Abstract
Pathogenic variants (PVs) in ATM are relatively common, but the scope and magnitude of risk remains uncertain. This study aimed to estimate ATM PV cancer risks independent of family cancer history. This analysis included patients referred for hereditary cancer testing with a multi-gene panel (N=627,742). Cancer risks for ATM PV carriers (N=4,607) were adjusted for family history using multivariable logistic regression and reported as odds ratios (ORs) with 95% confidence intervals (CIs). Sub-analyses of the c.7271T>G missense PV were conducted. Moderate-to-high risks for pancreatic (OR 4.21; 95% CI 3.24–5.47), prostate (OR 2.58; 95% CI 1.93–3.44), gastric (OR 2.97; 95% CI 1.66–5.31) and invasive ductal breast (OR 2.03; 95% CI 1.89–2.19) cancers were estimated for ATM PV carriers. Notably, c.7271T>G was associated with higher invasive ductal breast cancer risk (OR 3.76, 95% CI 2.76–5.12) than other missense and truncating ATM PVs. Low-to-moderate risks were seen for ductal carcinoma in situ (OR 1.80, 95% CI 1.61–2.02), male breast cancer (OR 1.72, 95% CI 1.08–2.75), ovarian cancer (OR 1.57; 95% CI 1.35–1.83), colorectal cancer (OR 1.49; 95% CI 1.24–1.79) and melanoma (OR 1.46; 95% CI 1.18–1.81). ATM PVs are associated with multiple cancer risks and, while professional society guidelines support that carriers are eligible for increased breast and pancreatic cancer screening, increased screening for prostate and gastric cancer may also be warranted. c.7271T>G is associated with high risk for breast cancer, with a three to four-fold risk increase that supports consideration of strategies for prevention and/or early detection.
Keywords: ATM, pathogenic variant, breast cancer, pancreatic cancer
INTRODUCTION
Germ-line pathogenic variants (PVs) in the ataxia telangiectasia mutated (ATM) gene are prevalent (approximately 0.35%) in the population [1,2]. ATM PVs are known to be associated with health risks in both an autosomal dominant and autosomal recessive fashion. Rare individuals homozygous or compound heterozygous for a germ-line ATM PV develop ataxia telangiectasia (A-T, prevalence ~1/40,000–1/100,000), a childhood-onset disorder typically characterized by global immunodeficiency, cerebellar neuro-degeneration, radiation sensitivity, and death in late adolescence [3]. Previous estimates of adult-onset breast cancer risk in women heterozygous for germline ATM PVs range from a 2- to 5-fold increased risk compared to women without ATM PVs [2,4–9]. It has been suggested that there may be substantially higher risks associated with specific ATM variants, such as c.7271T>G (p.Val2424Gly) where the risk of developing breast cancer by age 70 has been estimated at 52–60% [10–13]. Current guidelines from the National Comprehensive Cancer Network (NCCN) advise female ATM PV carriers to initiate annual mammographic screening at age 40, with consideration of annual breast MRI, which may be best accomplished at a high-risk breast cancer clinic [14].
Germ-line ATM PVs have also been suggested to increase risks for developing several other adult-onset cancers. Like the increased risk of breast cancer, these are generally regarded as low to moderately increased lifetime cancer risks. Multiple studies have documented an association of germline ATM PVs with pancreatic cancer, although risk estimates vary [15,16]. There is limited evidence for additional malignancies including gastro-esophageal cancer,[17,18] colorectal cancer [19,20], ovarian cancer [7,21], melanoma [22,23], prostate cancer [22,24], thyroid cancer [22], gastric cancer [17], and head and neck cancers [22]. Estimates of the varied cancer risks associated with ATM PVs have been limited by studies with small participant sample sizes leading to imprecise estimates of cancer risks, sequencing of limited loci, and ascertainment biases in selection of participants.
Improved precision of estimates of cancer risks associated with ATM PVs is critical because ATM PVs are common in individuals of varied ancestry undergoing genetic testing for hereditary cancer risk [25,26], and even modest risk increases may warrant modified recommendations for cancer screening and prevention. We used multi-gene sequencing results, personal and family cancer history data from a large sample of individuals undergoing hereditary cancer risk evaluation to obtain odds ratios (ORs) and penetrance estimates, adjusted for family history, for many cancer types. The differential impact of mutation type (nonsense/frameshift versus missense PV), and the previously described c.7271T>G missense variant were specifically examined.
Penetrance estimates adjusted for family history represent the magnitude of genetic risk that is independent of family history. Adjusted penetrance may be estimated in an unbiased manner from clinical populations where factors related to ascertainment are well captured [7,27,28]. These adjusted penetrance estimates are applicable to women with and without family history of cancer and may inform personalized assessments that combine genetic risks with environmental and lifestyle risk factors.
METHODS
Cohort and Hereditary Cancer Testing
The study included individuals who underwent testing with a multi-gene hereditary cancer panel including ATM between September 2013 and July 2019. All tested individuals were ascertained by healthcare providers for suspicion of hereditary cancer risk based on personal and/or family histories of cancer meeting professional society criteria for genetic testing. During this time, the number of genes on the panel expanded from 25 (2013–2016) to 29 (2016–2019) to 35 (2019). Individuals were eligible for analysis if they carried a single (heterozygous) PV in ATM or if they had no PV in any gene sequenced nor any uncertain variants (VUS) in the ATM gene. Variant classification was performed by the testing laboratory based on American College of Medical Genetics guidelines at a minimum, as previously described [29]. Variants with a laboratory classification of deleterious or suspected deleterious were considered to be PVs for this analysis.
Genetic testing data were correlated with demographic, personal, and family cancer history data from a test requisition form that accompanied each test kit. Individuals with missing data on gender or age of testing were excluded. Finally, patients from states that restrict the use of de-identified genetic data after completion of testing were excluded from analysis.
Statistical Methods
All analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria; Version 3.6.1). Multivariable logistic regression modeling was used to quantify ATM cancer risks for 11 different cancers in terms of ORs adjusted for age, ancestry, and personal and family cancer history. These ORs are a measure of cancer risks associated with ATM PVs and can be interpreted as relative risks independent of family history (e.g. an OR of 2 indicates that risk is doubled for a PV carrier versus a non-carrier with an identical family history of cancer). A separate analysis was performed for each ATM PV subgroup [all ATM, truncating (nonsense and frameshift), missense, and the c.7271T>G PV] using PV status as the dependent variable. Independent variables included age, sex, ancestry, and personal and family cancer histories associated with HBOC, and Lynch and adenomatous polyposis cancer syndromes. For history of breast cancer, categories included ductal invasive (coded as either triple-negative or hormone-positive), lobular invasive, ductal carcinoma in situ (DCIS), and male breast. Triple-negative breast cancer is a subset of ductal invasive for this cohort. All other categories are exclusive. Personal cancer variables were coded as binary (ever or never affected). Familial cancers were coded as numeric counts of diagnoses, weighted by degree of relatedness.
Cumulative invasive breast cancer risks were calculated according to the product-limit method [30] without adjustment for competing mortality. These calculations have been described in detail previously [7,31]. Briefly, age-specific incidence rates were estimated as products of ORs and general population incidences; ORs for PVs were calculated as specified above and were combined with age-specific SEER incidence rates from 2009 to 2013 in 5-year intervals to estimate absolute cancer risks [7,32,33].
Confidence intervals (CIs) and p-values were calculated using Wald statistics. All p-values are reported as two-sided. The study was reviewed by the Fox Chase Cancer Center Institutional Review Board (IRB) and was determined to be Human Subject Exempt and therefore written informed consent was not required.
RESULTS
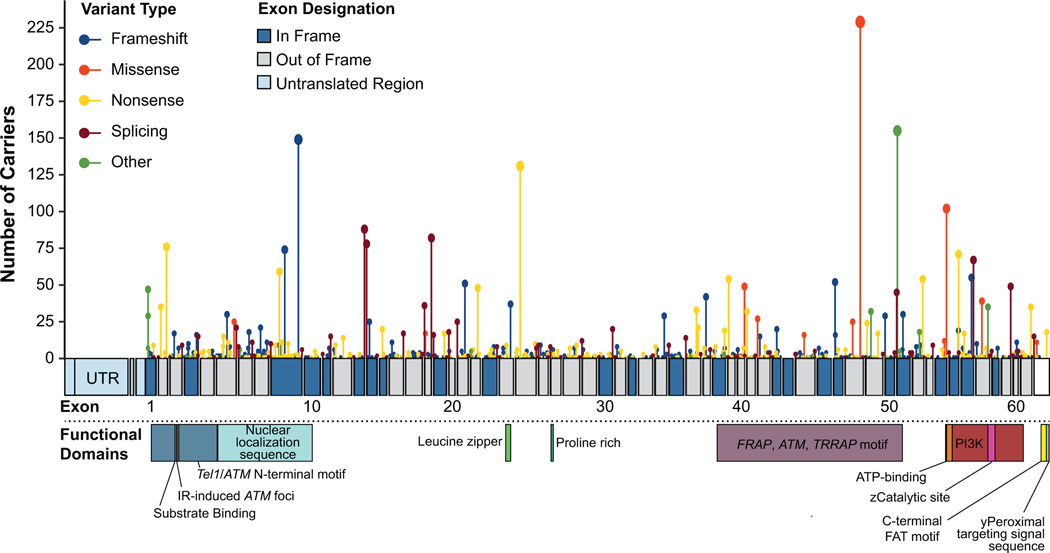
We identified 627,742 eligible patients (Table 1), including 4,607 (0.7%) individuals with an ATM PV and 623,135 (99.3%) individuals with no ATM PV, ATM VUS, or PV in another hereditary cancer risk gene. The majority of ATM PVs identified were truncating (nonsense and frameshift; 58.3%) and the remaining were missense (7.1%) or other (29.7%). The c.7271T>G variant was the most frequent individual PV observed (5.0%, 229/4607). All observed PV types and their locations on ATM are shown in Figure 1.
Table 1:
Demographics and personal cancer history by ATM PV carrier status
| Characteristic |
ATM pathogenic variant (PV) (N=4,607) |
No ATM PVa (N=623,135) |
Total (N=627,742) |
|---|---|---|---|
| Age at testing - median (IQR) | 49 (39, 59) | 48 (38, 58) | 48 (38, 58) |
| Sex | |||
| Female | 4,378 (95.0) | 601,246 (96.5) | 605,624 (96.5) |
| Male | 229 (5.0) | 21,889 (3.5) | 22,118 (3.5) |
| Race/Ethnicity | |||
| White/Caucasian | 2,889 (62.9) | 358,097 (57.5) | 360,996 (57.5) |
| Black/African | 289 (6.3) | 51,324 (8.2) | 51,613 (8.2) |
| Latino/Hispanic | 288 (6.3) | 46,713 (7.5) | 47,001 (7.5) |
| Asian | 100 (2.2) | 14,224 (2.3) | 14,324 (2.3) |
| Ashkenazi Jewish | 34 (0.7) | 10,302 (1.7) | 10,336 (1.6) |
| Native American | 31 (0.7) | 5,141 (0.8) | 5,172 (0.8) |
| Middle Eastern | 21 (0.5) | 3,428 (0.6) | 3,449 (0.5) |
| Multiple | 270 (5.9) | 41,021 (6.6) | 41,291 (6.6) |
| Other/None specified | 675 (14.7) | 92,885 (14.9) | 93,560 (14.9) |
| Personal cancer history | |||
| Total cancers | |||
| 0 cancer | 2,243 (48.7) | 385,932 (61.9) | 388,175 (61.8) |
| 1 cancer | 1,928 (41.8) | 203,668 (32.7) | 205,596 (32.8) |
| 2+ cancers | 436 (9.5) | 33,535 (5.4) | 33,971 (5.4) |
| Any Cancer | 2,364 (51.3) | 237,203 (38.1) | 239,567 (38.2) |
| Breast – Ductal Invasiveb | 1,461 (33.4) | 132,465 (22.0) | 133,926 (22.1) |
| Breast – Triple-negativeb | 52 (1.2) | 19,711 (3.3) | 19,763 (3.3) |
| Breast – Lobular Invasiveb | 68 (1.6) | 10,633 (1.8) | 10,701 (1.8) |
| Breast - DCISb | 351 (8.0) | 28,906 (4.8) | 29,257 (4.8) |
| Breast - Malec | 20 (8.7) | 1,909 (8.7) | 1,929 (8.7) |
| Ovarian cancerb | 192 (4.4) | 24,616 (4.1) | 24,808 (4.1) |
| Colorectal cancer | 135 (2.9) | 14,527 (2.3) | 14,662 (2.3) |
| Endometrial cancerb | 85 (1.9) | 11,877 (2.0) | 11,962 (2.0) |
| Melanoma | 88 (1.9) | 7,426 (1.2) | 7,514 (1.2) |
| Prostate cancerc | 75 (32.8) | 4,436 (20.3) | 4,511 (20.4) |
| Pancreatic cancer | 64 (1.4) | 2,085 (0.3) | 2,149 (0.3) |
| Gastric cancer | 12 (0.3) | 518 (0.1) | 530 (0.1) |
Patients with no PV in any gene tested, including ATM, and no VUS in ATM;
Female Only;
Male Only; Triple-negative breast cancer is a subset of Ductal Invasive for this cohort. All other categories are exclusive.
Figure 1.
Map of ATM and all variants discovered in this study. This map shows the types and frequency of ATM variants discovered within this patient cohort. The circle size/line length reflects the number of times a variant was observed at that location. The exons are numbered, with in-frame exons being dark blue and out-of-frame exons being grey. The untranslated region (UTR) is reflected in light blue. The functional regions of the gene are also shown. Tan: Tel1/ATM N-Terminal motif; NLS: nuclear localization sequence; LZ: leucine zipper; Prch: Proline rich; FAT: FRAP, ATM, TRRAP motif; CS: ZCatalytic site; PTS1: yPeroxisomal targeting signal sequence.
The study cohort was predominantly female (96.5%) and over half of individuals reported White (non-Hispanic) ancestry (57.5%; Table 1). Median age of testing was 58 years (range 6, ≥90) for the full cohort. More than one-third (38.2%) of individuals reported a personal history of at least one cancer, and patients with an ATM PV were proportionally more likely to report a personal history of cancer than those without an ATM PV. Ductal invasive breast cancer was the most common cancer reported. Similarly, ductal invasive breast cancer was by far the most common family history reported, followed by ovarian and colorectal cancers (Supplemental Table 1).
Because previous research has identified variability in breast cancer risk by mutation type, with several studies reporting high breast cancer risks associated with the missense c.7271T>G PV in ATM, we examined rates of personal and family history of breast cancer reported by individuals with a truncating (nonsense and frameshift) PV, missense PV, and the c.7271T>G missense PV (Supplementary Table 2). Among carriers of ATM PVs, 38.7% (120/310) of missense mutation carriers, 45.3% (101/223) of c.7271T>G PV carriers, and 32.8 % (837/2548) of truncating (frameshift and nonsense) PV carriers had a personal history of invasive breast cancer. No differences in age of cancer diagnosis were detected by mutation.
Multivariable models were used to estimate the family history-adjusted risk of developing cancer in the presence of any ATM PV. Results for any ATM PV are reported in Table 2 and show a two-fold increased risk (OR 2.03, 95% CI 1.89–2.19) of ductal invasive breast cancer for women, a 1.80-fold increased risk of DCIS (95% CI 1.61–2.02), and a 1.72-fold increased risk of male breast cancer (95% CI 1.08–2.75). Increased risks of several other cancers were also seen in ATM PV carriers compared to non-carriers. The largest increase in risk was for pancreatic cancer (OR 4.21, 95% CI 3.24–5.47), but gastric cancer (OR 2.97, 95% CI 1.66–5.31) and prostate cancer (OR 2.58, 95% CI 1.93–3.44) also showed marked elevations in risk. The risks of ovarian cancer (OR 1.57, 95% CI 1.35–1.83), colorectal cancer (OR 1.49, 95% CI 1.24–1.79), and melanoma (OR 1.46, 95% CI 1.18–1.81) were overall low-to-moderately increased among ATM PV carriers.
Table 2:
Odds ratios for cancer risk for all ATM pathogenic variants
| Cancer type | All ATM pathogenic variants | ||
|---|---|---|---|
| Odds ratio | 95% CI | P-value | |
| Breast - Ductal Invasive | 2.03 | 1.89–2.19 | <0.0001 |
| Breast - DCIS | 1.80 | 1.61–2.02 | <0.0001 |
| Breast – Lobular Invasive | 0.94 | 0.74–1.20 | 0.6229 |
| Breast - Male | 1.72 | 1.08–2.75 | 0.0233 |
| Ovarian | 1.57 | 1.35–1.83 | <0.0001 |
| Colorectal | 1.49 | 1.24–1.79 | <0.0001 |
| Endometrial | 1.10 | 0.88–1.36 | 0.4079 |
| Melanoma | 1.46 | 1.18–1.81 | 0.0006 |
| Prostate | 2.58 | 1.93–3.44 | <0.0001 |
| Pancreatic | 4.21 | 3.24–5.47 | <0.0001 |
| Gastric | 2.97 | 1.66–5.31 | 0.0002 |
Note: Odds ratio are adjusted for personal (coded as binary, affected/unaffected, variables) and family (numeric counts weighted by degree of relation) cancer histories of breast (invasive, ductal, DCIS, and male), ovarian, colorectal, melanoma, gastric, pancreatic, prostate, endometrial, and colon (polyps), as well as age, sex and ancestry.
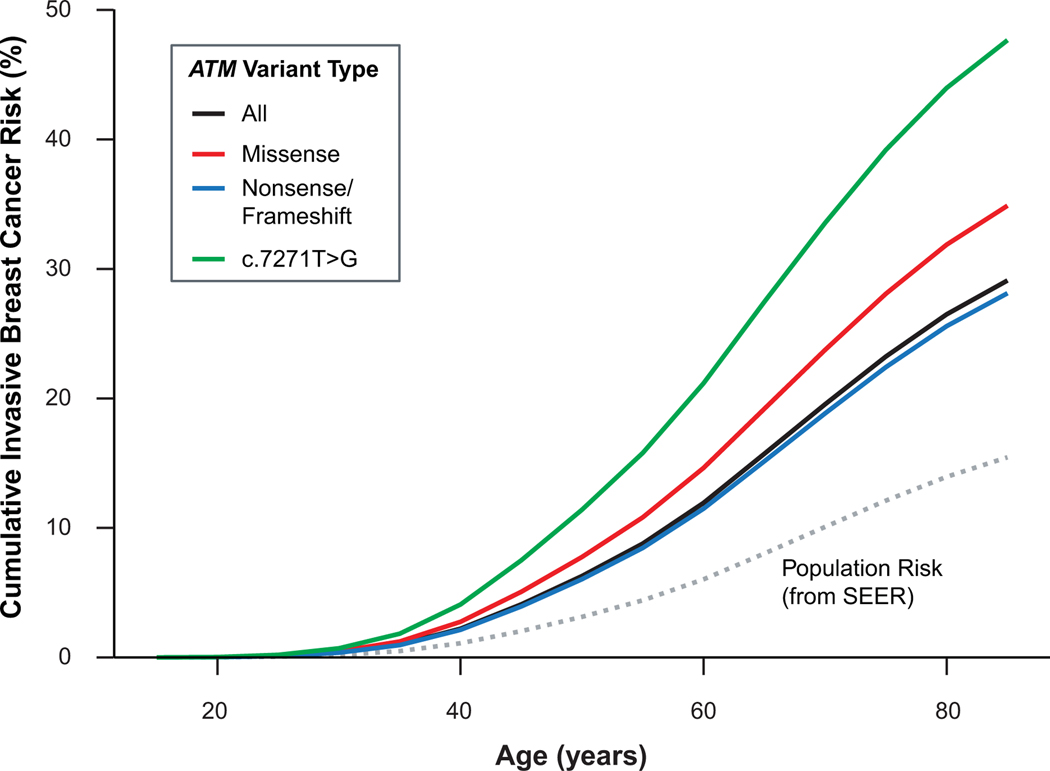
Family history-adjusted risk of breast cancer was also examined by mutation type and specifically in carriers of the c.7271T>G missense PV (Supplementary Table 3). The number of c.7271T>G carriers was insufficient to generate variant-specific risk estimates for cancers other than breast. Missense ATM PVs (OR 2.52; 95% CI 1.94–3.28) and truncating (nonsense and frameshift) ATM PVs (OR 1.95; 95% CI 1.77–2.14) were associated with similar risks of ductal invasive breast cancer. Risk estimates were significantly higher for c.7271T>G carriers (OR 3.76, 95% CI 2.76–5.21) compared to all other ATM PVs. Nonsense/frameshift PVs were also associated with an increased risk of DCIS (OR 2.05; 95% CI 1.78–2.36). The c.7271T>G PV was associated with an increased risk of DCIS (OR 1.70; 95% CI 1.03–2.81) and male breast cancer (OR 8.31, 95% CI 1.46–47.27). Cumulative risk of developing invasive breast cancer compared to population risk was modeled over an 80-year lifespan separately for each mutation type (Figure 2).
Figure 2:
Invasive Breast Cancer Cumulative Risk by Variant Type. Groups except for All are exclusive.
DISCUSSION
These results provide comprehensive estimates of the multiple cancer risks attributable to PVs in ATM, including close to two-fold elevated risks of invasive breast cancer and DCIS in female PV carriers. Additionally, moderate to high risks of pancreatic cancer (greater than four-fold increase), gastric cancer (three-fold increase), and prostate cancer (two to three-fold increase), and modestly yet significantly elevated risk of several other common tumors including colorectal cancer (1.5-fold increase) were observed. Of the cancers evaluated, only endometrial cancer did not show evidence of significant association with ATM PVs. Further, we demonstrate through analyses of the common c.7271T>G PV that there is site-specific variability in cancer risks with ATM PVs, constituting a genotype-phenotype association. While not all previously reported risks were confirmed by these analyses due to small numbers of reported cancers (e.g. thyroid cancer, head and neck cancers), it remains possible that such associations might be detectable in a larger, more diverse sample. Our estimates build on previous studies suggesting multiple risks of cancer for ATM PV carriers, [17,22] and offer new precision to the magnitude of those risks after controlling for confounding factors.
The importance of precise and comprehensive estimates of cancer risks associated with ATM PVs is supported by the high prevalence of ATM PVs in the general population, [1,2] the recognition of variability in cancer risk by PV type and position, [10–13] and the variety of common adult tumors like breast cancer, CRC, and prostate cancer whose risks may be moderately to highly increased by a germline ATM PV. It has been 30 years since Swift first published data detailing an increased risk of cancer in Ataxia-Telangiectasia families, [2] and understanding of the genetic risks underlying common cancers has advanced enormously. As genetic testing uptake increases, the identification of germline ATM PVs may contribute to reducing cancer incidence across a number of disease sites. Moreover, the development of poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors for tumors with homologous recombination deficiency due to disrupted function of BRCA1/2 and other Fanconi Anemia pathway-associated genes suggests that germline and tumor testing for ATM PVs may ultimately guide treatment selection [34,35].
Previous studies have demonstrated variability in breast cancer risk depending on the type of ATM PV (truncating versus missense) [6]. The c.7271T>G missense variant constituted almost 8% of all ATM PVs identified in this study. Previous research has strongly suggested that this variant increases cancer risk through a dominant-negative effect causing higher cancer risks in c.7271T>G carriers compared to other ATM PVs [10,12,13,36–38]. Our estimate of family history-adjusted ductal invasive breast cancer risk associated with the c.7271T>G PV [OR 3.76 (95% CI 2.76–5.12)] is similar to that estimated for BRCA2 PVs using the same methodology [7]. The family history-adjusted estimates presented here should be slightly lower than previously published unadjusted estimates, because the risk attributable to family history and ATM PV status is not double-counted. However, our estimates fall within the ranges reported from previous studies, all of which had wide confidence intervals. These include estimates from Goldgar et al. [OR 8.0 (95% CI 2.3–27.4)] [12], Southey et al. [OR 11.0 (95% CI 1.4–85.7)] [11], Bernstein et al. [8.6-fold increase risk (95% CI 3.9–18.9)] [13], and Chenevix-Trench et al. [13.7-fold increased risk (95% CI 5.1–36.6-fold)] [10]. Compared to these other studies, our study offers the advantage of a methodology that has been shown to produce unbiased results, and 10-fold more individuals genotyped and found to carry c.7271T>G non-carriers. ATM is a large gene comprised of 62 coding exons and 3056 amino acids (Figure 1), and plays a variety of roles in cellular processes including regulation of DNA damage response, DNA repair through multiple pathways, genotoxic stress response, and others [3]. Therefore, even rarer high-risk PV like c.7271T>G may be scattered throughout this complex gene, which have to date eluded discovery.
The current results demonstrate that ATM PV carriers have increased risk of pancreatic cancer (OR 4.21), prostate cancer (OR 2.58), and gastric cancer (2.97). ATM PV carriers were not included in the initial high-risk guidelines for pancreatic cancer published by the International Cancer of the Pancreas Screening (CAPS) Consortium in 2013 [39]; but since that time evidence has emerged demonstrating that germline ATM PVs are the most common, or second most common after BRCA2 PVs, findings in pancreatic cancer patients [15,40]. A recent study estimated a 5.7 OR for pancreatic cancer in ATM PV carriers, very close to what was calculated in the same study for BRCA2 (OR 6.2) [15]. Although ATM PV carriers are included in the CAPS5 pancreatic cancer screening study (NCT02000089), they are treated as lower risk than carriers of PVs in BRCA2 and PALB2. Future study design should consider the updated relative risk estimates for ATM from this and other studies.
The high prevalence of prostate cancer in the US population, as well as the challenge of differentiating prostate cancers associated with high morbidity and mortality from more indolent tumors, highlights the importance of identifying molecular and genetic factors associated with risk, treatment and outcomes. The prostate cancer history data in this analysis did not include Gleason score, so we could not determine whether ATM PVs are associated with more aggressive disease. Germline ATM PVs have previously been associated with more aggressive prostate cancers and shown to be susceptible to PARP inhibitor therapy [35,41]. Finally, our estimates of gastric cancer risk are consistent with those of Helgason et al. who estimated four to five-fold increased risks of gastric cancer [17], often early-onset, in carriers of germline loss-of-function ATM PVs. Though gastric cancer is rare in the US (~28,000 new cases annually), morbidity is exceedingly high (5-year survival ~30%) [32], highlighting the value of risk stratification through predictive genetic testing to promote risk factor mitigation (e.g. treatment of H. pylori infection, smoking cessation) and determine potential eligibility for gastric cancer screening, as performed in individuals with Lynch syndrome [42]. Notably, no differences in age at diagnosis were observed for prostate and gastric cancers based on whether an ATM PV was present or not. Further exploration of how ATM PV status may affect current screening guidelines is warranted for both of these tumors.
A recently published study examining data from a large sample of patients with diverse personal and cancer histories tested by multigene panel testing through a single laboratory reported ORs for ATM PV carriers for breast cancer (OR 2.91 95% CI 2.48–3.42), ovarian cancer (1.91, 95% CI 1.37–2.65), pancreatic cancer (OR 5.88, 95% CI 3.64–9.4), colorectal cancer (OR 2.6, 95% CI 1.53–4.3), endometrial cancer (OR 1.44, 95% CI 0.62 – 3.2) and melanoma (OR 2.69, 95% CI 1.32–5.23) [43]. Methodologies were notably different from those employed in our study, with the authors comparing ATM PVs in Caucasian breast cancer patients referred for hereditary cancer testing against PV frequencies observed in non-Finnish European controls from the gnomAD database. This methodology may significantly overestimate risk, since clinically ascertained cases are likely to be enriched for mutations over general population cases [44]. Despite this methodological difference, risk estimates are similar to those we report here.
Our study has several important limitations. Personal and family cancer history data were collected from a standard test requisition form; thus cancer pathology and age of diagnosis have not been verified. However, we have previously shown through sensitivity analyses that over- or under-reporting of family history would have modest or no impact on the risk estimates calculated using multivariable logistic regression [7]. In addition, despite the large sample size examined here, the relative rarity of missense ATM PVs and the c.7271T>G PV limited statistical power to estimate risks for cancer types beyond breast cancer. Similarly, small samples limited statistical power to estimate cancer risks precisely in rarer tumor types, such as triple-negative breast cancer and male breast cancer. In addition, our sample is notably female predominant (97%) reflective of the historically strong association of genetic testing for hereditary cancer risk with female breast cancer despite the broadening of testing indications in recent years to include prostate cancer and male-dominant cancers such as pancreatic cancer. Additionally, we may not have been able to detect situations where the risks for certain cancers, e.g. pancreatic, differ between men and women. Nonetheless, it should be noted that our proportionally smaller male population includes over 22,000 male patients and 229 male ATM PV carriers, as well as family history data in both male and female relatives among study participants. In conclusion, through analyses including multivariable modeling in a very large sample of individuals undergoing multi-gene panel testing for hereditary cancer risk, we demonstrate that PVs in ATM are associated with moderate- to high-risks for multiple adult-onset cancers, independent of family history. High risks of prostate, gastric, and notably pancreatic cancer may warrant screening in ATM PV carriers. Further, these results confirm that the c.7271T>G missense PV is associated with high risk for ductal invasive ductal breast cancer, with a three to four-fold risk increase that is similar to that of BRCA2 PVs and supports consideration of similar strategies for prevention and/or early detection.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge Stephanie Meek and Gabby Iorg for their assistance in manuscript preparation.
Conflicts of Interest: Dr. Hall receives research support through Core Grant funding from the National Cancer Institute to Fox Chase Cancer Center (NCI P30-CA006927) (PI: Richard I. Fisher). Dr. Kurian receives research support from the Suzanne Pride Bryan Fund for Breast Cancer Research, the Jan Weimer Faculty Chair in Breast Oncology, and the BRCA Foundation; she has also previously received funding from Myriad Genetics, Inc. for an unrelated project (2017–2019). At the time of this work, all other authors were employed by Myriad Genetics, Inc.
Footnotes
Prevention Relevance Statement: This study estimated risks for multiple cancers associated with ATM pathogenic variants independent of family history. These results indicate some common variants may be associated with higher breast cancer risks than previously appreciated and increased screening for prostate and gastric cancer may be warranted for carriers of ATM pathogenic variants.
REFERENCES
- 1.Swift M, Morrell D, Cromartie E, Chamberlin AR, Skolnick MH, Bishop DT. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet 1986;39(5):573–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Swift M, Reitnauer PJ, Morrell D, Chase CL. Breast and Other Cancers in Families with Ataxia-Telangiectasia. N Engl J Med 1987;316(21):1289–94 doi 10.1056/NEJM198705213162101. [DOI] [PubMed] [Google Scholar]
- 3.Rothblum-Oviatt C, Wright J, Lefton-Greif MA, McGrath-Morrow SA, Crawford TO, Lederman HM. Ataxia telangiectasia: a review. Orphanet J Rare Dis 2016;11(1):159 doi 10.1186/s13023-016-0543-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swift M, Morrell D, Massey RB, Chase CL. Incidence of Cancer in 161 Families Affected by Ataxia–Telangiectasia. N Engl J Med 1991;325(26):1831–6 doi 10.1056/NEJM199112263252602. [DOI] [PubMed] [Google Scholar]
- 5.Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet 2006;38(8):873–5 doi 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- 6.Tavtigian SV, Oefner PJ, Babikyan D, Hartmann A, Healey S, Le Calvez-Kelm F, et al. Rare, Evolutionarily Unlikely Missense Substitutions in ATM Confer Increased Risk of Breast Cancer. The American Journal of Human Genetics 2009;85(4):427–46 doi 10.1016/j.ajhg.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurian AW, Hughes E, Handorf EA, Gutin A, Allen B, Hartman A-R, et al. Breast and Ovarian Cancer Penetrance Estimates Derived From Germline Multiple-Gene Sequencing Results in Women. JCO Precision Oncology 2017(1):1–12 doi 10.1200/po.16.00066. [DOI] [PubMed] [Google Scholar]
- 8.Hauke J, Horvath J, Groß E, Gehrig A, Honisch E, Hackmann K, et al. Gene panel testing of 5589 BRCA1/2-negative index patients with breast cancer in a routine diagnostic setting: results of the German Consortium for Hereditary Breast and Ovarian Cancer. Cancer Medicine 2018;7(4):1349–58 doi 10.1002/cam4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H-M, Li S, Black MH, Lee S, Hoiness R, Wu S, et al. Association of Breast and Ovarian Cancers With Predisposition Genes Identified by Large-Scale Sequencing. JAMA Oncol 2019;5(1):51–7 doi 10.1001/jamaoncol.2018.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chenevix-Trench G, Spurdle AB, Gatei M, Kelly H, Marsh A, Chen X, et al. Dominant Negative ATM Mutations in Breast Cancer Families. JNCI: Journal of the National Cancer Institute 2002;94(3):205–15 doi 10.1093/jnci/94.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Southey MC, Goldgar DE, Winqvist R, Pylkäs K, Couch F, Tischkowitz M, et al. PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS. J Med Genet 2016;53(12):800–11 doi 10.1136/jmedgenet-2016-103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldgar DE, Healey S, Dowty JG, Da Silva L, Chen X, Spurdle AB, et al. Rare variants in the ATM gene and risk of breast cancer. Breast cancer research : BCR 2011;13(4):R73-R doi 10.1186/bcr2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein JL, Teraoka S, Southey MC, Jenkins MA, Andrulis IL, Knight JA, et al. Population-based estimates of breast cancer risks associated with ATM gene variants c.7271T>G and c.1066–6T>G (IVS10–6T>G) from the Breast Cancer Family Registry. Hum Mutat 2006;27(11):1122–8 doi 10.1002/humu.20415. [DOI] [PubMed] [Google Scholar]
- 14.Daly MB, Pilarski R, Berry M, Buys SS, Friedman S, Garber JE, et al. 2019 February 20, 2019. NCCN Clinical Practice Guidelines in Oncology, Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 3.2019). In NCCN Clinical Practice Guidelines in Oncology. Accessed 2019 February 20, 2019. [Google Scholar]
- 15.Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018;319(23):2401–9 doi 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, et al. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. J Clin Oncol 2017;35(30):3382–90 doi 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helgason H, Rafnar T, Olafsdottir HS, Jonasson JG, Sigurdsson A, Stacey SN, et al. Loss-of-function variants in ATM confer risk of gastric cancer. Nat Genet 2015;47(8):906–10 doi 10.1038/ng.3342. [DOI] [PubMed] [Google Scholar]
- 18.Huang D-S, Tao H-Q, He X-J, Long M, Yu S, Xia Y-J, et al. Prevalence of deleterious ATM germline mutations in gastric cancer patients. Oncotarget 2015;6(38):40953–8 doi 10.18632/oncotarget.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol 2017;3(4):464–71 doi 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yurgelun MB, Allen B, Kaldate RR, Bowles KR, Judkins T, Kaushik P, et al. Identification of a Variety of Mutations in Cancer Predisposition Genes in Patients With Suspected Lynch Syndrome. Gastroenterology 2015;149(3):604–13.e20 doi 10.1053/j.gastro.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurian AW, Ward KC, Howlader N, Deapen D, Hamilton AS, Mariotto A, et al. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J Clin Oncol 2019;37(15):1305–15 doi 10.1200/JCO.18.01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dombernowsky SL, Weischer M, Allin KH, Bojesen SE, Tybjjrg-Hansen A, Nordestgaard BG. Risk of Cancer by ATM Missense Mutations in the General Population. J Clin Oncol 2008;26(18):3057–62 doi 10.1200/JCO.2007.14.6613. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein AM, Xiao Y, Sampson J, Zhu B, Rotunno M, Bennett H, et al. Rare germline variants in known melanoma susceptibility genes in familial melanoma. Hum Mol Genet 2017;26(24):4886–95 doi 10.1093/hmg/ddx368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med 2016;375(5):443–53 doi 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenthal ET, Bernhisel R, Brown K, Kidd J, Manley S. Clinical testing with a panel of 25 genes associated with increased cancer risk results in a significant increase in clinically significant findings across a broad range of cancer histories. Cancer genetics 2017;218–219:58–68 doi 10.1016/j.cancergen.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Couch FJ, Shimelis H, Hu C, Hart SN, Polley EC, Na J, et al. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol 2017;3(9):1190–6 doi 10.1001/jamaoncol.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman KJ, Greenland S, Lash T. Modern epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. 95–7 p. [Google Scholar]
- 28.Rajamani S ELIMINATING BIAS IN CANCER RISK ESTIMATES A SIMULATION STUDY: Univeristy of Utah; 2016. 53 p. [Google Scholar]
- 29.Eggington JM, Bowles KR, Moyes K, Manley S, Esterling L, Sizemore S, et al. A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clinical genetics 2014;86(3):229–37 doi 10.1111/cge.12315. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 1958;53(282):457–81 doi 10.2307/2281868. [DOI] [Google Scholar]
- 31.Satagopan JM, Offit K, Foulkes W, Robson ME, Wacholder S, Eng CM, et al. The lifetime risks of breast cancer in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2001;10(5):467–73. [PubMed] [Google Scholar]
- 32.Surveillance Epidemiology, and Results End (SEER) Program Populations (1969–2014). National Cancer Institute, DCCPS Surveillance Research Program, Surveillance Systems Branch, released March 2016. [Google Scholar]
- 33.Rainville I, Hatcher S, Rosenthal E, Larson K, Bernhisel R, Meek S, et al. High risk of breast cancer in women with biallelic pathogenic variants in CHEK2. Breast Cancer Res Treat 2020. doi 10.1007/s10549-020-05543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weston VJ, Oldreive CE, Skowronska A, Oscier DG, Pratt G, Dyer MJS, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood 2010;116(22):4578–87 doi 10.1182/blood-2010-01-265769. [DOI] [PubMed] [Google Scholar]
- 35.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015;373(18):1697–708 doi 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gatti RA, Tward A, Concannon P. Cancer Risk in ATM Heterozygotes: A Model of Phenotypic and Mechanistic Differences between Missense and Truncating Mutations. Mol Genet Metab 1999;68(4):419–23 doi 10.1006/mgme.1999.2942. [DOI] [PubMed] [Google Scholar]
- 37.Waddell N, Jonnalagadda J, Marsh A, Grist S, Jenkins M, Hobson K, et al. Characterization of the breast cancer associated ATM 7271T>G (V2424G) mutation by gene expression profiling. Genes Chromosomes Cancer 2006;45(12):1169–81 doi 10.1002/gcc.20381. [DOI] [PubMed] [Google Scholar]
- 38.Marabelli M, Cheng S-C, Parmigiani G. Penetrance of ATM Gene Mutations in Breast Cancer: A Meta-Analysis of Different Measures of Risk. Genet Epidemiol 2016;40(5):425–31 doi 10.1002/gepi.21971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley J-W, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62(3):339–47 doi 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaffee KG, Oberg AL, McWilliams RR, Majithia N, Allen BA, Kidd J, et al. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genetics in medicine : official journal of the American College of Medical Genetics 2018;20(1):119–27 doi 10.1038/gim.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Na R, Zheng SL, Han M, Yu H, Jiang D, Shah S, et al. Germline Mutations in ATM and BRCA1/2 Distinguish Risk for Lethal and Indolent Prostate Cancer and are Associated with Early Age at Death. Eur Urol 2017;71(5):740–7 doi 10.1016/j.eururo.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Provenzale D, Gupta S, Ahnen DJ, Blanco AM, Bray TH, Chung DC, et al. 2018 August 1, 2018. NCCN Clinical Practice Guidelines in Oncology, Genetic/Familial High-Risk Assessment: Colorectal (Version 1.2018). In NCCN Clinical Practice Guidelines in Oncology. August 1, 2018. [Google Scholar]
- 43.LaDuca H, Polley EC, Yussuf A, Hoang L, Gutierrez S, Hart SN, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med 2019. doi 10.1038/s41436-019-0633-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlesselman JJ. Case control studies: design, conduct, analysis. New York, NY: Oxford University Press; 1982. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.