Abstract
Background:
Ischemia with no obstructive coronary artery disease (INOCA) is prevalent in women and is associated with increased risk of developing heart failure with preserved ejection fraction (HFpEF); however, the mechanism(s) contributing to this progression remains unclear. Given that diastolic dysfunction is common in women with INOCA, defining mechanisms related to diastolic dysfunction in INOCA could identify therapeutic targets to prevent HFpEF.
Methods:
Cardiac MRI was performed in 65 women with INOCA and 12 reference controls. Diastolic function was defined by left ventricular early diastolic circumferential strain rate (eCSRd). Contributors to diastolic dysfunction were chosen a priori as coronary vascular dysfunction (myocardial perfusion reserve index [MPRI]), diffuse myocardial fibrosis (extracellular volume [ECV]), and aortic stiffness (aortic pulse wave velocity [aPWV]).
Results:
Compared to controls, eCSRd was lower in INOCA (1.61±0.33/s vs. 1.36±0.31/s, P=0.016); however, this difference was not exaggerated when the INOCA group was sub-divided by low and high MPRI (P>0.05) nor was ECV elevated in INOCA (29.0±1.9% vs. 28.0±3.2%, control vs. INOCA; P=0.38). However, aPWV was higher in INOCA vs. controls (8.1±3.2m/s vs. 6.1±1.5m/s; P=0.045), and was associated with eCSRd (r = −0.50, P < 0.001). By multivariable linear regression analysis, aPWV was an independent predictor of decreased eCSRd (standardized β = −0.39, P=0.003), as was having an elevated left ventricular mass index (standardized β = −0.25, P=0.024) and lower ECV (standardized β = 0.30, P=0.003).
Conclusions:
These data provide mechanistic insight into diastolic dysfunction in women with INOCA, identifying aortic stiffness and ventricular remodeling as putative therapeutic targets.
Keywords: Diastolic function, coronary vascular dysfunction, aortic stiffness, pulse wave velocity, myocardial perfusion reserve, MRI
Introduction
Ischemia with no obstructive coronary artery disease (INOCA) is prevalent in women, and is associated with increased risk of major adverse cardiovascular events, including heart failure with preserved ejection fraction (HFpEF) [1–3]. The mechanism(s) contributing to progression to HFpEF in INOCA however, remains to be elucidated. One common trait consistently observed in both populations, and the most conspicuous and unifying hemodynamic finding of HFpEF, is left ventricular diastolic dysfunction, characterized by impaired early diastolic relaxation and elevated end-diastolic pressures [4–11]. Identifying the mechanism(s) contributing to diastolic dysfunction in INOCA is therefore critically important for understanding disease progression and developing new therapeutic interventions.
Multiple mechanisms have been suggested as contributors to left ventricular diastolic dysfunction in INOCA, including: (a) coronary vascular dysfunction-mediated impairment in active, energy-dependent, myocardial relaxation [12], (b) diffuse myocardial fibrosis [13, 14], and/or (c) aortic stiffness-mediated impairment in ventricular-arterial coupling [15, 16]. To evaluate the contribution of each of these proposed mechanisms on left ventricular diastolic dysfunction, we performed comprehensive cardiac magnetic resonance imaging (cMRI) to evaluate myocardial perfusion reserve, left ventricular tissue properties, and aortic stiffness.
Methods
Sixty-five women with suspected INOCA from the Women’s Ischemia Syndrome Evaluation – Coronary Vascular Dysfunction continuation study (NCT02582021), enrolled between October 2015 - June 2019, were included in the current investigation. INOCA was defined as having signs and symptoms of ischemia but no obstructive coronary disease (<50% coronary artery stenosis in any coronary artery) confirmed by angiography. Twelve reference control women were also studied, who had no symptoms, cardiovascular risk factors, or evidence of ischemic heart disease, confirmed by a standard 12-lead ECG treadmill stress test. All study subjects provided written informed consent prior to evaluation, and the study protocol was approved by the Institutional Review Board at Cedars-Sinai Medical Center.
All medications were withdrawn prior to experimental visits with long-acting nitrates, short-acting calcium-channel blockers, α-blockers, β-blockers, and angiotensin-converting enzyme-I/angiotensin-II-receptor antagonists held for 24 hours, and long-acting calcium-channel blockers held for 48 hours before CRT. Sublingual nitroglycerin was not taken within 4 hours of testing, and participants were caffeine-free and nicotine-free for 24 hours before testing. cMRI was performed on a 3T scanner (Siemens Healthineers, Erlangen, Germany), with ECG-gating and a phased-array receiver coil (CP Body Array Flex; Siemens Healthineers). Heart rate and blood pressure were measured and recorded throughout the study. Detailed imaging paramters are provided in the Online Supplement. Breifly, left ventricular mass, volume and systolic/diastolic function were assessed using a series of short-axis steady-state free-precession cine images spanning the entire left ventricle, along with 2- and 4-chamber long-axis images. To assess aortic pulse wave velocity (aPWV), two separate through-plane phase-contrast images were acquired: (1) at the level of the ascending aorta, and (2) ~10 cm distal, along the descending aorta. The distance between the ascending and descending images was manually determined from a sagittal image of the aortic arch. To evaluate myocardial perfusion reserve index (MPRI), basal, mid and distal short-axis first-pass myocardial perfusion images were acquired under resting conditions and in response to intra-venous adenosine (140 μg/kg/min over ~4 min; Adenoscan, Astellas Pharma US, Inc., Northbrook, IL) infusion, with use of a gadolinium-based contrast agent (0.05 mmol/kg Gadavist, Bayer HealthCare Pharmaceuticals) also administered intravenously (at 4 mL/s) as previously described [17, 18]. Coronary vascular dysfunction was defined as an MPRI <1.84, based on previously established cut-offs [17]. Prior to first-pass perfusion imaging a mid-ventricular short-axis T1 relaxation image (vendor provided MOLLI 5[3]3) was acquired in mid-diastole for assessment of myocardial native T1 relaxation time. After completion of first-pass perfusion imaging, an additional 0.1mmol/kg of contrast was administered (total gadolinium dose 0.2 mmol/kg), and post-contrast T1 images were acquired after waiting 12 minutes following the final gadolinium bolus, for calculation of extracellular volume (ECV). To challenge the myocardial oxygen supply-demand relationship, participants performed 5–7 minutes of continuous isometric handgrip exercise, at 30% of maximal voluntary contraction, using an MRI compatible handgrip dynamometer (Smedley, Stoelting Company, Wood Dale, Illinois). Left ventricular volume and function during handgrip was assessed by repeating the 2- and 4-chamber long-axis images, together with a mid-ventricular short-axis image. Individuals with an insufficient increase in the hemodynamic stress associated with isometric handgrip (defined as Δ heart rate <10 bpm and Δ mean arterial pressure <10 mmHg) were not considered for rest-stress handgrip comparisons.
All image analysis was performed using commercially available software (CVI42 version 5.6.8; Circle Cardiovascular Imaging Inc., Calgary, AB, Canada). Resting left ventricular mass and volumes were measured using the method of disks by manually tracing the endocardial and epicardial boarders, of the short-axis series, at end-diastole and end-systole. For rest-handgrip comparisons, left ventricular volumes were assessed using the biplane method by manually delineating the endocardial boarder, of the 2- and 4-chamber images, at end-diastole and end-systole. Left ventricular mass and volumes were indexed to body surface area. End-systolic elastance was calculated as (0.9 × peak brachial systolic blood pressure)/end-systolic volume and effective arterial elastance was calculated as (0.9 × peak brachial systolic blood pressure)/stroke volume [19]. Rate pressure product was calculated as the product of peak brachial systolic blood pressure and heart rate, and referred to as a surrogate measure of myocardial oxygen demand throughout [20].
Left ventricular circumferential and longitudinal strain and strain rates in systole and diastole were assessed by myocardial feature tracking, as previously described [10]. The endocardial and epicardial boarders were manually traced at end-diastole, on both short-axis and long-axis cine images, before applying the feature tracking algorithm across the remaining cardiac phases. Short-axis slices close to luminal obliteration (lumen diameter <2cm), and slices which included left ventricular outflow tract, were excluded as previously described [10]. Patients with insufficient tracking quality were excluded from the final analyses.
The distance between ascending and distal portion of descending aorta were measured between the precise locations where the through-plane phase-contrast images were collected using an oblique sagittal image through the thoracic aorta. The aortic transit time was calculated as the average time difference between the systolic up-slope of the ascending and descending aortic flow curves. aPWV was calculated as the distance between the ascending and descending aorta, divided by the transit time between the two aortic locations.
Myocardial perfusion reserve index (MPRI) was calculated as the average relative up-slope from the three first-pass perfusion images collected during adenosine stress divided by the average relative up-slope from the three resting images, normalized to the left ventricular luminal blood pool up-slopes at rest and during stress, respectively [17].
The endocardial and epicardial borders of the mid short-axis native and post-contrast T1 images were conservatively drawn taking care to exclude partial volume effects from both the blood pool and the surrounding tissues. The average T1 from the entire mid-wall slice of the myocardium from the native and post-contrast images were used to calculate ECV, as previously described [21].
Tonometer-derived carotid-femoral pulse wave velocity (central PWV) was also performed in all participants in order to provide an alternative measure of pulse-wave velocity beyond the MRI-based approach described herein [22]. With participants laying in the supine position, central PWV was assessed by placing a piezoelectric tonometer on the carotid artery, with the arterial blood pressure waveform in the femoral artery detected by a cuff placed around the thigh (SphygmoCor AtCor Medical, Australia). Sequential 10–20 second recordings of the arterial pressure waveforms, gated to the electrocardiogram, were taken at each location, and the PWV was calculated as the distance between each measurement location divided by the transit time derived from the R-wave of the ECG [23].
All statistical analyses were performed using SPSS Version 25 for Windows, as described in detail in the Online Supplement. Breifly, cross-sectional group differences and characteristics between women with INOCA and reference controls were tested with independent samples t-tests. Group comparisons involving more than two groups were assessed by one-way ANOVA for normally distributed variables and the Kruskal-Wallis test for non-normally distributed variables. LSD post-hoc comparisons were performed for variables with significant group main effects. Assessment of group differences in dependent variables in response to handgrip exercise were performed with repeated measures ANOVA. Pearson and Spearman rank-order correlations were computed to assess correlations among the study variables.
The association between diastolic function [defined a priori as early diastolic circumferential strain rate, based on our previous observations [6, 10, 24]] and other cMRI and hemodynamic variables were examined by univariable and multiple linear regression analyses in the entire sample of reference controls and women with INOCA. Variables with P ≤ 0.10 during univariable analysis were selected as predictors for the multivariable analysis. Standardized β-coefficients, 95% confidence intervals, and P-values are reported for all predictors considered for both univariable and multivariable analyses. Categorical variables are summarized using counts and percentages and compared using the Pearson chi-square test. All parametric data are expressed as means ± SD, and non-parametric as median (interquartile range). The study alpha was set to 0.05.
Results
Reference control subjects and women with suspected INOCA were well matched for age and anthropometric indices (Table 1). As expected, women with suspected INOCA reported both signs and symptoms of ischemia (Seattle Angina Questionnaire), with moderate frequency and burden of clinical symptoms (Kansas City Cardiomyopathy Questionnaire).
Table 1.
Baseline characteristics and left ventricular function in women with INOCA and controls.
| Reference Control (n = 12) | INOCA (n = 65) | P-value | |
|---|---|---|---|
| Anthropometrics and Hemodynamics | |||
| Age (years) | 50 ± 5 | 55 ± 11 | 0.14 |
| Height (cm) | 161 ± 6 | 164 ± 6 | 0.79 |
| Weight (kg) | 69.4 ± 8.9 | 71.4 ± 15.1 | 0.64 |
| BSA (m2) | 1.73 ± 0.11 | 1.77 ± 0.16 | 0.80 |
| SBP (mmHg) | 120 ± 14 | 114 ± 15 | 0.20 |
| DBP (mmHg) | 64 ± 11 | 63 ± 10 | 0.79 |
| Heart Rate (bpm) | 63 ± 9 | 62 ± 8 | 0.77 |
| Medical History | |||
| Hypertension n (%) | 0 (0) | 19 (29) | 0.03 |
| Diabetes n (%) | 0 (0) | 2 (3) | 0.54 |
| Hypercholesteremia n (%) | 0 (0) | 8 (12) | 0.71 |
| Smoking History n (%) | 1 (8) | 16 (25) | 0.04 |
| Medications | |||
| Beta-Blockers n (%) | 0 (0) | 18 (28) | - |
| ACEi n (%) | 0 (0) | 19 (29) | - |
| ARB n (%) | 0 (0) | 4 (6) | - |
| Statin n (%) | 0 (0) | 38 (58) | - |
| Health Status | |||
| SAQ - Exertional Capacity | n/a | 62 ± 23 | - |
| SAQ - Anginal Stability | n/a | 49 ± 22 | - |
| SAQ - Anginal Frequency | n/a | 49 ± 27 | - |
| SAQ - Disease Perception | n/a | 47 ± 21 | - |
| SAQ - Treatment Satisfaction | n/a | 72 ± 19 | - |
| KCCQ - Clinical Summary Score | n/a | 69 ± 19 | - |
| KCCQ - Overall Summary Score | n/a | 64 ± 22 | - |
| LV Mass & Volumes | |||
| EDVi (mL/m2) | 64 ± 8 | 67 ± 10 | 0.18 |
| ESVi (mL/m2) | 23 ± 6 | 25 ± 6 | 0.28 |
| SVi (mL/m2) | 41 ± 4 | 42 ± 6 | 0.34 |
| EF (%) | 64 ± 5 | 63 ± 5 | 0.62 |
| COi (L/min/m2) | 2.6 ± 0.3 | 2.7 ± 0.5 | 0.35 |
| LV Mass Index (g/m2) | 40.3 ± 3.6 | 43.2 ± 6.3 | 0.12 |
| Concentricity (g/mL) | 0.63 ± 0.06 | 0.65 ± 0.10 | 0.59 |
| LA Volume Index (mL/m2) | 36 ± 4 | 36 ± 7 | 0.89 |
| Mitral Inflow | |||
| E Velocity (cm/s) | 70 ± 15 | 69 ± 19 | 0.92 |
| A Velocity (cm/s) | 56 ± 12 | 56 ± 17 | 0.98 |
| E/A Ratio | 1.31 ± 0.40 | 1.37 ± 0.61 | 0.78 |
| LV Strain and Strain Rate | |||
| Circumferential Strain (%) | −24.8 ± 1.67 | −24.3 ± 2.5 | 0.51 |
| Circumferential Systolic SR (/s) | −1.14 ± 0.15 | −1.13 ± 0.19 | 0.84 |
| Circumferential Early Diastolic SR (/s) | 1.61 ± 0.33 | 1.36 ± 0.31 | 0.02 |
| Circumferential Late Diastolic SR (/s) | 0.70 ± 0.17 | 0.74 ± 0.21 | 0.51 |
| Longitudinal Strain (%) | −21.6 ± 2.4 | −20.9 ± 2.6 | 0.42 |
| Longitudinal Systolic SR (/s) | −0.98 ± 0.18 | −0.93 ± 0.31 | 0.57 |
| Longitudinal Early Diastolic SR (/s) | 1.23 ± 0.36 | 1.06 ± 0.25 | 0.04 |
| Longitudinal Late Diastolic SR (/s) | 0.88 ± 0.22 | 0.83 ± 0.23 | 0.52 |
| Myocardial Tissue Characteristics and Perfusion Reserve | |||
| Native T1 (ms) | 1246 ± 38 | 1253 ± 72 | 0.75 |
| Post-Contrast T1 (ms) | 456 ± 24 | 446 ± 59 | 0.61 |
| ECV (%) | 29.0 ± 1.9 | 28.0 ± 3.2 | 0.38 |
| MPRI | 1.89 ± 0.29 | 1.72 ± 0.30 | 0.08 |
| Late Gadolinium Enhancement | |||
| Ischemic Pattern (n/%) | 0/0 | 1/2 | |
| Non-Ischemic Pattern (n/%) | 0/0 | 9/14 | |
| Aortic Stiffness | |||
| aPWV (m/s) | 6.1 ± 1.5 | 8.1 ± 3.2 | 0.05 |
INOCA – ischemia with no obstructive coronary artery disease; BSA – body surface area; SBP – systolic blood pressure; DBP – diastolic blood pressure; LV – left ventricular; SAQ – Seattle angina questionnaire; KCCQ – Kansas City cardiomyopathy Questionnaire; EDVi – end-diastolic volume index; ESVi – end-systolic volume index; SVi – stroke index; EF – ejection fraction; COi – cardiac index; LA – left atrial; SR – strain rate; ECV – extracellular volume; MPRI – myocardial perfusion reserve index; aPWV – aortic pulse wave velocity. Mean ± SD.
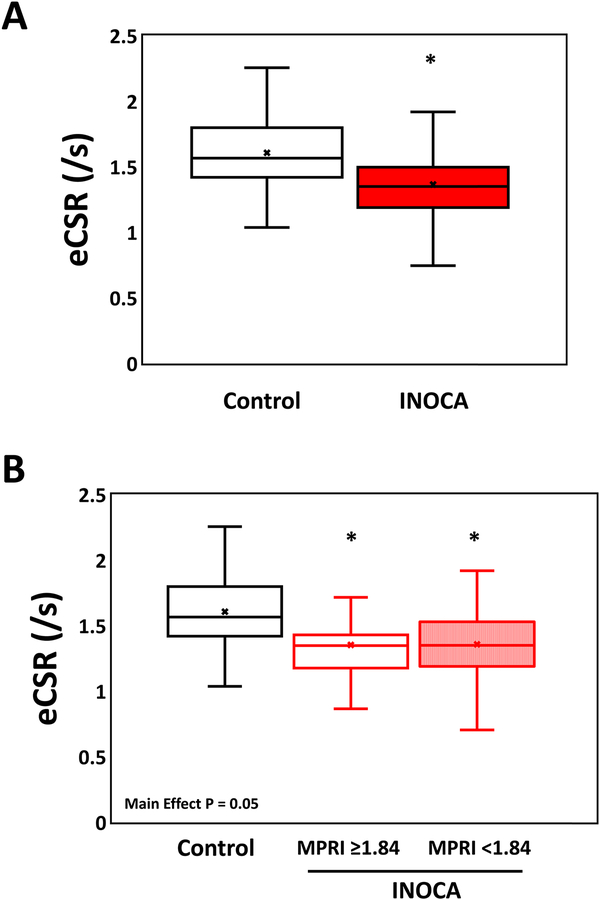
Consistent with previous reports from our group, early diastolic circumferential strain rate was lower in INOCA compared to reference controls (Figure 1A), as was early diastolic longitudinal strain rate (Table 1). To explore contributors to diastolic dysfunction, we evaluated three potential mechanisms: (a) coronary vascular dysfunction, (b) diffuse myocardial fibrosis, and/or (c) aortic stiffness.
Figure 1.
Left ventricular early diastolic circumferential strain rate (eCSR) is reduced in women with INOCA compared to reference controls (A). However, this difference is not exacerbated by splitting the women with INOCA by high and low myocardial perfusion reserve index (MPRI; B). * - indicated significantly different from controls (P < 0.05).
Coronary vascular dysfunction
To evaluate the influence of coronary vascular dysfunction on left ventricular diastolic function, the women with suspected INOCA were sub-divided by either low (<1.84; n = 41) or high (≥1.84; n = 22) MPRI, and compared to reference controls. Two women with INOCA did not receive adenosine and therefore were not considered for this analysis. Participant characteristics and cardiovascular measures did not differ between women with suspected INOCA grouped by MPRI (Supplemental Table 1). While early diastolic circumferential and longitudinal strain rate remained lower in INOCA compared to controls, having a lower MPRI did not exacerbate diastolic dysfunction (Figure 1B) and MPRI was not related to either early diastolic circumferential strain rate (r = −0.02, P = 0.90) or early diastolic longitudinal strain rate (r = 0.04, P = 0.76).
To further explore whether coronary vascular dysfunction contributes to diastolic dysfunction in INOCA, a subset of participants performed isometric handgrip exercise, to challenge the oxygen supply-demand relationship. As summarized in Supplemental Table 2, no stress induced group differences were observed in left ventricular early diastolic relaxation.
Diffuse myocardial fibrosis
To explore whether diastolic dysfunction in INOCA is associated with diffuse myocardial fibrosis, we measured both native T1 and post-contrast T1, in order to assess myocardial tissue properties and calculate ECV. Compared to reference controls, women with suspected INOCA had similar native T1 (1246 ± 38 ms vs. 1253 ± 72 ms, control vs. INOCA, respectively; P = 0.75) and calculated ECV (29.0 ± 1.9% vs. 28.0 ± 3.2%, control vs. INOCA, respectively; P = 0.38). However, contrary to our hypothesis, ECV was positively related to early diastolic circumferential (r = 0.36, P = 0.004) and longitudinal strain rate (r = 0.26, P = 0.041).
Aortic Stiffness
To explore whether aortic stiffness contributes to diastolic dysfunction in INOCA, we examined the relationship between aPWV and indices of diastolic function. Image quality prevented aPWV measurements in six women with suspected INOCA. Compared to reference controls, aPWV was higher in women with suspected INOCA (6.1 ± 1.5 m/s vs. 8.1 ± 3.2 m/s, control vs. INOCA, respectively; P = 0.045; Table 1), and was associated with decreased early diastolic circumferential (r = −0.50, P < 0.001; Figure 2A) and longitudinal strain rate (r = −0.35, P = 0.003). To further explore this observation, we sub-divided women with suspected INOCA by either low (<7.4 m/s; n = 29) or high (≥7.4 m/s; n = 30) aPWV; defined by the median value in the INOCA group (Supplemental Table 3). Consistent with the correlation data, INOCA women with high aPWV had the worst early diastolic circumferential (P = 0.003; Figure 2B), and longitudinal strain rates (P = 0.04) and were over-reliant on late diastolic strain rates (both P < 0.001), compared to either INOCA women with low aPWV or reference controls (Supplemental Table 3). Moreover, INOCA women with high aPWV had elevated systolic blood pressure (114 ± 15 mmHg vs. 113 ± 11 mmHg vs. 124 ± 14 mmHg, controls vs. INOCA low aPWV vs. INOCA high aPWV, respectively; P = 0.003), and lower ECV (29.0 ± 1.9% vs. 28.8 ± 3.3% vs. 27.1 ± 3.1%, control vs. INOCA low aPWV vs. INOCA high aPWV, respectively; P = 0.04, Supplemental Table 3).
Figure 2.
Aortic pulse wave velocity (aPWV) is inversely correlated with left ventricular early diastolic circumferential strain rate (eCSR) in all three groups (A). INOCA women with high aPWV have significantly lower eCSR compared to INOCA women with low aPWV and reference controls (B). * - indicated significantly different from controls (P < 0.01); † - indicates significantly different from INOCA women with low aortic pulse wave velocity (P < 0.05).
Notably, aPWV was correlated with tonometer-based carotid-femoral pulse wave velocity, and sub-dividing the women with suspected INOCA by the median carotid-femoral pulse wave velocity resulted in similar group differences in left ventricular diastolic dysfunction (Supplemental Table 4 and Supplemental Figure 1).
Predictors of early diastolic dysfunction
Univariable and multivariable logistic regression models for the determinants of early diastolic circumferential strain rate are summarized in Table 2. Age, systolic blood pressure, left ventricular mass index, aPWV, and ECV were all significantly (all P < 0.05) associated with early diastolic circumferential strain rate upon univariable analyses, and were therefore all included in the multivariable model. Upon multivariable linear regression left ventricular mass index (standardized β = −0.25 [−0.03 to 0.00 95%CI], P = 0.024), aPWV (standardized β = −0.39 [−0.59 to −0.13 95%CI], P = 0.003), and ECV (standardized β = 0.30 [0.01 to 0.05 95%CI], P = 0.003) were all significant independent predictors of early diastolic circumferential strain rate.
Table 2.
Cardiac MRI and hemodynamic variables associated with early diastolic circumferential strain rate.
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Standardized β (95% CI) | P-value | Standardized β (95% CI) | P-value | |
| Age | −0.52 (−0.02 to −0.01) | < 0.001 | −0.11 (−0.01 to 0.00) | 0.38 |
| BMI† | 0.06 (−2.77 to 13.3) | 0.59 | ||
| BSA† | 0.07 (−1.13 to 2.01) | 0.58 | ||
| SBP | −0.33 (−0.01 to −0.00) | 0.004 | 0.05 (0.00 to 0.01) | 0.65 |
| DBP | −0.11 (−0.01 to 0.00) | 0.33 | ||
| Heart rate | −0.04 (−0.01 to 0.00) | 0.74 | ||
| EF | 0.03 (−0.01 to 0.02) | 0.79 | ||
| LV mass index | −0.24 (−0.03 to 0.00) | 0.04 | −0.20 (−0.03 to 0.00) | 0.045 |
| aPWV‡ | −0.56 (−0.71 to −0.34) | < 0.001 | −0.43 (−0.62 to −0.16) | 0.001 |
| ECV | 0.35 (0.01 to 0.06) | 0.003 | 0.37 (0.02 to 0.06) | 0.001 |
| MPRI | −0.01 (−0.26 to 0.24) | 0.93 | ||
- variable was transformed by 1/(variable) to approximate a normal distribution.
- variable was transformed by ln(aPWV) to approximate a normal distribution. BMI – body mass index; BSA – body surface area; SBP – systolic blood pressure; DBP – diastolic blood pressure; EF – ejection fraction; LV – left ventricular; aPWV – aortic pulse wave velocity; ECV – extracellular volume; MPRI – myocardial perfusion reserve; CI – confidence intervals.
Discussion
Using a comprehensive cMRI approach, this study systematically evaluated three potential contributors thought to be responsible for the development of diastolic dysfunction in women with suspected INOCA; an observation frequently reported by our group and others [5–7, 11, 25]. Together, the data show that aPWV and left ventricular mass index are direct indpendent predictors of diastolic dysfunction in women with suspected INOCA, while left ventricular ECV being inversly predictive, with no discernible contribution from impaired myocardial perfusion reserve.
Women with INOCA are at increased risk of developing HFpEF, yet the mechanism driving disease progression remains incompletely understood. Women with INOCA often have diastolic dysfunction [5–7, 11, 25]; a common trait also frequently observed in HFpEF [8, 26]. Moreover, women with INOCA often have coronary vascular dysfunction [17, 25, 27, 28], and HFpEF patients with coronary vascular dysfunction have worse diastolic function then HFpEF without [12]. Together, this has led to the hypothesis that coronary vascular dysfunction both directly (via energy dependent active relaxation, [29]) and indirectly (via diffuse myocardial fibrosis, [30]) leads to left ventricular diastolic dysfunction, and heart failure progression. Moreover, it is believed that vascular dysfunction seen in the coronary arteries also manifests in the systemic circulation, and therefore may contribute to diastolic dysfunction indirectly through ventricular-arterial uncoupling. Here, using a comprehensive cMRI approach, we systematically evaluate each of these putative mechanistic pathways.
That grouping women with suspected INOCA according to MPRI did not differentiate between normal and abnormal diastolic function argues against the impaired myocardial perfusion reserve hypothesis. Because this comparison was performed using magnetic resonance images collected under resting conditions, it is possible that diastolic dysfunction may only be unmasked under conditions of increased oxygen demand (i.e. physiological stress leading to an oxygen supply-demand mismatch). To test this, isometric handgrip was performed in a subset of participants. In contrast to our hypothesis, however, isometric handgrip failed to exacerbate diastolic dysfunction in suspected INOCA. While we did not directly assess myocardial ischemia in this investigation, our group has documented isometric handgrip induced myocardial ischemia previously in this patient cohort [31]. Moreover, inclusion of participants in this sub-analysis was limited only to those individuals who achieved a robust hemodynamic stress response (i.e. greatest increase in rate pressure product), with INOCA sub-divided according to myocardial perfusion reserve index.
Our group has also observed frequent episodes of ST segment depression in women with suspected INOCA with ambulatory monitoring [32], and increased prevalence of focal scar lesions [33]. These observations, together with >2 decades of evidence showing a high prevalence of coronary vascular dysfunction in INOCA, has led to the hypothesis that women with INOCA experience repeat episodes of acute myocardial ischemia, which in turn could lead to the expansion of the extracellular matrix and diffuse/patchy myocardial fibrosis. In contrast to this hypothesis however, we did not observe any difference in native T1 or post-contrast ECV between INOCA and reference controls. That native T1 was not elevated in INOCA is inconsistent with a previous report from our group [18]. Though it remains unclear why these two observations are inconsistent, differences in sample size, and/or the extent/pattern of coronary vascular dysfunction may have contributed. Directionally inconsistent with our original hypothesis, we also found that low ECV was independently associated with low early diastolic circumferential strain rate. One possible explanation for this observation could be that myocyte hypertrophy is occurring independent of extracellular volume expansion, in this relatively early stage clinical population. Indeed, left ventricular mass index was negatively associated with early diastolic strain rate, the same way ECV was positively associated. Similar observations have been seen in response to physiological adaptations in athletes [34]. Future investigations are however required to substantiate this observation. Regardless, these data suggest that negative alterations to myocardial tissue characteristics (i.e. increased fibrosis) are unlikely to be playing a dominant role in the development of diastolic dysfunction in suspected INOCA.
Ventricular-arterial coupling has long been recognized as an important contributor to cardiac mechanics and hemodynamics. Alterations in the stiffness of the central and peripheral vascular system elevate cardiac afterload and compromise cardiac efficiency [15, 16]. The data herein are the first to show that women with suspected INOCA have elevated aPWV compared to reference controls and that aPWV is inversely related to, and an independent predictor of, left ventricular early diastolic strain rate. Furthermore, after sub-dividing the INOCA group by low and high aPWV, we found that those with the highest aPWV had the worst left ventricular diastolic function. A similar relationship between pulse wave velocity and diastolic function was also observed using tonometer-based carotid-femoral pulse wave velocity (Supplemental Data), increasing the external validity of this primary finding, and supporting the overall interpretation of these results. While the exact mechanism by which increased conduit artery stiffness causes diastolic dysfunction remains incompletely understood, we speculate that increased left ventricular afterload, along with decreased coronary perfusion, may have contributed [19]. Indeed, proximal aortic impedance, shorter time to the arrival of the reflected wave, and total compliance of the peripheral arterial tree all contribute to increased aortic stiffness. These mechanisms challenge left ventricular diastolic function by elevating aortic systolic pressure [16], which attenuates passive recoil of titin during isovolumic relaxation [35] and challenges myocyte calcium handling [36]. Moreover, the earlier arrival of the reflected wave also leads to reduced aortic diastolic pressure which compromises left ventricular diastolic function via coronary hypoperfusion [37]. These data are the first to suggest that aortic stiffness, and associated ventricular-arterial uncoupling, contribute to left ventricular diastolic dysfunction in suspected INOCA.
This study is not without limitation. Due to the cross-sectional nature of this study, we are unable to establish causality. Future investigations evaluating changes in diastolic function in women with suspected INOCA over time or in response to targeted therapy are therefore needed. While the sample size was relatively low, we were adequately powered to detect differences in each of our primary endpoints. Coronary vascular dysfunction was defined by semi-quantitative MPRI, consistent with previous work from our group [17, 38]; however, fully quantitative approaches may allow for greater discrimination between subjects, and should be considered in future investigations. Moreover, while inclusion of isometric handgrip is a strength of this investigation, evaluation of myocardial ischemia and/or myocardial perfusion during isometric handgrip was not included, limiting the interpretation of these results. Furthermore, the study design did not allow for the direct assessment of left ventricular chamber compliance and thus we cannot completely rule out chamber stiffness as a contributing mechanism to diastolic dysfunction in INOCA. However, given that we saw no differences in native T1 or ECV between INOCA and controls, suggests that chamber stiffness is unlikely to be playing a major role. Finally, an elevated left ventricular afterload irrespective of changes in aPWV could itself lead to reduced early diastolic strain rate, and could therefore be a confounding factor in the interpretation of these data. However, that systolic blood pressure was not a significant independent predictor of reduced diastolic strain rate suggests that afterload may not be a primary mediator of diastolic dysfunction in this cohort.
Conclusion
This is the first study to systematically evaluate key mechanisms thought to be responsible for the development of diastolic dysfunction in INOCA. Using a comprehensive MRI approach, we identified increased aortic stiffness and left ventricular mass index, together with lower extracellular volume, to be important determinants of left ventricular diastolic dysfunction. We interpret these data to suggest that aortic stiffness leads to myocardial hypertrophy and diastolic dysfunction in suspected INOCA, representing a novel therapeutic target in this at-risk population.
Supplementary Material
Highlights.
Ischemia with no obstructive coronary artery disease (INOCA) is prevalent in women and is associated with increased risk of developing heart failure with preserved ejection fraction (HFpEF).
The mechanism(s) contributing to heart failure progression in women with INOCA remains unclear.
Using a comprehensive cardiac MRI approach, this study found that aortic pulse wave velocity, left ventricular mass index, and left ventricular extracellular volume are independent predictors of diastolic dysfunction in women with INOCA.
Acknowledgments
Sources of Funding: This work was supported by the National Institutes of Health, nos. N01-HV-68164, N01-HV-68163, N01-HV-68162, N01-HV-6816, U01 HL649241, U01 HL649141, R00 HL124323, UL1TR000124, T32 HL69751, K23HL127262, K23HL105787, MO1-RR00425, K23HL125941, U01 64829, R03 AG032631, and UL1TR000064, and grants from the Women’s Guild of Cedars-Sinai Medical Center; the Gustavus and Louis Pfeiffer Research Foundation; the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center; the Ladies Hospital Aid Society of Western Pennsylvania; QMED, Inc., the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles; the American Heart Association (16SDG27260115, 18PRE33960358), and the Harry S. Moss Heart Trust.
Footnotes
Disclosures
CNBM: Abbott Diagnostics, Sanofi Vascular, iRhythm.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. [DOI] [PubMed] [Google Scholar]
- [2].Bakir M, Nelson MD, Jones E, Li Q, Wei J, Sharif B, et al. Heart failure hospitalization in women with signs and symptoms of ischemia: A report from the women’s ischemia syndrome evaluation study. Int J Cardiol. 2016;223:936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bakir M, Wei J, Nelson MD, Mehta PK, Haftbaradaran A, Jones E, et al. Cardiac magnetic resonance imaging for myocardial perfusion and diastolic function-reference control values for women. Cardiovasc Diagn Ther. 2016;6:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nelson MD. Left ventricular diastolic dysfunction in women with nonobstructive ischemic heart disease: insights from magnetic resonance imaging and spectroscopy. Am J Physiol Regul Integr Comp Physiol. 2017;313:R322–R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nelson MD, Szczepaniak LS, Wei J, Haftabaradaren A, Bharadwaj M, Sharif B, et al. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging. 2014;7:510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wei J, Mehta PK, Shufelt C, Yang Y, Gill E, Kahlon R, et al. Diastolic dysfunction measured by cardiac magnetic resonance imaging in women with signs and symptoms of ischemia but no obstructive coronary artery disease. Int J Cardiol. 2016;220:775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pfeffer MA, Shah AM, Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res. 2019;124:1598–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nelson MD, Sharif B, Shaw JL, Cook-Wiens G, Wei J, Shufelt C, et al. Myocardial tissue deformation is reduced in subjects with coronary microvascular dysfunction but not rescued by treatment with ranolazine. Clin Cardiol. 2017;40:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zamani SK, Samuel TJ, Wei J, Thomson LEJ, Tamarappoo B, Sharif B, et al. Left atrial function is preserved in women with ichemia but no obstructive coronary artery disease. Clin Cardiol. 2020;E-Pub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang JH, Obokata M, Reddy YNV, Redfield MM, Lerman A, Borlaug BA. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2019. [DOI] [PubMed] [Google Scholar]
- [13].Alsaied T, Niss O, Tretter JT, Powell AW, Chin C, Fleck RJ, et al. Left atrial dysfunction in sickle cell anemia is associated with diffuse myocardial fibrosis, increased right ventricular pressure and reduced exercise capacity. Sci Rep. 2020;10:1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Niss O, Fleck R, Makue F, Alsaied T, Desai P, Towbin JA, et al. Association between diffuse myocardial fibrosis and diastolic dysfunction in sickle cell anemia. Blood. 2017;130:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985). 2008;105:1342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–7. [DOI] [PubMed] [Google Scholar]
- [17].Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shaw JL, Nelson MD, Wei J, Motwani M, Landes S, Mehta PK, et al. Inverse association of MRI-derived native myocardial T1 and perfusion reserve index in women with evidence of ischemia and no obstructive CAD: A pilot study. Int J Cardiol. 2018;270:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ikonomidis I, Aboyans V, Blacher J, Brodmann M, Brutsaert DL, Chirinos JA, et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail. 2019;21:402–24. [DOI] [PubMed] [Google Scholar]
- [20].Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–56. [DOI] [PubMed] [Google Scholar]
- [21].White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, et al. T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging. 2013;6:955–62. [DOI] [PubMed] [Google Scholar]
- [22].Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507–32. [DOI] [PubMed] [Google Scholar]
- [23].Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LE, et al. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol. 2016;310:H14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. [DOI] [PubMed] [Google Scholar]
- [27].AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol. 2019;73:684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, et al. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation. 2017;135:566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mahmod M, Pal N, Rayner J, Holloway C, Raman B, Dass S, et al. The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2018;20:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–35. [DOI] [PubMed] [Google Scholar]
- [32].Roy R, Aldiwani H, Darouian N, Sharma S, Torbati T, Wei J, et al. Ambulatory and silent myocardial ischemia in women with coronary microvascular dysfunction: Results from the Cardiac Autonomic Nervous System study (CANS). Int J Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wei J, Bakir M, Darounian N, Li Q, Landes S, Mehta PK, et al. Myocardial Scar Is Prevalent and Associated With Subclinical Myocardial Dysfunction in Women With Suspected Ischemia But No Obstructive Coronary Artery Disease: From the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study. Circulation. 2018;137:874–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McDiarmid AK, Swoboda PP, Erhayiem B, Lancaster RE, Lyall GK, Broadbent DA, et al. Athletic Cardiac Adaptation in Males Is a Consequence of Elevated Myocyte Mass. Circ Cardiovasc Imaging. 2016;9:e003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ikonomidis I, Katsanos S, Triantafyllidi H, Parissis J, Tzortzis S, Pavlidis G, et al. Pulse wave velocity to global longitudinal strain ratio in hypertension. Eur J Clin Invest. 2019;49:e13049. [DOI] [PubMed] [Google Scholar]
- [36].Gorski PA, Ceholski DK, Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab. 2015;21:183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tritakis V, Tzortzis S, Ikonomidis I, Dima K, Pavlidis G, Trivilou P, et al. Association of arterial stiffness with coronary flow reserve in revascularized coronary artery disease patients. World J Cardiol. 2016;8:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shufelt CL, Thomson LE, Goykhman P, Agarwal M, Mehta PK, Sedlak T, et al. Cardiac magnetic resonance imaging myocardial perfusion reserve index assessment in women with microvascular coronary dysfunction and reference controls. Cardiovasc Diagn Ther. 2013;3:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.