Abstract
Background/Objectives
Myopia progression is of great concern because of its association with an increased risk of sight-threatening conditions. This study aims to determine whether certain clinical and optic disc features are associated with the rate of myopia progression.
Subjects/Methods
In this retrospective longitudinal observational study, we reviewed the medical records of 95 patients aged 6–11 years with myopia (spherical equivalent refractive error (SER) at baseline ≤ −0.5 D) and collected data regarding medical history, comprehensive ophthalmologic examination, and fundus photography. Using fundus photographs, we measured the ratio of horizontal to vertical disc diameter (HVDR), ratio of maximum β-zone peripapillary atrophy (β-PPA) width to vertical disc diameter (PVDR), and optic disc torsion. Outcome measurements included 2-year myopia progression (D/year) and overall myopia progression during the entire follow-up with a mean of 51 months.
Results
Mean age at initial visit was 7.67 ± 1.50 years and mean SER at baseline was −2.91 ± 1.68 D (range, −5.75 to −0.50 D). In the univariate analysis, age, parental myopia, SER at baseline, HVDR, and PVDR were significantly associated with myopia progression (P < 0.05). In the multivariate analysis, however, only age at initial visit and PVDR were significant factors associated with both 2-year and overall myopia progression.
Conclusions
Children with younger age and smaller β-PPA at baseline showed a faster myopia progression. This study suggests that the width of β-PPA, regardless of SER, might be used as a quantitative parameter to predict the potential for further myopia progression associated with scleral stretching.
Subject terms: Paediatrics, Refractive errors, Paediatrics, Refractive errors
Introduction
Myopia has emerged as one of the major public health issues worldwide, particularly affecting up to 80–90% of young adults in East Asia [1–7]. Although myopia can be corrected using spectacles, contact lenses, or refractive surgery, pathologic myopia is associated with sight-threatening complications, such as myopic macular degeneration, retinal detachment, glaucoma, and choroidal neovascularization [8–10].
Increasing public health care burden of myopia has urged studies to focus on the risk factors for myopia progression and control of myopia. Genetic factors including ethnic background and parental myopia, and environmental risk factors such as educational attainment, near work, and outdoor activities have been reported to be associated with the development and progression of myopia [11, 12]. Various interventions have become available for myopia control and treatment; among such interventions, topical atropine has been suggested as a safe and effective means for inhibiting myopia progression [13–17].
In addition to the genetic and environmental factors, ocular structural factors, such as the morphologic characteristics of the optic disc, are also associated with myopia. A myopic optic disc is characterized by a variety of optic disc abnormalities including tilted disc, disc torsion, and peripapillary atrophy, and these optic disc features tend to worsen with myopia progression [18–22]. It could be said, therefore, that the optic disc changes can reflect the degree of myopia. However optic disc changes have not been considered as a prognostic factor for myopia progression.
In the present study, we aimed to determine whether myopic optic disc features, such as tilted optic disc, disc torsion, and peripapillary atrophy, are associated with future course of myopia.
Methods
This study is a retrospective clinical investigation conducted in myopic children who were diagnosed and followed regularly every 6 months between 2012 and 2018 at the myopia clinic of the Asan Medical Center, University of Ulsan in Seoul, Korea. This study was approved by the Institutional Review Board of the Asan Medical Center and adhered to the tenets of the Declaration of Helsinki.
Children aged 6–11 years with myopia of spherical equivalent refractive error (SER) ≤ –0.50 and >–6.00 diopters (D) in both eyes at baseline were included in this study. Exclusion criteria were as follows: (1) best-corrected visual acuity worse than 0.3 logMAR; (2) other concurrent ocular diseases such as strabismus, congenital cataract, glaucoma, or retinal disease; (3) a history of traumatic ocular injury or ocular surgery; (4) any previous treatment for myopia control; (5) systemic or other neurological diseases that might affect the measurement of manual refraction; (6) genetic diseases such as Stickler syndrome or Marfan syndrome; (7) follow-up duration < 2 years; and (8) no fundus photographs obtained at the initial visit.
We reviewed the patient medical records and collected data pertaining to medical history, family history, and comprehensive ophthalmological examinations including best-corrected visual acuity, cycloplegic refraction, and fundus photography. Cycloplegia was induced by 3 cycles of 1 drop of 1% cyclopentolate and 1 drop of 1% tropicamide, administered 5 min apart, following corneal anesthesia with 0.5% proparacaine hydrochloride. Manual retinoscopic refraction was performed 30 min after the last cycle of cycloplegic eye drops.
We assessed the optic disc characteristics using standard fundus photographs that were taken at the initial visit. We measured the vertical and horizontal disc diameters and maximum β-zone peripapillary atrophy (β-PPA) width using ImageJ software (version 1.51; National Institutes of Health, Rockville, MD, available at http://rsb.info.nih.gov/ij/index.html) [23]. β-PPA was defined as an area of visible sclera adjacent to the disc margin without the retinal pigment epithelium [24]. Considering various photographic factors that could influence the accuracy of quantitative measurements, we used the ratio of horizontal to vertical disc diameter (HVDR) and the ratio of maximum β-PPA width to vertical disc diameter (PVDR) as measurement parameters of the optic disc [18]. In addition, we measured the optic disc torsional angle between vertical disc axis and vertical meridian to the reference line connecting fovea and disc center [22, 25]. A positive torsional angle indicates inferotemporal torsion, whereas a negative torsional angle indicates superonasal torsion. All measurements were performed twice independently by single examiner (YM), from which the mean values were obtained. The intraclass coefficient value of the parameters was 0.935.
The primary outcome was the 2-year myopia progression rate, which was defined as the mean annual changes in the SER during the first 2 years of follow-up. The secondary outcome was the overall myopia progression rate, which was calculated as the overall changes in SER between initial and final visit divided by the whole follow-up duration in years. The mean duration of follow-up was 51.09 ± 25.96 months (range, 24–133 months).
Statistical analysis
Strong correlations between right and left eye SER were observed at baseline, 2-year visit, and final visit (0.675, 0.725, and 0.783, respectively, P < 0.001, Pearson correlation test). Therefore, analyses were performed using data form the right eye only. All statistical analyses were performed using SPSS program version 21.0 (SPSS Inc., Chicago, IL). Continuous variables were expressed by means ± standard deviations, and categorical variables were presented as frequencies. Mann–Whitney U-test and Jonckheere–Terpstra test were used respectively to determine whether the rate of myopia progression would differ based on gender or parental myopia status. Univariate linear regression analysis was first applied to each continuous independent variable and only those (P < 0.2) were considered for inclusion in the full regression model. Then, a multivariate regression analysis was performed to identify the factors significantly associated with a continuous dependent variable, the rate of myopia progression. Among our independent variables, parental myopia was registered as a categorical variable having 3 levels (0, 1, or 2 myopic parents), and as such was converted into dummy variable. Since our dependent variable is continuous and the independent variables consisted of both continuous and categorical variables, we used a multiple linear regression analysis with dummy coding for the regression analysis. All statistical tests were performed considering 5% as the level of statistical significance.
Results
Overall, 95 children with myopia were enrolled in the present study. Mean age of these children at the initial visit was 7.67 ± 1.50 years, and 61.1% of them (n = 55) were female. Mean SER at baseline and mean follow-up period were −2.91 ± 1.68 D (range, −5.75 to −0.50 D) and 51.09 ± 25.96 months, respectively. Table 1 summarizes the baseline demographics and ophthalmologic examination data of all patients.
Table 1.
Baseline demographic and ophthalmologic examination data.
| Mean ± standard deviation | |
|---|---|
| Age (years) | 7.67 ± 1.50 |
| Sex (M:F) | 40: 55 |
| Parental myopia (0:1:2) | 39:16:22 |
| SER (D) | −2.91 ± 1.68 |
| Astigmatism (D) | 1.15 ± 1.32 |
| Anisometropia (D) | 0.45 ± 1.74 |
| HVDR | 0.91 ± 0.12 |
| PVDR | 0.08 ± 0.11 |
| Optic disc torsion | −0.77 ± 9.85 |
| Follow-up periods (months) | 51.09 ± 25.96 |
M male, F female, SER spherical equivalent refractive error, D diopter, HVDR ratio of horizontal to vertical diameter, PVDR ratio of maximum β-zone peripapillary atrophy width to vertical diameter.
There was no significant difference in the rate of myopia progression depending on gender. However, depending on the number of parents with myopia, the 2-year myopia progression rate varied significantly in our analysis when using Jonckheere–Terpstra test. Children with two myopic parents showed a significantly faster myopia progression than those with either zero or one myopic parent over a 2-year period. However, this difference was not significant in the overall yearly rate of myopia progression in our subjects (Table 2).
Table 2.
Comparison of the myopia progression rate according to sex and parental myopia.
| 2-year myopia progression rate | P-value | Overall myopia progression | P value | |
|---|---|---|---|---|
| Sexa | 0.397 | 0.287 | ||
| Male (n = 40) | −0.69 ± 0.48 | −0.62 ± 0.37 | ||
| Female (n = 55) | −0.69 ± 0.50 | −0.66 ± 0.37 | ||
| Parental myopiab | 0.006 | 0.132 | ||
| 0 (n = 39) | −0.51 ± 0.42 | −0.59 ± 0.38 | ||
| 1 (n = 16) | −0.68 ± 0.51 | −0.68 ± 0.49 | ||
| 2 (n = 22) | −0.91 ± 0.58 | −0.70 ± 0.35 |
Bold values indicate statistical significance p < 0.05.
aMann−Whitney U-test; bJonckheere−Terpstra test.
In the univariate linear regression analysis, the age, SER, HVDR, and PVDR at baseline were significant factors associated with the 2-year myopia progression rate. The data illustrated that the children with younger age of myopia onset, less myopic SER, larger HVDR, and smaller PVDR at baseline showed a faster rate of myopia progression. By contrast, in the multivariate linear regression analysis, only the age at onset and PVDR remained statistically significant, where the younger age of onset and smaller PVDR were significantly associated with the faster myopia progression. Likewise, as for the overall myopia progression (annual rate of myopia progression over the entire follow-up period), the age and PVDR at baseline were significant factors in the multivariate regression analysis (Table 3).
Table 3.
Association between multiple factors and myopia progression using univariate and multivariate linear regression analysis.
| 2-year myopia progression | Overall myopia progression | |||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Age | 0.295 (0.002) | 0.363 (0.005) | 0.303 (0.001) | 0.410 (0.001) |
| Parental myopia | ||||
| 1 vs 0 | −0.090 (0.459) | −0.043 (0.710) | ||
| 2 vs 0 | −0.147 (0.229) | −0.028 (0.808) | ||
| SER | −0.177 (0.043) | −0.093 (0.531) | −0.245 (0.008) | −0.140 (0.315) |
| Astigmatism | −0.022 (0.417) | −0.067 (0.261) | ||
| Anisometropia | −0.095 (0.181) | −0.130 (0.296) | −0.029 (0.390) | |
| HVDR | −0.247 (0.016) | −0.022 (0.876) | −0.254 (0.007) | 0.021 (0.884) |
| PVDR | 0.305 (0.001) | 0.275 (0.029) | 0.335 (<0.001) | 0.334 (0.006) |
| Optic disc torsion | 0.129 (0.106) | 0.145 (0.241) | 0.075 (0.234) | |
Bold values indicate statistical significance p < 0.05.
SER spherical equivalent refractive error, HVDR ratio of horizontal to vertical diameter, PVDR ratio of maximum β-zone peripapillary atrophy width to vertical diameter.
In our study, the baseline SER and PVDR were correlated to each other, which was consistent with previous research [18]. The Pearson correlation coefficient was −0.581 (P < 0.001). Eyes with higher levels of myopia tended to have a larger β-PPA. Furthermore, several studies have also reported that the baseline SER may act as a significant risk factor for myopia progression [26, 27]. As such, we conducted additional analysis to determine whether the PVDR has an independent effect on the rate of myopia progression, regardless of the levels of baseline SER.
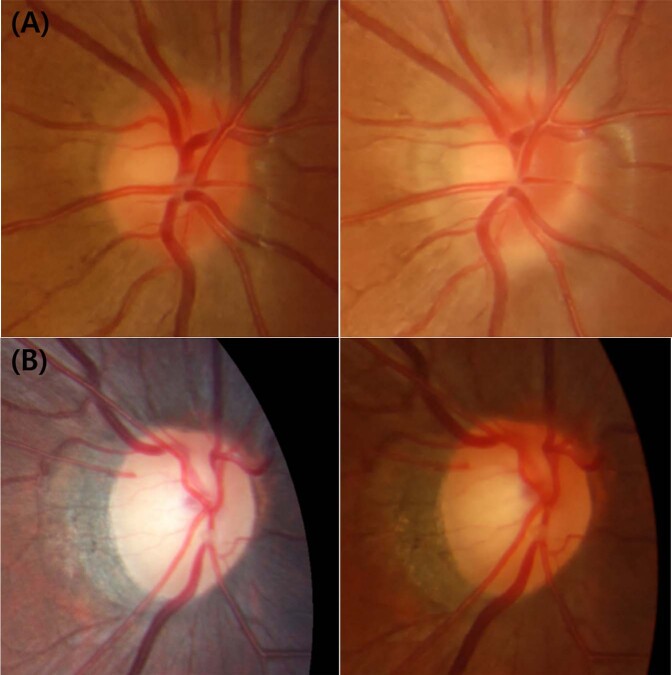
To statistically control the effect of SER, we classified the patients into two groups using linear regression model. Based on the linear relationship between SER and PVDR, which was computed as an equation of “PVDR = −0.012 − 0.033 × SER”, patients with a PVDR larger than “−0.012 − 0.033 × SER” were classified as group L (large), while those with a PVDR smaller than “−0.012 − 0.033 × SER” as group S (small). Mean SER of group L and group S was −2.86 ± 1.87 D and −2.95 ± 1.55 D, respectively (P = 0.799). Although the difference in SER was not statistically significant, the rate of 2-year myopia progression was significantly different between group L and S (−0.54 ± 0.46 D/year and −0.79 ± 0.49 D/year, respectively, P = 0.014). Similarly, the overall myopia progression rate was also significantly different between group L and S (−0.53 ± 0.35 D/year and −0.72 ± 0.53 D/year, respectively, P = 0.017). The group S, patients with a smaller PVDR at baseline, experienced a faster myopia progression compared with the group L, under circumstances where the baseline SER variable was controlled (Fig. 1).
Fig. 1. Longitudinal changes in the optic disc of group L and S children over 2 years.
a Disc photographs of a patient in group S (left; baseline, right; 2-year visit). He was 7.3-year-old boy and his spherical equivalent refractive error (SER) was −1.25 D at baseline. After 2 years, his SER changed to −4.00 D and β-zone peripapillary atrophy enlarged. b Disc photographs of a patient in group L (left; baseline, right; 2-year visit). He was 6.4-year-old boy and his SER was −1.00 D at baseline. After 2 years, his myopia remained unchanged and his optic disc and β-PPA also maintained stable.
Discussion
The present study showed that the age of myopia onset and PVDR of the optic disc could be considered as significant predictive factors for myopia progression in young school children. Earlier onset age and smaller β-PPA were associated with faster rate of myopia progression.
Several studies have already addressed the possible difference in the rate of myopia progression according to age. Some previous research conducted on school children aged 6 years or older reported a faster myopia progression in younger aged children [28, 29]. On the other hand, for children aged 6 years or younger, the literature showed inconsistent results regarding the effect of age on the rate of myopia progression [26, 30]. In the present study on children aged 6–11 years, younger baseline age was associated with faster rate of myopia progression. This inconsistency across the various studies might be attributable to differences in methods of refractive error measurement, race/ethnicity of study subjects, and inclusion criteria, i.e., as to whether the study included only myopic children or both hyperopic and myopic children. Our finding was in agreement with several previous reports that children with younger-onset myopia had a higher risk of rapid myopia progression [28, 30, 31]. This suggests that the speed of myopia progression may be the highest at the beginning or at the early stage of myopia and decrease over later childhood. Therefore, it would be reasonable to say that detecting myopia at an early stage is a crucial factor in the prevention of myopia progression in young school children.
In the present study, we observed that β-PPA, rather than baseline SER, was a predictive factor of myopia progression in a multivariate regression analysis and additional subgroup analysis. A recent longitudinal study by Kim et al. has nicely demonstrated the development and enlargement of β-PPA around the optic disc with the progression of myopia, where the inner surface of the scleral canal is exposed as the temporal scleral ring is stretched [18]. In tree shrews treated with a −5D lenses to induce myopia, the rate of myopic axial elongation (the slow, time-dependent extension) was found to display a bi-directional pattern representing a sudden increase and gradual decrease [32]. Based on these results and ours, it is conceivable that the β-PPA may reflect to some degree the severity or stage of myopia. As such, if the size of β-PPA is small, it seems highly likely that the myopia of the eye is at a relatively early stage in the course of progression, allowing for possible future progression of the myopia. All other factors being equal, the eye with a smaller β-PPA is expected to experience a faster future progression of myopia (Fig. 1).
In consistent with our results, a 2-year prospective cohort study reported that a group of patients whose optic disc had no obvious β-PPA at baseline showed a significantly faster myopia progression, compared with the other groups of patients who had an obvious β-PPA at baseline [19]. However, it should also be noted that a minor proportion of the children with minimal or very small area of β-PPA at baseline could have a mild or moderate rate of myopia progression during follow-up. Although the average rate of myopia progression was as high as −0.79 D/year in group S (having small β-PPA compared with the eye’s SER), as shown in this study, 14 patients (24.6%) in the group S exhibited only a moderate rate of myopia progression less than −0.50 D/year. In addition, in group L (having large β-PPA compared with the eye’s SER), a small proportion of patients (6 of 38 patients, 15.8%) underwent a fast rate of myopia progression higher than −1.00 D/year, despite having the large β-PPA. Therefore, it is noteworthy that, although the β-PPA may be considered as a potential independent predictive factor for myopia progression, it should not be considered as a sole factor determining the rate of myopia progression. Further studies are needed to elucidate the relationship between morphologic changes in the myopic optic disc and the rate of myopia progression and to identify the factors that slow down or stimulate the myopia progression in childhood.
It would be worthwhile at this point to mention that the term ‘β-PPA’ used in this study refers to the conventional ophthalmoscopic beta-zone PPA. Recent spectral-domain optical coherence tomography (OCT) imaging studies newly classified beta-PPA into two subsets that are hardly distinguishable by clinical examination: a zone with intact Bruch’s membrane, termed beta-PPA, and a zone lacking Bruch’s membrane, termed gamma-PPA [24, 33, 34]. The gamma-PPA is now considered to be associated with myopia, representing anatomical changes resulting from axial globe elongation, whereas beta-PPA more associated with glaucoma [34, 35]. As the globe elongation progressed with a myopia progression, the temporal border tissue of the optic disc is stretched and retracted nasally in relation to the temporal opening of the Bruch’s membrane in OCT images. This would result in a bared scleral region devoid of Bruch’s membrane on the temporal border of the optic disc. This bared zone in the temporal parapapillary region is called the gamma-PPA. Since the present study did not have the OCT images of the optic disc, we could not utilize this new classification of PPA. Although our assessment of the β-PPA was not based on OCT but on digital optic disc image, it could be reasonably inferred that the small β-PPA referred to in the present study implies either small new β-PPA or small Ɣ-PPA, or both, because conventional β-PPA is composed of new β-PPA and Ɣ-PPA. As such, the small β-PPA, in a conventional sense, only if Ɣ-PPA is present within the β-PPA, could be said to be predictive of further globe elongation with myopia progression, especially when considering the notion that Ɣ-PPA is related to the scleral stretching occurring with myopia progression. Similarly, a large β-PPA at baseline would be more likely associated with the eyes that had already underwent myopic optic disc changes and myopia progression.
One thing that also needs to be mentioned is that, unlike with PVDR, the degree of SER at baseline was not a significant covariate affecting myopia progression after multivariate analysis in our study. Although the initial degree of myopia has been regarded as a risk factor for myopia progression, the exact relationship between initial SER and the rate of myopia progression is still inconclusive [26, 27]. While a recent large study conducted in UK showed a higher baseline SER to be associated with faster myopia progression [36], the converse was true for the other large study conducted in China [26]. Furthermore, it is also uncertain whether the degree of initial SER is associated with the myopic changes in the optic disc, such as the development and enlargement of β-PPA. In a previous study, initial SER was not found to be significantly associated with PVDR, the width ratio of β-PPA. The significant factor associated with the PVDR in that study was the degree of change in SER during follow-up rather than initial SER itself, which was highly consistent with our findings [18]. The initial SER appears to be a covariate that might be affected by other individual, environmental, and genetic factors in regression analysis for myopia progression.
The number of parents with myopia was found to be significantly associated with the rate of myopia progression in our univariate analysis, but the association did not remain statistically insignificant after multivariate regression analysis. Previous studies have reported that a parental history of myopia is a predictive factor for the development and progression of myopia with variable relative risks [37–40]. However, it must be said that the methods of evaluating parental myopia history is crucial in this type of analysis to determine the effect on myopia progression. The present study collected the parental history of myopia based on self-report, not direct measures of the parents’ SER. The methods of assessing parental myopia status were variable across studies. Parental myopia is regarded as a marker for not only genes but also a shared family exposure to myopigenic environment. Myopic parents are more likely to create family environments causing myopia progression, such as intensive education or more time spent indoors. The effect of parental myopia on the progression of myopia is still inconclusive.
To the best of our knowledge, this is the first study to evaluate the direct correlation between optic disc features and the rate of myopia progression, which is considered to be the main strength of this study. However, several limitations should also be acknowledged regarding this study. First, owing to the retrospective nature of the study, we were unable to completely evaluate various factors, such as outdoor activities, near work, and environmental factors that can affect myopia progression. Second, as mentioned above, the self-reporting method used for collecting parental data might be a source of inaccuracy or uncertainty in evaluating the relationship between parental myopia and myopia progression in their children, because we could not assess the actual severity of parental myopia. Hence, to better identify factors associated with myopia progression, a well-designed prospective study should be conducted including a comprehensive ophthalmologic examination and environmental survey on both children and their parents. Third, the present study was conducted in a single institution and included a relatively smaller number of subjects. Finally, last but not least, we were unable to measure axial length elongation owing to the retrospective design. Axial length would more clearly reflect the anatomical changes of the eye with the progression of myopia. Therefore, further research including prospective serial assessments of axial length and refractive error would be worthwhile.
In conclusion, children with younger age of myopia onset and smaller β-PPA showed a faster rate of myopia progression. The β-PPA may serve as a predictive factor for future myopia progression in young childhood.
Summary
What was known before
Myopic disc is characterized by tilted optic disc, disc torsion, and peripapillary atrophy, and these changes deteriorate with myopia progression. Therefore, optic disc changes can reflect the degree of myopia. However optic disc changes have not been approved as a prognostic factor for myopia progression.
What this study adds
Younger children and children with smaller beta-zone peripapillary atrophy (beta-PPA) showed a faster myopia progression. Considering that myopia gradually decelerates, this finding indicates that beta-PPA can be considered as a surrogate marker for early stage myopia and a predictive factor for rapid myopia progression.
Supplementary information
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41433-020-0945-6) contains supplementary material, which is available to authorized users.
References
- 1.He M, Huang W, Li Y, Zheng Y, Yin Q, Foster PJ. Refractive error and biometry in older Chinese adults: the Liwan eye study. Investig Ophthalmol Vis Sci. 2009;50:5130–6. doi: 10.1167/iovs.09-3455. [DOI] [PubMed] [Google Scholar]
- 2.Jung SK, Lee JH, Kakizaki H, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in seoul, South Korea. Investig Ophthalmol Vis Sci. 2012;53:5579–83. doi: 10.1167/iovs.12-10106. [DOI] [PubMed] [Google Scholar]
- 3.Kuang TM, Tsai SY, Liu CJ, Ko YC, Lee SM, Chou P. Seven-year incidence of uncorrected refractive error among an elderly Chinese population in Shihpai, Taiwan: the Shihpai Eye Study. Eye. 2016;30:570–6. doi: 10.1038/eye.2015.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 5.Sawada A, Tomidokoro A, Araie M, Iwase A, Yamamoto T, Tajimi Study G. Refractive errors in an elderly Japanese population: the Tajimi study. Ophthalmology. 2008;115:363–70 e363. doi: 10.1016/j.ophtha.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Zhou J, Zhao P, Lian J, Zhu H, Zhou Y, et al. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Investig Ophthalmol Vis Sci. 2012;53:7504–9. doi: 10.1167/iovs.11-8343. [DOI] [PubMed] [Google Scholar]
- 7.Wong TY, Foster PJ, Hee J, Ng TP, Tielsch JM, Chew SJ, et al. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Investig Ophthalmol Vis Sci. 2000;41:2486–94. [PubMed] [Google Scholar]
- 8.Goldschmidt E. Ocular morbidity in myopia. Acta Ophthalmol Suppl. 1988;185:86–87. doi: 10.1111/j.1755-3768.1988.tb02673.x. [DOI] [PubMed] [Google Scholar]
- 9.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–91. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–5. doi: 10.1016/S0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 11.Galvis V, Tello A, Camacho PA, Parra MM, Merayo-Lloves J. Bio-environmental factors associated with myopia: an updated review. Arch Soc Esp Oftalmol. 2017;92:307–25. doi: 10.1016/j.oftal.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–48. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123:697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285–91. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 15.Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123:391–9. doi: 10.1016/j.ophtha.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126:113–24. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Sankaridurg P, Tran HDM. The lowdown on low-concentration atropine for myopia progression. Ophthalmology. 2019;126:125–6. doi: 10.1016/j.ophtha.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Kim TW, Kim M, Weinreb RN, Woo SJ, Park KH, Hwang JM. Optic disc change with incipient myopia of childhood. Ophthalmology. 2012;119:21-26 e21–23.. doi: 10.1016/j.ophtha.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Kim M, Choung HK, Lee KM, Oh S, Kim SH. Longitudinal changes of optic nerve head and peripapillary structure during childhood myopia progression on OCT: Boramae Myopia Cohort Study Report 1. Ophthalmology. 2018;125:1215–23. doi: 10.1016/j.ophtha.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Nonaka A, Hangai M, Akagi T, Mori S, Nukada M, Nakano N, et al. Biometric features of peripapillary atrophy beta in eyes with high myopia. Investig Ophthalmol Vis Sci. 2011;52:6706–13. doi: 10.1167/iovs.11-7580. [DOI] [PubMed] [Google Scholar]
- 21.Samarawickrama C, Mitchell P, Tong L, Gazzard G, Lim L, Wong TY, et al. Myopia-related optic disc and retinal changes in adolescent children from singapore. Ophthalmology. 2011;118:2050–7. doi: 10.1016/j.ophtha.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Kim JA, Kim TW, Lee EJ, Hwang JM. Development of optic disc torsion in children. Korean J Ophthalmol. 2019;33:173–80. doi: 10.3341/kjo.2018.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Y, Jonas JB, Huang H, Wang M, Sun X. Microstructure of parapapillary atrophy: beta zone and gamma zone. Invest Ophthalmol Vis Sci. 2013;54:2013–8. doi: 10.1167/iovs.12-11255. [DOI] [PubMed] [Google Scholar]
- 25.Lee JE, Sung KR, Park JM, Yoon JY, Kang SY, Park SB, et al. Optic disc and peripapillary retinal nerve fiber layer characteristics associated with glaucomatous optic disc in young myopia. Graefes Arch Clin Exp Ophthalmol. 2017;255:591–8. doi: 10.1007/s00417-016-3542-4. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Ding X, Long W, He M, Yang X. Longitudinal changes in spherical equivalent refractive error among children with preschool myopia. Investig Ophthalmol Vis Sci. 2019;60:154–60. doi: 10.1167/iovs.18-24862. [DOI] [PubMed] [Google Scholar]
- 27.Shih YF, Ho TC, Hsiao CK, Lin LL. Long-term visual prognosis of infantile-onset high myopia. Eye. 2006;20:888–92. doi: 10.1038/sj.eye.6702035. [DOI] [PubMed] [Google Scholar]
- 28.Hyman L, Gwiazda J, Hussein M, Norton TT, Wang Y, Marsh-Tootle W, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123:977–87. doi: 10.1001/archopht.123.7.977. [DOI] [PubMed] [Google Scholar]
- 29.Jung SI, Han J, Kwon JW, Kim DG, Kim DH, Lim HT. Analysis of myopic progression in childhood using the Korea National Health and Nutrition Examination Survey. J Korean Ophthalmological Soc. 2016;57:1430–4. doi: 10.3341/jkos.2016.57.9.1430. [DOI] [Google Scholar]
- 30.Fan D, Cheung E, Lai R, Kwok A, Lam D. Myopia progression among preschool Chinese children in Hong Kong. Ann Acad Med Singap. 2004;33:39–43. [PubMed] [Google Scholar]
- 31.Chua SYL, Sabanayagam C, Cheung Y-B, Chia A, Valenzuela RK, Tan D, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016;36:388–94. doi: 10.1111/opo.12305. [DOI] [PubMed] [Google Scholar]
- 32.Siegwart JT, Jr., Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vis Res. 1999;39:387–407. doi: 10.1016/S0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 33.Jonas JB, Jonas SB, Jonas RA, Holbach L, Dai Y, Sun X, et al. Parapapillary atrophy: histological gamma zone and delta zone. PLoS ONE. 2012;7:e47237. doi: 10.1371/journal.pone.0047237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim M, Kim T-W, Weinreb RN, Lee EJ. Differentiation of parapapillary atrophy using spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1790–7. doi: 10.1016/j.ophtha.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi K, Tomidokoro A, Lee KYC, Konno S, Saito H, Mayama C, et al. Spectral-domain optical coherence tomography of b-zone peripapillary atrophy: influence of myopia and glaucoma. Investig Ophthalmol Vis Sci. 2012;53:1499–505. doi: 10.1167/iovs.11-8572. [DOI] [PubMed] [Google Scholar]
- 36.Wong K, Dahlmann-Noor A. Myopia and its progression in children in London, UK: a retrospective evaluation. J Optom. 2020. 10.1016/j.optom.2019.06.002. [DOI] [PMC free article] [PubMed]
- 37.O’Donoghue L, Kapetanankis VV, McClelland JF, Logan NS, Owen CG, Saunders KJ, et al. Risk factors for childhood myopia: findings from the NICER Study. Investig Ophthalmol Vis Sci. 2015;56:1524–30. doi: 10.1167/iovs.14-15549. [DOI] [PubMed] [Google Scholar]
- 38.Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children’s refractive error. Investig Ophthalmol Vis Sci. 2002;43:3633–40. [PubMed] [Google Scholar]
- 39.Ip JM, Huynh SC, Robaei D, Rose KA, Morgan IG, Smith W, et al. Ethnic differences in the impact of parental myopia: findings from a population-based study of 12-year-old Australian children. Investig Ophthalmol Vis Sci. 2007;48:2520–8. doi: 10.1167/iovs.06-0716. [DOI] [PubMed] [Google Scholar]
- 40.Wu MM, Edwards MH. The effect of having myopic parents: an analysis of myopia in three generations. Optom Vis Sci. 1999;76:387–92. doi: 10.1097/00006324-199906000-00018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.