Abstract
Purpose
High myopia is associated with blinding ocular morbidities. Identifying novel biomarkers may provide clues on pathogenic pathways that are currently unknown. We aimed to identify serum metabolic biomarkers and investigate the metabolic alterations in relation to high myopia.
Methods
Forty adults with high myopia and 40 with low myopia aged 60 years or older from the Weitang Geriatric Diseases study were included in the case-control study. Refractive error was determined by autorefraction followed by subjective refraction. We performed the metabolomic analysis of serum samples from patients with high myopia and age- and sex- matched controls with low myopia, using a nontargeted gas chromatography coupled to time-of-flight mass spectrometer. The area under the receiver operating characteristic curve (AUC) was computed to assess the discrimination capacities of each metabolite marker. Databases including KEGG and MetaboAnalyst were utilized to search for the potential pathways of metabolites.
Results
Serum metabolomic profiles could well distinguish high myopia from low myopia. Twenty metabolic biomarkers were identified as potential serum biomarkers for high myopia, yielding AUC values of 0.59–0.71. Metabolic pathways in relation to high myopia, mainly characterized by increased energy metabolism, increased oxidative stress, abnormal amino acid metabolism, and altered biotin metabolism, provide a foundation to support myopia progression.
Conclusions
This study identified valuable metabolic biomarkers and pathways that may facilitate an improved understanding of the disease pathogenesis. The finding holds translational value in the development of new therapeutic measures for high myopia-related complications.
Subject terms: Epidemiology, Biomarkers
Introduction
High myopia, usually defined as spherical equivalent (SE) of less than −6.0 diopters (D), is known to be linked with quite a few ocular morbidities such as retinal detachment [1], cataract [2–4], and glaucoma [3, 5] and is a major cause for visual impairment and blindness, especially in Asian populations [6, 7]. With the global prevalence of myopia rising rapidly in recent decades [8–10], the public health burden of high myopia would thus increase correspondingly. It is estimated that nearly 1000 million people would be affected by high myopia throughout the world by the year 2050 [11]. It is still debatable whether high myopia is driven by genetic or environmental factors. A traditional view supports that genetic factors might have a greater impact on high myopia while environmental factors may play a more important role in mild or moderate myopia [12]. However, this view has been contradicted by recent evidence, which demonstrated that a significant increase in the prevalence of high myopia, with about 20% of the younger cohort in East Asia being affected [13, 14]. As genetic pools are impossible to change significantly in decades of time, the increase in the prevalence of high myopia suggests that environmental factors may also be important for the development of high myopia. From a public health perspective, it is important to identify novel biomarkers which could characterize the different developmental phenotypes of high myopia as these biomarkers may provide clues on pathogenic pathways that are currently unknown, and may allow population-based risk stratification of patients for high myopia.
Metabolomics is a widely used tool to assess biomarkers and provide molecular information of disease phenotype since metabolites are ultimate product of gene, mRNA, and protein activity [15]. Variations in the metabolome represent the interplay of genetic and environmental factors and are in relation to disease states, which may reveal new knowledge in disease mechanism and pathophysiology [16]. Metabolomics has been extensively used in the research of eye disorders including diabetic retinopathy, age-related macular degeneration, and glaucoma [17]. Although genome-wide-association studies (GWAS) have revealed several genetic loci associated with refractive phenotypes [18, 19], high myopia remains a complex disorder where findings from GWAS are far from conclusive. We hypothesize that a distinct metabolic signature of high myopia exists as one previous study has identified metabolite markers of high myopia in the aqueous humor [20]. However, aqueous humor sampling is invasive and the findings are difficult to be replicated in other studies. Moreover, it limits the translational potential of any biomarkers identified from aqueous fluid. In metabonomic studies, blood sample such as serum remains the biofluid of choice and understanding metabolite markers of high myopia is crucial for high myopia screening from a public health perspective. To the best of our knowledge, few studies have focused on the serum metabolite markers of high myopia. To address this gap of knowledge, we aimed to identify novel metabolite markers of high myopia using serum samples in this study.
Materials and methods
Study participants
A nested community-based case-control study on old adults selected from banked serum collected as a part of the Weitang Geriatric Diseases study. Cases were 40 participants with high myopia (SE < −0.6D) in either eye while equal numbers of samples were selected from participants with mild myopia (0 < SE < −3.0D) in both eyes and were treated as controls. Cases and controls were matched by age (difference within 2 years) and sex. In addition, considering that cataract may serve as a potential confounder, participants with a nuclear opacity score of more than 4 were not included in samples.
Protocol of the Weitang Geriatric Diseases study
The Weitang Geriatric Diseases study was a community-based study on people aged 60 years or older in the Weitang town located in Suzhou in eastern China. The study method has been reported elsewhere [21–24]. In brief, 4611 older Chinese adults eventually attended the clinical examinations between August 2014 and February 2015. Complete data on interviewer-administered questionnaires and blood samples were obtained for 4579 individuals. Participants had a comprehensive examination with standardized questionnaire, systemic and ocular examination, and had blood samples collected and stored. Objective refraction was measured using an autorefractor (Canon RK-5 Auto Ref-Keratometer, Canon Inc. Ltd, Tokyo, Japan). Manual subjective refraction was then used to refine vision, using the results of the objective refraction as the starting point. Slit-lamp examination (model SL-1E; Topcon) was performed on both eyes in each study participant and included a clinical grading of lens opacity using the Lens Opacities Classification System III [25]. Blood samples were collected using K2EDTA tubes and were transported to the laboratory of the Medical College of Soochow University within the same day. Each tube was centrifuged for 10 min in order to separate serum from whole blood and all aliquots were stored in the refrigerators at the temperatures of −80 °C.
The Weitang Geriatric Diseases study was conducted following the tenets of the Helsinki Declaration and was approved by the Institutional Review Board of Soochow University. All participants gave written informed consent at the recruitment stage of the study.
Metabonomic profiling and data processing
First, derivatization of the blood samples was performed according to standardized protocols. Then, all samples were analysed by gas chromatograph system coupled with a Pegasus HT time-of-flight mass spectrometer (GC-TOF-MS). GC-TOF-MS analysis was performed using an Agilent 7890 GC-TOF-MS. A 1 μL aliquot of the analyte was injected in a splitless mode. Helium was used as the carrier gas, the front inlet purge flow was 3 mL per minute, and the gas flow rate through the column was 1 mL per minute. The initial temperature was kept at 50 °C for 1 min, then raised to 310 °C at a rate of 20 °C per minute and was kept for 6 min at 310 °C. The injection, transfer line, and ion source temperatures were 280 °C, 280 °C, and 250 °C, respectively.
Chroma TOF 4.3X software of LECO Corporation and LECO-Fiehn Rtx5 database were used for raw peaks exacting, the data baselines filtering and calibration of the baseline, peak alignment, deconvolution analysis, peak identification, and integration of the peak area. Both of mass spectrum match and retention index match were considered in metabolites identification. We removed peaks detected in <50% of quality control (QC) samples or relative standard deviation >30% in QC samples.
Statistical analyses
First of all, peaks could be left through interquartile range denoising method. Then the missing values of raw data were filled up by half of the minimum value. A multivariate analysis was performed using the SIMCA14.1 software package (V14.1, Sartorius Stedim Data Analytics AB, Umea, Sweden). An unsupervised model of principal component analysis (PCA) with unit variance scaling was applied to show the distribution of origin data [26]. In order to obtain a higher level of group separation and get a better understanding of variables responsible for classification, supervised orthogonal projections to latent structures-discriminate analysis (OPLS-DA) were applied [27]. To refine this analysis, the first principal component of variable importance in the projection (VIP) was obtained. The VIP values exceeding 1 were first selected as changed metabolites. In addition, these selected metabolites were further validated at a critical P value of 0.05 using two sided student’s t test. The area under the receiver operating characteristic curve (AUC) was computed to assess the discrimination capacities of each metabolite marker. Databases including KEGG [28] (http://www.genome.jp/kegg/) and MetaboAnalyst [29] (http://www.metaboanalyst.ca/) were utilized to search for the pathways of metabolites.
Results
The mean age was 69.5 and 69.6 years in participants with high and low myopia, respectively. Women accounted for 78% of the study sample. The mean SE was −7.7D and −1.7D in the high and low myopia groups, respectively.
Serum metabolic profiles
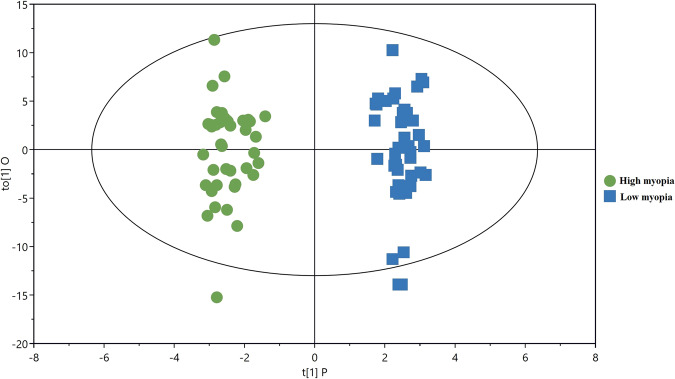
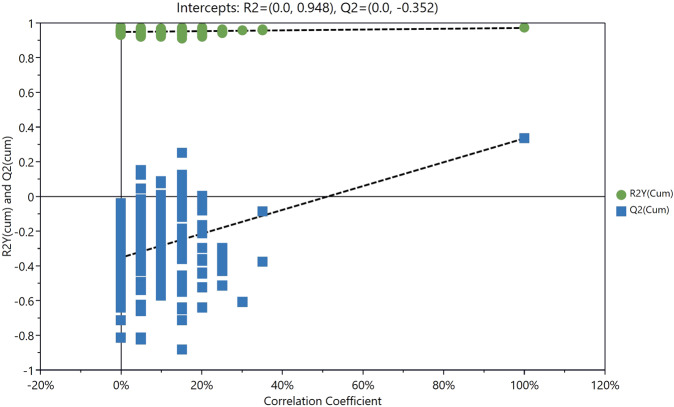
The PCA score plot showed that the QC samples were tightly clustered, supporting the robustness of the metabolic profiling platform (data not shown). The supervised OPLS-DA model was established to understand the holistic metabolic differences between high and low myopia. As can be seen from the OPLS-DA score plot, excellent separation between high and low myopia could be achieved (Fig. 1). The validation plot strongly supported the validity of the model, as all permuted R2 and Q2 values on the left were lower than the original points on the right (Fig. 2).
Fig. 1.
PLS-DA score plots for discriminating high myopia and low myopia.
Fig. 2.
Validation plots for the OPLS-DA model.
Identification of potential biomarkers
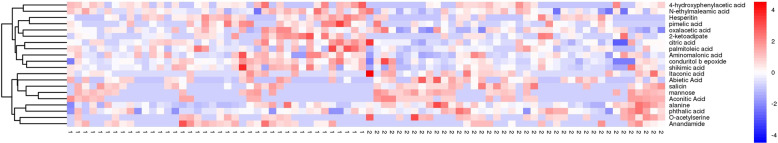
Following the successful establishment of the OPLS-DA model, potential metabolic biomarkers were selected using the criteria of VIP > 1.0 and P < 0.05. Finally, 20 metabolites were successfully selected and identified as potential biomarkers of high myopia (Table 1). Compared with participants with low myopia, eight metabolites were found to be decreased in those with high myopia, including alanine, mannose, itaconic acid, aconitic acid, O-acetylserine 1, phthalic acid, abietic acid, and salicin. By contrast, 12 metabolites, including citric acid, aminomalonic acid, palmitoleic acid, conduritol b epoxide, shikimic acid, 4-hydroxyphenylacetic acid, hesperitin, anandamide, oxalacetic acid, oxalacetic acid, pimelic acid, 2-ketoadipate, and N-ethylmaleamic acid, were increased in participants with high myopia compared with those with low myopia (Fig. 3). Those metabolite markers showed the potential to discriminate between high myopia and mild myopia, with AUC values ranging from 0.59 to 0.71 (Table 1).
Table 1.
Potential serum metabolic biomarkers identified for high myopia.
| ID | Metabolite | VIP | P value | Fold change | AUC |
|---|---|---|---|---|---|
| 1 | Alanine | 1.61 | 2.31E−3 | 0.72 | 0.71 |
| 2 | Citric acid | 1.66 | 6.66E−3 | 1.25 | 0.69 |
| 3 | Aminomalonic acid | 1.27 | 1.61E−2 | 1.36 | 0.65 |
| 4 | Mannose | 2.09 | 1.48E−2 | 0.50 | 0.65 |
| 5 | Palmitoleic acid | 1.10 | 9.91E−3 | 1.59 | 0.64 |
| 6 | Conduritol b epoxide | 1.76 | 5.36E−3 | 1.31 | 0.71 |
| 7 | Shikimic acid | 1.51 | 3.81E−3 | 1.49 | 0.70 |
| 8 | Itaconic acid | 1.69 | 1.74E−2 | 0.45 | 0.67 |
| 9 | 4-Hydroxyphenylacetic acid | 1.20 | 3.99E−2 | 1.60 | 0.59 |
| 10 | Aconitic acid | 1.96 | 2.34E−2 | 0.49 | 0.64 |
| 11 | O-Acetylserine | 1.66 | 9.28E−3 | 0.46 | 0.68 |
| 12 | Hesperitin | 2.49 | 8.94E−3 | 1.78 | 0.63 |
| 13 | Phthalic acid | 1.25 | 3.11E−3 | 0.71 | 0.68 |
| 14 | Abietic acid | 1.02 | 4.34E−2 | 0.61 | 0.64 |
| 15 | Anandamide | 1.79 | 1.46E−2 | 1.98 | 0.61 |
| 16 | Oxalacetic acid | 1.66 | 3.91E−2 | 1.24 | 0.60 |
| 17 | Salicin | 1.82 | 2.45E−2 | 0.57 | 0.64 |
| 18 | Pimelic acid | 1.69 | 3.67E−3 | 2.41 | 0.63 |
| 19 | 2-Ketoadipate | 2.02 | 2.46E−2 | 1.94 | 0.64 |
| 20 | N-Ethylmaleamic acid | 1.40 | 4.86E−2 | 1.20 | 0.62 |
VIP variable importance in the projection, AUC area under the receiver operating characteristic curve.
Fig. 3.
A heatmap showing the concentrations of 20 metabolite markers between high myopia and low myopia (1: high myopia, 2: low myopia).
Pathway analysis for potential biomarkers
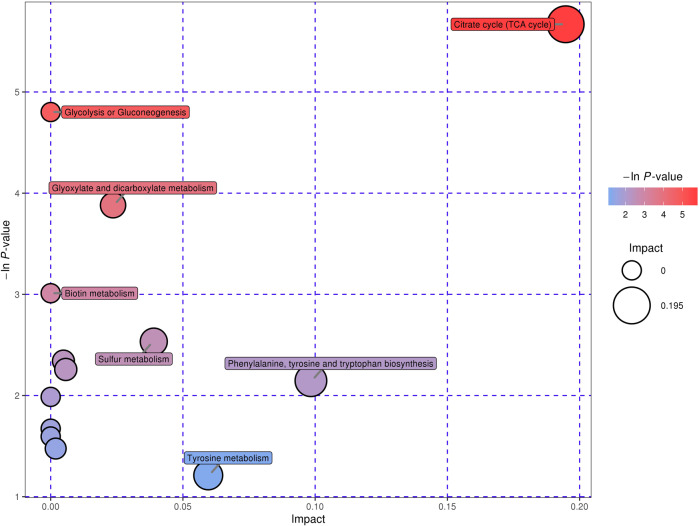
Pathway analysis, including enrichment analysis and pathway topology analysis, was further performed to understand the metabolic pathways that these potential biomarkers are involved in. A total of seven pathways were significantly enriched at the significance level of 0.10, namely citrate cycle; selenoamino acid metabolism; alanine, aspartate and glutamate metabolism; glycolysis or gluconeogenesis; glyoxylate and dicarboxylate metabolism; cysteine and methionine metabolism; and biotin metabolism (Fig. 4). Specially, citrate cycle was highly impacted, implying that these metabolic markers play important roles in the regulation of this pathway.
Fig. 4.
Enrichment analysis and pathway topology analysis for potential metabolic biomarkers of high myopia.
Discussion
In this study, we systematically explored the serum metabolic differences between high and low myopia. Twenty metabolites were ultimately identified as potential biomarkers that had the potential to distinguish high myopia from low myopia. The identification of novel metabolite markers for high myopia provides insights into potential new pathogenic pathways for this ocular condition and holds translational value in risk stratification and the development of new therapeutic measures.
Two important intermediates in energy metabolism, including citric acid and oxalacetic acid, were found to be increased between high myopia and low myopia. Increased energy metabolism has been suggested to result in the increase of extracellular adenosine [30, 31], which in turn activates the expression of adenosine receptors. Studies have demonstrated that adenosine receptors of all subtypes play a role in the regulation of eye growth [32]. Therefore, the pharmaceutical intervention targeting adenosine receptors may reduce myopia progression. Indeed, the development of form deprivation myopia in guinea pigs was reduced by the nonselective adenosine antagonist 7-methylxanthine [33]. In a clinical trial, 7-methylxanthine has also been shown to reduce the axial eye elongation rate and myopia progression rate of myopic children [34]. From this perspective, other pharmaceutical interventions targeting energy metabolism may deserve investigation in the future.
The phenomenon that aminomalonic acid and palmitoleic acid were elevated in high myopia compared with mild myopia probably suggested increased oxidative stress associated with high myopia. Aminomalonic acid has been suggested to reflect oxidative damage to proteins caused by free radicals and/or ionizing radiation [35]. According to a recent study, oxidative stress induced by carbon tetrachloride could be indicated by increase of palmitoleic acid [36]. By contrast, three metabolites that were increased in patients with high myopia all exhibited antioxidant properties or protective effects against oxidative stress, including shikimic acid [37], 4-hydroxyphenylacetic acid [38], and anandamide [39]. We speculate that these alterations could be attributed to a feedback mechanism in response to an excessive oxidative stress in the body. Numerous pieces of evidence show that oxidative stress plays a role in myopia progression [40]. Oxidative damage can alter the neuromodulation that nitric oxide and dopamine have in eye growth. Furthermore, radical superoxide or peroxynitrite production damage retina, vitreous, and lens, which eventually contributes to the appearance of retinopathies, retinal detachment and cataracts.
Both alanine and O-acetylserine were decreased in patients with high myopia. Alanine is a common nonessential amino acid and O-acetylserine is an acylated amino acid derivative. Our findings are consistent with the study by Wuu et al.’s study, which reported that the levels of amino acids (e.g., alanine, threonine, valine, isoleucine, and malic acid) decreased in form-deprived guinea pig [41]. However, this contrasts with those reported in Wuu et al.’s study, where concentrations of most of amino acids were higher in serum in patients with extreme myopia than in the normal subjects [42]. Different types of controls used in these studies may partially account for the differences. These amino acid declines in the blood may reflect increases in protein synthesis or conversions to metabolites involved in energy-conserving pathways. Elevated levels of pimelic acid were detected in patients with high myopia. As the precursor of biotin synthesis pathway, increased pimelic acid might suggest the metabolic dysregulation of the biotin biosynthesis. Biotin can be oxidized into the retina which functions as the active component of the visual cycle [43]. Biotin has also been shown to decrease retinal apoptosis and induces eye malformations in the early chick embryo [44]. Therefore, disturbed biotin metabolism may contribute to the progress of myopia.
Two previous metabolomic studies had explored the metabolic biomarkers associated with high myopia [45, 46]. A nontargeted metabolomic study collected aqueous humor samples from 40 cataract patients including 20 high myopia patients and 20 controls and found that 29 metabolites were significantly changed between the two groups [46]. The findings of this study could partly accord with ours, as some metabolic biomarkers identified in this study were replicated in ours, including oxalacetic acid, salicin, conduritol b epoxide, and 2-ketoadipate. However, due to the differences in biological samples (aqueous humors vs. serums), there were also different and complementary metabolic biomarkers between the two studies. In a more recent study, serum metabolomics profiling was investigated on 30 high myopia cases and 30 controls (without myopia) using liquid chromatography quadrupole time-of-flight mass spectrometry (LC-TOF-MS) [45]. Nine metabolites were found to be closely correlated with high myopia, among which, eight were confirmed in a validation analysis [45]. The study also indicated that oxidative stress and inflammation plays an important role in the pathogenesis of high myopia, which is consistent with our study. However, the reference group was a bit different between the two studies. Our study treated individuals with mild to moderate myopia as the controls while the controls were people without myopia in the study by Dai et al. This difference might explain the relatively larger magnitude of effect estimate of some metabolites detected in the study by Dai et al. as compared with ours. Moreover, our study employed GC-TOF-MS to acquire metabolic signatures, which was different from the study by Dai et al. that utilized LC-TOF-MS. Different analytical platforms might result in the differences of metabolites detected in these two studies [47].
To the best of our knowledge, our study represents the first one which performed the metabolomic analysis of serum samples to discriminate between high myopia and low myopia. Furthermore, this study adopted a well-matched case-control design, which excluded the confounding effects of age and sex to the maximum extent. One main limitation of this study was the small sample size, which may have prevented changes in certain metabolites from being apparent. Prospective cohort studies are needed to establish the causal relationship between metabolite markers and the incidence of high myopia. In addition, the study participants were older adults and it is unclear whether the findings could be directly extrapolated to younger generations.
In summary, this study investigated the serum metabolic signatures associated with high myopia based on a well-matched case-control design. As a result, 20 metabolites were identified as potential biomarkers that could discriminate between high myopia and low myopia. These metabolites, mainly involved in energy metabolism, biotin metabolism, and amino acid metabolism, were found to play important roles in the development and progression of high myopia. Overall, this study identified valuable metabolic biomarkers for high myopia and shed new lights on the disease pathogenesis. Validation studies are warranted to confirm the findings in other populations.
Summary
What was known before
High myopia is associated with blinding ocular morbidities.
A distinct metabolic signature of high myopia exists as one previous study has identified metabolite markers of high myopia in the aqueous humor.
What this study adds
Serum metabolomic profiles could well distinguish high myopia from low myopia.
Metabolic pathways in relation to high myopia, mainly characterized by increased energy metabolism, increased oxidative stress, abnormal amino acid metabolism, and altered biotin metabolism, provide a foundation to support myopia progression.
Acknowledgements
This study was funded by the National Natural Science Foundation of China under grant no. 81973061 and no. 81970800. The sponsor or funding organization had no role in the design or conduct of this research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chaofu Ke, Hua Xu
References
- 1.Alio JL, Ruiz-Moreno JM, Shabayek MH, Lugo FL, Abd El Rahman AM. The risk of retinal detachment in high myopia after small incision coaxial phacoemulsification. Am J Ophthalmol. 2007;144:93–98. doi: 10.1016/j.ajo.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 2.Pan CW, Cheng CY, Saw SM, Wang JJ, Wong TY. Myopia and age-related cataract: a systematic review and meta-analysis. Am J Ophthalmol. 2013;156:1021–33.e1. doi: 10.1016/j.ajo.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Pan CW, et al. Differential associations of myopia with major age-related eye diseases: the Singapore Indian Eye Study. Ophthalmology. 2013;120:284–91. doi: 10.1016/j.ophtha.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 4.Pan CW, et al. Myopia, axial length, and age-related cataract: the Singapore Malay eye study. Investig Ophthalmol Vis Sci. 2013;54:4498–502. doi: 10.1167/iovs.13-12271. [DOI] [PubMed] [Google Scholar]
- 5.Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–94.e2. doi: 10.1016/j.ophtha.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Wong YL, Saw SM. Epidemiology of pathologic myopia in asia and worldwide. Asia-Pac J Ophthalmol. 2016;5:394–402. doi: 10.1097/APO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 7.Resnikoff S, Pascolini D, Mariotti SP, Pokharel GP. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ. 2008;86:63–70. doi: 10.2471/BLT.07.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 9.Pan CW, Dirani M, Cheng CY, Wong TY, Saw SM. The age-specific prevalence of myopia in Asia: a meta-analysis. Optom Vis Sci. 2015;92:258–66. doi: 10.1097/OPX.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 10.Rudnicka AR, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2015-307724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden BA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016 doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24:1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, et al. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Investig Ophthalmol Vis Sci. 2012;53:7504–9. doi: 10.1167/iovs.11-8343. [DOI] [PubMed] [Google Scholar]
- 14.Jung SK, Lee JH, Kakizaki H, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in seoul, South Korea. Investig Ophthalmol Vis Sci. 2012;53:5579–83. doi: 10.1167/iovs.12-10106. [DOI] [PubMed] [Google Scholar]
- 15.Kell DB, Goodacre R. Metabolomics and systems pharmacology: why and how to model the human metabolic network for drug discovery. Drug Discov Today. 2014;19:171–82. doi: 10.1016/j.drudis.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–7. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, et al. Recent advances in the applications of metabolomics in eye research. Anal Chim Acta. 2018;1037:28–40. doi: 10.1016/j.aca.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 18.Kiefer AK, et al. Genome-wide analysis points to roles for extracellular matrix remodeling, the visual cycle, and neuronal development in myopia. PLoS Genet. 2013;9:e1003299. doi: 10.1371/journal.pgen.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhoeven VJ, et al. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45:314–8. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbas-Bernardos C, et al. Looking into aqueous humor through metabolomics spectacles—exploring its metabolic characteristics in relation to myopia. J Pharm Biomed Anal. 2016;127:18–25. doi: 10.1016/j.jpba.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Song E, Sun HP, Xu Y, Pan CW. Cigarette smoking and pterygium: a propensity score matching analysis. Optom Vis Sci. 2016;93:466–70. doi: 10.1097/OPX.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 22.Pan CW, et al. Visual impairment among older adults in a rural community in eastern China. J Ophthalmol. 2016;2016:9620542. doi: 10.1155/2016/9620542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan CW, Liu H, Sun HP, Xu Y. Increased difficulties in managing stairs in visually impaired older adults: a community-based survey. PLoS ONE. 2015;10:e0142516. doi: 10.1371/journal.pone.0142516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, et al. Myopia and depressive symptoms among older Chinese adults. PLoS ONE. 2017;12:e0177613. doi: 10.1371/journal.pone.0177613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chylack LT, Jr., et al. The lens opacities classification system III. The longitudinal study of cataract study group. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 26.Trygg J, Holmes E, Lundstedt T. Chemometrics in metabonomics. J Proteome Res. 2007;6:469–79. doi: 10.1021/pr060594q. [DOI] [PubMed] [Google Scholar]
- 27.Trygg J, Wold S. Orthogonal projections to latent structures (O‐PLS) J Chemom. 2002;16:119–28. doi: 10.1002/cem.695. [DOI] [Google Scholar]
- 28.Kanehisa M, et al. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42:D199–205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong J, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:W486–94. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribelayga C, Mangel SC. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci. 2005;25:215–22. doi: 10.1523/JNEUROSCI.3138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui D, Trier K, Munk Ribel-Madsen S. Effect of day length on eye growth, myopia progression, and change of corneal power in myopic children. Ophthalmology. 2013;120:1074–9. doi: 10.1016/j.ophtha.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Cui D, et al. Adenosine receptor protein changes in guinea pigs with form deprivation myopia. Acta Ophthalmol. 2010;88:759–65. doi: 10.1111/j.1755-3768.2009.01559.x. [DOI] [PubMed] [Google Scholar]
- 33.Cui D, et al. Effects of 7-methylxanthine on the sclera in form deprivation myopia in guinea pigs. Acta Ophthalmol. 2011;89:328–34. doi: 10.1111/j.1755-3768.2009.01688.x. [DOI] [PubMed] [Google Scholar]
- 34.Trier K, Munk Ribel-Madsen S, Cui D, Brogger Christensen S. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. J Ocul Biol Dis Inform. 2008;1:85–93. doi: 10.1007/s12177-008-9013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Copley SD, Frank E, Kirsch WM, Koch TH. Detection and possible origins of aminomalonic acid in protein hydrolysates. Anal Biochem. 1992;201:152–7. doi: 10.1016/0003-2697(92)90188-d. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Nagata Y, Katsurada M, Sato S, Ohori Y. Changes in rat plasma-free fatty acid composition under oxidative stress induced by carbon tetrachloride: decrease of polyunsaturated fatty acids and increase of palmitoleic acid. Redox Rep. 1996;2:121–5. doi: 10.1080/13510002.1996.11747038. [DOI] [PubMed] [Google Scholar]
- 37.Rabelo TK, et al. In vitro neuroprotective effect of shikimic acid against hydrogen peroxide-induced oxidative stress. J Mol Neurosci. 2015;56:956–65. doi: 10.1007/s12031-015-0559-9. [DOI] [PubMed] [Google Scholar]
- 38.Jung Y-S, et al. Synthesis and evaluation of 4-hydroxyphenylacetic acid amides and 4-hydroxycinnamamides as antioxidants. Bioorg Med Chem Lett. 2002;12:2599–602. doi: 10.1016/S0960-894X(02)00479-1. [DOI] [PubMed] [Google Scholar]
- 39.Harvey BS, Ohlsson KS, Maag JL, Musgrave IF, Smid SD. Contrasting protective effects of cannabinoids against oxidative stress and amyloid-beta evoked neurotoxicity in vitro. Neurotoxicology. 2012;33:138–46. doi: 10.1016/j.neuro.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Francisco BM, Salvador M, Amparo N. Oxidative stress in myopia. Oxid Med Cell Longev. 2015;2015:750637. doi: 10.1155/2015/750637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, et al. Changes in retinal metabolic profiles associated with form deprivation myopia development in guinea pigs. Sci Rep. 2017;7:2777. doi: 10.1038/s41598-017-03075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wuu JA, Wen LY, Chuang TY, Chang GG. Amino acid concentrations in serum and aqueous humor from subjects with extreme myopia or senile cataract. Clin Chem. 1988;34:1610–3. doi: 10.1093/clinchem/34.8.1610. [DOI] [PubMed] [Google Scholar]
- 43.Wishart DS, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46:D608–17. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valenciano AI, Mayordomo R, de La Rosa EJ, Hallbook F. Biotin decreases retinal apoptosis and induces eye malformations in the early chick embryo. Neuroreport. 2002;13:297–9. doi: 10.1097/00001756-200203040-00010. [DOI] [PubMed] [Google Scholar]
- 45.Dai LL, et al. Serum metabolomics profiling and potential biomarkers of myopia using LC-QTOF/MS. Exp Eye Res. 2019;186:107737. 10.1016/j.exer.2019.107737 [DOI] [PubMed]
- 46.Ji YH, et al. Metabolic characterization of human aqueous humor in relation to high myopia. Exp Eye Res. 2017;159:147–55. doi: 10.1016/j.exer.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Ke C, Pan CW, Zhang Y, Zhu X, Zhang Y. Metabolomics facilitates the discovery of metabolic biomarkers and pathways for ischemic stroke: a systematic review. Metabolomics. 2019;15:152. doi: 10.1007/s11306-019-1615-1. [DOI] [PubMed] [Google Scholar]