Abstract
Pathogenic CACNA1A gene variants are associated with a spectrum of disorders including migraine with or without hemiplegia, ataxia, epilepsy, and developmental disability. We present a case of a pathogenic variant (c.4046G>A, p.R1349Q) in the CACNA1A gene associated with a clinical phenotype of global developmental delay, left hemiparesis, epilepsy, and stroke-like episodes. Longitudinal neuroimaging demonstrates hemispheric encephalomalacia with mismatched perfusion and angiographic imaging, in addition to progressive cerebellar atrophy.
Keywords: CACNA1A, Hemiplegic Migraines, Episodic Ataxia
Introduction
The CACNA1A gene encodes the Cav2.1 voltage-gated calcium channel that is expressed in the central nervous system (CNS). In adults, pathogenic variants in this gene are associated with a clinical spectrum of disorders including famililal or sporadic hemiplegic migraine (FHM or HM respectively) with or without progressive ataxia, episodic ataxia type 2 (EA2), spinocerebellar ataxia type 6 (SCA6), and developmental and epileptic encephalopathy type 42 (DEE42) [1], [2]–3]. Thus, recognition of the clinical phenotype may be difficult and there is also a paucity of published neuroradiological studies, especially in individuals reported with the CACNA1A R1349Q variant.
Children found to carry this specific CACNA1A variant have variable presentations. These include encephalopathy with myoclonic epilepsy, hemiplegic migraine, coma, stroke, and eye movement disorders [1,[4], [5], [6], [7], [8]]. Associated brain MRI studies have frequently shown cerebellar atrophy and progressive atrophy of white matter [1,4,6,8], but there are no characteristic diagnostic findings.
This case report follows the disease course of a pediatric patient with infantile onset of motor delay and nystagmus followed by multiple episodes of intractable seizures, strokes, and hemiplegic migraine throughout childhood. The patient was ultimately found to carry a pathogenic variant (R1349Q) in CACNA1A. This case provides educational value given the image-rich longitudinal brain findings via MRI and adds to the emerging literature for this rare pathogenic variant.
Case report
A 10-month-old patient presented to the neurology clinic on referral from his general pediatrician in consultation for hypotonia and global developmental delays. His neurologic history began at 4 months of age when he was noticed to have head lag and poor tracking, normally present at 2 months of age. At 5 months, he demonstrated tremors of his head and of his hands when holding objects. At 6 months, he showed nystagmus which was confirmed by an ophthalmologist at 10-months of age. On the recommendation of an herbalist, the patient was fed rice protein containing amino acids with resultant improvement of the tremor, according to the mother.
The patient had a normal birth history, without surgeries. Other than rice protein and flaxseed diet supplementation, the patient received B12 and magnesium vitamin supplementation without other medication.
Metabolic/genetic workup demonstrated normal cerebrospinal fluid (CSF) neopterin and biopterin neurotransmitter profile, serum amino acids, lactate and pyruvate, very long chain fatty acids, carnitine, acylcarnitine profile, TSH, B12 levels, urine amino and organic acids, mucopolysaccharide spot test, chromosome microarray, and electromyelography.
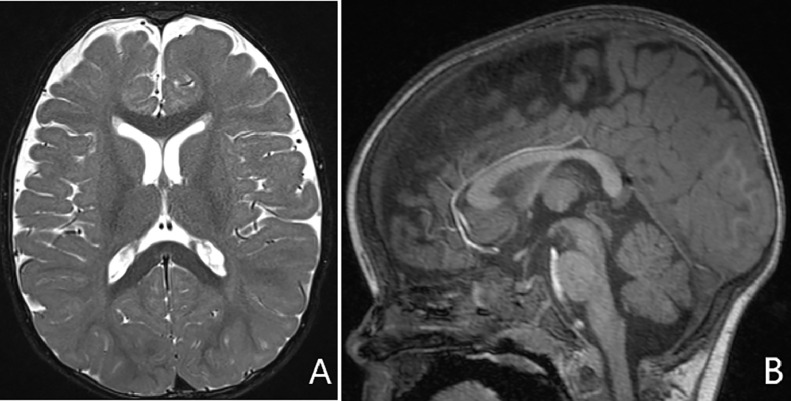
An outside hospital MRI performed at 6 months of age (unavailable) was reported as normal. MRI at 10 months of age (Fig. 1) revealed mild white volume loss but was otherwise normal for age.
Fig. 1.
10 months of age. (A) Axial T2 image of the brain shows prominent ventricles and sulci indicating mild volume loss. (B) Sagittal T1 image shows normal appearing cerebellar vermis and brainstem.
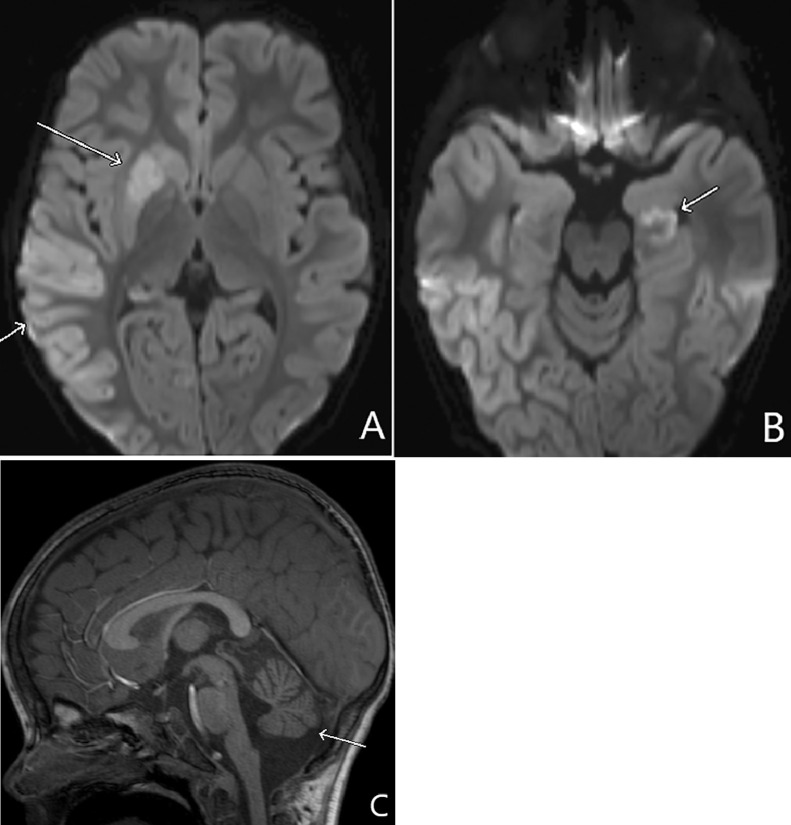
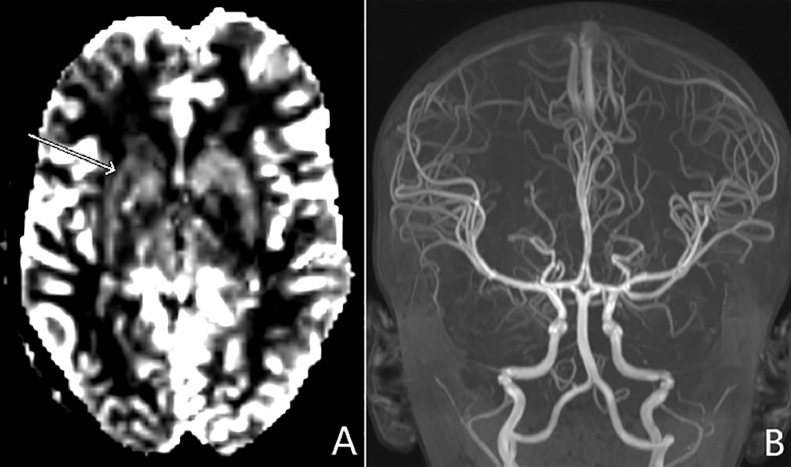
The patient next presented to a local emergency department at 31 months of age with respiratory failure, status epilepticus, and new onset left-sided hemiplegia. Brain MRI findings revealed diffuse right sided cortical swelling with decreased diffusion, left hippocampal decreased diffusion, and mild cerebellar vermian atrophy (Fig. 2). Perfusion imaging and MR arteriography showed decreased cerebral blood flow of the right basal ganglia with normal patency of the intracranial vessels (Fig. 3). An infectious workup including CSF was negative. Seizures were treated with levetiracetam and physical therapy was initiated for treatment of left hemiplegia. He had developmental regression after this event as he had left sided weakness, but could roll over and babbles. Prior to this event, he was able to sit independently, crawl, point and use 5-10 words.
Fig. 2.
Thirty-one months of age. (A) Axial diffusion weighted imaging (DWI) shows right basal ganglia and right temporal cortex decreased diffusion from cytotoxic injury (arrows). (B) The left hippocampal cortex is also involved (arrow) which may be from ischemia or acute seizure sequela. (C) Sagittal T1 shows interval atrophy of the cerebellar vermis (arrow).
Fig. 3.
Thirty-one of age. (A) Cerebral blood flow map from dynamic susceptibility contrast perfusion imaging shows decreased perfusion of the right basal ganglia (arrow). Maximum intensity projection (MIP) in the coronal plane of MR arteriography shows normal caliber vessels without stenosis of the right anterior circulation.
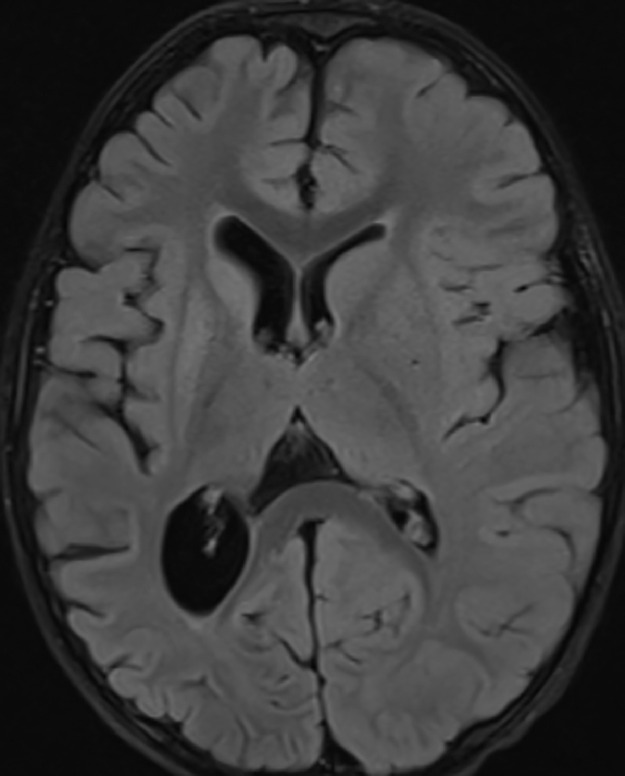
At 34 months, the patient had a follow-up MRI (Fig. 4) showing interval atrophy of the right cerebral hemisphere gray and white matter involving all lobes with secondary ex vacuo dilatation of the ventricles and interval volume loss of the right caudate and putamen. This was thought to be related to evolution of cytotoxic edema previously seen in these areas. During this time, the patient experienced breakthrough seizures. Mitochondrial DNA sequencing was performed on blood but was negative.
Fig. 4.
Thirty-four months of age. Axial FLAIR image shows interval encephalomalacia and atrophy of the right cerebral hemisphere and basal ganglia with resulting ex-vacuo dilatation of the right lateral ventricle.
At 4 years old, the patient was still having seizures as well as uncontrolled movements of his arms, head and neck. In addition, he reported headache. He was started on lamotrigine which helped to reduce the frequency of seizures. Because of the high suspicion of a metabolic etiology for the recent stroke and seizures despite previous negative testing, he underwent whole exome sequencing and was found to have a mutation in the CACNA1A calcium channel gene (c. 4046 G>A, p.R1249Q). Given this diagnosis, verapamil was started. Despite the addition of lamotrigine, the patient had poor seizure control with 2-3 grand mal seizures a day. Over the next three years, anticonvulsants were increased or added without full control of seizures.
At 7 years of age, the patient presented acutely to the hospital with fever, vomiting and seizure like activity. He had decreased responsiveness and right sided hemiplegia. An infectious workup was negative, and patient received an MRI which showed left sided decreased diffusion in the subcortical white matter of the occipital and temporal lobes. MR arteriography showed normal patency of the intracranial vessels (Fig. 5). The patient was treated with IV verapamil and corticosteroids and discharged to home after supportive treatment. The patient continues taking verapamil and a combination of valproic acid, topiramate and levetiracetam for seizure control and movement disorder. Over time, he has shown improvement in his right sided weakness; however, he has not regained baseline function prior to this last event.
Fig. 5.
Seven years of age. (A) Axial DWI shows decreased diffusion in the left occipital lobe (arrow). (B) Axial MIP of MRA of the head shows normal caliber vessels. (C) Sagittal T1 image shows further atrophy of the cerebellar vermis (arrow).
Discussion
This case report describes the longitudinal history of a boy with developmental delay, refractory epilepsy, hemiplegic migraines, and two episodes of stroke-like events. A pathogenic variant, R1349Q, was found in the CACNA1A gene. To our knowledge, this is the only case that demonstrates longitudinal neuroimaging of a pediatric patient with this specific variant associated with epilepsy, hemiplegic migraines, and stroke.
CACNA1A encodes for the alpha-1a subunit of a voltage-dependent calcium channel widely expressed throughout the central nervous system. Although the pathophysiology is not fully understood at this time, calcium channels play an important role in neuronal excitation and neurotransmitter release. In addition, specific mutations of CACNA1A have been implicated in cerebral vascular smooth muscle dysfunction with vasospasm as a possible contributor [9].
CACNA1A pathogenic variants are associated with varied phenotypes as mentioned earlier. These phenotypes may overlap in symptomatology. Expansion of CAG trinucleotide repeats are associated with spinocerebellar ataxia type 6 whereas numerous distinct point mutations cause other phenotypes in the spectrum of CACNA1A-related disorders [2,10].
There are few descriptions of the R1349Q variant in the literature. In three existing cases, pediatric patients with familial or spontaneous hemiplegic migraine have been discovered to carry this variant. These patients have all presented with stroke-like events after mild to moderate head trauma [7,11,12]. These reports stress the importance of genetic testing in pediatric patients who experience stroke and stroke-like events as well as symptoms of ataxia and migraine. They also offer limited discussion of treatment options, such as verapamil [7] or corticosteroids [10], but they lack the extensive longitudinal neuroimaging that our case provides.
With enough clinical suspicion, pediatric patients with global delay, seizures, strokes, or other neurological problems will undergo brain imaging. There is currently a paucity of literature regarding what to expect on brain imaging in patients with this genetic mutation and phenotype. There are reports of cerebellar atrophy on CT and MRI in patients with CACNA1A mutations [1,6,8], and also in patients with hemiplegic migraines that are associated with these variants [5]. Other case studies have demonstrated progressive white-matter atrophy with periventricular signal change on MRI in a child with epilepsy and cognitive impairment due to this mutation [4] as well as repeatedly normal MRIs in an adult patient with a CACNA1A mutation associated with episodic ataxia type 2 [13].
Serial MRIs of this patient identify the sequelae of cytotoxic injury as a result of the CACNA1A variant. Gray and white matter atrophy with ex vacuo dilatation of the lateral ventricle, as seen in this patient, has also been reported in another child with this mutation [4]. During a stroke-like event of the right hemisphere, there was decreased perfusion with normal MRA, similar to what has previously been described in hemiplegic migraine perfusion imaging [14]. The extensive longitudinal neuroimaging in our report shows in one child the evolution of structural changes representing this spectrum of CACNA1A-related injury. Given these imaging findings, a specific test for hemiplegic migraines should be considered, with mitochondrial disease such as mitochondrial encephalopathy lactic acidosis and stroke like episodes in the differential. Whole exome and mitochondrial DNA sequencing tests would be an efficient method to detect and differentiate these entities to avoid a delay in diagnosis.
In conclusion, we present a rare case of the pathogenic variant R1394Q in the calcium channel CACNA1A gene with longitudinal imaging demonstrating progressive vermian atrophy, and two stroke-like events with hypoperfusion despite normal angiographic imaging. With a clinical background of developmental delay, seizures, hemiplegic migraines and ataxia, appropriate genetic testing should be performed to identify the likelihood of this specific gene mutation.
This report is in accordance with the declaration of Helsinki. No patient identifiers were included in this report.
The authors have no conflicts of interest to disclose.
References
- 1.Jodice C, Mantuano E, Veneziano L, Trettel F, Sabbadini G, Calandriello L. Episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6) due to CAG repeat expansion in the CACNA1A gene on chromosome 19p. Hum Mol Genet. 1997;6(11):1973–1978. doi: 10.1093/hmg/6.11.1973. [DOI] [PubMed] [Google Scholar]
- 2.Grieco GS, Gagliardi S, Ricca I, Pansarasa O, Neri M, Gualandi F. New CACNA1A deletions are associated to migraine phenotypes. J Headache Pain. 2018;19(1):75. doi: 10.1186/s10194-018-0891-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Online Mendelian Inheritance in Man, OMIM®. Johns Hopkins University, Baltimore, MD. MIM Number: 601011: 11/19/2020: https://www.omim.org/entry/601011?search=cacna1a&highlight=cacna1a. Accessed January 21, 2021
- 4.Hayashida T, Saito Y, Ishii A, Yamada H, Itakura A, Minato T. CACNA1A-related early-onset encephalopathy with myoclonic epilepsy: A case report. Brain Dev. 2018;40(2):130–133. doi: 10.1016/j.braindev.2017.08.006. doi: 10.1016/S1474-4422(11)70048-5. [DOI] [PubMed] [Google Scholar]
- 5.Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011;10(5):457–470. doi: 10.1016/S1474-4422(11)70048-5. [DOI] [PubMed] [Google Scholar]
- 6.Vahedi K, Denier C, Ducros A, Bousson V, Levy C, Chabriat H. CACNA1A gene de novo mutation causing hemiplegic migraine, coma, and cerebellar atrophy. Neurology. 2000;55(7):1040–1042. doi: 10.1212/wnl.55.7.1040. [DOI] [PubMed] [Google Scholar]
- 7.Knierim E, Leisle L, Wagner C, Weschke B, Lucke B, Bohner G. Recurrent stroke due to a novel voltage sensor mutation in Cav2.1 responds to verapamil. Stroke. 2011;42(2):e14–e17. doi: 10.1161/STROKEAHA.110.600023. [DOI] [PubMed] [Google Scholar]
- 8.Tantsis EM, Gill D, Griffiths L, Gupta S, Lawson J, Maksemous N. Eye movement disorders are an early manifestation of CACNA1A mutations in children. Dev Med Child Neurol. 2016;58(6):639–644. doi: 10.1111/dmcn.13033. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki S, Ikeno K, Abe T, Tohyama J, Adachi Y. Hemiconvulsion-hemiplegia-epilepsy syndrome associated with CACNA1A S218L mutation. Pediatr Neurol. Sep 2011;45(3):193–196. doi: 10.1016/j.pediatrneurol.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Carreño O, Corominas R, Serra SA, Sintas C, Fernandez-Castillo N, Vila-Pueyo M. Screening of CACNA1A and ATP1A2 genes in hemiplegic migraine: clinical, genetic, and functional studies. Mol Genet Genomic Med. 2013;1(4):206–222. doi: 10.1002/mgg3.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malpas TJ, Riant F, Tournier-Lasserve E, Vahedi K, Neville BG. Sporadic hemiplegic migraine and delayed cerebral oedema after minor head trauma: a novel de novo CACNA1A gene mutation. Dev Med Child Neurol. 2010;52(1):103–104. doi: 10.1111/j.1469-8749.2009.03493.x. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Albisua I, Schoning M, Jurkat-Rott K, Lerche H. Possible effect of corticoids on hemiplegic attacks in severe hemiplegic migraine. Pediatr Neurol. 2013;49(4):286–288. doi: 10.1016/j.pediatrneurol.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs DA, Bradshaw MJ, Brown K, Hedera P. Case report of novel CACNA1A gene mutation causing episodic ataxia type 2. SAGE Open Med Case Rep. 2017;5 doi: 10.1177/2050313X17706044. 2050313X17706044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rath C, He J, Nordling M, Wienecke T. Acute Intravenous Calcium Antagonist for Suspected Hemiplegic Migraine – A Case Story. Case Rep Neurol. 2017;9:98–105. doi: 10.1159/000474934. [DOI] [PMC free article] [PubMed] [Google Scholar]