Abstract
Objectives:
To understand the differential neuroanatomical substrates underlying apathy and depression in Frontotemporal dementia (FTD).
Methods:
T1-MRIs and clinical data of patients with behavioral and aphasic variants of FTD were obtained from an open database. Cortical thickness was derived, its association with apathy severity and difference between the depressed and not depressed were examined with appropriate covariates.
Results:
Apathy severity was significantly associated with cortical thinning of the lateral parts of the right sided frontal, temporal and parietal lobes. The right sided orbitofrontal, parsorbitalis and rostral anterior cingulate cortex were thicker in depressed compared to patients not depressed.
Conclusions:
Greater thickness of right sided ventromedial and inferior frontal cortex in depression compared to patients without depression suggests a possible requisite of gray matter in this particular area for the manifestation of depression in FTD. This study demonstrates a method for deriving neuroanatomical patterns across non-harmonized neuroimaging data in a neurodegenerative disease.
Keywords: frontotemporal dementia, cortical thickness, structural MRI, apathy, depression, right frontal cortex
Introduction
Frontotemporal Dementia (FTD) is a neurodegenerative disorder constituting a prevalence of 10.2% and 2.7% of all cases of dementia below and above 65 years of age respectively1 with the Behavioral variant (bvFTD) being more common than the language variants i.e. Semantic variant Primary Progressive Aphasia (svPPA) and the Non-fluent/agrammatic variant Primary Progressive Aphasia (nfvPPA).2 Due to the co-existence of a multitude of behavioral symptoms in FTD in addition to cognitive decline, FTD renders itself as an excellent substrate to study the neurobiological underpinnings of numerous behavioral phenomena that overlap significantly with primary psychiatric disorders.3,4 Thus, FTD provides a good opportunity to measure and investigate potential neurological substrates underlying the fundamental behavioral phenotypes that also characterize major psychiatric disorders.
Apathy is defined as a quantitative reduction of voluntary, goal-directed behaviors.5 Apathy is the most common behavioral symptom in FTD resulting in high caregiver distress6 and substantial functional disability.7 Depression is an affective disorder characterized by pervasive sad mood and/or markedly diminished interest or pleasure in all activities with other accompanying symptoms (Diagnostic and Statistical Manual/DSM-5). Depression can also occur in FTD.8 While apathy and depression overlap in a decreased interest in previously pursued activities, the behavioral constructs of apathy and depression can be distinguished,9,10 though they may share some common external manifestations. Apathy is more often associated with disinhibition and aberrant motor behavior whereas depression is more commonly associated with anxiety, irritability and agitation in neurodegenerative disorders.9 Though the neurobiology of apathy in FTD has been studied,11–14 studies focusing on the potential mechanisms underlying depression in FTD15 are limited in the scientific literature. As both apathy and depression occur frequently in FTD, it is an appropriate domain to study the differential neuroanatomical involvement underlying the 2 symptoms. This particular approach of studying the neuroanatomical correlates of co-existing symptom dimensions across different variants of FTD has been undertaken for the domains of apathy and impulsivity16 but the same underlying apathy and depression needs to be studied. Understanding the possible mechanisms of these 2 distinct syndromes is clinically relevant as it can inform our understanding of the neuroanatomical bases of apathy vs. depression in other neurodegenerative disorders. We designed a study involving open source structural neuroimaging data on FTD to explore the differential neuroanatomical involvement of these 2 fundamental symptom dimensions. Our study had 2 objectives: first to determine those areas of the brain that are significantly associated with apathy and to verify those findings in light of existing FTD findings. Second was to determine whether FTD patients with and without depression have different neuroanatomical signatures in the brain, and to examine whether these areas are distinct from those implicated in apathy.
Methodology
Data Source
Structural T1 Magnetic Resonance Imaging (MRI) scans, demographic and clinical data of FTD subjects were downloaded on 01/18/2018 from Frontotemporal Lobar Degeneration Neuroimaging Initiative (FTLDNI) after complying with the appropriate data usage agreement policies as mentioned by FTLDNI. FTLDNI started in 2010 through the National Institute of Aging with goals of identifying neuroimaging modalities and methods of analysis for tracking frontotemporal lobar degeneration and to assess the diagnostic value of imaging versus other biomarkers. Neuroimaging in Frontotemporal Dementia (NIFD) is the nickname for FTLDNI, the Principal Investigator is Dr. Howard Rosen, MD at the University of California, San Francisco. The data is the result of collaborative efforts at 3 sites in North America i.e. University of California San Francisco (UCSF), Mayo clinic Rochester and Massachusetts General Hospital (MGH). For up-to-date information on participation and protocol, see http://memory.ucsf.edu/research/studies/nifd.
Clinical and Demographic Data
Figure 1 demonstrates the process of arriving at the final sample of FTD subjects which was used in the analysis. Data on behavioral symptoms was available in the form of Neuropsychiatric Inventory—Questionnaire (NPI-Q)17 ratings and the clinical stage of dementia through Clinical Dementia Rating (CDR) scores.18 We included only those subjects who scored “Yes” to the domain question of “Apathy/Indifference” on the NPI-Q for the analysis concerning apathy (N = 97). Based on the response to the domain question of “Depression/Dysphoria” we categorized 103 subjects into depressed (scored “Yes,” FTD-Depressed, N = 36) versus not depressed (scored “No,” FTD-Not-Depressed, N = 67). Six subjects who had only depression and no apathy were not included in the analysis concerning apathy and also were not treated as a separate group (depression without apathy) to compare with the other 2 groups (apathy with depression & apathy without depression) due to the very low sample size and the resulting unbalanced comparisons. Severity ratings of apathy and depression were available in the range of 1–3. Age, gender, variant of FTD, NPI-Q symptom ratings, CDR scores (total, box score, behavioral and language subscores) and Mini Mental Status Examination (MMSE) scores were also tabulated. Standard published consensus criteria were used for the diagnosis of variants of FTD.19,20 We included bvFTD (N = 56), svPPA (N = 29) and nfvPPA (N = 18). While prominent neuropsychiatric symptoms, including apathy and depression, are most characteristic of bvFTD and svPPA, nfvPPA patients can frequently demonstrate these symptoms as well, and were included in the analyses
Figure 1.
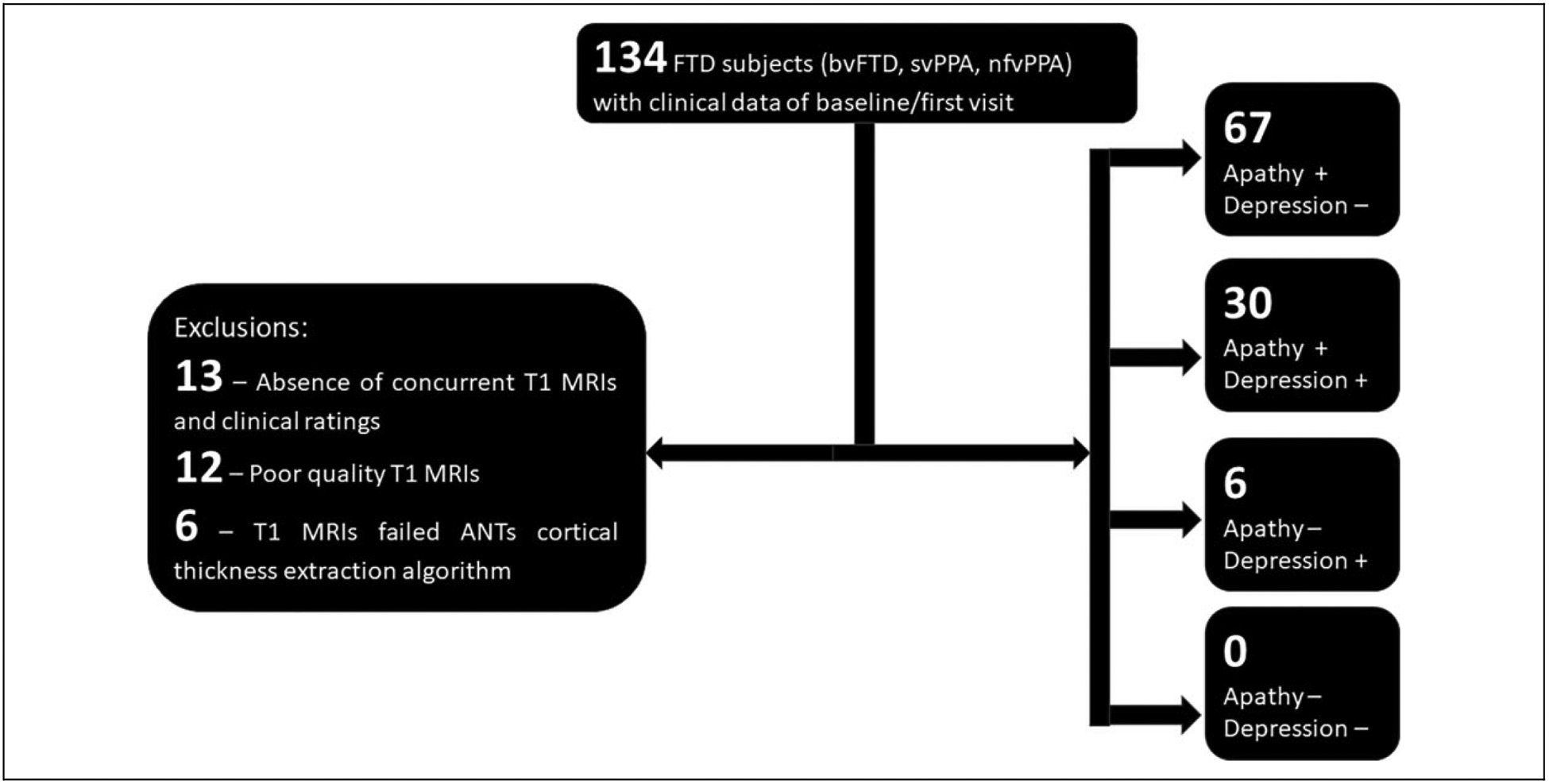
Clinical and imaging data acquisition. Structural T1 MRI scans, demographic and clinical data obtained from the open public neuroimaging database, Frontotemporal Lobar Degeneration Neuroimaging Initiative (FTLDNI) http://memory.ucsf.edu/research/studies/nifd. The process of arrival at the final sample for the analysis along with exclusions have been described. FTD: Frontotemporal Dementia, bvFTD: behavioral variant FTD, svPPA: semantic variant Primary Progressive Aphasia, nfvPPA: non-fluent variant PPA, ANTs: Advanced Normalization Tools (software used for analyzing cortical thickness).
Structural MRI Analysis
We considered only cortical areas in this study and analyzed the measure of cortical thickness. We acquired cortical thickness measurements from structural T1-weighted MRIs using the cortical thickness pipeline developed by Advanced Normalization Tools (ANTs) version 2.2. ANTs is an open source biological image processing software and has excellent performance, especially with non-harmonized imaging data, to obtain thickness measurements. One shell script in the ANTs toolbox, called antsCorticalThickness, streamlines the entire cortical thickness estimation process. This script automatically performs image preprocessing steps such as N4 bias field correction,21 brain extraction and tissue segmentation using the Atropos algorithm.22 ANTs utilizes a hybrid brain extraction process that includes registering initial T1 images with an age-matched template from the OASIS dataset.23 After preprocessing, we gathered cortical thickness estimations using a diffeomorphic registration based cortical thickness (DiReCT) measure, a robust algorithmic technique that encodes volumetric surface data based on white-mater to gray-matter and estimated gray-matter to cerebrospinal fluid diffeomorphic mapping.
After the algorithm was completed, we utilized another ANTs script, antsImageMath, to multiply the initial T1 image to the brain mask that was generated from antsCorticalThickness. From there, the masked T1 image acted as the moving image in a diffeomorphic co-registration method, while the OASIS template served as the fixed image. Finally, we warped, the output structural image from antsCorticalThickness into Open Access Series of Imaging Studies (OASIS) space using WarpImageMultiTransform. At this point, the structural T1 image is now in OASIS space, and we extracted the cortical thickness estimations using Freesurfer’s mri_segstats.24 Thickness values of totally 48 regions of interest for each subject were used in the analyses (Tables 1 and 2). A visual quality control inspection was performed to confirm the accuracy of the ANTs derived cortical thickness values (Supplemental materials “Visual QC 1 & 2”). We have not examined subcortical structures as we intended to restrict our analysis to thickness as opposed to volume (which arises out of both surface area and thickness) as thickness and surface area are phenotypically and genetically independent entities.25
Table 1.
Apathy and Cortical Thickness.
| Left | Right | |||||
|---|---|---|---|---|---|---|
| Structure | β (95% CI) | #P | ES | β (95% CI) | #P | ES |
| Superior Frontal | −0.13 (−0.18 to 0.04) | 0.181 | 0.230 | −0.17 (−0.21 to 0.001) | 0.053 | 0.321 |
| Caudal Middle Frontal | −0.16 (−0.2 to 0.01) | 0.08 | 0.303 | −0.2 (−0.22 to −0.01) | 0.03 | 0.372 |
| Rostral Middle Frontal | −0.07 (−0.19 to 0.08) | 0.436 | 0.135 | −0.18 (−0.25 to 0.002) | 0.054 | 0.340 |
| Paropercularis | −0.14 (−0.22 to 0.03) | 0.147 | 0.255 | −0.23 (−0.26 to−0.03) | 0.013 | 0.425 |
| Parsorbitalis | −0.10 (−0.21 to 0.06) | 0.286 | 0.191 | −0.22 (−0.32 to −0.02) | 0.025 | 0.411 |
| Parstriangularis | −0.14 (−0.22 to 0.03) | 0.142 | 0.260 | −0.18 (−0.25 to 0.001) | 0.051 | 0.338 |
| Caudal Anterior Cingulate | −0.11 (−0.22 to 0.05) | 0.232 | 0.207 | −0.16 (−0.25 to 0.02) | 0.099 | 0.294 |
| Rostral Anterior Cingulate | −0.14 (−0.39 to 0.06) | 0.145 | 0.256 | −0.12 (−0.38 to 0.08) | 0.210 | 0.224 |
| Lateral Orbitofrontal | −0.10 (−0.24 to 0.08) | 0.316 | 0.190 | −0.15 (−0.3 to 0.04) | 0.130 | 0.274 |
| Medial Orbitofrontal | −0.13 (−0.3 to 0.07) | 0.209 | 0.234 | −0.15 (−0.35 to 0.05) | 0.141 | 0.269 |
| Insula | −0.03 (−0.19 to 0.14) | 0.761 | 0.061 | 0.24 (0.41 to −0.03) | 0.021 | 0.446 |
| Superior Temporal | −0.07 (−0.17 to 0.08) | 0.472 | 0.129 | −0.22 (−0.25 to −0.02) | 0.025 | 0.417 |
| Middle Temporal | −0.07 (−0.2 to 0.1) | 0.497 | 0.127 | −0.17 (−0.27 to 0.03) | 0.106 | 0.318 |
| Inferior Temporal | 0.05 (−0.14 to 0.22) | 0.634 | 0.082 | −0.15 (−0.31 to 0.04) | 0.121 | 0.283 |
| Transverse Temporal | −0.17 (−0.17 to 0.01) | 0.094 | 0.313 | −0.22 (−0.19 to −0.01) | 0.028 | 0.412 |
| Parahippocampal | 0.002 (−0.13 to 0.14) | 0.980 | 0.005 | −0.15 (−0.25 to 0.03) | 0.130 | 0.281 |
| Entorhinal | 0.02 (−0.18 to 0.97) | 0.792 | 0.042 | −0.13 (−0.48 to 0.09) | 0.171 | 0.237 |
| Fusiform | 0.07 (−0.08 to 0.18) | 0.418 | 0.132 | −0.09 (−0.2 to 0.07) | 0.358 | 0.162 |
| Superior Parietal | −0.16 (−0.12 to 0.01) | 0.104 | 0.303 | −0.24 (−0.15 to −0.01) | 0.019 | 0.439 |
| Inferior Parietal | −0.02 (−0.11 to 0.09) | 0.855 | 0.037 | −0.20 (−0.21 to 0.01) | 0.062 | 0.380 |
| Posterior Cingulate | −0.11 (−0.13 to 0.04) | 0.264 | 0.211 | −0.24 (−0.22 to −0.03) | 0.014 | 0.453 |
| Isthmus Cingulate | −0.09 (−0.12 to 0.05) | 0.407 | 0.159 | −0.16 (−0.18 to 0.02) | 0.122 | 0.298 |
| Supramarginal | −0.14 (−0.14 to 0.02) | 0.166 | 0.252 | 0.2 (−0.17 to −0.004) | 0.041 | 0.369 |
| Precuneus | −0.19 (−0.15 to 0.01) | 0.081 | 0.354 | −0.16 (−0.15 to 0.02) | 0.122 | 0.299 |
Multiple linear regression results with cortical thickness of individual areas as dependent variable, apathy severity as the independent variable, age, gender and variant of FTD as covariates.
β (95% CI): Standardized co-efficient of apathy severity score in the multiple linear regression model with 95% confidence interval.
p value of apathy severity in the regression model, significant at ≤ 0.05.
ES: Effect Size expressed as Cohen’s d.
Table 2.
Comparison of Cortical Thickness Between FTD-Depressed and FTD-Not-Depressed.
| Left | Right | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Structure | *FTD-D, Mean (SD) | *FTD-ND, Mean (SD) | β (95% CI) | #p | ES | *FTD-D, Mean (SD) | *FTD-ND, Mean (SD) | β (95% CI) | #p | ES |
| Caudal_Anterior_Cingulate | 1.74 (0.6) | 1.54 (0.52) | 0.17 (−0.04 to 0.38) | 0.117 | 0.292 | 1.85 (0.55) | 1.66 (0.55) | 0.16 (−0.05 to 0.37) | 0.125 | 0.277 |
| Caudal_Middle_Frontal | 1.49 (0.48) | 1.4 (0.43) | 0.05 (−0.11 to 0.22) | 0.509 | 0.116 | 1.54 (0.43) | 1.44 (0.44) | 0.07 (−0.09 to 0.22) | 0.413 | 0.143 |
| Entorhinal | 2.7 (1.25) | 2.78 (1.2) | −0.07 (−0.58 to 0.43) | 0.77 | 0.058 | 3.27 (1.09) | 3.13 (1.23) | 0.14 (−0.34 to 0.63) | 0.556 | 0.117 |
| Fusiform | 2.55 (0.63) | 2.69 (0.53) | −0.13 (−0.37 to 0.1) | 0.253 | 0.226 | 2.86 (0.55) | 2.9 (0.55) | −0.04 (−0.26 to 0.18) | 0.728 | 0.068 |
| Inferior_Parietal | 1.84 (0.31) | 1.92 (0.36) | −0.1 (−0.23 to 0.04) | 0.163 | 0.267 | 2.1 (0.4) | 2.1 (0.39) | −0.02 (−0.17 to 0.12) | 0.737 | 0.06 |
| Inferior_Temporal | 2.54 (0.72) | 2.57 (0.74) | −0.05 (−0.35 to 0.25) | 0.742 | 0.066 | 2.81 (0.61) | 2.79 (0.69) | 0 (−0.28 to 0.28) | 0.986 | 0.004 |
| Isthmus_Cingulate | 2.33 (0.3) | 2.35 (0.32) | −0.04 (−0.15 to 0.08) | 0.517 | 0.117 | 2.45 (0.37) | 2.4 (0.39) | 0.03 (−0.12 to 0.18) | 0.68 | 0.076 |
| Lateral_Orbitofrontal | 1.9 (0.51) | 1.69 (0.64) | 0.16 (−0.07 to 0.39) | 0.165 | 0.256 | 2.05 (0.47) | 1.73 (0.66) | 0.26 (0.03 to 0.48) | 0.024 | 0.403 |
| Medial_Orbitofrontal | 2.01 (0.58) | 1.75 (0.74) | 0.18 (−0.08 to 0.45) | 0.164 | 0.254 | 2.31 (0.59) | 1.91 (0.84) | 0.33 (0.04 to 0.62) | 0.027 | 0.409 |
| Middle_Temporal | 2.15 (0.55) | 2.21 (0.57) | −0.09 (−0.32 to 0.13) | 0.419 | 0.159 | 2.29 (0.45) | 2.17 (0.58) | 0.08 (−0.14 to 0.29) | 0.475 | 0.138 |
| Parahippocampal | 1.71 (0.54) | 1.83 (0.55) | −0.11 (−0.34 to 0.11) | 0.323 | 0.196 | 2.08 (0.54) | 2.17 (0.55) | −0.1 (−0.32 to 0.13) | 0.392 | 0.172 |
| Parsopercularis | 1.42 (0.49) | 1.28 (0.52) | 0.11 (−0.07 to 0.3) | 0.235 | 0.211 | 1.5 (0.49) | 1.36 (0.49) | 0.1 (−0.07 to 0.28) | 0.238 | 0.202 |
| Parsorbitalis | 1.67 (0.52) | 1.48 (0.54) | 0.16 (−0.03 to 0.35) | 0.107 | 0.284 | 1.88 (0.46) | 1.58 (0.64) | 0.25 (0.04 to 0.47) | 0.019 | 0.412 |
| Parstriangularis | 1.49 (0.51) | 1.36 (0.54) | 0.1 (−0.1 to 0.29) | 0.324 | 0.173 | 1.61 (0.45) | 1.46 (0.56) | 0.11 (−0.09 to 0.3) | 0.284 | 0.193 |
| Posteriorcingulate | 1.94 (0.29) | 1.95 (0.33) | −0.01 (−0.13 to 0.1) | 0.808 | 0.044 | 2.17 (0.36) | 2.12 (0.4) | 0.03 (−0.11 to 0.17) | 0.676 | 0.075 |
| Precuneus | 1.88 (0.3) | 1.95 (0.29) | −0.08 (−0.2 to 0.03) | 0.149 | 0.276 | 2.02 (0.3) | 1.97 (0.33) | 0.03 (−0.09 to 0.15) | 0.571 | 0.102 |
| Rostral_Anterior_Cingulate | 2.44 (0.81) | 2.12 (0.95) | 0.21 (−0.12 to 0.54) | 0.203 | 0.224 | 2.42 (0.82) | 1.96 (0.93) | 0.36 (0.02 to 0.7) | 0.036 | 0.381 |
| Rostral_Middle_Frontal | 1.88 (0.52) | 1.71 (0.57) | 0.13 (−0.07 to 0.34) | 0.192 | 0.232 | 1.96 (0.45) | 1.74 (0.54) | 0.18 (−0.01 to 0.37) | 0.06 | 0.335 |
| Superior_Frontal | 1.72 (0.46) | 1.58 (0.46) | 0.11 (−0.06 to 0.27) | 0.215 | 0.219 | 1.75 (0.42) | 1.56 (0.47) | 0.16 (−0.01 to 0.33) | 0.064 | 0.328 |
| Superior_Parietal | 1.37 (0.28) | 1.38 (0.25) | −0.03 (−0.12 to 0.07) | 0.6 | 0.096 | 1.35 (0.3) | 1.32 (0.27) | 0.01 (−0.1 to 0.11) | 0.918 | 0.018 |
| Superior_Temporal | 1.61 (0.49) | 1.63 (0.48) | −0.04 (−0.23 to 0.15) | 0.667 | 0.081 | 1.73 (0.43) | 1.62 (0.5) | 0.09 (−0.09 to 0.27) | 0.315 | 0.186 |
| Supramarginal | 1.74 (0.31) | 1.7 (0.33) | 0.02 (−0.1 to 0.14) | 0.697 | 0.07 | 1.74 (0.35) | 1.63 (0.34) | 0.08 (−0.04 to 0.21) | 0.178 | 0.237 |
| Transverse_Temporal | 1.05 (0.37) | 1.04 (0.36) | 0 (−0.14 to 0.14) | 0.984 | 0.004 | 0.95 (0.4) | 0.91 (0.32) | 0.04 (−0.09 to 0.17) | 0.533 | 0.113 |
| Insula | 2.01 (0.58) | 1.97 (0.6) | −0.01 (−0.24 to 0.22) | 0.941 | 0.013 | 2.29 (0.69) | 2.06 (0.71) | 0.19 (−0.08 to 0.45) | 0.172 | 0.252 |
Analysis of Covariance (ANCOVA) model comparing cortical thickness between the 2 groups with age, gender, variant of FTD and CDR-Total score as covariates.
FTD-D: Frontotemporal Dementia-Depressed, FTD-ND: Frontotemporal Dementia-Not-Depressed.
p of group (FTD-D v/s FTD-ND) in the ANCOVA model, significant at ≤ 0.05.
β (95%CI): Standardized co-efficient of group (FTD-D v/s FTD-ND) in the ANCOVA model with 95% confidence interval.
Cortical thickness in millimeters.
ES=Effect Size expressed as Cohen’s d.
Statistical Analysis
To determine those areas of the brain that are significantly associated with apathy we performed a multiple linear regression with cortical thickness of each of the 48 areas as the dependent variable, apathy severity as the independent variable, age, gender and variant of FTD as covariates in the model (N = 97). Age, gender, variant of FTD, NPI-Q symptom ratings, CDR scores (total, box score, behavioral and language subscores) and MMSE scores were compared between FTD-Depressed (N = 36) and FTD-Not-Depressed (N = 67) using independent sample t-test and chi-square test accordingly. Pearson’s correlations were performed to examine the relationship between NPI-Q symptom severity ratings, CDR and MMSE scores. To determine the differences between the 2 groups in cortical thickness of the 48 areas we performed an Analysis of Covariance (ANCOVA) with the covariates, age, gender, variant of FTD and CDR-Total score as a measure of severity of dementia. Assumptions for linear regression and normality of data were tested (apathy severity had a normal distribution). The level of alpha was fixed at 0.05. Adjusted p-values (for multiple co-variates) are provided without correction for multiple brain areas to minimize false negatives given the novel exploratory objective of the study.26 p-values, confidence intervals of the standard regression coefficients and effect sizes (Cohen’s d) are provided for all brain areas examined.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was determined to be exempt of review by the Institutional Review Board (Reference number: IRB-AAAS6975).
Data Statement
Data is not provided as the NIFD data use agreement (https://ida.loni.usc.edu/collaboration/access/appLicense.jsp) does not allow further disclosure of these data beyond the uses outlined in the agreement and redistribution of data in any manner is prohibited. However, data will be shared by request from any qualified investigator after checking with NIFD if such a sharing is allowed and if yes it shall be shared after obtaining their due permissions.
Software
Cortical thickness values were obtained from ANTs version 2.2. All statistical analyses were performed on R Studio version 3.6.0. R Studio version 3.6.0, MATLAB version 2018a, FreeSurfer v6.0 and Microsoft PowerPoint were used for creating figures.
Results
Clinical and Demographic Data
The 2 groups FTD-Depressed and FTD-Not-Depressed did not differ from each other with respect to age, gender, variant of FTD, NPI-Q symptom severity scores (except depression), CDR and MMSE scores (Table 3).
Table 3.
Clinical and Demographic Data.
| FTD-depressed (N = 36) | FTD-not-depressed (N = 67) | t/X2 | P | |
|---|---|---|---|---|
| #Age(years) | 63.66(7.60) | 63.43(6.82) | 0.154 | 0.878 |
| *Gender(Males) | 58.33 | 63.3 | 0.0051 | 0.943 |
| *Variant of FTD | bvFTD = 44.45 | bvFTD = 50.0 | 2.243 | 0.326 |
| svPPA = 33.33 | svPPA = 33.33 | |||
| nfvPPA = 22.22 | nfvPPA = 16.67 | |||
| #Delusions | 1.83 (0.41) | 1.55 (0.69) | 1.082 | 0.297 |
| hallucinations | 2.5 (0.7) | 2.0 (0.0) | 1.00 | 0.5 |
| #Agitation/Aggression | 1.87 (0.63) | 1.75 (0.69) | 0.687 | 0.495 |
| #Depression/Dysphoria | 1.39 (0.49) | 0.0(0) | 16.855 | 2.2e-16 |
| #Anxiety | 1.62 (0.67) | 1.61 (0.5) | 0.058 | 0.954 |
| #Elation/Euphoria | 1.78 (0.65) | 1.71 (0.66) | 0.323 | 0.749 |
| #Apathy/lndifference | 1.83(0.75) | 2.00 (0.78) | 1.003 | 0.3202 |
| #Disinhibition | 1.82 (0.77) | 2.02 (0.66) | −1.137 | 0.261 |
| #Irritability/Lability | 1.67 (0.76) | 1.86 (0.75) | −0.998 | 0.323 |
| #Motor Disturbance | 1.96 (0.76) | 2.04 (0.7) | −0.437 | 0.664 |
| #Nighttime Behaviors | 1.74 (0.65) | 1.85 (0.73) | −0.527 | 0.601 |
| #Appetite/Eating | 2.00 (0.62) | 1.94 (0.7) | 0.375 | 0.709 |
| #CDR-Total score | 0.82 (0.5) | 0.93 (0.54) | −1.061 | 0.292 |
| #CDR-Box score | 4.28 (2.75) | 5.14 (3.01) | −1.458 | 0.149 |
| #CDR-Language score | 0.53 (1.54) | 0.95 (0.71) | −1.551 | 0.128 |
| #CDR-Behavior score | 0.86 (1.65) | 1.27 (0.79) | −1.409 | 0.166 |
| #MMSE-Total | 24.64 (5.92) | 23.00 (6.92) | 1.261 | 0.211 |
Symptom severities of the 12 items of the Neuropsychiatric Inventory—Questionnaire.
FTD = Frontotemporal Dementia, bvFTD = behavioral variant Frontotemporal Dementia, svPPA = semantic variant Primary Progressive Aphasia, nfvPPA = non-fluent variant Primary Progressive Aphasia, CDR = Clinical Dementia Rating, MMSE = Mini Mental Status Examination.
Mean (Standard Deviation).
Percentage.
Apathy and Cortical Thickness
Multiple linear regression demonstrated that apathy severity was significantly inversely associated with cortical thickness of the following areas: right parsopercularis (p = 0.013), right parsorbitalis (p = 0.025), right caudal middle frontal cortex (p = 0.03), right insula (p = 0.021), right superior temporal cortex (p = 0.025), right transverse temporal cortex (p = 0.028), right posterior cingulate cortex (p = 0.014), right superior parietal cortex (p = 0.019) and right supramarginal cortex (p = 0.041) (N = 97) (Figure 2, Table 1). As apathy severity and CDR-Total scores were highly collinear (supplemental table 1) we did not incorporate CDR as a covariate in the model
Figure 2.
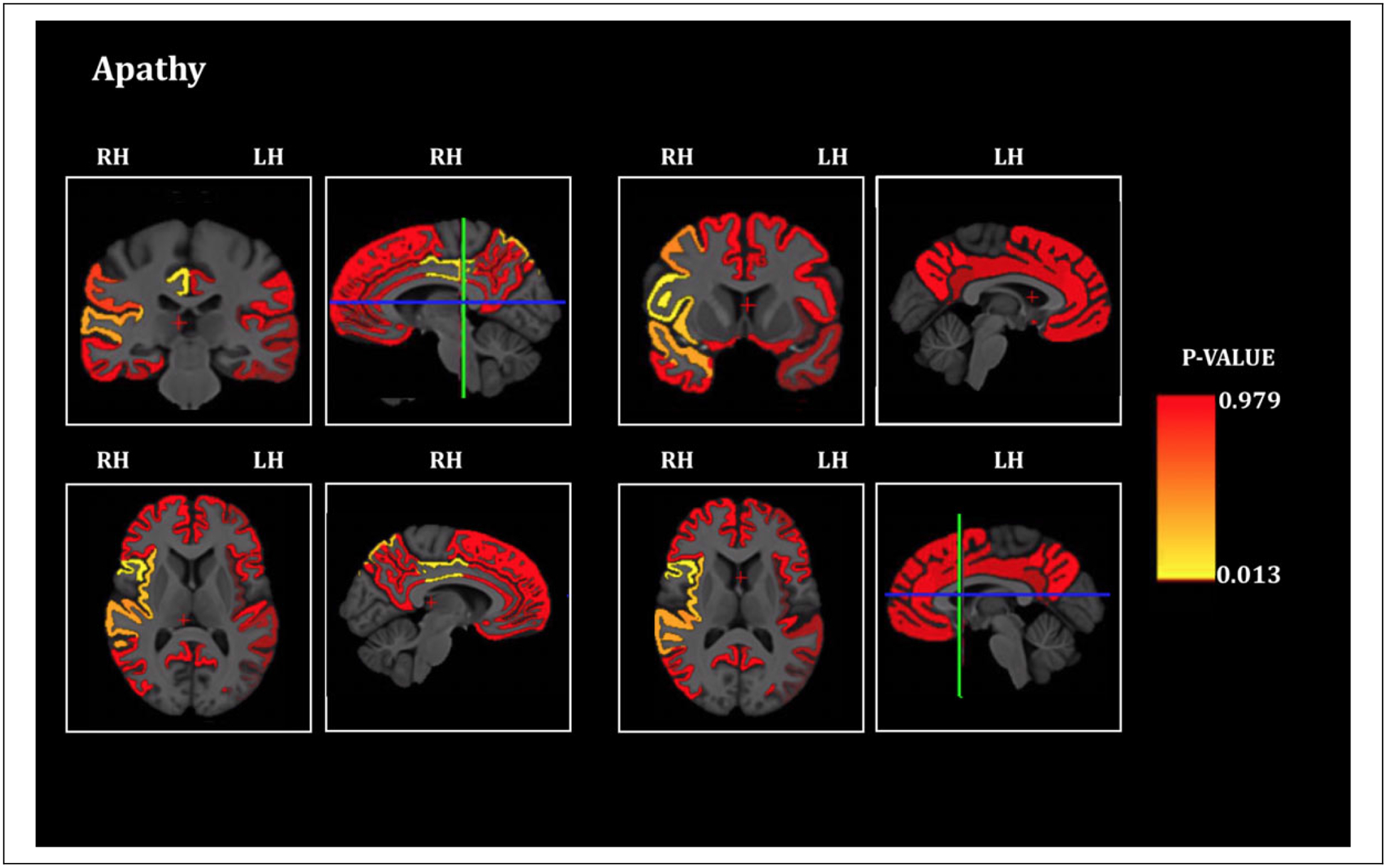
Apathy and cortical thickness in FTD. Brain sections showing the statistical significance of the associations of cortical thickness of Regions of Interest and apathy severity score (significant at p ≤ 0.05, all significant areas are inversely associated), RH = Right Hemisphere, LH = Left Hemisphere. The blue-green intersecting lines indicate the plane at which the coronal and axial sections are depicted.
Comparison of Cortical Thickness Between FTD-Depressed and FTD-Not-Depressed
ANCOVA demonstrated that the FTD-Depressed group had greater cortical thickness of the right lateral orbitofrontal cortex (p = 0.024), right medial orbitofrontal cortex (p = 0.027), right parsorbitalis (p = 0.019) and right rostral anterior cingulate cortex (p = 0.036) compared to FTD-Not-Depressed (Figure 3, Figure 4, Table 2). No other areas were significantly different between the 2 groups. Apathy severity was not included as a covariate in the ANCOVA as it was highly collinear with CDR-Total score (supplemental Table 1). Of the 2 we chose CDR-Total score as a co-variate in the model as it is an overall clinical indicator of the severity of dementia
Figure 3.
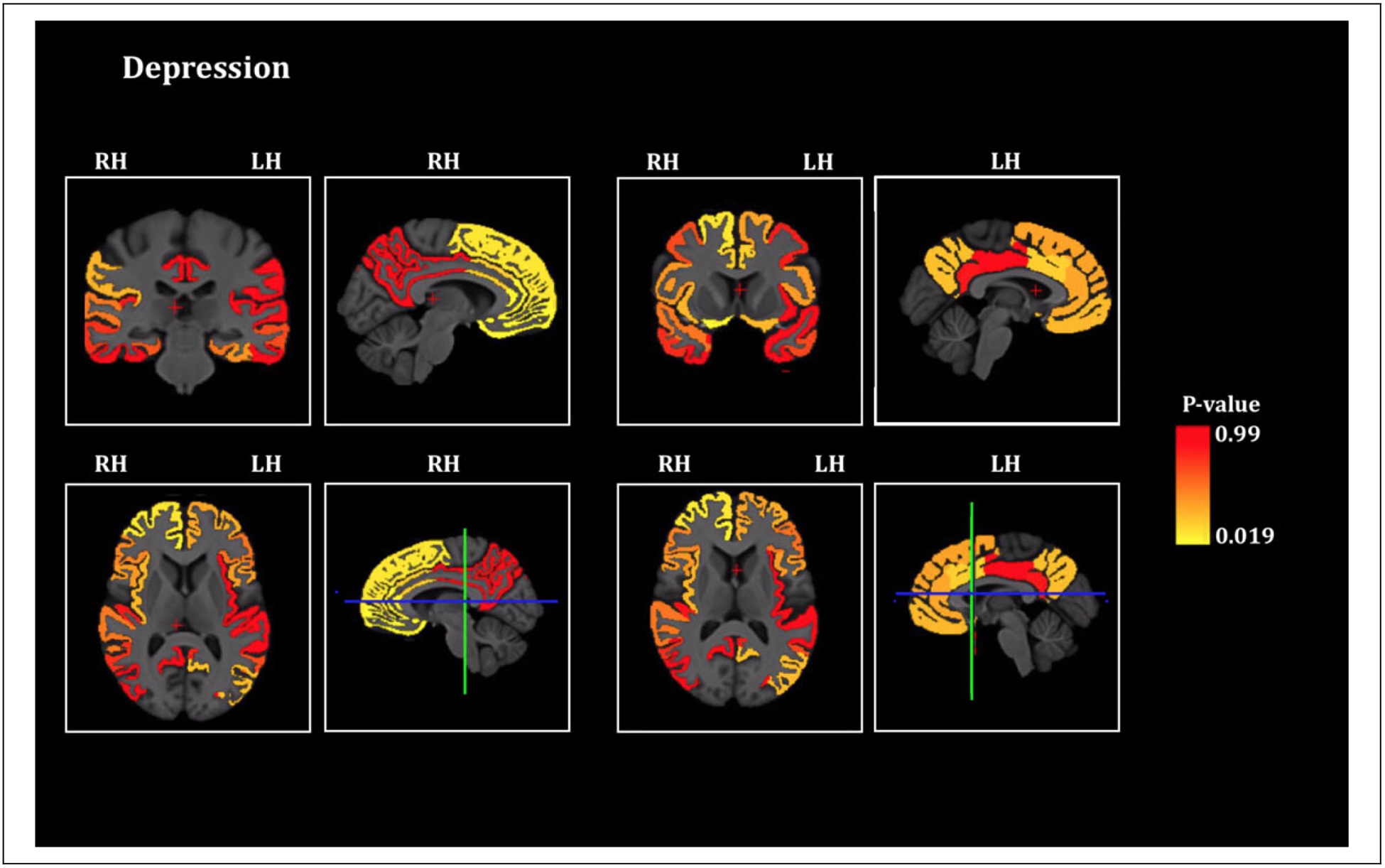
Depression and cortical thickness in FTD. Brain sections showing the statistical significance of the differences of cortical thickness of Regions of Interest between the depressed and the non-depressed FTD patients (significant at p ≤ 0.05, all significant areas are thicker in depressed), RH = Right Hemisphere, LH = Left Hemisphere. The blue-green intersecting lines indicate the plane at which the coronal and axial sections are depicted.
Figure 4.
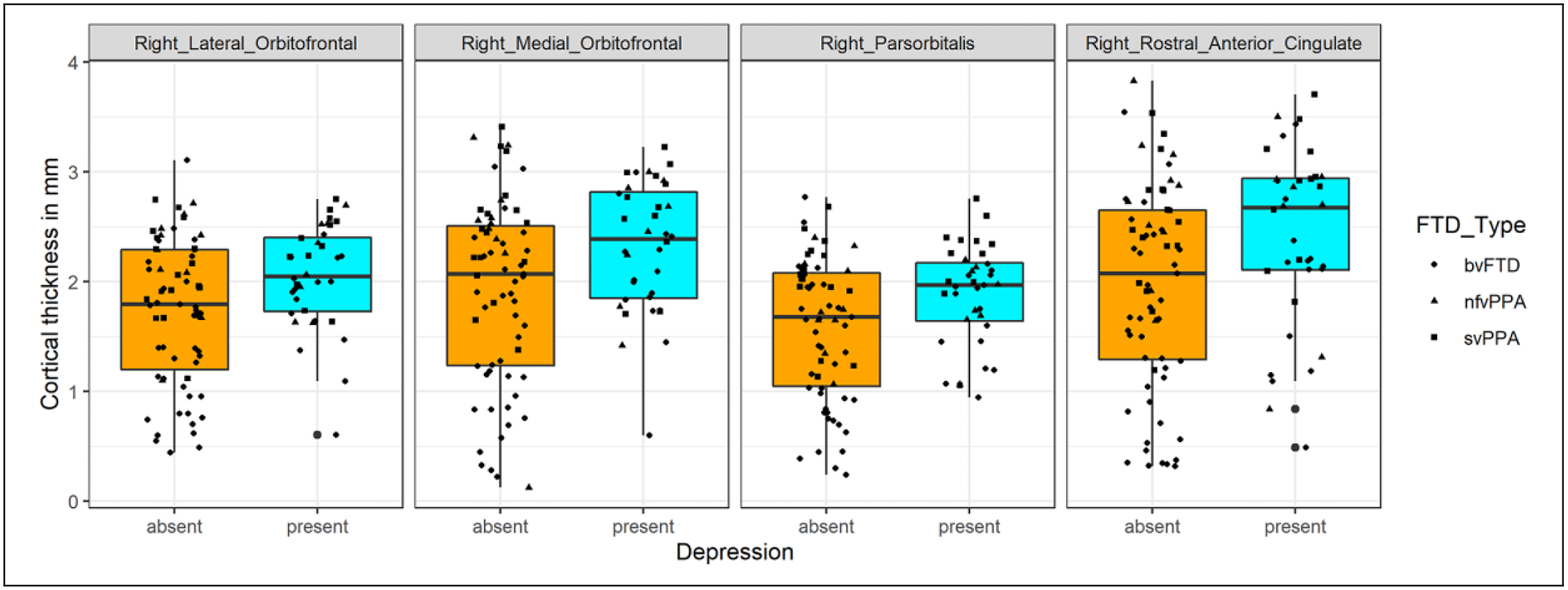
Cortical thickness of the right sided inferior and ventromedial frontal cortex in FTD patients with and without depression. Boxplots with a scatter of cortical thickness values of brain areas that are significantly different (p < 0.05) between FTD-Depressed and the FTD-Non-Depressed, mm = millimeter, bvFTD = behavioral variant Frontotemporal Dementia, svPPA = semantic variant Primary Progressive Aphasia, nfvPPA = non-fluent variant Primary Progressive Aphasia.
Discussion
Through this study we have demonstrated severity of apathy to be significantly associated with cortical thinning of parts of the frontal lobe (middle and inferior frontal gyrus), temporal lobe (superior and transverse temporal gyrus), parietal lobe (superior parietal, posterior cingulate and supramarginal gyrus) and the insula, all on the right side. Previous studies on the neural correlates of apathy12,13 in FTD have implicated different parts of the frontal lobes including the dorsolateral prefrontal cortex (DLPFC),27–29 ventromedial superior frontal gyrus,30 anterior cingulate cortex (ACC)27,29 and orbitofrontal cortex (OFC),28–30 predominantly on the right side.12 In our study we found only the lateral parts of the PFC on the right side to be significantly associated with apathy whereas none of the medial structures reached significance. Our study also reveals a significant association between apathy and certain parts of the temporal lobe, parietal lobe and insula, on the right side. Generally, studies which have also included the aphasic variants of FTD (svPPA and nfvPPA) in addition to bvFTD as in our study, have implicated the right temporoparietal junction, right middle and posteroinferior temporal gyri, and insula27,31 in apathy, in addition to the frontal lobes.
FTD patients who are depressed have a thicker right sided inferior and medial frontal cortex compared to those who are not depressed. Unlike apathy severity, the severity of depression was dichotomous (1 or 2) as none scored 3. Therefore, we did not perform a linear regression with depression, instead elected to categorize patients based on presence or absence of depression, and compared the cortical thickness between the 2 groups. We observe a relative preservation of thickness of the right sided inferior and medial frontal cortical areas associated with the symptom domain of depression.
There is evidence indirectly substantiating these findings demonstrated by studies in neurodegenerative disorders. SvPPA patients were significantly more depressed than bvFTD probably due to a universal lack of insight in bvFTD.32 SvPPA had a preserved display of wide range of emotions including sadness, irritability and aggression, demonstrated more emotional insightfulness, displayed high distress and anxiety.33 When FTD patients were classified into frontal and temporal variants through relative volumetric measurements by structural MRIs, it was observed that the temporal variant had significantly more depression than the frontal variant. This was associated with decreased volume of the right amygdala and right anterior temporal cortex.15 Studies which have attempted to explore the behavioral differences between Alzheimer’s dementia (AD) and FTD have demonstrated higher levels of depression and lower levels of apathy characterizing AD compared to FTD.34 Apathy was associated with more depressive symptoms of dysphoria, tearfulness, worthlessness, hopelessness, burdensomeness and suicidal thinking in AD compared to FTD.35 Higher expression of depression and its accompanying symptoms in the variant of FTD characterized by a relatively lesser reduction of the frontal lobes compared to temporal lobes, and in Alzheimer’s disease, which is characterized by a relative preservation of the ventral brain structures save for later stages of disease, point toward the association of depressive behavior and relative preservation of ventral frontal lobar gray matter. A study which examined psychiatric symptoms in pre-clinical carriers of Microtubule Associated Protein Tau (MAPT) mutations, a mutation associated with the development of bvFTD with high penetrance, demonstrated only isolated symptoms of depression in the absence of a full syndrome.36 Major Depressive Disorder (MDD) is a syndrome characterized by a constellation of a wide variety of symptoms of which depressed mood and diminished interest or pleasure are the principle symptoms accompanied by changes in sleep, weight, energy levels, psychomotor retardation/agitation, difficulty in concentration, depressive cognitions and suicidality (Diagnostic and Statistical Manual/DSM-5). This implies that degeneration of the frontal lobes might be exclusive of the production of MDD, yet still contribute to emotional blunting or apathy which starts as the disease progresses.36 In a series of patients with head injury, bilateral ventromedial PFC (vmPFC) lesions conferred resistance to depression with lower prevalence of cognitive (guilt, hopelessness, worthlessness, helplessness, suicidality) and affective (sad mood) aspects of depression, compared to bilateral dorsolateral PFC lesions, which in contrast increased the vulnerability for depression.37 The vmPFC comprises of the Brodmann areas 11, 12, 25, subgenual portion of 32 and the lower medial portion of area 1038 which overlaps with our results of significant areas of differential cortical thickness between the depressed and the not depressed. vmPFC lesions in human beings have resulted in profound changes in emotional expression like blunted affect, lack of physiological reactions to emotionally evocative stimuli, lack of feelings of regret, decrease in the subjective feeling of negative emotions, impaired affective information processing, reduced affective empathy38 and a deficit in self-insight.37 The vmPFC can be implied to be a critical neuroanatomical site for the expression of these phenomena, a pathological exaggeration of which characterizes depression. Some ablative surgeries which are performed for treatment resistant depression have involved ventral and medial structures of the PFC, and these procedures carry the risk of causing apathy as one of the adverse effect,39 again implying the importance of this area in expression of depression and the loss being associated with apathy. If FTD can serve as a model of the continuum of dysfunction as evidenced by alterations in frontal cortical thickness, the phase of relatively preserved right inferior and medial frontal cortical thickness may manifest as depression compared to extreme thinning of these areas presenting as apathy. A longitudinal study with serial brain volumetric measurements and mapping of psychopathology to the brain volumes in FTD can clarify these findings and is currently being performed (ALLFTD).
Some of the strengths of the study are that the imaging data is derived from an open large and varied database representing a consolidation of data from multiple sites and hence is representative of a broader FTD population in contrast to more selective convenient sampling in individual small studies. Additionally, we have demonstrated the results across all variants of FTD, we did not individually examine the variants as the sample size of svPPA and nfvPPA were small. As a post-hoc when we restricted the analysis to an anatomically homogenous entity and the variant rich with behavioral symptoms i.e. bvFTD alone, we obtained the same results (Supplemental Figure 1). The relatively greater cortical thickness in the depressed group cannot be attributed to a possibly less severe or a milder stage of dementia in the depressed compared to the non-depressed group, as both groups are comparable on the CDR-Total score, and the ANCOVA group differences are significant despite including the score as a covariate in the model. One of the limitations of this study is that the duration of illness, a potential confounder is unavailable but the information on stage of dementia through CDR scores possibly overcomes this limitation. The NPI-Q is a very brief form of measuring psychiatric phenomena in FTD patients. Though the information on NPI-Q is obtained from collaterals it has been demonstrated to have significant independent concurrence with the clinician’s diagnosis of depression.40 By using ratings from collaterals there is a possibility of retrospective misclassification of caregiver’s own depression versus patient’s ratings, however, the correlations between the different NPI-Q symptom severity scores are in line with the general trends of behavioral phenomena in neurological disorders i.e. apathy severity was significantly inversely correlated with depression severity but anxiety severity was significantly directly correlated with depression severity (supplemental Table 1). These expected directionalities of the correlations validate the NPI-Q ratings though they are from the caregiver. The NPI-Q was used in place of Geriatric Depression Scale (GDS) as GDS has been demonstrated to be most suitable for assessing depression only in the cognitively intact and mild to moderate cognitive impairment41 and it was available only in a smaller subset of patients compared to NPI-Q. Ratings on apathy were not available on any scale other than NPI-Q. We intended to study differential involvement of brain regions in apathy and depression which warranted measurement of both symptoms on the same scale such as NPI-Q. This avoids heterogeneity in ratings which would inevitably be introduced if apathy is rated on a caregiver instrument and depression on a self-rated tool and can influence imaging results as shown previously.16 Also, this study lacks a pathological confirmation of FTD diagnosis. Future studies should explore the biological underpinnings of symptom ratings in FTD obtained through different modes (caregiver, patient, rater)16 and elaborate rating scales including measurements of insight in larger samples.
Apathy in FTD is associated with extensive cortical thinning in multiple areas of the frontal, temporal and parietal lobes only on the right side. The results are unique in demonstrating that in FTD, a certain threshold of right sided ventral and inferior frontal lobar mass is associated with manifestation of emotional disorders like depression and the reduction of same underlies emotional blunting/apathy.
Supplementary Material
Acknowledgments
Data for this study was obtained from the Frontotemporal Lobar Degeneration Neuroimaging Initiative (FTLDNI) database (http://4rtni-ftldni.ini.usc.edu/). The Frontotemporal Lobar Degeneration Neuroimaging Initiative (National Institutes of Health Grant R01 AG032306) funded the data collection and sharing for this project. The University of California, San Francisco, Memory and Aging Centre coordinates the FTLDNI and data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. The investigators at NIFD/FTLDNI were involved in the design and implementation of FTLDNI and data gathering and readying of the same for open availability, but did not participate in analysis or writing of this report.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Dr. Rakshathi Basavaraju reports no disclosures. Dr. Xinyang Feng is currently a Research Scientist at Facebook Inc. He participated in this study when he was a PhD student in the Department of Biomedical Engineering, Columbia University. Ms. Jeanelle France reports no disclosures. Dr. Edward D. Huey reports no disclosures. Dr. Frank A. Provenzano is a consultant for and has equity in IMIJ technologies.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Hogan DB, Jette N, Fiest KM, et al. The prevalence and incidence of frontotemporal dementia: a systematic review. Can J Neurol Sci. 2016;43(Suppl 1):S96–S109. [DOI] [PubMed] [Google Scholar]
- 2.Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onyike CU, Huey ED. Frontotemporal dementia and psychiatry. Int Rev Psychiatry. 2013;25(2):127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galimberti D, Dell’Osso B, Altamura AC, Scarpini E. Psychiatric symptoms in frontotemporal dementia: epidemiology, phenotypes, and differential diagnosis. Biol Psychiatry. 2015;78(10): 684–692. [DOI] [PubMed] [Google Scholar]
- 5.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006; 16(7):916–928. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee A, Biswas A, Roy A, Biswas S, Gangopadhyay G, Das SK. Behavioural and psychological symptoms of dementia: correlates and impact on caregiver distress. Dement Geriatr Cogn Dis Extra. 2017;7(3):354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor CM, Clemson L, Hornberger M, et al. Longitudinal change in everyday function and behavioral symptoms in frontotemporal dementia. Neurol Clin Pract. 2016;6(5):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuring JK, Mathias JL, Ward L. Prevalence of depression, anxiety and PTSD in people with dementia: a systematic review and meta-analysis. Neuropsychol Rev. 2018;28(4):393–416. [DOI] [PubMed] [Google Scholar]
- 9.Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10(3): 314–319. [DOI] [PubMed] [Google Scholar]
- 10.Marin RS, Firinciogullari S, Biedrzycki RC. Group differences in the relationship between apathy and depression. J Nerv Ment Dis. 1994;182(4):235–239. [DOI] [PubMed] [Google Scholar]
- 11.Raimo S, Santangelo G, D’Iorio A, Trojano L, Grossi D. Neural correlates of apathy in patients with neurodegenerative disorders: an activation likelihood estimation (ALE) meta-analysis. Brain Imaging Behav. 2019;13(6):1815–1834. [DOI] [PubMed] [Google Scholar]
- 12.Kos C, van Tol MJ, Marsman JB, Knegtering H, Aleman A. Neural correlates of apathy in patients with neurodegenerative disorders, acquired brain injury, and psychiatric disorders. Neurosci Biobehav Rev. 2016;69:381–401. [DOI] [PubMed] [Google Scholar]
- 13.Ducharme S, Price BH, Dickerson BC. Apathy: a neurocircuitry model based on frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2018;89(4):389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, et al. White matter change with apathy and impulsivity in frontotemporal lobar degeneration syndromes. Neurology. 2018;90(12):e1066–e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62(5):742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, et al. Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain. 2017;140(6):1792–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–239. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 19.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011; 76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3): 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open access series of imaging studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19(9):1498–1507. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 25.Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53(3): 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 27.Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology. 2008;71(10):736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massimo L, Powers C, Moore P, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massimo L, Powers JP, Evans LK, et al. Apathy in frontotemporal degeneration: neuroanatomical evidence of impaired goal-directed behavior. Front Hum Neurosci. 2015;9:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(Pt 11):2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M. Apathy in frontotemporal dementia: behavioral and neuroimaging correlates. Behav Neurol. 2012;25(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2000;69(2):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001; 70(3):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy ML, Miller BL, Cummings JL, Fairbanks LA, Craig A. Alzheimer disease and frontotemporal dementias. Behavioral distinctions. Arch Neurol. 1996;53(7):687–690. [DOI] [PubMed] [Google Scholar]
- 35.Chow TW, Binns MA, Cummings JL, et al. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Arch Neurol. 2009;66(7): 888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheran G, Silverman H, Manoochehri M, et al. Psychiatric symptoms in preclinical behavioural-variant frontotemporal dementia in MAPT mutation carriers. J Neurol Neurosurg Psychiatry. 2018;89(5):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28(47): 12341–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider B, Koenigs M. Human lesion studies of ventromedial prefrontal cortex. Neuropsychologia. 2017;107:84–93. [DOI] [PubMed] [Google Scholar]
- 39.Volpini M, Giacobbe P, Cosgrove GR, Levitt A, Lozano AM, Lipsman N. The history and future of ablative neurosurgery for major depressive disorder. Stereotact Funct Neurosurg. 2017; 95(4):216–228. [DOI] [PubMed] [Google Scholar]
- 40.Chopra MP, Sullivan JR, Feldman Z, Landes RD, Beck C. Self-, collateral- and clinician assessment of depression in persons with cognitive impairment. Aging Ment Health. 2008;12(6):675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balsamo M, Cataldi F, Carlucci L, Padulo C, Fairfield B. Assessment of late-life depression via self-report measures: a review. Clin Interv Aging. 2018;13:2021–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


