Abstract
While evidence suggests an increasing incidence of tongue cancer in young adults, published findings regarding the prognostic role of age at diagnosis are inconsistent. We performed a meta‐analysis of the literature to highlight key points that might help in understanding the association between age of oral tongue cancer patients at diagnosis and their prognosis. According to age at diagnosis, a systematic literature review of all published cohort studies assessing the recurrence risks and mortality associated with tongue cancer was conducted. We compared the risk estimates between patients aged >45 years and those aged <45 years at diagnosis. Random‐effects models were used to calculate summary relative risk estimates (SRRs) according to different clinical outcomes and sources of between‐study heterogeneity (I2) and bias. We included 31 independent cohort studies published between 1989 and 2019; these studies included a total of 28,288 patients. When risk estimations were not adjusted for confounders, no significant association was found between age at diagnosis and overall survival (OS). Conversely, after adjustment for confounders, older age at diagnosis was associated with a significantly increased risk of mortality. The difference between SRRs for adjusted and unadjusted estimates was significant (p < 0.01). Younger patients had a significantly higher risk of local recurrence. Younger patients with oral tongue cancer have better OS but a greater risk of recurrence than older patients. These findings should be validated in a large prospective cohort study which considers all confounders and prognostic factors.
Keywords: age, meta‐analysis, oral cavity cancer, survival, tongue cancer, young adults
Although oral tongue cancer is generally considered to affect older patients, increasing evidence suggests an increasing incidence in younger patients. The study results illustrated that age at diagnosis had varying effects on the risks of poor outcomes and local recurrence. We suggest that younger patients with oral tongue cancer should receive personalized follow‐up plans to improve prognosis by identifying disease recurrence at an early stage.

1. INTRODUCTION
Oral cavity cancer (OCC) is the most common malignancy of the head and neck region, as reported by the Global Cancer Observatory (GLOBOCAN). 1 The incidence of new OCC is expected to exceed 29/100,000 persons worldwide by 2030 in both men and women at all ages (http://gco.iarc.fr/tomorrow). 2
In light of these epidemiological data, Authors show an increasing interest about OCC and oral tongue squamous cell cancer (OTSCC), because of the crucial role of this anatomical region and the impact of therapies and treatments on swallowing and respiration. 2 , 3 , 4 , 5 , 6 More recently, the authors have voiced concern about an increasing trend of the incidence of OSTCC among the “young population,” these data are reported for Scotland, the US, and Northern Europe in addition to India, China, and South Korea, although an unequivocal definition of this subgroup of patients has not been released yet and globally accepted. 3 , 7 , 8 , 9 , 10 , 11 , 12 In the “young population,” the female group under the age of 45 seems more affected, with gender differences between female and male patients regarding the age at diagnosis and tumor site 3 , 12 , 13 Focusing in the whole female group, older women (>70 years) seem to be more affected than younger women, suggesting a cumulative etiological effect leading to cancer. Male patients with an age of 51 to 60 years are mostly affected by buccal mucosa carcinoma, while female patients mainly develop oral tongue tumors. However, there are no differences in OS between male and female patients, suggesting that patients with OSCC have similar survival conditions. 13
Typically, alcohol consumption, cigarette smoking, and betel nut chewing are prominently males' habits. This could be one explanation for the earlier age at diagnosis of OSCC in male patients, compared with females. 14 , 15
Tongue cancer has always been considered to affect primarily middle‐aged men, and it has been associated with tobacco and alcohol use. 10 , 16 In Southeast Asia, OSCC is associated with chewing betel nut, a traditional habit. 10 , 16 Nevertheless, compared with older patients, young patients have no history of tobacco or alcohol abuse. Furthermore, the time of exposure to these well‐known risk factors may not have been sufficient to promote malignant transformation, suggesting the involvement of other external or internal factors in the development of tongue cancer in this population. 17 , 18 , 19 , 20 , 21 In the past years, an increasing incidence of TSCC has been registered in those countries where the primary prevention campaign to stop smoking and drinking has been active, thus suggesting the possible emerging role of new etiological or genetic factors driving carcinogenesis. 5 , 10 , 11 , 22 , 23 , 24
More recently, the authors have investigated other independent risk factors for OSCC in young patients as chronic mucosal trauma, poor oral hygiene, or inadequate dental status. 25 , 26
By studying the genomic profiles, similar mechanisms of tumorigenesis were discovered to be very similar among young and elderly OTSCC patients. 27 , 28 , 29
Nowadays, the role of human papillomavirus (HPV) for oral tongue cancer appears undefined, but yet different from the oropharynx. 30 , 31 Dietary nutrients, specifically fruits and vegetables, have been consistently associated with lower oral cancer risk. 32
In summary, exposure to tobacco, excessive alcohol consumption, and a diet with low intake of fruits and vegetables combined with poor oral hygiene, significantly increase the risk of oral cancer in the whole population.
The women subgroup OTSCC is not often associated with HPV infection, tobacco, and alcohol consumption. Since this disease does not appear to be related to dietary habits, it may be considered a new emerging and distinct clinical entity. 13 , 23
In young adults, the role of prognostic factors such as genetic or histopathological alterations has been studied. 33 , 34 , 35 In this regard, the discussion has focused on the role of unknown risk and prognostic factors in young patients with OTSCC. Several authors have reported that compared with older patients, younger patients have worse outcomes in overall survival (OS), disease‐free survival (DFS), or recurrence rate, 17 , 18 , 19 , 36 , 37 with women having a higher risk of poor outcomes than men. 19 , 23 For instance, Park et al. revealed that patients aged <45 years at diagnosis of advanced‐stage oral cancer have a worse overall regional recurrence and DFS rates than their older counterparts. 38 Conversely, other authors have reported that younger patients have similar or better prognoses than older patients. 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 Therefore, the predictive value of age must be studied to optimize the treatment of OTSCC in young patients.
In the present study, we performed a comprehensive meta‐analysis of all published data regarding the prognosis of younger patients (≤45 years) versus older patients (>45 years) to better understand the role of age at diagnosis in the prognosis of OTSCC.
2. METHODS
We performed a systematic literature review and searched for studies that assessed the impact of age at diagnosis on the prognosis of patients with OTSCC. We conducted a meta‐analysis to evaluate the association of age at diagnosis with DFS (local, regional, or distant recurrence) and OS. This study was conducted according to the Meta‐analysis of Observational Studies in Epidemiology guidelines.
2.1. Information sources and search strategy
Scientific literature published up to June 2019 was searched using the Medline, Embase, and Web of Science databases by three independent reviewers (MT, LS, and RDB) to identify papers, and relevant data were extracted. Disagreements among reviewers were resolved by discussion. The search strategy was consistent across the databases, and it was performed using the following keywords: “Tongue Neoplasms” [MeSH Major Topic] AND (young OR younger OR older OR elderly OR age). To ensure that all studies assessing any outcome of interest were captured, no selective keywords referring to the outcome were introduced in the search strategy. Cross‐referencing from relevant studies was performed to confirm the retrieval of all possible studies. There were no restrictions in the search in terms of the year of publication or language.
2.2. Selection of articles
The inclusion criteria for this meta‐analysis were as follows:
-
▪
Studies that investigated the prognosis of oral tongue cancer, with the anatomical sites being the anterior two‐thirds, dorsal surface, tip, and lateral border of the tongue.
-
▪
Studies must be independent to avoid giving double weight to estimates derived from the same study.
-
▪
Studies must present risk estimates or data to extract risk estimates by age.
-
▪
Studies must include data on at least one of the following clinical outcomes: local recurrence‐free survival (LRFS), regional recurrence‐free survival (RRFS), distant recurrence‐free survival (DRFS), DFS, disease‐specific survival (DSS), or OS.
-
▪
Studies must present adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) comparing outcomes among age groups: younger patients (≤45 years) versus older patients (>45 years); if adjusted HRs were not presented, then eligible studies had to provide sufficient information to estimate either the HR or the relative risk (RR) and the corresponding standard errors and 95% CIs.
-
▪
Studies wherein all patients were treated according to their tumor stage and in accordance with internationally recognized guidelines. 49
2.3. Exclusion criteria
The exclusion criteria for this meta‐analysis were as follows:
-
▪
Studies with an unspecified definition of “tongue cancer,” with no distinction between the base of the tongue and mobile tongue
-
▪
Studies examining the base of the tongue because cancer at this location is formally considered oropharyngeal tongue cancer
2.4. Statistical analysis
All association and corresponding 95% CIs were log‐transformed, and the corresponding variance and standard error were calculated using the formula proposed by Greenland. 50 When estimates were not available from the paper, we calculated them from the published crude data in terms of RRs. Woolf's formula was used to obtain the standard error of log RR. Finally, if only survival curves were presented, then HR and its log‐transformed value and standard error were indirectly extracted using Parmar's method. 51
The association of age group with outcomes across studies was computed as summary RRs (SRRs) with 95% CIs by pooling the study‐specific estimates using a random‐effects model fitted using the R statistical software (version 3.6.0). These models provided estimates adjusted for the potential correlation within studies as well as the heterogeneity among studies.
The homogeneity of the effect across studies for large samples was assessed using Cochrane's Q test, which is approximately distributed as χ2 statistic, for which p < 0.10 was used to indicate the lack of homogeneity among effects. I2 statistic was also calculated to quantify the percentage of total variation across studies attributable to the study's heterogeneity rather than to the chance. 52 The methods reported by Sterne et al. was used to assess publication bias. 53
Sensitivity, subgroup, and meta‐regression analyses were conducted to investigate heterogeneity among studies focusing on the study characteristics, such as country, the cutoff for age, main tumor stage of included patients, year of publication, and adjustment for confounders and other prognostic factors.
3. RESULTS
3.1. Description of the retrieved studies
A total of 3678 studies were retrieved using the aforementioned keywords, and of these, 279 were related to OTSCC, young age, and prognosis and were considered for further scrutiny. We excluded studies that did not meet the previously defined inclusion criteria.
In particular, we filtered out studies that did not specifically address mobile oral cancer, studies that had a cutoff age at diagnosis of OTSCC other than 40 or 45 years, review articles, case reports, and editorials. After applying these inclusion/exclusion criteria, 44 studies were considered potentially eligible. Two additional studies that used an age cutoff of 30 years were forced to be included because of the reported data's strong validity. We then excluded studies with insufficient statistical information to estimate the risk of recurrence or mortality according to age (15 full‐text articles were excluded). Details of the excluded studies are summarized in PRISMA flowchart (Figure 1).
FIGURE 1.
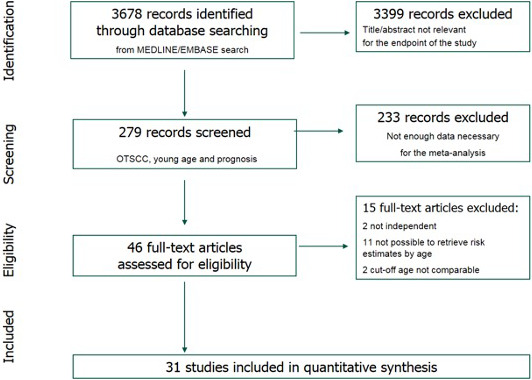
PRISMA flow chart.
Finally, we included 31 independent studies published between 1989 and 2019; these studies included a total of 28,288 patients (Table 1). Of the 31 studies, six were prospective, and the remaining were retrospective. The studies were conducted in different regions as follows: 5, 9, 9, 3, 2, 1, 1, and 1 in Europe, the USA, Asia, Israel, Australia, Saudi Arabia, Canada, and Brazil, respectively. The largest study was also the most recent one, four conducted in 22,930 patients in the USA. Some studies presented risk estimates adjusted for confounders or other prognostic factors [sex, tumor–node–metastasis (TNM) stage, smoking status, alcohol consumption, ethnicity, adjuvant therapy, lymphatic and vascular invasion, surgical treatment, comorbidities, and surgical margins].
TABLE 1.
Descriptive characteristics of the included cohort studies
| First author, PY | Study design | Country | Cutoff age (years) | No. of patients | T stage | N stage | Outcomes |
|---|---|---|---|---|---|---|---|
| Jones, 1989 54 | NA | Canada | 40 | 71 | NA | NA | OS |
| Siegelman, 1998 48 | R | USA | 45 | 87 | Any | Any | OS, DFS |
| Friedlander, 1998 42 | R | USA | 40 | 72 | Any | Any | DFS |
| Yoshida, 1999 55 | NA | Japan | 40 | 568 | T1, T2 | N0 | OS, DSS |
| Al Rajhi, 2000 56 | NA | Saudi Arabia | 45 | 85 | T1, T2 | N0 | OS, DSS |
| Vargas, 2000a 46 | R | USA | 40 | 34 | Any | Any | OS, DFS |
| Pitman, 2000 43 | R | USA | 40 | 272 | Any | Any | DFS |
| Davidson, 2001 57 | P | USA | 40 | 819 | NA | NA | OS, DSS |
| Veness, 2003 47 | R | Australia | 40 | 164 | Any | Any | OS, DFS |
| Hyam, 2003 36 | R | Australia | 40 | 144 | Any | Any | OS |
| Popovtzer, 2004 39 | P/R | Israel | 45 | 48 | T1, T2 | Any | OS, DSS |
| Liao, 2006 45 | P | Korea | 40 | 296 | Any | Any | OS, DSS, DFS |
| Garavello, 2007 17 | P | Italy | 40 | 138 | Any | Any | OS, DSS, DFS |
| Lee, 2007 58 | R | Taiwan | 45 | 40 | Any | Any | OS, DSS |
| Lim, 2007 59 | R | Korea | 45 | 32 | T2 | N0 | OS, DSS |
| Yang, 2008 60 | P | China | 40 | 229 | Any | Any | OS |
| Park, 2010 38 | R | Korea | 40 | 85 | Any | Any | OS, DSS |
| Soudry, 2010 44 | R | Israel | 30 | 85 | Any | Any | OS, DSS, DFS |
| Kies, 2012 61 | R | USA | 40 | 23 | T2, T3 | N0–N2 | OS, DSS |
| Kabeya, 2012 62 | R | Japan | 40 | 32 | Any | Any | OS, DFS |
| Hilly, 2013 18 | R | Israel | 30 | 78 | Any | Any | OS, DSS, DFS |
| Chen, 2016 63 | P | Taiwan | 40 | 128 | Any | Any | DSS |
| Sgaramella, 2015 64 | NA | Italy, Sweden | 40 | 129 | Any | Any | OS |
| Santana, 2017 21 | R | Brazil | 45 | 82 | Any | Any | DSS, DFS |
| Mroueh, 2017 41 | R | Finland | 40 | 563 | Any | Any | OS, DSS |
| Cassidy, 2017 65 | R | USA | 45 | 180 | Any | N0 | OS |
| Blanchard, 2017 40 | R | France | 40 | 100 | Any | Any | OS, DSS |
| Knopf, 2015 66 | R | Germany | 45 | 276 | Any | Any | OS, DSS |
| Chen, 2018 67 | R | China | 45 | 101 | Any | Any | OS |
| Farquhar, 2018 68 | R | USA | 45 | 397 | Any | Any | OS, DSS |
| Oliver, 2019 4 | R | USA | 40 | 22,930 | Any | Any | OS |
PY, Publication year; P, prospective; R, retrospective; NA, not available; DFS, disease‐free survival; DSS, disease‐specific survival; OS, overall survival. DFS may also include local recurrence‐free survival, regional recurrence‐free survival, and distant recurrences‐free survival.
Study included only women.
3.2. Meta‐analysis results
Overall, we found no association of age with OS and DSS, but pooled estimates were significantly different between crude and adjusted HRs (forest plots in Figures 2 and 3; p < 0.01, Table 2). Summary HR estimates from multivariate models adjusted for confounders indicated a nearly twofold increase in the risk of mortality in older patients versus younger patients (SRR = 1.93, 95% CI = 1.52–2.45), with no significant between‐study heterogeneity (I2 = 41.5%). Although statistically insignificant, the inverse association was found for unadjusted risk estimates, with older age being associated with an approximately 10% reduction in the risk of mortality (SRR = 0.88, 95% CI = 0.71–1.09, I2 = 35.5%), and a similar trend was found for DFS.
FIGURE 2.
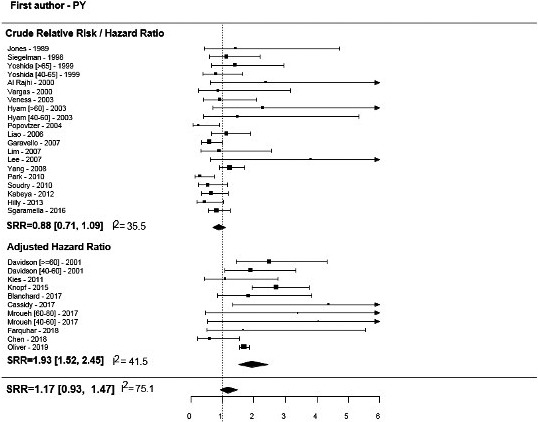
Overall survival for older versus younger OTSCC patients.
FIGURE 3.
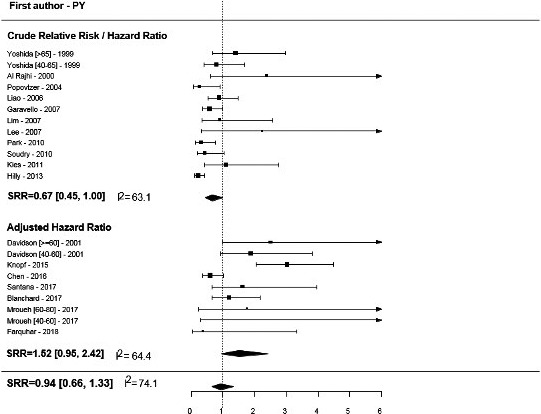
Disease‐specific free survival for older versus younger OTSCC patients.
TABLE 2.
Summary relative risk for older vs. younger patients from random‐effects models by tumor outcome and after adjustment for confounders of the risk estimates
| No. of estimates | SRR | Lower 95% CI | Upper 95% CI | p‐value | I2 (%) | ||
|---|---|---|---|---|---|---|---|
| OS | Overall | 31 | 1.17 | 0.93 | 1.47 | 76 | |
| Crude | 20 | 0.88 | 0.71 | 1.09 | <0.01 | 35 | |
| Adjusted | 11 | 1.93 | 1.52 | 2.45 | 41 | ||
| DSS | Overall | 21 | 0.94 | 0.66 | 1.33 | 74 | |
| Crude | 12 | 0.67 | 0.45 | 1.00 | 0.01 | 63 | |
| Adjusted | 9 | 1.52 | 0.95 | 2.42 | 64 | ||
| DFS | Crude | 11 | 0.76 | 0.6 | 0.95 | 31 | |
| LRFS | Crude | 12 | 0.76 | 0.63 | 0.92 | 0 | |
| RRFS | Crude | 12 | 0.75 | 0.48 | 1.17 | 73 | |
| DRFS | Crude | 12 | 0.60 | 0.27 | 1.35 | 66 | |
P‐value from meta‐regression
SRR, summary risk estimate; CI, confidence interval; OS, overall survival; DSS, disease‐specific survival; DFS, disease‐free survival; LRFS, local recurrence‐free survival; RRFS, regional recurrence‐free survival; DRFS, distant recurrence‐free survival
Overall, 11 studies presented unadjusted estimates for DFS (forest plot in Figure 4), and the SRR estimate indicated a 24% reduction in the risk of local recurrence in older patients, with no between‐study heterogeneity (SRR = 0.76, 95% CI = 0.63–0.92, I2 = 0). Similar results were found for the summary unadjusted RR/HR estimates of DFS with low heterogeneity (I2 = 31%). In contrast, the summary estimates for DRFS and RRFS, despite displaying similar patterns in terms of the point estimate, were found to be statistically insignificant (Table 2 and Figure 5A,B,C).
FIGURE 4.
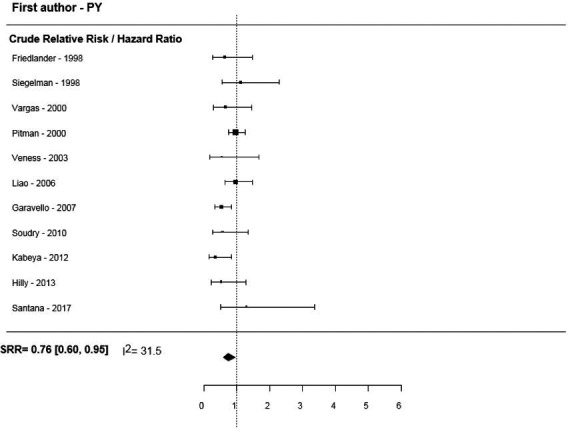
Disease‐free survival for older versus younger OTSCC patients.
FIGURE 5.
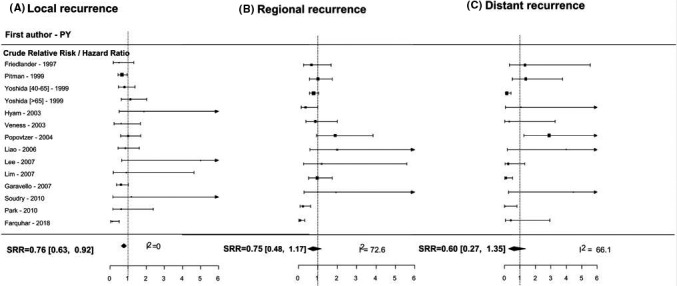
(A) Local recurrence for older versus younger OTSCC patients. (B) Regional recurrence for older versus younger OTSCC patients. (C) Distant recurrence for older versus younger OTSCC patients.
Regarding sensitivity analyses, exclusion of the most recent and largest cohort study, which accounted for 80% of the total patient cohort included in the OS analyses, did not affect the results (SRR = 2.02, 95% CI = 1.48–2.74). 4 The majority of the studies compared outcomes by age using 40 or 45 years as the cutoff, 4 , 17 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 except for studies by Hilly et al. and Soudry et al. that used 30 years old as the cutoff. 18 , 44 Both these studies presented non‐adjusted risk measures and indicated that older patients had lower risks of recurrence and mortality than their younger counterparts. The sensitivity analysis of the crude summary estimate for OS confirmed that no association existed after excluding these two studies (SRR =0.94, 95% CI = 0.76–1.16). Besides, the summary estimates for local recurrence did not change (data not shown).
Hyam et al., Yoshida et al., and Davidson et al. presented estimates for two cutoff points: “very old” (>60–65 years) versus “younger” (<40–45 years) and “middle age” (between 40–50 and 60–65 years) versus “younger” (<40–45 years). 36 , 55 , 57 These studies, except for Mroueh et al., 41 recorded a higher risk of recurrence of mortality for very old patients versus younger patients and very old patients versus middle‐aged patients (forest plots in Figures 2 and 3).
Other sources of between‐study heterogeneity were investigated using subgroup analyses and meta‐regression (Table 3). We found a significant difference (p = 0.03) in risk estimates by country, such that older patients had a significantly higher risk of mortality in the USA or Australia. In contrast, the inverse association was observed in East Asian and Middle Eastern countries, albeit without significance in some cases. We found a significantly reduced risk of local recurrence for the same countries, but none of the estimates was adjusted for other prognostic factors.
TABLE 3.
Subgroup analyses and results of meta‐regression models
| Outcome | No. of studies | SRR | Lower 95% CI | Upper 95% CI | p‐value | ||
|---|---|---|---|---|---|---|---|
|
OS |
pT | T1–T2 only | 6 | 0.97 | 0.87 | 1.45 | 0.60 |
| Any pT | 22 | 1.13 | 0.85 | 1.49 | |||
| pN | N0 only | 5 | 1.32 | 0.85 | 2.06 | 0.37 | |
| Any pN | 23 | 1.03 | 0.78 | 1.37 | |||
| Region | Europe | 6 | 1.45 | 0.77 | 2.75 | 0.03 | |
| USA/Australia | 12 | 1.69 | 1.55 | 1.84 | |||
| East Asia | 9 | 0.88 | 0.65 | 1.20 | |||
| Middle East | 4 | 0.57 | 0.75 | 1.17 | |||
| DSS | pT | T1–T2 only | 6 | 0.97 | 0.65 | 1.45 | 0.69 |
| Any pT | 13 | 0.81 | 0.50 | 1.18 | |||
| pN | N0 only | 4 | 1.12 | 0.72 | 1.74 | 0.31 | |
| Any pN | 15 | 0.75 | 0.47 | 1.21 | |||
|
Region |
Europe | 5 | 1.41 | 0.67 | 2.96 | 0.09 | |
| USA/Australia | 5 | 1.64 | 1.08 | 2.48 | |||
| East Asia | 7 | 0.78 | 0.55 | 1.09 | |||
| Middle East | 4 | 0.44 | 0.46 | 1.18 | |||
|
DFS |
pT | T1–T2 only | 0 | ‐ | ‐ | ‐ | |
| Any pT | 11 | 0.76 | 0.60 | 0.95 | ‐ | ||
| pN | N0 only | 0 | ‐ | ‐ | ‐ | ||
| Any pN | 11 | 0.76 | 0.60 | 0.95 | ‐ | ||
|
Region |
Europe | 1 | 0.53 | 0.35 | 0.84 | 0.14 | |
| USA/Australia | 6 | 0.93 | 0.75 | 1.15 | |||
| East Asia | 2 | 0.64 | 0.25 | 1.65 | |||
| Middle East | 2 | 0.56 | 0.31 | 1.02 | |||
|
Local RFS |
pT | T1–T2 only | 4 | 0.96 | 0.70 | 1.31 | 0.06 |
| Any pT | 10 | 0.67 | 0.52 | 0.84 | |||
| pN | N0 only | 3 | 0.94 | 0.64 | 1.38 | 0.21 | |
| Any pN | 11 | 0.71 | 0.57 | 0.88 | |||
|
Region |
Europe | 1 | 0.61 | 0.37 | 1.02 | 0.15 | |
| USA/Australia | 5 | 0.58 | 0.34 | 0.98 | |||
| East Asia | 6 | 0.93 | 0.68 | 1.28 | |||
| Middle East | 2 | 1.01 | 0.61 | 1.69 | |||
|
Regional RFS |
pT | T1–T2 only | 2 | 0.79 | 0.58 | 1.06 | 0.74 |
| Any pT | 10 | 0.72 | 0.41 | 1.25 | |||
| pN | N0 only | 2 | 0.79 | 0.58 | 1.06 | 0.74 | |
| Any pN | 10 | 0.72 | 0.41 | 1.25 | |||
|
Region |
Europe | 1 | 0.96 | 0.53 | 1.75 | 0.57 | |
| USA/Australia | 5 | 0.51 | 0.24 | 1.08 | |||
| East Asia | 5 | 0.94 | 0.44 | 2.01 | |||
| Middle East | 1 | 1.93 | 0.28 | 13.35 | |||
|
Distant RFS |
pT | T1–T2 only | 1 | 0.16 | 0.06 | 0.42 | 0.21 |
| Any pT | 11 | 0.73 | 0.32 | 1.67 | |||
| pN | N0 only | 1 | 0.16 | 0.06 | 0.42 | 0.21 | |
| Any pN | 11 | 0.73 | 0.32 | 1.67 | |||
| Region | Europe | 1 | 0.25 | 0.05 | 1.31 | 0.13 | |
| USA/Australia | 5 | 1.01 | 0.50 | 2.04 | |||
| East Asia | 4 | 1.33 | 0.23 | 7.78 | |||
| Middle East | 2 | 0.08 | 0.02 | 0.35 |
P‐value from meta‐regression for differences between subgroups
SRR, summary risk estimate; CI, confidence interval; OS, overall survival; DSS, disease‐specific survival; DFS, disease‐free survival; RFS, recurrence‐free survival; pT, pathological tumor; pN, pathological node.
SRR was estimated for older patients vs. younger patients.
No publication bias, as measured by the method of Sterne et al. 53 was observed for any outcome (crude OS, p = 0.51; adjusted OS, p = 0.90; DSFF, p = 0.55; DFS, p = 0.19; LRFS, p = 0.61; RRFS, p = 0.78; and DRFS, p = 0.87).
4. DISCUSSION
The present meta‐analysis aimed to reveal the difference in prognosis between younger and older patients with OTSCC.
In the vast majority of published studies, the “young group” is not uniformly defined, or its definition is based on an arbitrary judgment, with cutoffs ranging from 20 to 30, 40, or 45 years. In addition, differences in the median/mean age among age groups ranged from a minimum of 12 years to a maximum of 42 years. 44 , 58 Among the 31 selected studies, the “younger patients” group, in terms of age at diagnosis, was defined as <30, <40, and <45 years in 3, 15, and 9 studies, respectively, whereas “older patients” was defined with consecutive ranges in the remaining studies. We used the 45‐year cutoff for the current analysis to include all the studies mentioned above with age ranges of 30, 40, and 45 years, to maximize the possible study population, and consider that the definition of "young adult" is still not universal defined.
The increasing interest for “young patients” affected by oral cancer, is related to the different etiological factors which might differentiate this subset of patients, and indirectly influence their prognosis, response to treatments and prompt recovery compared to aged patients. Prognosis and function rehabilitation is closely related to the quality of life in all patients affected by oral cancer. Nevertheless, this is even more crucial for younger patients since the sequelae of OTSCC treatments and surgery impair patients’ future personal and professional perspectives and social skills. Nowadays, researchers’ main challenge is to identify an ideal treatment that might optimize the cost‐benefit ratio and provide good prognosis with minimal functional impairment. Moreover, besides smoke and alcohol consumption, identifying new risk factors could lead to the establishment of prevention measures against tongue cancer especially in young patients and redefine patients’ risk stratification for tailored surveillance and follow‐up programs. 19
Although the “infectious aspect” of tumors in younger patients is currently being investigated, the relationship between HPV infection and tongue cancer risk in young patients remains debatable. Compared with that observed for oropharyngeal cancer, viral infection is not strongly related to tongue cancer. 69 , 70 , 71 However, abnormal dentition in terms of persistent trauma and chronic inflammation and a diet low in fruit and vegetables have been studied at length, providing consistent results. 25 , 32 , 72 Finally, the role of genetic alterations such as an altered expression (overexpression) of the epidermal growth factor receptor in young adults has been reported to be related to a worse prognosis. 34 , 35
The literature is very heterogeneous with regards to the prognosis by age group, ranging from a worse prognosis for young people to a significantly better prognosis. 4 , 36
In the current analysis, younger patients exhibited better prognosis in terms of OS but a higher risk of recurrence, even if the estimations were not adjusted for confounders and other prognostic factors. Conversely, older patients exhibited a lower risk of local recurrence and worse DSS according to estimates that were not adjusted for other prognostic factors and possible confounding factors such as TNM stage, sex, and treatment type. This trend was also confirmed in other studies which presented two different cutoffs for age: Mroueh et al. and Davidson et al. reported that older patients (>60 years at diagnosis) had worse prognoses than patients in the reference group (40–60 years at diagnosis). 41 , 57
The findings of a higher risk of local recurrence and improved OS in young adults could be related to the assumption that younger patients benefit from a higher number of available therapeutic options, such as major surgery and first‐line chemotherapy. Generally, younger patients have fewer comorbidities and better resilience. Therefore, they may have lower probabilities of postoperative complications, thereby increasing their survival chance. 73 Moreover, the increased recurrence rates may be associated with non‐cancer‐related mortality in older patients before relapse occurs. 73
Furthermore, an interesting aspect of this study was represented by the heterogeneity of the survival outcomes concerning geographical areas: older patients seemed to have a significantly higher risk of mortality in the USA or Australia, whereas an inverse association was observed in East Asian and Middle Eastern countries. These differences can be justified by differences in health care systems among these countries and relative ease to access to medical care. In Asian, Middle Eastern countries as well as Latin America, limited access to medical care could be the cause of a delayed diagnosis and worse prognosis in younger people compared to the elderly when considering both OS and DSS. 74 Countries with limited access to cure suffer from late diagnosis, treatments might be inadequate, with higher mortality and risk of relapse due to advanced‐stage disease.
The authors debate on the geographical prognostic differences between the elderly and young people also mirrored the incidence of disease: it seems that in Asia, Africa, and the Middle East, tongue cancer incidence among young population is highest. One explanation could be the lower life expectancy in these countries or an overestimated number of young patients because of the reluctance of elderly to attend a hospital. 3
Our study has some limitations: it is not easy to clarify these aspects using published retrospective studies because the reported incidence of OTSCC in young adults is at <5%. 40 , 75 Moreover, different studies have presented discordant data, making it difficult to identify and establish the real impact of age on tongue cancer prognosis. 7 The disparity of these results could be attributed to the small sample size of younger patients as well as the heterogeneity among studies (matched/unmatched studies, early/advanced tumor stage, different treatments reported, and cancer at different subsites [i.e., oral tongue cancer or cancer of the base of the tongue] considered in the same patient group). 69 , 76 The disparity could also be partly associated with the different definitions of “young” patients and different inclusion criteria regarding the anatomical tumor site. In many studies, the base of the tongue was included, although it is considered part of the oropharynx, which differs from tongue cancer for staging and prognosis. 76 , 77 Furthermore, the analyzed studies were conducted over an extremely long period during which the knowledge, diagnostics, and therapies in these fields have considerably changed. The majority of the included patients were part of a single recently published study in the USA. However, the effects of confounding factors were statistically lower. 4
5. CONCLUSION
This systematic review and meta‐analysis revealed that younger patients with OTSCC have a better OS but a higher risk of recurrence than elderly patients. Therefore, they should receive personalized follow‐up plans to improve their prognosis, by identifying disease recurrence at an early stage and ensuring adequate oncological radicality with as much as possible functional conservative treatment.
Further prospective international studies with uniform inclusion criteria are strongly needed to corroborate our findings. Prospective studies could focus on different trends in women and teenagers, where the hormonal, genetic and molecular factors are potentially crucial.
CONFLICT OF INTEREST
Nothing to declare.
ETHICAL STATEMENT
Ethical approval is not be required because this study retrieves and synthesises data from already published studies.
ACKNOWLEDGEMENTS
The authors have declared no conflicts of interest.
Funding information
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5 × 1000 funds.
Contributor Information
Marta Tagliabue, Email: marta.tagliabue@ieo.it.
Rita De Berardinis, Email: rita.deberardinis@ieo.it.
DATA AVAILABILITY STATEMENT
Data are available upon request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer . Cancer tomorrow. Available from: https://gco.iarc.fr/tomorrow/graphic‐isotype?type=0&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0; [accessedonFebruary2020].
- 3. Hussein AA, Helder MN, de Visscher JG, et al. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: a systematic review. Eur J Cancer. 2017;82:115‐127. [DOI] [PubMed] [Google Scholar]
- 4. Oliver JR, Wu SP, Chang CM, et al. survival of oral tongue squamous cell carcinoma in young adults. Head Neck. 2019;41(9):2960‐2968. [DOI] [PubMed] [Google Scholar]
- 5. Annertz K, Anderson H, Biörklund A, et al. incidence and survival of squamous cell carcinoma of the tongue in Scandinavia, with special reference to young adults. Int J Cancer. 2002;101(1):95‐99. [DOI] [PubMed] [Google Scholar]
- 6. Tota JE, Anderson WF, Coffey C, et al. Rising incidence of oral tongue cancer among white men and women in the united states, 1973–2012. Oral Oncol. 2017;67:146‐152. [DOI] [PubMed] [Google Scholar]
- 7. Paderno A, Morello R, Piazza C. Tongue carcinoma in young adults: a review of the literature. Acta Otorhinolaryngol Ital. 2018;38(3):175‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi SW, Moon E‐K, Park JY, et al. Trends in the incidence of and survival rates for oral cavity cancer in the Korean population. Oral Dis. 2014;20(8):773‐779. [DOI] [PubMed] [Google Scholar]
- 9. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309‐316. [DOI] [PubMed] [Google Scholar]
- 10. Michmerhuizen NL, Birkeland AC, Bradford CR, et al. Genetic determinants in head and neck squamous cell carcinoma and their influence on global personalized medicine. Genes & Cancer. 2016;7(5–6):182‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ng JH, Iyer NG, Tan MH, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck. 2017;39(2):297‐304. [DOI] [PubMed] [Google Scholar]
- 12. Satgunaseelan L, Allanson BM, Asher R, et al. The incidence of squamous cell carcinoma of the oral tongue is rising in young non‐smoking women: an international multi‐institutional analysis. Oral Oncol. 2020;110:104875. [DOI] [PubMed] [Google Scholar]
- 13. Nan‐Chin L, Jui‐Ting H, Kuo‐Yang T. Difference between female and male patients with oral squamous cell carcinoma: a single‐center retrospective study in Taiwan. Int J Environ Res Public Health. 2020;17(11):3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kruse AL, Bredell M, Grätz KW. Oral cancer in men and women: are there differences? Oral Maxillofac Surg. 2011;15:51‐55. [DOI] [PubMed] [Google Scholar]
- 15. Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. J Natl Cancer Inst. 2007;99:777‐789. [DOI] [PubMed] [Google Scholar]
- 16. IARC . Monographs on evaluation of carcinogenic risks to humans. (2015). Retrieved June 10, 2016, from the International Agency for Research on Cancer. Available from: http://monographs.iarc.fr/ENG/Preamble/instructions.php; [accessed on January 2020].
- 17. Garavello W, Spreafico R, Gaini RM. Oral tongue cancer in young patients: a matched analysis. Oral Oncol. 2007;43(9):894‐897. [DOI] [PubMed] [Google Scholar]
- 18. Hilly O, Shkedy Y, Hod R, et al. Carcinoma of the oral tongue in patients younger than 30 years: comparison with patients older than 60 years. Oral Oncol. 2013;49(10):987‐990. [DOI] [PubMed] [Google Scholar]
- 19. Kourelis K, Tsue T, Girod D, Tawfik O, Sykes K, Shnayder Y. Negative prognostic factors for head and neck cancer in the young. J BUON. 2013;18(2):459‐464. [PubMed] [Google Scholar]
- 20. Strindlund K, Troiano G, Sgaramella N, et al. Patients with high c‐MYC‐expressing squamous cell carcinomas of the tongue show better survival than those with low‐ and medium‐expressing tumours. J Oral Pathol Med. 2017;46(10):967‐971. [DOI] [PubMed] [Google Scholar]
- 21. Santana T, Sa MC, de Moura SE, Galvao HC, Coletta RD, Freitas RA. DNA base excision repair proteins APE‐1 and XRCC‐1 are overexpressed in oral tongue squamous cell carcinoma. J Oral Pathol Med. 2017;46(7):496‐503. [DOI] [PubMed] [Google Scholar]
- 22. National Health Survey . First Results, 2017–18. Australia: Australian Bureau of Statistics; 2018. Available from: https://www.abs.gov.au/statistics/health/health‐conditions‐and‐risks/national‐health‐survey‐first‐results/latest‐release; [accessed on January 2021].
- 23. Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29(11):1488‐1494. [DOI] [PubMed] [Google Scholar]
- 24. Vettore AL, Ramnarayanan K, Poore G, et al. Mutational landscapes of tongue carcinoma reveal recurrent mutations in genes of therapeutic and prognostic relevance. Genome Med. 2015;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singhvi HR, Malik A, Chaturvedi P. The role of chronic mucosal trauma in oral cancer: a review of literature. Indian J Med Paediatr Oncol. 2017;38(1):44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenquist K, Wennerberg J, Schildt E‐B, et al. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population‐based case‐control study in southern Sweden. Acta Otolaryngol. 2005;125:1327‐1336. [DOI] [PubMed] [Google Scholar]
- 27. O'Regan EM, Toner ME, Smyth PC, et al. Distinct array comparative genomic hybridization profiles in oral squamous cell carcinoma occurring in young patients. Head Neck. 2006;28(4):330‐338. [DOI] [PubMed] [Google Scholar]
- 28. Benevenuto TG, Nonaka CFW, Pinto LP, et al. Immunohistochemical comparative analysis of cell proliferation and angiogenic index in squamous cell carcinomas of the tongue between young and older patients. Appl Immunohistochem Mol Morphol. 2012;20(3):291‐297. [DOI] [PubMed] [Google Scholar]
- 29. Pickering CR, Zhang J, Neskey DM, et al. Squamous cell carcinoma of the oral tongue in young non‐smokers is genomically similar to tumors in older smokers. Clin Cancer Res. 2014;20(14):3842‐3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Søland TM, Bjerkli IH, Georgsen JB, et al. High‐risk human papillomavirus was not detected in a Norwegian cohort of oral squamous cell carcinoma of the mobile tongue. Clin Exp Dent Res. 2020;7(1):70‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tagliabue M, Mena M, Maffini F, et al. Role of human papillomavirus infection in head and neck cancer in Italy: the HPV‐AHEAD study. Cancers (Basel). 2020;12(12):3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta B, Bray F, Kumar N, et al. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: a case‐control study from India. Cancer Epidemiol. 2017;51:7‐14. [DOI] [PubMed] [Google Scholar]
- 33. Bello IO, Almangush A, Heikkinen I, et al. Histological characteristics of early‐stage oral tongue cancer in young versus older patients: a multicenter matched‐pair analysis. Oral Dis. 2020;26(5):1081‐1085. 10.1111/odi.13288. [DOI] [PubMed] [Google Scholar]
- 34. Gamez ME, Kraus R, Hinni ML, et al. Treatment outcomes of squamous cell carcinoma of the oral cavity in young adults. Oral Oncol. 2018;87:43‐48. [DOI] [PubMed] [Google Scholar]
- 35. Thomas L, Moore EJ, McGree ME, et al. Prognostic features, human papillomavirus status, and epidermal growth factor receptor expression in oral squamous cell carcinoma in young adults. Am J Otolaryngol. 2012;33(6):650‐656. [DOI] [PubMed] [Google Scholar]
- 36. Hyam DM, Conway RC, Sathiyaseelan Y, et al. Tongue cancer: do patients younger than 40 do worse? Aust Dent J. 2003;48(1):50‐54. [DOI] [PubMed] [Google Scholar]
- 37. Zhang YY, Wang DC, Su JZ, Jia LF, Peng X, Yu GY. Clinicopathological characteristics and outcomes of squamous cell carcinoma of the tongue in different age groups. Head Neck. 2017;39(11):2276‐2282. [DOI] [PubMed] [Google Scholar]
- 38. Park JO, Sun DI, Cho KJ, Joo YH, Yoo HJ, Kim MS. Clinical outcome of squamous cell carcinoma of the tongue in young patients: a stage‐matched comparative analysis. Clin Exp Otorhinolaryngol. 2010;3(3):161‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Popovtzer A, Shpitzer T, Bahar G, Marshak G, Ulanovski D, Feinmesser R. Squamous cell carcinoma of the oral tongue in young patients. Laryngoscope. 2004;114(5):915‐917. [DOI] [PubMed] [Google Scholar]
- 40. Blanchard P, Belkhir F, Temam S, et al. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults: a single‐institution case‐matched analysis. Eur Arch Otorhinolaryngol. 2017;274(3):1683‐1690. [DOI] [PubMed] [Google Scholar]
- 41. Mroueh R, Haapaniemi A, Grénman R, et al. Improved outcomes with oral tongue squamous cell carcinoma in Finland. Head Neck. 2017;39(7):1306‐1312. [DOI] [PubMed] [Google Scholar]
- 42. Friedlander PL, Schantz SP, Shaha AR, Yu G, Shah JP. Squamous cell carcinoma of the tongue in young patients: a matched‐pair analysis. Head Neck. 1998;20(5):363‐368. [DOI] [PubMed] [Google Scholar]
- 43. Pitman KT, Johnson JT, Wagner RL, Myers EN. Cancer of the tongue in patients less than forty. Head Neck. 2000;22(3):297‐302. [DOI] [PubMed] [Google Scholar]
- 44. Soudry E, Preis M, Hod R, et al. Squamous cell carcinoma of the oral tongue in patients younger than 30 years: clinicopathologic features and outcome. Clin Otolaryngol. 2010;35(4):307‐312. [DOI] [PubMed] [Google Scholar]
- 45. Liao C‐T, Wang H‐M, Hsieh L‐L, et al. Higher distant failure in young age tongue cancer patients. Oral Oncol. 2006;42(7):718‐725. [DOI] [PubMed] [Google Scholar]
- 46. Vargas H, Pitman KT, Johnson JT, Galati LT. More aggressive behavior of squamous cell carcinoma of the anterior tongue in young women. Laryngoscope. 2000;110(10):1623‐1626. [DOI] [PubMed] [Google Scholar]
- 47. Veness MJ, Morgan GJ, Sathiyaseelan Y, Gebski V. Anterior tongue cancer: age is not a predictor of outcome and should not alter treatment. ANZ J Surg. 2003;73(11):899‐904. [DOI] [PubMed] [Google Scholar]
- 48. Siegelmann‐Danieli N, Hanlon A, Ridge JA, Padmore R, Fein DA, Langer CJ. Oral tongue cancer in patients less than 45 years old: institutional experience and comparison with older patients. J Clin Oncol. 1998;16(2):745‐753. [DOI] [PubMed] [Google Scholar]
- 49. National Comprehensive Cancer Network guidelines. Available from: http://www.nccn.org/professionals/physician_gls/pdf/head‐and‐neck.pdf; [accessed on February 2020].
- 50. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1‐30. [DOI] [PubMed] [Google Scholar]
- 51. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815‐2834. [DOI] [PubMed] [Google Scholar]
- 52. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 53. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta‐analysis. BMJ. 2001;323(7304):101‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jones JB, Lampe HB, Cheung HW. Carcinoma of the tongue in young patients. J Otolaryngol. 1989;18(3):105‐108. [PubMed] [Google Scholar]
- 55. Yoshida K, Koizumi M, Inoue T, et al. Radiotherapy of early tongue cancer in patients less than 40 years old. Int J Radiat Oncol Biol Phys. 1999;45(2):367‐371. [DOI] [PubMed] [Google Scholar]
- 56. Al‐Rajhi N, Khafaga Y, El‐Husseiny J, et al. Early stage carcinoma of oral tongue: prognostic factors for local control and survival. Oral Oncol. 2000;36(6):508‐514. [DOI] [PubMed] [Google Scholar]
- 57. Davidson BJ, Root WA, Trock BJ. Age and survival from squamous cell carcinoma of the oral tongue. Head Neck. 2001;23(4):273‐279. [DOI] [PubMed] [Google Scholar]
- 58. Lee CC, Ho HC, Chen HL, Hsiao SH, Hwang JH, Hung SK. Squamous cell carcinoma of the oral tongue in young patients: a matched‐pair analysis. Acta Otolaryngol. 2007;127(11):1214‐1217. [DOI] [PubMed] [Google Scholar]
- 59. Lim YC, Choi EC. Unilateral, clinically T2N0, squamous cell carcinoma of the tongue: surgical outcome analysis. Int J Oral Maxillofac Surg. 2007;36(7):610‐614. [DOI] [PubMed] [Google Scholar]
- 60. Yang AK, Liu TR, Chen FJ, et al. Survival analysis of 229 patients with advanced squamous cell carcinoma of the oral tongue. Ai Zheng. 2008;27(12):1315‐1320. [PubMed] [Google Scholar]
- 61. Kies MS, Boatright DH, Li G, et al. Phase II trial of induction chemotherapy followed by surgery for squamous cell carcinoma of the oral tongue in young adults. Head Neck. 2012;34(9):1255‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kabeya M, Furuta R, Kawabata K, Takahashi S, Ishikawa Y. Prevalence of human papillomavirus in mobile tongue cancer with particular reference to young patients. Cancer Sci. 2012;103(2):161‐168. [DOI] [PubMed] [Google Scholar]
- 63. Chen H‐C, Yang C‐M, Cheng J‐T, et al. Global DNA hypomethylation is associated with the development and poor prognosis of tongue squamous cell carcinoma. J Oral Pathol Med. 2016;45(6):409‐417. [DOI] [PubMed] [Google Scholar]
- 64. Sgaramella N, Lindell Jonsson E, Boldrup L, et al. High expression of podoplanin in squamous cell carcinoma of the tongue occurs predominantly in patients </=40 years but does not correlate with tumour spread. J Pathol Clin Res. 2015;2(1):3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cassidy RJ, Switchenko JM, Jegadeesh N, et al. Association of lymphovascular space invasion with locoregional failure and survival in patients with node‐negative oral tongue cancers. JAMA Otolaryngol Head Neck Surg. 2017;143(4):382‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Knopf A, Lempart J, Bas M, Slotta‐Huspenina J, Mansour N, Fritsche MK. Oncogenes and tumor suppressor genes in squamous cell carcinoma of the tongue in young patients. Oncotarget. 2015;6(5):3443‐3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen SW, Zhang Q, Guo ZM, et al. Trends in clinical features and survival of oral cavity cancer: fifty years of experience with 3362 consecutive cases from a single institution. Cancer Manag Res. 2018;10:4523‐4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Farquhar DR, Tanner AM, Masood MM, et al. Oral tongue carcinoma among young patients: an analysis of risk factors and survival. Oral Oncol. 2018;84:7‐11. [DOI] [PubMed] [Google Scholar]
- 69. Li H, Torabi SJ, Yarbrough WG, Mehra S, Osborn HA, Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg. 2018;144(6):519‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morbini P, Alberizzi P, Ferrario G, et al. The evolving landscape of human papillomavirus‐related oropharyngeal squamous cell carcinoma at a single institution in northern Italy. Acta Otorhinolaryngol Ital. 2019;39(1):9‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Castellsague X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403. [DOI] [PubMed] [Google Scholar]
- 72. Merlano MC, Abbona A, Denaro N, Garrone O. Knowing the tumour microenvironment to optimize immunotherapy. Acta Otorhinolaryngol Ital. 2019;39(1):2‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van Gestel YRBM, Lemmens VEPP, de Hingh IHJT, et al. Influence of comorbidity and age on 1‐, 2‐, and 3‐month postoperative mortality rates in gastrointestinal cancer patients. Ann Surg Oncol. 2013;20(2):371‐380. [DOI] [PubMed] [Google Scholar]
- 74. Cohen Goldemberg D, de Araújo LHL, Antunes HS, et al. Tongue cancer epidemiology in Brazil: incidence, morbidity and mortality. Head Neck. 2018;40(8):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 75. Amin MB, Edge S, Greene FL, Byrd DR, Brookland RK, Washington MK. AJCC Cancer Staging Manual. Springer International Publishing; 2017. [Google Scholar]
- 76. Pike LRG, Royce TJ, Mahal AR, et al. Outcomes of HPV‐associated squamous cell carcinoma of the head and neck: impact of race and socioeconomic status. J Natl Compr Canc Netw. 2020;18(2):177‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muller S, Pan Y, Li R, Chi AC. Changing trends in oral squamous cell carcinoma with particular reference to young patients: 1971–2006. The Emory university experience. Head Neck Pathol. 2008;2(2):60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.


