Abstract
Lipase-catalyzed glycerolysis was recently shown to be a viable technique to structure cottonseed and peanut oils into structural fats by converting native triacylglycerols into partial glycerides without changing overall fatty acid composition. Here, this approach was extended to a variety oils of differing fatty acid compositions. Reactions were performed at 65 °C for 48 h at a triacylglycerol:glycerol molar ratio of 1:1, using the non-regiospecific Candida antarctica lipase B. In all oil systems, a 20 °C increase in crystallization onset temperature was observed following glycerolysis. Solid fat content increases resulting from glycerolysis were greatest for oils containing >10% saturated fat along with a high oleic acid content. The solid fat content of tigernut oil at 5 °C increased from 8% to 34% following glycerolysis. Tigernut glycerolysis product was used to make margarine with plasticity similar to commercial margarine and butter. This research demonstrates that glycerolysis is a general strategy to convert liquid oils into structural fats used in food applications, and thus replace palm oil and hydrogenated fats.
Keywords: Glycerolysis, Enzymatic, Monoacylglycerol, Diacylglycerol, Triacylglycerol, Crystallization
Graphical abstract
Highlights
-
•
Edible oil fatty acid content affects the composition of crystalline material.
-
•
High oleic content enhances structuring in oils of intermediate saturated fat levels.
-
•
Highly saturated monoacylglycerol crystal material melts gradually.
-
•
Partial glycerol crystals of high oleic oils showed a two-segment melting profile.
-
•
Glycerolysis-structured oils produced margarine comparable to commercial products.
1. Introduction
Trans fatty acid (TFA) consumption has been linked to several negative health effects stemming from their effect on serum cholesterol levels (Katan et al., 1995). Consequently, partially hydrogenated oils (PHOs), which are high in TFAs, were banned from food products, a ban which came into effect in 2018. While this presented the opportunity to replace PHOs with healthful alternatives, for the most part fats high in saturated fatty acids (SFAs) have been used as replacements. SFA consumption is associated with elevated serum total cholesterol and low-density lipoprotein cholesterol levels (Mensink et al., 2003; Mensink, 2016). Fully hydrogenating oil converts all unsaturated fatty acids into SFAs. Often for food applications, a fully hydrogenated oil will be interesterified with a liquid vegetable oil, thereby increasing the saturated fat content of the vegetable oil. Furthermore, palm oil is also frequently used in the food industry. Palm oil is able to solve some of the functionality issues related to trans fat removal because it contains nearly 50% SFAs. Besides the negative health effects associated with palm oil due to its high SFA content, the lack of sustainability of palm oil production needs to be considered. Although yields of palm oil per hectare are higher than any other oil crop, the African oil palm tree only grows in tropical climates (Austin et al., 2017; Coral Medina et al., 2019). This means that in order to make way for increased palm oil production, habitat and environmental destruction must take place in some of the most biodiverse regions of the world. Since the 1980’s, extensive rainforest destruction has been taking place in South East Asia and more recently, plans to increase palm oil production in South America and China, the world’s largest importer of palm oil, are underway. Although sustainable palm oil production initiatives have been beneficial thus far, these can only go so far (Carlson et al., 2018). Currently there are 321 species threatened due to palm oil production (Meijjard et al., 2020). Based on the total area of production numbers from 2014 (18.9 Mha), palm oil threatens 17.0 species per Mha. This is substantially higher than even coconut oil (5.3 species threatened per Mha). Other common oils such as soybean and cottonseed oil threaten only 0.6 and 1.1 species/Mha, respectively, because they are capable of growing in less biodiverse regions of the world. Additionally, palm oil is the sole material obtained from the oil palm tree, while other oil crops provide multiple end-products and in many cases the oil is obtained as a by-product. Soybean oil is a by-product of soymeal production, cottonseed oil a by-product of cotton production, rice bran oil a by-product of rice production. Furthermore, as citizens become more focussed on sustainability and factors influencing climate change, the need for sustainable fat sources will only grow. Therefore, the need to find suitable ways of structuring liquid oils, allowing for a wide variety of edible oils to be used in food applications, without increasing the SFA content, has never been greater.
Research into fat mimetic strategies has been ongoing for nearly two decades in an effort to improve the nutritional quality and functionality of edible oils. This originally began as a means of removing PHOs from food products, but they have yet to make a great impact in the food industry. Amongst the fat mimetic strategies that have been investigated, three main categories exist: i) mono and mixed component oleogels, ii) structured biphasic systems, and iii) polymer oleogels (Marangoni et al., 2020). Mono and mixed component oleogels include strategies such as long-chain saturated fatty acids, fatty alcohols, wax esters, natural waxes, oryzanol, and partial acylglycerols. Of these strategies, oleogels formulated with natural waxes seem to be attracting the most attention as of late. However, several issues arise with waxes in food applications, these include a residual waxy mouthfeel, due to the high melting point of the waxes as well as a low emulsion stability in margarines and spreads (Patel et al., 2020). Structured biphasic systems represent a class of emulsions stabilized through different means of gelation. Within this class, structured emulsions prepared with crystallized monoacylglycerol multi-bilayers, have shown success (Batte et al., 2007; Marangoni et al., 2007). Furthermore, ethylcellulose (EC) has received a lot of attention as a polymer gelator, given that EC and chitin are the only food-grade polymers to act as oil gelators that can be directly dispersed in oil (Marangoni et al., 2020). One major problem with EC oleogels, which also happens to be a problem with most fat mimetics, is their brittleness. This was recently addressed by Gravelle and coworkers, in which EC oleogels were formulated with a (70:30 w/w) mix of stearyl alcohol-stearic acid in order to reduce oleogel brittleness, thereby increasing plasticity (Gravelle et al., 2017). Plasticity is a crucial property that must be considered when attempting to formulate a fat mimetic to be used in food applications, particularly spreads.
Monoacylglycerols (MAGs) and diacylglycerols (DAGs) represent a means of structuring liquid oils into solid fats. Previously, Ojijo and coworkers have demonstrated the effectiveness of using MAGs to form stable network structures in olive oil (Ojijo et al., 2004a, 2004b). Additionally, Terentjev’s group has studied the structuring properties of MAGs and polymorphic stability of MAGs in oil (Chen and Terentjev, 2009, 2018). But besides using MAGs and DAGs for the purpose of producing a fat mimetic, these components have the potential to solidify liquid vegetable oils into structural fats because of differences in their crystallization behaviour from that of TAGs. Partial acylglycerols crystallize at higher temperatures than TAGs, even when the same fatty acid composition is present. Furthermore, this crystallization point is higher for MAGs compared to DAGs (Belitz et al., 2009).
When present in TAG solutions, MAGs and DAGs have been shown to act as a template for TAG nucleation and later act to accelerate crystal growth (Goh and Timms, 1985; Fredrick et al., 2008; Tavernier et al., 2019). Wright and co-workers demonstrated that milk fat DAGs accelerated both the nucleation and crystal growth of isolated milk fat TAGs (Wright et al., 2000; Wright and Marangoni 2002), with a particularly strong effect from the 1-palmitoyl-2-oleoyl-glycerol. This effect is, however, strongly dependent on the structure of the interacting DAGs and TAGs, and generalizations are difficult to make. Furthermore, the influence of MAG/DAG blends on the solid fat content (SFC) of vegetable oil systems was examined by Vereecken and coworkers (Vereecken et al., 2009a, Vereecken et al., 2009b). MAGs were determined to increase the SFC to a greater extent compared to DAGs and a higher saturated fatty acid content within the partial acylglycerols produced larger increases in the SFC. Exploiting the crystallization properties of partial acylglycerols could therefore be used as a means of structuring a variety of plant-based oils.
Within the research by Vereecken and coworkers, partial acylglycerols were added to the vegetable oils, thereby altering their fatty acid composition. However, glycerolysis can be used to produce partial acylglycerols within a liquid oil, without affecting the fatty acid composition (i.e., no change in the SFA content). Furthermore, glycerolysis is frequently used to produce MAGs and DAGs for the purpose of using them as food ingredients, and not to structure the oil itself. MAGs and DAGs are the most frequently used emulsifiers in the food industry and as such the glycerolysis process has been well researched in an effort to improve the production of these molecules (McNeill et al., 1991; Noureddini and Harmeier, 1998; Elfman-Borjesson and Harrod, 1999; Kristensen et al., 2005; Fregolente et al., 2008). We have recently optimized the crystallization behaviour and SFC increases resulting from the glycerolysis process in order to improve the functionality of plant-based oils (Nicholson and Marangoni, 2020). In our previous work, cottonseed and peanut oils were successfully structured through glycerolysis. Here, the enzymatic glycerolysis process will be extended to a variety of vegetable oils and the effect of fatty acid composition on the physical properties of the glycerolysis-structured systems will be discussed. Furthermore, the applicability of these structured lipids in food systems, specifically margarine, will be explored.
2. Materials and methods
2.1. Materials
Tigernut oil was generously provided by a local producer (The Chufa Co.; Toronto, ON, Canada). Cottonseed oil was purchased online (Bass Pro Shops; Springfield, MO, USA). Peanut, olive, soybean, canola oils were purchased from a local supermarket (Wal-mart Canada Corp.). Rice bran oil was purchased from New Directions Aromatics (New Directions Inc.; Mississauga, ON, Canada). High-oleic canola oil (HOCO) was obtained through a supplier (Bunge Limited; Chesterfield, MO, USA). Sesame oil was provided by Soybean Crushing Co. & Derivatives (SOYA; Saudi Arabia). High-oleic algal oil (HOAO) was a gift from Solazyme Inc. (San Francisco, CA, USA). Glycerol (99.9% Glycerol, Fisher Scientific; Ottawa, ON, Canada) was obtained through a scientific material supplier. Candida antarctica lipase B immobilized on Immobead 150 was purchased from Sigma-Aldrich (Sigma-Aldrich; St. Louis, MO, USA).
2.2. Fatty acid composition
Fatty acid composition for each of the oils was determined with an Agilent 6890-series gas chromatograph (Agilent Technologies, Inc.; Wilmington, DE, USA) equipped with a 7683-series auto-sampler. A BPX70 (SGE Inc.; Austin, TX, USA) GC column (60 m × 0.22 mm internal diameter; 0.25 μm film thickness) was used. Oven temperature increased from 110 to 230 °C (4 °C/min) and was maintained at 230 °C for 18 min. The injector was set to 250 °C, operating at 20.1 psi and a flow of 17.7 mL/min. A carrier gas (high-purity helium) flowing at a velocity of 25 cm/s was used. A flame ionization detector was used (255 °C; 450 mL/min air flow; 50 mL/min helium flow). The GC peaks obtained were analyzed with Open LAB software (Agilent Technologies). Fatty acid composition was determined through comparison of the retention times with those of internal standards.
2.3. Glycerolysis reaction
Glycerolysis reactions were performed in 125 mL Erlenmeyer flask reaction vessels. Reactions were performed with tigernut, peanut, cottonseed, rice bran, HOCO, soybean, sesame, canola, and HOAO oils as the reaction substrates. Glycerol was added to oil (30 g) at a glycerol to triacylglycerol molar ratio of 1:1. The glycerol mass was determined based on molecular weights for glycerol and TAG molecules. Glycerol has a molecular weight of 92 g/mol, while TAG molecular weight was calculated using the equation: MW = 3 × (56 000/SV). Where MW represents the TAG molecular weight and SV represents the saponification value of the oil. Deionized water was added at a concentration of 3.5 wt% relative to the glycerol. Reactions were enzymatically catalyzed using the non-regiospecific (random) Candida antarctica lipase B, immobilized on Immobead 150, added at 2 wt% relative to the oil. The reaction vessel was lightly shaken by hand to disperse the enzyme particles within the mixture immediately prior to insertion into the shaking water bath (Precision Reciprocal Shaking Bath, ThermoScientific; Waltham, MA, USA). Glycerolysis reactions were run for 48 h at 65 °C and 130 rpm reciprocating speed. Upon completion of the reaction, the contents were centrifuged (Model CL, International Equipment Co.; Chattanooga, TN., USA) for 5 min at medium speed to separate the oil from the immobilized lipase, glycerol, and water.
2.4. Monoacylglycerol (MAG) and diacylglycerol (DAG) content
MAG and DAG contents were determined using a Waters Alliance model 2690 high performance liquid chromatograph (HPLC) equipped with a refractive index detector (Waters model 2410, Waters, Milford, MA, USA). Chromatographic separation of the MAGs, DAGs, and TAGs was obtained with a Waters xbridge C18 (Waters Limited, Mississauga, ON, Canada) column (4.6 mm × 250 mm internal diameter; 5 μm particle size). Isocratic elution at a flow rate of 1 mL/min of degassed acetone/acetonitrile 60/40 (v/v) was applied. Column and detector temperatures were set to 40 °C. The data obtained was analyzed using Millennium 32 (K&K Testing, LLC, Decatur, GA, USA). Internal standards were used for the identification of the different classes of partial acylglycerols.
2.5. X-ray diffraction
X-ray diffraction spectra measurements were performed using a Multiflex powder X-ray diffractometer (Rigaku MSC Inc.; Toronto, ON, Canada). Glycerolysis reaction product samples, previously crystallized and stored at 5 °C were transferred onto metal sample holders. A copper X-ray tube (CuKα1; λ = 1.54 Å) was used as the X-ray source. Spectra were acquired at 25 °C in the 2θ 1.1–35° diffraction angle region at a 0.3° min−1 acquisition speed with a divergence and scattering slit of 0.5°, and a 0.3 mm receiving slit.
2.6. Margarine preparation
Margarine samples were prepared in 100 g batches from tigernut oil following glycerolysis performed under optimal reaction conditions. The oil phase represented 81 wt% of the margarine. Sodium chloride (1.8 wt%) and 0.1 wt% potassium sorbate (Sigma-Aldrich) were dispersed in water (17.1 wt%). The water phase was added to the oil phase and mixed with an immersion blender until completely homogenous. Samples were then spread on a stainless-steel tempering table maintained at 5 °C and sheared for several minutes with a plastic bench scraper in order to induce crystallization. Margarine samples were then stored at 5 °C for 1 week.
2.7. Solid fat content melting profile
Solid fat content (SFC) was measured through pulsed nuclear magnetic resonance (p-NMR) (minispec mq20, Bruker Corp.; Milton, ON, Canada). Glycerolysis reaction products and margarine samples were transferred to standard NMR tubes (h: 180 mm; d: 9 mm) and stored at 5 °C for 1 week prior to performing measurements. SFC was measured over a temperature range of 5–50 °C at 5 °C intervals, with 30 min equilibration time at each temperature (AOCS Official Method Cd 16b-93).
2.8. Differential scanning calorimetry
Differential scanning calorimetry (DSC) (DSC 1 Star System, Mettler Toledo; Scherwerzenbach, Switzerland) was used to investigate crystallization and melting behaviors. Each of the oils and the optimized glycerolysis reaction products were heated to 100 °C and held for 15 min to ensure the crystal structure was completely melted. Samples were then cooled to −80 °C at 5 °C/min and held for 15 min, before being heated to 100 °C at 5 °C/min. Furthermore, margarine samples prepared with the tigernut oil reacted under optimal glycerolysis reaction conditions, which had previously been crystallized and stored at 5 °C for 1 week, were equilibrated at 5 °C for 2 h before being heated to 50 °C. A commercially, available soft-tub margarine was also tested in the same fashion. In all cases, 8–10 mg of sample was placed in aluminum crucibles that were hermetically sealed prior to testing.
2.9. Polarized light microscopy
Microstructure was characterized by polarized light microscopy with a digital PLAN trinocular infinity polarizing lab microscope (M838PL-C180U3, OMAX; Kent, WA, USA). Prior to imaging, previously prepared glycerolysis reaction product samples were melted at 80 °C and transferred to glass microscope slides stored at 40 °C. Glass coverslips (40 °C) were then placed over the samples and they were stored at 5 °C for 1 week. Furthermore, the stored experimental margarine sample was transferred to the microscope slide and imaging was performed immediately. Images were captured with an 18 MP digital camera (OMAX; Kent, WA, USA) using the ToupView software package (v3.7, ToupTek Photonics; Hangzhou, China).
2.10. Margarine back extrusion
Back extrusion was performed with a texture analyzer (TA.HD.plus, Stable Micro Systems, Texture Technologies Corp.; Scarsdale, NY, USA) equipped with a 30 Kg load cell. Prior to testing, margarine samples were transferred to glass test tubes (h: 125 mm; d: 14 mm) and stored for 1 week at 5 °C. A cylindrical stainless-steel probe (h: 89 mm; d: 9.20 mm) with a truncated semi-spherical tip (h: 6.75 mm; d: 10.20 mm) penetrated samples to a distance of 20 mm at a speed of 1.5 mm/s.
2.11. Data analysis
Three replicates (n = 3) were performed for all experiments. Data was analyzed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). A one-way analysis of variance (ANOVA) was performed along with a Tukey’s multiple comparison test to determine differences between treatments. Treatments were taken as significantly different when P < 0.05.
3. Results and discussion
3.1. Acylglycerol composition
Optimal glycerolysis reaction conditions were previously determined to be 48 h with a 1:1 glycerol:TAG molar ratio when 2 w/w% enzyme was used as the catalyst in cottonseed oil (Nicholson and Marangoni, 2020). Following glycerolysis under these conditions, the cottonseed system contained 34.8% monoacylglycerols (MAGs), 49.6% diacylglycerols (DAGs), and 17.1% triacylglycerols (TAGs). To characterize the production of partial acylglycerols, glycerolysis was performed under optimized reaction conditions with tigernut, peanut, rice bran, and soybean oils. Subsequently, the MAG, DAG, and TAG concentrations were determined. After glycerolysis, the tigernut system contained 31.2% MAGs, 42.3% DAGs, and 26.5% TAGs. The peanut glycerolysis product was composed of 31.6% MAGs, 46.8% DAGs, and 21.6% TAGs. The rice bran system contained 31.4% MAGs, 47.8% DAGs, and 20.8% TAGs, and the soybean glycerolysis product contained 33.2% MAGs, 44.8% DAGs, and 22.0% TAGs. It must be noted that although free fatty acids (FFA) were not quantified for each of the oil systems examined, they were measured in the peanut oil system. The FFA content was approximately 0.3% in the native oil and remained below 2% following the glycerolysis reaction. A peak for the FFAs was not observed during the HPLC analysis of the MAG, DAG, and TAG concentrations. Given the presence of a small amount of FFA, it must be recognized that the total MAG, DAG, TAG portion of the glycerolysis reaction products is representative of the remainder of the total material (>98%). These results demonstrate that when glycerolysis is performed under defined conditions, similar acylglycerol levels will be produced regardless of the reacted oil. Therefore, it can be expected that any vegetable oil, when reacted with 2% w/w Candida antarctica lipase B in the presence of a 1:1 glycerol:TAG molar ratio for 48 h, will contain upwards of 30% MAGs, 40–50% DAGs, with as low as 20% TAGs remaining. For this reason, glycerolysis was performed under these conditions on several additional vegetable oils to further study the physical properties of the glycerolysis reaction products, without quantification of partial acylglycerols. Furthermore, the fatty acid composition was determined for each of the unaltered oils. Results are shown in Table 1.
Table 1.
Average fatty acid compositions for each of the unaltered oils used in this study. Values indicate the mean and standard deviation of three replicates and represented as a mass percentage (% w/w).
| Fatty Acid | Tigernut | Peanut | Cottonseed | Rice Bran | Olive | HOCO | Soybean | Sesame | Canola | HOAO |
|---|---|---|---|---|---|---|---|---|---|---|
| 14:0 | – | – | 0.88 ± 0.00 | 0.32 ± 0.02 | – | – | – | – | – | 0.41 ± 0.00 |
| 16:0 | 13.47 ± 0.03 | 9.40 ± 0.01 | 23.59 ± 0.04 | 18.76 ± 0.01 | 11.49 ± 0.01 | 3.86 ± 0.01 | 10.61 ± 0.01 | 10.00 ± 0.02 | 4.14 ± 0.01 | 1.64 ± 0.01 |
| 16:1 | – | – | 0.56 ± 0.01 | 0.22 ± 0.01 | 0.83 ± 0.01 | – | – | – | – | – |
| 18:0 | 6.60 ± 0.02 | 2.87 ± 0.01 | 2.75 ± 0.01 | 2.09 ± 0.00 | 2.65 ± 0.01 | 1.75 ± 0.01 | 4.67 ± 0.02 | 5.94 ± 0.01 | 1.82 ± 0.01 | 0.71 ± 0.00 |
| 18:1 | 68.14 ± 0.03 | 58.35 ± 0.01 | 18.22 ± 0.02 | 41.61 ± 0.03 | 75.07 ± 0.02 | 72.54 ± 0.03 | 21.63 ± 0.04 | 39.72 ± 0.01 | 63.13 ± 0.04 | 92.01 ± 0.04 |
| 18:2 | 10.30 ± 0.01 | 21.38 ± 0.01 | 53.44 ± 0.03 | 33.95 ± 0.05 | 8.99 ± 0.01 | 16.58 ± 0.02 | 54.40 ± 0.06 | 43.69 ± 0.01 | 20.00 ± 0.03 | 4.28 ± 0.01 |
| 18:3 | 0.78 ± 0.00 | – | 0.30 ± 0.00 | 0.90 ± 0.01 | 0.97 ± 0.01 | 3.70 ± 0.02 | 8.12 ± 0.01 | 0.66 ± 0.00 | 9.74 ± 0.01 | 0.95 ± 0.01 |
| 20:0 | 0.13 ± 0.01 | 1.44 ± 0.01 | – | 0.76 ± 0.03 | – | – | 0.22 ± 0.01 | – | – | – |
| 21:0 | 0.17 ± 0.01 | 1.47 ± 0.01 | – | 0.56 ± 0.01 | – | 1.26 ± 0.01 | 0.35 ± 0.01 | – | 1.17 ± 0.01 | – |
| 22:0 | 0.14 ± 0.01 | 3.38 ± 0.01 | 0.15 ± 0.01 | 0.31 ± 0.00 | – | – | – | – | – | – |
| 22:1 | – | – | – | – | – | 0.31 ± 0.01 | – | – | – | – |
| 24:0 | 0.27 ± 0.00 | 1.69 ± 0.01 | 0.11 ± 0.01 | 0.50 ± 0.02 | – | – | – | – | – | – |
3.2. Physical properties
3.2.1. Differential scanning calorimetry
The production of MAGs and DAGs through glycerolysis altered the crystallization and melting behaviour of each of the oils, as measured by differential scanning calorimetry (DSC). Crystallization curves for each of the vegetable oils, before and after the optimized glycerolysis reaction, are shown in Fig. 1. For each oil tested, a completely different crystallization profile was obtained following glycerolysis. In all cases, the presence of new molecular species produced a crystallization peak with a crystallization onset that was approximately 20 °C higher than that displayed for the unaltered oil systems. For glycerolysis systems containing greater than 10% saturated fatty acids (SFAs) this was observed as a new high temperature crystallization peak separate from the bulk of the material. Tigernut (Fig. 1A), peanut (Fig. 1B), cottonseed (Fig. 1C), rice bran (Fig. 1D), olive (Fig. 1E), soybean (Fig. 1G), and sesame (Fig. 1H) fall into this category. For lipid systems containing low levels of SFAs (i.e., HOCO (Fig. 1F), canola (Fig. 1I), and HOAO (Fig. 1J)), there was simply a dramatic shift in the bulk crystallization.
Fig. 1.
Differential scanning calorimetry crystallization profiles for glycerolysis reaction products (black lines) and their respective oils (gray lines). (A) tigernut; (B) peanut; (C) cottonseed; (D) rice bran; (E) olive (F) high oleic canola oil (HOCO); (G) soybean; (H) sesame; (I) canola; (J) high oleic algal oil (HOAO).
Several additional points and similarities are worth highlighting. First, the highest crystallization onset temperature was observed with the tigernut glycerolysis product and tigernut and peanut glycerolysis products were the only systems that displayed high temperature crystallization peaks above 20 °C. The profile for the olive glycerolysis product resembled that of the tigernut glycerolysis product, however the high temperature peak was shifted to a lower temperature, likely the result of a lower SFA content (14.1% vs 20.8%), but otherwise similar fatty acid profiles. Furthermore, the high temperature crystallization peaks observed for the cottonseed and rice bran glycerolysis products were proportionally very large compared to each of the other systems. This was potentially due to their SFA contents (27.5% and 23.3%, respectively), which happen to be the highest of the oils studied. Striking similarities between the crystallization behaviour of HOCO and canola glycerolysis systems were evident, however, crystallization onset in the HOCO system began at a slightly elevated temperature. This likely would have been due to the higher oleic acid content of HOCO (72.5% vs 63.1%), given that the SFA contents are practically identical. Finally, HOAO, which is composed mostly of triolein, had a single, sharp crystallization peak below −40 °C. Following production of MAGs and DAGs through glycerolysis, this peak was shifted to a higher temperature by around 20 °C. It should be noted that since no high temperature crystallization peaks were obtained, no solids were present in the HOAO glycerolysis product when stored at 5 °C. Consequently, x-ray diffraction (XRD) spectra and micrographs could not be obtained for the HOAO system. Corresponding melting profiles obtained through DSC are shown in the supplementary material (Supplementary Figure 1).
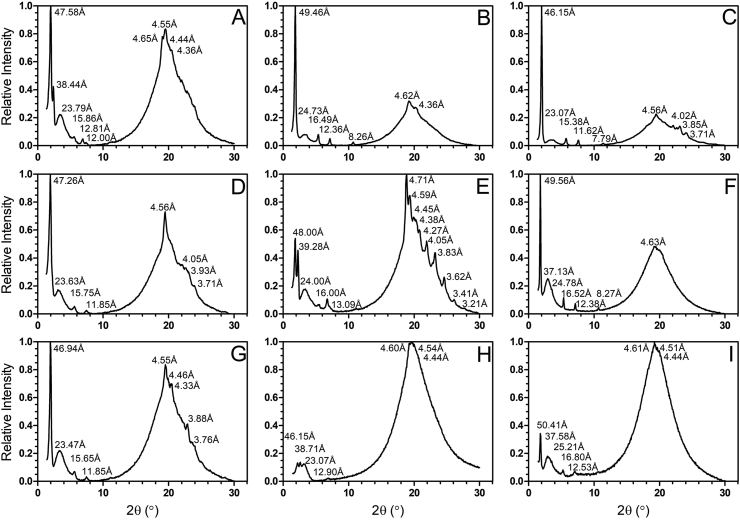
3.2.2. X-ray diffraction
XRD spectra for the optimized glycerolysis products, crystallized statically and stored for a minimum of 1 week at refrigeration temperatures, are shown in Fig. 2. According to Chen and Terentjev, MAG crystals in the β-crystalline state are characterized by a strong peak at 4.5–4.6 Å in the short-spacing (wide-angle) region (Chen and Terentjev, 2009). Peanut and HOCO glycerolysis products displayed short-spacings peaks just above 4.6 Å (4.62 and 4.63 Å, respectively), while each of the other systems, displayed short-spacings peaks between 4.5 and 4.6 Å. This demonstrates the presence of MAG crystals in the β polymorphic form. In each system, except the olive glycerolysis product, this peak dominated the wide-angle region, indicating that the β-form MAG crystals were the primary structuring material. For the olive system, the peak of greatest intensity was located at 4.71 Å. This peak, along with the peak at 3.83 Å correspond with the short-spacings of 1,3-diolein crystals (Daubert and Lutton, 1947). Therefore, diolein crystals were the primary structuring material of the olive glycerolysis product, while MAG crystals acted as secondary crystals in the structural matrix.
Fig. 2.
X-Ray diffraction spectra obtained for glycerolysis reaction products of (A) tigernut oil; (B) peanut oil; (C) cottonseed oil; (D) rice bran oil; (E) olive oil (F) high oleic canola oil (HOCO); (G) soybean oil; (H) sesame oil; (I) canola oil. Measurements were performed after crystallization and storage at 5 °C for 1 week.
The diffraction peak corresponding to the (001) crystal plane in the long-spacing region (small-angle) was positioned at between 46 and 50 Å for each of the systems. Higher order reflections, namely (002), (003) and (004), were also evident in the spectra, and are characteristic of lamellar phases, with relative positions at 1:1/2:1/3:1/4 in real space or 1:2:3:4 in inverse space (q = 2π/d). The position of the 001 peak for each of the systems is dependent on the composition of the MAGs making up the crystals. In their pure forms, monopalmitin and monostearin β crystals are known to display peaks at 45.8 Å and 50.0 Å, respectively (Malkin and Riad El Shurbagy, 1936). Additionally, pure β crystals of monoolein have been observed with a peak at 49.52 Å (Vereecken et al., 2009a, Vereecken et al., 2009b). The presence of MAGs containing long-chain saturated fatty acids (i.e., 20:0, 21:0, 22:0, 24:0) would also influence the position of the 001 diffraction peak. Finally, although monolinolein (and sometimes monolinolenin) would be present in substantial quantities in many of the systems, these MAG species have crystallization temperatures well below the crystallization and storage temperature that these samples were subjected to and as a result, are unlikely to have been incorporated into the crystal network. Furthermore, 1,3-diolein crystals are known to have a long-spacing of 39.3 Å (Daubert and Lutton, 1947). A peak in this region was present in the tigernut, olive, HOCO, sesame, and canola systems following glycerolysis, indicating that 1,3-diolein co-crystallized with the MAGs. However, it was only within the olive system that diolein was the dominant crystal within the network, as was determined from the wide-angle region. In all other systems, the diolein crystals were secondary to the MAG crystals.
The MAG crystal peak position for the cottonseed glycerolysis product (46.15 Å) corresponds with crystals composed almost entirely of monopalmitin. This stands to reason, as cottonseed oil contained approximately 23.6% palmitic acid with less than 3% stearic acid and has the lowest oleic acid content (18.2%) of any of the oils. The sesame glycerolysis product also had a long-spacing of 46.15 Å, again indicating MAG crystals composed mainly of monopalmitin. However, the amplitude of this peak was extremely low, demonstrating that there was very little MAG crystal matter present in the system. Furthermore, MAG peaks for soybean and rice bran systems were located at 46.94 and 47.26 Å, respectively. These long-spacings again indicate that monopalmitin is the major MAG species incorporated into these crystals. Since the oleic acid concentration (21.6%) for soybean oil was low, the increase in the long-spacing was likely due to the incorporation of stearic acid within the MAG crystals. For the rice bran system, the higher oleic acid concentration (41.6%) would have played an important role. The long-spacing increased further to 47.58 Å for the tigernut glycerolysis product. While this value remains closer to that of monopalmitin than to monoolein or monostearin, increased concentrations of both oleic acid (68.1%) and stearic acid (6.6%) contributed to the observed increase. The long-spacing of the olive glycerolysis product was 48.00 Å. This MAG crystal value is now closer to that of monoolein than to monopalmitin, meaning that a lower proportion of monopalmitin was present in the crystal structure compared to the combination of monoolein and monostearin. The peanut glycerolysis product MAG crystals had a long-spacing of 49.46 Å. Peanut oil had an interesting fatty acid composition, with SFAs containing ≥20 carbons making up 8.0%. This combined with the stearic acid content (2.9%) was greater than the palmitic acid content (9.4%). Additionally, peanut oil contained a high amount of oleic acid (58.4%) and given that pure monoolein crystals have an almost identical long-spacing to this system, monoolein likely contributed substantially. The HOCO glycerolysis product had a very similar long-spacing (49.56 Å). A major contribution from the high oleic acid content (72.5%) and only minor contributions from SFAs (6.9%) resulted in a long-spacing very close to that of pure monoolein. Similarly, canola had a low SFA content (7.1%), meaning that monoolein contributed substantially to the MAG crystals (50.41 Å).
3.2.3. Microstructure
Microstructures for each of the glycerolysis reaction products in which crystalline material was present are depicted in Fig. 3. The similarities between the crystal morphology of the cottonseed (Fig. 3C), rice bran (Fig. 3D), and soybean (Fig. 3G) glycerolysis products are easily noticeable. Each containing needle-like crystals of 50–100 μm diameter. In contrast, the tigernut oil glycerolysis product (Fig. 3A) formed a crystal network composed of smaller platelet-like crystals (20–50 μm) which aggregated to form larger crystal structures. The crystals of the olive glycerolysis product (Fig. 3E) had a similar morphology, containing aggregates of platelet-like crystals. However, the platelets within the olive system were larger for the most part, while the aggregated structures were smaller. Furthermore, the peanut glycerolysis product (Fig. 3B) contained platelet-like crystals of a similar size to those within the tigernut system, but they had arranged themselves in a different manner. Within the sesame glycerolysis product (Fig. 3H), again were platelet-like crystals, but these crystals were large (20–70 μm diameter), round, and tightly packed in small clusters of several crystals. Finally, the crystalline material within both the HOCO (Fig. 3F) and canola (Fig. 3I) glycerolysis products formed extensive structures, of similar morphologies, within the oil phase. It is important to note that in the bulk sample, stored at 5 °C, the liquid and crystalline materials formed two distinct phases in both the HOCO and canola systems, while each of the other glycerolysis products showed no phase separation.
Fig. 3.
Micrographs for the glycerolysis reaction products of (A) tigernut oil; (B) peanut oil; (C) cottonseed oil; (D) rice bran oil; (E) olive oil (F) high oleic canola oil (HOCO); (G) soybean oil; (H) sesame oil; (I) canola oil. Scale bar represents 100 μm. Imaging of the glycerolysis product samples was performed after crystallization and storage for 1 week at 5 °C.
The fatty acid composition along with the crystallization behaviour and XRD spectra help to explain some of the similarities and differences among the microstructures of the oil systems. To begin with, the extensive structures extending throughout the liquid phase were unique to the HOCO and canola systems. Also unique to the HOCO and canola systems was that the starting oils contained less than 10% SFAs (6.9% and 7.1%, respectively) and greater than 60% oleic acid (72.5% and 63.1%, respectively). This produced MAG crystals, with long-spacings close to that of neat monoolein crystals, and diolein crystals in both systems. As a result, neither the HOCO nor the canola glycerolysis products displayed high temperature crystallization peaks. This means that when stored at 5 °C monoolein and diolein crystal growth would have occurred from a small number of nucleation sites, resulting in the very large crystal structures seen within these systems.
Cottonseed, rice bran, and soybean glycerolysis products each possessed similar needle-like morphologies. The cottonseed and rice bran systems showed relatively similar crystallization behaviours (proportionally large high-temperature crystallization peak with an onset just below 20 °C), not shared by the soybean glycerolysis product. However, all three oils contained low levels of oleic acid relative to palmitic acid (their SFA of highest proportion). Cottonseed, rice bran, and soybean had oleic:palmitic ratios of 0.77, 2.22, and 2.04, respectively. Sesame oil had the next lowest ratio (3.97). Rice bran oil did have a substantially higher overall oleic acid content (41.6%) compared to cottonseed (18.2%) and soybean (21.6%) oils, which may have affected the crystal composition, given that the rice bran system had a greater long-spacing (47.26 Å) compared to the cottonseed (46.15 Å) and soybean (46.94 Å) systems. But this evidently did not affect crystal morphology. Furthermore, these long-spacings demonstrated that monopalmitin was the primary molecular species in the MAG crystals of each system and XRD did not indicate the presence of DAG crystals, both factors would have contributed to the similar microstructures. Meanwhile, the peanut glycerolysis product, which was the only other system with an XRD pattern indicating the network was composed of only MAG crystals, had a long-spacing (49.46 Å) to indicate monopalmitin was not the primary species within the crystalline material. Peanut oil contained 58.4% oleic acid along with 18.8% SFAs, of which palmitic acid represented only half (9.4%). MAGs containing oleic acid and more importantly, long-chain SFAs unique to peanut oil, therefore played an important role in determining the properties of the peanut glycerolysis product and led to a high crystallization onset temperature, numerous nucleation sites, and small crystals.
Furthermore, tigernut and olive systems contained similar platelet-like crystals with similar MAG crystal compositions. This is evident from their similar long-spacings (47.58 and 48.00 Å, respectively). The fatty acid compositions of tigernut and olive oil share some similarities. Both contain high levels of oleic acid (68.1% and 75.1%, respectively) along with moderate levels of SFAs (20.8% and 14.1%, respectively). Evidently, the higher oleic acid and lower saturated fatty acid contents of the olive system resulted in the diolein crystals being the dominant crystal in the structural matrix of the olive glycerolysis product, while they were secondary to the MAG crystals in the tigernut glycerolysis product. This key difference likely factored into the less aggregated structure within the olive system.
While diolein crystals would generally be considered to have a positive contribution to the overall crystal network, they have perhaps reduced aggregation in the olive system. Additionally, diolein crystals may have been disruptive to the MAG crystals of the sesame network. The microstructure of the sesame glycerolysis product does not match any of the other systems tested, however, the crystallization curve for sesame resembles that of soybean, with peak positions for the sesame system shifted to higher temperatures by several degrees. These two systems also have similar XRD peak positions, except the sesame system contains a diolein peak, while the soybean system does not. Furthermore, in the sesame system, the amplitude of both the MAG and DAG peaks are low, demonstrating that although both MAG and DAG crystals were formed, neither crystal species were present in large quantities. It is possible that the secondary crystallization of small amounts of diolein in the sesame glycerolysis product may have been detrimental to the formation of MAG crystals, leading to its apparent lack of crystalline material.
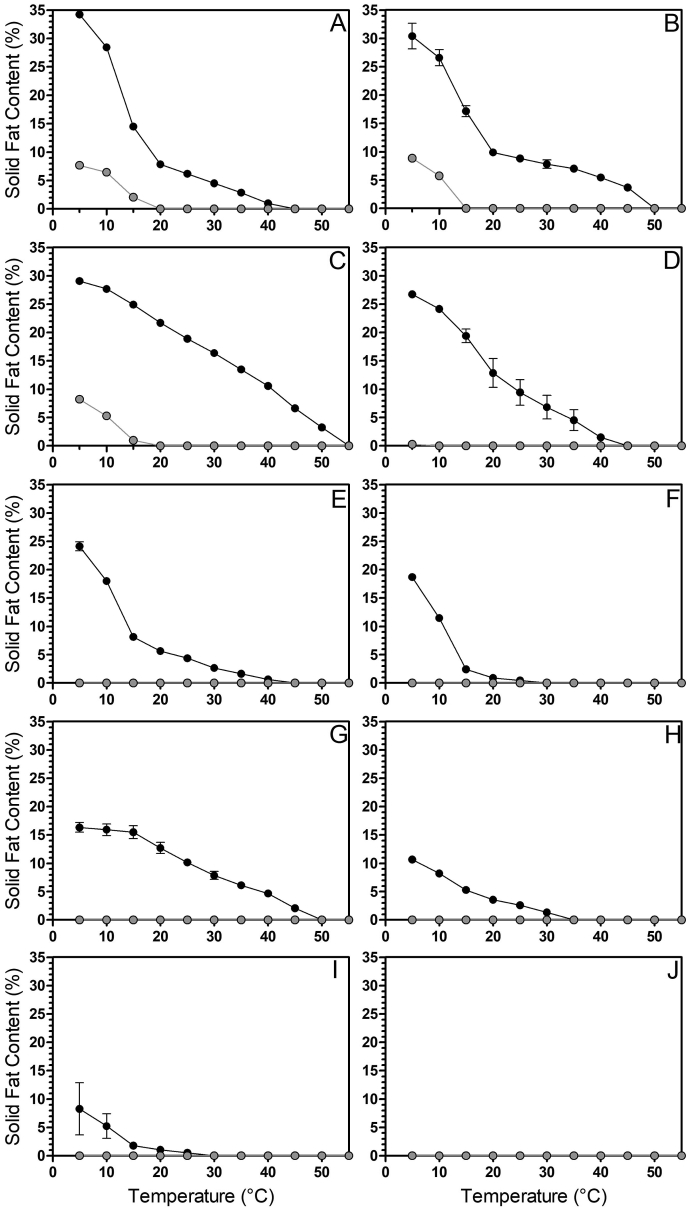
3.2.4. Solid fat content
In each of the vegetable oils studied, the changes in the crystallization behaviour of the glycerolysis reaction products resulted in increases in the solid fat content (SFC). Fig. 4 depicts the SFC-temperature profiles for each of the ten oils studied. When crystallized and stored at 5 °C for one week, the tigernut glycerolysis product (Fig. 4A) had a SFC of 34.3%, compared to 7.7% solids in the unaltered tigernut oil. Following glycerolysis, the SFC of the peanut system (Fig. 4B) was increased from 8.9% to 30.4%, while the cottonseed system (Fig. 4C) underwent an increase from 8.2% to 29.1%. The next highest SFC was observed for the rice bran glycerolysis product (26.7%; Fig. 4D). This was the last of the samples tested in which there was crystalline material in the starting oil (0.3%). Next, the olive glycerolysis product (Fig. 4E) had a SFC of 24.1%. SFC values of 18.7%, 16.3%, 10.7%, and 8.3% were observed for the HOCO (Fig. 4F), soybean (Fig. 4G), sesame (Fig. 4H), and canola (Fig. 4I) glycerolysis products, respectively. Fig. 4J depicts HOAO oil before and after glycerolysis. As mentioned earlier, the HOAO glycerolysis product did not possess any solids at 5 °C despite the increase in crystallization temperature as measured through DSC.
Fig. 4.
Solid fat content (SFC) melting profiles for glycerolysis reaction products (black lines) and their respective oils (gray lines). (A) tigernut; (B) peanut; (C) cottonseed; (D) rice bran; (E) olive (F) high oleic canola oil (HOCO); (G) soybean; (H) sesame; (I) canola; (J) high oleic algal oil (HOAO). Testing was performed after crystallization and storage at 5 °C for 1 week. Error bars represent the standard error of three replicates. Actual values for each data point are included as supplementary material (Supplementary Table 1).
Vereecken and coworkers had previously determined that the saturated fatty acid (SFA) content and the oleic-linoleic (monounsaturated-polyunsaturated) fatty acid (MUFA:PUFA) ratio of MAGs and DAGs affected the SFC of model lipid systems (Vereecken et al., 2009a, Vereecken et al., 2009b). In the present study, it is evident that these characteristics both factored into the SFC of the glycerolysis products. The SFC of the cottonseed system was high due to a high SFA content (27.5%), even while the MUFA:PUFA ratio was low (0.35). Meanwhile, the tigernut and peanut systems displayed higher SFC values (5 °C) than the cottonseed system even though they contained lower levels of SFAs (20.8% and 18.8%, respectively). This highlights the importance of the MUFA:PUFA ratio, as ratios within tigernut oil (6.14) and peanut oil (2.73) were greater than in cottonseed oil. This is again exemplified by a comparison with the rice bran system, which had a higher SFA content (23.3%), but a lower SFC at 5 °C than tigernut and peanut, because rice bran oil had a MUFA:PUFA ratio of only (1.20). Furthermore, olive oil had the highest MUFA:PUFA ratio (7.62) but contained only 14.1% SFAs and did not achieve a SFC as high as the rice bran system. This demonstrates the importance of both SFAs and the MUFA:PUFA ratio. HOCO had a low SFA content (6.9%) paired with a relatively high MUFA:PUFA ratio (3.59), which allowed this system to achieve a marked increase in SFC. Evidently, the MUFA:PUFA ratio of canola oil (2.12) was not high enough to substantially boost the SFC of the canola system even though the SFA content (7.1%) was similar to HOCO. In this situation, individual PUFA contents may have been a factor. The only system comparison that could not be explained by the SFAs and MUFA:PUFA ratio was between soybean and sesame. These two systems contained identical SFA contents (15.9%), while sesame had a greater MUFA:PUFA ratio (0.89 vs 0.35). However, the SFC of the soybean system is 16.3%, while the sesame glycerolysis product only achieved 10.7%. This again suggests that crystallization was inhibited in the sesame system. To summarize, when the MUFA:PUFA ratio was low, the increase in SFC above what would be expected from the SFA contribution alone was also small. A high MUFA:PUFA ratio allowed the SFC to rise to a value much greater than simply what the SFAs contribute. However, this did not always hold true at low SFA contents. This demonstrates that SFAs and oleic acid contribute to the SFC of the lipid systems (at 5 °C) with the contribution of oleic acid being greatest when the PUFA content is at a minimum. Lower PUFA concentrations result in less dilution of the oleic acid containing species, thereby minimizing the reduction in their crystallization points.
As the sample temperature was increased and the SFC decreased, two distinctive SFC-temperature profiles were evident. In the first scenario, the glycerolysis products underwent a two-segment decrease, with substantial decreases in SFC as the sample temperature was increased from 5 °C to 20 °C, before a gradual decrease as the temperature was increased further. The other SFC-temperature profile depicted a gradual decrease in SFC throughout the entire temperature range. The two-segment profile was observed for the tigernut, peanut, olive, HOCO, and canola glycerolysis products. As a result, the SFC of the tigernut system decreased from 34.3% at 5 °C to 7.9% at 20 °C, while the peanut glycerolysis product decreased from 30.4% at 5 °C to 9.9% at 20 °C. For the olive, HOCO, and canola systems, this rapid SFC decrease took place between 5 °C and 15 °C. The olive glycerlysis product decreased from 24.1% to 8.2% and HOCO and canola systems decreased from 18.7% to 2.4% and 8.3% to 1.8%, respectively. A high oleic acid content was a commonality amongst these lipid systems. Each contained over 50% oleic acid and more than three times as much oleic acid as SFAs. Since the peanut system falls within this category, it demonstrates that even when diolein crystals were not present, the overall oleic acid content factors into the SFC-temperature behaviour. This was due to the substantial contribution of monoolein to the MAG crystals. It is for this reason that the SFC-temperature profile for the rice bran glycerolysis product appeared to transition between these two categories, since it contained the next highest oleic acid content and was the only other system with a MAG long-spacing of ≥47 Å.
Furthermore, cottonseed, soybean, and sesame glycerolysis products demonstrated gradual decreases in SFC as the temperature increased. This was most obvious with the cottonseed and soybean glycerolysis products, in which the respective SFC values decreased from 29.1% to 21.7% and from 16.3% to 12.7% as the temperature was increased from 5 °C to 20 °C. The sesame system underwent a decrease in SFC from 10.7% at 5 °C to 3.6% at 20 °C. While this SFC decrease was greater than that of the cottonseed and soybean systems, it was not nearly as pronounced a decrease as observed for the systems described previously. Consistent among each of these lipid systems is an oleic acid content of <40% (18.2%, 21.6%, and 39.7%, respectively). Additionally, each displayed MAG long-spacings of ≤47 Å, indicative of large amounts of monopalmitin in the MAG crystals. This resulted in the presence of less material that would melt at temperatures between 5 and 20 °C, leading to a gradual decrease in SFC and a higher remaining level of solids at high temperatures (i.e., cottonseed and soybean glycerolysis products). This latter phenomenon was also observed for the peanut glycerolysis product because of the high concentration of long-chain saturated fatty acids unique to that system.
Evidently, SFAs are necessary in the starting oil to achieve a high SFC following glycerolysis. At least 10% SFAs are necessary, otherwise in the case of the canola system, very little solids will be present following the reaction; or the moderate level of solids produced will decrease substantially as the temperature is increased, as was the case with HOCO. Furthermore, increasing the SFA content is beneficial to the structuring ability of the reaction (i.e., increasing the SFC above that of the oil). This is true at least until the starting lipid system has a high enough SFA content that it becomes structured in its native triacylglycerol state (Nicholson and Marangoni, 2020). However, since one of the goals of these glycerolysis reactions is to reduce the saturated fat content of food products, it is most desirable to choose a lipid system that is lower in saturated fat while still able to achieve a high SFC. While cottonseed oil may still represent a desirable base oil given that its SFA content is nearly half that of palm oil, other oil systems tested showed similar or greater SFC values at 5 °C, even with lower levels of SFAs. Additionally, the oleic acid content is important. When low amounts of oleic acid are present in the system, the crystals formed contain mostly SFAs and undergo a very gradual reduction in the SFC as the temperature is increased. This could potentially lead to negative sensory aspects since high levels of solids may remain at in-mouth temperatures. When oleic acid represents a portion of the crystal structure, either as monoolein or diolein, the lipid system will undergo a more substantial reduction in the SFC at the low-end temperatures, leading to a lower SFC at in-mouth temperatures. The ideal situation, which is to achieve a high level of solids at 5 °C, that melt substantially at temperatures less than that of the mouth, is achievable when moderate amounts of SFAs (14%–25%) are present in the oil system along with high levels of oleic acid (>60%).
3.3. Applications in food
The tigernut glycerolysis product had the highest SFC when measured at 5 °C, a property which is very important for the structure within a variety of food applications. In addition, this system demonstrated a two-segment SFC-temperature profile with a pronounced reduction in SFC with respect to temperature. These characteristics make the tigernut glycerolysis product an ideal candidate for refrigerated soft-tub margarine and fat spreads. Furthermore, a margarine product was successfully produced with the tigernut glycerolysis product. This margarine product is depicted one week after production in Fig. 5A. The experimental margarine had a high stability demonstrating minimal phase separation after 10 months storage at 5 °C, as is evident from Fig. 5B. Furthermore, the microstructure of the tigernut margarine sample after 10 months storage is shown in Fig. 5C, and while there are several larger droplets of water within the sample, as would be expected after 10 months time, most of the water droplets visible within the fat crystal network are less than 20 μm in diameter.
Fig. 5.
Images of an experimental margarine sample prepared with tigernut glycerolysis product (A) 1 week and (B) 10 months after sample preparation. Sample was stored at 5 °C in both cases. (C) Microstructure of the tigernut glycerolysis product margarine after 10 months storage at 5 °C.
Fig. 6A shows the force-deformation profiles for commercially available butter and soft-tub margarine, along with the margarine prepared with tigernut glycerolysis product. Testing was performed at the expected temperatures of use for each sample; butter at 22 °C (room temperature) and the soft-tub and experimental margarines at 5 °C (refrigeration temperature). The margarine formulated with the tigernut glycerolysis product demonstrated a force-deformation profile similar to those obtained for commercially available soft-tub margarine and butter. For applications such as margarine and spreads, plasticity of the material is crucial to the spreading properties. A fat product that undergoes brittle fracture will be difficult to spread (i.e., butter straight out of the fridge), making it undesirable for this sort of application. Whether a material undergoes brittle fracture or demonstrates plastic flow behaviour during deformation can be determined through back extrusion based on whether the material produces a jagged and choppy profile or smooth curve, without stress accumulation (Gravelle et al., 2017; Macias-Rodriguez et al., 2018). As would be expected given the ease with which these common products spread, commercially available soft-tub margarine and butter each displayed a smooth force-deformation curve without any stress accumulation, characteristic of plastic flow behaviour. This profile was also successfully achieved with the tigernut glycerolysis product margarine. Furthermore, although β̍ crystals are often desired during the production of margarine because they are associated with a high plasticity, the shear induced reduction in the crystal size of the tigernut glycerolysis product produced a margarine that had adequate plasticity.
Fig. 6.
(A) Back extrusion force deformation profiles for margarine produced with tigernut glycerolysis product along with commercially available soft-tub margarine and butter. (a) butter, (b) tigernut glycerolysis product margarine, (c) commercial margarine. Experimental and commercially available margarines were tested at 5 °C, while butter was tested at room temperature 22 °C. Each profile was taken as an average of three replicates. Error bars were omitted for clarity. (B) Differential scanning calorimetry and (C) solid fat content melting profiles for margarine produced with tigernut glycerolysis product (gray line) and a commercially available soft-tub margarine (black line).
Examining the force required to deform the material (firmness), we notice that butter was firmer than the soft-tub margarine. More importantly, it is evident that the deformation curve of the experimental margarine lay in between the commercially available products, performing more similarly to room temperature butter. These results indicate that tigernut oil structured through glycerolysis can be used to prepare margarine with plastic behaviour similar to that of commercially available soft-tub margarine and butter, while producing a margarine product with a firmness that more closely resembles that of butter compared to the commercially available soft-tub margarine tested here. Given that the goal when producing margarine is to formulate a product that can be used as a substitute for butter, the tigernut glycerolysis product was successful in producing a margarine product as far as firmness is concerned.
The melting profiles of the experimental margarine produced with the tigernut glycerolysis product are pictured in Fig. 6. DSC thermograms (Fig. 6B) demonstrated a gradual melting of the experimental margarine product from the 5 °C start temperature until approximately 26 °C. A distinctive melting peak was then visible at approximately 32 °C. This melting behaviour is very promising for the eating characteristics of the margarine, as the melting peak occurred at a temperature just below that which is typically associated with in-mouth temperatures. Fig. 6C depicts the SFC-temperature profile. These melting characteristics described translated to a gradual reduction in the SFC of the margarine with increasing temperature. Perhaps most importantly, 3.2% solids remained when the margarine samples were tested at 30 °C (just below the temperature of the mouth) and 0% solids were present when the sample was measured at 35 °C. Therefore, no solids remained at in-mouth temperatures, thereby demonstrating that the margarine formulated with the tigernut glycerolysis product yielded nearly optimal melting characteristics. Interestingly, the commercially available soft-tub margarine had a lower SFC when measured at 5 °C, which explains the lower firmness compared to the tigernut margarine. Perhaps more importantly, the SFC of this margarine decreased much more slowly with increasing temperature and over 4% solids remained at 35 °C. The DSC melting profile confirmed the presence of a melting peak just below 40 °C. This indicates that edible oils structured through glycerolysis, can produce margarines with proper melting characteristics, without a lingering waxy mouthfeel. In some cases, these glycerolysis structured oils may even perform better than commercially available margarines prepared with palm oil.
Margarine and spreads are common food products that are often produced through the addition of palm oil and/or the use of hydrogenation and interesterification reactions. They are also food products that have been difficult to produce using oleogels or other fat mimetics, while maintaining adequate product stability, melting properties, and plasticity. Here we have demonstrated that glycerolysis is a viable means of structuring plant-based oils with potential to be used in the production of margarine and spreads. Using these structured oils would improve the fatty acid profile and sustainability of the product as palm oil would no longer be a required ingredient. Additionally, this would also clean up the ingredients’ list as hydrogenated oils would no longer be necessary, thus increasing consumer acceptance of the product.
The potential oils which could be structured through glycerolysis and used for margarine and spread production do not stop at tigernut oil. Many other oils that contain an intermediate SFA content and high oleic acid concentration represent potential options to be used for margarine formulation. The versatility of the glycerolysis process for improving the functionality of oils means that the oil of many oilseed crops already in production for other purposes around the world could be used in a greater variety of food applications. This would reduce the need to produce more palm oil to meet demand for functional oils, while improving the healthfulness of food products. Besides spreads and margarines, partially crystalline oils, such as the HOCO, soybean, sesame, and canola glycerolysis products, could be used as a self-emulsifying system due to the high MAG content. Moreover, the presence of crystals could help stabilize emulsions, both W/O and O/W via Pickering mechanisms without requiring added emulsifiers. The clean label advantage of this is obvious.
Furthermore, using glycerolysis to structure oils improves the overall healthfulness of the system. In addition to the healthier fatty acid profiles of liquid oils structured through glycerolysis compared to palm oil or other means of improving the functionality of a lipid system (i.e., SFA addition). Glycerolysis-structured oils also bring with them the health benefits that have been associated with the consumption of DAGs in place of TAGs. Due to differences in the metabolic pathway of 1,3-DAGs compared to TAGs, consumption of DAGs rather than TAGs has been shown to reduce body fat stores and serum TAG levels along with low-density lipoprotein cholesterol and total cholesterol levels (Flickinger and Matsuo, 2003; Matsuo 2004; Teramoto et al., 2004; Yanai et al., 2007). This makes glycerolysis-structured oils a healthy, functional fat source.
4. Conclusions
This research has shown that through glycerolysis, the SFC of liquid vegetable oils can be increased, structuring them into solid fats without altering their fatty acid profile. The effectiveness of glycerolysis at structuring edible oils is dependent on the fatty acid composition of the liquid oil. The potential structuring power of the glycerolysis reaction is greatest when SFAs are present at intermediate concentrations (14–25%) and the oleic acid content is high. This oil composition was also beneficial to the melting characteristics of the structured system. When used to formulate margarine, glycerolysis-structured oils provide a solution to the lack of plasticity often observed with fat mimetics and when the appropriate oil system is used, namely tigernut oil, solves the melting behaviour issues commonly experienced with wax-based oleogel systems. In some cases, margarine prepared with glycerolysis-structured oils perform better than commercial margarine prepared with palm oil. Using plant-based oils structured through glycerolysis, provides a sustainable way to reduce demand for palm oil, which would help to end further habitat and environmental destruction throughout the world’s tropical regions. Glycerolysis-structured oils offer a healthful solution to blending liquid oils with high SFA fats to improve the functionality of an oil.
Data availability statement
The datasets for this study are available upon request.
CRediT authorship contribution statement
Reed A. Nicholson: Conceptualization, Methodology, Validation, Investigation, Data curation, Formal analysis, Data curation, Writing – original draft, Visualization, Project administration. Alejandro G. Marangoni: Conceptualization, Resources, Writing – review & editing, Visualization, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
Special thanks go to Dr. Saeed Mirzaee Ghazani for determining the fatty acid composition of the vegetable oils.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2021.03.005.
Contributor Information
Reed A. Nicholson, Email: rnicho01@uoguelph.ca.
Alejandro G. Marangoni, Email: amarango@uoguelph.ca.
Funding
The authors acknowledge the financial support of the Government of Ontario, the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Research Chairs (CRC) Program, the Ontario Ministry of Agriculture and Food (OMAF), and the Barrett Foundation. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Austin K.G., Mosnier A., Pirker J., McCallum I., Fritz S., Kasibhatla P.S. Shifting patterns of oil palm driven deforestation in Indonesia and implications for zero-deforestation commitments. Land Use Pol. 2017;69:41–48. [Google Scholar]
- Belitz H.D., Grosch W., Schieberle P. Lipids. In: Belitz H.D., Grosch W., Schieberle P., editors. Food Chemistry. fourth ed. Springer; 2009. pp. 158–245. [Google Scholar]
- Batte H.D., Wright A.J., Rush J.W., Idziak S.H.J., Marangoni A.G. Effect of processing conditions on the structure of monostearin–oil–water gels. Food Res. Int. 2007;40:982–988. [Google Scholar]
- Carlson K.M., Heilmayr R., Gibbs H.K., Noojipady P., Burns D.N., Morton D.C., Walker N.F., Paoli G.D., Kremen C. Effect of oil palm sustainability certification on deforestation and fire in Indonesia. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115(1):121–126. doi: 10.1073/pnas.1704728114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.H., Terentjev E.M. Aging and metastability of monoglycerides in hydrophobic solutions. Langmuir. 2009;25(12):6717–6724. doi: 10.1021/la9002065. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Terentjev E.M. Monoglycerides in oils. In: Marangoni A.G., Garti N., editors. Edible Oleogels. second ed. AOCS Press; 2018. pp. 103–131. [Google Scholar]
- Coral Medina J.D., Magalhaes A.I., Zamora H.D., Quijano Melo J.D. Oil palm cultivation and production in South America: status and perspectives. Biofuels, Bioprod. Bioref. 2019;13:1202–1210. [Google Scholar]
- Daubert B.F., Lutton E.S. X-Ray diffraction analyses of synthetic unsaturated monacid diglycerides. J. Am. Chem. Soc. 1947;69:1449–1451. doi: 10.1021/ja01198a055. [DOI] [PubMed] [Google Scholar]
- Elfman-Borjesson I., Harrod M. Synthesis of monoglycerides by glycerolysis of rapeseed oil using immobilized lipase. J. Am. Oil Chem. Soc. 1999;76(6):701–707. [Google Scholar]
- Flickinger B.D., Matsuo N. Nutritional characteristics of DAG oil. Lipids. 2003;38(2):129–132. doi: 10.1007/s11745-003-1042-8. [DOI] [PubMed] [Google Scholar]
- Fredrick E., Foubert I., Van De Sype J., Dewettinck K. Influence of monoglycerides on the crystallization behaviour of palm oil. Cryst. Growth Des. 2008;8(6):1833–1839. [Google Scholar]
- Fregolente P.B.L., Fregolente L.V., Pinto G.M.F., Batistella B.C., Wolf-Maciel M.R., Filho R.M. Monoglycerides and diglycerides synthesis in a solvent-free system by lipase-catalyzed glycerolysis. Appl. Biochem. Biotechnol. 2008;146:165–172. doi: 10.1007/s12010-008-8133-3. [DOI] [PubMed] [Google Scholar]
- Goh E.M., Timms R.E. Determination of mono- and diglycerides in palm oil, olein and stearin. J. Am. Oil Chem. Soc. 1985;62(4):730–734. [Google Scholar]
- Gravelle A.J., Davidovich-Pinhas M., Barbut S., Marangoni A.G. Influencing the crystallization behavior of mixtures of stearyl alcohol and stearic acid (SOSA) using ethylcellulose. Food Res. Int. 2017;91:1–10. doi: 10.1016/j.foodres.2016.11.024. [DOI] [PubMed] [Google Scholar]
- Katan M.B., Zock P.L., Mensink R.P. Trans fatty acids and their effects on lipoproteins in humans. Annu. Rev. Nutr. 1995;15:473–493. doi: 10.1146/annurev.nu.15.070195.002353. [DOI] [PubMed] [Google Scholar]
- Kristensen J.B., Xu X., Mu H. Process optimization using response surface design and pilot plant production of dietary diacylglycerols by lipase-catalyzed glycerolysis. J. Agric. Food Chem. 2005;53:7059–7066. doi: 10.1021/jf0507745. [DOI] [PubMed] [Google Scholar]
- Macias-Rodriguez B.A., Ewolt R.H., Marangoni A.G. Nonlinear viscoelasticity of fat crystal networks. Rheol. Acta. 2018;57:251–266. [Google Scholar]
- Malkin T., Riad El Shurbagy M. An x-ray and thermal examination of the glycerides. Part II. The α-monoglycerides. J. Chem. Soc. 1936:1628–1634. [Google Scholar]
- Marangoni A.G., Idziak S.H.J., Vega C., Batte H., Ollivon M., Jantzi P.S., Rush J.W.E. Encapsulation-stucturing of edible oil attenuates acute elevation of blood lipids and insulin in humans. Soft Matter. 2007;3:183–187. doi: 10.1039/b611985a. [DOI] [PubMed] [Google Scholar]
- Marangoni A.G., van Duynhoven J.P.M., Acevedo N.C., Nicholson R.A., Patel A.R. Advances in our understanding of the structure and functionality of edible fats and fat mimetics. Soft Matter. 2020;16:289–306. doi: 10.1039/c9sm01704f. [DOI] [PubMed] [Google Scholar]
- Matsuo N. Nutritional characteristics and health benefits of diacylglycerol in foods. Food Sci. Technol. Res. 2004;10(2):103–110. [Google Scholar]
- McNeill G.P., Shimizu S., Yamane T. High-yield enzymatic glycerolysis of fats and oils. J. Am. Oil Chem. Soc. 1991;68(1):1–5. [Google Scholar]
- Meijjard E., Abrams J.F., Juffe-Bignoli D., Voight M., Sheil D. Coconut oil, conservation, and the conscientious consumer. Curr. Biol. 2020;30:R737–R758. doi: 10.1016/j.cub.2020.05.059. [DOI] [PubMed] [Google Scholar]
- Mensink R.P. World Health Organization; 2016. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: a Systematic Review and Regression Analysis. [Google Scholar]
- Mensink R.P., Zock P.L., Kester A.D.M., Katan M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Nicholson R.A., Marangoni A.G. Enzymatic glycerolysis converts vegetable oils into structural fats with the potential to replace palm oil in food products. Nature Food. 2020;1:684–692. doi: 10.1038/s43016-020-00160-1. [DOI] [PubMed] [Google Scholar]
- Noureddini H., Harmeier S.E. Enzymatic glycerolysis of soybean oil. J. Am. Oil Chem. Soc. 1998;75(10):1359–1365. [Google Scholar]
- Ojijo N.K.O., Kesselman E., Shuster V., Eichler S., Eger S., Neeman I., Shimoni E. Changes in microstructural, thermal, and rheological properties of olive oil/monoglyceride networks during storage. Food Res. Int. 2004;37(4):385–393. [Google Scholar]
- Ojijo N.K.O., Neeman I., Eger S., Shimoni E. Effects of monoglyceride content, cooling rate and shear on the rheological properties of olive oil/monoglyceride gel networks. J. Sci. Food Agric. 2004;84:1585–1593. [Google Scholar]
- Patel A.R., Nicholson R.A., Marangoni A.G. Applications of fat mimetics for the replacement of saturated and hydrogenated fat in food products. Curr. Opin. Food Sci. 2020;33:61–68. [Google Scholar]
- Tavernier I., Moens K., Heyman B., Danthine S., Dewettinck K. Relating crystallization behaviour of monoacylglycerol-diacylglycerol mixtures to the strength of their crystalline network in oil. Food Res. Int. 2019;120:504–513. doi: 10.1016/j.foodres.2018.10.092. [DOI] [PubMed] [Google Scholar]
- Teramoto T., Watanabe H., Ito K., Omata Y., Furukawa T., Shimoda K., Hoshino M., Nagao T., Naito S. Significant effects of diacylglycerol on body fat and lipid metabolism in patients on hemodialysis. Clin. Nutr. 2004;23(5):1122–1126. doi: 10.1016/j.clnu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Vereecken J., Foubert I., Meeussen W., Lesaffer A., Dewettinck K. Fat structuring with partial acylglycerols: effect on solid fat profiles. Eur. J. Lipid Sci. Technol. 2009;111:259–272. [Google Scholar]
- Vereecken J., Meeussen W., Foubert I., Lesaffer A., Wouters J., Dewettinck K. Comparing the crystallization and polymorphic behaviour of saturated and unsaturated monoglycerides. Food Res. Int. 2009;42:1415–1425. [Google Scholar]
- Wright A.J., Hartel R.W., Nurine S.S., Marangoni A.G. The effect of minor components on milk fat crystallization. J. Am. Oil Chem. Soc. 2000;77(5):463–475. [Google Scholar]
- Wright A.J., Marangoni A.G. Effect of DAG on milk fat TAG crystallization. J. Am. Oil Chem. Soc. 2002;79:395–402. [Google Scholar]
- Yanai H., Tomono Y., Ito K., Furutani N., Yoshida H., Tada N. Diacylglycerol oil for the metabolic syndrome. Nutr. J. 2007;6(43):1–6. doi: 10.1186/1475-2891-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets for this study are available upon request.