycaemic control and inhibitors of the renin‐angiotensin system (RAS), residual risk for remains high, as shown in the STENO‐2 trial. 5 Glomerular hyperfiltration has long been proposed to be a major contributing factor in the pathogenesis of DKD 6 and numerous studies reported on the association between whole‐kidney hyperfiltration and onset and progression of albuminuria. 7 , 8 , 9 In contrast, large‐sized studies that estimated GFR did not detect such an association, which may relate to definitions and methods of renal function measurement. 10 , 11 , 12 While glomerular hyperfiltration is often referred to as elevated whole‐kidney GFR (eg, >2 SD above mean for age and sex), the number of functioning nephrons should also be taken into account, especially in elderly people with T2D. 13 In this setting, glomerular hyperfiltration and increased intraglomerular pressure at the single‐nephron level may occur while whole‐kidney GFR is normal or even reduced due to extensive nephron loss. Thus, they are misclassified as being normofilterers based on their apparently normal whole‐kidney eGFR. 3 , 14 Perhaps the strongest evidence for a role of glomerular hypertension in the pathogenesis of DKD is the fact that interventions that modulate renal haemodynamic function by lowering intraglomerular pressure seem most effective in reducing the DKD burden, including the sodium‐glucose cotransporter (SGLT)2 inhibitors.
2. NEPHROPROTECTION BY SGLT2 INHIBITION: LOWERING OF GLOMERULAR PRESSURE?
SGLT2 inhibitors were initially designed to lower plasma glucose concentrations by blocking reabsorption of filtered glucose in the proximal tubule, thereby inducing glycosuria. 15 The glucose‐lowering efficacy of SGLT2 inhibitors is directly related to GFR and degree of hyperglycaemia. 16 , 17 In addition, SGLT2 inhibitors improve several renal risk factors independent of GFR and degree of hyperglycaemia, including reductions in blood pressure (BP), uric acid, body weight and urinary albumin‐to‐creatinine ratio (UACR). 18 Despite the beneficial effects of SGLT2 inhibition on these renal risk factors, the results from three completed cardiovascular (CV) safety trials with SGLT2 inhibitors in people with T2D 19 , 20 , 21 : EMPA‐REG OUTCOME (empagliflozin), CANVAS Program (canagliflozin) and DECLARE‐TIMI 58 (dapagliflozin) were nevertheless surprising. In these trials (Table 1), SGLT2 inhibition lowered renal endpoints by 44% in people with established atherosclerotic disease (hazard ratio [HR], 0.56; 95% CI, 0.47 to 0.67; P < .0001) and by 46% in people with multiple CV risk factors (HR, 0.54; 95% CI, 0.42‐0.71 < P < .0001). 22 In the dedicated DKD trial CREDENCE (canagliflozin), hard renal outcomes (kidney failure, doubling of serum creatinine, or renal or cardiovascular death) were improved with a relative risk reduction of 34% (HR, 0.66; 95% CI, 0.53‐0.81; P < .001) and corresponding number needed to treat (NNT) of 22 (95% CI, 15‐38). 23
TABLE 1.
Renal outcomes of large trials with SGLT2 inhibitors, RAS blockers and CCB in people with T2D
| Trial | Year | Treatment arms | Patient population | Number of patients | Median follow‐up | Renal outcome |
|---|---|---|---|---|---|---|
| SGLT2 inhibitors | ||||||
| EMPA‐REG OUTCOME 19 | 2015 | Empagliflozin vs placebo | T2D with established CVD | 7020 | 3.1 years | Secondary: composite (macroalbuminuria, dSCr, ESKD, renal death); HR 0.61; 95% CI 0.53‐0.70 |
| CANVAS Program 20 | 2017 | Canagliflozin vs placebo | T2D who had or were at high risk for atherosclerotic CVD | 10 142 | 3.6 years | Secondary: composite (macroalbuminuria, dSCr, ESKD, renal death); HR 0.58; 95% CI 0.50‐0.67 |
| DECLARE‐TIMI 21 | 2018 | Dapagliflozin vs placebo | T2D who had or were at high risk for atherosclerotic CVD | 17 160 | 4.2 years | Secondary: composite (>40% decrease in eGFR to <60 mL/min per 1.73 m 2 , ESKD, renal death); HR 0.53; 95% CI 0.43‐0.66 |
| CREDENCE 23 | 2019 | Canagliflozin vs placebo | T2D + nephropathy | 4401 | 2.6 years | Composite (dSCr, ESKD, renal death); HR 0.66; 95% CI 0.53‐0.81 |
| RAS inhibitors | ||||||
| FACET 47 | 1997 | Amlodipine vs fosinopril | Hypertensive T2D | 380 | 3.5 years |
Albuminuria change from baseline: Fosinopril −8%; 95% CI ‐11 to −5 |
| MICRO‐HOPE 48 | 2000 | Ramipril vs placebo | T2D ± microalbuminuria | 3577 | 4.5 years | Overt nephropathy; RRR 24%; 95% CI 3‐40 |
| IDNT 49 | 2001 | Irbesartan vs amlodipine vs placebo | T2D + nephropathy | 1715 | 2.6 years | Composite: (dSCr, ESKD, death from any cause); RR 0.80; 95% CI 0.66‐0.97) vs placebo; RR 0.77; 95% CI 0.63‐0.93) vs amlodipine |
| IRMA‐2 50 | 2001 | Irbesartan vs placebo | T2D + microalbuminuria | 590 | 2 years |
Time to onset of diabetic nephropathy: 150 mg; HR 0.61; 95% CI 0.34‐1.08 300 mg; HR 0.30; 95% 0.14‐0.61 |
| RENAAL 51 | 2001 | Losartan vs conventional therapy | T2D + nephropathy | 1513 | 3.4 years | dSCr; RRR 25%; 95% CI 8‐39ESKD; RRR 27%; 95% CI 11‐42 |
| BENEDICT‐A 52 | 2004 | Trandolapril vs verapamil vs combination vs placebo | Hypertensive T2D | 1204 | 3.6 years |
Time to onset of microalbuminuria: Trandolapril monotherapy; HR 0.47; 95% CI 0.26‐0.83 Trandolapril + verapamil; HR 0.39; 95% 0.19‐0.80 |
| BENEDICT‐B 53 | 2011 | Trandolapril vs trandolapril/verapamil | Hypertensive T2D + microalbuminuria | 281 | 4.5 years | Trandolapril normalized albuminuria independent of verapamil |
| ADVANCE 54 | 2008 | Perindopril/indapamide vs placebo | T2D + micro or macrovascular disease or risk factor | 11 140 | 4.3 years | Risk of new or worsening nephropathy; RRR 18%; 95% Ci −1‐32; time to onset of microalbuminuria RRR 21%; 95% CI 14‐27 |
| ROADMAP 55 | 2011 | Olmesartan vs placebo | T2D + preserved kidney function | 4447 | 3.2 years | Time to onset of microalbuminuria; HR 0.77; 95% CI 0.63‐0.94 |
| VA NEPHRON D 56 | 2013 | Losartan + lisinopril vs monotherapy | T2D + macroalbuminuria | 1448 | 2.2 years | Prematurely stopped owing to safety concerns |
| CCB | ||||||
| FACET 47 | 1997 | Amlodipine vs fosinopril | Hypertensive T2D | 380 | 3.5 years |
Albuminuria change from baseline: Amlodipine: −11, 95% CI ‐14 to −8 |
| IDNT 49 | 2001 | Amlodipine vs Irbesartan vs placebo | T2D + nephropathy | 1715 | 2.6 years | Composite: (dSCr, ESKD, death from any cause); RR 1.04 95% CI 0.86‐1.25) vs placebo |
| BENEDICT‐A 52 | 2004 | Verapamil vs trandolapril vs combination vs placebo | Hypertensive T2D | 1204 | 3.6 years |
Time to onset of microalbuminuria: Verapamil monotherapy; HR 0.83; 95% CI 0.45‐1.51 |
| BENEDICT‐B 53 | 2011 | Trandolapril vs trandolapril/verapamil | T2D ± microalbuminuria | 281 | 4.5 years | Verapamil added on trandolapril did not improve renal outcomes compared to trandolapril or placebo |
Abbreviations: CCB, calcium channel blocker; CVD, cardiovascular disease; dSCr, doubling of serum creatinine; eGFR estimated glomerular filtration rate; ESKD, end‐stage kidney disease; P/I perindopril/indapamide; RAS renin‐angiotensin system; SGLT‐2 sodium glucose cotransporter‐2; T2D type 2 diabetes; UACR, urinary albumin creatinine ratio.
The mechanisms by which SGLT2 inhibitors attenuate renal events remain under extensive discussion and have been reviewed in detail elsewhere. 24 One of the haemodynamic factors that could impact renal outcomes is a reduction in blood pressure. SGLT2 inhibitors lower blood pressure in hypertensive adults with T2D regardless of baseline renal function. 25 Accordingly, the blood pressure‐lowering effects are preserved in the setting of impaired renal function in several separate analyses in T2D patients. 26 Until now, it is not yet established how blood pressure lowering occurs with SGLT2 inhibition. Yet, several factors may be involved, including natriuresis, osmotic diuretic effects and a contraction in plasma volume. 27 Other factors beyond renal haemodynamic actions have also been proposed to underlie the renoprotective effects of SGLT2 inhibitors, including reduction of serum uric acid, amelioration of renal hypoxia and reductions in glucose levels, however, this is beyond the scope of this review.
Besides blood pressure lowering, a reduction in intraglomerular pressure remains a leading hypothesis for renal protection. This is due to the observation that in clinical practice, SGLT2 inhibitors induce an acute ‘dip’ in GFR in people with T2D, an effect that occurs even after a single dose, 28 and is reversible after drug discontinuation. 31 It has been hypothesized that the drop in GFR is caused by an activation of tubuloglomerular feedback (TGF) response due to higher presence of sodium and chloride at the level of the macula densa. In rodent models of type 1 diabetes (T1D) and in people with T1D with glomerular hyperfiltration (GFR > 135 mL/min/1.73 m2) this led to vasoconstriction of the preglomerular arteriole and GFR lowering. 29 , 30 , 31 , 32 On the other hand, in people with T2D that were older, similar decreases in GFR were induced by post‐glomerular vasodilatory effects in two separate studies rather than preglomerular vasoconstriction (Figure 1), indicating genuine renal haemodynamic differences between people with T1D and T2D as well as different effects of SGLT2 inhibition. 33 , 34 , 35 As such, these studies showed a reduction in renal vascular resistance (RVR) and post‐glomerular resistance, while ERPF was not reduced. The results of these studies in adults with T2D were unexpected, as post‐glomerular vasodilation has been linked to RAS inhibitors with respect to renal haemodynamic actions, contributing to their renal protective properties (as discussed below).
FIGURE 1.
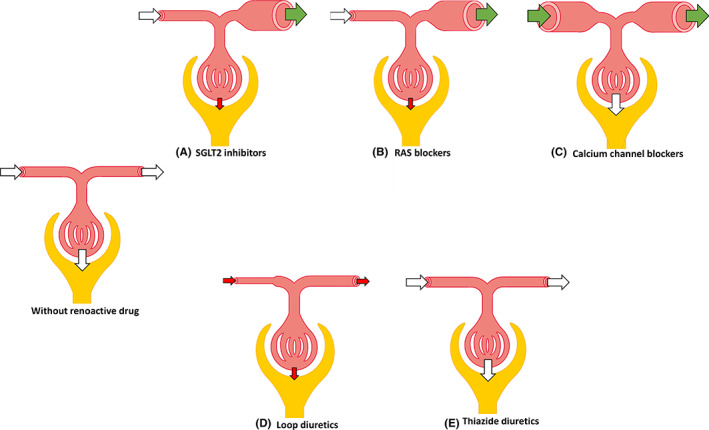
Evidence of different renoactive drugs on kidney haemodynamics in people with type 2 diabetes. A, SGLT2 inhibitors, B, RAS blockers, C, calcium channel blockers, D, loop diuretics, E, thiazide diuretics. White arrows indicate no change in glomerular resistance or GFR, green arrows indicate reduction in glomerular resistance, red arrows indicate increase in glomerular resistance or reduction in GFR. GFR glomerular filtration rate; RAS renin‐angiotensin system; SGLT2, sodium‐glucose cotransporter 2
3. RENAL HAEMODYNAMIC EFFECTS OF SGLT2 INHIBITION: INTERACTION WITH OTHER DRUGS
A logical question that arises from the previous observations, is how SGLT2 inhibitors might interact with RAS inhibitors regarding renal haemodynamic function, efficacy and safety; questions that could also be broadened to other commonly‐prescribed medication. 36 In a recent post‐hoc analysis of the EMPA‐REG OUTCOME study, Mayer et al investigated whether relevant interactions between SGLT2 inhibitor empagliflozin and RAS inhibitors, calcium channel blockers (CCB) and diuretics occurred. 36 They reported no significant interactions between these drugs with respect to efficacy and safety, however, the study had several limitations including its post‐hoc and exploratory design, small subgroups and potential bias by indication. 37
When assessing potential interactions with respect to renal haemodynamic actions of different drugs in people with T2D, it is important to understand the actions of each individual drug class, specifically studied in this population, as baseline characteristics strongly drive response to drug therapy. 35 , 38 In previous literature, many conclusions regarding the renal haemodynamic actions of drug classes have been based on data from animal studies or from human studies comprising different populations, including adults with hypertension or T1D. 37 In addition, not all studies have assessed renal haemodynamic function with gold standard methods for GFR and ERPF. Thus, in the present review, we critically review the effects of drugs commonly prescribed to people with T2D, including angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), CCBs and diuretics, on renal haemodynamic function. Although other actions of SGLT2 inhibitors might be involved in the cardiorenal protection of these drugs, this was beyond the scope of this review. We focused only on clinical studies in adults with T2D in whom renal haemodynamic function was assessed with one of the following gold‐standard substances: for GFR: iohexol, inulin, thiosulfate sodium, iothalamate, 99mTc‐diethylenetriaminepentaacetic acid (DTPA), 51 Cr‐ethylenediaminetetraacetic acid (EDTA), technetium pentetate and for ERPF: para‐aminohippuric acid (PAH), iodohippurate, 131‐I hippuran, 123‐I orthohippurate or 125‐I iodohippurate. Based on these data, we speculate on interactions with SGLT2 inhibitors, both concerning efficacy and safety.
4. RAS INHIBITORS
The renin‐angiotensin‐aldosterone system (RAAS) regulates blood volume, blood pressure, electrolyte balance, as well as systemic vascular resistance and is mainly activated in times of hypoperfusion as measured by the juxtaglomerular cells and baroreceptors in the carotids and aortic branch. 39 Through renin secretion and modulation by ACE, the important effector of the RAAS angiotensin (Ang)‐II is activated, leading to vasoconstriction and aldosterone secretion, which stimulates sodium retention. 40 , 41 , 42 Furthermore, experimental studies showed that Ang‐II plays an important role in the regulation of GFR and renal blood flow (RBF) by constricting renal arterioles. 43 , 44 Although both pre‐ and post‐glomerular arterioles are constricted by Ang‐II, the efferent arteriole has a greater increase in resistance due to a smaller basal diameter. 45 The three major classes of drugs that target the RAAS are the ACE inhibitors, the ARB and aldosterone antagonists. ACE inhibitors block the enzyme ACE and therefore reduce the production of Ang‐II. 46 In contrast to the ACE inhibitors, ARB do not reduce Ang II concentrations, but produce a selective, dose‐dependent blockade of the AT1 receptor, independently of the non‐ACE pathways of Ang‐II generation. 42 Despite these theoretical differences, the net effect of both ACE inhibitors and ARB is a decrease in total body sodium, total body water and vascular tone, resulting in comparable clinical actions. 46 As there is no information on interaction between SGLT2 inhibitors and aldosterone antagonists (see the section on potassium‐sparing diuretics), we will focus on interaction between SGLT2 inhibitors with ACE inhibitors and ARB, collectively termed RAS inhibitors.
4.1. Results of RAS inhibitors on renal outcome trials and actions on renal haemodynamic function
Based on their interference with Ang‐II production or blockade of the receptor, RAS inhibitors lower blood pressure effectively. In line with this reduction, these agents also reduce CV morbidity and mortality. In addition, in several trials they were shown to improve renal outcomes as well (Table 1). Since the beneficial renal effects are beyond the impact of blood pressure lowering, RAS inhibitors are suggested to exert beneficial pleiotropic renal effects which have also been linked to a renal haemodynamic phenomenon, in line with the biological effects of Ang‐II activation. 57 , 58 Given the actions of Ang‐II, post‐glomerular vasodilation reported in experimental research has been widely thought to contribute to these renoprotective effects. Indeed, data from studies have demonstrated that treatment with RAS inhibitors is associated with an initial fall in eGFR or increase in serum creatinine concentrations in adults with T2D, 59 , 60 but whether this reduction in GFR is relevant has been debated ever since. A post‐hoc analysis conducted by Clase et al showed an association between an acute RAS‐induced GFR decline over the first 2 weeks and higher risk of doubling of creatinine or dialysis, and did not support the long‐term stabilization of renal function. 61 Notably, the degree of GFR decline appears to be clinically relevant as well. As such, two meta‐analyses reported that the risk of developing kidney failure was higher in patients with a larger estimated GFR decline compared to a smaller or no estimated GFR decline. 62 , 63 In contrast, other studies found that in patients using RAS inhibitors a large initial fall in eGFR showed a more stable long‐term estimated GFR course compared with patients with a moderate fall or an increase in initial eGFR. 64 , 65 Indeed, since this drop has been found to be reversible after discontinuation of the drug, there is no doubt that this phenomenon is of haemodynamic origin due to reduction of intraglomerular pressure rather than treatment‐induced damage to functioning nephrons. 66 , 67
The impact of RAS inhibitors on renal haemodynamic function in people with T2D has been investigated in at least 15 studies using gold‐standard methods, as shown in Table 2. The majority of the included studies investigated ACE inhibitors, only two studies were using ARB as investigational product. The study population varied between participants who were normotensive with normoalbuminuria and participants with hypertension and macroalbuminuria, and the number of participants as well as the study duration also differed widely among the included trials. Despite this heterogeneity, all studies showed a reduction or no significant change in GFR, beside two studies that reported an increase in GFR, possibly attributed to the opening of additional glomeruli and capillary loops or increased flow. 68 , 75 Nearly, all studies showed a reduction in mean arterial pressure (MAP). If GFR would have been reduced by an increase in preglomerular resistance, an increase in RVR would have been expected. However, most studies showed either a reduction or no change in renal vascular resistance (RVR), without decreasing renal RBF. Thus, these results seem to support the proposed mechanism of a post‐glomerular vasodilative effect of RAS inhibitors in people with T2D (Figure 1).
TABLE 2.
Effect of ACE inhibitors and ARB on renal haemodynamic function in people with T2D measured with gold‐standard methods
| Effect of RAS blocker | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Treatment arms | Patient population | Intervention | Method (GFR/ERPF) | MAP | GFR | ERPF/RBF | RVR |
| Price et al 68 | 1999 | Irbesartan vs placebo | Hypertensive T2D + macroalbuminuria (n = 12) | Single dose | Inulin/PAH | ↓ | ↔ | ↑ | ↔ |
| De'Oliveira et al 69 | 1997 | Enalapril vs placebo | Hypertensive T2D (n = 19) | 3 days | Inulin/PAH | ↓ | ↔ | ↑ | ↔ |
| New et al 70 | 1998 | Trandolapril vs placebo | Normotensive T2D (n = 29) | 10 days | 51Cr‐EDTA/125‐I iodophippurate |
0.5 mg: ↔ 4.0 mg: ↔ |
↔ ↔ |
↔ ↔ |
↓ a ↑ a |
| Stornello et al 71 | 1989 | Captopril vs nicardipine vs both | Hypertensive T2D + macroalbuminuria (n = 12) | 4 weeks cross‐over | 99mTc‐DTPA/I‐131 hippuran | ↓ | ↔ | ↔ | ↓ |
| Baba et al 72 | 1989 | Enalapril vs nicardipine | T2D + microalbuminuria (n = 7) | 4 weeks cross‐over | Thiosulfate sodium/PAH | ↓ | ↔ | ↔ | ↔ |
| Ruggenenti et al 73 | 1999 | Perindopril vs nitrendipine | Hypertensive T2D + macroalbuminuria (n = 9) | 10 weeks cross‐over | Inulin/PAH | ↓ | ↔ | ↔ | ↔ |
| Fliser et al. 74 | 2005 | Olmesartan vs placebo | Normotensive T2D (n = 35) | 12 weeks | Inulin/PAH | ↓ | ↔ | ↑ | ↓ |
| Marre et al 75 | 1987 | Enalapril vs placebo | Normotensive T2D ± microalbuminuria (n = 20) | 6 months | Iothalamate/ I‐131 hippuran | ↓ | ↑ | ↑ | ↓ |
| Valvo et al 76 | 1988 | Captopril vs placebo | Hypertensive T2D + microalbuminuria (n = 12) | 6 months | Iothalamate/ I‐131 hippuran | ↔ | ↔ | ↔ | ↔ |
| Romero et al 77 | 1992 | Captopril vs nifedipine | T2D + macroalbuminuria (n = 20) | 6 months | Iothalamate/ I‐131 hippuran | ↓ | ↓ | ↔ | ↓a |
| Bakris et al 78 | 1992 | Lisinopril vs verapamil vs both vs hydrochloorthiazide | T2D + macroalbuminuria (n = 30) | 12 months | 99mTc‐DTPA /PAH | ↓ | ↓ | ↑ | ↓a |
| Capek et al 79 | 1994 | Captopril vs placebo | T2D + microalbuminuria (n = 15) | 12 months | 61Cr‐EDTA/123‐I orthohippurate | ↔ | ↔ | ↔ | ↔ a |
| Vora et al 80 | 1996 | Captopril vs placebo | T2D + microalbuminuria (n = 8) | 12 months | 51Cr‐EDTA/125‐I iodophippurate | ↓ | ↔ | ↔ | ↓ a |
| Ruggenenti et al. 81 | 1994 | Enalapril vs nitrendipine | Hypertensive T2D + biopsy proven nephropathy (n=16) | 98 days and 1 year | Inulin/PAH |
Short term: ↓ Long term: ↓ |
↔ ↑ |
↔ ↔ |
NA NA |
| Slataper et al 82 | 1993 | Lisinopril vs diltiazem vs furosemide + atenolol | Hypertensive T2D (n = 30) | 18 months | Technetium pentetate/ Iodohippurate | ↓ | ↔ | ↔ | ↓ a |
Abbreviations: ACE angiotensin‐converting enzyme, ARB angiotensin II receptor blocker, T2D type 2 diabetes, GFR glomerular filtration rate, ERPF effective renal plasma flow, RAS renin‐angiotensin system, MAP mean arterial pressure, RVR renal vascular resistance, DTPA diethylenetriaminepentaacetic acid, PAH para‐amino hippuric acid, EDTA ethylenediamineetetraacetic acid, NA not able to calculate.
RVR not reported in manuscript, manually calculated as follows: MAP divided by RBF (not statistically tested).
Only abstract available.
4.2. Combining RAS inhibitors and SGLT2 inhibitors
Two mechanistic trials investigating renal haemodynamic effects of SGLT2 inhibition using gold standard techniques in people with T2D both suggested post‐glomerular vasodilation (ie, a reduction in GFR without increasing RVR). 33 , 34 As there is enough evidence confirming the proposed mechanism of RAS inhibitors in people with T2D, combining these two drugs might possibly have an additive effect regarding renal haemodynamic function, as they both would exert their effect on the efferent arteriole. On the other hand, one could hypothesize that due to overlapping pathways the effects of SGLT2 inhibitors on a RAS inhibitor background are reduced, which would be called sub‐additive. However, in both a mechanistic clinical trial and in a post‐hoc analysis investigating the effect of SGLT2 inhibitors on cardiorenal risk factors in people with T2D with and without RAS inhibitors, the results were not modulated by the use of RAS inhibitors, suggesting the pre‐SGLT2 inhibitor effect of RAS inhibitors to be much less. 33 , 83 Similar observations were performed in the large outcome trials and outcomes were not affected by concomitant RAS treatment. However, most patients used RAS inhibitors at baseline (>90%). Consequently, it is difficult to conclude from the outcome trials what the precise effects of SGLT2 inhibition monotherapy are and whether there are relevant interactions when using both drugs. Therefore, trials comparing and/or combining both renoactive drugs (eg, NCT04238702 and NCT03078101) are necessary to put the beneficial effects seen in the outcome trials into perspective. Finally, no additional safety findings were reported in patients treated with SGLT2 inhibitors and RAS inhibitors, such as acute kidney injury (AKI), which seems encouraging from a safety perspective.
5. CALCIUM CHANNEL BLOCKERS
Calcium channel blockers inhibit the inward movement of extracellular calcium through ion‐specific channels that span the cell membrane. Various types of such channels have been identified, however, almost all of them preferentially or exclusively block the L‐type voltage‐gated calcium channel. The two major classes of L‐type‐selective CCB are the non‐dihydropyridines (eg, verapamil, diltiazem) and the dihydropyridines (eg, amlodipine, nifedipine, lercanidipine). When the influx of calcium is blocked, vascular smooth muscle cells relax, resulting in vasodilation and lowering of blood pressure and heart rate. 84
5.1. Results of CCB on renal outcome trials in people with T2D and their actions on renal haemodynamic function
Currently, ACE inhibitors and ARB have been the first choice for treatment of hypertension in people with diabetes or DKD. However, most of these patients require two or more drugs to optimize blood pressure levels, and therefore CCB, are suggested as add‐on therapy, mainly because of their strong BP‐lowering properties and good tolerability. 85 , 86
In particular non‐dihydropyridines CCB (ndCCB) are associated with a reduction in proteinuria by 30%, independent of blood pressure lowering, compared to baseline in hypertensive patients with proteinuric renal disease. 87 However, the use of CCB to reduce albuminuria in DKD has been discussed, since different results regarding the effect on renal outcomes in people with T2D have been observed (Table 1). The renoprotective effect of RAS inhibitors does not seem to be enhanced by the addition of verapamil, as shown in the BENEDICT study. 52 In this primary prevention trial, trandolapril plus verapamil and trandolapril alone decreased the incidence of microalbuminuria to a similar extent. However, the TRAVEND study, where the effect of antihypertensive combinations on metabolic control (HbA1c and blood glucose levels) and albuminuria in people with T2D was investigated, showed that the combination of verapamil plus trandolapril allowed better metabolic control than enalapril plus hydrochlorothiazide (HCTZ). 88
These uncertain renoprotective effects may be due to the presence of L‐type calcium channels at the afferent but not efferent arterioles of the kidney. 89 L‐type CCB cause afferent vasodilation which would theoretically increase GFR and intraglomerular pressure in case of unaffected efferent resistance. This adverse action counteracts their ability to attenuate glomerular hypertension through the systemic reduction in BP and therefore may not always be beneficial in people with CKD. 90 On the other hand, other CCB, such as mibefradil and efonidipine, block both T‐ and L‐types calcium channels and dilate both afferent and efferent arterioles. 91 In addition, combined L‐/N‐type CCB, such as cilnidipine, inhibit norepinephrine release from the sympathetic nerve terminal by blockade of N‐type calcium channels which innervates both afferent and efferent arterioles, resulting in a decrease in intraglomerular pressure as demonstrated in rat kidney arterioles. 92 , 93
At least 10 studies using gold‐standard methods have investigated renal haemodynamic function effects of CCB in people with T2D, as shown in Table 3. The patient populations varied widely among the studies, as well as the used CCB and study duration. In general, the majority of the trials did not report significant changes in GFR and RBF, while all showed significant reductions in both MAP and RVR, suggesting concomitant post‐glomerular dilation to maintain stable glomerular pressure (Figure 1). As these trials only used L‐type CCB, these findings are in contrast with the hypothesized mechanism of action, that is, selective preglomerular vasodilation, since, in case of unchanged efferent vascular resistance, an increase in GFR and RBF then would have been expected. 97 Only two studies indeed reported an increase in GFR and RBF and reduction in MAP and RVR. 81 , 95
TABLE 3.
Effect of CCBs on renal haemodynamic function in people with T2D measured with gold‐standard methods
| Effect of CCB | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Treatment arms | Patient population | Intervention | Methods (GFR/ERPF) | MAP | GFR | ERPF/RBF | RVR |
| Baba et al 72 | 1989 | Enalapril vs nicardipine | T2D + microalbuminuria (n = 7) | 4 weeks cross‐over | Thiosulfate sodium/PAH | ↓ | ↔ | ↔ | ↓ |
| Stornello et al 71 | 1989 | Captopril vs nicardipine vs both | Hypertensive T2D + macroalbuminuria (n = 12) | 4 weeks cross‐over | DTPA/I‐131 hippuran | ↓ | ↔ | ↔ | ↓ |
| Baba et al 94 | 1990 | Nicardipine vs placebo | Hypertensive T2D ± microalbuminuria or overt nephropathy (n = 18) | 4 weeks | Thiosulfate sodium/PAH |
Normoalbuminuria: ↓ Microalbuminuria:↓ Overt nephropathy: ↓ |
↔ ↔ ↔ |
↔ ↔ ↑ |
↓ ↓ ↓ |
| Ruggenenti et al. 73 | 1999 | Perindopril vs nitrendipine | Hypertensive T2D + macroalbuminuria (n = 9) | 10 weeks cross‐over | Inulin/PAH | ↓ | ↔ | ↔ | ↔ |
| Baba et al 95 | 1986 | Nicardipine vs placebo | Hypertensive T2D + mild‐to‐moderate or severe DN (n = 12) | 6 months | Thiosulfate sodium/PAH |
Mild‐to‐moderate DN: ↓ Severe DN: ↓ |
↑ ↔ |
↑ ↔ |
↓ ↔ |
| Romero et al 77 | 1992 | Captopril vs nifedipine | T2D + macroalbuminuria (n = 20) | 6 months | Iothalamate/I‐131 hippuran | ↓ | ↔ | ↔ | ↔ a |
| Ruggenenti et al 81 | 1994 | Enalapril vs nitrendipine | Hypertensive T2D + biopsy proven nephropathy | 98 days and 12 months | Inulin/PAH |
Short term: ↓ Long term: ↓ |
↔ ↑ |
↔ ↑ |
NA NA |
| Bakris et al 78 | 1992 | Lisinopril vs verapamil vs both vs HCTZ | T2D + macroalbuminuria (n = 30) | 12 months | DTPA/PAH | ↓ | ↔ | ↔ | ↓ a |
| Slataper et al 82 | 1993 | Lisinopril vs diltiazem vs furosemide + atenolol | Hypertensive T2D (n = 30) | 18 months | Technetium pentetate/iodohippurate | ↓ | ↔ | ↔ | ↓ a |
| Smith et al 96 | 1998 | Diltiazem vs nifedipine | Hypertensive T2D + macroalbuminuria (n = 21) | 21 months | Inulin/PAH | Both: ↓ | ↔ | ↔ | ↓ a |
Abbreviations: CCB calcium channel blocker, T2D type 2 diabetes, GFR glomerular filtration rate, ERPF effective renal plasma flow, PAH para‐amino hippuric acid, MAP mean arterial pressure, RVR renal vascular resistance, DTPA diethylenetriaminepentaacetic acid, HCTZ hydrochlorothiazide, NA not able to calculate.
RVR not reported in manuscript, manually calculated as follows: MAP divided by RBF (not statistically tested).
Only abstract available.
5.2. The potential combination of CCB and SGLT2 inhibitors
One could speculate that L‐type CCB might counteract the beneficial renal haemodynamic effects caused by SGLT2 inhibitors due to preglomerular vasodilation counteracting the effects of SGLT2 inhibition to reduce GFR, however, CCB's have shown mostly neutral effects on GFR due to concomitant post‐glomerular vasodilation. Of course, the small sample sizes and fairly different study designs have to be taken into account when making conclusions in this regard. Moreover, the individual effect of ndCCB, alone or in combination with SGLT2 inhibitors, is likely to be small, since the effect of SGLT2 inhibition on renal haemodynamics was not modulated by CCB and data suggest that the effect of CCB on renal outcomes is modest. 33 , 36
6. DIURETICS
6.1. Proposed mechanism of action
Diuretics are a heterogeneous group of drugs, and with the exception of vasopressin receptor antagonists and mannitol, their primary action is to impair sodium reabsorption at different sites along the tubule, thereby increasing urinary excretion of sodium and chloride and inducing increased water excretion. 98 , 99 Different types of diuretics are available and this review will focus on loop, thiazide and potassium‐sparing diuretics (PSD).
Loop diuretics, such as furosemide, act on the Na‐K‐2Cl (NKCC2) co‐transporter type 2 at the apical surface of the thick ascending limb cells along the loop of Henle. This transporter reabsorbs up to 25% of filtered salt and its blockade is responsible for most natriuretic effects of loop diuretics. 100 Besides their natriuretic effect, however, it is thought that the blood pressure‐lowering effect caused by loop diuretics may be, at least partly, due to a direct vasodilative effect. 101
Thiazide and related diuretics inhibit sodium reabsorption by blocking the sodium‐chloride co‐transporter (NCC) transporter on the apical membrane in the proximal segment of the distal convoluted tubule (DCT), 62 , 63 where approximately 5% to 10% of the filtered sodium normally is reabsorbed. 102
Potassium‐sparing diuretics were developed to reduce the risk of cardiac arrhythmias, which can be induced by loop and thiazide diuretics due to increased potassium excretion. 99 These drugs either compete with aldosterone for intracellular cytoplasmic receptor sites (eg, spironolactone, eplerenone and finerenone), or directly block epithelial sodium channels (ENaC) in the distal parts of the nephron (eg, amiloride). 103
6.2. Results of diuretics on renal outcome trials in people with T2D and their actions on renal haemodynamic function
Diuretics play a major role in the treatment of hypertension and heart failure (HF), 104 two common comorbidities associated with T2D and CKD, 105 , 106 and are used either as monotherapy or as part of any combined therapy. 107 Thus far, there is no evidence from large outcome studies or meta‐analyses that diuretics are superior to other classes of antihypertensive drugs in reducing CV risk in hypertensive individuals and potential benefits in people with T2D or renal failure remain unclear. 108 Yet, in a post‐hoc analysis of the ALLHAT study, data could be extracted from a T2D subgroup, which showed no significant differences in the incidence of total mortality or kidney failure for patients assigned to thiazide‐type diuretic chlorthalidone compared to amlodipine or lisinopril. 109 In another study, albuminuria appeared more reduced in patients treated with ACE inhibitor/diuretic (benazepril/HCT) group compared to the ACE inhibitor/CCB (benazepril/amlodipine) group, but this difference was explained by a significantly greater fall in GFR in the benazepril/HCTZ group, resulting in a relatively greater fall in albuminuria. 110
Steroidal mineralocorticoid receptor antagonists (MRA), such as spironolactone and eplerenone, are highly efficacious for further reducing albuminuria when added to RAS inhibitors. 111 , 112 , 113 Blockade of mineralocorticoid receptors has proven clinical efficacy in patients with HF with reduced ejection fraction (HFrEF), hypertension and in CKD with or without T2D. 114 , 115 , 116 However, steroidal MRAs can cause significant elevation of serum potassium. 117 , 118 Therefore, novel, selective non‐steroidal MRAs, such as finerenone, are being developed and revealed a lower risk for hyperkalaemia. Finerenone has had promising results with respect to renal endpoints in phase 2 trials. The minerAlocorticoid Receptor Antagonist Tolerability Study (ARTS) found a reduction in UACR in patients with HFrEF, while adverse events were mild. The next, phase 2b, ARTS‐Diabetic Nephropathy trial showed a dose‐dependent reduction in UACR, with the largest reduction (−48%) in the group receiving 20 mg. Currently, two large renal/CV outcome trials are conducted in patients with or without T2D, the FIDELIO and FIGARO trials. 119 , 120
However, whether diuretics per se alter renal haemodynamics in people with T2D is not completely understood as only limited data are available. To our knowledge, only two studies have investigated the long‐term effects of diuretic drugs on renal haemodynamic function using gold‐standard methods. The first study, conducted in 1993 by Slataper et al, 82 investigated the effect of loop diuretic furosemide in 30 hypertensive people with T2D, and showed a decrease in GFR, RPF and MAP after 18 months of treatment (Figure 1). As the NKCC2 transporter is located at the luminal membrane of the macula densa cells, stimulating renin secretion, 121 these results imply a tubuloglomerular feedback (TGF) response of the kidney in these individuals, which also has been reported by others, 122 but would not have been expected. These effects may be both harmful and beneficial, since elevated plasma renin activity increases angiotensin II, while blocking TGF helps to preserve GFR in individuals with glomerular hyperfiltration. 123 In the second study, thiazide‐type diuretic, HCTZ, did not alter GFR and RPF, while MAP was decreased, in 30 people with T2D after 12 months of treatment. 78 These results are in line with the proposed mechanism of action, since the NCC transporter is located distally to the macula densa, which would not impair the TGF response by blockade of this transporter (Figure 1). 122
The effect of potassium‐sparing diuretics on renal haemodynamic function has not been assessed in people with T2D.
6.3. The potential combination of diuretics and SGLT2 inhibitors
As stated previously, the underlying mechanisms for the renal benefits of SGLT2 inhibitors remain unclear, but the same can be stated for their beneficial effects on HF outcomes. 19 , 20 , 21 , 124 A central hypothesis is that SGLT2 inhibitors induce systemic haemodynamic actions including a diuretic effect, secondary to glycosuria and natriuresis, leading to contraction of plasma and possibly interstitial volume. Therefore, given that both drugs induce natriuresis, interactions could be plausible. Indeed, in the CANVAS program, a marked reduction in the composite CV endpoint was reported with canagliflozin in patients using diuretics at baseline compared to no reduction in patients without a diuretic background therapy. 125 In contrast, although results of the DECLARE‐TIMI 58 trial also suggest a potential interactive effect of diuretics, the observed renal benefits appeared to be the greatest among those who were not on diuretics at baseline. 126 These results are in contrast with findings of the EPMA‐REG OUTCOME trial, in which reductions in HF or renal outcomes were irrespective of diuretic use at baseline. 46 However, the multiple and post‐hoc analyses performed for these trials had limited statistical power to test for interactions, and the risk of missing real differences or observing spurious chance differences is high. Therefore, the clinical significance of these differences remains unclear and these post‐hoc subgroup analyses were exploratory at the most.
The effect on volume status of diuretics, alone or compared to SGLT2 inhibitors, in people with T2D remains incompletely understood. A mechanistic study assessed the effects of dapagliflozin, HCTZ and placebo on blood pressure, plasma volume and renal function and concluded that both drugs reduced day‐time blood pressure and GFR to the same extent, whereas only dapagliflozin lowered plasma volume by 7%. 37 Weber et al investigated the blood pressure‐lowering effect of SGLT2 inhibitors in inadequately controlled hypertensive T2D patients and showed that for patients already taking RAS inhibitors and diuretics, the reduction in placebo‐adjusted blood pressure with dapagliflozin was smaller compared with those receiving RAS inhibitors and beta‐blockers or CCB. 127 Given the view that SGLT2 inhibitors have diuretic‐like properties, this finding may have been anticipated, since the additive effect of this drug in patients already receiving thiazide diuretics might be modest. In addition, combining two drugs with diuretic‐like properties and a different mechanism (eg, SGLT2 inhibitors and loop diuretics) could increase the risk for volume depletion, although proof for this potential interaction is currently lacking. SGLT2 inhibitors seem to reduce the risk for AKI across trials 128 , 129 and no interaction with diuretic use has been observed. 46 The recently published RECEDE‐CHF trial investigated the diuretic and natriuretic effect of empagliflozin in combination with loop diuretics in patients with T2D and HF and showed a significant increase in 24‐hour urine volume at day 3 and week 6, while the combined effects on renal haemodynamic function remain unknown as measured GFR and effective renal plasma flow have not been measured. 130 , 131
7. CONCLUSIONS AND FUTURE PERSPECTIVES
Inhibitors SGLT2and RAS are the most effective renoprotective drugs and have most prominent effects on estimated intraglomerular pressure (Figure 2). However, as most patients with T2D need more than one drug to reach optimal control of renal risk factors, interactions with respect to renal haemodynamic function with different background medication might occur. We have summarized the studies that quantified renal haemodynamic function for the most commonly used drugs in this population. In patients with T2D, SGLT2 inhibitors induce post‐glomerular vasodilation, which also seems the mode of action of RAS inhibitors. Whether this same mechanism of action is favourable or results in lower efficacy is unclear at present. CCB do not seem to strongly affect renal outcomes, while at renal haemodynamic level they seem to induce both pre and post‐glomerular vasodilation, with no net effect on GFR. Although loop diuretic drugs appear to impact TGF and sodium excretion, their interaction with SGLT2 inhibitors seems limited based on available data from large outcome trials. Despite the fact that both MRA and other glucose‐lowering drugs, such as GLP‐1 receptor agonists, might affect renal outcomes, mechanistic studies in combination with SGLT2 in T2D are lacking.
FIGURE 2.
Key messages
Several studies are needed in the future. Mechanistic cross‐over design studies with gold‐standard quantification of renal haemodynamic function may shed light on interactions that these drugs have. Moreover, with the completion of several large‐sized outcome trials, post‐hoc analyses of these studies, despite several limitations generally associated with such analyses, could reveal certain signals. Finally, with the implementation of these drugs in clinical practice, real‐world data could be informative. Ultimately, these studies could (a) help to maximize the benefits of these renoactive drugs by choosing the best regimen for a particular patient and (b) help to identify adverse effects such as AKI, volume depletion and other side effects potentially associated with renal haemodynamic drug actions.
CONFLICT OF INTEREST
Petter Bjornstad has acted as a consultant for AstraZeneca, Bayer, Bristol‐Myers Squibb, Boehringer Ingelheim, Sanofi, Novo Nordisk and Horizon Pharma. Petter Bjornstad serves on the advisory boards of XORTX and Boehringer Ingelheim. David Z. I. Cherney has received honoraria from Abbvie, AstraZeneca, Bayer, BMS, Boehringer Ingelheim‐Lilly, Janssen, Merck, Mitsubishi‐Tanabe, Novo‐Nordisk, Prometic and Sanofi and has received operational funding for clinical trials from AstraZeneca, Boehringer Ingelheim‐Lilly, Janssen, Merck, Novo‐Nordisk and Sanofi. Daniël H. van Raalte has acted as a consultant and received honoraria from Boehringer Ingelheim and Lilly, Merck, Novo Nordisk, Sanofi and AstraZeneca and has received research operating funds from Boehringer Ingelheim‐Lilly Diabetes Alliance, AstraZeneca and Novo Nordisk; all honoraria are paid to his employer (AUMC, location VUMC). All other authors reports nothing to disclosure.
Scholtes RA, van Baar MJB, Kok MD, et al. Renal haemodynamic and protective effects of renoactive drugs in type 2 diabetes: Interaction with SGLT2 inhibitors. Nephrology. 2021;26:377–390. 10.1111/nep.13839
[Correction added on 20 February, after first online publication: The reference citations have been updated.]
REFERENCES
- 1. Collins AJ, Foley RN, Chavers B, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end‐stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl 1):A7, e1‐e420. [DOI] [PubMed] [Google Scholar]
- 2. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muskiet MH, Tonneijck L, Smits MM, Kramer MH, Heerspink HJ, van Raalte DH. Pleiotropic effects of type 2 diabetes management strategies on renal risk factors. Lancet Diabetes Endocrinol. 2015;3(5):367‐381. [DOI] [PubMed] [Google Scholar]
- 4. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164‐176. [DOI] [PubMed] [Google Scholar]
- 5. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580‐591. [DOI] [PubMed] [Google Scholar]
- 6. Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl 2):64‐78. [DOI] [PubMed] [Google Scholar]
- 7. Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F. Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta‐analysis. Diabetologia. 2009;52(4):691‐697. [DOI] [PubMed] [Google Scholar]
- 8. Ruggenenti P, Porrini EL, Gaspari F, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35(10):2061‐2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almeida JC, Mello VD, Canani LH, Gross JL, Azevedo MJ. Role of dietary lipids in diabetic nephropathy. Arq Bras Endocrinol Metabol. 2009;53(5):634‐645. [DOI] [PubMed] [Google Scholar]
- 10. Molitch ME, Gao X, Bebu I, et al. Early glomerular Hyperfiltration and long‐term kidney outcomes in type 1 diabetes: the DCCT/EDIC experience. Clin J Am Soc Nephrol. 2019;14(6):854‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas MC, Moran JL, Harjutsalo V, et al. Hyperfiltration in type 1 diabetes: does it exist and does it matter for nephropathy? Diabetologia. 2012;55(5):1505‐1513. [DOI] [PubMed] [Google Scholar]
- 12. Ficociello LH, Perkins BA, Roshan B, et al. Renal hyperfiltration and the development of microalbuminuria in type 1 diabetes. Diabetes Care. 2009;32(5):889‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010;53(10):2093‐2104. [DOI] [PubMed] [Google Scholar]
- 14. Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28(4):1023‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 inhibition in the diabetic kidney‐from mechanisms to clinical outcome. Clin J Am Soc Nephrol. 2017;12(4):700‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369‐384. [DOI] [PubMed] [Google Scholar]
- 18. Petrykiv S, Sjostrom CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12(5):751‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 20. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018. [DOI] [PubMed] [Google Scholar]
- 21. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 22. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. [DOI] [PubMed] [Google Scholar]
- 23. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. [DOI] [PubMed] [Google Scholar]
- 24. Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond). 2018;132(12):1329‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGurnaghan SJ, Brierley L, Caparrotta TM, et al. The effect of dapagliflozin on glycaemic control and other cardiovascular disease risk factors in type 2 diabetes mellitus: a real‐world observational study. Diabetologia. 2019;62(4):621‐632. [DOI] [PubMed] [Google Scholar]
- 26. Cherney DZI, Cooper ME, Tikkanen I, et al. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018;93(1):231‐244. [DOI] [PubMed] [Google Scholar]
- 27. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bjornstad P, Laffel L, Tamborlane WV, et al. Acute effect of empagliflozin on fractional excretion of sodium and eGFR in youth with type 2 diabetes. Diabetes Care. 2018;41(8):e129‐e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587‐597. [DOI] [PubMed] [Google Scholar]
- 30. Arakawa K, Ishihara T, Oku A, et al. Improved diabetic syndrome in C57BL/KsJ‐db/db mice by oral administration of the Na(+)‐glucose cotransporter inhibitor T‐1095. Br J Pharmacol. 2001;132(2):578‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R75‐R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malatiali S, Francis I, Barac‐Nieto M. Phlorizin prevents glomerular hyperfiltration but not hypertrophy in diabetic rats. Exp Diabetes Res. 2008;2008:305403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Bommel EJM, Muskiet MHA, van Baar MJB, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post‐glomerular vasodilatation rather than pre‐glomerular vasoconstriction in metformin‐treated patients with type 2 diabetes in the randomized, double‐blind RED trial. Kidney Int. 2020;97(1):202‐212. [DOI] [PubMed] [Google Scholar]
- 34. Ott C, Jung D, Kannenkeril D, Striepe A, Bosch M, Korn P, et al. Disparate effects of the combined therapy of empagliflozin and linagliptin versus metformin and insulin glargine on renal haemodynamics in type 2 diabetes. [Abstract EASD]. In Press, 2019.
- 35. van Bommel EJM, Lytvyn Y, Perkins BA, et al. Renal hemodynamic effects of sodium‐glucose cotransporter 2 inhibitors in hyperfiltering people with type 1 diabetes and people with type 2 diabetes and normal kidney function. Kidney Int. 2020;97(4):631‐635. [DOI] [PubMed] [Google Scholar]
- 36. Mayer GJ, Wanner C, Weir MR, et al. Analysis from the EMPA‐REG OUTCOME((R)) trial indicates empagliflozin may assist in preventing the progression of chronic kidney disease in patients with type 2 diabetes irrespective of medications that alter intrarenal hemodynamics. Kidney Int. 2019;96(2):489‐504. [DOI] [PubMed] [Google Scholar]
- 37. van Baar MJB, Scholtes RA, van Raalte DH. SGLT2 inhibitors' interaction with other renoactive drugs in type 2 diabetes patients: still a lot to learn. Kidney Int. 2019;96(2):283‐286. [DOI] [PubMed] [Google Scholar]
- 38. Bjornstad P, Nelson RG, Pavkov ME. Do sodium‐glucose cotransporter‐2 inhibitors affect renal hemodynamics by different mechanisms in type 1 and type 2 diabetes? Kidney Int. 2020;97(1):31‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fountain JH, Lappin SL. Physiology. Treasure Island (FL): Renin Angiotensin System. StatPearls; 2020. [PubMed] [Google Scholar]
- 40. Robles NR, Cerezo I, Hernandez‐Gallego R. Renin‐angiotensin system blocking drugs. J Cardiovasc Pharmacol Ther. 2014;19(1):14‐33. [DOI] [PubMed] [Google Scholar]
- 41. Uehara Y, Miura S, Yahiro E, Saku K. Non‐ACE pathway‐induced angiotensin II production. Curr Pharm Des. 2013;19(17):3054‐3059. [DOI] [PubMed] [Google Scholar]
- 42. Ruilope LM, Rosei EA, Bakris GL, et al. Angiotensin receptor blockers: therapeutic targets and cardiovascular protection. Blood Press. 2005;14(4):196‐209. [DOI] [PubMed] [Google Scholar]
- 43. Ichikawi I, Harris RC. Angiotensin actions in the kidney: renewed insight into the old hormone. Kidney Int. 1991;40(4):583‐596. [DOI] [PubMed] [Google Scholar]
- 44. Heyeraas KJ, Aukland K. Interlobular arterial resistance: influence of renal arterial pressure and angiotensin II. Kidney Int. 1987;31(6):1291‐1298. [DOI] [PubMed] [Google Scholar]
- 45. Denton KM, Fennessy PA, Alcorn D, Anderson WP. Morphometric analysis of the actions of angiotensin II on renal arterioles and glomeruli. Am J Physiol. 1992;262(3 Pt 2):F367‐F372. [DOI] [PubMed] [Google Scholar]
- 46. Hanif K, Bid HK, Konwar R. Reinventing the ACE inhibitors: some old and new implications of ACE inhibition. Hypertens Res. 2010;33(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 47. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597‐603. [DOI] [PubMed] [Google Scholar]
- 48. Heart Outcomes Prevention Evaluation Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Lancet. 2000;355(9200):253‐259. [PubMed] [Google Scholar]
- 49. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin‐receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851‐860. [DOI] [PubMed] [Google Scholar]
- 50. Parving HH, Lehnert H, Brochner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870‐878. [DOI] [PubMed] [Google Scholar]
- 51. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861‐869. [DOI] [PubMed] [Google Scholar]
- 52. Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941‐1951. [DOI] [PubMed] [Google Scholar]
- 53. Ruggenenti P, Fassi A, Ilieva A, et al. Effects of verapamil added‐on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT‐B randomized trial. J Hypertens. 2011;29(2):207‐216. [DOI] [PubMed] [Google Scholar]
- 54. Group AC , Patel A, Mac Mahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 55. Haller H, Ito S, Izzo JL Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907‐917. [DOI] [PubMed] [Google Scholar]
- 56. Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892‐1903. [DOI] [PubMed] [Google Scholar]
- 57. Bavishi C, Bangalore S, Messerli FH. Renin angiotensin aldosterone system inhibitors in hypertension: is there evidence for benefit independent of blood pressure reduction? Prog Cardiovasc Dis. 2016;59(3):253‐261. [DOI] [PubMed] [Google Scholar]
- 58. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456‐1462. [DOI] [PubMed] [Google Scholar]
- 59. Tarnow L, Rossing P, Jensen C, Hansen BV, Parving HH. Long‐term renoprotective effect of nisoldipine and lisinopril in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2000;23(12):1725‐1730. [DOI] [PubMed] [Google Scholar]
- 60. Bjorck S, Mulec H, Johnsen SA, Norden G, Aurell M. Renal protective effect of enalapril in diabetic nephropathy. BMJ. 1992;304(6823):339‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Clase CM, Barzilay J, Gao P, et al. Acute change in glomerular filtration rate with inhibition of the renin‐angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney Int. 2017;91(3):683‐690. [DOI] [PubMed] [Google Scholar]
- 62. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end‐stage renal disease and mortality. JAMA. 2014;311(24):2518‐2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heerspink HJL, Greene T, Tighiouart H, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta‐analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7(2):128‐139. [DOI] [PubMed] [Google Scholar]
- 64. Heerspink HJ, Holtkamp FA, de Zeeuw D, Ravid M. Monitoring kidney function and albuminuria in patients with diabetes. Diabetes Care. 2011;34(Suppl 2):S325‐S329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long‐term renal function. Kidney Int. 2011;80(3):282‐287. [DOI] [PubMed] [Google Scholar]
- 66. Hansen HP, Rossing P, Tarnow L, Nielsen FS, Jensen BR, Parving HH. Increased glomerular filtration rate after withdrawal of long‐term antihypertensive treatment in diabetic nephropathy. Kidney Int. 1995;47(6):1726‐1731. [DOI] [PubMed] [Google Scholar]
- 67. Apperloo AJ, de Zeeuw D, de Jong PE. A short‐term antihypertensive treatment‐induced fall in glomerular filtration rate predicts long‐term stability of renal function. Kidney Int. 1997;51(3):793‐797. [DOI] [PubMed] [Google Scholar]
- 68. Price DA, Porter LE, Gordon M, et al. The paradox of the low‐renin state in diabetic nephropathy. J Am Soc Nephrol. 1999;10(11):2382‐2391. [DOI] [PubMed] [Google Scholar]
- 69. De'Oliveira JM, Price DA, Fisher ND, et al. Autonomy of the renin system in type II diabetes mellitus: dietary sodium and renal hemodynamic responses to ACE inhibition. Kidney Int. 1997;52(3):771‐777. [DOI] [PubMed] [Google Scholar]
- 70. New JP, Marshall SM, Bilous RW. Renal autoregulation is normal in newly diagnosed, normotensive, NIDDM patients. Diabetologia. 1998;41(2):206‐211. [DOI] [PubMed] [Google Scholar]
- 71. Stornello M, Valvo EV, Scapellato L. Hemodynamic, renal, and humoral effects of the calcium entry blocker nicardipine and converting enzyme inhibitor captopril in hypertensive type II diabetic patients with nephropathy. J Cardiovasc Pharmacol. 1989;14(6):851‐855. [DOI] [PubMed] [Google Scholar]
- 72. Baba T, Murabayashi S, Takebe K. Comparison of the renal effects of angiotensin converting enzyme inhibitor and calcium antagonist in hypertensive type 2 (non‐insulin‐dependent) diabetic patients with microalbuminuria: a randomised controlled trial. Diabetologia. 1989;32(1):40‐44. [DOI] [PubMed] [Google Scholar]
- 73. Ruggenenti P, Mosconi L, Sangalli F, et al. Glomerular size‐selective dysfunction in NIDDM is not ameliorated by ACE inhibition or by calcium channel blockade. Kidney Int. 1999;55(3):984‐994. [DOI] [PubMed] [Google Scholar]
- 74. Fliser D, Wagner KK, Loos A, Tsikas D, Haller H. Chronic angiotensin II receptor blockade reduces (intra)renal vascular resistance in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(4):1135‐1140. [DOI] [PubMed] [Google Scholar]
- 75. Marre M, Leblanc H, Suarez L, Guyenne TT, Menard J, Passa P. Converting enzyme inhibition and kidney function in normotensive diabetic patients with persistent microalbuminuria. Br Med J (Clin Res Ed). 1987;294(6585):1448‐1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Valvo E, Bedogna V, Casagrande P, et al. Captopril in patients with type II diabetes and renal insufficiency: systemic and renal hemodynamic alterations. Am J Med. 1988;85(3):344‐348. [DOI] [PubMed] [Google Scholar]
- 77. Romero R, Salinas I, Lucas A, Teixido J, Audi L, Sanmarti A. Comparative effects of captopril versus nifedipine on proteinuria and renal function of type 2 diabetic patients. Diabetes Res Clin Pract. 1992;17(3):191‐198. [DOI] [PubMed] [Google Scholar]
- 78. Bakris GL, Barnhill BW, Sadler R. Treatment of arterial hypertension in diabetic humans: importance of therapeutic selection. Kidney Int. 1992;41(4):912‐919. [DOI] [PubMed] [Google Scholar]
- 79. Capek M, Schnack C, Ludvik B, Kautzky‐Willer A, Banyai M, Prager R. Effects of captopril treatment versus placebo on renal function in type 2 diabetic patients with microalbuminuria: a long‐term study. Clin Investig. 1994;72(12):961‐966. [DOI] [PubMed] [Google Scholar]
- 80. Vora JP, Leese GP, Peters JR, Owens DR. Longitudinal evaluation of renal function in non‐insulin‐dependent diabetic patients with early nephropathy: effects of angiotensin‐converting enzyme inhibition. J Diabetes Complications. 1996;10(2):88‐93. [DOI] [PubMed] [Google Scholar]
- 81. Ruggenenti P, Mosconi L, Bianchi L, et al. Long‐term treatment with either enalapril or nitrendipine stabilizes albuminuria and increases glomerular filtration rate in non‐insulin‐dependent diabetic patients. Am J Kidney Dis. 1994;24(5):753‐761. [DOI] [PubMed] [Google Scholar]
- 82. Slataper R, Vicknair N, Sadler R, Bakris GL. Comparative effects of different antihypertensive treatments on progression of diabetic renal disease. Arch Intern Med. 1993;153(8):973‐980. [PubMed] [Google Scholar]
- 83. Scholtes RA, van Raalte DH, Correa‐Rotter R, et al. The effects of dapagliflozin on cardio‐renal risk factors in patients with type 2 diabetes with or without renin‐angiotensin system inhibitor treatment: a post hoc analysis. Diabetes Obes Metab. 2020;22(4):549‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hockerman GH, Peterson BZ, Johnson BD, Catterall WA. Molecular determinants of drug binding and action on L‐type calcium channels. Annu Rev Pharmacol Toxicol. 1997;37:361‐396. [DOI] [PubMed] [Google Scholar]
- 85. Boner G, Cao Z, Cooper ME. Combination antihypertensive therapy in the treatment of diabetic nephropathy. Diabetes Technol Ther. 2002;4(3):313‐321. [DOI] [PubMed] [Google Scholar]
- 86. Kloke HJ, Branten AJ, Huysmans FT, Wetzels JF. Antihypertensive treatment of patients with proteinuric renal diseases: risks or benefits of calcium channel blockers? Kidney Int. 1998;53(6):1559‐1573. [DOI] [PubMed] [Google Scholar]
- 87. Bakris GL, Weir MR, Secic M, Campbell B, Weis‐McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004;65(6):1991‐2002. [DOI] [PubMed] [Google Scholar]
- 88. Fernandez R, Puig JG, Rodriguez‐Perez JC, Garrido J, Redon J, Group TS . Effect of two antihypertensive combinations on metabolic control in type‐2 diabetic hypertensive patients with albuminuria: a randomised, double‐blind study. J Hum Hypertens. 2001;15(12):849‐856. [DOI] [PubMed] [Google Scholar]
- 89. Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T‐ and L‐type voltage‐dependent calcium channels in renal resistance vessels. Circ Res. 2001;89(7):630‐638. [DOI] [PubMed] [Google Scholar]
- 90. Ando K, Ueshima K, Tanaka S, et al. Comparison of the antialbuminuric effects of L‐/N‐type and L‐type calcium channel blockers in hypertensive patients with diabetes and microalbuminuria: the study of assessment for kidney function by urinary microalbumin in randomized (SAKURA) trial. Int J Med Sci. 2013;10(9):1209‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Homma K, Hayashi K, Yamaguchi S, Fujishima S, Hori S, Itoh H. Renal microcirculation and calcium channel subtypes. Curr Hypertens Rev. 2013;9(3):182‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Konno Y, Kimura K. Vasodilatory effect of cilnidipine, an L‐type and N‐type calcium channel blocker, on rat kidney glomerular arterioles. Int Heart J. 2008;49(6):723‐732. [DOI] [PubMed] [Google Scholar]
- 93. Zhou X, Ono H, Ono Y, Frohlich ED. N‐ and L‐type calcium channel antagonist improves glomerular dynamics, reverses severe nephrosclerosis, and inhibits apoptosis and proliferation in an l‐NAME/SHR model. J Hypertens. 2002;20(5):993‐1000. [DOI] [PubMed] [Google Scholar]
- 94. Baba T, Tomiyama T, Murabayashi S, Takebe K. Renal effects of nicardipine, a calcium antagonist, in hypertensive type 2 (non‐insulin‐dependent) diabetic patients with and without nephropathy. Eur J Clin Pharmacol. 1990;38(5):425‐429. [DOI] [PubMed] [Google Scholar]
- 95. Baba T, Ishizaki T, Ido Y, Aoyagi K, Murabayashi S, Takebe K. Renal effects of nicardipine, a calcium entry blocker, in hypertensive type II diabetic patients with nephropathy. Diabetes. 1986;35(11):1206‐1214. [DOI] [PubMed] [Google Scholar]
- 96. Smith AC, Toto R, Bakris GL. Differential effects of calcium channel blockers on size selectivity of proteinuria in diabetic glomerulopathy. Kidney Int. 1998;54(3):889‐896. [DOI] [PubMed] [Google Scholar]
- 97. Dalal R, Bruss ZS, Sehdev JS. Physiology. Treasure Island (FL): Renal Blood Flow and Filtration. StatPearls; 2020. [PubMed] [Google Scholar]
- 98. Hendry BM, Ellory JC. Molecular sites for diuretic action. Trends Pharmacol Sci. 1988;9(11):416‐421. [DOI] [PubMed] [Google Scholar]
- 99. Ali SS, Sharma PK, Garg VK, Singh AK, Mondal SC. The target‐specific transporter and current status of diuretics as antihypertensive. Fundam Clin Pharmacol. 2012;26(2):175‐179. [DOI] [PubMed] [Google Scholar]
- 100. Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol. 2015;10(4):676‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Biamino G, Wessel HJ, Noring J, Schroder R. Plethysmographic and in vitro studies of the vasodilator effect of furosemide (Lasix). Int J Clin Pharmacol Biopharm. 1975;12(3):356‐368. [PubMed] [Google Scholar]
- 102. Tamargo J, Segura J, Ruilope LM. Diuretics in the treatment of hypertension. Part 1: thiazide and thiazide‐like diuretics. Expert Opin Pharmacother. 2014;15(4):527‐547. [DOI] [PubMed] [Google Scholar]
- 103. Horisberger JD, Giebisch G. Potassium‐sparing diuretics. Ren Physiol. 1987;10(3‐4):198‐220. [DOI] [PubMed] [Google Scholar]
- 104. Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther. 2014;19(1):5‐13. [DOI] [PubMed] [Google Scholar]
- 105. Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380(9841):601‐610. [DOI] [PubMed] [Google Scholar]
- 106. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 107. Grossman E, Verdecchia P, Shamiss A, Angeli F, Reboldi G. Diuretic treatment of hypertension. Diabetes Care. 2011;34(Suppl 2):S313‐S319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Turnbull F. Blood Pressure Lowering Treatment Trialists C. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet. 2003;362(9395):1527‐1535. [DOI] [PubMed] [Google Scholar]
- 109. Whelton PK, Barzilay J, Cushman WC, et al. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med. 2005;165(12):1401‐1409. [DOI] [PubMed] [Google Scholar]
- 110. Bakris GL, Toto RD, McCullough PA, et al. Effects of different ACE inhibitor combinations on albuminuria: results of the GUARD study. Kidney Int. 2008;73(11):1303‐1309. [DOI] [PubMed] [Google Scholar]
- 111. Morales E, Millet VG, Rojas‐Rivera J, et al. Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol Dial Transplant. 2013;28(2):405‐412. [DOI] [PubMed] [Google Scholar]
- 112. Saklayen MG, Gyebi LK, Tasosa J, Yap J. Effects of additive therapy with spironolactone on proteinuria in diabetic patients already on ACE inhibitor or ARB therapy: results of a randomized, placebo‐controlled, double‐blind, crossover trial. J Investig Med. 2008;56(4):714‐719. [DOI] [PubMed] [Google Scholar]
- 113. Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double‐masked, cross‐over study. Diabetes Care. 2005;28(9):2106‐2112. [DOI] [PubMed] [Google Scholar]
- 114. Selvaraj S, Claggett B, Shah SJ, et al. Prognostic value of albuminuria and influence of spironolactone in heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11(11):e005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Filippatos G, Anker SD, Bohm M, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37(27):2105‐2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Furumatsu Y, Nagasawa Y, Tomida K, et al. Effect of renin‐angiotensin‐aldosterone system triple blockade on non‐diabetic renal disease: addition of an aldosterone blocker, spironolactone, to combination treatment with an angiotensin‐converting enzyme inhibitor and angiotensin II receptor blocker. Hypertens Res. 2008;31(1):59‐67. [DOI] [PubMed] [Google Scholar]
- 117. Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;4:CD007004. [DOI] [PubMed] [Google Scholar]
- 118. Bianchi S, Bigazzi R, Campese VM. Long‐term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70(12):2116‐2123. [DOI] [PubMed] [Google Scholar]
- 119. Ruilope LM, Agarwal R, Anker SD, et al. Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol. 2019;50(5):345‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bakris GL, Agarwal R, Anker SD, et al. Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease Trial. Am J Nephrol. 2019;50(5):333‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Martinez‐Maldonado M, Gely R, Tapia E, Benabe JE. Role of macula densa in diuretics‐induced renin release. Hypertension. 1990;16(3):261‐268. [DOI] [PubMed] [Google Scholar]
- 122. Gutsche HU, Brunkhorst R, Muller‐Ott K, Franke H, Niedermayer W. Effect of diuretics on the tubuloglomerular feedback response. Can J Physiol Pharmacol. 1984;62(4):412‐417. [DOI] [PubMed] [Google Scholar]
- 123. Wang H, D'Ambrosio MA, Ren Y, et al. Tubuloglomerular and connecting tubuloglomerular feedback during inhibition of various Na transporters in the nephron. Am J Physiol Renal Physiol. 2015;308(9):F1026‐F1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995‐2008. [DOI] [PubMed] [Google Scholar]
- 125. Perkovic V, Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinology. 2018. [DOI] [PubMed] [Google Scholar]
- 126. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 127. Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4(3):211‐220. [DOI] [PubMed] [Google Scholar]
- 128. Nadkarni GN, Ferrandino R, Chang A, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity‐matched analysis. Diabetes Care. 2017;40(11):1479‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gilbert RE, Thorpe KE. Acute kidney injury with sodium‐glucose co‐transporter‐2 inhibitors: A meta‐analysis of cardiovascular outcome trials. Diabetes Obes Metab. 2019;21(8):1996‐2000. [DOI] [PubMed] [Google Scholar]
- 130. Mordi NA, Mordi IR, Singh JS, et al. Renal and Cardiovascular Effects of sodium‐glucose cotransporter 2 (SGLT2) inhibition in combination with loop Diuretics in diabetic patients with Chronic Heart Failure (RECEDE‐CHF): protocol for a randomised controlled double‐blind cross‐over trial. BMJ Open. 2017;7(10):e018097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE‐CHF trial. Circulation. 2020;142:1713‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]


