Checkpoint blockade on lymphocyte surfaces has revolutionized cancer treatment. In particular, blockade of programmed cell death protein-1 by therapeutic antibodies is remarkably effective to reactivate T cells for the treatment of several cancer types.1,2 Immune checkpoint inhibitors that promote natural killer (NK) cell effector function have also recently demonstrated significant efficacy.3,4 Notably however, one of the main challenges of these clinical immunotherapies is that the majority of patients are resistant to immune checkpoint inhibitors.5 One practical approach to address this is identifying novel molecular targets and manipulating them to enhance antitumor immunity.
Adenosine is evidently a critical immunosuppressive metabolite that can limit the antitumor effects of cytotoxic lymphocytes.6 Aspects of the tumor microenvironment such as hypoxia and extracellular pressure effectively induce ATP conversion to adenosine by ectonucleotidase CD73 and CD39. Adenosine transmits inhibitory signals by activating adenosine receptor family molecules.7 In the current study the gene expression profiles of tumor-infiltrating NK cells from hepatocellular carcinoma (HCC) patients were analyzed using SMART-seq2 data (EGAS00001003449).8 Greater expression of adenosine A3 receptor (A3AR, also known as ADORA3) was detected in tumor-infiltrating NK cells than in peripheral NK cells (Supplementary Fig. 1A–C).
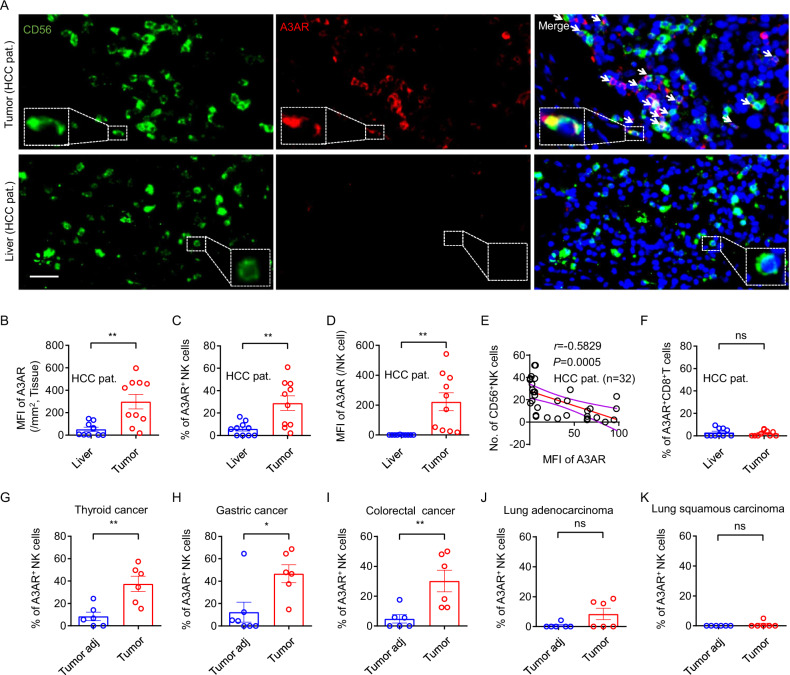
To verify enhanced expression of A3AR in the tumor-infiltrating NK cells, multicolor immunohistochemistry staining was used to assess A3AR expression in CD56+ NK cells in intratumoral and peritumoral regions in HCC patients. Locally, tumor tissues expressed higher levels of A3AR than matched paracancer liver tissues (Fig. 1B). Microimaging depicted increased expression of A3AR in tumor-infiltrating NK cells (Fig. 1A). The cumulative percentage of intratumoral A3AR+ NK cells was significantly greater than that of peritumoral NK cells (Fig. 1C). The expression level assessment in single cell indicated that the mean fluorescence intensity of A3AR in intratumoral NK cells was significantly greater than that in peritumoral NK cells (Fig. 1D). Notably, the level of A3AR expression was negatively correlated with the number of NK cells localized within the tumor (Fig. 1E). These data indicate increased expression of A3AR in intratumoral NK cells in HCC patients, and suggest that A3AR expression is related to the abundance of intratumoral NK cells.
Fig. 1.
Upregulated A3AR expression in tumor-infiltrating NK cells in multiple types of tumors. A–F Multicolor immunohistochemistry staining to assess A3AR expression in CD56+ NK cells and CD8+ T cells in intratumoral and peritumoral regions in HCC patients. A Imaging depicting co-localization of CD56 and A3AR in regions. The scale bar represents 20 μm. B Regional fluorescence intensity representing A3AR expression levels. C Proportion of CD56+ NK cells that were also positive for A3AR. D Fluorescence intensity of A3AR in individual CD56+ NK cells. E Correlational analysis of A3AR expression levels and CD56+ NK cell counts. F Proportion of CD8+ T cells that were also positive for A3AR. G–K Multicolor immunohistochemistry staining to assess A3AR expression in CD56+ NK cells in intratumoral and peritumoral regions of (G) thyroid cancer, (H) gastric cancer, (I) colorectal cancer, (J) lung adenocarcinoma, and (K) lung squamous carcinoma. The proportions of CD56+ NK cells that were also positive for A3AR are shown. Two-tailed unpaired Student’s t tests between two groups. Data represent the mean ± SEM. P < 0.05 was considered significant. *p < 0.05; **p < 0.01
A3AR expression was then analyzed in CD8+ T cells in HCC patients. A3AR were almost undetectable in intratumoral and peritumoral CD8+ T cells (Supplementary Fig. 2 and Fig. 1F). These data suggest that increased A3AR expression is confined to tumor-infiltrating cytotoxic NK cells.
Additional tumor types were then investigated to assess A3AR expression in tumor-infiltrating NK cells. Microimaging indicated that the cumulative percentage of intratumoral A3AR+ NK cells was significantly increased compared with that of peritumoral NK cells in thyroid cancer, gastric cancer and colorectal cancer (Fig. 1G–I). The upregulated expression is not obvious in lung cancer (Fig. 1J, K). These data suggest that the upregulated A3AR expression is not confined to HCC.
Collectively the results of the current study indicate that there is increased A3AR expression in tumor-infiltrating NK cells in multiple types of tumors. A3AR is reportedly involved in the inhibition of neutrophil degranulation in tissue injury.9 In addition, A2A receptors—another member of the adenosine receptor family—reportedly inhibit the antitumor effects of cytotoxic lymphocytes, and terminating its signals can markedly enhance antitumor immunity.10 Therefore, A3AR is worth studying as an NK cell related marker in tumors.
Supplementary information
Supplementary Figures and Materials and Methods
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0710802) and the Natural Science Foundation of China (reference number 81872318, 82071768).
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-020-00632-1) contains supplementary material.
References
- 1.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 2.Zhou, F., Qiao, M. & Zhou, C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell. Mol. Immunol.10.1038/s41423-020-00577-5 (2020). [DOI] [PMC free article] [PubMed]
- 3.André P, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731–1743.e1713. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruf, B., Heinrich, B. & Greten, T. F. Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells. Cell. Mol. Immunol.10.1038/s41423-020-00572-w (2020). [DOI] [PMC free article] [PubMed]
- 5.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 7.Young A, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78:1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179:829–845.e820. doi: 10.1016/j.cell.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Fishman P, Bar-Yehuda S. Pharmacology and therapeutic applications of A3 receptor subtype. Curr. Top. Med. Chem. 2003;3:463–469. doi: 10.2174/1568026033392147. [DOI] [PubMed] [Google Scholar]
- 10.Willingham SB, et al. A2AR antagonism with CPI-444 induces antitumor responses and augments efficacy to anti-PD-(L)1 and anti-CTLA-4 in preclinical models. Cancer Immunol. Res. 2018;6:1136–1149. doi: 10.1158/2326-6066.CIR-18-0056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Materials and Methods