Abstract
Despite the life-extending success of antiretroviral pharmacotherapy in HIV infection (HIV), the prevalence of mild cognitive impairment in HIV remains high. Near-normal life expectancy invokes an emerging role for age–infection interaction and a potential synergy between immunosenescence and HIV-related health factors, increasing risk of cognitive and motor impairment associated with degradation in corticostriatal circuits. These neural systems are also compromised in Parkinson’s disease (PD), which could help model the cognitive deficit pattern in HIV. This cross-sectional study examined three groups, age 45–79 years: 42 HIV, 41 PD, and 37 control (CTRL) participants, tested at Stanford University Medical School and SRI International. Neuropsychological tests assessed executive function (EF), information processing speed (IPS), episodic memory (MEM), visuospatial processing (VSP), and upper motor (MOT) speed and dexterity. The HIV and PD deficit profiles were similar for EF, MEM, and VSP. Although only the PD group was impaired on MOT compared with CTRL, MOT scores were related to cognitive scores in HIV but not PD. Performance was not related to depressive symptoms, socioeconomic status, or CD4+ T-cell counts. The overlap of HIV-PD cognitive deficits implicates frontostriatal disruption in both conditions. The motor-cognitive score relation in HIV provides further support for the hypothesis that these processes share similar underlying mechanisms in HIV infection possibly expressed with or exacerbated by ageing.
Introduction
Highly active antiretroviral therapy (HAART) has greatly extended the life expectancy for people living with HIV infection and substantially decreased the incidence of HIV-related dementia (Cole et al., 2017; Heaton et al., 2011). Despite these advances, mild-to-moderate cognitive and motor deficits endure, with 20% (Sacktor, 2002; Sacktor et al., 2001, 2016) to upwards of 50% of individuals with HIV infection demonstrating deficits (Heaton et al., 2011; Smail & Brew, 2018). Although the prevalence of asymptomatic neurocognitive impairment without functional impairment may have been overestimated in the presence of too lenient neuropsychological guidelines (Gisslen, Price, & Nilsson, 2011), it has become evident over the years that there is considerable heterogeneity in pattern and severity of cognitive and motor deficits in the HIV population. Some individuals demonstrate moderate deficits in multiple domains, whereas others demonstrate little to no observable deficits (Nookala, Mitra, Chaudhari, Hegde, & Kumar, 2017). Further, a new challenge looms as individuals living with HIV infection age, with older individuals at greater risk of developing cognitive and motor deficits than are older individuals without HIV infection (Elicer, Byrd, Clark, Morgello, & Robinson-Papp, 2018; Goodkin et al., 2017; Sheppard, Woods, et al., 2015; Smail & Brew, 2018).
HIV has direct and indirect effects on the nervous system. Concentration of the virus is higher in selective brain regions including basal ganglia structures (Kumar, Ownby, Waldrop-Valverde, Fernandez, & Kumar, 2011; Schier et al., 2017), an integral part of the frontostriatal system, which is associated with executive functions, motor processes, and information processing speed (Ances, Ortega, Vaida, Heaps, & Paul, 2012; Hakkers et al., 2017). Even after the introduction of HAART and in individuals whose viral level is well controlled, mild-to-moderate cognitive and motor deficits subserved by frontostriatal systems persist (Ipser et al., 2015) with a threefold risk of HIV-associated neurocognitive disorder (HAND) in older people with HIV infection (Milanini et al., 2019; Valcour et al., 2004).
Immunosenescence and HIV infection together may increase central nervous system (CNS) vulnerability for neurological complications and neurocognitive decline (Appay et al., 2011). Although motor impairment in individuals with HIV infection has become less common in the ART era with few individuals meeting clinical diagnostic criteria for parkinsonism (Dehner, Spitz, & Pereira, 2016), motor dysfunctions in older people with HIV on HAART occur beyond levels expected in healthy ageing (Lau, Esmaeili-Firidouni, Wendelken-Riegelhaupt, & Valcour, 2015). There has been speculation that the motor-related symptoms observed in HIV might result from disease-associated neurotoxicity to dopaminergic terminals in the basal ganglia (Kumar et al., 2011; Liu, Shi, Liu, & Wang, 2014; Silvers et al., 2006). Indeed, motor-related symptoms, reported in middle-aged individuals with HIV infection, are akin to characteristic motor deficits in Parkinson’s disease (PD), which affects the basal ganglia structures and frontostriatal systems and are characterized by bradykinesia, resting tremor, muscular rigidity, and postural instability (Tisch & Brew, 2009).
Early studies highlighted the relevance of considering HIV infection as a primary cause of parkinsonism or parkinsonian features (Nath & Jankovic, 1989). Shared neural and neurotransmitter system involvement between HIV infection and PD have led to the speculation that the cognitive and motor deficit profile in older adults with HIV infection may resemble that observed in individuals with PD (DeVaughn, Müller-Oehring, Markey, Brontë-Stewart, & Schulte, 2015; Valcour et al., 2008). Cognitive deficits among Parkinson’s patients are common (Dubois & Pillon, 1997), vary broadly (Goldman & Litvan, 2011), and affect multiple domains (Broeders et al., 2013), commonly involving executive functions, visuospatial skills, and memory (Hendershott, Zhu, Llanes, & Poston, 2017). Similar to individuals with PD who show a faster rate of cognitive decline, particularly in attention and psychomotor speed, compared with healthy ageing (Muslimovic, Post, Speelman, De Haan, & Schmand, 2009), accelerated cognitive decline (Smith, de Boer, Brul, Budovskaya, & van Spek, 2012), and slowed psychomotor processing speed have been observed in individuals with HIV infection (Vance, Fazeli, Ross, Wadley, & Ball, 2012).
As people with HIV age, reflective of the frontostriatal dysfunction and dopaminergic changes characteristic of the disease, cognitive, and motor symptoms (Agarwal, Aujla, Gupta, & Kumar, 2020) similar to those observed in PD may emerge. Indeed, the interaction of age, chronic neuroinflammation, and living on ART long-term (Ances et al., 2012; Hakkers et al., 2017) might exacerbate this phenotype (Rubin et al., 2018). Herein, we assessed older individuals with HIV infection, individuals with PD, and older healthy controls on five cognitive and motor domains to examine pattern, extent, severity, and disease overlap of deficits. It was hypothesized that 1) older adults with HIV infection and individuals with PD would demonstrate a similar pattern of cognitive deficits on tasks associated with frontostriatal functioning including executive functions, processing speed, memory, and visuospatial processes, and 2) a subset of older adults with HIV would exhibit a pattern of motor compromise comparable, albeit not at the severity level, to individuals with PD.
Methods
Participants
Participants included 42 individuals who were HIV seropositive (HIV), 41 individuals who met criteria for mild-to-moderate idiopathic Parkinson’s disease (Litvan et al., 2012), and 37 healthy controls (CTRL) (Table 1). HIV participants were recruited from community physicians and HIV treatment centres. PD participants were recruited through regional PD events, including the Michael J. Fox trial finder and the University Neurology Clinic. CTRL participants were recruited through web-postings and flyers distributed throughout the local community. All participants were at least 45 years of age. Written informed consent was obtained from all participants at study initiation. All procedures were approved by the Institutional Review Boards of the University and the Research Institute, research was completed in accordance with the Helsinki Declaration, and participants received a modest financial remuneration for their time.
Table 1.
Demographic characteristics of subject groups
| Group | Sex | Age (years) | Education (years) | WTAR IQ* | Dementia Rating Scale | Disease Duration (years) | SES† |
|---|---|---|---|---|---|---|---|
| CTRL (n = 37) | 19 M, 18 F | 61.4 | 16.4 | 116.4 | 140.8 | 22.7 | |
| (8.8) | (2.5) | (9.5) | (2.0) | n/a | (10.5) | ||
| 45 to 77 | 12 to 21 | 84 to 126 | 137 to 144 | 11 to 47 | |||
| HIV (n = 42) | 25 M, 17 F | 60.0 | 14.2 | 98.1 | 136.7 | 25.1 | 37.7 |
| (7.0) | (2.2) | (20.2) | (5.9) | (7.8) | (14.6) | ||
| 47 to 78 | 10 to 19 | 59 to 125 | 114 to 144 | 6 to 40 | 11 to 69 | ||
| PD (n = 41) | 26 M, 15 F | 66.0 | 16.7 | 113.1 | 138.5 | 5.2 | 20.4 |
| (7.5) | (2.0) | (10.5) | (3.6) | (3.0) | (8.2) | ||
| 49 to 79 | 12 to 21 | 84 to 126 | 129 to 144 | 2 to 14 | 11 to 40 | ||
| Group differences | p = .001 | p = .0001 | p = .001 | p = .002 | p = .0001 | p = .0001 | |
| Post hoc 2 group comparisons | PD> CTRL, HIV | HIV < CTRL, PD | HIV < CTRL, PD | HIV, PD < CTRL | HIV> PD | HIV> CTRL, PD |
HIV, HIV infection; PD, Parkinson’s disease, and CTRL, controls (mean, standard deviation, range).
WTAR – Wechsler Test of Adult Reading.;
SES – Socioeconomic Status (higher scores = lower SES).
All participants were assessed using the Structured Clinical Interview for DSM-IV (SCID-IV) (First, Spitzer, Gibbon, & Williams, 1998), and a neurological examination by a board certified neurologist confirming none of the participants had sensory neuropathy. All participants also underwent a semi-structured timeline follow-back interview (Skinner & Sheu, 1982) to quantify lifetime alcohol consumption. Severity of depressive symptoms was assessed with the Beck Depression Inventory-II (Beck, Steer, & Brown, 1996). Blood testing confirmed HIV-positive serostatus in individuals enrolled in the HIV group and negative serostatus in PD and CTRL participants.
All HIV participants were on a continuous regimen of ART for at least 2 months prior to testing, consisting of two or more nucleoside reverse transcriptase inhibitors (NRTIs) combined with integrase inhibitors (II) (NRTIs + II n = 15), protease inhibitors (PI) (NRTIs + PI n = 12), non-nucleoside reverse transcriptase inhibitors (NNRTI) (NRTIs + NNRTI n = 6), or a combination thereof (NRTIs + PI+II n = 4; NRTIs + NNRTI+PI n = 2; NRTIs + NNRTI+II n = 3). For all HIV participants, health status was regularly monitored by a physician; one participant was on a physician-scheduled drug holiday starting 10 days before testing. On average, ART adherence was 95% over the past month (median = 100%).
Participants were excluded if they had fewer than 8 years of education or a history of psychiatric (e.g. schizophrenia or bipolar disorder), neurological (other than PD), or medical (e.g. stroke, diabetes) condition potentially affecting the CNS other than HIV, or MRI contraindications. All individuals were initially screened for dementia using the Dementia Rating Scale (DRS-2) (Jurica, Leitten, & Mattis, 2004). The cut-off score on the DRS-2 for CTRL participants was 136/144 (Springate, Tremont, Papandonatos, & Ott, 2014).
Inclusion of patients with mild-to-moderate PD was based on disease duration ≥ 2 years, Hoehn and Yahr stages < 4 off-dopaminergic medication (Hoehn & Yahr, 1967, 2001), and improvement on medication assessed by the Movement Disorder Society–UPDRS part III scores (Goetz et al., 2008), as determined by a neurologist. In accordance with recommended guidelines (Litvan et al., 2012), PD participants were on dopaminergic medication during neuropsychological testing to minimize the influence of motor disturbance on cognitive scores. No PD participant had prior neurosurgery. No CTRL participant tested positive for HIV or hepatitis C virus (HCV) or had an abnormal neurological examination at time of testing.
Self-reported use of alcohol and drugs indicated a history of past substance use in a greater number of HIV participants than PD or CTRL participants. This substance use was in full remission for at least 3½ years for all substances, except for cannabis (n = 4 HIV) and nicotine (n = 15 HIV, n = 2 CTRL). Past history of alcohol use disorder was documented in 13 HIV and 2 PD participants; all were in full remission (range 3.6 to 46 years). Cocaine was misused in the past by 17 HIV participants and 1 PD participant; all were in full remission (range 4 to 39 years).
Analysis of variance (ANOVA) indicated that groups differed in age, education, and socioeconomic status (SES) (Hollingshead, 1965), with the PD group being older than the HIV and CTRL groups and the HIV group having fewer years of education and lower SES than the PD or CTRL groups (TABLE 1). Participants with HIV had undetectable viral loads, average CD4+ T-cell counts of 747.4 cells/mm3 (median = 788 cells/mm3, range = 221-1576 cells/mm3), and CD4+ nadir of 181.9 cells/mm3 (median = 171.5 cells/mm3, range = 0-600 cells/mm3); 27 HIV participants had been diagnosed with AIDS (i.e. having had an AIDS-defining event and/or CD4+ blood T-cell count less than 200 cells/mm3) at any time since HIV infection. Years since diagnosis were, on average, 25 years for the HIV group. Although all three subject groups had an average DRS-2 score ≥ 136, group differences were observed (F (2,116) = 9.49, p = .002), with the HIV and PD groups scoring lower than the CTRL group (HIV = 136.7; PD = 138.5; CTRL = 140.8). Groups further differed on MDS-UPDRS part III scores (F(2,112) = 63.8, p = .0001; ANCOVA controlling for age) with HIV and PD groups showing more motor symptoms than the CTRL group (p = .05 and p = .0001, respectively), although parkinsonian motor signs in the HIV group were mild and at a subclinical level. Groups differed on BDI-II scores (F (2,116) = 11.92, p < .0001), with HIV and PD reporting greater depressive symptomatology than CTRL, but not differing between each other.
The HIV group had more African American participants (n = 17) than the CTRL (n = 5) or PD groups (n = 0)(χ2 = 23.53, p = .0001). The PD group had more Caucasian participants (n = 37) than the CTRL (n = 25) or HIV (n = 26) groups (χ2 = 9,43, p = .009). The CTRL group had more Asian participants (n = 4) than the HIV (n = 0) or PD (n = 1) groups (χ2 = 6.22, p = .045). Groups did not differ in any other ethnic category (Hispanic, Pacific Islander, Native American).
Test measures and cognitive and motor domains
All participants underwent cognitive and motor testing. Theoretically derived domains (Fama, Sullivan, Sassoon, Pfefferbaum, & Zahr, 2016; Sullivan, Fama, Rosenbloom, & Pfefferbaum, 2002) assessed included executive functioning, information processing speed, episodic memory, visuospatial processing, and upper motor speed and dexterity.
Executive functioning (EF)
Word-Color Interference (subtest of Stroop Test) (Golden & Freshwater, 1978). Participants were instructed to say the colour of ink a colour word was printed in rather than reading the word itself. This required an individual to inhibit an initial response (reading of the word) and respond with a less automatic response (the colour of ink in which the word was printed). Subjects were given 45-sec, and score was the number of correct responses.
Digit Span Backward (subtest of Wechsler Memory Scale-Revised) (Wechsler, 1987). Participants recited in backwards order an increasingly longer string of digits the tester presents aloud (digit spans range from 2 to 7 digits). There were 2 items per span, and the test was discontinued when both items in a span were incorrect. The score was number of correct items (max = 12).
Information processing speed (IPS)
Color Naming (subtest of Stroop Test) (Golden & Freshwater, 1978). Participants said the colour of ink (red, blue, yellow) in which stimuli (rows of XXXXs) were printed. This assessed speed of processing of simple stimuli. Time limit was 45-sec, and score was number of correct responses.
Symbol Digit Modalities Test – Oral Version (Smith, 1982). Participants were shown a key with symbols in the upper boxes and numbers (1-9) in the lower boxes. They were then presented with rows of symbols and asked to say aloud what number was paired with each symbol, with the key showing the symbol–digit pairings kept in view. Score was total number of correct responses in 90 sec.
Episodic memory (MEM)
List Learning (Trials 1-5) from the California Verbal Learning Test-II (CVLT-II) (Delis, 2000). An examiner read a list of 16 words, consisting of 4 words in each of 4 categories (i.e. animals, furniture, vegetables, transportation), to the participant, who then was asked to recall as many words as possible from the list. Score was number of words recalled over five learning trials (max = 80).
Long Delay Free Recall (CVLT-II) (Delis, 2000). After the five learning trials, a second word list, short delay free and cued recall of the initial word list, and a 20-minute delay, the participants were asked to recall the initial word list, the one that had been presented 5 times. Score was number of correct words recalled (max = 16).
Visuospatial processing (VSP)
Judgment of Line Orientation (Benton, Hamsher, Varney, & Spreen, 1983). Participants matched the orientation of two lines presented to a target card consisting of 11 equidistant lines forming a semi-circle. There were 30 trials, and the score was number of items in which both lines were correctly identified (max = 30).
Hooper Visual Organization Test (Hooper, 1983). Participants identified and named objects depicted in segments requiring mental rotation and integration. There were 30 items, and the score was number of items correctly identified.
Upper motor speed and dexterity (MOT)
Fine Finger Movement Test (Corkin, Growdon, & Sullivan, 1981). Participants used their thumb and index finger to turn a knurled spindle for 30-sec trials. Score was the average of three left- and right-hand trials.
Alternated Finger Tapping Test (Sullivan et al., 2002). Participants depressed a key, as many times as possible for 15-sec, alternating between the right and left index finger. The number of key presses by the left hand and right hand over the three trials was averaged separately, and then, these two scores were averaged for an overall finger tapping score.
Statistical analyses
All test scores were age- and education-corrected based on the CTRL group; Z-scores were calculated such that the mean of the CTRL group was 0 with a standard deviation of 1. Using a single group of controls to standardize across all test measures circumvents issues associated with using disparate normative groups taken from individual test manuals. The benefit of using age- and education-corrected Z-scores from our CTRL group is that everyone had the exact same test procedures and test conditions in the laboratory according to best practice guidelines for research. Whenever higher test scores indicated worse performance (e.g. time to complete a task), scores were multiplied by −1, so that lower Z-scores always indicated worse performance.
Theoretically based composites were created – Executive Function (EF), Information Processing Speed (IPS), Episodic Memory (MEM), Visuospatial Processing (VSP), and Upper Motor Speed and Dexterity (MOT). Each domain consisted of two test scores from measures demonstrated and reported in previous publications to assess each of these domains. Z-scores for these composites were calculated by averaging the age and education Z-scores for the test measures that comprised each of these domains.
Group differences were examined with two-tailed ANOVAs, with follow-up t-tests to assess two-group comparisons as no score met the definition of being an outlier – defined here as at least 4 standard deviations away from the mean of the group. Cohen’s d was calculated to assess effect size. In cases, where there was a participant who scored 3 or more standard deviation away from the mean of the group, non-parametric analyses were conducted to confirm parametric results. Pearson’s chi-square and Fisher’s exact test were used to assess whether a greater number of participants in one group scored below a certain cut-off point – here defined as −1.5 SD below the mean of the CTRL group – on each of the cognitive domains compared with the other groups.
Relations between demographic and disease-related variables and cognitive and motor scores were assessed with Pearson product–moment correlations. Spearman rank order correlations were used for variables with a non-normal distribution or restricted range. To reduce the risk of Type I error for analyses examining relations between variables, a false discovery rate (FDR) correction (Benjamini & Hochberg, 1995) was used. Multiple regression analyses were conducted to identify independent predictors of composite scores when applicable.
To relate our impairment criterion of −1.5 SD below our control group to the Frascati criteria for HIV-associated neurocognitive disorder (HAND) definitions (Antinori et al., 2007), we calculated Z-scores based on individual test manual-derived demographically corrected normative data. The benefit of using normative scores from test manuals is the comparability with clinical practice for the classification of HAND. T-scores and percentiles in normative tables were transformed into Z-scores in order to calculate standard composite scores across tests for each domain. Individuals with HIV infection were grouped into categories related to severity of impairment according to the Frascati criteria – asymptomatic neurocognitive impairment (ANI): at least mild impairment (−1 SD) in 2 or more domains with no demonstrable functional impairment, mild neurocognitive disorder (MND): at least mild impairment (−1 SD) in 2 or more domains with at least mild functional impairment, and HIV-associated dementia (HAD): at least moderate impairment (−2 SD) on 2 or more cognitive domains with concomitant functional impairment. To document functional impairment, we examined difficulties in performing instrumental activities of daily living (ADL-i; Lawton & Brody, 1969; Martinez-Martin et al., 2003). Pearson’s chi-square test was used to test whether the number of participants with neurocognitive impairment differed among groups.
Results
Between group analyses (HIV, PD, CTRL)
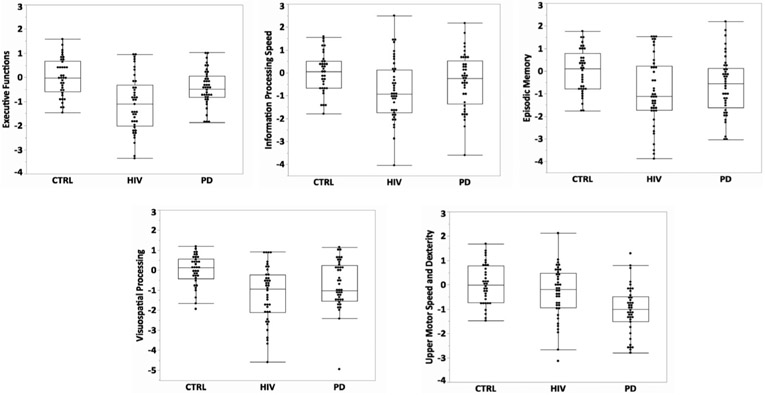
Group differences were observed on all five composite Z-scores: Executive Function (EF): (F(2,116) = 14.95, p < .0001), Information Processing Speed (IPS): F(2,114) = 3.98, p = .02), Episodic Memory (MEM): F(2,117) = 4.96, p = .009; Visuospatial Processing (VSP): F(2,115) = 10.27, p = .0001), and Upper Motor Speed and Dexterity (MOT): F (2,112) = 12.04, p = .0001) (Figure 1).
Figure 1.
Boxplots depicting cognitive and motor composite scores for the HIV, PD, and CTRL participants. Follow-up t-tests indicated a graded group effect for EF, with both HIV and PD scoring lower than CTRL [HIV vs. CTRL: t(76) = 5.39, p = .0001, Cohen’s d = 1.24; PD vs. CTRL: t(76) = 2.06, p = .04, Cohen’s d = .47] and HIV scoring even lower than PD [t(80) = 3.42, p = .001, Cohen’s d = .76]. For IPS, HIV scored lower than CTRL [HIV vs. CTRL: t(74) = 2.82, p = .006, Cohen’s d = .66]. Both HIV and PD scored lower than CTRL on MEM [HIV vs. CTRL: t(77) = 3.08, p = .003, Cohen’s d = .70; PD vs. CTRL: t (76) = 2.20, p = .03] and VSP [HIV vs. CTRL: t(75) = 4.52, p = .0001, Cohen’s d = 1.04; PD vs. CTRL: t (75) = 2.71, p = .008, Cohen’s d = .63]. PD scored lower than CTRL and HIV on MOT [PD vs. CTRL: t (73) = 4.77, p = .0001, Cohen’s d = 1.12 PD vs. HIV: t(78) = 3.31, p = .001, Cohen’s d = .75].
Follow-up t-tests indicated a graded group effect for EF, with both HIV and PD scoring lower than CTRL and HIV scoring even lower than PD. Although the IPS scores were numerically graded, the only significant difference indicated lower scores for the HIV group relative to CTRL. Both HIV and PD scored lower than CTRL on MEM and VSP. PD scored lower than CTRL and HIV on MOT.
One PD participant scored greater than 3 standard deviations below the PD group mean on the VSP composite. Non-parametric analyses without this PD participant continued to demonstrate a group effect (chi-square = 17.37, p = .0002), with the same pattern of 2-group comparison results, HIV and PD scoring lower than CTRL. Raw scores for all individual test measures included in the composites are presented in Table 2.
Table 2.
Individual test raw score means (standard deviation)
| HIV | PD | CTRL | |
|---|---|---|---|
| Stroop: Color word | 34.8 | 36.1 | 42.2 |
| (12.0) | (8.8) | (9.2) | |
| Digits backwards (max = 12) | 5.8 | 7.1 | 8.1 |
| (2.6) | (2.1) | (2.2) | |
| Stroop: Color | 64.6 | 64.8 | 70.2 |
| (14.4) | (13.5) | (11.5) | |
| Oral symbol digit total | 46.5 | 46.7 | 51.9 |
| (11.1) | (11.0) | (8.4) | |
| CVLT: Trials 1-5 (max = 80) | 43.1 | 46.5 | 51.9 |
| (12.5) | (11.6) | (9.1) | |
| CVLT: Long delay recall (max = 16) | 9.1 | 10.4 | 12.4 |
| (3.8) | (3.4) | (2.7) | |
| Judgement of line orientation (max = 30) | 23.0 | 24.1 | 27.1 |
| (4.7) | (4.2) | (2.9) | |
| Hooper visual organization (max = 30) | 24.0 | 24.6 | 26.1 |
| (3.5) | (3.3) | (2.4) | |
| Fine finger movement | 71.4 | 54.4 | 74.2 |
| (18.4) | (14.1) | (14.6) | |
| Alternated finger tapping | 56.0 | 50.4 | 57.9 |
| (0.5) | (9.8) | (9.3) |
Group differences were confirmed with Z-scores based on individual test manual-derived demographically corrected normative data for EF (F(2,116) = 11.20, p < .0001), MEM (F(2,116) = 8.51, p < .0001), VSP (F(2,115) = 7.68, p = .001), and MOT scores (F (2,112) = 12.77, p < .0001).
Within group analyses
Impairment at −1.5 SD below age- and education-corrected composite scores
Defining impairment as −1.5 standard deviations below expected score based on our control group adjustment for age and education indicated that in the HIV group: 16 participants were impaired on EF (39.0%), 13 were impaired on IPS (31.7%), 15 were impaired on MEM (35.7%), 16 were impaired on VSP (39.0%), and 6 were impaired on MOT (15%). As a comparison, in the PD group, 4 participants were impaired on EF (9.8%), 9 were impaired on IPS (22.0%), 11 were impaired on MEM (26.8%), 12 were impaired on VSP (29.3%), and 10 were impaired on MOT (25%).
Fisher’s exact tests used to compare the number of participants in each group (HIV, PD, CTRL) who scored below −1.5 standard deviation from the mean indicated group differences on all composite scores (EF: p < .0001; IPS: p = .0028; MEM: p = .0025; VSP: p = .0014; MOT: p = .0035). The HIV and PD groups differed from the CTRL group, but did not differ between each other in the proportion of participants impaired on IPS, MEM, VSP, and MOT composite scores. HIV and PD groups did differ in proportion of participants impaired on EF, with a higher proportion of HIV participants scoring below −1.5 SD than the PD group.
Differences in the number of participants in each group scoring below −1 standard deviation based on manual-derived demographically corrected normative data were confirmed for all composite scores (EF: p < .0001; IPS: p = .033; MEM: p < .00001; VSP: p = .002; MOT: p < .00001), with a similar pattern noted for −1.5 SD below norms (EF: p = .012; IPS: p = .004; MEM: p < .00001; trend for VSP: p = .056; MOT: p = .00001).
Correlations among composite scores
Correlational analyses were conducted to identify relations among cognitive and motor scores in the HIV and PD groups (Table 3). A false discovery rate correction based on 4 correlational analyses for each composite score was instituted, making the starting significant level p < .0125.
Table 3.
Pearson correlations among composite scores in the HIV and PD groups
| EF | IP | MEM | VSP | MOT | |
|---|---|---|---|---|---|
| HIV group | |||||
| Executive function (EF) | |||||
| Information processing (IP) | .633 | - | |||
| Memory (MEM) | .381 | .645 | - | ||
| Visuospatial (VSP) | .534 | .584 | .482 | - | |
| Upper motor (MOT) | .475 | .496 | .087 | .414 | - |
| PD group | |||||
| Executive function (EF) | - | ||||
| Information processing (IP) | .520 | - | |||
| Memory (MEM) | .391 | .364 | - | ||
| Visuospatial (VSP) | .386 | .361 | .266 | - | |
| Upper motor (MOT) | .046 | .265 | −.004 | .163 | - |
FDR – false discovery rate initial p value = .0125 (4 comparisons within each group for each composite score). Bold values represent significant correlations, FDR-corrected.
In the HIV group, cognitive composite scores were correlated with each other and all but MEM score was correlated with MOT score. By contrast, in the PD group, although all cognitive composite scores were correlated with each other, with the exception of MEM score and VSP score, no cognitive composite score was correlated with MOT score. Comparing correlation coefficients for EF and MOT scores indicated that the correlation between EF and MOT score was significantly stronger in the HIV group than in the PD group (z = 2.06, p = .039) (Figure 2).
Figure 2.
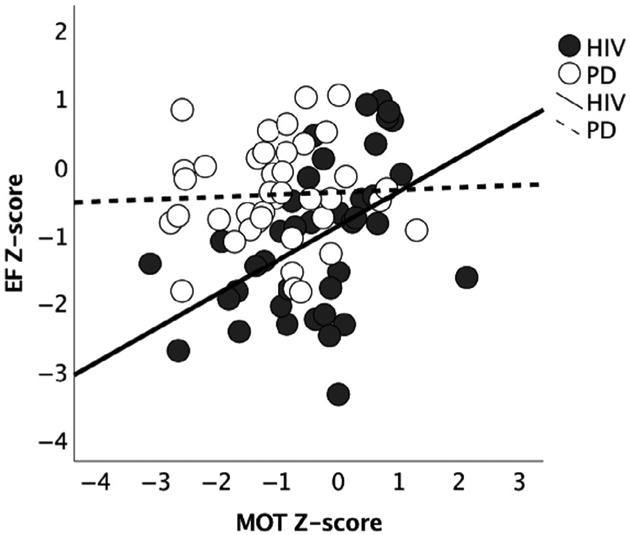
Correlation graphs depicting the relationship between EF and MOT in HIV and PD groups. Age- and education-corrected Z-scores denote deviation from CTRL mean = 0 (± 1 SD).
Multiple regression analyses identified independent predictors of the composite scores on which both the HIV and PD groups were impaired: EF, MEM, and VSP. Each of these domains was modelled on the remaining 4 domain scores (e.g. predicting EF score from IPS, MEM, VSP, and MOT). Results indicated that IPS score was an independent predictor of MEM score in the HIV group (p = .002), whereas IPS score was an independent predictor of EF score in the PD group (p = .012).
To ensure that history of alcohol use disorder did not account for the relation between IPS and MEM scores in HIV, the multiple regression analyses predicting MEM score from the other composites scores were rerun without the HIV participants with a past alcohol use disorder history. Even with the resultant reduced statistical power due to the smaller sample IPS score remained an independent predictor of MEM score in HIV (t Ratio = 4.36, p = .0003). Additionally, separate post hoc analyses excluding HIV with a history of cocaine diagnosis or excluding HIV with a history of cannabis diagnosis did not change the finding that IPS score was an independent predictor of MEM score in the HIV group.
Associations to neurocognitive impairment (NCI) for HAND definition
Using the Frascati criteria, 21 HIV participants met criteria for HAND (6 ANI, 12 MND, 0 HAD, 3 participants met criteria of −1 SD below norm on two or more domains, but were missing ADL-i data precluding subtype differentiation). Using these criteria, 12 PD participants (9 ANI, 3 MND,) and 1 CTRL participant (ANI) would be flagged as neurocognitively impaired. Groups differed in the number of subjects with neurocognitive impairment (Pearson chi-square = 25.06, p < .0001); the HIV and PD groups differed from the CTRL group (p < .0001 and p = .001, respectively), but not between each other in the proportion of participants impaired. For comparison, our impairment criteria of −1.5 SD below the CTRL group flagged about the same number of subjects (20 HIV, 12 PD, and 1HC) as being impaired on two or more composite scores.
Associations among demographic and disease variables and composite scores in HIV
Age, BDI-II score, amount of lifetime alcohol drunk, CD4+ T-cell count, nadir CD4+, and duration of HIV diagnosis were not correlated with any of the age- and education-corrected composite scores in the HIV group. AIDS status and type of combination ART (NRTIs + II n = 15, NRTIs + PI n = 12, NRTIs + other n = 15) were also not associated with any significant group differences in cognitive and motor composite scores (ANOVAs, all p’s> 0.05). Years of education was correlated with EF (r = .64, p < .0001), IPS (r = .33, p = .033), and MOT (r = .38, p = .017) scores. When added to the model to predict MEM scores in the HIV group, both IPS score (p = .0003) and years of education (p = .018)were independent predictors.
Discussion
Taken together, this study showed a similar pattern of deficits in HIV infection and PD in a number of cognitive domains – executive functioning, episodic memory, and visuospatial processing – relative to healthy ageing controls. Nonetheless, differences between the HIV and PD groups were also evident, with only the HIV group demonstrating information processing speed deficits and only the PD group demonstrating upper motor speed and dexterity deficits. This is not to say that all individuals in the HIV group did not demonstrate deficits on the motor domain or that all individuals in the PD group did not demonstrate deficits in information processing speed; as a subset of both of these groups did demonstrate such deficits.
The neuropsychological profile of impaired executive functioning, episodic memory, and speed of information processing in this HIV sample represent three of the five domains included in the diagnosis of mild forms of HIV-associated neurocognitive disorder (HAND) (Antinori et al., 2007). In our sample of older HIV participants, 50% met Frascati criteria for mild impairment, with 29% reporting difficulties in activities of daily living (MND). Although recommended, motor symptoms – a sensitive correlate of HAD (Berger & Arendt, 2000; Valcour et al., 2008) – are no longer required for the diagnosis of HAND (Eggers et al., 2017). This likely reflects the fact that the prevalence of HAD and extrapyramidal motor signs in HIV has decreased (Smail & Brew, 2018) and the disease course altered by ART, resulting in less severe neurocognitive impairment earlier during the course of HIV infection (Heaton et al., 2010; Heaton et al., 2011; Sacktor et al., 2001; Simioni et al., 2010; but see Gisslen et al., 2011). Here, 14% of our older HIV participants showed at least mild motor impairment, suggesting that motor symptoms remain a functional consequence of HIV infection (Dehner et al., 2016; Montoya et al., 2019) with older age (Valcour et al., 2008).
These data are also in line with previous reports of impaired functions associated with the effects of ageing and HIV infection on frontostriatal and thalamocortical circuits (Iudicello, Woods, Deutsch, Grant, & Group, 2012). Here our sample of older HIV-infected adults showed a neuropsychological profile characteristic of both subcortical and cortical neuropathology, including a more classically defined cortical presentation with learning and memory dysfunctions (Ciccarelli et al., 2017; Iudicello et al., 2012). This deficit profile supports reports that the neuropsychological deficit profile in the ART era has undergone a shift from what was previously characterized as a classical subcortical profile to a more cortical profile with memory and executive dysfunction (Kieburtz et al., 1996; Ragin et al., 2005).
These results further highlight the occurrence of deficits associated with visuospatially based processes in HIV. Although visuospatial deficits have not received as much attention as executive function, information processing speed, and memory deficits, a number of studies have reported such deficits in the HIV populations. Indeed, a recent study noted that the relevance of visuospatial processes may be one of the most predictive deficits of HAND (Agarwal et al., 2020). Historically, visuospatial processing deficits in HIV have been noted in older studies (e.g., Dolan et al., 2003), although they have received less attention relative to other cognitive domains.
Examination of the component processes associated with deficits shared by the HIV and PD groups (executive functioning, episodic memory, and visuospatial processing) indicated that slower information processing speed was an independent predictor of memory in HIV, whereas slower information processing speed was an independent predictor of executive functioning in PD. Slowed speed of information processing and associate learning and memory deficits have been related to decreased dopamine levels – on average by 45% – in the substantia nigra in HIV individuals on ART treatment (Kumar et al., 2011). Similar to our findings, Ciccarelli et al. (2017) reported comparable profiles of learning and memory in HIV and PD. Thus, slowness of information processing in HIV may be a factor underlying episodic memory deficits, possibly through nigrostriatal–posterior cortical pathway pathology (Goodkin et al., 2017).
In the HIV group, upper motor performance was related to all cognitive domains except episodic memory, whereas motor performance was not related to any cognitive composite score in the PD group. These associations suggest that a common mechanism may underlie cognitive and motor processes in HIV. In support of this, recent reports indicate that indices of bradyphrenia and bradykinesia, derived from the MDS-UPDRS motor examination (Goetz et al., 2008), were related in a smaller non-demented HIV sample (Sundaram et al., 2019). To the extent that information processing speed (IPS) indexes bradyphrenia and upper motor speed and dexterity (MOT) indexes bradykinesia, evidence for slowness of thought and movement may herald more severe forms of HAND (Rumbaugh & Tyor, 2015). Taken together, these results raise the possibility that compromised subcortical dopaminergic neurotransmission within corticostriatal circuits may underlie the cardinal clinical symptom presentation of bradyphrenia and bradykinesia in HIV.
Visuospatial functional impairment in our HIV sample is consistent with earlier published research reporting posterior parietal cortical involvement after the inception of the ART era (Kieburtz et al., 1996). Cortical posterior brain pathology has also been associated with visuospatial impairment in PD (Garcia-Diaz et al., 2018; Pereira et al., 2009). Frontostriatal circuit disruption in HIV infection (Ipser et al., 2015) similar to PD (Darvas, Henschen, & Palmiter, 2014) may extend to mediotemporal and parietal projections (Muller-Oehring et al., 2020), eventually manifesting in episodic memory and visuospatial impairment and may even increase the risk for Mild Cognitive Impairment (Sheppard, Iudicello, et al., 2015) especially as individuals with HIV infection age.
Heterogeneity in extent, pattern, and severity of cognitive and motor deficits was evident within the HIV and PD groups, with some individuals showing no cognitive or motor deficits, whereas others exhibit deficits across a number of domains examined. Analyses at the individual level indicated that upwards of 1/3 of the HIV group demonstrated at least mild deficits in most cognitive domains assessed (executive functions, information processing speed, episodic memory, and visuospatial processing). The proportion of HIV participants with a deficit in upper motor speed and dexterity did not differ from the proportion of PD participants meeting the cutoff threshold, albeit to a lesser severity level. Thus, although not all HIV participants exhibit cognitive and motor deficits, a substantial number do, highlighting clinical relevance in identifying selective impairments that are likely to interfere with activities of daily living and quality of life (Goodkin et al., 2017; Sheppard, Iudicello, et al., 2015).
Despite adjustment for years of formal education, higher levels of education were, nonetheless, related to better executive functioning, faster information processing speed, and greater upper motor speed and dexterity in the HIV group. We speculate that these relations may be due to cognitive reserve (Richards & Deary, 2005) in individuals with higher education and SES levels and stresses a potential role of resources and their availability in mitigating impairment (Chang, Holt, Yakupov, Jiang, & Ernst, 2013; Marin-Webb, Jessen, Kopp, Jessen, & Hahn, 2016). Earlier studies reported that an AIDS diagnosis and a low nadir CD4 were predictors of neurocognitive impairment (Ellis et al., 2011; Munoz-Moreno et al., 2008); however, neither an AIDS diagnosis nor nadir CD4 cell count accounted for lower cognitive scores in any of the domains assessed in our cohort of older adults with HIV infection who were adherent on ART and had current CD4+ T-cell counts in the normal range. A possible explanation is that HAART has considerably altered the disease course such that the pathogenic virus–brain interaction is less direct (Eggers et al., 2017) and the association between past HIV-related CNS injury and later neurocognitive impairment is less common.
Individuals with HIV infection often have comorbidities of alcohol and drug abuse, psychiatric disorders, or both as occurred in our HIV cohort. Despite positive histories, these comorbidities were remote and did not explain impairment presence or severity. It is plausible, however, that comorbidities – even when they were in the remote past and in full remission as in our cohort – may play a role in rate of functional decline with ageing or treatment non-compliance or recovery with ART and sustained viral suppression. In addition, partly due to the epidemiological nature related to the prevalence and incidence of HIV and PD, demographic characteristics are disparate between groups. Although statistical methods were employed to control for demographic discrepancies between groups, the degree of relevance of these characteristics to the cognitive and motor processes cannot be determined limiting the interpretation of the results. Nonetheless, our results were confirmed when using individual test manual-derived demographically corrected normative data. Finally, a limitation of this study is its cross-sectional nature, because without longitudinal analyses it is impossible to detect change in performance with ageing or other influential factors.
Conclusion
This study highlights the cognitive and upper motor performance profile of deficits associated with older adults living with HIV infection. The notable overlap in the profile of impairments in several cognitive domains observed in HIV and PD supports a common frontostriatal substrate of these functions. In addition, marked deficits in executive functioning, episodic memory, and visuospatial processing define a constellation of domains contributing to an operational definition of HAND. Consideration of performance on an individual level indicated that not all participants in either the HIV or PD group were impaired on all domains showing group impairment and lends evidence to the hypothesis that affected individuals may be at heightened risk of developing dementia or dementia-like syndromes as they accrue additional domain impairments in older age. Lastly, these data may have clinical relevance and be useful in the management of HIV in highlighting functions such as visuospatial abilities and upper motor speed and dexterity processes that may not be being adequately assessed as they are not generally deemed processes affected in HIV.
Acknowledgements
This work was supported by National Institutes of Health (NIH)/National Institute on Alcohol Abuse and Alcoholism Grants R01 AA023165 (Schulte), U01 AA017347 (Pfefferbaum), and R37 AA010723 (Sullivan); NIH/National Institute of Neurological Disorders and Stroke Grant K23 NS075097 (Poston); and the Michael J. Fox Foundation for Parkinson’s Research (Poston). Portions of these data have been presented at Annual Meetings of the International Neuropsychological Society (INS; 2017, 2018, 2020), American Academy of Clinical Neuropsychology (AACN; 2018), Research Society on Alcoholism (RSA; 2019), and Society for Neuroscience (SfN; 2019). The current study extends previous reports (Müller-Oehring et al., 2020; Sundaram et al., 2019) by comparing impairment in component executive functions, episodic memory, visuospatial processing, and upper motor speed and dexterity among HIV and PD participants relative to healthy ageing. We thank Ryan Goodcase, Aditi Bhatnagar, Priya Asok, Joshua Karpf, and Stephanie Sassoon for assistance with recruitment, screening, clinical interviewing, and testing of study participants, and Weiwei Chu for assistance with data management.
Footnotes
Conflict of interest
All authors declare no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Agarwal R, Aujla RS, Gupta A, & Kumar M (2020). Determining the Neurocognitive Status and the Functional Ability of Patients to Screen for HIV-Associated Neurocognitive Disorder (HAND). Dement Neurocogn Disord, 19(1), 19–27. 10.12779/dnd.2020.19.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, & Paul R (2012). Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of Acquired Immune Deficiency Syndromes, 59, 469–477. 10.1097/QAI.0b013e318249db17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, … Wojna VE (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69, 1789–1799. 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Fastenackels S, Katlama C, Ait-Mohand H, Schneider L, Guihot A, … Sauce D (2011). Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS, 25, 1813–1822. 10.1097/QAD.0b013e32834640e6 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory-II. San Antonio, 78, 490–498. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Benton AL, Hamsher K, Varney NR, & Spreen O (1983). Judgment of line orientation. New York, NY: Oxford University Press. [Google Scholar]
- Berger JR, & Arendt G (2000). HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol, 14, 214–221. 10.1177/026988110001400304 [DOI] [PubMed] [Google Scholar]
- Broeders M, Velseboer DC, de Bie R, Speelman JD, Muslimovic D, Post B, … Schmand B (2013). Cognitive change in newly-diagnosed patients with Parkinson’s disease: a 5-year follow-up study. Journal of the International Neuropsychological Society, 19, 695–708. 10.1017/S1355617713000295 [DOI] [PubMed] [Google Scholar]
- Chang L, Holt JL, Yakupov R, Jiang CS, & Ernst T (2013). Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiology of Aging, 34, 1240–1253. 10.1016/j.neurobiolaging.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli N, Limiti S, Fabbiani M, Baldonero E, Milanini B, Lamonica S, … Silveri MC (2017). Verbal list learning and memory profiles in HIV-infected adults, Alzheimer’s disease, and Parkinson’s disease: An evaluation of the "cortical hypothesis" of NeuroAIDS. Applied Neuropsychology: Adult, 24(5), 410–419. 10.1080/23279095.2016.1189424 [DOI] [PubMed] [Google Scholar]
- Cole JH, Underwood J, Caan MW, De Francesco D, van Zoest RA, Leech R, … Collaboration, C (2017). Increased brain-predicted aging in treated HIV disease. Neurology, 88, 1349–1357. 10.1212/WNL.0000000000003790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S, Growdon JH, & Sullivan EV (1981). Dissociation of sensorimotor functions in Alzheimers-disease. In: AGE (Vol. 4, pp. 146–146). 2129 Providence Avenue, Chester, PA 19013: American Aging Association. [Google Scholar]
- Darvas M, Henschen CW, & Palmiter RD (2014). Contributions of signaling by dopamine neurons in dorsal striatum to cognitive behaviors corresponding to those observed in Parkinson’s disease. Neurobiology of Diseases, 65, 112–123. 10.1016/j.nbd.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehner LF, Spitz M, & Pereira JS (2016). Parkinsonism in HIV infected patients during antiretroviral therapy - data from a Brazilian tertiary hospital. The Brazilian Journal of Infectious Diseases, 20, 499–501. 10.1016/j.bjid.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC (2000). CVLT-II: California verbal learning test: adult version. San Antonio, TX: Psychological Corporation. [Google Scholar]
- DeVaughn S, Müller-Oehring EM, Markey B, Brontë-Stewart HM, & Schulte T (2015). Aging with HIV-1 infection: motor functions, cognition, and attention–A comparison with Parkinson’s Disease. Neuropsychology Review, 25, 424–438. 10.1007/s11065-015-9305-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan S, Montagno A, Wilkie S, Aliabadi N, Sullivan M, Zahka N, … Grinspoon S (2003). Neurocognitive function in HIV-infected patients with low weight and weight loss. Journal of Acquired Immune Deficiency Syndromes, 34, 155–164. 10.1097/00126334-200310010-00005 [DOI] [PubMed] [Google Scholar]
- Dubois B, & Pillon B (1997). Cognitive deficits in Parkinson’s disease. Journal of Neurology, 244 (1), 2–8. [DOI] [PubMed] [Google Scholar]
- Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E, … Association German of Neuro A. u. N.-I. (2017). HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. Journal of Neurology, 264, 1715–1727. 10.1007/s00415-017-8503-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elicer MI, Byrd D, Clark US, Morgello S, & Robinson-Papp J (2018). Motor function declines over time in human immunodeficiency virus and is associated with cerebrovascular disease, while HIV-associated neurocognitive disorder remains stable. J Neurovirol, 24, 514–522. 10.1007/s13365-018-0640-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, … Group, C (2011). CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS, 25(14), 1747–51. 10.1097/QAD.0b013e32834a40cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Sullivan EV, Sassoon SA, Pfefferbaum A, & Zahr NM (2016). Impairments in component processes of executive function and episodic memory in alcoholism, HIV infection, and HIV infection with alcoholism comorbidity. Alcoholism, Clinical and Experimental Research, 40, 2656–2666. 10.1111/acer.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, & Williams J (1998). Structured clinical interview for DSM-IV axis I disorders: patient edition. Washington, DC: American Psychiatric Press. [Google Scholar]
- Garcia-Diaz AI, Segura B, Baggio HC, Uribe C, Campabadal A, Abos A, … Junque C (2018). Cortical thinning correlates of changes in visuospatial and visuoperceptual performance in Parkinson’s disease: A 4-year follow-up. Parkinsonism & Related Disorders, 46, 62–68. 10.1016/j.parkreldis.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Gisslen M, Price RW, & Nilsson S (2011). The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infectious Diseases, 11, 356. 10.1186/1471-2334-11-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, … Movement Disorder Society, U. R. T. F (2008). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement Disorders, 23, 2129–2170. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM (2002). The stroop color and word test: A manual for clinical and experimental uses. Chicago, IL: Stoelting. [Google Scholar]
- Goldman JG, & Litvan I (2011). Mild cognitive impairment in Parkinson’s disease. Minerva Medica, 102, 441–459. [PMC free article] [PubMed] [Google Scholar]
- Goodkin K, Miller EN, Cox C, Reynolds S, Becker JT, Martin E, … Multicenter, A. C. S. (2017). Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV, 4, e411–e422. 10.1016/S2352-3018(17)30098-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkers CS, Arends JE, Barth RE, DuPlessis S, Hoepelman AI, & Vink M (2017). Review of functional MRI in HIV: effects of aging and medication. J Neurovirol, 23(1), 20–32. 10.1007/s13365-016-0483-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, … Grant I (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 17(1), 3–16. 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F … Group, C (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–96. 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershott TR, Zhu D, Llanes S, & Poston KL (2017). Domain-specific accuracy of the Montreal Cognitive Assessment subsections in Parkinson’s disease. Parkinsonism & Related Disorders, 38, 31–34. 10.1016/j.parkreldis.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, & Yahr MD (1967). Parkinsonism: onset, progression and mortality. Neurology, 17, 427–472. 10.1212/wnl.17.5.427 [DOI] [PubMed] [Google Scholar]
- Hoehn MM, & Yahr MD (2001). Parkinsonism: onset, progression, and mortality. 1967. Neurology, 57(10 Suppl 3), S11–S26. 10.1067/mcp.2001.116328 [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (1965). Two factor index of social position. 1957. New Haven, CT: Yale University. [Google Scholar]
- Hooper EH (1983). Hooper visual organization test (VOT). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Ipser JC, Brown GG, Bischoff-Grethe A, Connolly CG, Ellis RJ, Heaton RK, & Grant I (2015). HIV infection is associated with attenuated frontostriatal intrinsic connectivity: a preliminary study. Journal of the International Neuropsychological Society, 21, 203–213. 10.1017/s1355617715000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Deutsch R, Grant I & The HIV Neurobehavioral Research Pr (2012). Combined effects of aging and HIV infection on semantic verbal fluency: a view of the cortical hypothesis through the lens of clustering and switching. Journal of Clinical and Experimental Neuropsychology, 34, 476–488. 10.1080/13803395.2011.651103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica P, Leitten C, & Mattis S (2004). DRS-2 dementia rating scale-2: Professional manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, … Caine ED (1996). Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Archives of Neurology, 53, 155–158. 10.1001/archneur.1996.00550020059016 [DOI] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, & Kumar M (2011). Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol, 17(1), 26–40. 10.1007/s13365-010-0003-4 [DOI] [PubMed] [Google Scholar]
- Lau EK, Esmaeili-Firidouni P, Wendelken-Riegelhaupt L, & Valcour V (2015). Parkinsonism motor findings in the University of California San Francisco Over Sixty Cohort. Hawai’i Journal of Medicine & Public Health, 74(9 Suppl 2), 51.25755913 [Google Scholar]
- Lawton MP, & Brody EM (1969). Assessment of older people: self-maintaining and instrumental activities of daily living. The gerontologist, 9(3 Part 1), 179–186. 10.1097/JCN.0b013e3181a80faf [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, … Emre M (2012). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Movement Disorders, 27, 349–356. 10.1002/mds.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Shi Z, Liu J, & Wang Y (2014). HIV transactivator of transcription enhances methamphetamine-induced Parkinson’s-like behavior in the rats. NeuroReport, 25, 860–864. 10.1097/WNR.0000000000000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Webb V, Jessen H, Kopp U, Jessen AB, & Hahn K (2016). Validation of the international HIV dementia scale as a screening tool for HIV-associated neurocognitive disorders in a German-Speaking HIV Outpatient Clinic. PLoS One, 11, e0168225. 10.1371/journal.pone.0168225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Martin P, Benito-Leon J, Alonso F, Catalan MJ, Pondal M, Tobias A, & Zamarbide I (2003). Patients’, doctors’, and caregivers’ assessment of disability using the UPDRS-ADL section: are these ratings interchangeable? Movement Disorders, 18, 985–992. 10.1002/mds.10479 [DOI] [PubMed] [Google Scholar]
- Milanini B, Samboju V, Cobigo Y, Paul R, Javandel S, Hellmuth J, … Valcour V (2019). Longitudinal brain atrophy patterns and neuropsychological performance in older adults with HIV-associated neurocognitive disorder compared with early Alzheimer’s disease. Neurobiology of Aging, 82, 69–76. 10.1016/j.neurobiolaging.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Campbell LM, Paolillo EW, Ellis RJ, Letendre SL, Jeste DV, & Moore DJ (2019). Inflammation relates to poorer complex motor performance among adults living With HIV on suppressive antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes, 80(1), 15–23. 10.1097/QAI.0000000000001881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Hong JY, Hughes RL, Kwon D, Brontë-Stewart HM, Poston KL, & Schulte T (2020). Alterations of brain signal oscillations in older individuals with HIV infection and Parkinson’s Disease. Journal of Neuroimmune Pharmacology: the Official Journal of the Society on NeuroImmune Pharmacology. 10.1007/s11481-020-09914-x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Moreno JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, … Clotet B (2008). Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Research and Human Retroviruses, 24, 1301–1307. 10.1089/aid.2007.0310 [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, De Haan RJ, & Schmand B (2009). Cognitive decline in Parkinson’s disease: a prospective longitudinal study. Journal of the International Neuropsychological Society, 15, 426–437. 10.1017/S1355617709090614 [DOI] [PubMed] [Google Scholar]
- Nath A, & Jankovic J (1989). Motor disorders in patients with human immunodeficiency virus infection. Progress in AIDS Pathology, 1, 159–166. 10.1056/NEJM199610103351506 [DOI] [PubMed] [Google Scholar]
- Nookala AR, Mitra J, Chaudhari NS, Hegde ML, & Kumar A (2017). An overview of human immunodeficiency virus Type 1-associated common neurological complications: does aging pose a challenge? Journal of Alzheimer’s Disease, 60(s1), S169–S193. 10.3233/JAD-170473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bargallo N, & Tolosa E (2009). Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. Movement Disorders, 24, 1193–1199. 10.1002/mds.22560 [DOI] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, & Epstein LG (2005). Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. Journal of Neurovirology, 11, 292–298. 10.1080/13550280590953799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, & Deary IJ (2005). A life course approach to cognitive reserve: a model for cognitive aging and development? Annals of Neurology, 58, 617–622. 10.1002/ana.20637 [DOI] [PubMed] [Google Scholar]
- Rubin LH, Sacktor N, Creighton J, Du Y, Endres CJ, Pomper MG, & Coughlin JM (2018). Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS, 32, 1661–1667. 10.1097/QAD.0000000000001858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh JA, & Tyor W (2015). HIV-associated neurocognitive disorders: Five new things. Neurology: Clinical Practice, 5, 224–231. 10.1212/CPJ.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N (2002). The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. Journal of Neurovirology, 8, 115–121. 10.1080/13550280290101094 [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, … Multicenter, A. C. S. (2001). HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology, 56, 257–260. 10.1212/wnl.56.2.257 [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, … Miller E (2016). Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology, 86, 334–340. 10.1212/WNL.0000000000002277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier CJ, Marks WD, Paris JJ, Barbour AJ, McLane VD, Maragos WF, … Hauser KF (2017). Selective vulnerability of striatal D2 versus D1 dopamine receptor-expressing medium spiny neurons in HIV-1 Tat Transgenic Male Mice. Journal of Neuroscience, 37, 5758–5769. 10.1523/JNEUROSCI.0622-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Iudicello JE, Bondi MW, Doyle KL, Morgan EE, Massman PJ, … Woods SP (2015). Elevated rates of mild cognitive impairment in HIV disease. Journal of NeuroVirology, 21, 576–584. 10.1007/s13365-015-0366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Woods SP, Bondi MW, Gilbert PE, Massman PJ, & Doyle KL (2015). Does older age confer an increased risk of incident neurocognitive disorders among persons living with HIV disease? Clin Neuropsychol, 29, 656–677. 10.1080/13854046.2015.1077995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenov MY, Aksenova MV, Beckley J, Olton P, Mactutus CF, & Booze RM (2006). Dopaminergic marker proteins in the substantia nigra of human immunodeficiency virus type 1-infected brains. Journal of Neurovirology, 12, 140–145. 10.1080/13550280600724319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, … Du Pasquier RA (2010). Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS, 24, 1243–1250. 10.1097/QAD.0b013e3283354a7b [DOI] [PubMed] [Google Scholar]
- Skinner HA, & Sheu WJ (1982). Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. Journal of Studies on Alcohol, 43, 1157–1170. 10.15288/jsa.1982.43.1157 [DOI] [PubMed] [Google Scholar]
- Smail RC, & Brew BJ (2018). HIV-associated neurocognitive disorder. Handbook of Clinical Neurology, 152, 75–97. 10.1016/B978-0-444-63849-6.00007-4 [DOI] [PubMed] [Google Scholar]
- Smith A (1982). Digit symbol modalities test (SDMT) manual [revised]. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Smith RL, de Boer R, Brul S, Budovskaya Y, & van Spek H (2012). Premature and accelerated aging: HIV or HAART? Front Genet, 3, 328. 10.3389/fgene.2012.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springate BA, Tremont G, Papandonatos G, & Ott BR (2014). Screening for mild cognitive impairment using the dementia rating scale-2. Journal of Geriatric Psychiatry and Neurology, 27, 139–144. 10.1177/0891988714522700 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, & Pfefferbaum A (2002). A profile of neuropsychological deficits in alcoholic women. Neuropsychology, 16(1), 74–83. 10.1037/0894-4105.16.1.74 [DOI] [PubMed] [Google Scholar]
- Sundaram S, Müller-Oehring EM, Fama R, Brontë-Stewart HM, Poston KL, Goodcase R, … Schulte T (2019). Information processing deficit in older adults with HIV infection: A comparison with Parkinson’s disease. Neuropsychology, 33, 157–168. 10.1037/neu0000500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch S, & Brew B (2009). Parkinsonism in hiv-infected patients on highly active antiretroviral therapy. Neurology, 73, 401–403. 10.1212/WNL.0b013e3181b04b0d [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, … Sacktor N (2004). Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology, 63, 822–827. 10.1212/01.wnl.0000134665.58343.8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, & Shikuma C (2008). Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J Neurovirol, 14, 362–367. 10.1080/13550280802216494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Ross LA, Wadley VG, & Ball KK (2012). Speed of processing training with middle-age and older adults with HIV: a pilot study. Journal of the Association of Nurses in AIDS Care, 23, 500–510. 10.1016/j.jana.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1987). WMS-R: Wechsler memory scale-revised. San Antonio, TX: The Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.