Abstract
BACKGROUND & AIMS:
We aimed to compare safety and effectiveness of vedolizumab to tumor necrosis factor (TNF)-antagonist therapy in ulcerative colitis in routine practice.
METHODS:
A multicenter, retrospective, observational cohort study (May 2014 to December 2017) of ulcerative colitis patients were treated with vedolizumab or TNF-antagonist therapy. Propensity score weighted comparisons for development of serious adverse events and achievement of clinical remission, steroid-free clinical remission, and steroid-free deep remission. A priori determined subgroup comparisons in TNF-antagonist–naïve and –exposed patients, and for vedolizumab against infliximab and subcutaneous TNF-antagonists separately.
RESULTS:
A total of 722 (454 vedolizumab, 268 TNF antagonist) patients were included. Vedolizumab- treated patients were more likely to achieve clinical remission (hazard ratio [HR], 1.651; 95% confidence interval [CI], 1.229–2.217), steroid-free clinical remission (HR, 1.828; 95% CI, 1.135–2.944), and steroid-free deep remission (HR, 2.819; 95% CI, 1.496–5.310) than those treated with TNF antagonists. Results were consistent across subgroup analyses in TNF-antagonist–naïve and –exposed patients, and for vedolizumab vs infliximab and vs subcutaneous TNF-antagonist agents separately. Overall, there were no statistically significant differences in the risk of serious adverse events (HR, 0.899; 95% CI, 0.502–1.612) or serious infections (HR, 1.235; 95% CI, 0.608–2.511) between vedolizumab-treated and TNF antagonist–treated patients. However, in TNF-antagonist–naïve patients, vedolizumab was less likely to be associated with serious adverse events than TNF antagonists (HR, 0.192; 95% CI, 0.049–0.754).
CONCLUSIONS:
Treatment of ulcerative colitis with vedolizumab is associated with higher rates of remission than treatment with TNF-antagonist therapy in routine practice, and lower rates of serious adverse events in TNF-antagonist–naïve patients.
Keywords: Health Outcomes, Comparative Research, Biologics
Treatment with biologic therapy in ulcerative colitis (UC) has been shown to result in disease remission and to prevent hospitalization and progression to colectomy.1 Current guidelines allow for a choice between vedolizumab or tumor necrosis factor (TNF) antagonist therapy.2–5 A recent phase 3 clinical trial demonstrated that vedolizumab is superior to adalimumab for achievement of clinical and endoscopic remission in UC.6 The superiority of vedolizumab was predominately seen in patients naive to TNF antagonist therapy. Although informative in helping to understand the relative positioning of these biologics, nearly 75% of UC patients seen in routine practice do not qualify for these phase 3 trial programs.7 Direct routine practice comparative studies are needed to fully inform treatment decisions.
We studied the comparative safety and effectiveness of vedolizumab and TNF antagonist therapy in adult patients with UC by using a multicenter propensity score weighted cohort study. We used patient-level data from medical records and compared the relative risk for developing serious infections or serious adverse events and the relative effectiveness of each therapy for achieving disease remission.
Methods
We performed a Hypothesis Evaluating Treatment Effectiveness study to understand the optimal biologic treatment for UC, with the a priori hypothesis that vedolizumab is associated with a lower risk of serious infections than TNF antagonist therapy in UC, and that vedolizumab is superior to TNF antagonist therapy for achieving clinical remission in UC8–12 (Supplementary Methods). All authors had access to the study data and reviewed and approved the final manuscript.
Protocol and Reporting
We followed Good ReseArch for Comparative Effectiveness (GRACE) principles and good practice recommendations from the joint International Society for Pharmacoeconomics and Outcomes Research and the International Society for Pharmacoepidemiology for real-world data comparative effectiveness studies.13,14 The study protocol was posted to the Health Services Research Projects in Progress website (https://wwwcf.nlm.nih.gov/hsr_project/home_proj.cfm), and the results are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies and reporting guidelines for propensity score analyses.15,16
Ethical Approval Statement
Institutional Review Board approval was obtained from each site for ongoing data collection and transfer.
Study Design and Data Source
This study presents a retrospective review of a North American–based consortium registry.17,18 In brief, this is a multicenter collaborative research group that pools outcomes for consecutive UC patients treated with biologics. The current analysis represents data collected between May 2014 and December 2017.
Participants
Consecutive patients from the consortium registry were included in the current analysis if they had (1) a confirmed diagnosis of UC based on clinical, endoscopic, and/or histologic data; (2) active clinical symptoms attributed to UC before biologic therapy; and (3) at least one clinical or endoscopic follow-up after biologic initiation, irrespective of response status after induction. No predefined period of follow-up or treatment was required for inclusion. Data on variables of interest collected are reported in Supplementary Methods.
Outcomes
The comparative safety outcomes were time to serious adverse events and serious infections. The comparative effectiveness outcomes were time to clinical remission, steroid-free clinical remission, deep remission (achieving both clinical and endoscopic remission), and steroid-free deep remission (achieving both steroid-free clinical remission and endoscopic remission) (Supplementary Methods19–25).
Statistical Analyses
Because of the nonrandomized nature of this study, inverse probability weighting (IPW) with propensity score was used to estimate the average treatment effect (ATE) of vedolizumab vs TNF antagonists in our study population.26–28 The Cox proportional cause-specific hazards model was used, with time to surgery treated as a competing risk.28–30 Time-to-event analyses were chosen because of routine practice variability in follow-up timing recommendations and access for assessment of response and known variation in time to response across key subgroups (ie, TNF antagonist exposed). Sensitivity analyses were performed by using optimal full match to assess for consistency in estimates across methodologies.27,31–33
Propensity score model.
Propensity scores were calculated by using R package “twang,” a toolkit for weighting and analysis of nonequivalent groups,34 which estimates propensity score by using boosted regression as the predicted probability of starting treatment with vedolizumab vs TNF antagonist therapy conditional on the measured baseline variables thought to be confounders or predictors for the outcome of interest. Investigators used a combination of prior published literature, clinical experience, and data availability within the current dataset to generate a list of potential prognostic variables for consideration. An investigator-driven approach for confounding evaluation was chosen on the basis of causal knowledge.35 In addition, care was taken not to include those variables that were strongly correlated with exposure but only weakly correlated with outcome.36–38
Stabilized weights were obtained and further trimmed to be within (0.1, 10), if necessary, before they were used for IPW approaches.15 Adequacy of the propensity score model was examined by plotting propensity score distributions in the vedolizumab vs TNF antagonist groups and by the standardized mean difference of each covariate before and after weighting. The propensity score model was fit separately for all comparisons of effectiveness and safety between vedolizumab and TNF antagonists and for subgroup analyses39,40 (Supplementary Methods).
Subgroup Analyses
A priori subgroup analyses were specified for the comparison of vedolizumab with TNF antagonist therapy in TNF antagonist–naive and TNF antagonist–exposed patients and for vedolizumab vs infliximab and vedolizumab vs subcutaneous TNF antagonists (adalimumab or golimumab) separately. Subgroup comparisons in TNF antagonist–naive and TNF antagonist–exposed groups were performed because of prior evidence supporting an increased risk of serious infections and reduction in effectiveness with vedolizumab in TNF antagonist–exposed individuals.17,41,42 Subgroup comparisons to infliximab and subcutaneous TNF antagonists were conducted separately to assess for the potential influence of medical (vedolizumab or infliximab) or pharmacy (subcutaneous TNF antagonists) benefits and market access as a determinant of treatment choice and comparative estimates.43
Results
Baseline Demographics
A total of 722 UC patients with a median follow-up of 333 days (interquartile range, 167–494 days) were included (Table 1, Supplementary Results, Supplementary Tables 1–3). The variables for TNF antagonist exposure and number of prior TNF antagonists used were the most imbalanced in distribution between the vedolizumab and TNF antagonist cohorts at baseline. After weighting, distributions of variables were well-balanced as assessed by standardized mean difference, with the exception of number of prior TNF antagonists (Figure 1). None of the observations were trimmed to avoid extreme weights.
Table 1.
Baseline Patient Characteristics
| Vedolizumab (n = 454) | Infliximab (n = 165) | Subcutaneous TNF antagonists (n = 103) | |
|---|---|---|---|
| Age at biologic, y, mean (SD) | 42.08 (17.13) | 38.47 (15.97) | 40.11 (15.28) |
| Age at diagnosis, y, mean (SD) | 32.71 (15.88) | 32.27 (14.77) | 31.52 (15.56) |
| Female sex, n (%) | 226 (49.8) | 87 (52.7) | 48 (46.6) |
| BMI, kg/m2, mean (SD) | 25.76 (5.94) | 24.70 (6.57) | 26.39 (6.34) |
| Smoking status | |||
| Current, n (%) | 11 (2.4) | 6 (3.6) | 6 (5.8) |
| Former, n (%) | 113 (24.9) | 50 (30.3) | 25 (24.3) |
| Never, n (%) | 330 (72.7) | 109 (66.1) | 72 (69.9) |
| Disease duration, y, mean (SD) | 6 (11) | 3 (6) | 6 (11) |
| Ever hospitalized for UC flare? | |||
| Never, n (%) | 213 (46.9) | 72 (43.6) | 57 (55.3) |
| Yes (in the last year), n (%) | 114 (25.1) | 70 (42.4) | 18 (17.5) |
| Yes (not in the last year), n (%) | 127 (28.0) | 23 (13.9) | 28 (27.2) |
| CRP, mean (SD) | 1.9 (6.15) | 2.6 (15.28) | 0.41 (0.95) |
| Albumin, mean (SD) | 3.92 (0.54) | 3.67 (0.65) | 4.16 (0.46) |
| Rheumatic EIM, n (%) | 79 (17.4) | 27 (16.4) | 15 (14.6) |
| Ophthalmologic EIM, n (%) | 3 (0.7) | 3 (1.8) | 2 (1.9) |
| Dermatologic EIM, n (%) | 17 (3.7) | 8 (4.8) | 3 (2.9) |
| Hepatic EIM, n (%) | 20 (4.4) | 3 (1.8) | 5 (4.9) |
| Disease extent | |||
| E1, n (%) | 22 (4.9) | 6 (3.7) | 5 (4.9) |
| E2, n (%) | 161 (35.5) | 63 (38.7) | 30 (29.1) |
| E3, n (%) | 270 (59.6) | 94 (57.7) | 68 (66.0) |
| Disease severity | |||
| Mild, n (%) | 52 (11.5) | 5 (3.0) | 8 (7.8) |
| Moderate, n (%) | 253 (55.7) | 82 (49.7) | 57 (55.3) |
| Severe, n (%) | 149 (32.8) | 78 (47.3) | 38 (36.9) |
| Endoscopic severity | |||
| Mild, n (%) | 48 (13.6) | 10 (7.9) | 14 (19.2) |
| Moderate, n (%) | 166 (47.2) | 42 (33.1) | 34 (46.6) |
| Severe, n (%) | 138 (39.2) | 75 (59.1) | 25 (34.2) |
| Concomitant mesalamine, n (%) | 236 (52) | 83 (50) | 52 (51) |
| Concomitant IM, n (%) | 149 (32.8) | 71 (43.0) | 43 (41.7) |
| Concomitant steroids, n (%) | 243 (53.5) | 99 (60.0) | 46 (44.7) |
| Steroid dependency, n (%) | 216 (47.6) | 60 (36.4) | 23 (22.3) |
| TNF antagonist exposed, n (%) | 311 (68.5) | 51 (30.9) | 57 (55.3) |
| Number TNF antagonists exposed | |||
| 0, n (%) | 143 (31.5) | 114 (69.1) | 46 (44.7) |
| 1, n (%) | 205 (45.2) | 48 (29.1) | 48 (46.6) |
| 2, n (%) | 93 (20.5) | 3 (1.8) | 8 (7.8) |
| 3, n (%) | 13 (2.9) | 0 (0.0) | 1 (1.0) |
| TNF antagonist failure, n (%) | 260 (57.3) | 31 (18.8) | 30 (29.1) |
NOTE. Subcutaneous TNF antagonists: n = 87 adalimumab; n = 16 golimumab.
BMI, body mass index; CRP, C-reactive protein; EIM, extraintestinal manifestation; IM, immunomodulator (azathioprine, 6-mercaptopurine, methotrexate); SD, standard deviation; TNF antagonist, tumor necrosis factor antagonist.
Figure 1.
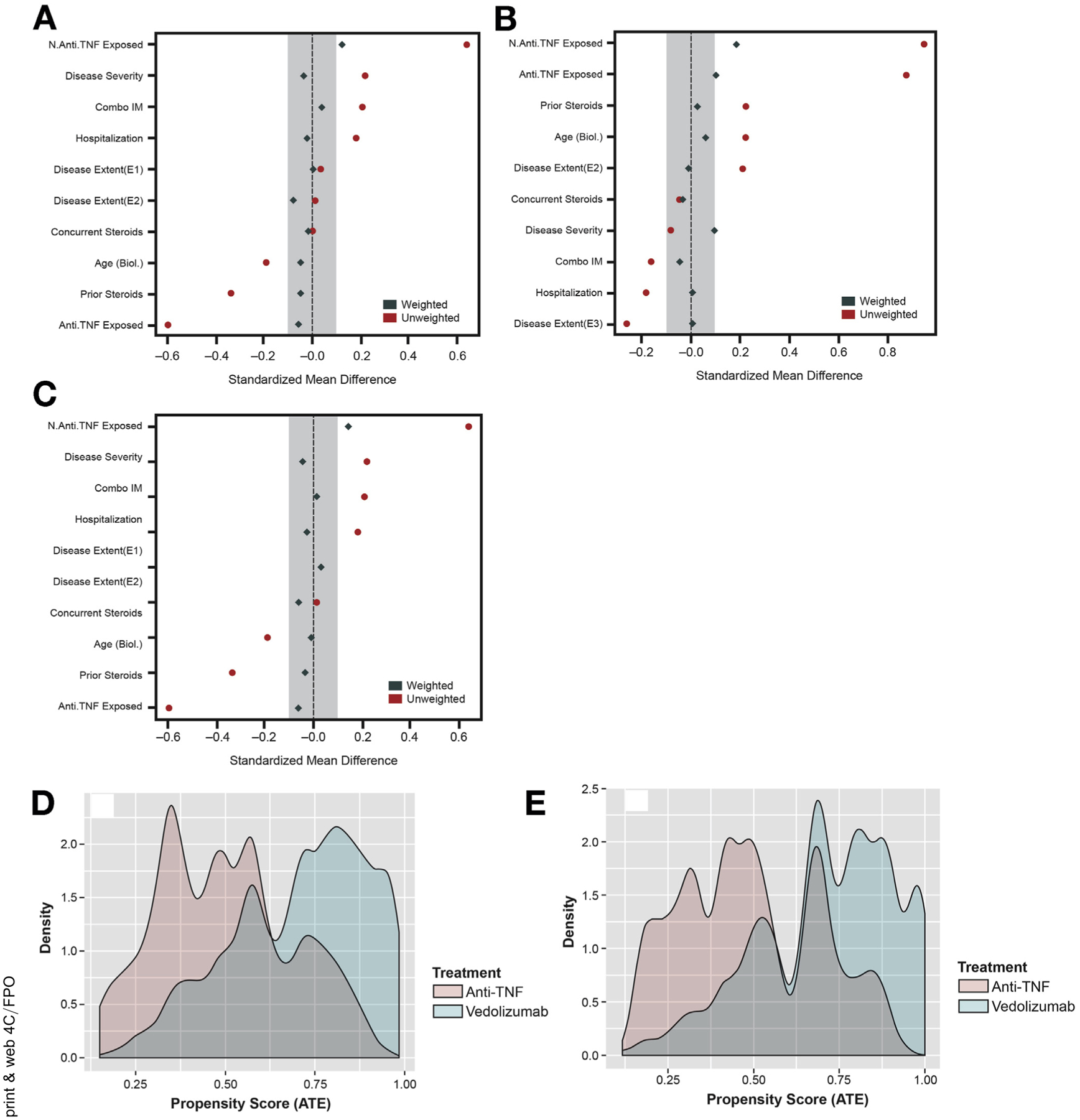
Standardized mean difference and distribution of propensity scores. Standardized mean difference before (red) and after (blue) weighting for (A) serious infections, serious adverse events, clinical remission, and deep remission; (B) deep remission limited to subset with moderate-severe endoscopic inflammation at baseline; and (C) steroid-free clinical remission and steroid-free deep remission. Distribution of propensity scores between cohorts for (D) serious infections, serious adverse events, clinical remission, and deep remission; (E) steroid-free clinical remission and steroid-free deep remission. Anti-TNF, tumor necrosis factor antagonist; ATE, average treatment effect; IM, immunomodulatory; SAE, serious adverse event.
Safety Events
A total of 21 vedolizumab-treated and 27 TNF antagonist–treated patients developed serious infections (Supplementary Table 4). The most common serious infections were Clostridium difficile (n = 7 vedolizumab, n = 12 TNF antagonist), respiratory infections (n = 6 vedolizumab, n = 4 TNF antagonist), and shingles (n = 0 vedolizumab, n = 5 TNF antagonist). A total of 5 vedolizumab-treated patients and 19 TNF antagonist–treated patients developed noninfectious serious adverse events. For vedolizumab-treated patients, these serious adverse events were severe joint pain requiring therapy discontinuation (n = 3), squamous cell cancer of the hand (n = 1), and colon cancer (n = 1). For TNF antagonist–treated patients, these serious adverse events included severe infusion reactions (n = 9), drug-induced lupus (n = 4), drug-induced psoriasis (n = 2), severe liver enzyme abnormalities (n = 1), lung cancer (n = 1), head and neck cancer (n = 1), and metastatic adenocarcinoma of unknown primary source (n = 1).
Comparative Safety
In the overall cohort, there were no statistically significant differences in the risk of serious adverse events (hazard ratio [HR], 0.899; 95% confidence interval [CI], 0.502–1.612) or serious infections (HR, 1.235; 95% CI, 0.608–2.511) between vedolizumab-treated and TNF antagonist treated patients (Figure 2). Among TNF antagonist–naive patients, treatment with vedolizumab was associated with a significantly lower risk for serious adverse events (HR, 0.192; 95% CI, 0.049–0.754), with nonsignificant trends toward lower risk for serious infection (HR, 0.320; 95% CI, 0.078–1.322) (Figure 2). Among TNF antagonist–exposed patients, treatment with vedolizumab was associated with a nonsignificant increased risk for serious adverse events (HR, 2.495; 95% CI, 0.988–6.301) and a significant increased risk for serious infections (HR, 4.295; 95% CI, 1.091–16.897). Subgroup analyses confirmed these trends (Supplementary Tables 4 and 5).
Figure 2.
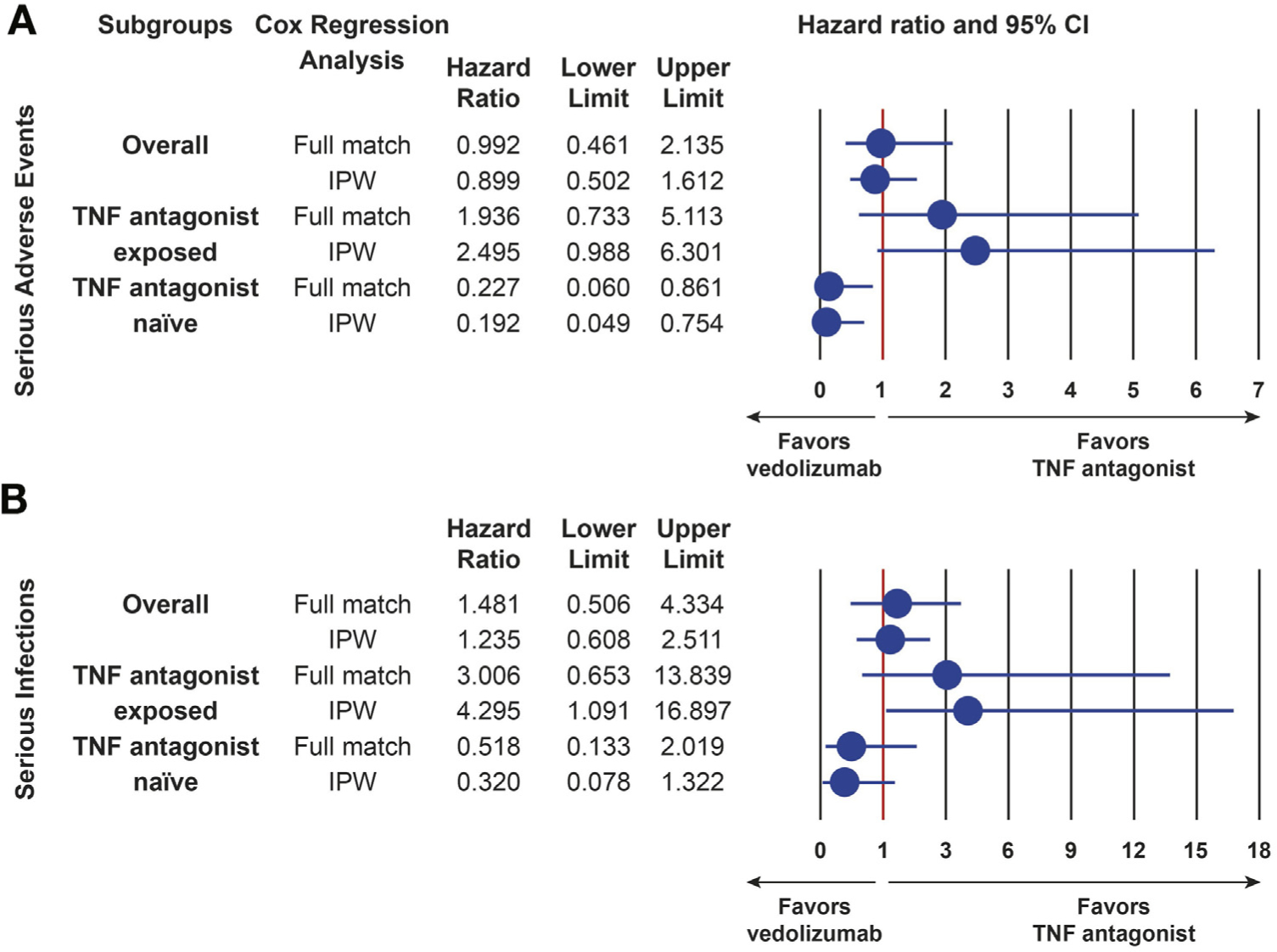
Comparative safety of vedolizumab to TNF antagonist therapy in UC. (A) Comparative safety of vedolizumab to TNF antagonist therapy for serious adverse events. (B) Comparative safety of vedolizumab to TNF antagonist therapy for serious infections. CI, confidence interval; IPW, inverse probability weighting; TNF antagonist, tumor necrosis factor antagonist (infliximab, adalimumab, golimumab). Serious infections or adverse events defined as infections or non-infection adverse events requiring antibiotics, antivirals, antifungals, discontinuation of therapy, hospitalization, or resulting in death.
Comparative Effectiveness
Clinical remission.
Patients treated with vedolizumab were more likely to achieve clinical remission than those treated with TNF antagonist therapy (HR, 1.651; 95% CI, 1.229–2.217). The comparative effectiveness for clinical remission was comparable in TNF antagonist–naive (HR, 1.676; 95% CI, 1.157–2.428) and TNF antagonist–exposed (HR, 1.689; 95% CI, 1.507–2.700) groups separately (Figure 3). Subgroup analyses confirmed vedolizumab to be associated with a higher probability for achieving clinical remission than infliximab (HR, 1.810; 95% CI, 1.225–2.675) and subcutaneous TNF antagonist agents (HR, 1.693; 95% CI, 1.091–2.627) separately (Supplementary Tables 4 and 5).
Figure 3.
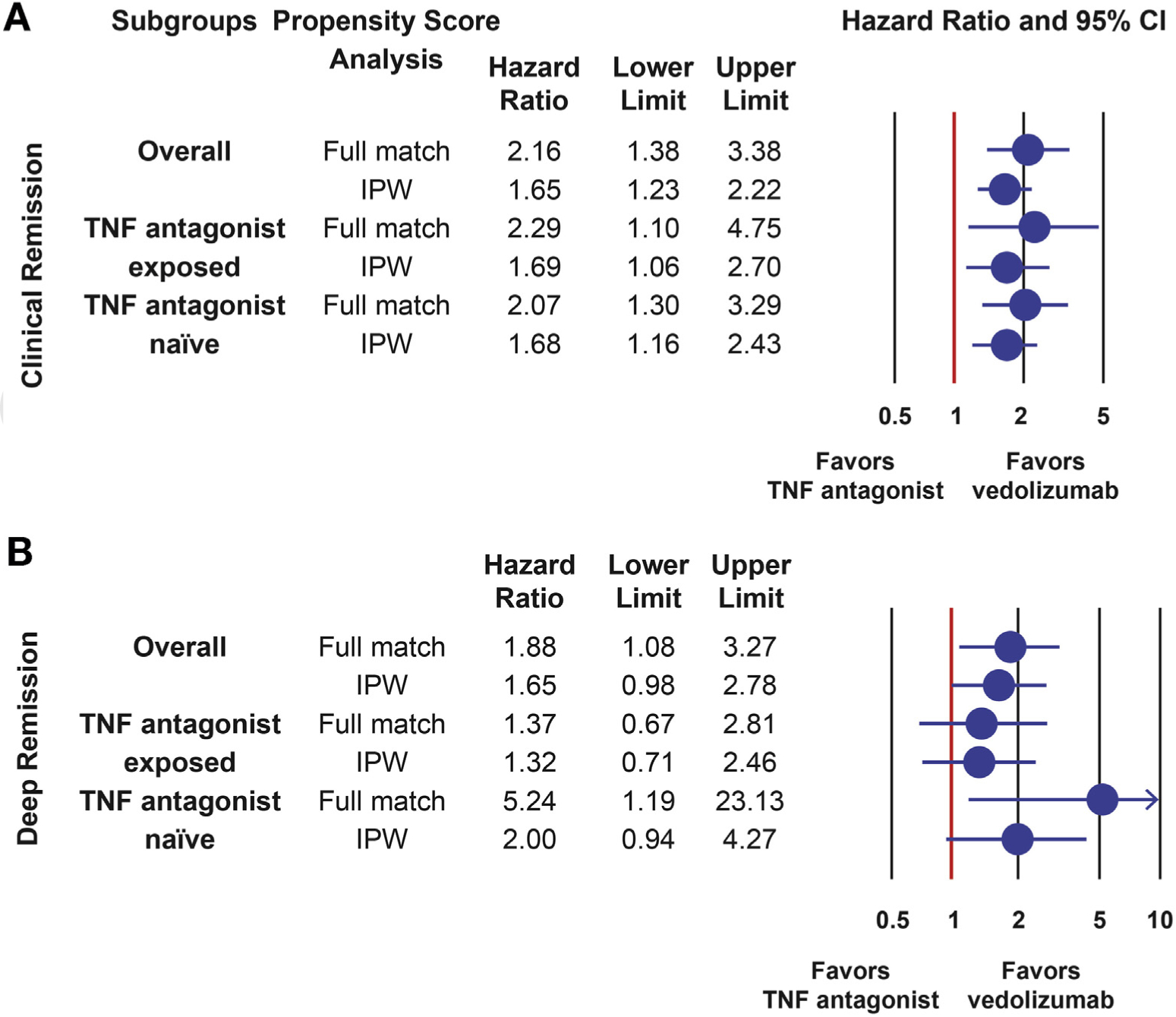
Comparative effectiveness of vedolizumab vs TNF antagonist therapy in UC for achieving clinical remission and deep remission. (A) Comparative effectiveness of vedolizumab vs TNF antagonist therapy for clinical remission. (B) Comparative effectiveness of vedolizumab vs TNF antagonist therapy for deep remission (clinical + endoscopic remission). CI, confidence interval; IPW, inverse probability weighting; TNF antagonist, tumor necrosis factor antagonist (infliximab, adalimumab, golimumab); UC, ulcerative colitis. Deep remission was defined as achieving clinical remission (resolution of diarrhea, rectal bleeding, and/or urgency) and endoscopic remission (Mayo endoscopic subscore 0 or 1).
Deep remission.
Patients treated with vedolizumab were more likely to achieve deep remission than those treated with TNF antagonist therapy (HR, 1.653; 95% CI, 0.978–2.794; P = .06); however, this did not reach statistical significance using IPW ATE. Using optimal full match, this comparison reached significance in favor of vedolizumab (HR, 1.878; 95% CI, 1.082–3.259). Using optimal full match, the comparison of vedolizumab to infliximab (HR, 3.576) and subcutaneous TNF antagonist agents (HR, 2.127) reached significance, as well as the comparison of vedolizumab to TNF antagonist therapy in TNF antagonist–naive patients (HR, 5.244; 95% CI, 1.186–23.193). The comparison of vedolizumab to TNF antagonist therapy in TNF antagonist–exposed patients was not significant across both propensity score matching approaches (Figure 3, Supplementary Tables 4 and 5).
Steroid-free clinical remission and corticosteroid-free deep remission.
Patients treated with vedolizumab were more likely to achieve steroid-free clinical remission (HR, 1.828; 95% CI, 1.135–2.944) and steroid-free deep remission (HR, 2.819; 95% CI, 1.496–5.310) than those treated with TNF antagonist therapy. Point estimates were comparable across propensity score approaches and subgroups; however, statistical significance was not reached in all subgroup analyses (Figure 4, Supplementary Tables 4 and 5).
Figure 4.
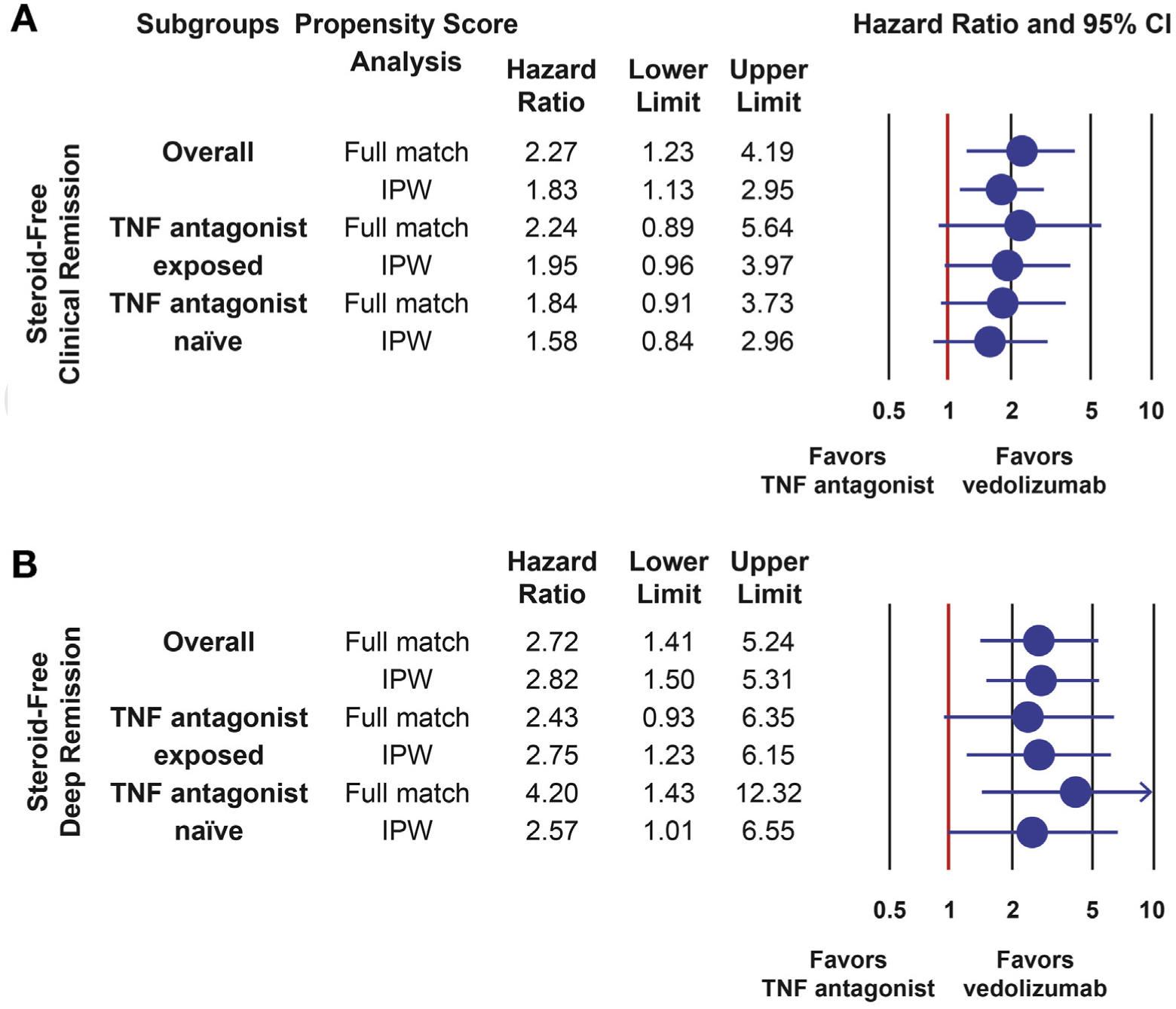
Comparative effectiveness of vedolizumab vs TNF antagonist therapy in UC for achieving steroid-free clinical remission and steroid-free deep remission. (A) Comparative effectiveness of vedolizumab vs TNF antagonist therapy for steroid-free clinical remission. (B) Comparative effectiveness of vedolizumab vs TNF antagonist therapy for steroid-free deep remission (steroid-free clinical + endoscopic remission). CI, confidence interval; IPW, inverse probability weighting; TNF antagonist, tumor necrosis factor antagonist (infliximab, adalimumab, golimumab); UC, ulcerative colitis. Analyses restricted to patients on steroids at baseline. Steroid-free deep remission was defined as achieving clinical remission, endoscopic remission, tapering off steroids, and no repeat steroid prescription for at least 4 weeks.
Discussion
In this routine practice, propensity score weighted, multicenter cohort study of 722 patients with UC, we observed that treatment with vedolizumab was associated with significantly lower rates of serious adverse events and significantly higher rates of disease remission in TNF antagonist–naive UC patients. Among TNF antagonist–exposed patients, although vedolizumab was associated with significantly higher rates of disease remission, it was also associated with significantly higher rates of serious infections. Together, these data suggest that the optimal positioning of vedolizumab in UC is as first-line therapy before exposure to TNF antagonist therapy.12
Confidence in the comparative effectiveness observation can be taken from the fact that point estimates were consistent across propensity score methodologies and subgroups, and our comparative effectiveness estimates for achieving clinical remission (HR, 1.651; 95% CI, 1.229–2.217) are comparable to results from a head-to-head phase 3 clinical trial of vedolizumab vs adalimumab (odds ratio, 1.568; 95% CI, 1.137–2.164; the VARSITY study; NCT02497469).6 Our study extends findings from the phase 3 trial by creating generalizability to routine practice for patients often not eligible for phase 3 programs and through the observed superiority in safety depending on positioning. In addition, our study analyzed the comparative safety and efficacy of vedolizumab to both subcutaneous and intravenous TNF antagonists, a subgroup not assessed in the VARSITY study.
On the basis of our comparative safety assessment, vedolizumab-treated UC patients with no prior TNF antagonist exposure had a nearly 80% reduction in any serious adverse events and a nearly 68% reduction in risk for serious infections relative to TNF antagonist–treated UC patients. The GEMINI clinical trial programs had previously demonstrated that TNF antagonist exposure was a risk factor for development of serious infections in vedolizumab-treated UC patients41; however, the comparative safety to TNF antagonist therapy in these patients had not been addressed and is now addressed in the current study. We observed that the comparative safety of vedolizumab to TNF antagonist therapy was substantially impacted by prior TNF antagonist exposure. The exact reasons for this observation are unknown and may be related to differences in concomitant steroid utilization, patient characteristics, or differences in rapidity of effectiveness of vedolizumab in TNF antagonist–naive vs –exposed patients. The consistency in observation that prior TNF antagonist exposure impacts the safety of vedolizumab in both the clinical trial long-term safety studies and this routine practice comparative effectiveness study warrants further consideration and evaluation for mechanisms driving this observation.
Although our study has several strengths, including the routine practice multicenter nature of data extraction and the utilization of propensity score methods with consistency in estimates across propensity score approaches, it also has important limitations. The retrospective observational academic center–based nature of data review may impact confidence in comparative estimates or generalizability to routine community practice. However, it is reassuring that our point estimates for comparative effectiveness using measured confounders are comparable to those achieved in a phase 3 clinical trial program that balanced measured and unmeasured confounders through randomization.6 This would therefore suggest that residual confounding for unmeasured variables is unlikely to have been a substantial factor in our cohort study. Although we were able to account for several measures of disease severity at baseline, we were not able to account for comorbidities that may have influenced choice of therapy or risk of adverse event, and we could not adequately account for practice variations when managing patients after biologic initiation, particularly with regard to steroid tapering protocols or disease monitoring approaches. This is an inherent limitation of such observational cohort studies where protocolized treatment and observation are not performed, but it also represents a strength by allowing for natural routine practice variation when comparing agents to create more generalizable estimates of comparative effectiveness and safety. It is also worth noting that the phase 3 trial of vedolizumab vs adalimumab similarly had no predefined steroid tapering protocol. We did not have detailed information on histology to allow for an assessment of the comparative effectiveness for evolving definitions of mucosal healing that incorporate both endoscopic and histologic remission. There were also patients with missing data for certain variables and a differential attrition rate for the assessment of endoscopic remission. Although we limited the cohort to those with documented moderate-severe endoscopic inflammation within 4 weeks of treatment initiation, which is in line with clinical trial recruitment criteria, this limited our ability to confidently estimate the comparative effectiveness for this important outcome. Finally, the low event rate and wide CIs for the safety comparisons, particularly among subgroups, limit the confidence in these comparisons. These subgroup analyses should therefore be considered exploratory in nature, and further population-based comparisons are likely needed.
In conclusion, using a well-phenotyped routine clinical practice multicenter cohort of more than 700 UC patients treated with biologics, we observed that treatment with vedolizumab was associated with lower adverse events and higher disease remission rates relative to TNF antagonist therapy in TNF antagonist–naive patients. It is currently estimated that 20% of the U.S. population requires at least one prior biologic failure before treatment with vedolizumab for the indication of UC, whereas infliximab and adalimumab are available for nearly 100% of the population without any prior biologic exposure.43 Data generated from this routine clinical practice comparative study may help to bridge gaps in understanding the appropriate positioning of biologic therapies in UC. Although cost-effectiveness will need to be assessed, particularly considering the emerging availability of biosimilars in the U.S. market, our work could be of substantial value for informing policy decisions to ensure equal access to safe and effective therapies for all patients.
Supplementary Material
Funding
Parambir S. Dulai and Ronghui Xu had independence from funders for conduct of this study, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. Takeda Pharmaceuticals provided funding for statistical support to analyze the data. Takeda Pharmaceuticals and associated employees had no access to patient level data and were not involved in data analyses. All data analyses were performed at the University of California San Diego by consortium investigators or statisticians, independent of Takeda Pharmaceuticals. Parambir S. Dulai is supported by an American Gastroenterology Association Research Scholar Award.
Conflicts of interest
These authors disclose the following: Dana Lukin reports consulting for AbbVie, Abgenomics, Celgene, Pfizer, Prometheus, and Takeda and educational grant support from AbbVie and Takeda. David Faleck reports travel support from Takeda. Jenna L. Koliani-Pace reports travel support from Takeda. Joseph Meserve reports travel support from Takeda. Brigid Boland receives research support from Takeda and Janssen. Siddharth Singh reports research support from AbbVie, Pfizer; consulting for Pfizer, AbbVie, Takeda, and AMAG Pharmaceuticals; and career development awards from the American College of Gastroenterology and Crohn’s and Colitis Foundation. Robert Hirten reports speaking or advisory boards for Takeda and Janssen. Ryan Ungaro is a consulting and/or advisory board member for Takeda, Pfizer, and Janssen.
Karen Lasch is an employee of Takeda Pharmaceuticals, USA, Inc. Eugenia Shmidt reports travel support from Takeda. Vipul Jairaith reports consulting fees from AbbVie, Janssen, Takeda, Sandoz, Ferring, Pfizer, GlaxoSmithKline, Robarts Clinical Trials, Eli Lilly and Company, and Arena and speaker fees from Takeda, Ferring, Janssen, and Shire. David Hudesman reports consulting fees from AbbVie, Takeda, Janssen, and Pfizer. Shannon Chang reports consulting fees from Oshi. Arun Swaminath reports fellowship support from Janssen, AbbVie, and Pfizer and grant support from Pfizer. Bo Shen reports consulting for Janssen, Salix, AbbVie, Takeda, Theravance, and Robarts Clinical Trials. Sunanda Kane reports consulting for AbbVie, Merck, Spherix Health, Seres Pharmaceuticals, and Samsung Bioepis. Edward V. Loftus reports consulting for Janssen, Takeda, AbbVie, UCB, Amgen, Pfizer, Salix, Mesoblast, Eli Lilly and Company, Celgene, and CVS Caremark and research support from Janssen, Takeda, AbbVie, UCB, Amgen, Pfizer, Genentech, Gilead, Receptos, Celgene, MedImmune, Seres Therapeutics, and Robarts Clinical Trials. Bruce E. Sands reports consulting/advisory board or honorarium from 4D Pharma, AbbVie, Allergan Sales, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Capella BioScience, Celgene, EnGene, Ferring, Gilead, Janssen, Eli Lilly and Company, Lyndra, MedImmune, Oppilan Pharma, Otsuka, Palatin Technologies, Pfizer, Progenity, Rheos Pharmaceuticals, Seres Therapeutics, Synergy Pharmaceuticals, Takeda, Target PharmaSolutions, Theravance Biopharma R&D, Inc, TiGenix, Vivelix Pharmaceuticals, and WebMD. Jean-Frederic Colombel reports consultant or advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Arena Pharmaceuticals, Celgene Corporation, Celltrion, Enterome, Eli Lilly and Company, Ferring Pharmaceuticals, Genentech, Janssen, Medimmune, Merck & Co, Nextbiotix, Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Development & Commercialization, Inc, Pfizer, Protagonist, Second Genome, Gilead, Seres Therapeutics, Shire, Takeda, and Theradiag; speaker for AbbVie, Ferring, Takeda, and Celgene Corporation; stock options from Intestinal Biotech Development and Genfit; and research grants from AbbVie, Takeda, and Janssen. Corey A. Siegel reports consulting/advisory board for AbbVie, Amgen, Celgene, Eli Lilly and Company, Janssen, Sandoz, Pfizer, Prometheus, Sebela, and Takeda; speaker for AbbVie, Janssen, Pfizer, and Takeda; grant support from Crohn’s and Colitis Foundation, AHRQ (1R01HS021747-01), AbbVie, Janssen, Pfizer, and Takeda; intellectual property from MiTest Health, LLC, and ColonaryConcepts, LLC; and equity interest from MiTest Health and ColonaryConcepts. William J. Sandborn reports personal fees from Kyowa Hakko Kirin, Millennium Pharmaceuticals, Celgene Cellular Therapeutics, Santarus, Salix Pharmaceuticals, Catabasis Pharmaceuticals, Vertex Pharmaceuticals, Warner Chilcott, Cosmo Pharmaceuticals, Ferring Pharmaceuticals, Sigmoid Biotechnologies, Tillotts Pharma, Am Pharma BV, Dr August Wolff, Avaxia Biologics, Zyngenia, Ironwood Pharmaceuticals, Index Pharmaceuticals, Nestle, Lexicon Pharmaceuticals, UCB Pharma, Orexigen, Luitpold Pharmaceuticals, Baxter Healthcare, Ferring Research Institute, Novo Nordisk, Mesoblast Inc, Shire, Ardelyx Inc, Actavis, Seattle Genetics, MedImmune (AstraZeneca), Actogenix NV, Lipid Therapeutics Gmbh, Eisai, Qu Biologics, Toray Industries Inc, Teva Pharmaceuticals, Eli Lilly and Company, Chiasma, TiGenix, Adheron Therapeutics, Immune Pharmaceuticals, Celgene, Arena Pharmaceuticals; personal fees from Ambrx Inc, Akros Pharma, Vascular Biogenics, Theradiag, Forward Pharma, Regeneron, Galapagos, Seres Health, Ritter Pharmaceuticals, Theravance, Palatin, Biogen, and University of Western Ontario (owner of Robarts Clinical Trials); grants and personal fees from Prometheus Laboratories, AbbVie, Gilead Sciences, Boehringer Ingelheim, Amgen, Takeda, Atlantic Pharmaceuticals, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Pfizer, Nutrition Science Partners, Receptos, and Amgen; grants, personal fees, and non-financial support from Janssen; and grants from Broad Foundation, American College of Gastroenterology, and Exact Sciences. Parambir S. Dulai is on the steering committee for Takeda; does consulting for Takeda and Janssen; honorarium for speaker events from Takeda; travel support from Takeda and Janssen; and grant support from Takeda and Pfizer. The remaining authors disclose no conflicts.
Abbreviations used in this paper:
- ATE
average treatment effect
- CI
confidence interval
- GRACE
Good ReseArch for Comparative Effectiveness
- HR
hazard ratio
- IPW
inverse probability weighting
- TNF
tumor necrosis factor
- UC
ulcerative colitis
Footnotes
Supplementary Data
Note: to access the supplementary materials accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at 10.1016/j.cgh.2020.10.003.
References
- 1.Mao EJ, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 2017;45:3–13. [DOI] [PubMed] [Google Scholar]
- 2.Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology 2015; 148:1035–1058.e1033. [DOI] [PubMed] [Google Scholar]
- 3.Rubin DT, Ananthakrishnan AN, Siegal CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019; 114:384–413. [DOI] [PubMed] [Google Scholar]
- 4.Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis: part 2—current management. J Crohns Colitis 2017;11:769–784. [DOI] [PubMed] [Google Scholar]
- 5.Feuerstein JD, Isaacs KL, Schneider Y, et al. American Gastroenterological Association Institute clinical guideline on the management of moderate to severe ulcerative colitis. Gastroenterology 2020;158:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019;381:1215–1226. [DOI] [PubMed] [Google Scholar]
- 7.Ha C, Ullman TA, Siegel CA, et al. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012;10:1002–1007, quiz e1078. [DOI] [PubMed] [Google Scholar]
- 8.Cowan K The James Lind Alliance: tackling treatment uncertainties together. J Ambul Care Manage 2010; 33:241–248. [DOI] [PubMed] [Google Scholar]
- 9.Hart AL, Lomer M, Verjee A, et al. What are the top 10 research questions in the treatment of inflammatory bowel disease? a priority setting partnership with the James Lind Alliance. J Crohns Colitis 2017;11:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese S, Fiorino G, Peyrin-Biroulet L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med 2014; 160:704–711. [DOI] [PubMed] [Google Scholar]
- 11.Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2016;14:1385–1397.e1310. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Fumery M, Sandborn WJ, et al. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther 2018;47:162–175. [DOI] [PubMed] [Google Scholar]
- 13.Dreyer NA, Bryant A, Velentgas P. The GRACE checklist: a validated assessment tool for high quality observational studies of comparative effectiveness. J Manag Care Spec Pharm 2016; 22:1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the Joint ISPOR-ISPE Special Task Force on Real-World Evidence in Health Care Decision Making. Value Health 2017;20:1003–1008. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 16.Yao XI, Wang X, Speicher PJ, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst 2017;109:djw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narula N, Peerani F, Meserve J, et al. Vedolizumab for ulcerative colitis: treatment outcomes from the VICTORY Consortium. Am J Gastroenterol 2018;113:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meserve J, Aniwan S, Koliani-Pace JL, et al. Retrospective analysis of safety of vedolizumab in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018; 17:1533–1540.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taleban S, Colombel JF, Mohler MJ, et al. Inflammatory bowel disease and the elderly: a review. J Crohns Colitis 2015; 9:507–515. [DOI] [PubMed] [Google Scholar]
- 20.Dulai PS, Thompson KD, Blunt HB, et al. Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol 2014;12:1443–1451, quiz e88–e89. [DOI] [PubMed] [Google Scholar]
- 21.Murthy SK, Steinhart AH, Tinmouth J, et al. Impact of Clostridium difficile colitis on 5-year health outcomes in patients with ulcerative colitis. Aliment Pharmacol Ther 2012;36:1032–1039. [DOI] [PubMed] [Google Scholar]
- 22.Jodorkovsky D, Young Y, Abreu MT. Clinical outcomes of patients with ulcerative colitis and co-existing Clostridium difficile infection. Dig Dis Sci 2010;55:415–420. [DOI] [PubMed] [Google Scholar]
- 23.Navaneethan U, Mukewar S, Venkatesh PG, et al. Clostridium difficile infection is associated with worse long term outcome in patients with ulcerative colitis. J Crohns Colitis 2012; 6:330–336. [DOI] [PubMed] [Google Scholar]
- 24.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis: part 1—definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–670. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J Statistical power analysis for the behavioral sciences. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers, 1988. [Google Scholar]
- 26.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013; 32:2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC, Schuster T. The performance of different propensity score methods for estimating absolute effects of treatments on survival outcomes: a simulation study. Stat Methods Med Res 2016;25:2214–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33:1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolkewitz M, Cooper BS, Bonten MJ, et al. Interpreting and comparing risks in the presence of competing events. BMJ 2014;349:g5060. [DOI] [PubMed] [Google Scholar]
- 30.Koller MT, Raatz H, Steyerberg EW, et al. Competing risks and the clinical community: irrelevance or ignorance? Stat Med 2012;31:1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirracchio R, Carone M, Rigon MR, et al. Propensity-score estimators for the average treatment effect and the average treatment effect on the treated may yield very different estimates. Stat Methods Med Res 2016;25:1938–1954. [DOI] [PubMed] [Google Scholar]
- 32.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin PC, Stuart EA. Optimal full matching for survival outcomes: a method that merits more widespread use. Stat Med 2015;34:3949–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods 2004;9:403–425. [DOI] [PubMed] [Google Scholar]
- 35.Hernan MA, Hernandez-Diaz S, Werler MM, et al. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002; 155:176–184. [DOI] [PubMed] [Google Scholar]
- 36.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006; 163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adelson JL, McCoach DB, Rogers HJ, et al. Developing and applying the propensity score to make causal inferences: variable selection and stratification. Front Psychol 2017;8:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patrick AR, Schneeweiss S, Brookhart MA, et al. The implications of propensity score variable selection strategies in pharmacoepidemiology: an empirical illustration. Pharmacoepidemiol Drug Saf 2011;20:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyss R, Girman CJ, LoCasale RJ, et al. Variable selection for propensity score models when estimating treatment effects on multiple outcomes: a simulation study. Pharmacoepidemiol Drug Saf 2013;22:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green KM, Stuart EA. Examining moderation analyses in propensity score methods: application to depression and substance use. J Consult Clin Psychol 2014;82:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barre A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther 2018;47:896–905. [DOI] [PubMed] [Google Scholar]
- 43.Dulai PS, Osterman MT, Lasch K, et al. Market access analysis of biologics and small-molecule inhibitors for inflammatory bowel disease among US health insurance policies. Dig Dis Sci 2019;64:2478–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


