Highlights
-
•
High-risk HPV infection is less frequently detected in HIV-positive non-metastatic ASCC compared with HIV-negative cases.
-
•
Mutational profile identified in the non-metastatic ASCC cohort revealed expected similarities with metastatic ASCC.
-
•
High PD-L1 expression levels are associated with high CD3 and CD8 TIL density, complete response to treatment, and good survival outcome of ASCC patients.
-
•
PD-L1 is a reliable predictive and prognostic biomarker that allows the stratification in low- vs. high-risk ASCC patients at their initial therapeutic approach.
Keywords: ASCC, HIV, HPV, TIL, PD-L1, HER2/NEU, Targeted DNA sequencing
Abstract
Anal squamous cell carcinoma (ASCC) is a rare gastrointestinal malignancy associated with high-risk Human papillomavirus (HPV) infection. Despite improved outcomes in non-metastatic ASCC, definitive chemoradiotherapy constitutes the standard treatment for localized disease. Evidences for predictive and prognostic biomarkers are limited. Here, we performed a viral, immune, and mutational characterization of 79 non-metastatic ASCC patients with complete definitive chemoradiotherapy. HPV-16 was detected in 91% of positive cases in single infections (78%) or in coinfections with multiple genotypes (22%). Fifty-four percent of non-metastatic ASCC cases displayed mutations affecting cancer driver genes such as PIK3CA (21% of cases), TP53 (15%), FBXW7 (9%), and APC (6%). PD-L1 expression was detected in 57% of non-metastatic ASCC. Increased PD-L1 positive cases (67%) were detected in patients with complete response compared with non-complete response to treatment (37%) (p = 0.021). Furthermore, patients with PD-L1 positive tumors were significantly associated with better disease-free survival (DFS) and overall survival (OS) compared with patients with PD-L1 negative tumors (p = 0.006 and p = 0.002, respectively). PD-L1 expression strongly impacts CR rate and survival of non-metastatic ASCC patients after standard definitive chemoradiotherapy. PD-L1 expression could be used to stratify good versus poor responders avoiding the associated morbidity with abdominal perineal resection.
Introduction
Anal squamous cell carcinoma (ASCC) is a rare disease accounting for approximately 2.5% of all gastrointestinal malignancies, although their incidence is increasing across the developed world [1]. Anal infection with Human papillomavirus (HPV) is the seminal cause in nearly 90% of ASCC, and most HPV-associated cases are caused by HPV-16 followed by other high-risk genotypes (e.g., HPV-18, -31, -33, -45) [2]. As other HPV-driver malignancies, anal cancer risk factors such as HIV-related immunosuppression, number of sexual partners, anal intercourse, and tobacco smoking have been described [3]. However, HPV infection alone is not enough to cause tumorigenesis and additional immune and host-cell genetic factors are involved. Within host tumor cells, HPV oncoproteins are immunogenic and can trigger an anti-tumor immune response by recruiting tumor-infiltrating lymphocytes (TIL) [4]. TIL density has been proposed as a risk factor to stratify HPV-positive ASCC. Low TIL density was found to be significantly associated with a decreased relapse-free rate (63%) compared with ASCC patients with high TIL density (92%) [5]. Mutations affecting cancer driver genes such as PIK3CA and TP53 were also reported in ASCC [6–8]. However, molecular signatures with predictive or prognostic value have not been clearly defined.
Approximately three-quarters of all ASCC patients are diagnosed with localized disease, being definitive chemoradiotherapy (CRT) the primary treatment [9]. CRT based on 5-fluorouracil (5-FU) and mitomycin C remains the standard of care, resulting in high complete response (CR) in 80% of cases with 5-year overall survival (OS) [10,11]. The relapse pattern is mostly locoregional, while distant metastases are seen in approximately 10% of ASCC cases [12]. Clinical prognostic factors associated with poor ASCC outcomes include gender, tumor stage, and incomplete radiotherapy doses [13]. In addition to high-risk HPV infections, TP53 wild-type status and p16 protein overexpression have been proposed as predictive biomarkers of a better response to standard therapy in oropharyngeal and anogenital SCC [14,15]. Nevertheless, not all HPV-positive patients achieve a CR, and local recurrence is reported in approximately 25% of ASCC cases [13]. In other words, we still have no understanding on why only some ASCC respond to CRT whereas others appear not to do requiring an abdominiperineal resection.
The development of targeted therapies and immune checkpoint blockade (ICB) during the last decade has significantly impacted clinical oncology, transforming treatment for various cancer types. The ICB of the PD-1/PD-L1 interaction and the cytotoxic T-lymphocyte antigen 4 (CTLA-4) has achieved significant results in anticancer therapy of melanoma, non-small cell lung cancer, and squamous cell carcinoma of the head and neck (HNSCC) [16,17]. Clinical studies of ICB in ASCC have mainly focused on using the anti-PD-1 antibodies nivolumab and pembrolizumab in patients with chemotherapy-refractory disease with promising results [18,19]. Ongoing chemoimmunotherapy studies are evaluating the combination of docetaxel, cisplatin, and 5-FU with or without the anti-PD-L1 in advanced ASCC. However, ICB can also induce autoimmune-related adverse events [16], and for that, there is a great clinical need to identify those patients how may benefit from this option to avoid overtreatment. In this sense, multifactorial integrative analyses will facilitate a better molecular understanding of rare and complex diseases, and the development of new stratification criteria's and treatment strategies for ASCC patients. This single-institution retrospective study aimed to elucidate the viral (HPV/HIV infection), immune (TIL and PD-L1 expression), and mutational factors of local response and survival for non-metastatic ASCC patients who had undergone definitive CRT treatment.
Materials and methods
ASCC cohort, treatment and follow-up
This retrospective study included eligible consecutive non-metastatic ASCC patients referred to the Oncology Unit at the Gastroenterology Hospital “Dr. Carlos Bonorino Udaondo” (CABA, Argentina) between October 2008 and April 2019. Inclusion criteria were available pre-treatment formalin-fixed paraffin-embedded (FFPE) biopsy, histologically confirmed squamous cell carcinoma, clinical stage I-III TNM disease, and completed definitive CRT as their primary therapeutic approach. CR was determined according to RECIST v1.1 from clinical, ano-rectoscopy and radiological images at 6 months. Patients were staged by anoscopy and digital anal examination, thorax-abdomen CT scan and pelvic MRI. Clinical data collected from medical records included age at diagnosis, gender, disease staging per the American Joint Committee on Cancer (AJCC, 8th edition), HIV-status, type of CRT regimen, treatment response assessment at 6 months, recurrence data, relapse patterns and survival status.
3D-conformal pelvic radiotherapy (50.4 to 54 Gy) was delivered as 28 daily fractions over 5.5 weeks. The three chemotherapy regimens delivered concomitantly with radiotherapy were: 1) mitomycin 12 mg/m² IV bolus, day 1 (maximum dose 20 mg) with 5-FU 1,000 mg/m² on days 1–4 and 29–32 by continuous 24-h IV infusion; 2) mitomycin 12 mg/m² IV bolus, day 1 (maximum dose 20 mg) with capecitabine 825 mg/m² twice daily on each radiotherapy treatment day, and 3) cisplatin 60 mg/m² on days 1 and 29, with 5-FU 1000 mg/m² on days 1–4 and 29–32 by continuous 24-h IV infusion. All HIV-positive patients simultaneously received highly active antiretroviral therapy (HAART).
Clinical follow-up included digital anal examination, anoscopy, and a clinical exam, monthly for 3 months, then every 3 months for the first two years and every 6 months for 3 to 5 years. A thorax CT and abdominal-pelvic MRI imaging were performed every 6 months during follow-up period. Abdominal perineal resection was performed in non-CR patients.
DNA purification and HPV detection and genotyping
DNA was extracted from FFPE primary tumor biopsies using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). DNA quality was evaluated based on the absorbance ratios of A260/280 and A260/230 using a NanoDropTM 2000c Spectrophotometer (Thermo Fisher, MA, USA). DNA quantity was determined using the Qubit® 2.0 Fluorometer with the Qubit® dsDNA HS Assay Kit (Thermo Fisher).
HPV detection was performed using PCR with biotinylated Broad-Spectrum General Primers BSGP5+/GP6+ designed to amplify a 140 bp fragment of the HPV-L1 gene. Genotyping was carried out by reverse line blot hybridization (RLB) which identifies 36 HPV genotypes (Artisan technique, validated by WHO HPV LabNet) [20,21]. Briefly, the denaturated biotynilated amplicons were hybridized with genotype-specific oligonucleotide probes immobilized as parallel lines on membrane strips.
Targeted DNA sequencing and bioinformatic analysis
DNA libraries were prepared with 100 ng of genomic DNA using the AmpliSeqTM for Illumina Cancer Hotspot Panel v2 Kit that allow the detection of 2800 COSMIC mutations from 50 oncogenes and tumor suppressor genes. The DNA library was measured using Qubit® 2.0 Fluorometer with the Qubit® dsDNA HS Assay Kit (Thermo Fisher). All libraries were above the minimum concentration requirement of 2 nM for further sequencing in an Illumina MiSeq platform. Quality control of sequencing data was performed in all samples using the Real-Time Analysis software sequence pipeline from Illumina. Short reads were aligned against the human reference genome (build hs37d5, based on NCBI GRCh37) using the Burrows-Wheeler aligner (BWA-MEM algorithm). Subsequent mutational analysis was performed at mean coverage depth ≥200 reads. Variants were filtered out when the alternative allele depth was lower than 10 reads. The GATK Mutect2 toolkit (https://gatk.broadinstitute.org) was used for single nucleotide variants (SNV) calling. Variant annotation was performed using several resources and databases such as: SnpEff, dbNSFP, PhyloP, SIFT, PolyPhen2, MutationTaster, LRT and CADD. The GnomAD resource (https://gnomad.broadinstitute.org/) was used to evaluate variant frequency in the global population. All mutations were evaluated using the Integrative Genomics Viewer (https://software.broadinstitute.org/software/igv/). In addition, we used CovCopCan software to detect copy number alterations in ASCC samples on the basis of targeted DNA sequencing data (https://git.unilim.fr/merilp02/CovCopCan/). Integrative visualization of genomics and clinical data was performed with Oviz-Bio LandScape resource (https://bio.oviz.org/).
Immunohistochemistry analysis
Immunostaining was performed using a Roche Benchmark XT system and anti-CD3 (Clone 2GV6, Ventana - Roche), anti-CD8 (Clone SP57, Ventana - Roche), anti-HER2/Neu (Clone 4B5, Ventana - Roche) and anti-PD-L1 (Clone SP263, Ventana - Roche) antibodies. Immunostaining were evaluated by two independent qualified pathologists. In four cases of discrepancy, an additional assessment was performed by a third senior pathologist. For CD3 and CD8, average values were obtained from examining all intra and peritumoral areas. A semi-quantitative score was defined; CD3 and CD8 expression was classified according to the percentage of total tumor-related lymphocyte (peritumoral and intratumorally) staining: low (0–34%), moderate (35–64%) and high (65–100%). PD-L1 was evaluated using the Combined Positive Score (CPS) established for gastric/gastroesophageal junction adenocarcinoma [22]. HER2/Neu scoring was performed according to the College of American Pathologists (CAP), which describes three categories: HER2-negative (0;1+), HER2-equivocal (2+) and HER2-positive (3+) [23].
Statistical analysis
The Shapiro-Wilk test was employed for the assessment of normality. To compare categorical data between groups, the Chi-square test or Fisher's exact test were used. Continuous data were compared with the Wilcoxon rank-sum test. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Endpoints were CR rate, DFS and OS. DFS was defined as the time from the first day of CRT until clinical or radiological disease recurrence or death from any cause. OS was defined as the time from treatment initiation to death from any cause. Cox proportional-hazards models were used to determine the association between OS or DFS and predictive variables and were expressed as hazard ratios (HR) with their corresponding 95% confidence intervals (CI). Two-tailed p-values were calculated and p-values < 0.05 were considered as significant. All statistical data analyses were performed using R Statistical Software version 3.6.1.
Results and discussion
Patient cohort and treatment response
Seventy-nine FFPE samples of non-metastatic ASCC patients were analyzed for HPV infection, immune-related markers and their mutational profile. Clinical and demographic data are summarized in Table 1. Thirty patients were treated with mitomycin-capecitabine (38%), 27 patients received cisplatin-5-FU (34%), and 22 patients received mitomycin-5-FU (28%), concomitantly with 3D-pelvic radiotherapy. Fifty-five patients reported CR (70%) at 6 months after initiation of CRT, while partial response was reported in 25 patients (30%). No significant differences were found between mitomycin and cisplatin regimens according to CR rate and DFS (p >0.05). Median follow-up after treatment completion was 35 months (range 6–149 months). None of the clinical variables evaluated (age, gender, stage, differentiation grade or HIV status) were associated with treatment response or disease relapse (p >0.05). Female and HIV-positive patients were significantly associated with worse OS (p = 0.004 and p = 0.018, respectively).
Table 1.
Clinical and demographics data of the ASCC cohort.
| Patient Characteristics | Number of Patients (%)* |
|---|---|
| Median age at diagnosis | 59 years (26–87) |
| Gender | |
| Female | 58 (73%) |
| Male | 21 (27%) |
| T stage | |
| T1 / T2 | 42 (53%) |
| T3 / T4 | 37 (47%) |
| N stage | |
| Positive | 47 (59%) |
| Negative | 32 (41%) |
| Tumor differentiation | |
| Well differentiated | 17 (22%) |
| Moderately differentiated | 39 (49%) |
| Poorly differentiated | 19 (24%) |
| Not available | 4 (5%) |
| HIV infection status | |
| Positive | 11 (14%) |
| Negative | 68 (86%) |
Unless Otherwise Stated.
HPV infection status
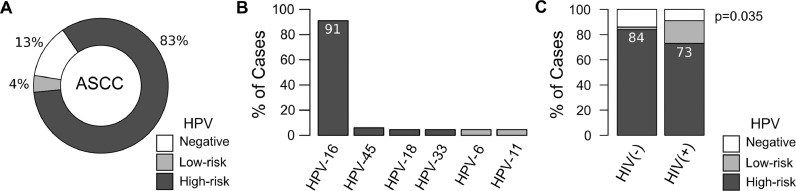
DNAs from 76 out of 79 ASCC samples were suitable for HPV detection and genotyping. HPV-DNA was detected in 87% of ASCC cases (66 out of 76) (Fig. 1a). Whereas high-risk HPV-DNA (types -16, -18, -33 or -45) was detected in 95% of positive samples (63 out of 66), the remaining infected samples were positive for low-risk HPV genotypes (types -6, -11, -42 or -44). The HPV-16 was the most frequent viral genotype, comprising 91% (60 out of 66) of the infected samples (Fig. 1b). Co-infection with two or three HPV genotypes were less frequent than single infection, comprising 18% (12 out of 66) and 3% (2 out of 66) of the infected samples respectively. HPV-16 was detected in 93% of the co-infected samples (13 out of 14). HPV status was not associated with clinicopathological variables, CRT treatment response or survival (p>0.05). However, high-risk HPV infection was less frequent in HIV-positive (73%) than HIV-negative (84%) ASCC cases (p = 0.035) (Fig. 1c).
Fig. 1.
HPV infection among non-metastatic ASCC. (A) Donut chart representing the percentage of HPV-negative and HPV-positive cases (low- and high-risk groups); (B) Distribution of HPV genotypes among ASCC HPV-positive cases. About 22% of the patients harboring HPV-16 were infected with multiple genotypes; (C) Prevalence of HPV-negative, high-risk and low-risk HPV infection according to HIV status.
The high prevalence of HPV infection and HPV-16 genotype in our non-metastatic ASCC cohort are in agreement with previously reported studies [14,[24], [25], [26], [27]]. A very low frequency of other high-risk (HPV-45, -18, -33) and low-risk (HPV-6/11) HPV genotypes were also described in other ASCC series [14,24,27]. HPV-6 and -11 were detected in three samples, confirming that low-risk HPV genotypes might be occasionally associated with anal cancer, as observed in a laser capture microdissection study [28].
The role of HPV infection as a prognostic factor associated with better response to CRT treatment or survival was not confirmed in our ASCC cohort as was reported by other studies [13,15]. This could be associated with the reduce number of ASCC patients without HPV infection (13%) detected in our cohort. Interestingly, high-risk HPV and HPV-16 infections were more frequently associated with HIV-negative patients such as was previously reported [26]. Furthermore, HIV status was not associated with treatment response, but was associated with worse long-term outcomes as was previously demonstrated [29,30].
Mutational profile
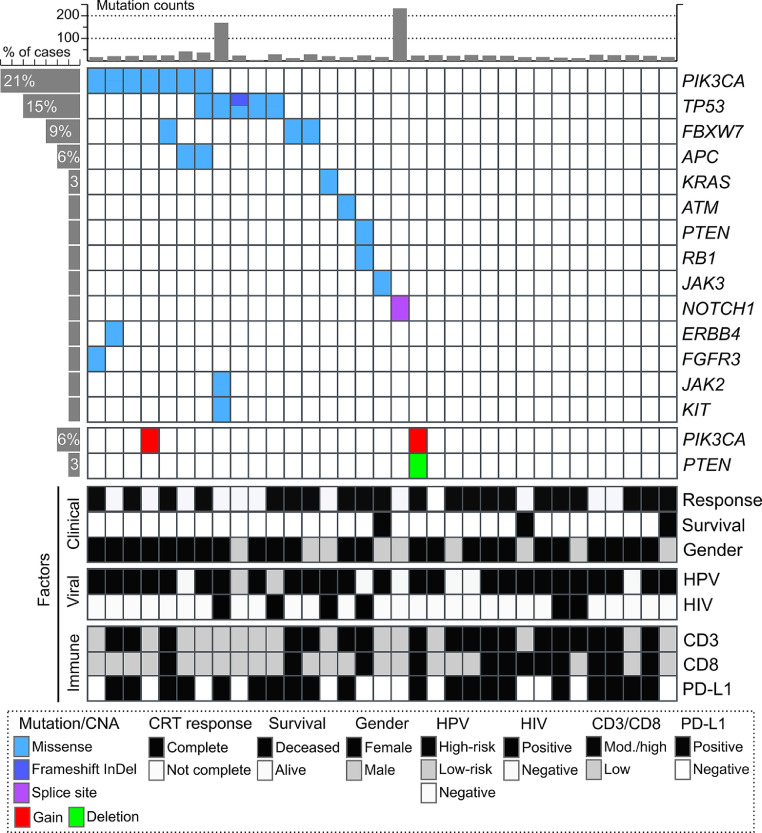
Targeted DNA sequencing was performed in pretreatment biopsies from 44 patients using the Illumina Cancer Hotspot Panel v2, that allow the identification of 2800 COSMIC somatic mutations across 50 cancer driver genes. Thirty-three out of 44 cases had at least 50x coverage and were included for subsequent mutational analysis. We detected 30 somatic mutations among 54% of ASCC cases (18 out of 33) including 28 nonsynonymous substitution, a frameshift InDel and a mutation at splicing sites (Supplementary Data 1). Among these, we detected mutations affecting PIK3CA (21%), TP53 (15%), FBXW7 (9%), APC (6%) and single cases of mutations affecting KRAS, ATM, PTEN, RB1, JAK2, JAK3, NOTCH1, ERBB4, FGFR3 and KIT (Fig. 2). Furthermore, copy number alterations (CNA) were estimated using Targeted-seq data to predict amplicons of genomic amplification or homozygous deletion. Regions of potential focal amplification of PIK3CA and PTEN deletion were identified in only two ASCC cases (Fig. 2), one of these cases harboring both CNAs (Supplementary Fig. 1). No significant CNAs were detected for the remaining ASCC cases using the referenced method.
Fig. 2.
Tile plot showing recurrent altered cancer driver genes (mutated or with CNVs) in non-metastatic ASCC according to CRT treatment response, survival, HPV and HIV infections, and gender. All included cases were HER2- ASCC.
A total of 10 PIK3CA alterations were observed in 8 samples, with two samples harboring multiple alterations (two mutations or mutation and gene amplification), and the majority were known oncogenic mutations (E545K, E542K). While, 7 TP53 alterations were observed in 6 samples, all described as pathogenic or likely pathogenic variants. Almost all PIK3CA altered cases were females patients with HPV-16 infection (7 out of 8), while HPV-low risk genotypes were detected among TP53 mutated cases (2 out of 5). No significant association with clinical variables or response to treatment were observed (p>0.05). However, 71% of PIK3CA/TP53 non-altered cases (15 out of 21) showed complete treatment response, while only 50% of PIK3CA/TP53 altered cases showed complete response (6 out of 12). Interestingly, mutations previously reported by Exome-seq in metastatic ASCC [7] were no detected in an Exome-seq study of localized ASCC [8] and in our non-metastatic ASCC cohort, such as AKT1, BRAF, EGFR, FGFR2 and NRAS. While other mutations affecting FBXW7, ATM, and NOTCH1 appears to be detected in non-metastatic cases (Fig. 2) [8].
In agreement with previous studies, we identified an average low mutation burden in non-metastatic ASCC [6–8], except for two cases with a higher mutational burden, one of them with a TP53 mutation and in which no mutations were detected in the MLH1 mismatch repair gene. These results are also consistent with the somatic mutation rate reported in other HPV-related malignancies such as cervical cancer and HPV-positive head and neck squamous cell carcinoma (HNSCC) [31]. PIK3CA, TP53 and FBXW7 were among the most common mutated gene in agreement with previous ASCC studies [6–8]. Moreover, PIK3CA gene amplification and PTEN homozygous deletion were previously reported as recurrent CNA among ASCC cases [6]. Interestingly, PIK3CA and FBXW7 mutations have also been reported in cervical squamous cell carcinomas and HPV-positive HNSCC [31]. HPV-driven cancers show shared genomic alterations and deregulated signaling pathways. Disruption of the PI3K/AKT/mTOR, TP53 and NOTCH signaling pathways have been reported to occur in both HPV-positive and HPV-negative cases, involving different cancer driver genes and mechanism among groups. For instance, inactivation of the wild type TP53 and RB1 proteins by the HPV oncoproteins E6 and E7 has been found to be common in HPV-positive cases, while inactivation of TP53 and RB1 by mutations are common in HPV-negative cases. Conversely, FBXW7 is a tumor suppressor gene which expression increases following TP53 activation, causes tumorigenesis in wild-type TP53 and FBXW7 mutated mice [32]. In this sense, FBXW7 mutations were exclusively detected in HPV-positive and TP53 wild-type ASCC cases (Fig. 2). No less relevant, FBXW7 causes proteasome-mediated degradation of NOTCH1, mTOR and several cancer driver genes such as CMYC, CCNE and JUN [32]. Mutational inactivation of FBXW7 may promote proliferative and survival signaling through PI3K/AKT/mTOR, TP53 and NOTCH pathways in non-metastatic ASCC. It is important to note that PI3K/AKT/mTOR pathway has been also described as one of the mechanisms that induces activation of the PD1 signaling by PD-L1 upregulation in tumor cells [33]. Interestingly, recent studies suggest that PTEN not only maintains the genomic integrity and controls the PI3K/AKT/mTOR oncogenic pathway but also may be involved in modulating the immune responses independently of PI3K signaling [34]. Further studies need to be performed to elucidate the role of PTEN alterations in the context of the ASCC immune response.
Immune markers, response to treatment and survival
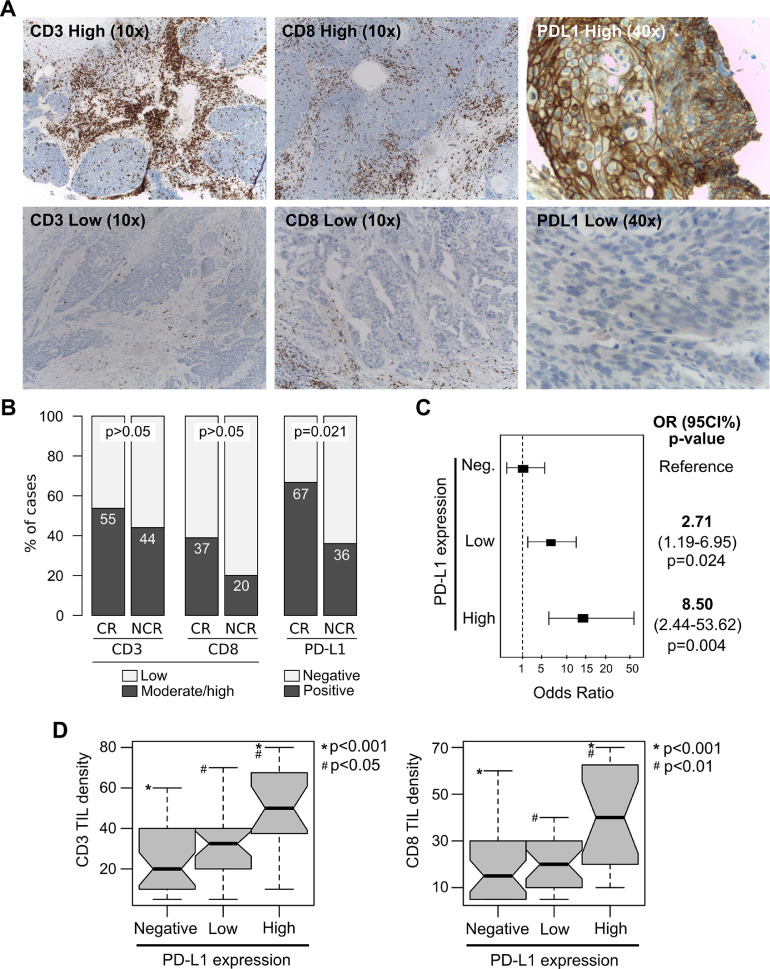
CD3 and CD8 tumor-infiltrating lymphocytes (TILs) were evaluated in all ASCC samples by IHC (Fig. 3a). CD3 and CD8 TILs density was moderate to high in 51% (40 out of 79) and 32% (25 out of 79) of ASCC respectively. Fifty-five percent of patients with CR showed moderate to high CD3 TILs density respect to 44% of NCR cases (p>0.05). While, 37% of patients with CR showed moderate to high CD8 TILs density respect to 20% of NCR cases (p>0.05) (Fig. 3b). When the CD3 and CD8 TIL distribution patterns were evaluated, a peritumoral infiltrating pattern was predominantly detected among moderate to high CD3 and CD8 tumors (80% and 64%, respectively) compared with the intratumoral / mixed patterns (20% and 36%, respectively). No statistically significant differences were detected between the CD3 and CD8 TIL patterns and the CRT treatment response (p>0.05). In addition, not significant association were detected between CD3 and CD8 TIL density and DFS and OS of ASCC patients (p>0.05). Although there was a significant trend towards better DFS based on CD3 TIL median density (p= 0.046).
Fig. 3.
CD3 and CD8 TILs density, and PD-L1 expression analyses among non-metastatics ASCC according to CRT treatment response. (A) Representative immunohistochemistry results for high and low CD3, CD8 and PD-L1 expression (up and down panels respectively); (B) Comparative analysis of immunological markers and response to treatment. CR: Complete Response, NCR: Non-Complete Response; (C) Logistic regression model showing the relationship between high or low PD-L1 expression levels based on CPS grading and the likelihood to achieve CR compared with PD-L1 negative tumors. (D) Box plot of CD3 and CD8 TIL density among high, low and PD-L1 negative cases.
The PD-L1 expression status was evaluated in all ASCC cases using the Combined Positive Score (CPS). PD-L1 positivity (CPS>1%) was detected in 57% of ASCC cases (45 out of 79). High PD-L1 expression levels (CPS>10%) were detected in 42% of positive cases (19 out of 45), while the remaining samples showed low PD-L1 expression levels (CPS<10%) (26 out of 45). Similar PD-L1 expression levels have been previously described in metastatic disease [18,19]. An extremely high PD-L1 expression (CPS>50%) was observed in 13% of positive samples (6 out of 45). Interestingly, high PD-L1 expression was more frequently detected in women (31%, 18 out of 58) than in men (5%, 1 out of 21) (p= 0.016). Respect to the pattern of PD-L1 expression, 49% of samples showed immunostaining in tumor cells only (22 out of 45), 31% in immune cells only (14 out of 45), and 20% showed mixed pattern (9 out of 45) with predominant staining in tumor cells (Fig. 3a). Similar results were previously reported demonstrating that PD-L1 protein was more expressed by tumor cells than in immune cells respect to the local Immune response to ASCC [35]. When the frequency of PD-L1 positive cases was compared considering the CRT response at 6 month, significant differences were detected between CR (67%, 36 out of 54) and NCR patients (36%, 9 out of 25) (p= 0.021) (Fig. 3b). Furthermore, patients with high PD-L1 expression levels (CPS≥10%) showed an 8-fold increased likelihood of having a CR compared to those with PD-L1 negative cases (OR=8.50, 95% CI=2.44-53.62; p= 0.004) (Fig. 3c). While patients with low PD-L1 expression levels (CPS<10%) showed a 2.7-fold increased likelihood of having a CR compared with reference cases (OR=2.71 95%CI=1.19-6.95; p=0.024). Moreover, increased CD3 and CD8 TILs were detected in high PD-L1 expressing tumors compared with low PD-L1 (p<0.05) and PD-L1 negative (p<0.001) tumors (Fig. 3d). A previous study in metastatic ASCC patients demonstrated that anti-PD-1 responders had higher percentages of CD8 TIL density and granzyme B, than non-responders. Similarly, PD-L1 was expressed on more than 40% of tumor cells analyzed from the patients who responded to anti-PD-1, higher than non-responders [19].
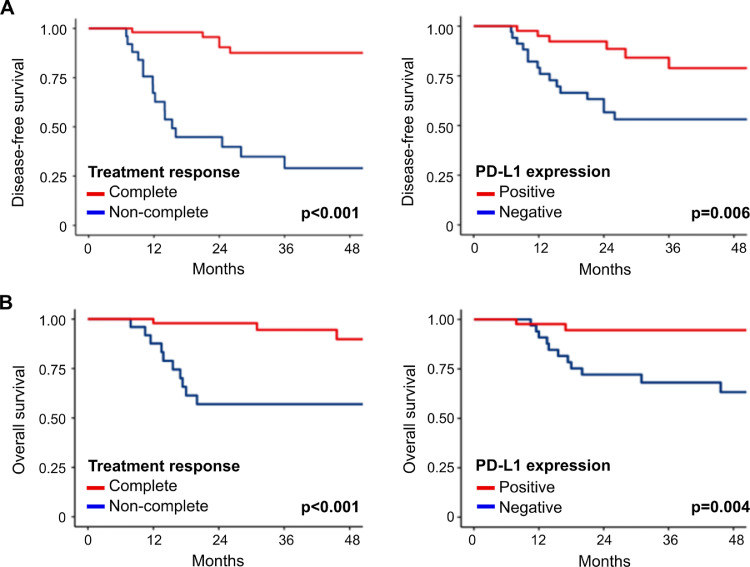
The median follow-up after treatment completion was 35 months. As expected, the complete response to treatment was a reliable surrogate marker of DFS (HR=0.10 95%CI=0.04-0.28; p<0.001) and OS (HR=0.12 95%CI=0.03-0.45; p<0.001) (Fig. 4a). More importantly, patients with PD-L1 positive tumors (CPS>1%) showed a significantly better DFS (HR=0.28 95%CI=0.11-0.73; p= 0.006) and OS (HR=0.15 95%CI=0.03-0.68; p<0.004) compared with patients with PD-L1 negative tumors (Fig. 4b). These results showed that PD-L1 was a strong predictive and prognostic biomarker for ASCC in agreement with previous studies [27,34], and suggest that immune checkpoints inhibitors may be considered as molecular targeted therapy for non-metastatic ASCC cases. Finally, When HER2/Neu immunodetection was assessed, a single case of expression was detected in a patient without residual tumor after definitive CRT (1 out 79 cases) (Supplementary Fig. 2), which has not been previously described in ASCC.
Fig. 4.
Survival analysis of non-metastatic ASCC patients according to the treatment response and PD-L1 expression. (A) DFS analysis of ASCC patients according to the CRT treatment response and PD-L1 expression. (B) OS analysis of ASCC patients according to CRT treatment response and PD-L1 expression. Survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Cox proportional-hazards models were used to determine the association between OS or DFS and predictive variables and were expressed as hazard ratios (HR) with their corresponding 95% confidence intervals (CI).
Conclusions
Comprehensive characterization of non-metastatic ASCC at viral, immune, and mutational levels allowed us to identify the most relevant changes in the context of CRT response and survival. A comparison of the mutational profile identified in this non-metastatic cohort with previous studies revealed expected similarities with metastatic ASCC. More importantly, our finding indicates that high PD-L1 expression levels were associated with high CD3 and CD8 TIL density, complete response to treatment, and good survival outcome of ASCC patients. Given the severe impact of the abdominal perineal resection, there is a great clinical need to identify those patients with no benefit from the primary therapeutic approach to avoid side effects that may severely impair their quality of life. Thus, PD-L1 could be an upfront reliable predictive and prognostic biomarker to stratify low- vs. high-risk patients at the initial therapeutic approach in non-metastatic ASCC. Finally, the increased PD-L1 expression on tumor cells of ASCC provides the rationale for implementing therapeutic strategies targeting the PD-1/PD-L1 axis.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethic Committee of the Gastroenterology Hospital “Dr. Carlos Bonorino Udaondo” (V.2.0 10-03-19). Informed consents were obtained for all patients included in this study (V.1.0 10-03-19).
Authors’ contributions
All the authors have directly participated in the preparation of this manuscript and have read and approved the final version submitted and declare no ethical conflicts of interest.
S.I., M.G., J.R., G.R., R.S., M.P., E.R.: Conceptualization; S.I., M.G., J.R., R.S., A.C., M.P., J.G., J.B.,J.M.: methodology; S.I., M.G., J.M., M.C.A.: validation, visualization and formal analysis; S.I., M.G., J.R., G.R., J.M., M.P., M.C.: investigation and data curation; S.I., M.G., J.R., J.M., M.C.A., F.G, J.V.G., J.B, M.A.P, M.C, M.E.: writing- original draft preparation; S.I., M.G., J.R., G.R., F.G., J.M., R.S., A.C, M.E, J.V.G., J.B., M.A.P, G.M., M.C., E.R., G.M., M.C., M.C.A.: writing- review and editing; S.I., M.C.A., E.R.: funding acquisition.
Funding
This research was funded by Foundation Nelia and Amadeo Barletta (FNAB) and NIH grant CA221208.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the patients who participated in this research and their relatives for their time, altruism, and generosity. We also thank Dr. Esteban Cvitkovic for the critical reading of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101084.
Contributor Information
Soledad Iseas, Email: mabba@med.unlp.edu.ar.
Martin C. Abba, Email: mcabba@gmail.com.
Appendix. Supplementary materials
References
- 1.Siegel R., Ward E., Brawley O., Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.De Martel C., Plummer M., Vignat J., Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Martel C., Shiels M.S., Franceschi S., Simard E.P., Vignat J., Hall H.I., Engels E.A., Plummer M. Cancers attributable to infections among adults with HIV in the United States. AIDS. 2015;29:2173. doi: 10.1097/QAD.0000000000000808. (London, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth M.J., Dunn G.P., Schreiber R.D. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert D.C., Serup-Hansen E., Linnemann D., Høgdall E., Bailey C., Summers J., Havsteen H., Thomas G.J. Tumor-infiltrating lymphocyte scores effectively stratify outcomes over and above p16 post chemo-radiotherapy in anal cancer. Br. J. Cancer. 2016;114:134–137. doi: 10.1038/bjc.2015.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung J.H., Sanford E., Johnson A., Klempner S.J., Schrock A.B., Palma N.A., Erlich R.L., Frampton G.M., Chalmers Z.R., Vergilio J. Comprehensive genomic profiling of anal squamous cell carcinoma reveals distinct genomically defined classes. Ann. Oncol. 2016;27:1336–1341. doi: 10.1093/annonc/mdw152. [DOI] [PubMed] [Google Scholar]
- 7.Morris V., Rao X., Pickering C., Foo W.C., Rashid A., Eterovic K., Kim T., Chen K., Wang J., Shaw K. Comprehensive genomic profiling of metastatic squamous cell carcinoma of the anal canal. Mol. Cancer Res. 2017;15:1542–1550. doi: 10.1158/1541-7786.MCR-17-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trilla-Fuertes L, Ghanem I, Maurel J, Laura G, Mendiola M, Peña C, López-Vacas R, Prado-Vázquez G, López-Camacho E, Zapater-Moros A, Heredia V. Comprehensive characterization of the mutational landscape in localized anal squamous cell carcinoma. Transl. Oncol. 2020;13 doi: 10.1016/j.tranon.2020.100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glynne-Jones R., Nilsson P.J., Aschele C., Goh V., Peiffert D., Cervantes A., Arnold D. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25 doi: 10.1093/annonc/mdu159. iii10-20. [DOI] [PubMed] [Google Scholar]
- 10.James R.D., Glynne-Jones R., Meadows H.M., Cunningham D., Myint A.S., Saunders M.P., Maughan T., McDonald A., Essapen S., Leslie M. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomized, phase 3, open-label, 2×2 factorial trial. Lancet Oncol. 2013;14:516–524. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 11.Northover J., Glynne-Jones R., Sebag-Montefiore D., James R., Meadows H., Wan S., Jitlal M., Ledermann J. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomized UKCCCR anal cancer trial (ACT I) Br. J. Cancer. 2010;102:1123–1128. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shakir R., Adams R., Cooper R., Downing A., Geh I., Gilbert D., Jacobs C., Jones C., Lorimer C., Namelo W.C. Patterns and predictors of relapse following radical chemoradiation therapy delivered using intensity modulated radiation therapy with a simultaneous integrated boost in anal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020;106:329–339. doi: 10.1016/j.ijrobp.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parwaiz I., MacCabe T.A., Thomas M.G., Messenger D.E. A systematic review and meta-analysis of prognostic biomarkers in anal squamous cell carcinoma treated with primary chemoradiotherapy. Clin. Oncol. 2019;31:e1–e13. doi: 10.1016/j.clon.2019.06.013. (R Coll Radiol) [DOI] [PubMed] [Google Scholar]
- 14.Serup-Hansen E., Linnemann D., Skovrider-Ruminski W., Høgdall E., Geertsen P.F., Havsteen H. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on cancer stages I to III carcinoma of the anal canal. J. Clin. Oncol. 2014;32:1812–1817. doi: 10.1200/JCO.2013.52.3464. [DOI] [PubMed] [Google Scholar]
- 15.Soares P.C., Abdelhay E.S., Thuler L.C.S., Soares B.M., Demachki S., Ferro G.V.R., Assumpção P.P., Lamarão L.M., Ribeiro Pinto L.F., Burbano R.M.R. HPV positive, wild type TP53, and p16 overexpression correlate with the absence of residual tumors after chemoradiotherapy in anal squamous cell carcinoma. BMC Gastroenterol. 2018;18:30. doi: 10.1186/s12876-018-0758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieder T, Eigentler T, Brenner E, Röcken M. Immune checkpoint blockade therapy. J. Allergy Clin. Immunol. 2018;142:1403–1414. doi: 10.1016/j.jaci.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 17.von der Grün J, Rödel F, Brandts C, Fokas E, Guckenberger M, Rödel C, Balermpas P. Targeted therapies and immune-checkpoint inhibition in head and neck squamous cell carcinoma: where do we stand today and where to go? Cancers. 2019;11(4):472. doi: 10.3390/cancers11040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ott P.A., Piha-Paul S.A., Munster P., Pishvaian M.J., van Brummelen E.M.J., Cohen R.B., Gomez-Roca C., Ejadi S., Stein M., Chan E. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann. Oncol. 2017;28:1036–1041. doi: 10.1093/annonc/mdx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris V.K., Salem M.E., Nimeiri H., Iqbal S., Singh P., Ciombor K., Polite B., Deming D., Chan E., Wade J.L. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicenter, single-arm, phase 2 study. Lancet Oncol. 2017;18:446–453. doi: 10.1016/S1470-2045(17)30104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt M., Dondog B., Waterboer T., Pawlit M. Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5+ and GP6+ primers. J. Clin. Microbiol. 2008;46:1050–1059. doi: 10.1128/JCM.02227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eklund C., Forslund O., Wallin K.L., Dillner J. Continuing global improvement in human papillomavirus DNA genotyping services: the 2013 and 2014 HPV LabNet international proficiency studies. J. Clin. Virol. 2018;101:74–85. doi: 10.1016/j.jcv.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Technologies Agilent. PD-L1 IHC 22C3 pharmDx is FDA-approved for in vitro diagnostic use, Interpretation manual – gastric or gastroesophageal junction adenocarcinoma. DAKO Agil. Pathol. Solut. 2018:1–71. [Google Scholar]
- 23.Bartley A.N., Christ J., Fitzgibbons P.L., Hamilton S.R., Kakar S., Shah M.A., Tang L.H., Troxell M.L. Template for reporting results of HER2 (ERBB2) biomarker testing of specimens from patients with adenocarcinoma of the stomach or esophagogastric junction. Arch. Pathol. Lab. Med. 2015;139:618–620. doi: 10.5858/arpa.2014-0395-CP. [DOI] [PubMed] [Google Scholar]
- 24.Abramowitz L., Jacquard A.C., Jaroud F., Haesebaert J., Siproudhis L., Pradat P., Aynaud O., Leocmach Y., Soubeyrand B., Dachez R. Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study. Int. J. Cancer. 2011;129:433–439. doi: 10.1002/ijc.25671. [DOI] [PubMed] [Google Scholar]
- 25.Valmary-Degano S., Jacquin E., Prétet J.L., Monnien F., Girardo B., Arbez-Gindre F., Joly M., Bosset J.F., Kantelip B., Mougin C. Signature patterns of human papillomavirus type 16 in invasive anal carcinoma. Hum. Pathol. 2013;44:992–1002. doi: 10.1016/j.humpath.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Lin C., Franceschi S., Clifford G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 2018;18:198–206. doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wessely A., Heppt M.V., Kammerbauer C., Steeb T., Kirchner T., Flaig M.J., French L.E., Berking C., Schmoeckel E., Reinholz M. Evaluation of PD-L1 expression and HPV genotyping in anal squamous cell carcinoma. Cancers. 2020;12:2516. doi: 10.3390/cancers12092516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornall A.M., Roberts J.M., Garland S.M., Hillman R.J., Grulich A.E., Tabrizi S.N. Anal and perianal squamous carcinomas and high-grade intraepithelial lesions exclusively associated with “low-risk” HPV genotypes 6 and 11. Int. J. Cancer. 2013;133:2253–2258. doi: 10.1002/ijc.28228. [DOI] [PubMed] [Google Scholar]
- 29.Camandaroba M.P.G., Iseas S., Taboada R.G., Oliveira C.P., Mauro C.D.C., Xerfan M.P., Barros M., Jesus V.H.F.de, Felismino T.C., Mello C.A. Timing to achieve complete response (CR) after definitive chemoradiotherapy (ChRT) in patients with squamous cell carcinoma of the anal (SCCAC) with and without HIV infection: a multicenter retrospective study. Ann. Oncol. 2019;30:v204. doi: 10.1093/annonc/mdz246.015. [DOI] [Google Scholar]
- 30.Camandaroba M.P.G., de Araujo R.L.C., Silva V.S.E., de Mello C.A.L., Riechelmann R.P. Treatment outcomes of patients with localized anal squamous cell carcinoma according to HIV infection: systematic review and meta-analysis. J. Gastrointest. Oncol. 2019;10:48–60. doi: 10.21037/jgo.2018.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuna M., Amos C.I. Next generation sequencing and its applications in HPV associated cancers. Oncotarget. 2017;8:8877. doi: 10.18632/oncotarget.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh C.H., Bellon M., Nicot C. FBXW7: a critical tumor suppressor of human cancers. Mol. Cancer. 2018;17:115. doi: 10.1186/s12943-018-0857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y., Chen W., Xu Z.P., Gu W. PD-L1 distribution and perspective for cancer immunotherapy—blockade, knockdown, or inhibition. Front Immunol. 2019;10:2022. doi: 10.3389/fimmu.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vidotto T., Melo C.M., Castelli E., Koti M., Dos Reis R.B., Squire J.A. Emerging role of PTEN loss in evasion of the immune response to tumors. Br. J. Cancer. 2020;122:1732–1743. doi: 10.1038/s41416-020-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanik E.L., Kaunitz G.J., Cottrell T.R., Succaria F., McMiller T.L., Ascierto M.L., Esandrio J., Xu H., Ogurtsova A., Cornish T. Association of HIV status with local immune response to anal squamous cell carcinoma: implications for immunotherapy. JAMA Oncol. 2017;3:974–978. doi: 10.1001/jamaoncol.2017.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.