Chronic inflammation in the tumor microenvironment can promotes tumorigenesis. Various immune cells, including macrophages, can infiltrate into the tumor microenvironment and secrete abundant inflammatory cytokines.1 Interleukin-6 (IL-6) is a major inflammatory cytokine that is widely involved in the tumorigenesis of many cancers, including nasopharyngeal carcinoma (NPC).2 In NPC patients, serum IL-6 was elevated and positively correlated with poor prognosis, suggesting that IL-6 may play a critical role in NPC progression.2,3 Indeed, previous studies demonstrated that IL-6 could activate signal transducer and activator of transcription 3 (STAT3) and promote the proliferation, migration, and invasion of NPC cells.2 However, the mechanism underlying the upregulation of IL-6 in NPC remains elusive.
Within solid tumors, tumor-associated macrophages (TAMs) are the most abundant immune cell type that can be affected by tumor factors and produce abundant inflammatory cytokines, including IL-6, to support tumorigenesis.4 For example, human hepatocellular carcinoma (HCC) cells could significantly enhance macrophages to produce IL-6, which subsequently induced the expansion of HCC stem cells.5 Moreover, colorectal cancer cells could secrete CCL2 to recruit TAMs, which further produced abundant IL-6.6 This evidence highlights the protumoral role of macrophages in the tumor microenvironment by producing IL-6. However, it is still not clear whether IL-6-producing macrophages in the tumor microenvironment are involved in NPC development and progression.
Exosomes are endosome-originating small extracellular membrane vesicles (20–200 nm) that mediate intercellular communications. Cancer cells generate abundant exosomes into the tumor microenvironment to mediate tumor-host crosstalk.7 Through transferring pathogenic signals from cancer cells to immune cells, tumor-derived exosomes can modulate tumor immunity by enhancing opsonization, regulating antigen presentation, and inducing immune activation or suppression.8 NPC-derived exosomes (NPC-Exos) can also regulate tumor immunity. NPC-Exos can hamper antitumor immunity by preventing Th1 differentiation through secretion of immunomodulatory exosomal miRNAs and proteins to T cells.9 In addition, NPC-Exos can induce and recruit human regulatory T cells to support immune evasion.10 However, it is unknown whether NPC-Exos can modulate macrophages to produce IL-6. In this study, we investigated the role of NPC-Exos in IL-6 production from macrophages and their subsequent effects on NPC tumorigenesis.
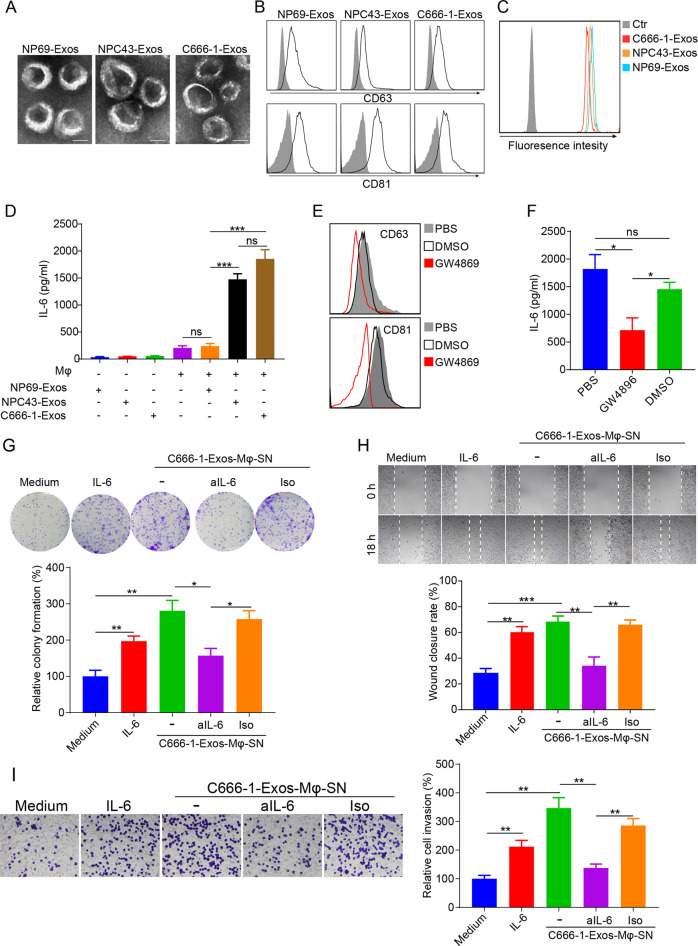
Exosomes of an immortalized normal nasopharyngeal epithelial cell line (NP69-Exos) and two undifferentiated NPC cell lines (NPC43-Exos and C666-1-Exos) were isolated from conditioned culture medium using differential ultracentrifugation. Electron microscopy revealed that the ultracentrifuged vesicles from NP69, NPC43, and C666-1 had similar cup-shaped morphology with ranges of ~20–200 nm (Fig. 1a), which are the typical characteristics of exosomes. For further characterization, the ultracentrifuged vesicles were bound to latex beads and subjected to analysis of exosomal markers using flow cytometry. The analysis identified robust expression of exosomal markers CD63 and CD81 on all three types of vesicles (Fig. 1b), indicating the successful isolation of exosomes. To compare the uptake efficiencies of NP69-Exos, NPC43-Exos, and C666-1-Exos by macrophages, the exosomes were labeled with CFSE and then added to the culture medium of THP-1-macrophages for 18 h. Pellets isolated from nonconditioned exosome-free medium by differential ultracentrifugation were used as controls. The results indicated that all three types of exosomes were efficiently taken up by macrophages (Fig. 1c). Then, we determined the effects of NPC-Exos on IL-6 production from macrophages. The same amounts of NP69-Exos, NPC43-Exos, and C666-1-Exos were used to treat THP-1 macrophages. Twenty-four hours later, the supernatant was harvested for IL-6 detection. NP69-Exos, NPC43-Exos, or C666-1-Exos only contained a very small amount of IL-6 (Fig. 1d). NP69-Exos could not stimulate macrophages to produce IL-6, while both NPC43-Exos and C666-1-Exos induced macrophages to produce a large quantity of IL-6 (Fig. 1d), indicating that exosomes derived from NPCs were more capable of inducing macrophages to produce IL-6 than exosomes from normal epithelial cells. GW4869, an exosome secretion inhibitor, could block exosome secretion from the NPC cell line C666-1 (Fig. 1e). Compared with C666-1 alone or DMSO-treated C666-1 cells, GW4869 significantly hampered the capacity of C666-1 cells to induce IL-6 production by macrophages (Fig. 1f), supporting the importance of exosomes from NPCs in the induction of IL-6 production in macrophages. Taken together, these findings demonstrated that NPC cells can secrete exosomes to induce macrophages to produce IL-6. As IL-6 is a protumoral cytokine for NPC,2,11 it may further promote the tumorigenesis of NPC. To confirm this, we harvested the supernatant from C666-1-Exos-treated macrophages (C666-1-Exos-Mφ-SN) and determined its effects on the malignant behaviors of NPC cells. Medium alone or recombinant IL-6 treatment was used as a control. Similar to IL-6 treatment, C666-1-Exos-Mφ-SN significantly promoted the malignant behaviors of NPC cells, including colony formation, migration, and invasion (Fig. 1g–i). More importantly, neutralization of IL-6 in C666-1-Exos-Mφ-SN cells using an anti-IL-6 blocking antibody significantly attenuated its protumoral effects on NPC cells (Fig. 1g–i), suggesting that the protumoral activity of C666-1-Exos-Mφ-SN was mainly mediated by IL-6.
Fig. 1.
NPC-Exos induce IL-6 production from macrophages to promote tumorigenesis. a The morphology of NP69-Exos, NPC43-Exos, and C666-1-Exos was determined by transmission electron microscopy. Scale bar, 50 nm. b Expression of exosomal markers of CD63 and CD81 on exosomes was measured by flow cytometry. c CFSE-labeled exosomes were cultured with THP-1 macrophages for 18 h. The CFSE-positive cells were then detected by flow cytometry. d Expression of IL-6 in exosomes (NP69-Exos, NPC43-Exos, C666-1-Exos) or the supernatant from THP-1 macrophages treated with exosomes for 24 h. e Expression of CD63 and CD81 on ultracentrifuged vesicles isolated from the conditioned culture medium of C666-1 cells pretreated with GW4896, DMSO, or PBS for 6 h. f Expression of IL-6 in the supernatant from THP-1 macrophages treated with ultracentrifuged vesicles isolated from C666-1 cells that had been preincubated with GW4896, DMSO, or PBS for 6 h. The cell proliferation (g), migration (h) and invasion (i) of NPC cells (C666-1) treated with medium, recombinant human IL-6, supernatant from macrophages pretreated with C666-1-Exos, or supernatant from C666-1-Exos-pretreated macrophages plus anti-IL-6 (aIL-6) blocking antibody or its isotype (Iso) were determined by colony formation, wound-healing, and transwell invasion assays. Data are expressed as the means ± SEM and representative of four independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. NS not significant, Exos exosomes, Mφ macrophages, SN supernatant
Although exosomes derived from some tumors have been found to induce inflammatory cytokines from tumor-infiltrated immune cells,12 no studies are available on the effects of NPC-Exos on IL-6 production in macrophages. In addition, the effect of IL-6 secreted from macrophages on NPC tumorigenesis remains unknown. In this study, we demonstrated for the first time that exosomes derived from NPC cells significantly induced IL-6 production from macrophages, which urged us to determine its subsequent effect on NPC tumorigenesis because IL-6 can serve as a growth factor for many tumor types. Indeed, enhanced IL-6 signaling has been found to promote NPC tumorigenesis both in vitro and in vivo through STAT3 activation.2 More importantly, stable EBV infection in nasopharyngeal epithelial cells potentiated their responses to IL-6-induced STAT3 activation and promoted malignant properties, suggesting that the activation of IL-6 signaling might also be involved in the early stage of NPC development.2 Here, we found that NPC-Exos-induced IL-6 production from macrophages significantly increased the malignant behaviors of NPC cells. These results emphasize the importance of tumor-derived exosomes on NPC development and progression by promoting the production of inflammatory cytokines. Furthermore, we found that only NPC-Exos could induce IL-6 production from macrophages, whereas NP69-Exos did not. Since NPC-Exos had similar morphology and expression of exosomal markers to NP69-Exos and both NPC-Exos and NP69-Exos had similar uptake efficiencies by THP-1-macrophages, our results suggested that the difference in the ability of NPC-Exos to induce IL-6 production in macrophages was probably due to their distinct exosomal contents, such as mRNA, proteins, microRNA, metabolites, and DNA. To further clarify the detailed mechanisms of how NPC-Exos induce IL-6 from macrophages, more efforts should be made to provide comprehensive information about the contents of NPC-Exos. Although novel therapeutic approaches against EBV-associated tumors are developing rapidly,13 progress in NPC is relatively slow. Considering the protumoral activity of NPC-Exos through inducing IL-6 production from macrophages, targeting tumor exosomes may be an effective therapeutic approach for NPC treatment and warrants future investigation.
Acknowledgements
This work was supported in part by the General Research Fund, Research Grants Council of Hong Kong (17122519, 17126317, 17115015, 17121214), Hong Kong SAR, China.
Competing interests
The authors declare no competing interests.
References
- 1.Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell. Mol. Immunol. 2020;17:1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang G, et al. Enhanced IL-6/IL-6R signaling promotes growth and malignant properties in EBV-infected premalignant and cancerous nasopharyngeal epithelial cells. PLoS ONE. 2013;8:e62284. doi: 10.1371/journal.pone.0062284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao Q, et al. Analysis of the contribution of nasopharyngeal epithelial cancer cells to the induction of a local inflammatory response. J. Cancer Res. Clin. Oncol. 2012;138:57–64. doi: 10.1007/s00432-011-1066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan S, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei C, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer. 2019;18:64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins VR, Dias MS, Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr. Opin. Oncol. 2013;25:66–75. doi: 10.1097/CCO.0b013e32835b7c81. [DOI] [PubMed] [Google Scholar]
- 8.Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin. Cancer Res. 2011;17:959–964. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye SB, et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J Pathol. 2016;240:329–340. doi: 10.1002/path.4781. [DOI] [PubMed] [Google Scholar]
- 10.Mrizak D, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl. Cancer Inst. 2015;107:363. doi: 10.1093/jnci/dju363. [DOI] [PubMed] [Google Scholar]
- 11.Zergoun AA, et al. IL-6/NOS2 inflammatory signals regulate MMP-9 and MMP-2 activity and disease outcome in nasopharyngeal carcinoma patients. Tumour Biol. 2016;37:3505–3514. doi: 10.1007/s13277-015-4186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altevogt P, Bretz NP, Ridinger J, Utikal J, Umansky V. Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin. Cancer Biol. 2014;28:51–57. doi: 10.1016/j.semcancer.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Xiang Z, et al. Targeted activation of human Vgamma9Vdelta2-T cells controls epstein-barr virus-induced B cell lymphoproliferative disease. Cancer Cell. 2014;26:565–576. doi: 10.1016/j.ccr.2014.07.026. [DOI] [PubMed] [Google Scholar]