Abstract
Prior observational studies have suggested that medications targeting the renin–angiotensin system, such as angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs), may be associated with decreased PTSD symptoms. Given known sex differences in PTSD prevalence and cardiovascular disease, here we tested whether the effects of ACE-I/ARB status on PTSD differ by sex. We also expanded these observations with replication analyses in a large biorepository database. Participants in the initial sample included 840 trauma-exposed individuals recruited as part of the Grady Trauma Project. The Modified PTSD Symptom Scale (M-PSS) was administered and ACE-I/ARB status was determined by self-report. Replication analyses were conducted using a large biorepository database (Partners Healthcare Biobank, N = 116,389) with diagnoses and medication status based on available electronic health records. Among individuals treated with ACE-Is/ARBs in the initial sample, women had significantly higher M-PSS total and Re-experiencing severity compared to men (p’s < 0.05). Analyses with the large biorepository sample robustly replicated the overall effects of ACE-I/ARB medication associated with lower rate of PTSD diagnosis (p < 0.001). We also demonstrated that this effect may be specific to the renin–angiotensin system as it did not replicate for beta-blockers, calcium channel blockers, or diuretics. When we examined more specific drug classes, results indicated that the ACE-I/ARB effect on PTSD may be driven more by ARBs (e.g., Losartan) than by ACE-Is. Post-hoc analyses indicated that racial differences may exist in these effects. Overall, our results replicate and extend prior observations that the renin–angiotensin system is associated with PTSD. Medications targeting this system may be worthy of further investigation for PTSD treatment. Our findings suggest that sex and race effects should be considered in future treatment research.
Subject terms: Stress and resilience, Post-traumatic stress disorder
Introduction
Posttraumatic stress disorder (PTSD) is a highly impairing psychiatric disorder that affects ~8% of the population. Notably, its prevalence is twice as high in women compared to men [1]. Symptoms of PTSD include unwanted re-experiencing of the traumatic event, mental and behavioral avoidance of trauma-related reminders, negative changes in cognition and mood, and hyperarousal [2]. One of the enduring phenotypes of PTSD is an overactive fear response resulting from exaggerated sympathetic arousal (e.g., increased sweating, heart rate, blood pressure) coupled with a decreased ability to regulate this response due to poor parasympathetic control (e.g., low heart rate variability; see Michopoulos et al. [3] for a review). It is thought that these autonomic deficits underlie the increased risk of cardiovascular disease (CVD) that has been observed in PTSD [4–6]. Specifically, individuals with PTSD demonstrate higher rates of stroke, heart failure, renal dysfunction, hypertension, and angina compared to healthy and trauma-exposed controls [7]. Notably, increased CVD and hypertension may also lead to increased prescriptions for anti-hypertensive medications in those with PTSD.
The renin–angiotensin system has recently been directly implicated in the PTSD-CVD link due to its involvement in sympathetic arousal. This hormone system is particularly involved in blood pressure regulation through its release of renin. When stress activates the sympathetic nervous system, renin is released into the bloodstream and activates angiotensinogen, which cleaves to form angiotensin I. The angiotensin-converting enzyme (ACE) converts angiotensin I to angiotensin II, and it is this peptide that causes vascular resistance and increases blood pressure. Importantly, angiotensin II also increases norepinephrine release and prevents its reuptake, further increasing sympathetic activity [8–11]. Thus, given the well-established sympathetic overactivation observed in PTSD, the renin–angiotensin system is an important target for better understanding the increased risk of CVD in PTSD.
Recent studies probing the renin–angiotensin system in trauma-exposed samples provide further support for its potential role in PTSD and CVD. For example, circulating levels of renin appear to be higher among those with trauma exposure compared to healthy controls, and highest among those with PTSD [12]. Blockade of the renin–angiotensin system via ACE-Inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) has been associated with decreased anxiety and stress [13, 14], and retrospective studies from our group found that trauma-exposed individuals taking ACE-Is or ARBs (generally for hypertension) report fewer PTSD symptoms than those who were not on such medications [15, 16]. While these findings are promising from a treatment perspective, there are well-established sex differences in PTSD and no prior studies have examined the effect of sex on the association between the renin–angiotensin system and PTSD symptoms.
The current study sought to: (1) examine potential sex differences in the association between ACE-I/ARB drug status and PTSD in the previously examined cohort described above [15, 16], and (2) replicate these findings using a large psychiatric database (The Partners Healthcare Biobank, N = 116,389). In the original cohort, we tested the association between ACE-I/ARB status and PTSD separately within each sex, and then examined sex effects among the sub-sample of individuals who were taking ACE-Is/ARBs. In the replication database, we tested the association between ACE-I/ARB status and PTSD in the total sample (replication of the original Khoury et al. [15] and Nylocks et al. [16] findings), and then separately within each sex. Given that our original study demonstrated specificity of ACE-Is/ARBs and PTSD such that no significant associations were found for other hypertensive medications [15], we also examined the associations among PTSD diagnosis and three other drug classes in the replication database: beta-blockers, calcium channel blockers, and diuretics.
Methods
Primary sample
Participants and procedure
The primary sample consisted of 840 participants for whom medication data were available. Between 2006 and November 2010, participants between the ages of 18–65 were recruited from primary care, specialty, and emergency clinics of Grady Memorial Hospital as part of the Grady Trauma Project [17]. All procedures were approved by the Institutional Review Board at Emory University. See Nylocks et al. [16] for details outlining inclusion and exclusion criteria. Once recruited, all participants completed a battery of self-report measures. Pending completion of the initial study, participants were given the option of enrolling in subsequent studies, including additional self-report measures and structured clinical interviews.
Measures
Lifetime trauma exposure was indexed by the Traumatic Event Inventory (TEI) [18, 19], a 14-item screening tool that systematically assesses trauma type (e.g., natural disaster, combat), exposure type (e.g., experiencing, witnessing), and frequency of traumatic events throughout the individual’s lifetime. The Childhood Trauma Questionnaire (CTQ) [20] is a 28-item measure that was used to assess trauma exposure that occurred ≤18 years of age. The Modified PTSD Symptom Scale (M-PSS) [21] is a psychometrically sound 17-item self-report scale used to measure the presence and severity of current PTSD symptoms, providing both a total severity score and subtotals for each of the DSM-IV PTSD symptom clusters (Re-experiencing, Avoidance/Numbing, and Hyperarousal). The Beck Depression Inventory-II (BDI-II) [22] is a 21-item measure that was used to assess depression severity, with scores ≥20 indicating moderate-to-severe depression.
Medication data
Medication data were collected via self-report during the physician-administered medical exam. ACE-Is included Captopril, Enalapril, Fosinopril, Lisinopril, Quinapril, and Lisinopril/hydrochlorothiazide. ARBs included Losartan, Losartan/hydrochlorothiazide, and Valsartan. Participants who reported being on any of these medications were categorized into the ACE-I/ARB group.
Replication sample
Participants and procedure
Patients who consented to the Partners Healthcare Biobank between April 2010 and April 2020 and were 18 years of age or older were included in this analysis (N = 116,389). The Partners Healthcare Biobank is a biorepository that recruits patients throughout Partners Healthcare (e.g., Brigham and Women’s Hospital, Massachusetts General Hospital, McLean Hospital). Consent is provided when patients sign a Data and Sample Use Agreement, which allows Partners researchers to utilize their deidentified health records. Participants provide blood samples, complete health surveys, and consent to these samples and data being linked to clinical data from Electronic Health Records (EHR). Clinical data were collected from the Partners Healthcare Biobank Portal database.
PTSD and medication data
Diagnosis of PTSD was determined based on the presence of at least one ICD-10 code of PTSD in a patient’s EHR (i.e., based on clinical judgment and not necessarily a structured interview). Medication data were extracted from EHRs for patients ever prescribed ACE-Is, ARBs, beta-blockers, calcium channel blockers, diuretics, and other hypertensive medications.
Data processing and analysis
Primary sample
Chi-square tests were used to test differences in PTSD diagnosis by ACE-I/ARB status within each sex. Univariate analyses of covariance were used to test differences in M-PSS scores by sex, ACE-I/ARB status, and interaction effects. To be consistent with our prior methods, these analyses controlled for potential confounders of age, race, and substance abuse and were conducted with the same dataset [16]. All analyses were conducted using SPSS v.24 with a significance level of p < 0.05.
Replication sample
Chi-square tests were used to test differences in PTSD diagnosis by ACE-I/ARB status. This was conducted both across and within sexes. Consistent with the primary sample, all analyses were conducted using SPSS v.24 with a significance level of p < 0.05.
Results
Primary sample
See Table 1 for demographic characteristics of the primary sample (N = 840). Most participants were women (62.6%; n = 526) and identified as Black (89.6%; n = 753). The average age was 43.14 years. Prevalence of PTSD diagnosis among participants was 33.1% (n = 278), and 22.1% (n = 186) of participants reported taking ACE-Is/ARBs. Men reported significantly more lifetime trauma exposure on the TEI (F[1,700] = 29.11, p < 0.001), while women reported significantly more childhood abuse on the CTQ (F[1,798] = 19.53, p < 0.001) and worse PTSD symptoms on the M-PSS (F[1,762] = 4.79, p = 0.029).
Table 1.
Demographic characteristics: primary sample.
| Male (N = 299) | Female (N = 526) | Missing values | ||||
|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % |
| Discrete | ||||||
| ACE-I or ARB | 64 | 21.4 | 121 | 23.0 | ||
| Current PTSD Diagnosis | 89 | 29.8 | 189 | 35.9 | 64 | 7.6 |
| Race, Black | 268 | 89.6 | 485 | 92.2 | 21 | 2.5 |
| Ethnicity, Non-Hispanic | 180 | 60.2 | 393 | 74.7 | 248 | 29.5 |
| Education | 25 | 3.0 | ||||
| <12th grade | 56 | 18.7 | 142 | 27.0 | ||
| 12th or high school grad | 111 | 37.1 | 168 | 31.9 | ||
| GED | 23 | 7.7 | 28 | 5.3 | ||
| Some college/tech school | 64 | 21.4 | 119 | 22.6 | ||
| Tech school grad | 12 | 4.0 | 22 | 4.2 | ||
| College grad | 21 | 7.0 | 36 | 6.8 | ||
| Graduate school | 7 | 2.3 | 6 | 1.1 | ||
| Current substance abuse | 25 | 8.4 | 21 | 4.0 | 44 | 5.2 |
| Continuous | M | SD | M | SD | n | % |
| Age | 46.32 | 10.89 | 41.33 | 13.02 | 21 | 2.5 |
Sex data missing for n = 15 participants.
ACE-I angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker.
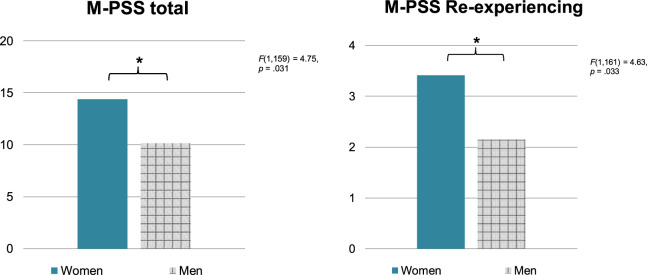
Chi-square analyses conducted separately within each sex did not reveal differences between ACE-I/ARB status and PTSD diagnosis among either men (X2 = 2.00, p = 0.157) or women (X2 = 1.61, p = 0.204). Furthermore, there were no significant differences in PTSD symptom severity (M-PSS scores) among women who were on ACE-Is/ARBs compared to those who were not, F(1,484) = 0.59, p = 0.442. However, men who were on ACE-Is/ARBs were found to have significantly lower M-PSS scores compared to those who were not, F(1,280) = 4.33, p = 0.038. This effect did not remain significant after controlling for age, race, and substance use, F(1,271) = 2.53, p = 0.113. Among individuals who were taking ACE-Is/ARBs, there was not a significant sex difference in PTSD diagnosis, but there was a significant effect for symptom severity, such that women had significantly higher M-PSS scores than men, F(1,168) = 4.22, p = 0.042. This effect remained statistically significant after controlling for age, race, and substance use, F(1,159) = 4.75, p = 0.031 (Fig. 1, left panel).
Fig. 1. PTSD among men vs. women on ACE-Is/ARBs.
Left panel: Total PTSD symptoms. M-PSS modified PTSD symptom scale; *p < 0.05. Right panel: PTSD Re-experiencing symptoms. M-PSS modified PTSD symptom scale; *p < 0.05.
With regard to M-PSS subscales, there were no significant differences in any subscale scores among women or men who were on ACE-Is/ARBs compared to those who were not. Within the group of individuals taking ACE-Is/ARBs, women demonstrated higher M-PSS Re-experiencing scores than men and this was statistically significant after controlling for age, race, and substance use, F(1,161) = 4.63, p = 0.033 (Fig. 1, right panel). There were no significant differences for the Avoidance/Numbing or Hyperarousal subscales.
Replication sample
See Table 2 for demographic characteristics of the Partners Biobank sample (N = 116,389). Most participants were women (56.0%; n = 65,142) and identified as White (84.8%; n = 98,711). The average age was 56.09 years. Prevalence of PTSD diagnosis among participants was 5.0% (n = 5,765) and 32.3% (n = 37,537) of participants were taking ACE-Is/ARBs.
Table 2.
Demographic characteristics: replication sample.
| Male (N = 51244) | Female (N = 65142) | Missing values | ||||
|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % |
| Discrete | ||||||
| ACE-I or ARB | 20,702 | 40.4 | 16,835 | 25.8 | 3 | 0.003 |
| Current PTSD Diagnosis | 1855 | 3.6 | 3910 | 6 | 3 | 0.003 |
| Race | 5872 | 5.05 | ||||
| African American or Black | 2118 | 4.1 | 3434 | 5.3 | ||
| White | 44,252 | 86.4 | 54,457 | 83.6 | ||
| Mixed or Other | 2368 | 4.6 | 3888 | 6.0 | ||
| Ethnicity, Non-Hispanic | 49,992 | 97.6 | 62,678 | 96.2 | 3 | 0.003 |
| Education | 65,494 | 56.3 | ||||
| <12th grade | 341 | 0.7 | 388 | 0.6 | ||
| 12th/high school grad/GED | 1699 | 3.3 | 2412 | 3.7 | ||
| Some college/tech school | 2228 | 4.3 | 3586 | 5.5 | ||
| College/tech school grad | 7530 | 14.7 | 12,963 | 19.9 | ||
| Graduate school | 8620 | 16.8 | 11,128 | 17.1 | ||
| Substance abuse | 12,446 | 24.3 | 11,433 | 17.6 | 3 | 0.003 |
| Continuous | M | SD | M | SD | n | % |
| Age | 58.55 | 17.1 | 54.15 | 17.6 | 3 | 0.003 |
ACE-I angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker.
Replicating the original findings of Khoury et al. [15], results of a chi-square test indicated that there was a significant univariate association between PTSD diagnosis and the presence of ACE-Is/ARBs. Specifically, 29.4% (n = 1696) of the 5765 individuals with PTSD were on ACE-I’s/ARBs, while 32.4% (n = 35,841) of the 110,624 individuals without PTSD were on ACE-Is/ARBs, X2 = 22.27, p < 0.001. When we broke these analyses down by more specific drug classes, we observed that significant effects remained for the ARB class, and Losartan in particular (in the same direction; p < 0.001), but not for ACE-Is (see Table 3 for a summary of all chi-square analyses).
Table 3.
Summary of Chi-square tests.
| PTSD | No PTSD | Analysis | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | df | χ2 | p | |
| Primary sample | |||||||
| ACE-Is/ARBS | |||||||
| Totala | 26 | 14.4 | 72 | 22.2 | 1 | 4.40 | 0.036* |
| Females | 37 | 19.6 | 74 | 24.5 | 1 | 1.61 | 0.102 |
| Males | 14 | 15.7 | 45 | 23.1 | 1 | 2.00 | 0.079 |
| Replication sample | |||||||
| ACE-Is/ARBS | |||||||
| Total | 1696 | 29.4 | 35841 | 32.4 | 1 | 22.27 | <0.001** |
| Females | 986 | 25.2 | 15849 | 25.9 | 1 | 0.85 | 0.356 |
| Males | 710 | 38.3 | 19992 | 40.5 | 1 | 3.61 | 0.058 |
| ACE-Is | |||||||
| Total | 1519 | 26.3 | 29461 | 26.6 | 1 | 0.23 | 0.636 |
| Females | 869 | 22.2 | 12748 | 20.8 | 1 | 4.39 | 0.036* |
| Males | 650 | 35.0 | 16713 | 33.8 | 1 | 1.15 | 0.283 |
| ARBs | |||||||
| Total | 514 | 8.9 | 13853 | 12.5 | 1 | 65.88 | <0.001** |
| Females | 335 | 8.6 | 6665 | 10.9 | 1 | 20.57 | <0.001** |
| Males | 179 | 9.6 | 7188 | 14.6 | 1 | 34.93 | <0.001** |
| Losartan | |||||||
| Total | 397 | 6.9 | 10063 | 9.1 | 1 | 32.73 | <0.001** |
| Females | 255 | 6.5 | 4929 | 8.0 | 1 | 11.72 | 0.001* |
| Males | 142 | 7.7 | 5134 | 10.4 | 1 | 14.53 | <0.001** |
| Beta-blockers | |||||||
| Total | 2894 | 50.2 | 45181 | 40.8 | 1 | 197.90 | <0.001** |
| Females | 1879 | 48.1 | 22464 | 36.7 | 1 | 202.99 | <0.001** |
| Males | 1015 | 54.7 | 22717 | 46.0 | 1 | 54.69 | <0.001** |
| Calcium channel blockers | |||||||
| Total | 1456 | 25.3 | 28117 | 25.4 | 1 | 0.08 | 0.784 |
| Females | 894 | 22.9 | 13414 | 21.9 | 1 | 1.97 | 0.161 |
| Males | 562 | 30.3 | 14703 | 29.8 | 1 | 0.24 | 0.626 |
| Diuretics | |||||||
| Total | 2102 | 36.5 | 37293 | 33.7 | 1 | 18.51 | <0.001** |
| Females | 1403 | 35.9 | 19327 | 31.6 | 1 | 31.60 | <0.001** |
| Males | 699 | 37.7 | 17966 | 36.4 | 1 | 1.32 | 0.251 |
ACE-I angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker.
*p < 0.05; **p < 0.001.
afrom Khoury et al., 2010, N = 505.
To address a potential sex difference in the effect of ACE-I/ARB status on PTSD diagnosis, we conducted chi-square analyses within each sex. Prevalence of PTSD diagnosis within women was 6.0% (n = 3910), while prevalence within men was 3.6% (n = 1855). Within women there was no significant association between PTSD diagnosis and ACE-I/ARB status: 25.2% (n = 986) of the 3910 women with PTSD were on ACE-Is/ARBs and 25.9% (n = 15,849) of the 61,232 women without PTSD were on ACE-Is/ARBs, X2 = 0.85, p = 0.356. Within men there was a trend-level association found between PTSD diagnosis and ACE-I/ARB status: 38.3% (n = 710) of the 1855 men with PTSD were on ACE-Is/ARBs, while 40.5% (n = 19,992) of the 49,392 men without PTSD were on ACE-Is/ARBs, X2 = 3.61, p = 0.058. When broken down by specific drug class, both men and women without PTSD were more likely to be on ARBs, including Losartan, compared to those with PTSD, p’s < 0.001. However, the opposite effect was observed for ACE-Is in women, such that those with PTSD were more likely to be on these medications than those without PTSD, X2 = 4.39, p = 0.036. No effect of ACE-Is was observed in men. Overall, these findings are consistent with those in the primary sample and suggest that sex should be considered in future analyses of ACE-Is/ARBs for PTSD.
While our original analyses demonstrated no significant associations between beta-blockers or diuretics and PTSD [15], analyses with the replication sample indicated the opposite effect of these medications compared to that of ACE-Is/ARBs (Table 3). Specifically, 50.2% (n = 2894) of the 5765 individuals with PTSD were on beta-blockers, while 40.8% (n = 45,181) of the 110,624 individuals without PTSD were on beta-blockers, X2 = 197.90, p < 0.001. Similarly, 36.5% (n = 2102) of the 5765 individuals with PTSD were on diuretics, while 33.7% (n = 37,293) of the 110,624 individuals without PTSD were on diuretics, X2 = 197.90, p < 0.001. We then ran these analyses by sex and both effects remained significant in women (p < 0.001), while only the effects of beta-blockers remained significant in men (p < 0.001). No significant effects were observed for calcium channel blockers. These findings suggest that there may be specificity in the ACE-I/ARB association with decreased PTSD symptoms, providing further evidence of the relevance of the renin–angiotensin system.
Post-hoc analyses
Given the high comorbidity of PTSD and depression [1, 23], as well as potential beneficial effects seen with ACE-Is/ARBs in depression [24], post-hoc analyses probed the association between ACE-I/ARB status and depression. In the Primary Sample, this was done using a dichotomous variable where BDI-II scores ≥20 were coded as “1” and lower scores were coded as “0.” In the Replication Sample, this was done by identifying individuals with a diagnosis of major depression in their medical record. The chi-square analysis in the Primary Sample was not significant (X2 = 2.32, p = 0.127), and the analysis in the Replication Sample was significant but in the opposite direction (X2 = 950.78, p < 0.001). Overall, this suggests that there may be more specificity of ACE-I/ARB medication for PTSD or anxiety symptoms over depression symptoms.
Post-hoc analyses were also conducted to probe racial differences in the Replication Sample (we were not sufficiently powered to do so in the Primary Sample). These analyses indicated that both the overall ACE-I/ARB finding and the finding in ARBs were significant in White but not Black individuals (male and female), and there were no differences in ACE-Is. The findings in beta-blockers were replicated across race and sex, suggesting that higher percentages of both White and Black individuals with PTSD were on beta-blockers compared to those without PTSD. There were no significant differences in calcium channel blockers or diuretics among Black individuals. It should be noted that the sample of Black individuals (n = 2133) was much smaller than that of White individuals (n = 32,490); therefore, these racial differences in effects should be interpreted with caution.
Discussion
In this study, we were able to replicate prior research in a much larger sample demonstrating a significant association between ACE-I/ARB status and PTSD. This effect was specific to the renin–angiotensin system given that it was not observed for other hypertension medications, and it may be driven more by ARBs (e.g., Losartan) than by ACE-Is. Furthermore, our findings suggest that sex and race should be considered in future studies of ACE-Is/ARBs and PTSD.
Analyses in our primary sample (see Khoury et al. [15] and Nylocks et al. [16]) suggested that the original finding regarding a beneficial effect of taking ACE-Is/ARBs may be moderated by sex, such that women on ACE-Is/ARBs exhibited worse overall PTSD severity and higher Re-experiencing symptoms compared to men. This finding is consistent with earlier research demonstrating that the Re-experiencing cluster is most strongly associated with fear inhibition in PTSD [25]. Although the Hyperarousal cluster indexes some aspects of sympathetic arousal (e.g., hypervigilance), the Re-experiencing cluster is most specific to PTSD and its underlying fear circuitry because it indexes emotional and psychophysiological responses to trauma reminders, as well as flashbacks and nightmares. A possible mechanism underlying the observed sex difference is the effect of estrogen. Specifically, low levels of estrogen have been associated with worse PTSD symptoms [26] and worse fear inhibition among women [27, 28], and estrogen levels significantly decrease following menopause [29]. Given that ACE-Is/ARBs are typically prescribed for hypertension, which is more common among older adults, it follows that women on ACE-Is/ARBs are significantly older than those who are not (this was the case in our primary and replication samples). Thus, these women are likely to have significantly lower estrogen levels than women who are not on such medications. It is therefore critical for future research to test sex differences in the association between ACE-Is/ARBs and PTSD, whether they are accounted for by estrogen, and how these medications work for women across the age spectrum. At a minimum, our results suggest that future research testing ACE-Is/ARBs for PTSD treatment should consider sex as a moderating factor as their efficacy may differ for men versus women.
Findings from our replication analyses were consistent with the original ACE-I/ARB finding in PTSD [15] and extend this work by demonstrating differential effects of ACE-Is versus ARBs. Using a large biorepository database (N = 116,389), we were able to replicate the observation that there is a significant association between ACE-I/ARB status and PTSD diagnosis, such that significantly more individuals without PTSD were on ACE-Is/ARBs compared to those with PTSD. We also demonstrated that this effect was driven by ARBs (e.g., Losartan) rather than ACE-Is, and that it was not observed for beta-blockers, calcium channel blockers, or diuretics. One possible explanation for the higher percentage of individuals with PTSD on beta-blockers is that this class of medication is often used off-label for PTSD treatment (e.g., propanolol). In contrast, diuretics, ACE-Is/ARBs, and calcium channel blockers are not generally used off-label for PTSD or stress-related disorders. Rather, we believe that ours and other data suggest that individuals with PTSD are more likely to have CVD, sympathetic arousal, and potentially hypertension, leading to increased prescription of anti-hypertensives. This may also suggest that while all three classes are likely to be used in PTSD, ACE-Is/ARBs could have a unique effect because of their mechanistic action. Whereas beta-blockers inhibit the action of epinephrine and norepinephrine (resulting in decreased sympathetic activity), ACE-Is/ARBs inhibit angiotensin II (resulting in vasodilation). Further, ACE-Is inhibit angiotensin II by blocking its synthesis by the ACE, while ARBs inhibit angiotensin II by blocking it from binding to the AT1 receptor. Work from our group has suggested that direct blockade of the AT1 receptor with Losartan (an ARB) decreases fear and increases extinction in mice, at least in part through effects directly within the brain [30]. Additional research probing AT1 receptor inhibition via ARBs will be needed in humans to further explore this mechanism and why it may have a unique role in PTSD.
Overall, our findings appear to have specificity and support our prior research showing that the renin–angiotensin system may be implicated in PTSD, making it a worthy target of future treatment investigations. Such trials are necessary to determine if these classes of medications are useful in treating PTSD, as well as mechanisms underlying their effects (e.g., reduced sympathetic arousal). The trend-level finding regarding sex differences in the replication sample suggests that future analyses of sex are warranted but these findings are insufficient to make a definitive claim that ACE-I/ARB efficacy differs by sex. It is noteworthy that men in the replication sample were significantly more likely to have hypertension in their medical record compared to women (X2 = 2242.19, p < 0.001). Thus, future analyses should also consider cardiovascular measures when examining possible sex effects of ACE-I/ARB efficacy.
These findings have clinical relevance in terms of PTSD treatment, though randomized controlled trials are needed to confirm our cross-sectional results. Our findings suggest that this class of medications is indeed worthy of investigation for PTSD treatment and that sex needs to be accounted for in clinical trials. Future studies are also needed to understand mechanisms underlying the effects of ACE-Is/ARBs, including a role for estrogen. In addition, the renin–angiotensin system was originally probed in PTSD due to its regulation of blood pressure and other aspects of sympathetic arousal, but no studies have tested whether individuals with PTSD on ACE-Is/ARBs exhibit decreased renin–angiotensin system activity or sympathetic arousal (beyond what would be expected given the intended effects of these medications). Rodent studies have demonstrated that renin–angiotensin system inhibition decreases stress hormone responding and improves fear inhibition [30–32], but these have yet to be translated to prospective human trials.
The primary limitation of the current study is its cross-sectional nature. This limits any causal inferences that could be made regarding the efficacy of ACE-Is/ARBs for PTSD, and it precludes us from determining if ACE-Is/ARBs were prescribed as treatment of PTSD, which is unlikely, or if those on ACE-Is/ARBs were less likely to develop PTSD as a result of being on this class of medications. In addition, while a large sample size is a strength of the replication sample, PTSD diagnostic data and medication data were obtained from medical charts and not from a formal clinical interview. We therefore cannot say with certainty that all PTSD diagnoses (or absence thereof) were valid. Similarly, we cannot say with certainty that individuals took the medications that were indicated in their medical charts. Among those with ACE-I prescriptions, 85.4% had at least one recurrence of the prescription in their chart, and among those with ARB prescriptions, 85.2% had at least one recurrence. This may indicate a greater likelihood that those individuals took their prescribed medication, but we still cannot make a definitive conclusion. Further, we were unable to determine the influence of CVD risk factors or treatment seeking/frequency of medical visits in our analyses. Another limitation of the replication sample is the lack of PTSD severity measures, which prohibited us from fully replicating our primary cohort analyses with the M-PSS. Finally, an important limitation is that our post-hoc analyses indicated a race-specific effect in the replication sample. The original ACE-I/ARB and PTSD finding was observed among a sample of predominantly Black individuals (the primary sample; see Khoury et al. [15]). However, the effect of ACE-I/ARB status on PTSD was not present among the relatively smaller subset of Black individuals in the replication sample (it was only significant among those who were White). While this could be due to large differences in sample size, it is also possible that there are important differences between these samples that affect the association between ACE-Is/ARBs and PTSD. For example, the primary sample was recruited from a highly traumatized population in Atlanta, GA, while the replication sample consisted of individuals from the Boston area who were not selected on the basis of trauma exposure. Further, participants in the replication sample were all patients of the Partners healthcare system, while those in the primary sample had more variable healthcare. Thus, several factors beyond race could explain the differential findings in these samples and should be considered in future replication studies.
In conclusion, results of the current study replicated prior research suggesting that there is an association between ACE-I/ARB medication status and PTSD. This effect may be stronger in men versus women, which should be considered in future treatment research. Other considerations for future research include the need to study mechanisms underlying these effects, such as estrogen levels, autonomic functioning, and renin–angiotensin system activity. Our findings support prior work demonstrating that the renin–angiotensin system is likely implicated in PTSD and provide further evidence that it may be a useful treatment target for PTSD, when considering the possible important effects of sex and race differences.
Funding and disclosure
AVS supported by AHA 20CDA35310031. KJR supported by NIH R21MH112956, P50MH115874, R01MH094757, and R01MH106595, the Frazier Foundation Grant for Mood and Anxiety Research, and Partners Healthcare Biobank. KJR and MBS supported by DoD W81XWH-15-2-0090. PJM supported by NIH 1R01HL137103-01A1 and 3R01HL137103-02S1. KJR has received consulting income from Alkermes, research support from NIH, Genomind and Brainsway, and he is on scientific advisory boards for Janssen and Verily, all of which is unrelated to the present work. MBS has received consulting income in the past 3 years from Acadia, Aptinyx, Bionomics, GW Pharma, and Janssen, and research support from NIH, the Department of Veterans Affairs, and the Department of Defense. CFG has received consulting income from Cohen Veterans Bioscience which is unrelated to the present work. AVS, LAD, JBM, VM, PJM have no biomedical financial interests or conflicts of interest.
Author contributions
AVS analyzed the data and wrote the manuscript. LAD analyzed the data and contributed to writing the manuscript. JBM, PJM, and MBS contributed to writing the manuscript. VM, CFG, and KJR conceived and executed the parent project, and contributed to editing the manuscript. All authors reviewed and edited the final manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- 3.Michopoulos V, Norrholm SD, Jovanovic T. Diagnostic biomarkers for posttraumatic stress disorder: promising horizons from translational neuroscience research. Biol Psychiatry. 2015;78:344–53. doi: 10.1016/j.biopsych.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J. 2013;166:806–14. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmondson D, von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4:320–9. doi: 10.1016/S2215-0366(16)30377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers B. Corticolimbic regulation of cardiovascular responses to stress. Physiol Behav. 2017;172:49–59. doi: 10.1016/j.physbeh.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spitzer C, Barnow S, Völzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosom Med. 2009;71:1012–7. doi: 10.1097/PSY.0b013e3181bc76b5. [DOI] [PubMed] [Google Scholar]
- 8.Byku M, Macarthur H, Westfall TC. Nerve stimulation induced overflow of neuropeptide Y and modulation by angiotensin II in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H2188–97. doi: 10.1152/ajpheart.00384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackins CJ, Kano S, Seyedi N, Schäfer U, Reid AC, Machida T, et al. Cardiac mast cell–derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Investig. 2006;116:1063–70.. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin II–salt hypertension in the rat. Exp Physiol. 2010;95:61–8. doi: 10.1113/expphysiol.2008.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid AC, Mackins CJ, Seyedi N, Levi R, Silver RB. Coupling of angiotensin II AT1 receptors to neuronal NHE activity and carrier-mediated norepinephrine release in myocardial ischemia. Am J Physiol Heart Circ Physiol. 2004;286:H1448–54. doi: 10.1152/ajpheart.01062.2003. [DOI] [PubMed] [Google Scholar]
- 12.Terock J, Hannemann A, Janowitz D, Freyberger HJ, Felix SB, Dörr M, et al. Associations of trauma exposure and post-traumatic stress disorder with the activity of the renin-angiotensin-aldosterone-system in the general population. Psychol Med. 2019;49:843–51. doi: 10.1017/S0033291718001496. [DOI] [PubMed] [Google Scholar]
- 13.Weber MA. Angiotensin-II receptor blockers for hypertension and heart failure: quality of life and outcomes. Manag Care Interface. 2005;18:47–54. [PubMed] [Google Scholar]
- 14.Zanchetti A, Elmfeldt D. Findings and implications of the Study on Cognition and Prognosis in the Elderly (SCOPE)—a review. Blood Press. 2006;15:71–79. doi: 10.1080/08037050600771583. [DOI] [PubMed] [Google Scholar]
- 15.Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73:849–55. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nylocks KM, Michopoulos V, Rothbaum AO, Almli L, Gillespie CF, Wingo A, et al. An angiotensin-converting enzyme (ACE) polymorphism may mitigate the effects of angiotensin-pathway medications on posttraumatic stress symptoms. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:307–15. doi: 10.1002/ajmg.b.32313. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–14. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv. 2005;56:212–5. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz AC, Bradley R, Penza KM, Sexton M, Jay D, Haggard PJ, et al. Pain medication use among patients with posttraumatic stress disorder. Psychosomatics. 2006;47:136–42. doi: 10.1176/appi.psy.47.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. Behav Ther. 1993;16:161–2. [Google Scholar]
- 22.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonia, TX: Psychological Corporation; 1996. [Google Scholar]
- 23.Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J Trauma Stress. 2013;26:299–309. doi: 10.1002/jts.21814. [DOI] [PubMed] [Google Scholar]
- 24.Vian J, Pereira C, Chavarria V, Köhler C, Stubbs B, Quevedo J, et al. The renin–angiotensin system: a possible new target for depression. BMC Med. 2017;15:144. doi: 10.1186/s12916-017-0916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167:151–60. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glover EM, Jovanovic T, Norrholm SD. Estrogen and extinction of fear memories: implications for posttraumatic stress disorder treatment. Biol Psychiatry. 2015;78:178–85. doi: 10.1016/j.biopsych.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiol Learn Mem. 2014;116:145–54. doi: 10.1016/j.nlm.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longcope C, Franz C, Morello C, Baker R, Johnston CC., Jr Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas. 1986;8:189–96. doi: 10.1016/0378-5122(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 30.Marvar PJ, Goodman J, Fuchs S, Choi DC, Banerjee S, Ressler KJ. Angiotensin type 1 receptor inhibition enhances the extinction of fear memory. Biol Psychiatry. 2014;75:864–72. doi: 10.1016/j.biopsych.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurt RC, Garrett JC, Keifer OP, Jr, Linares A, Couling L, Speth RC, et al. Angiotensin type 1a receptors on corticotropin-releasing factor neurons contribute to the expression of conditioned fear. Genes Brain Behav. 2015;14:526–33. doi: 10.1111/gbb.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saavedra JM, Benicky J. Brain and peripheral angiotensin II play a major role in stress. Stress. 2007;10:185–93. doi: 10.1080/10253890701350735. [DOI] [PubMed] [Google Scholar]