Abstract
The dopamine transporter (DAT) has been implicated in a variety of arousal-related processes including the regulation of motor activity, learning, motivated behavior, psychostimulant abuse, and, more recently, sleep/wake state. We previously demonstrated that DAT uptake regulates fluctuations in extracellular dopamine (DA) in the striatum across the light/dark cycle with DA levels at their highest during the dark phase and lowest during the light phase. Despite this evidence, whether fluctuations in DA uptake across the light/dark cycle are associated with changes in sleep/wake has not been tested. To address this, we employed a combination of sleep/wake recordings, fast scan cyclic voltammetry, and western blotting to examine whether sleep/wake state and/or light/dark phase impact DA terminal neurotransmission in male rats. Further, we assessed whether variations in plasma membrane DAT levels and/or phosphorylation of the threonine 53 site on the DAT accounts for fluctuations in DA neurotransmission. Given the extensive evidence indicating that psychostimulants increase DA through interactions with the DAT, we also examined to what degree the effects of cocaine at inhibiting the DAT vary across sleep/wake state. Results demonstrated a significant association between individual sleep/wake states and DA terminal neurotransmission, with higher DA uptake rate, increased phosphorylation of the DAT, and enhanced cocaine potency observed after periods of sleep. These findings suggest that sleep/wake state influences DA neurotransmission in a manner that is likely to impact a host of DA-dependent processes including a variety of neuropsychiatric disorders.
Subject terms: Circadian rhythms and sleep, Transporters in the nervous system
Introduction
The dopamine (DA) system has been implicated in a variety of arousal-related processes including the regulation of motor activity, learning, motivated behavior, psychostimulant abuse, and sleep/wake activity. Although numerous observations highlight the importance of DA neuron activity in regulating striatal DA levels [1, 2], recent advances highlight the influence of the axonal dopamine transporter (DAT) in controlling the intensity and duration of DA actions in the striatum [3–8].
The DAT is commonly regarded as a homeostatic regulator that governs extracellular DA in response to acute and long-term physiological demands. Increases in DAT function and phosphorylation have been observed in attention deficit hyperactivity disorder and substance use disorders while decreases in DAT binding has been observed in Parkinson’s disease, presumably to compensate for sustained dysregulation of DA neurotransmission [5, 9–11]. High fat diet and stress significantly decrease DAT function over extended periods of time [12–14]. In addition to these long-term alterations in function, DATs can also undergo rapid changes in response to environmental and extracellular challenges [15–17]. DAT substrates, blockers, and presynaptic receptor ligands, for example, can rapidly regulate DAT function by altering either cell surface expression or posttranslational modifications of the transporter [12, 18–22]. Together, these observations suggest that DATs behave in a malleable fashion on both long and short time scales to tightly control DA’s actions [23].
We previously demonstrated in rodents that DA uptake in the striatum fluctuates in a diurnal manner across the light/dark cycle with the least efficient uptake occurring in the dark phase and most efficient uptake during the light phase [24]. Importantly, these DAT fluctuations were inversely related to extracellular DA levels which could not be accounted for by variations in DA metabolism, tyrosine hydroxylase (TH) activity, or DA D2 receptor (D2R)/D3 receptor (D3R) function, suggesting that extracellular DA cycling was governed by fluctuations in rates of DA uptake [24].
Upon initial consideration, these prior observations could appear to suggest that DAT function may be tracking time of day and/or light/dark conditions. However, the 24 h light/dark cycle is comprised of recurring sequences of distinct sleep/wake states that integrate as greater time spent asleep during the light phase and greater time spent awake during the dark phase. Consequently, it is possible that instead of tracking time of day or light/dark phase, fluctuations in DA uptake observed in our prior work are associated with distinct patterns of sleep/wake architecture that occur across the 24 h cycle.
Here we hypothesize that DAT function tracks sleep/wake state activity such that higher uptake rates occur during periods of sleep and lower uptake rates occur during wakefulness. To test this hypothesis, we performed a series of experiments in rats to examine the degree to which sleep/wake state and light/dark cycle impact DA terminal neurotransmission and phosphorylation of DAT at Thr53 site using a combination of sleep/wake recordings, fast scan cyclic voltammetry (FSCV), and western blotting. Further, given that psychostimulants increase DA through interactions with the DAT, and since there is evidence that DA-dependent behaviors—including psychostimulant self-administration—are strongly influenced by diurnal cycles [25–29], we examined whether the degree of DA uptake inhibition produced by cocaine (cocaine potency) also varies across sleep/wake state. We predicted that sleep/wake state influences DA uptake regardless of light/dark phase via phosphorylation of the DAT at Thr53, and that these fluctuations impact cocaine potency.
Methods
Animal housing and conditions
Experiments were conducted in adult male Sprague-Dawley rats (325–350 g, Envigo, Frederick, MD, USA). Rats were pair-housed prior to surgery and subsequently single-housed in a temperature-controlled room (24 °C) on a 12 h light/dark cycle. To collect brains at the same time of the workday, but at different light/dark phases, lights were on from 3:00 a.m. to 3:00 p.m. for animals sacrificed at zeitgeber time 6 (ZT6; six h into the light phase) and lights were on from 3:00 p.m. to 3:00 a.m. for animals sacrificed at zeitgeber time 18 (ZT18; six h into the dark phase). Rats were given ad libitum access to food, water, and enrichment material. All protocols and animal procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals under the supervision of the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
Surgical procedures
All surgical procedures were performed stereotaxically under aseptic conditions, as described previously [30–32]. Rats were implanted with electroencephalographic/electromyographic (EEG–EMG) electrodes to determine sleep/wake state prior to assessing DA release and uptake via ex vivo FSCV or tissue quantification of key DA proteins. To obtain differential recordings of EEG activity, one screw electrode (Plastics One, Roanoke, VA, USA) was implanted above the frontal cortex (+1.3 mm A/P, +1.3 mm M/L, and −1.0 mm D/V) and a second electrode was implanted ipsilaterally over hippocampus (+2.4 mm A/P, +3.2 mm M/L, and −1.0 mm D/V). A third screw electrode was implanted in contralateral cortex (+1.3 mm A/P, −1.3 mm M/L, and −1.0 mm D/V) and served as an isolated ground. Additionally, two EMG wire electrodes were implanted into the dorsal neck muscle for recording EMG activity. EMG electrodes consisted of 100 mm insulated stainless-steel wires (Cooner Wire, Charsworth, CA, USA) with 2 mm of exposed wire in contact with muscle. Electrodes were routed through a connector (Plastics One) and cemented into place on the skull. Ketoprofen (5 mg/kg s.c. of 5 mg/ml) and Baytril (5 mg/kg s.c. of 5 mg/ml) were provided at the time of surgery and a second dose 12 h later. In addition, antibiotic/analgesic powder (Neopredef, Kalamazoo, MI) was applied around the cranial implant immediately after surgery. Rats recovered for 7 days before sleep recordings began.
Sleep recordings
Rats were placed in an acrylic testing chamber (14 × 14 × 20 inches) housed in a sound-attenuated outer chamber containing an LED light and a fan. Rats were supplied with food and water available ad libitum and connected to recording lines via a commutator which allowed unrestricted movement. After a 16 h acclimation period, EEG–EMG signals were recorded for 24 h. EEG (0.3–100 Hz bandpass) and EMG signals (1–50 Hz bandpass) were amplified, filtered, and recorded using Labchart 7 (AD Instruments, Colorado Springs, CO, USA; Fig. 1). Data were analyzed manually using Sirenia Sleep Pro (Pinnacle Technology, Lawrence, KS, USA) to determine the number of bouts, bout length, and percentage of time spent in wakefulness (Wake), rapid eye movement (REM) and non-REM (NREM) sleep, as previously described [30–32]. Briefly, EEG signals were scored in 10 s bins and sorted into individual frequency bands (Delta = 0.5–4.0 Hz; Theta = 5.5–8.5 Hz; Alpha, Beta, and Gamma = 8–44 Hz). NREM sleep was defined as high-voltage EEG consisting of >50% delta and low-voltage EMG; REM sleep was defined as low-voltage EEG consisting of >50% theta, combined with EMG activity of ~50% lower amplitude than that observed in NREM sleep; and Wake was defined as low-voltage EEG consisting of <40% delta and <20% theta with EMG activity of an average amplitude twice that observed in NREM. To be scored as a distinct epoch, the appropriate EEG and EMG activity patterns were required to persist for a minimum of three bins (30 s).
Fig. 1. Sleep/wake state across a 24 h period is organized into recurring bouts during which sleep primarily occurs during the light phase and wakefulness during the dark phase.
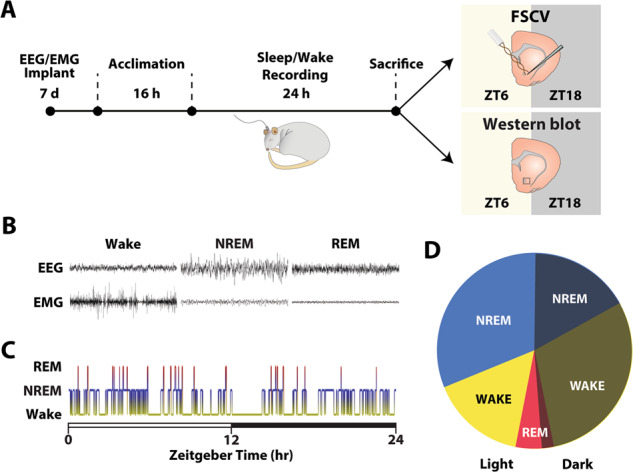
A Rats were implanted with EEG/EMG electrodes. After 7 days of recovery, rats were acclimated to recording chambers for 16 h prior to recording of sleep/wake activity for 24 h. Following sleep recordings, rats were sacrificed 6 h into the light phase (ZT6) or 6 h into the dark-phase (ZT18) and brains were prepared for FSCV recordings in the NAc core. A separate group of rats was subjected to the same sleep recording procedures except that after sacrifice, the NAc was dissected for western blot analysis of plasma membrane fractions of DAT and phosphorylated DAT. B Representative EEG and EMG recordings. C Example hypnogram across a 24 h period. White bar represents the light phase and the black bar represents the dark phase. D Mean percentage of time rats spent in Wake, NREM, and REM during the light and dark phase for the full 24 h recording (n = 18 rats).
To confirm that recordings were representative of normal sleep/wake patterns and consistent with prior reports, the mean percentage of time spent in Wake, NREM and REM during the light and dark phases was analyzed for the full 24 h recording (n = 18 rats; Fig. 1B–D).
Ex vivo slice FSCV procedures
Following sleep recordings, rats were anesthetized with 2.5% isoflurane for 5 min and then decapitated. These anesthesia procedures have been shown previously to have no effect on DA uptake [33]. To control for the effect of light/dark condition, one group of rats was decapitated 6 h into the light phase (ZT6; n = 14) and the other group was decapitated 6 h into the dark-phase (ZT18; n = 16). Animals remained in their corresponding light/dark phase during isoflurane anesthesia prior to decapitation. Brains were rapidly dissected and transferred to oxygenated, ice-cold artificial cerebral spinal fluid (aCSF) containing NaCl (126 mM), KCL (2.5 mM), NaH2PO4 (1.2 mM), CaCl2 (2.4 mM), MgCl2 (1.2 mM), NaHCO3 (25 mM), glucose (11 mM), L-ascorbic acid (0.4 mM), and pH adjusted to 7.4. A vibrating microtome was used to produce 400 µm-thick sections containing the nucleus accumbens (NAc) core. Slices rested at room temperature for 45 min before being transferred into a recording chamber flushed with aCSF (32 °C).
A bipolar stimulating electrode (Plastics One) was placed on the surface of the tissue and a carbon fiber microelectrode was implanted between stimulating electrode leads. DA release was evoked every 3 min using a single electrical pulse (~400 μA, 4 ms, monophasic) and measured using Demon Voltammetry and Analysis software [34]. Baseline DA release and uptake recordings were obtained for the subset of rats in which only single slices were recorded (ZT6, n = 6 slices from six rats; ZT18, n = 7 slices from seven rats) and the subset of rats in which two slices were recorded (ZT6, n = 14 slices from seven rats; ZT18, n = 18 slices and from nine rats). For rats in which two slices were recorded, DA release and uptake measures were averaged to obtain a single data point for those rats. Therefore, we analyzed and report baseline DA release and uptake data for 13 rats at ZT6 and 16 rats at ZT18. Once stable baselines were obtained for all slices (three stimulations with <10% variation), a subset of slices was then exposed to five concentrations of either cocaine (0.3–30 μM; ZT6, n = 9 slices from 9 rats; ZT18 n = 14 slices from 14 rats) or the D2R/D3R agonist quinpirole (3–300 nM; ZT6, n = 12 slices from 12 rats; ZT18 n = 12 slices from 12 rats) as previously described [13, 21, 33, 35–39]. In some cases, one slice was recorded shortly after rats were sacrificed (n = 26) and a second slice was recorded up to 7 h post sacrifice (n = 19). Supplementary Figure 1 demonstrates that the time of recording post sacrifice did not affect DA release or uptake.
DA concentrations were calculated by comparing currents at the peak oxidation potential for DA in consecutive voltammograms with electrode calibrations determined using an in situ calibration method as described previously [40, 41]. To determine if sleep/wake state influences DA terminal neurotransmission, we assessed stimulated DA release, DA uptake rate (Vmax), cocaine-induced DA uptake inhibition (app Km), and the effects of quinpirole on stimulated DA release across various sleep/wake states using a Michaelis–Menten based model [30, 33, 35, 42]. Baseline uptake was determined by setting Km values to 0.18 µM and all cocaine-induced alterations in uptake were attributed to changes in apparent Km. Cocaine inhibition constants (Ki) were determined to calculate the necessary cocaine concentration to produce 50% of DA uptake inhibition. Reductions in stimulated DA release following varying concentrations of quinpirole were expressed as a percent of baseline.
Subcellular fractionation and western blotting
Following sleep recordings, a separate cohort of rats was sacrificed for western blot analysis (ZT6; 13 rats and ZT18; 14 rats). Rats were decapitated, brains extracted, flash frozen in liquid nitrogen, and stored at −80 °C until the day of the assay. Briefly, the NAc core was dissected and subjected to subcellular fractionation methods according to previous work [43, 44]. Between 0.10 and 0.15 g of tissue was homogenized in 1.5 mL of ice-cold PBS containing sucrose (0.32 M) and HEPES (20 mM), EGTA (1 mM), EDTA (1 mM), and protease inhibitors. The homogenate was centrifugated at 1000 × g for 10 min and the pellet discarded. The supernatant (total protein extract) was spun at 12,500 × g for 20 min at 4 °C. Synaptosomes were lysed in hypotonic media on ice for 45 min and centrifuged at 2000 × g for 20 min to obtain the enriched plasma membrane fraction. Previous work using this subcellular fractionation protocol has shown high DAT levels, as well as other membrane proteins including Na+/K+ ATPase in the enriched plasma membrane fraction [43]. Thus, in the current western blot studies, we used the enriched plasma membrane fraction and refer to this fractions as plasma membrane DAT [43]. Protein concentrations were measured using the DCTM protein assay (Bio-Rad), and equal amounts of total protein were loaded on a 10% SDS PAGE gel and immunoblotted for specific antigens: rabbit anti-DAT polyclonal antibody (1:1000, AB2231, EMD Millipore Corp, RRID:AB_1586991), rabbit anti phospho-DAT polyclonal antibody (1:1000, P435-53, PhosphoSolutions, RRID:AB_2492078), and peroxidase-conjugated goat anti-rabbit IgG (H + L) (1:5000, Jackson Immuno Research, RRID:AB_2313586). GAPDH was used as a marker for plasma membrane in the same blots using rabbit anti GAPDH polyclonal antibody (1:5000, PA1-987, Invitrogen, RRID: AB_2107311). GAPDH has been validated as an ideal reference due to a lack of fluctuations in levels across sleep/wake states [45]. Western blot data were quantified by densitometry with ImageQuant LAS4000 (GE Healthcare Bio-Science) and presented as the ratio of total DAT over GAPDH, phosphorylated DAT (pDAT) over GAPDH, or pDAT/GAPDH over DAT/GAPDH [21].
Statistical analyses
Data were analyzed using IBM SPSS Statistics 24 (SPSS Inc, Chicago, IL). Prior in vitro studies indicate that the DAT can undergo rapid posttranslational modifications and trafficking on the order of seconds to minutes [16, 19, 20, 46, 47]. Together with the fast transitions from sleep to awake throughout the day (Fig. 1C), we hypothesized that relatively brief periods of specific sleep/wake states prior to sacrifice would predict differences in DA release and uptake rate. We used linear regressions to examine whether the percentage, bout number, or bout length of Wake, NREM or REM sleep that occurred in the 5, 15, and 30 min prior to sacrifice significantly predicted ex vivo FSCV measures of DA release and uptake (Fig. 2C–E, G–I and Supplementary Tables 1, 2). We refer to these periods of wakefulness and sleep prior to sacrifice as the 5-, 15-, and 30 min time periods (see Supplementary Fig. 2).
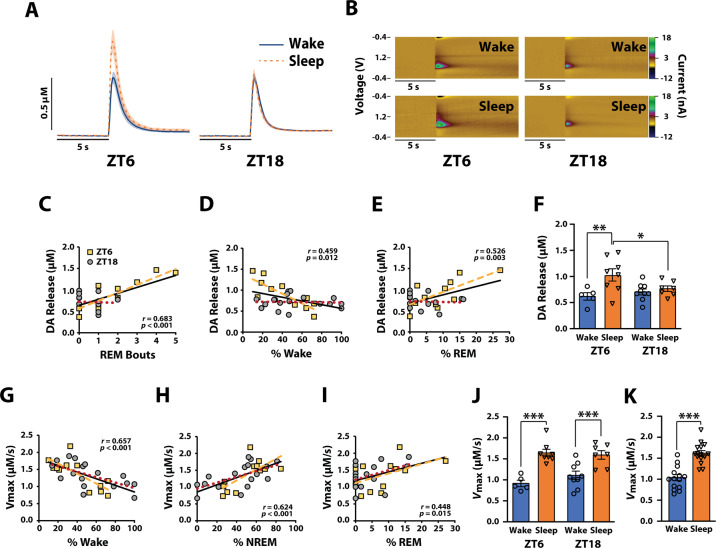
Fig. 2. Dopamine release and uptake fluctuate across sleep/wake state.
A Average current versus time plots and (B) example color plots for the Wake and Sleep groups during the light (ZT6) and dark (ZT18) phases. Shaded areas in current versus time plots represent SEM. Correlations between DA release and (C) REM bouts, (D) % of Wake, or (E) % of REM during the 30-min time period. F DA release for rats categorized into Wake and Sleep groups for ZT6 and ZT18 based on sleep/wake state during the 30 min time period. Correlations between DA uptake and (G) % of Wake, (H) % of NREM, or (I) % of REM during the 30 min time period. J DA uptake rate for rats categorized into Wake and Sleep groups for ZT6 and ZT18 based on sleep/wake state during the 30 min time period. K DA uptake rate for rats categorized into Wake and Sleep groups collapsed across ZT6 and ZT18. Wake (ZT6) n = 5 rats, Wake (ZT18) n = 9 rats, Sleep (ZT6) n = 8 rats, and Sleep (ZT18) n = 7 rats. Correlation coefficients associated p values, and black regression lines represent analyses on data collapsed across ZT6/ZT18. Yellow dashed lines depict regression lines for ZT6 data, and red dotted lines depict regression lines for ZT18 data. Note: data points in correlations appear different across panels due to overlaying of symbols. Data are shown as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
Based on our finding that sleep/wake activity during the 30 min prior to sacrifice (i.e., the 30 min time period) was strongly correlated with measures of DA release and/or uptake, we used a median split to dichotomize sleep/wake data into separate Wake and Sleep groups based on percentage of time spent awake. The median and any value above it was designated as the “Wake” group and every value below the median was designated as the “Sleep” group for each of the light/dark phases (light = ZT6, dark = ZT18). Two-way ANOVAs were then used to determine differences in DA release and uptake with time of day and sleep/wake state as between-subjects variables (Fig. 2F, J). Following the two-way ANOVAs, Sidak’s post hoc tests were used to determine whether DA release, DA uptake, and the effects of cocaine varied across sleep/wake state. Because of our a priori hypothesis that DAT function would vary with sleep/wake states regardless of light/dark phase, a subset of Sidak’s post hoc tests were conducted even though no significant interaction was observed in the two-way ANOVA. When no significant main effect was observed for a variable (e.g., time of day for DA uptake rate) data were collapsed across that variable and Student’s t tests were used to assess differences between the Wake and Sleep groups (Fig. 2K).
To examine if the effects of cocaine or quinpirole differed between Wake and Sleep groups, two-way mixed design ANOVAs were conducted with cocaine or quinpirole concentration as the within-subjects variable and sleep/wake state as the between-subjects variable. Cocaine Ki was determined by plotting the linear uptake inhibition effect of the 5 cumulative concentrations of cocaine and determining the slope of the linear regression. Ki values were calculated by the equation Km/slope [13, 48, 49]. Ki was compared between the Wake and Sleep groups using a Students’ t test. Differences in DAT and pDAT between the Wake and Sleep groups were assessed using a Students’ t tests (Fig. 4C–E).
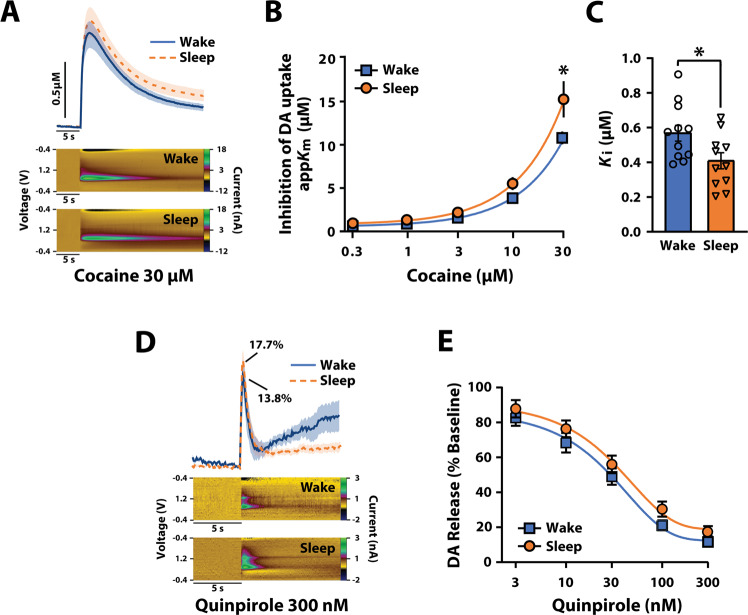
Fig. 4. Cocaine potency is higher after periods of sleep.
A Average current versus time plots and example color plots for rats categorized into Wake and Sleep groups after exposure to 30 µM cocaine. Shaded areas in current versus time plots represent SEM. B Inhibition of DA uptake following cumulative cocaine concentrations (0.3–30 μM) and (C) cocaine Ki for the Wake and Sleep groups. D Average current versus time plots and example color plots for the Wake and Sleep groups after exposure to 300 nM quinpirole. 13.8% and 17.7% represent average percent of baseline for stimulated release amplitudes. Shaded areas in current versus time plots represent SEM. E DA release following cumulative quinpirole concentrations (3–300 nM) for the Wake and Sleep groups. A–C Wake n = 12, Sleep n = 11. D, E Wake n = 12, Sleep n = 12. Data are shown as mean ± SEM. *p < 0.05.
Results
Sleep/wake activity is organized into bouts occurring on the time scale of minutes
To confirm that sleep/wake activity consists of recurring bouts of Wake, NREM, and REM during both the light- (ZT6) and dark-phase (ZT18), we monitored sleep/wake state for 24 h using EEG–EMG recordings in freely moving rats (Fig. 1). As a group, rats spent ~44.9% of the time awake (633.31 ± 67.5 min), 46.7% in NREM sleep (658.14 ± 68.30 min) and 8.3% in REM sleep (117.61 ± 21.10 min) over the 24 h period; 65.27% of NREM and 60.39% of REM occurred during the light phase while 65.33% of Wake occurred during the dark phase (Fig. 1B–D). Wake, NREM, and REM were organized into numerous bouts over the 24 h period indicating that sleep/wake states undergo several transitions across the day and within each light/dark phase (Fig. 1B–D; Supplementary Table 1). Importantly, the distribution of sleep/wake state percentages, bout number, and bout length were similar to published observations in adult rats [50–54].
Dopamine release fluctuates across sleep/wake state only during the light phase
To examine whether sleep/wake state predicts DA release, we analyzed EEG–EMG signals 5, 15, and 30 min prior to sacrificing rats and then assessed DA dynamics using FSCV in rat NAc slices obtained during the light (ZT6) or dark phase (ZT18; Figs. 1A, 2A–F). While no significant relationships were observed between bout length and DA release for any of the sleep/wake states, we did observe a significant relationship between the number of NREM and REM bouts during the 15 min time period and magnitude of DA release (Supplementary Table 2). However, the number of REM bouts (r = 0.683, p < 0.001) during the 30 min time period resulted in the strongest predictor of DA release, accounting for 46.6% of the variability (Fig. 2C; Supplementary Table 2). Although a significant relationship was observed between the percentage of Wake (r = 0.459, p = 0.012) and REM (r = 0.526, p = 0.003) during the 30 min time period and magnitude of DA release, this effect was comparatively modest, accounting for only 21.1% and 27.7% of the variability, respectively (Fig. 2D, E; Supplementary Table 2).
Despite these observations, the relationship between sleep/wake state and DA release appeared to be driven primarily by data obtained during the light phase (Fig. 2A–E). To test for this possibility, we separated our data into “Wake” and “Sleep” groups using a median split for the 30 min time period, during which we had observed the strongest relationship with DA release. Two-way ANOVA with sleep/wake state and light/dark phase as between-subjects variables revealed a significant effect of sleep/wake state (F(1,25) = 8.193, p = 0.008) and a significant interaction between sleep/wake state and light/dark phase on DA release (F(1,25) = 4.507, p = 0.044), but no significant effect of light/dark phase (F(1,25) = 1.149, p = 0.294). Sidak’s post hoc analysis revealed significantly greater DA release in the Sleep group during the light phase (ZT6; F(1,25) = 11.096, p = 0.003), but not during the dark phase (ZT18; F(1,25) = 0.307, p = 0.585; Fig. 2F). Additionally, there was significantly greater DA release in the Sleep group during the light phase (ZT6) compared to the dark phase (ZT18; F(1,25) = 5.530, p = 0.027), but no differences in DA release between light/dark phase in the Wake group (F(1,25) = 0.522, p = 0.477; Fig. 2F). These data suggest that DA release differs across sleep/wake state depending on the light/dark phase, with REM sleep as the best predictor of DA release.
Dopamine uptake varies across sleep/wake state regardless of light/dark phase
To examine whether sleep/wake state predicts DA uptake rate (Vmax; µM/s), we analyzed EEG–EMG signals for the 5, 15, and 30 min prior to sacrificing rats and then assessed DA dynamics using FSCV in rat NAc slices obtained during the light (ZT6) or dark phase (ZT18; Figs. 1A, 2G–K). Significant relationships were observed between the percentage of time in each sleep/wake state during the 5-, 15-, and 30 min time periods and the rate of DA uptake (Supplementary Table 3). However, the percentage of Wake (r = 0.657, p < 0.001) and NREM (r = 0.624, p < 0.001) at the 30 min time period resulted in the strongest predictor of DA uptake, accounting for 43.2% and 39.0% of the variability, respectively (Fig. 2G, H; Supplementary Table 3). Although a significant relationship between percentage of REM at the 30 min time period and DA uptake was also observed (r = 0.448, p = 0.015), this effect was comparatively modest (Fig. 2I; Supplementary Table 3). Neither the number of bouts nor bout length of any sleep/wake state were robust predictors of DA uptake at any time period (Supplementary Table 3).
To examine whether the relationship between sleep/wake state and DA uptake differs between the light (ZT6) and dark (ZT18) phase. We again used a median split to separate our data into Wake and Sleep groups for the 30 min time period. Two-way ANOVA with sleep/wake state and light/dark phase as between-subjects variables revealed a significant effect of sleep/wake state on DA uptake rate (F(1,25) = 37.300, p < 0.001) but no significant effect of light/dark phase (F(1,25) = 0.511, p = 0.481), nor sleep/wake state and light/dark phase interaction (F(1,25) = 1.456, p = 0.239). Because of our a priori hypothesis that DAT function would vary with sleep/wake state regardless of light/dark phase, we used a Sidak’s post hoc to test for differences in DA uptake across the Wake and Sleep groups at both the light and dark phases. As shown in Fig. 2J, DA uptake rate was significantly faster in the Sleep group during both the light (F(1,25) = 23.823, p < 0.001) and dark phases (F(1,25) = 13.689, p < 0.001). Further, a t test showed significantly faster DA uptake in the Sleep group when collapsed across light/dark phase (t27 = −6.005, p < 0.001; Fig. 2K). Together, these results suggest that DA uptake is more efficient after periods of sleep, independent of the light/dark phase.
DAT phosphorylation state likely accounts for fluctuations in dopamine uptake
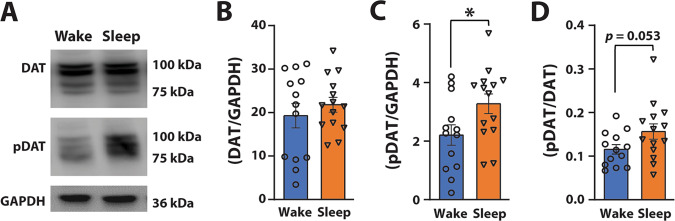
DAT trafficking and posttranslational modifications such as phosphorylation at the Thr53 site on the DAT (i.e., pDAT) can influence DA uptake through putative alterations in DAT conformational state [12, 22, 55–59]. To examine whether fluctuations in DA uptake across sleep/wake state could be a consequence of alterations in DAT protein levels, we conducted a western blot experiment in a separate cohort of rats. We analyzed EEG–EMG activity in the 30 min immediately prior to collecting NAc tissue for western blot analysis of plasma membrane DAT and pDAT during the light (ZT6) or dark phases (ZT18; Fig. 3). We again used a median split to separate our data into Wake and Sleep groups for the 30 min time period. A t test revealed that plasma membrane DAT did not differ across sleep/wake state, (t19.979 = 0.779, p = 0.445; Fig. 3B). However, pDAT levels were significantly higher in the Sleep group (t25 = 2.21, p = 0.036; Fig. 3C). Further, plasma membrane pDAT/DAT ratio showed a strong trend (t25 = 2.033, p = 0.053; Fig. 3D) for a significant difference between the Wake and Sleep groups, indicating the possibility that the proportion of transporters that are phosphorylated varies across sleep/wake state. These results are consistent with the higher DA uptake rate observed following periods of sleep, suggesting that fluctuations in DA uptake across sleep/wake state may be driven, in part, by levels of pDAT.
Fig. 3. Plasma membrane pDAT levels are higher after periods of sleep.
A Representative blots from adjacent lanes for NAc plasma membrane DAT, pDAT, and GAPDH. B Average plasma membrane DAT and (C) pDAT expressed as a ratio over GAPDH, and (D) pDAT/DAT expressed as a ratio over GAPDH for rats categorized into Wake and Sleep groups. B–D Wake n = 13, Sleep n = 14. Data are shown as mean ± SEM. *p < 0.05.
Cocaine potency fluctuates across sleep/wake state
In addition to enhancing basal DA uptake rate, phosphorylation of the DAT at Thr53 has been shown to enhance DAT sensitivity to cocaine analogs [22, 55, 60, 61], suggesting that fluctuations in pDAT could impact the effects of cocaine. To examine whether cocaine potency at the DAT fluctuates across sleep/wake state, we analyzed EEG–EMG signals in the 30 min prior to sacrificing rats and then examined cocaine-induced DA uptake inhibition using FSCV in rat NAc slices obtained during the light (ZT6) or dark period (ZT18; Figs. 1A, 4A–C). We used a median split to separate our data into Wake and Sleep groups for the 30 min time period. Two-way repeated measures ANOVA with sleep/wake state as the between-subjects variable and cocaine concentration as a within-subjects measures variable revealed a significant effect of sleep/wake state (F(1,10) = 5.63, p = 0.039), and cocaine concentration (Greenhouse–Geisser correction; F(1.082,10.818) = 128.210, p < 0.005), but no significant sleep/wake state × cocaine concentration interaction (Greenhouse–Geisser correction; F(1.034,10.34) = 3.998, p = 0.072). Because of our a priori hypothesis that DAT function would vary with sleep/wake state, we used Sidak’s post hoc tests to examine whether the effects of cocaine would differ across the Wake and Sleep groups. As shown in Fig. 4A, B, cocaine was significantly more effective at inhibiting DA uptake in the Sleep group at the 30 µM cocaine concentration compared to the Wake group (F(1,9) = 8.293, p = 0.018; Fig. 4A, B). Consistent with this, the cocaine concentration needed to inhibit 50% of uptake (Ki) was lower in the Sleep group indicating a significant increase in cocaine potency at the DAT (t20 = −2.512), p = 0.044; Fig. 4C). These results are consistent with our observations that both DA uptake rate and pDAT levels are enhanced after periods of sleep, suggesting the likelihood that underlying changes in DAT function are responsible for the observed fluctuations in cocaine potency.
D2R/D3R sensitivity does not account for the fluctuation in dopamine release
D2R/D3R in the striatum have been demonstrated to modulate DA release [13, 62–66] by hyperpolarization via voltage-dependent potassium channels [67] and by inhibiting calcium entry [68]. D2R/D3R are also known to regulate DA uptake via a direct interaction with DAT or through activation of downstream signaling molecules such as ERK1/2 and PKC which can regulate the surface expression of DAT and hence DA uptake [69–71]. These observations suggest the possibility that fluctuations in DA release, uptake, or DAT sensitivity to cocaine could be influenced by differences in D2R/D3R function. To test for this possibility, we analyzed EEG–EMG signals for the 30 min prior to sacrificing rats and then assessed the effects of the D2R/D3R agonist quinpirole using FSCV recordings in rat NAc slices obtained during the light (ZT6) or dark phase (ZT18; Figs. 1A, 4D, E). We used a median split to separate our data into Wake and Sleep groups for the 30 min time period. Two-way mixed design ANOVA with sleep/wake state as the between-subjects variable and quinpirole concentration as the within-subjects measures variable revealed a significant effect of quinpirole concentration (F(1,9) = 424.389, p < 0.0005), but no significant effect of sleep/wake state (F(1,9) = 1.571, p = 0.242) nor significant sleep/wake state × quinpirole concentration interaction (F(1,9) = 0.192, p = 0.672). Given the observation that quinpirole had a similar effect on DA release in the Wake and Sleep groups (Fig. 4D, E), it appears that D2R/D3R sensitivity is not likely to be the mechanism through which DA release and uptake fluctuate across sleep/wake state.
Discussion
The current experiments examined whether DA release and uptake fluctuate across sleep/wake state using ex vivo FSCV evaluation of DA dynamics and biochemical assessment of plasma membrane DAT and pDAT levels in the NAc core. We demonstrated that sleep/wake state predicts both DA release and uptake rate, but that only DA uptake fluctuated exclusively across sleep/wake activity and not light/dark phase. These variations in DA release and uptake do not appear to be dependent on changes in D2R/D3R, given that sensitivity to quinpirole did not fluctuate across sleep/wake state. Western blotting demonstrated that periods of sleep were associated with higher levels of phosphorylation of the DAT at Thr53 and not an increase in DAT levels on the plasma membrane. Consistent with this, the effects of cocaine at inhibiting DA uptake were more potent after periods of sleep.
Fluctuations in dopamine release across sleep/wake state are driven primarily by REM sleep
In the current studies, we observed that during the light phase (ZT6), DA release was highest after periods of sleep and lowest after periods of wakefulness. These findings are consistent with variations in DA neuron activity and NAc DA levels across sleep/wake state that are also observed during the light phase [72–74]. In contrast, we found that DA release did not differ between wakefulness and sleep during the dark phase (ZT18), which could suggest that light/dark condition exerts an important influence on DA release. This possibility does not appear likely given that (1) there was no significant effect of light/dark phase on DA release (Fig. 2F); and (2) previous evidence indicates that extracellular DA tone in the NAc fluctuates across a 24 h period even when rats were exposed to constant light or darkness—in other words independent of the light/dark phase [75]. Rather, we suggest that variations in DA release across sleep/wake state only during the light phase may be associated with marked differences in sleep architecture observed between the light and dark phases. In particular, we note that we observed more frequent and longer REM bouts during the light phase and few and brief REM bouts during the dark phase. Given that REM sleep has been associated with higher DA tone in the NAc and increased DA neuron activity in the ventral tegmental area [72–74]—in addition to our finding that REM is the best predictor of DA release—this increased REM activity during the light phase, positions REM sleep as a potentially critical participant in the regulation of DA release.
Dopamine uptake rate fluctuates across sleep/wake state, regardless of light/dark phase
Recent observations demonstrate that DA metabolism, D2R/D3R sensitivity, and TH expression are not the mechanisms driving diurnal variation in extracellular DA tone [24, 76]. Importantly, however, DAT function was shown to be a critical governor of this fluctuation with higher uptake rate during the light phase when animals are usually asleep and lower uptake during the dark phase when animals are usually awake [24]. Consistent with this, we observed that DATs are more efficient after periods of sleep compared to periods of wakefulness regardless of light/dark phase, suggesting that sleep/wake state is a stronger zeitgeber for DA uptake efficiency than light/dark phase.
High pDAT levels are consistent with increased dopamine uptake and exaggerated cocaine potency
DAT proteins are highly dynamic and continuously distributed between the plasma membrane to intracellular endosomal compartments [12]. This type of DAT trafficking can modulate DAT function in response to physiological conditions on a time scale of seconds to minutes [12, 16]. In spite of previous work showing higher total DAT levels during the dark phase and lower during the light period in the NAc [77], we observed no variations in plasma membrane DAT levels in our studies indicating that DA uptake fluctuations across sleep/wake state are likely not attributable to changes in DAT. This discrepancy between studies could be related to the fact that we measured plasma membrane DAT instead of total DAT number and that we control for both sleep/wake state and light/dark phase.
Numerous observations suggest that enhanced phosphorylation of DAT at Thr53 promotes DA uptake efficiency and sensitivity to psychostimulants [21, 55, 59, 60]. Consistent with these findings, we observed that pDAT was significantly higher following periods of sleep suggesting that the relationship between sleep/wake state and DA uptake observed in our FSCV experiments may be mediated by alterations in phosphorylation of DAT at Thr53. In addition to the importance of DAT phosphorylation state, numerous other mechanisms may be involved in altered DAT function. Indeed, several studies indicate that alterations in basal DA uptake rate may also be a product of changes in the balance of inward/outward facing DAT [78], changes in oligomer/monomer ratios [79, 80], dimerization with sigma receptor [81], or differential DAT phosphorylation states on serine sites [82, 83]. Thus, although our present findings indicate a potentially critical link between pDAT fluctuations across sleep/wake states that match alterations in DA uptake rate, other mechanisms may also be involved.
Emerging evidence posits that faster rates of DA uptake and high levels of pDAT increase cocaine potency at the DAT, which is manifested as higher cocaine-induced DA uptake inhibition [21, 55, 59]. Consistent with these findings, we observed that after periods of sleep—when DA uptake efficiency was at its highest—cocaine potency was exaggerated. These findings suggest the likelihood that fluctuations in DA uptake across sleep/wake state are due to alterations in DAT state that impact its ability to interact with cocaine, and possibly other psychostimulants.
Circadian and ultradian influences on dopamine neurotransmission
In addition to sleep/wake state, it is also possible that circadian oscillations may participate in the regulation of DA neurotransmission. For example, TH—the rate limiting step in DA synthesis—varies in a circadian-like fashion with significantly higher expression and activity in the NAc during the dark phase [24, 84]. Consistent with this finding, mutant mice without a functional circadian Clock gene show increased expression and phosphorylation of TH which is associated with enhanced firing of ventral tegmental area DA neurons [84, 85]. These influences on TH levels and activity do not appear to coincide with our present findings, however, given that we did not observe overall differences in DA release or uptake across time of day. Nevertheless, we did observe that DA uptake rate varied across sleep/wake state regardless of the time of the day suggesting that DAT function may be responsive to influences that occur on a shorter time scale.
Ultradian rhythms are repeated biological patterns that occur with periods shorter than 24 h. Well-recognized ultradian rhythms include sleep, body temperature, motor activity, heart rate, feeding, and many others [86–90]. In the context of our findings, observed fluctuations in DA uptake, and DAT phosphorylation are consistent with ultradian regulation, including by sleep/wake state as we propose here. However, the extent to which these DA changes are directly attributable to sleep/wake state, or possibly to other ultradian rhythms that closely follow sleep/wake patterns (e.g., motor activity or body temperature), remains unclear. Moreover, while we propose that fluctuations in sleep/wake state influence DA release and uptake, it is also possible that the relationship is reversed, with changes in DA responsible for fluctuations in sleep/wake state. In fact, recent observations indicate that DAT knockout mice have lengthened ultradian motor activity periods and display increase time spent awake [8, 91], and that chemogenetic or optogenetic activation of DA neuron activity enhances motor activity and reduces sleep [73, 91].
Behavioral implications
Decades of research indicate that the expression of numerous behaviors is exquisitely reliant on tight regulation of DA neurotransmission across the neuroaxis [92–98]. Considering the amassing evidence that dysregulation of DA-dependent processes may be a contributing factor to a variety of neuropsychiatric conditions [5, 9, 10, 99], the fluctuations in DA release and uptake observed in our studies are likely to have profound impacts on behavior in both normal and disease states.
In the context of goal-directed and reward-associated behaviors, evidence suggests that food and drug self-administration, locomotor sensitization, conditioned place preference, and drug craving are influenced by diurnal and/or circadian rhythms which have been posited to involve DA signaling [76, 85, 100–103]. Further, in the case of cocaine, potency at the DAT is known to influence behavior with high cocaine potency tied to increases in cocaine self-administration [21, 35, 49, 59, 104, 105], and reduced cocaine potency leading to reduced cocaine self-administration [49, 106]. Thus, cocaine potency fluctuations across sleep/wake state are expected to drive more pronounced cocaine intake after extended periods of sleep. This finding may help to explain prior research showing that rats exposed to a discrete trials procedure display nearly 100% probability of taking cocaine at the start of the dark phase, when they would have just awakened from an extended period of sleep [29]. Indeed, pharmacological manipulations known to impact DA uptake and cocaine potency disrupt cocaine intake under these discrete trial conditions [26].
When considered together, these observations suggest that the relationship between sleep/wake state and DA neurotransmission may be one explanation for why reward-associated behaviors have marked diurnal oscillations [73, 101–103, 107, 108]. Future work will attempt to disentangle the complex interactions between sleep/wake activity, general arousal state, and motivation for rewards, and the degree to which this involves dynamic alterations in DA neurotransmission.
Summary
In conclusion, our studies demonstrate a robust association between sleep/wake state and DA terminal neurotransmission, with higher DA uptake rate, increased pDAT, and enhanced cocaine potency after periods of sleep. In addition to providing a possible link between DA neurotransmission and diurnal variations in goal-directed behaviors across the day, our observations also suggest that fluctuations in DA neurotransmission may also explain diurnal changes in a host of DA-dependent behaviors and physiological processes. Finally, our findings also provide evidence for the need to carefully consider the potential impacts that sleep/wake state, time of day, and light/dark condition may have on measures of both neurotransmission and behavior across many areas of research.
Funding and disclosure
This work was supported by NIH grant DA031900 to RAE, MH106912 to SK, Fondecyt Initiation Fund N°11191049 to JAP, NIH grant DA038598 to GET, as well as the Drexel University Dean’s Fellowship for Excellence in Collaborative or Themed Research to IPA. The authors report no biomedical financial interests or potential competing interest.
Supplementary information
Acknowledgements
We would like to thank Wei Xu, PhD for his technical assistance with the western blot assays, and Phil J. Clark, MS and Bethan M. O’Connor for their thoughtful comments during the writing of this paper. We also thank the NIDA drug supply program for donating cocaine hydrochloride.
Author contributions
IPA, conceptualization and design of the work; acquisition, analysis, interpretation of data; writing the original draft; editing of intellectual content. JAP, acquisition and analysis of data; review and editing of intellectual content. SK, resources; editing of intellectual content. GET, resources; editing of intellectual content. RAE, conceptualization and design of the work; analysis and interpretation of data; writing-original draft; editing of intellectual content; resources and funding acquisition.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00879-2).
References
- 1.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 2.Trulson ME, Preussler DW. Dopamine-containing ventral tegmental area neurons in freely moving cats: activity during the sleep-waking cycle and effects of stress. Exp Neurol. 1984;83:367–77. doi: 10.1016/S0014-4886(84)90105-5. [DOI] [PubMed] [Google Scholar]
- 3.Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM, Caron MG. Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res Brain Res Rev. 1998;26:148–53. doi: 10.1016/s0165-0173(97)00063-5. [DOI] [PubMed] [Google Scholar]
- 4.Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, et al. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–55. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- 5.Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. J Neurochem. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- 6.Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC. High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. J Pharmacol Exp Ther. 1994;271:1678–85. [PubMed] [Google Scholar]
- 7.Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47(Suppl 1):80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, et al. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. J Neurosci. 2012;32:5385–97. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–92. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palermo G, Giannoni S, Frosini D, Morganti R, Volterrani D, Bonuccelli U. Dopamine transporter, age, and motor complications in Parkinson’s disease: a clinical and single-photon emission computed tomography study. Mov Disord. 2020;35:1028–36. doi: 10.1002/mds.28008. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–40. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 13.Brodnik ZD, Black EM, Clark MJ, Kornsey KN, Snyder NW, España RA. Susceptibility to traumatic stress sensitizes the dopaminergic response to cocaine and increases motivation for cocaine. Neuropharmacology. 2017;125:295–307. doi: 10.1016/j.neuropharm.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fordahl SC, Jones SR. High-fat-diet-induced deficits in dopamine terminal function are reversed by restoring insulin signaling. ACS Chem Neurosci. 2017;8:290–9. doi: 10.1021/acschemneuro.6b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. Eur J Pharmacol. 2003;479:139–52. doi: 10.1016/j.ejphar.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 16.Richardson BD, Saha K, Krout D, Cabrera E, Felts B, Henry LK, et al. Membrane potential shapes regulation of dopamine transporter trafficking at the plasma membrane. Nat Commun. 2016;7:10423. doi: 10.1038/ncomms10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur J Pharmacol. 2003;479:159–70. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 18.Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, Gnegy M. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci. 2009;29:3328–36. doi: 10.1523/JNEUROSCI.5386-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulley JM, Doolen S, Zahniser NR. Brief, repeated exposure to substrates down-regulates dopamine transporter function in Xenopus oocytes in vitro and rat dorsal striatum in vivo. J Neurochem. 2002;83:400–11. doi: 10.1046/j.1471-4159.2002.01133.x. [DOI] [PubMed] [Google Scholar]
- 20.Richards TL, Zahniser NR. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J Neurochem. 2009;108:1575–84. doi: 10.1111/j.1471-4159.2009.05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodnik ZD, Alonso IP, Xu W, Zhang Y, Kortagere S, España RA. Hypocretin receptor 1 involvement in cocaine-associated behavior: therapeutic potential and novel mechanistic insights. Brain Res. 2020;1731:145894. [DOI] [PubMed]
- 22.Challasivakanaka S, Zhen J, Smith ME, Reith MEA, Foster JD, Vaughan RA. Dopamine transporter phosphorylation site threonine 53 is stimulated by amphetamines and regulates dopamine transport, efflux, and cocaine analog binding. J Biol Chem. 2017;292:19066–75. doi: 10.1074/jbc.M117.787002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–13. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris MJ, España RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci USA. 2014;111:E2751–9. doi: 10.1073/pnas.1407935111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brebner K, Froestl W, Andrews M, Phelan R, Roberts DC. The GABA(B) agonist CGP 44532 decreases cocaine self-administration in rats: demonstration using a progressive ratio and a discrete trials procedure. Neuropharmacology. 1999;38:1797–804. doi: 10.1016/s0028-3908(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 26.España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–48. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.España RA, Scammell TE. Sleep neurobiology from a clinical perspective. Sleep. 2011;34:845–58. doi: 10.5665/SLEEP.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts DC, Andrews MM. Baclofen suppression of cocaine self-administration: demonstration using a discrete trials procedure. Psychopharmacology. 1997;131:271–7. doi: 10.1007/s002130050293. [DOI] [PubMed] [Google Scholar]
- 29.Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–9. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 30.Brodnik ZD, Bernstein DL, Prince CD, España RA. Hypocretin receptor 1 blockade preferentially reduces high effort responding for cocaine without promoting sleep. Behav Brain Res. 2015;291:377–84. doi: 10.1016/j.bbr.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 32.Berridge CW, España RA. Synergistic sedative effects of noradrenergic alpha(1)- and beta- receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience. 2000;99:495–505. doi: 10.1016/s0306-4522(00)00215-3. [DOI] [PubMed] [Google Scholar]
- 33.Brodnik ZD, España RA. Dopamine uptake dynamics are preserved under isoflurane anesthesia. Neurosci Lett. 2015;606:129–34. doi: 10.1016/j.neulet.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–64. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy KA, Brodnik ZD, Shaw JK, Perrey DA, Zhang Y, España RA. Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling. Psychopharmacology. 2017;234:2761–76. doi: 10.1007/s00213-017-4673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips PE, Johns JM, Lubin DA, Budygin EA, Gainetdinov RR, Lieberman JA, et al. Presynaptic dopaminergic function is largely unaltered in mesolimbic and mesostriatal terminals of adult rats that were prenatally exposed to cocaine. Brain Res. 2003;961:63–72. doi: 10.1016/s0006-8993(02)03840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett. 1994;176:259–63. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 38.Truong JG, Newman AH, Hanson GR, Fleckenstein AE. Dopamine D2 receptor activation increases vesicular dopamine uptake and redistributes vesicular monoamine transporter-2 protein. Eur J Pharmacol. 2004;504:27–32. doi: 10.1016/j.ejphar.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 39.Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J Neurosci. 2002;22:6272–81. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brodnik ZQ, Black EM, España RA. Accelerated development of cocaine-associated dopamine transients and cocaine use vulnerability following traumatic stress. Neuropsychopharmacology. 2020;45:472–81. doi: 10.1038/s41386-019-0526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts JG, Toups JV, Eyualem E, McCarty GS, Sombers LA. In situ electrode calibration strategy for voltammetric measurements in vivo. Anal Chem. 2013;85:11568–75. doi: 10.1021/ac402884n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6:138–46. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao A, Simmons D, Sorkin A. Differential subcellular distribution of endosomal compartments and the dopamine transporter in dopaminergic neurons. Mol Cell Neurosci. 2011;46:148–58. doi: 10.1016/j.mcn.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colon-Perez LM, Pino JA, Saha K, Pompilus M, Kaplitz S, Choudhury N, et al. Functional connectivity, behavioral and dopaminergic alterations 24 h following acute exposure to synthetic bath salt drug methylenedioxypyrovalerone. Neuropharmacology. 2018;137:178–93. doi: 10.1016/j.neuropharm.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KS, Alvarenga TA, Guindalini C, Andersen ML, Castro RM, Tufik S. Validation of commonly used reference genes for sleep-related gene expression studies. BMC Mol Biol. 2009;10:45. doi: 10.1186/1471-2199-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology. 2005;49:750–8. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Kahlig KM, Javitch JA, Galli A. Amphetamine regulation of dopamine transport. Combined measurements of transporter currents and transporter imaging support the endocytosis of an active carrier. J Biol Chem. 2004;279:8966–75. doi: 10.1074/jbc.M303976200. [DOI] [PubMed] [Google Scholar]
- 48.Calipari ES, Ferris MJ, Siciliano CA, Jones SR. Differential influence of dopamine transport rate on the potencies of cocaine, amphetamine, and methylphenidate. ACS Chem Neurosci. 2015;6:155–62. doi: 10.1021/cn500262x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR. Temporal pattern of cocaine intake determines tolerance vs. sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology. 2013;38:2385–92. doi: 10.1038/npp.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang X, Yang L, Sanford LD. Individual variation in sleep and motor activity in rats. Behav Brain Res. 2007;180:62–8. doi: 10.1016/j.bbr.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas AM, Schwartz MD, Saxe MD, Kilduff TS. Sleep/wake physiology and quantitative electroencephalogram analysis of the neuroligin-3 knockout rat model of autism spectrum disorder. Sleep. 2017;40:zsx138. [DOI] [PubMed]
- 52.Datta S, Hobson JA. The rat as an experimental model for sleep neurophysiology. Behav Neurosci. 2000;114:1239–44. doi: 10.1037//0735-7044.114.6.1239. [DOI] [PubMed] [Google Scholar]
- 53.Swift KM, Keus K, Echeverria CG, Cabrera Y, Jimenez J, Holloway J, et al. Sex differences within sleep in gonadally-intact rats. Sleep. 2020;43:zsz289. [DOI] [PMC free article] [PubMed]
- 54.Bernstein DL, Badve PS, Barson JR, Bass CE, España RA. Hypocretin receptor 1 knockdown in the ventral tegmental area attenuates mesolimbic dopamine signaling and reduces motivation for cocaine. Addict Biol. 2018;23:1032–45. [DOI] [PMC free article] [PubMed]
- 55.Foster JD, Yang JW, Moritz AE, Challasivakanaka S, Smith MA, Holy M, et al. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J Biol Chem. 2012;287:29702–12. doi: 10.1074/jbc.M112.367706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–38. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, et al. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem. 2003;278:2731–9. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- 59.Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017;8:13877. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foster JD, Vaughan RA. Phosphorylation mechanisms in dopamine transporter regulation. J Chem Neuroanat. 2017;83-84:10–8. doi: 10.1016/j.jchemneu.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci. 2013;34:489–96. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts C, Cummins R, Gnoffo Z, Kew JN. Dopamine D3 receptor modulation of dopamine efflux in the rat nucleus accumbens. Eur J Pharmacol. 2006;534:108–14. doi: 10.1016/j.ejphar.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Shin JH, Adrover MF, Alvarez VA. Distinctive modulation of dopamine release in the nucleus accumbens shell mediated by dopamine and acetylcholine receptors. J Neurosci. 2017;37:11166–80. doi: 10.1523/JNEUROSCI.0596-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Threlfell S, Lalic T, Platt Nicola J, Jennings Katie A, Deisseroth K, Cragg Stephanie J. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 65.Maina FK, Mathews TA. A functional fast scan cyclic voltammetry assay to characterize dopamine D2 and D3 autoreceptors in the mouse striatum. ACS Chem Neurosci. 2010;1:450–62. doi: 10.1021/cn100003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGinnis MM, Siciliano CA, Jones SR. Dopamine D3 autoreceptor inhibition enhances cocaine potency at the dopamine transporter. J Neurochem. 2016;138:821–9. doi: 10.1111/jnc.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martel P, Leo D, Fulton S, Berard M, Trudeau LE. Role of Kv1 potassium channels in regulating dopamine release and presynaptic D2 receptor function. PLoS ONE. 2011;6:e20402. doi: 10.1371/journal.pone.0020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phillips PE, Stamford JA. Differential recruitment of N-, P- and Q-type voltage-operated calcium channels in striatal dopamine release evoked by ‘regular’ and ‘burst’ firing. Brain Res. 2000;884:139–46. doi: 10.1016/s0006-8993(00)02958-9. [DOI] [PubMed] [Google Scholar]
- 69.Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, et al. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–32. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- 70.Chen R, Daining CP, Sun H, Fraser R, Stokes SL, Leitges M, et al. Protein kinase Cbeta is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J Neurochem. 2013;125:663–72. doi: 10.1111/jnc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, et al. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem. 2007;282:35842–54. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]
- 72.Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–41. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- 73.Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci. 2016;19:1356–66. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lena I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–9. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- 75.Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36:177–85. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 76.Sleipness EP, Jansen HT, Schenk JO, Sorg BA. Time-of-day differences in dopamine clearance in the rat medial prefrontal cortex and nucleus accumbens. Synapse. 2008;62:877–85. doi: 10.1002/syn.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 78.Liang YJ, Zhen J, Chen N, Reith ME. Interaction of catechol and non-catechol substrates with externally or internally facing dopamine transporters. J Neurochem. 2009;109:981–94. doi: 10.1111/j.1471-4159.2009.06034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siciliano CA, Saha K, Calipari ES, Fordahl SC, Chen R, Khoshbouei H, et al. Amphetamine reverses escalated cocaine intake via restoration of dopamine transporter conformation. J Neurosci. 2018;38:484–97. doi: 10.1523/JNEUROSCI.2604-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhen J, Antonio T, Cheng SY, Ali S, Jones KT, Reith ME. Dopamine transporter oligomerization: impact of combining protomers with differential cocaine analog binding affinities. J Neurochem. 2015;133:167–73. doi: 10.1111/jnc.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong WC, Yano H, Hiranita T, Chin FT, McCurdy CR, Su TP, et al. The sigma-1 receptor modulates dopamine transporter conformation and cocaine binding and may thereby potentiate cocaine self-administration in rats. J Biol Chem. 2017;292:11250–61. doi: 10.1074/jbc.M116.774075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moritz AE, Foster JD, Gorentla BK, Mazei-Robison MS, Yang JW, Sitte HH, et al. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. J Biol Chem. 2013;288:20–32. doi: 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms. 2009;24:465–76. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- 85.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377–81. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dowse H, Umemori J, Koide T. Ultradian components in the locomotor activity rhythms of the genetically normal mouse, Mus musculus. J Exp Biol. 2010;213:1788–95. doi: 10.1242/jeb.038877. [DOI] [PubMed] [Google Scholar]
- 87.Eastman CI, Mistlberger RE, Rechtschaffen A. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol Behav. 1984;32:357–68. doi: 10.1016/0031-9384(84)90248-8. [DOI] [PubMed] [Google Scholar]
- 88.Honma KI, Hiroshige T. Endogenous ultradian rhythms in rats exposed to prolonged continuous light. Am J Physiol. 1978;235:R250–6. doi: 10.1152/ajpregu.1978.235.5.R250. [DOI] [PubMed] [Google Scholar]
- 89.Gerkema MP, Daan S, Wilbrink M, Hop MW, van der Leest F. Phase control of ultradian feeding rhythms in the common vole (Microtus arvalis): the roles of light and the circadian system. J Biol Rhythms. 1993;8:151–71. doi: 10.1177/074873049300800205. [DOI] [PubMed] [Google Scholar]
- 90.Stein PK, Domitrovich PP, Lundequam EJ, Duntley SP, Freedland KE, Carney RM. Circadian and ultradian rhythms in heart rate variability. Biomed Tech. 2006;51:155–8. doi: 10.1515/BMT.2006.026. [DOI] [PubMed] [Google Scholar]
- 91.Blum ID, Zhu L, Moquin L, Kokoeva MV, Gratton A, Giros B. A highly tunable dopaminergic oscillator generates ultradian rhythms of behavioral arousal. Elife. 2014;3:e05105. doi: 10.7554/eLife.05105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiu NT, Lee BF, Yeh TL, Chen PS, Lee IH, Chen KC, et al. Relationship between striatal dopamine transporter availability and sleep quality in healthy adults. Mol Imaging Biol. 2011;13:1267–71. doi: 10.1007/s11307-010-0442-6. [DOI] [PubMed] [Google Scholar]
- 93.Darvas M, Fadok JP, Palmiter RD. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn Mem. 2011;18:136–43. doi: 10.1101/lm.2041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci USA. 2016;113:14835–40. doi: 10.1073/pnas.1616515114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryczko D, Dubuc R. Dopamine and the brainstem locomotor networks: from lamprey to human. Front Neurosci. 2017;11:295. doi: 10.3389/fnins.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu X, Ottenheimer D, DiLeone RJ. Activity of D1/2 receptor expressing neurons in the nucleus accumbens regulates running, locomotion, and food intake. Front Behav Neurosci. 2016;10:66. doi: 10.3389/fnbeh.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones SR, Gainetdinov RR, Caron MG. Application of microdialysis and voltammetry to assess dopamine functions in genetically altered mice: correlation with locomotor activity. Psychopharmacology. 1999;147:30–2. doi: 10.1007/s002130051137. [DOI] [PubMed] [Google Scholar]
- 99.Simms SL, Huettner DP, Kortagere S. In vivo characterization of a novel dopamine D3 receptor agonist to treat motor symptoms of Parkinson’s disease. Neuropharmacology. 2016;100:106–15. doi: 10.1016/j.neuropharm.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 100.Grippo RM, Tang Q, Zhang Q, Chadwick SR, Gao Y, Altherr EB, et al. Dopamine signaling in the suprachiasmatic nucleus enables weight gain associated with hedonic feeding. Curr Biol. 2020;30:196–208.e8. doi: 10.1016/j.cub.2019.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hanlon EC, Andrzejewski ME, Harder BK, Kelley AE, Benca RM. The effect of REM sleep deprivation on motivation for food reward. Behav Brain Res. 2005;163:58–69. doi: 10.1016/j.bbr.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 102.Smit AN, Patton DF, Michalik M, Opiol H, Mistlberger RE. Dopaminergic regulation of circadian food anticipatory activity rhythms in the rat. PLoS ONE. 2013;8:e82381. doi: 10.1371/journal.pone.0082381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Webb IC, Lehman MN, Coolen LM. Diurnal and circadian regulation of reward-related neurophysiology and behavior. Physiol Behav. 2015;143:58–69. doi: 10.1016/j.physbeh.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 104.Calipari ES, Siciliano CA, Zimmer BA, Jones SR. Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology. 2015;40:728–35. doi: 10.1038/npp.2014.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferris MJ, Mateo Y, Roberts DC, Jones SR. Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry. 2011;69:201–7. doi: 10.1016/j.biopsych.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siciliano CA, Jones SR. Cocaine Potency at the dopamine transporter tracks discrete motivational states during cocaine self-administration. Neuropsychopharmacology. 2017;42:1893–904. doi: 10.1038/npp.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Derryberry D, Rothbart MK. Arousal, affect, and attention as components of temperament. J Pers Soc Psychol. 1988;55:958–66. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- 108.Sleipness EP, Sorg BA, Jansen HT. Time of day alters long-term sensitization to cocaine in rats. Brain Res. 2005;1065:132–7. doi: 10.1016/j.brainres.2005.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.