Abstract
Transforming growth factor-beta (TGFβ) is a highly potent immunosuppressive cytokine. Although TGFβ is a tumor suppressor in early/premalignant cancer lesions, the cytokine has several tumor-promoting effects in advanced cancer; abrogation of the antitumor immune response is one of the most important tumor-promoting effects. As several immunoregulatory mechanisms have recently been shown to be targets of specific T cells, we hypothesized that TGFβ is targeted by naturally occurring specific T cells and thus could be a potential target for immunomodulatory cancer vaccination. Hence, we tested healthy donor and cancer patient T cells for spontaneous T-cell responses specifically targeting 38 20-mer epitopes derived from TGFβ1. We identified numerous CD4+ and CD8+ T-cell responses against several epitopes in TGFβ. Additionally, several ex vivo responses were identified. By enriching specific T cells from different donors, we produced highly specific cultures specific to several TGFβ-derived epitopes. Cytotoxic CD8+ T-cell clones specific for both a 20-mer epitope and a 9-mer HLA-A2 restricted killed epitope peptide were pulsed in HLA-A2+ target cells and killed the HLA-A2+ cancer cell lines THP-1 and UKE-1. Additionally, stimulation of THP-1 cancer cells with cytokines that increased TGFβ expression increased the fraction of killed cells. In conclusion, we have shown that healthy donors and cancer patients harbor CD4+ and CD8+ T cells specific for TGFβ-derived epitopes and that cytotoxic T cells with specificity toward TGFβ-derived epitopes are able to recognize and kill cancer cell lines in a TGFβ-dependent manner.
Keywords: Immune regulation, TGFbeta, cancer, T cells, adaptive immunity
Subject terms: Immune evasion, Tumour immunology, Tumour immunology
Introduction
The cytokine transforming growth factor-beta (TGFβ) is a highly pleiotropic cytokine that is involved in diverse important mechanisms, such as immune homeostasis, tissue regeneration, organogenesis, and both the suppression and development of cancer.1,2 In relation to the regulation of the immune response, TGFβ is highly implicated in immunosuppression that maintains inflammation. The immunosuppressive properties of TGFβ are orchestrated through several mechanisms. First, TGFβ inhibits the Th1 immune response through downregulation of the transcription factor T-bet, thereby reducing the T cell-mediated production of interferon-gamma (IFN-γ) and granzyme B.2 Additionally, TGFβ decreases the expression of the IL-12 receptor β2, thereby rendering T cells insensitive to proinflammatory IL-12 produced by activated dendritic cells (DCs). TGFβ decreases the density of costimulatory molecules on DCs, thereby producing tolerogenic DCs that may induce the formation of regulatory T cells (Tregs). Moreover, TGFβ may act directly on naive T cells and induce them to differentiate into immunosuppressive Tregs.3
In the setting of cancer, TGFβ has a dual role, as it acts both as a tumor suppressor and a tumor promoter. Studies indicate that TGFβ acts as a tumor suppressor during the early stages of tumorigenesis by decreasing intracellular c-myc and cycline-dependent kinase inhibitors as well as inducing apoptosis through the formation of death-associated protein kinase.4 However, these anticarcinogenic properties may be circumvented by transformed cells that have acquired a mutation in the TGFβ signaling pathway, which may reverse the effects of TGFβ, causing the cytokine to act as a powerful tumor-promoting cytokine. Hence, it has been shown in several models that preneoplastic cells that acquire a mutation in the TGFβ signaling pathway may undergo transformation into overt malignant cells. This transformation is mediated by several mechanisms; premalignant cells have partially lost their sensitivity to the anticarcinogenic properties of TGFβ, but perhaps more importantly, TGFβ has a significant impact on the tumor stroma. As such, TGFβ may induce local immune suppression in the tumor microenvironment (TME) and thereby thwart an effective antitumor immune response.5 Second, TGFβ can enhance tumor cell invasiveness and metastasis through the induction of epithelial-mesenchymal transition (EMT),6 and TGFβ may also induce the activation of cancer-associated fibroblasts (CAFs),7 which themselves induce local immune suppression as well as neoangiogenesis and fibrosis.8
In recent years, it has become increasingly clear that the immune system harbors anti-regulatory T cells that kill regulatory immune cells and thus enhance immune system reactivity.9 These anti-regulatory T cells target and kill both cancer cells and regulatory immune cells that express immunosuppressive proteins such as programmed death ligand-1 (PD-L1),10 PD-L2,11 indoleamine 2,3-dioxygenase 1 (IDO1),12 arginase-I (ARG1),13 and ARG2.14 These anti-regulatory T-cell responses may be enhanced by peptide vaccination,15 and therapeutic cancer vaccination against IDO1 and PD-L1 in advanced cancer has already shown promising results.16,17
Given that the immune system harbors T cells specifically implicated in several immunosuppressive mechanisms, we investigated the presence of TGFβ-specific T cells in healthy donors (HDs) and cancer patients. We show that both cancer patients and HDs exhibit strong and frequent responses against several epitopes in TGFβ, and we demonstrate that T cells are able to kill TGFβ-expressing cancer cells in a TGFβ-dependent manner, thus providing a preclinical rationale for immunomodulating vaccination against TGFβ.
Materials and methods
Patients and donors
Buffy coats from healthy anonymized blood donors were acquired from the blood bank in Rigshospitalet, Copenhagen, Denmark. According to the Danish Law on Research Ethics § 14, section 3, the usage of anonymized biological material does not require approval from an ethics committee. Buffy coats from cancer patients were acquired from the Department of Oncology, Copenhagen University Hospital, Herlev, Denmark. All patients provided informed consent in line with the Helsinki Declaration before study entry (Capital Region Ethics Committee approval number H-2-2011-043). The patient buffy coats were cryopreserved using the same method, but before cryopreservation, the patient PBMCs were depleted of monocytes. Thus, the cryopreserved patient cells were effectively peripheral blood lymphocytes (PBLs), but for clarity, patient PBLs are referred to as PBMCs below. PBMCs were isolated with Lymphoprep (Axis Shield, Oslo, Norway) and frozen in fetal calf serum with 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA).
Peptides
The entire TGFβ1 sequence was split into 20-mer epitopes, each of which had 10 overlapping amino acids, thus generating a library of 38 peptides spanning the entire TGFβ1 sequence. The peptides were provided by Pepscan (Lelystadt, Netherlands) and dissolved in DMSO at a concentration of 10 mM. After identifying the TGFβ1 lead epitopes, these peptides were obtained at a higher purity (>90%) from Schafer (Copenhagen, Denmark).
Enzyme-linked immunospot assay
The identification of immune responses against TGFβ1 epitopes was performed using enzyme-linked immunospot (ELISPOT) assays as previously described.10 In short, PBMCs were thawed and then stimulated with 2 µL of 10 mM peptides, and the cells were incubated in 500 µL X-VIVO (Lonza, Belgium) for 2 h at 37 °C in a humidified atmosphere with 5% CO2. After incubation, 1.5 mL of X-VIVO with 5% human serum was added to the cell cultures, and the cultures were incubated under the conditions described above. The next day, IL-2 (Novartis, Switzerland) was added to the cells at a concentration of 120 U/mL. The cells were then incubated for 12–14 days before being counted using a Countess II Automated Cell Counter (ThermoFisher). The cells were subjected to ELISPOT assays at the desired concentration and stimulated with peptide at a final concentration of 5 µM. The plates used for ELISPOT were PVDF membrane plates (Merck, Germany) coated with primary IFN-γ-specific antibody (Mabtech, Sweden). Plates were coated with primary antibody the day before use and allowed to incubate at room temperature. The next day, the wells were washed six times in 200 µL sterile PBS, and the wells were preconditioned with X-VIVO for a minimum of 2 h. Next, the X-VIVO was poured out, and PBMCs resuspended in X-VIVO at a concentration of 2–3 × 105 cells/well were plated. Next, PBMCs were stimulated with peptides as described above. The plates were incubated overnight. The next day, the cells were poured out, the wells were washed six times with 200 µL PBS, and the wells were coated with secondary antibody, streptavidin-ALP, and enzyme substrate according to the manufacturer’s protocol (Mabtech, Sweden) with appropriate washing of wells with PBS between each step. The ELISPOT plates were dried overnight and then analyzed and counted using the ImmunoSpot S6 Ultimate Analyzer (CTL Analyzers, Shaker Heights, OH, USA). The number of specific cells was defined as the mean number of spots in the peptide-stimulated wells minus the mean number of spots in the negative control wells. Statistical analysis was performed using the distribution-free resampling (DFR) method and the more conservative DFR2x method described by Moodie et al.18 Ex vivo ELISPOT assays were performed by resting thawed cells overnight and then plating the cells directly without any prior in vitro stimulation. The cells used for the ex vivo ELISPOT assays were incubated for 48 h in the plate to provide an appropriate amount of time for antigen processing.
Fluorescence-activated cell sorting (FACS) and intracellular cytokine staining (ICS)
The identification of responses in primary PBMC cultures using ICS and FACS was performed with our usual in vitro culture method for cell cultures destined for analysis by ELISPOT. However, instead of stimulating cells in the ELISPOT wells, the cells were analyzed as previously described.19 We used the following fluorochrome-tagged antibodies: anti-CD3-APC-H7, anti-CD4-PerCP or anti-CD4-FITC, anti-CD8-FITC or anti-CD8-PerCP, anti-IFN-γ-APC, anti-TNF-α-BV421, and anti-CD107a-PE (all BD Biosciences, San José, CA, USA). Dead cells were stained with Fixable Viability Stain 510 (BD Biosciences, San José, CA, USA). Another method used to identify activated T cells was by overnight stimulation of T cells with either antigen or target cells. After 18–24 h of stimulation, cells were stained with the surface antigen-specific antibodies and fixable viability stain mentioned above in combination with anti-CD107a-PE and anti-CD137-BV421 (BD Biosciences, San José, CA, USA). Donor PBMCs were analyzed for HLA-A2 by staining with anti-HLA-A2-FITC (BD Biosciences, San José, CA, USA) and an appropriate isotype control.
FACS of live cells for the establishment of specific T-cell cultures
Enrichment of specific T cells from a primary PBMC culture was performed by using our usual in vitro culture method for cell cultures destined for analysis by ELISPOT. Next, the cells were stimulated with antigen overnight, and the next day, the cells were washed twice in FACS buffer and then stained for 30 min with the following antibodies: LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (Waltham, MA, USA), anti-CD4-FITC, anti-CD8-PerCP, anti-CD107a-PE and anti-CD137-BV421 (BD Biosciences, San José, CA, USA). Cells were then washed twice and resuspended in FACS buffer. Next, the cells were sorted on a FACS ARIA flow cytometer with the appropriate application settings and compensation controls. Cell sorting was performed with the purity setting. After sorting, the cells were split into two fractions—half of the enriched cells were expanded using our rapid expansion protocol, and the other half of the cells were cloned using limiting dilution with seeding of three cells/well. Cloned cells were expanded using our rapid expansion protocol.
Magnetically activated cell sorting (MACS) and establishment of T-cell cultures specific for TGFβ-15
We used MACS to enrich for antigen-specific T cells both from primary cultures and from previously enriched cultures. Enrichment of specific T cells from a primary PBMC culture was performed by using our usua in vitro culture method for cell cultures destined for analysis by ELISPOT. Next, the cells were stimulated with antigen overnight, and the next day, the cells were enriched using the MACS CD137 enrichment kit (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer’s protocol. The enriched cells were expanded using our rapid expansion protocol, and to obtain a high proportion of T cells specific for TGFβ-15, we re-enriched the TGFβ-15-specific culture, which was then enriched for CD8+ T cells after an appropriate amount of time for expansion. The enriched CD8+ T cells were either cloned by a limiting dilution method or expanded directly. All enriched cultures and clones were expanded using our rapid expansion protocol.
Chromium-51 cytotoxicity assay and cytokine stimulation of target cells
We used the standard chromium-51 cytotoxicity assay to assess the killing potential of the specific T cells as described previously.20 To manipulate the expression of TGFβ in several cancer cell lines, the cancer cell lines were stimulated with IL-4 (100 or 200 U/mL) and TGFβ1 (2.5 or 5 ng/mL) (all Peprotech, Rocky Hill, New Jersey, USA) either alone or in combination for 48 h before the assay. All cancer cell lines were maintained in R10 medium (RPMI supplemented with 10% FCS) at all times during culture. Cr51 experiments were performed in R10 medium, whereas ELISPOT assays and ICS were performed in X-VIVO.
Lysing of cells and determination of the protein content in cell lysates
Cells destined for analysis by WB were washed twice using sterile PBS. The supernatant was carefully removed, and the pellet was kept at −80 °C. Cells were lysed using RIPA lysis solution (ThermoFisher) with a 1:100 dilution of Halt protease inhibitor cocktail (ThermoFisher). Cells were lysed with constant agitation at 4 °C for 15 min. Next, the cells were spun down, and the supernatant was carefully removed and transferred to a new Eppendorf tube. For equal loading of protein in the gel, the amount of protein in each lysate was determined using the BCA Protein Assay Kit (ThermoFisher) according to the manufacturer’s protocol.
Analysis of intracellular TGFβ using western blotting
Prior to loading the gels, lysates from each cell line were mixed with distilled water and BoltTM reducing agent (10× dilution) and BoltTM buffer (4× dilution) (both ThermoFisher Scientific) for a total loading volume of 50 µL. The amount of loaded protein varied from experiment to experiment, but the amount of loaded protein was always equal across all lanes in one gel. For quantification of the protein size, we used the BioRad Precision Plus ladder. After loading of the BoltTM 4–12% Bis-Tris Plus gel (ThermoFisher) with lysates, the gel was run for ~20 min at 200 V using a PowerPac HV (BioRad). Next, the gel was transferred to an iBlot 2 PVDF ministack (ThermoFisher), and blotting was performed using an iBlot Gel 2 Transfer device (ThermoFisher) at 20 V for 1 min, 23 V for 4 min and 25 V for 2 min. After transferring the protein, the membrane was cut in half, as the antibodies specific for TGFβ and the vinculin housekeeping protein required different blocking and incubation conditions. The membrane with the TGFβ protein was blocked for 1 h in blocking buffer containing TBS + 0.1% Tween + 3% BSA, whereas the membrane with vinculin was blocked for 1 h in TBS + 0.1% Tween +5% skimmed milk powder. After 1 h of blocking, the membrane with TGFβ was incubated overnight with constant agitation with the TGFβ specific antibody (Cell Signaling Technologies, CST3711) at a 1:2000 dilution in blocking buffer at 4 °C. The membrane with vinculin was incubated in TBST + 0.1% Tween overnight at 4 °C. On the following day, the membrane with vinculin was washed three times for 5 min each with TBST buffer and then stained with the vinculin-specific antibody SAB4200080 (Sigma-Aldrich) at a 1:1,000,000 dilution in blocking buffer for 1 h at room temperature with constant agitation. Next, both membranes were washed three times for 5 min each with the appropriate blocking buffer and then incubated with either anti-rabbit antibody (CST7074) at a 1:2000 dilution in blocking buffer (TGFβ membrane), whereas the membrane with vinculin was incubated with blocking buffer containing anti-mouse antibody (CST7076) at a 1:2000 dilution. Next, the membranes were washed three times in TBST and then incubated for 5 min at RT with the SuperSignalTM West Femto Maximum Sensitivity Substrate (ThermoFisher) before imaging using a BioRad Gel Doc gel reader (BioRad) at chemihigh resolution for visualization of the vinculin and TGFβ bands and by using colorimetric analysis for visualization of the ladder. The bands for TGFβ were acquired after ~10 s of exposure, whereas the bands for vinculin were acquired after ~80 s of exposure; however, this time varied from experiment to experiment. The band for TGFβ appeared universally at ~50 kDa, which is in accordance with the TGFβ-specific band detected in cell lysates by the manufacturer. The bands for the vinculin loading controls appeared at ~120 kDa. The relative expression of TGFβ in the different cell lysates was analyzed using ImageJ software.21
FACS analysis of HLA-I expression on THP-1 cells
For the analysis of HLA-I expression on THP-1 cells treated with different cytokines, 106 cells were isolated and split into two fractions—one for the isotype control and one for staining for HLA-I. As THP-1 is a myeloid cell line, we chose to use an Fc-blocker to decrease the amount of nonspecific binding. Blocking was performed by blocking 5 × 105 cells with 95 µL FACS buffer (PBS + 2% FCS) and 5 µL Fc-blocker. Cells were blocked for 10 min. Next, the cells were washed and stained with either 20 µL anti-HLA-I FITC-conjugated antibody (BD Biosciences, San José, USA) or the appropriate isotype control. Cells were stained for 30 min and washed twice. Just before FACS, cells were stained with 5 µL 7AAD to exclude dead cells from the FACS experiment.
siRNA-mediated TGFβ silencing
A set of three Stealth siRNA duplexes for targeted silencing of TGFβ (HSS110683, HSS110684, and HSS110685) and Stealth siRNA negative control duplexes were obtained from Invitrogen (Invitrogen/Life Technologies, Paisley, UK). The Stealth TGFβ siRNA duplexes consisted of the sense sequences 5′-GGUGGAAACCCACAACGAAAUCUAU-3′, 5′-UCCUGGCCCUGUACAACCAGCAUAA-3′ and 5′-CCACCUGCAAGACUAUCGACAUGGA-3′. In general, negative control siRNAs are most often used as nontargeting siRNAs and are designed not to target any gene. They are used to determine the nonspecific effects of siRNA delivery and to provide a baseline for comparison to siRNA-treated samples, and they are the most appropriate negative control for such experiments.
In regard to off-target effects, they occur when a siRNA has sufficient homology to an untargeted gene, thereby silencing it along with the intended target. It is well known that off-target effects can be problematic when using traditional siRNA since both the sense and antisense strands of an unmodified siRNA can enter the RNAi pathway. However, we used a 25-mer blunt dsRNA oligo that contains a chemically modified sense strand, which allows only the antisense strand to efficiently enter the RNAi pathway. This modification eliminates concerns about sense strand off-target effects. For TGFβ silencing experiments, THP-1 cells were transfected with TGFβ siRNA using electroporation parameters that were previously described.22,23 In short, 6.5 × 106 cells were washed three times in OPTIMEM and then resuspended in 400 µL OPTIMEM. Cells were transferred to a 4-mm-gap cuvette and transfected with 0.25 nmol of each of the three TGFβ siRNAs using one pulse of 500 V lasting for 2 mS. Directly after transfection, the cells were transferred into a warm medium and allowed to rest for 48 h before setup of the Cr51 cytotoxicity experiment. The remaining cells were pelleted and analyzed for intracellular TGFβ expression as described above. An appropriate transfection efficiency was confirmed prior to performing the functional experiments by transfecting 6.5 × 106 THP-1 cells with 3 µL BLOCK-iT fluorescent oligo (ThermoFisher) using the same transfection settings. At 24 h after transfection, the cells were analyzed by FACS for the expression of FITC to ensure the proper transfection efficiency (Supplementary Fig. 1). Mock-transfected cells were used as negative controls. The negative controls used for the functional experiments and western blotting were mock-transfected cells.
Results
TGFβ harbors several immunogenic epitopes that induce a response in both healthy donor and cancer patient T cells
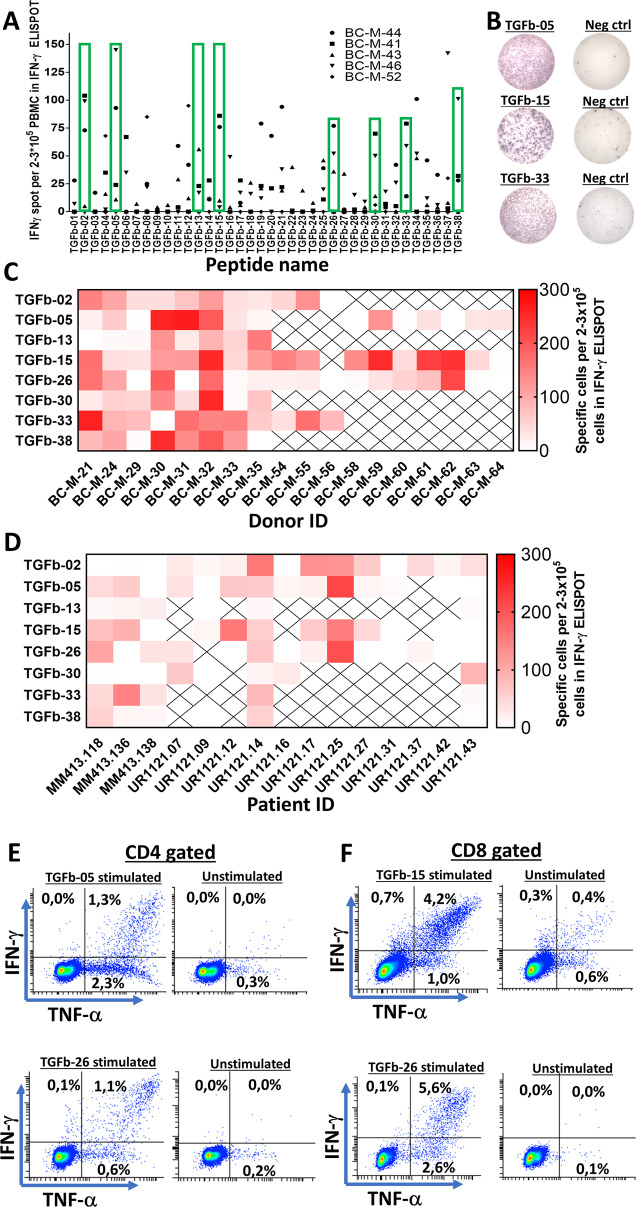
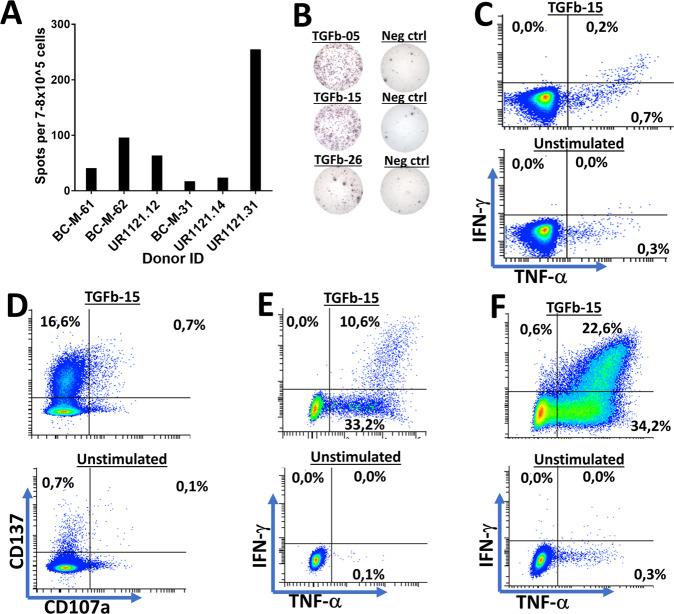
PBMCs from five HDs were tested to detect spontaneous immune responses against epitopes in TGFβ. We chose to investigate immune responses against all parts of TGFβ by dividing the entire TGFβ sequence into 20-mer peptides, each with 10 overlapping amino acids, thus generating a total of 38 peptides (Fig. 1). Responses were analyzed by in vitro interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) assays. The effective screening was performed by stimulation of PBMCs with three pooled peptides in vitro and then restimulation with each peptide individually after 7–10 days of in vitro culture. Responses were detected against several epitopes in TGFβ, and these epitopes were spread out over the entire TGFβ sequence (Fig. 2A). Based on the frequency and amplitude of the responses, we chose eight lead epitopes. To confirm the immunogenic potential of these epitopes, we tested PBMCs from additional HD, which were stimulated in vitro with each peptide individually. We confirmed strong and frequent responses to all eight lead epitopes, among which TGFβ-02, TGFβ-15, TGFβ-26, and TGFβ-33 produced the strongest and most frequent responses (Fig. 2B, C). As HD and cancer patients can show different patterns of immune responses toward epitopes24, we investigated the immunogenic potential of the selected epitopes in patients with metastatic melanoma and prostate cancer. The responses identified in cancer patients were not as strong as the responses identified in HDs, and not all epitopes incited a strong response in patient PBMCs. TGFβ-02, TGFβ-15, TGFβ-26, and TGFβ-33 appeared to be immunogenic in patients (Fig. 2D). Using intracellular cytokine staining (ICS), we showed that the selected epitopes induced both CD4+ (Fig. 2E) and CD8+ T-cell responses (Fig. 2F). Additionally, we established bulk cultures for several of the TGFβ lead epitopes using magnetically activated cell sorting (MACS), and these cultures harbored both CD4+ and CD8+ TGFβ-specific T cells (Supplementary Fig. 2), thus demonstrating the high immunogenic potential of several epitopes in TGFβ. Ex vivo responses against several epitopes were identified in both HD and cancer patient PBMCs. Cells were thawed and rested overnight before plating and stimulation for 48 h, and both HD and patient PBMCs stimulated ex vivo released significant amounts of IFN-γ (Fig. 3A, B). We detected a CD8+ T-cell response in ex vivo-plated PBMCs from a patient with prostate cancer (UR1121.14) after only 5 h of stimulation with the epitope TGFβ-15 (Fig. 3C). As this patient showed a strong response to TGFβ-15, our intention was to establish a specific T-cell culture against TGFβ-15 using PBMCs from this patient. First, we wanted to establish whether CD137 could be used as a marker for sorting specific T cells from this donor. Accordingly, we stimulated patient PBMCs with TGFβ-15 and maintained the cells in culture for 14 days. Next, the PBMCs were restimulated with TGFβ-15 for 18 h and then analyzed for the expression of CD137 and CD107a using FACS. By this experiment, we showed that CD137 would be a suitable marker for enriching for specific T cells, as 16.6% of CD8+ T cells were CD137+ after stimulation with the peptide (Fig. 3D). Thus, we enriched TGFβ-15-specific T cells by using the MACS CD137 enrichment kit and established a culture containing both CD4+ (Fig. 3E) and CD8+ TGFβ-15-specific T cells (Fig. 3F).
Fig. 1.

Amino acid sequences of all 20-mer epitopes in the TGFβ peptide library. Underlining of letters has been performed to ease tracking of the sequences. Below is a figure depicting the different parts of TGFβ. The signal peptide (SP), Amino acid (AA)
Fig. 2.
In vitro responses against several TGFβ1-derived epitopes. A Peripheral blood mononuclear cells from five healthy donors were analyzed for in vitro responses against 38 20-mer peptides derived from the TGFβ1 protein. Each peptide had 10 amino acids overlapping with the adjacent 20-mer peptide. Cells were stimulated once with a pool of three peptides and restimulated with each peptide individually in the ELISPOT assay after 7–9 days of incubation. Green rectangles depict the lead epitopes, which were chosen upon analyzing the immune responses in the five healthy donors. B Representative ELISPOT responses against the identified lead peptides. C Heatmap depicting the amplitude of the responses in PBMCs from healthy donors against the lead epitopes. X in the heatmap indicates that the peptide was not tested in the donor. The names on the x axis refer to the healthy donor ID. D Heatmap depicting the amplitude of the responses in cancer patient PBMCs against the lead epitopes. X in the heatmap indicates that the peptide was not tested in the patient. The names on the x axis refer to patient IDs. E CD4+ responses in T cells from a cancer patient against TGFβ epitopes using ICS. F CD8+ responses in T cells from a cancer patient against TGFβ epitopes using ICS
Fig. 3.
Ex vivo responses against several TGFβ1-derived epitopes. A Amplitude of responses in PBMCs from both cancer patients and healthy donor PBMCs in ex vivo ELISPOT. PBMCs were rested overnight after thawing and then plated directly in ELISPOT wells and stimulated with epitopes for 48 H in ELISPOT wells. The results shown demonstrate a response against TGFβ-05, TGFβ-15, and TGFβ-26. B Examples of ex vivo ELISPOT responses against several of the TGFβ1 lead epitopes. C CD8+ T-cell response identified against the epitope TGFβ-15 after only five H of stimulation using ICS in an ex vivo setting. D PBMCs from a patient with prostate cancer displaying a CD8+ T-cell response against the TGFβ-15 epitope after 18 H stimulation with the epitope with a prior 14 days of in vitro stimulation. E Gate set on CD4+ T cells from a TGFβ-15-specific T-cell culture from donor UR1121.14 cells stimulated with TGFβ-15. Cells were enriched twice using the MACS CD137 enrichment method as described in the Materials and Methods. F Gate set on CD8+ T cells in the TGFβ-15-specific T-cell culture described above
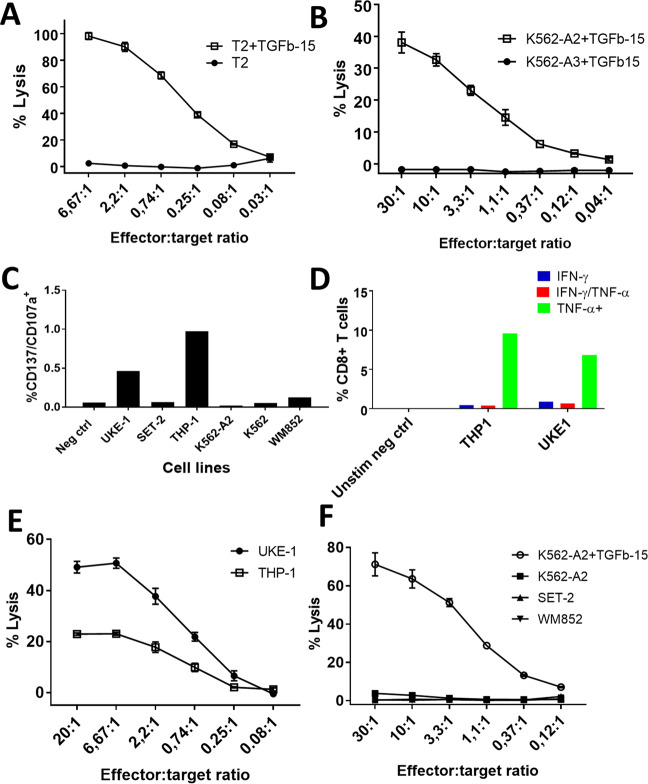
TGFβ-15-specific T cells recognize and kill peptide-pulsed HLA-A2+ target cells
Using limiting dilution, we established CD8+ TGFβ-15-specific clones from patient UR1121.14, and the clones showed high reactivity to TGFβ-15 (Supplementary Fig. 3). Staining of PBMCs with an HLA-A2+-specific antibody revealed that the donor UR1121.14 was HLA-A2+ (data not shown), and we next sought to establish whether the specific T cells could lyse peptide-pulsed HLA-A2+ target cells by using standard Cr51 cytotoxicity assays. As the TGFβ-15 epitope is a 20-mer, it cannot be presented in its full length on HLA-I molecules. Previous reports have shown that TAP-deficient HLA-A2+ T2 cells can efficiently cross-present long peptides.10,25 Thus, we examined whether the TGFβ-15 epitope could be processed and presented by T2 cells. Peptide-pulsed T2 cells were readily lysed by specific T cells, whereas nonpulsed T2 cells were not killed by specific T cells (Fig. 4A). As T2 cells are positive for other HLA alleles than HLA-A2, the killing could potentially be mediated by a match on another HLA allele. Thus, we used K562 cells as target cells. The original K562 cell line is completely HLA deficient,26 but we chose to work with two cell lines that were genetically modified to stably express either HLA-A2 or HLA-A3, ensuring that these were the only HLA alleles expressed by the respective cells. Hence, only peptide-pulsed HLA-A2+ cells were killed by TGFβ-15-specific clones, whereas nonpulsed HLA-A2+ cells and peptide-pulsed HLA-A3+ cells were not killed (Fig. 4B).
Fig. 4.
TGFβ-15-specific CD8+ T-cell clones kill target cells in an HLA-A2-restricted manner and kill cancer cell lines expressing TGFβ. A TGFβ-15-specific CD8+ T cells effectively lysed T2 cells pulsed with TGFβ-15. B To control that the TGFβ-15 response was indeed HLA-A2 restricted, it was demonstrated that HLA-A2+ target cells but not HLA-A3+ target cells pulsed with peptide were lysed. C Stimulation of TGFβ-15-specific clones with HLA-A2+ cancer cell lines and K562 cells with subsequent analysis of the expression of CD107a and CD137 on T cells. D Stimulation of T cells with UKE-1 and THP-1 in an ICS. E UKE-1 and THP-1 cells used as target cells in Cr51 release assays with TGFβ-15-specific CD8+ T cells as effector cells. F The HLA-A2+ and TGFβ-expressing cancer cell lines SET-2 and WM-852 were used as target cells in a Cr51 release assay with TGFβ-15-specific T cells as effector cells. K562-A2 loaded with TGFβ-15 peptide was included as a positive control
Next, we identified the minimal epitope in TGFβ-15 by dividing the TGFβ-15 epitope into a 10-mer peptide library of nine overlapping amino acids, thus generating 11 10-mer peptides. T cells from the TGFβ-15-specific bulk culture were plated for ELISPOT assays and stimulated with each of the 10-mer peptides, which revealed that the minimal epitope in the TGFβ-15 sequence was the epitope with the sequence VLLSRAELRL (TGFb-15-08) (Supplementary Fig. 4).
TGFβ-15-specific T cells kill TGFβ-expressing HLA-A2+ cancer cells
As TGFβ is heavily involved in creating a tumor-suppressive environment, we sought to identify whether TGFβ-15-specific T cells were able to recognize HLA-A2+ cancer cell lines in order to establish whether the immune system itself can kill immunosuppressive TGFβ-expressing cells. The cell lines UKE-1, SET-2, and THP-1, which are all derived from patients with acute myeloid leukemia, were used as target cells in concert with the HLA-A2+ melanoma cell lines WM-852 and K562 as well as HLA-A2+ K562 cells. TGFβ-15-specific T cells were stimulated overnight with the respective target cells at an effector target ratio of 3:1, and the next day, T cells were analyzed for the expression of CD137 and CD107a. Two cancer cell lines, THP-1 and UKE-1, were recognized by specific T cells, whereas the other cell lines did not activate T cells (Fig. 4C). These findings were confirmed using ICS (Fig. 4D). Next, we wanted to confirm that UKE-1 and THP-1 cells express TGFβ, so we determined the amount of intracellular TGFβ by western blotting (WB) of cell lysates. Lysates from UKE-1 and THP-1 cells were analyzed in concert with lysates from the myeloid leukemia cell lines HL60, SET-2, and MonoMac, the lymphoma cell line Daudi, the myeloma cell line RPMI8226, and the melanoma cell line WM-852. WB showed that all the analyzed cells expressed TGFβ (Supplementary Fig. 5). As both UKE-1 and THP-1 cells express TGFβ intracellularly, the activation of TGFβ-15-specific T cells by these cells was mediated by the recognition of TGFβ-derived epitopes presented by HLA-I on UKE-1 and THP-1 cells. Next, a Cr51 release assay demonstrated that both UKE-1 and THP-1 cells are readily killed by TGFβ-15-specific T cells (Fig. 4E), whereas neither SET-2 nor WM-852 cells were killed by T cells (Fig. 4F).
Increased T-cell recognition of THP-1 cells that express increased amounts of TGFβ
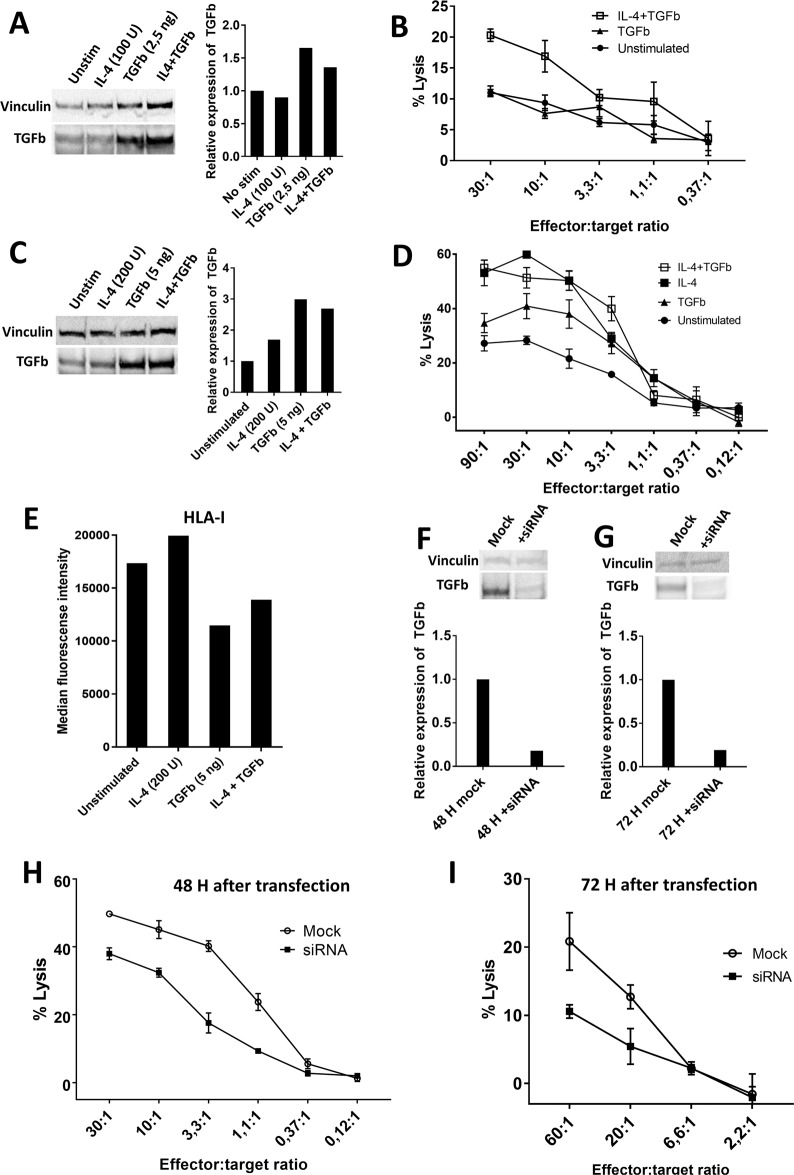
Treatment of the THP-1 cell line with different cytokines has been shown to impact the gene expression of THP-1 cells.27 As IL-4 is a cornerstone cytokine in the development of the Th2 response and TGFβ secretion is involved in the Th2 response,3 we speculated that treatment of THP-1 cells with IL-4 would enhance the expression of TGFβ. Additionally, since the stimulation of DCs with TGFβ is known to increase the expression of TGFβ, we hypothesized that treatment of THP-1 cells with TGFβ would increase the expression of TGFβ in THP-1 cells. Importantly, the TGFβ-15 epitope is located in the latency-associated peptide (LAP) of TGFβ and hence is not found in the active form of the TGFβ sequence. Consequently, any change in the recognition of THP-1 cells by TGFβ-15-specific T cells after treatment with exogenous active TGFβ will depend on a change in the expression of intracellular TGFβ, as exogenous active TGFβ does not contain the TGFβ-15 epitope. THP-1 cells were treated with either IL-4 (100 U/mL), TGFβ1 (2.5 ng/mL), or both cytokines together for 48 h. Then, the cells were harvested and analyzed for the expression of intracellular TGFβ using WB. Treatment with IL-4 did not increase the amount of intracellular TGFβ, whereas THP-1 cells treated with TGFβ alone or in combination with IL-4 displayed increased amounts of intracellular TGFβ (Fig. 5A). As we expected that the increased expression of TGFβ would result in increased killing of THP-1 cells, we used cytokine-treated THP-1 cells as target cells in a Cr51 release assay and demonstrated an increase in the fraction of killed cytokine-treated THP-1 cells (Fig. 5B). We then tested whether increased concentrations of IL-4 and TGFβ would enhance the production of TGFβ and hence the fraction of killed cells. THP-1 cells were treated with increased doses of IL-4 (200 U/mL), TGFβ (5 ng/mL), or both cytokines for 48 h. WB of the cell lysates showed that all three cytokine conditions increased the intracellular amount of TGFβ (Fig. 5C), and THP-1 cells treated with IL-4 or TGFβ alone or in combination were more readily killed (Fig. 5D). However, we wondered why IL-4-treated cells were more readily killed than TGFβ-treated cells, as the latter displayed a higher expression of TGFβ. Since TGFβ is a highly immunosuppressive cytokine, we speculated that THP-1 cells treated with TGFβ could possibly express lower amounts of HLA-I. Cytokine-treated THP-1 cells were analyzed for the expression of HLA-I using FACS, and cells treated with TGFβ showed considerably lower expression of HLA-I than untreated cells and IL-4-treated cells (Fig. 5E). Finally, to clarify whether decreased amounts of TGFβ would result in a lower fraction of killed cells, the production of TGFβ in THP-1 cells was silenced using siRNA oligonucleotides specific for TGFβ. Mock-transfected and siRNA-transfected cells were pelleted and lysed 48 and 72 h after transfection. WB of the lysates revealed markedly lower amounts of intracellular TGFβ in the siRNA-transfected cells at both time points (Fig. 5F, G), and a Cr51 release assay of siRNA-transfected THP-1 cells as target cells demonstrated a reduced fraction of killed siRNA-transfected cells compared to that of mock-transfected cells (Fig. 5H, I).
Fig. 5.
Recognition and killing of cancer cells by TGFβ-15-specific T cells depends on the expression of TGFβ by cancer cells. A Western blot (WB) analysis of intracellular expression of TGFβ in THP-1 cells that were stimulated for 48 h with either IL-4 (100 U/mL) or TGFβ (2.5 ng/mL) or both in combination. B Killing of THP-1 cells that were stimulated for 48 h with either TGFβ (2.5 ng/mL) or both with IL-4 (100 U/mL) and TGFβ (2.5 ng/mL). C Western blot analysis of intracellular expression of TGFβ in THP-1 cells that were stimulated for 48 h with either IL-4 (200 U/mL) or TGFβ (5 ng/mL) or both in combination. D Killing of THP-1 cells that were stimulated for 48 h with either IL-4 (200 U/mL) or TGFβ (5 ng/mL) or both in combination. E Expression of HLA-I in THP-1 cells treated with either IL-4 (200 U/mL) or TGFβ (5 ng/mL) or both in combination. F Western blot analysis of the expression of intracellular TGFβ in THP-1 cells after 48 h of transfection with TGFβ siRNA. G Western blot analysis of the expression of intracellular TGFβ in THP-1 cells after 72 h of transfection with TGFβ siRNA. H Cr51 release assay showing the killing of THP-1 cells mock-transfected or transfected with TGFβ siRNA at 48 H after transfection. I Cr51 release assay showing the killing of THP-1 cells mock-transfected or transfected with TGFβ siRNA at 72 H after transfection
A 9-mer epitope in the signal peptide of TGFβ is a target of specific T cells
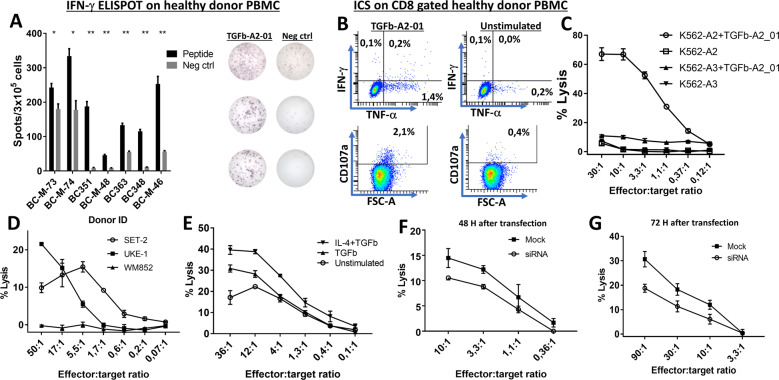
Given the high frequency of T-cell responses associated with several different sites in the TGFβ sequence, we set out to characterize an HLA-A2-restricted 9-mer epitope located in another part of the TGFβ sequence in more detail. By using the SYFPEITHI database of MHC ligands and peptide motifs28, we searched the entire TGFβ sequence for 9-mer epitopes with high binding affinity for HLA-A2, and the peptide sequence LLLLPLLWL, which is part of the TGFβ-01 peptide, was identified as the top binding 9-mer epitope with a binding affinity score of 30. Hence, we scrutinized spontaneous T-cell responses to this epitope, which was called TGFβ-A2-01, in HLA-A2+ healthy donor PBMCs. The majority of healthy donor PBMCs displayed a response against the epitope (Fig. 6A). Using ICS, we showed that the T-cell responses were indeed CD8+ T-cell responses (Fig. 6B). We then isolated TGFβ-A2-01-specific T cells from a healthy donor (BC363) with a strong response to TGFβ-A2-01 by performing a single in vitro stimulation of donor PBMCs followed by 14 days of culture. Next, cells were stimulated overnight with TGFβ-A2-01, and the specific cells were then enriched using FACS live cell sorting by gating on CD3+/CD8+/CD137+ cells. The enriched cells were expanded as described in the materials and methods section, and after 14 days of expansion, several T-cell lines derived from the enrichment showed high specificity for the TGFβ-A2-01 peptide (Supplementary Fig. 6). The specific T cells lysed the peptide-pulsed HLA-A2+ cells, whereas the nonpulsed HLA-A2+ and peptide-pulsed HLA-A3+ target cells were not lysed (Fig. 6C). As the WB analysis showed that the HLA-A2+ cell lines UKE-1, SET-2, and WM-852 all express TGFβ (Supplementary Fig. 5), we used these cells as target cells in a Cr51 release assay, and both UKE-1 and SET-2 cells were killed, whereas the WM-852 cells were not killed (Fig. 6D). Of note, SET-2 cells were not killed by the TGFβ-specific T-cells. We enhanced the expression of TGFβ by THP-1 cells by stimulating THP-1 cells for 48 h with TGFβ (5 ng/mL) either alone or in combination with IL-4 (200 U/mL). Cytokine-treated THP-1 cells were more readily killed than unstimulated target cells (Fig. 6E). Finally, THP-1 cells transfected with TGFβ siRNA were not as readily killed as mock-transfected cells 48 h (Fig. 6F) and 72 h (Fig. 6G) after transfection, showing that the killing of target cells depends on the expression level of TGFβ.
Fig. 6.
CD8+ T cells specific for an HLA-A2 binding 9-mer epitope in the TGFβ signal peptide sequence readily kill TGFβ-expressing HLA-A2+-specific cancer cell lines in an HLA-A2- and TGFβ-dependent manner. A Healthy donor PBMCs secrete IFN-γ upon stimulation with the HLA-A2 binding 9-mer epitope TGFβ-A2-01 after 14 days in vitro culture (left), representative ELISPOT responses (right). B Intracellular cytokine staining of healthy donor PBMCs stimulated with TGFβ-A2-01 gated on CD8+ T cells with analysis of IFN-γ and TFN-α expression (top) and expression of CD107a (bottom). C TGFβ-A2-01-specific CD8+ T cells from a healthy donor used as effector cells against TGFβ-A2-01-pulsed HLA-A2+ target cells, nonpulsed HLA-A2+ target cells, and peptide-pulsed HLA-A3+ target cells. D TGFβ-A2-01-specific CD8+ T cells were used as effector cells with the HLA-A2+ cells UKE-1, SET-2, and WM-852 as targets in a Cr51 release assay. E THP-1 cells that were stimulated for 48 h with either TGFβ (5 ng/mL) alone or combined with IL-4 (200 U/mL) were used as target cells in a Cr51 release assay with TGFβ-A2-01-specific T cells as target cells. F Killing of THP-1 cells mock-transfected or transfected with TGFβ siRNA at 48 H after transfection. I Killing of THP-1 cells mock-transfected or transfected with TGFβ siRNA at 72 H after transfection
Discussion
Perhaps the strongest tumor-promoting property of TGFβ is its robust immunosuppressive effect. TGFβ inhibits the formation of the Th1 transcription factor T-bet, thereby inhibiting the production of IFN-γ, granzyme B, and perforin in both CD8+ T cells and NK cells.29,30 Stimulation of DCs with TGFβ decreases the expression of HLA-II and costimulatory molecules, thus generating tolerogenic DCs.3 These DCs secrete TGFβ and IL-10, and additionally, the low expression of costimulatory molecules results in ineffective priming of naive T cells, which then turn into Tregs.3 Moreover, TGFβ has been shown to skew the differentiation of monocytes into non-M1/non-M2 macrophages, which phenotypically resemble myeloid-derived suppressor cells (MDSCs).31 These highly suppressive cells not only express TGFβ themselves but also express several other immunosuppressive substances, such as arginase and reactive oxygen species.32 The metastatic process is also influenced by TGFβ and MDSCs. In a murine lung cancer model, deletion of the TGFβ receptor on myeloid cells inhibited the formation of distal metastases, an effect that was dependent on the presence of CD8+ T cells.33 In another study, abrogation of TGFβ signaling in malignant cells resulted in an influx of MDSCs into the tumor site. These MDSCs secreted TGFβ and matrix metalloproteinases themselves, thereby enhancing local immune suppression and tumor cell invasiveness.34 These studies show how TGFβ produced by myeloid cells is an important factor for the suppression of the tumor-specific immune response, and as such, there is compelling evidence that TGFβ is a powerful tumor-promoting and immune-suppressing cytokine. Given the discovery of T cells specifically involved in several other immune regulatory mechanisms,9 it seemed logical for us to search for TGFβ-specific T cells in HDs and cancer patients.
In the current study, we found high levels of TGFβ-specific immune responses in both cancer patients and HDs. Screening of a 20-mer library spanning the entire sequence showed that TGFβ contains several highly immunogenic epitopes. In fact, the number of immunogenic epitopes may be underestimated by our method of pooling the peptides during in vitro stimulation, as this may lead to HLA competition between the different peptides. As such, the degree of the TGFβ-specific immune responses could be even higher than shown by our data. By stimulating PBMC cultures with nonpooled epitopes, we confirmed the immunogenic potential of the identified lead epitopes in both HDs and cancer patients. The responses in HDs were apparently stronger than the responses in patients with cancer. The reduced responses are most likely attributed to the absence of antigen-presenting cells in the patient samples, as patient PBMCs had been depleted of monocytes prior to cryopreservation. Of note, immunogenic epitopes were found in all parts of the TGFβ protein sequence, and we directly identified several responses against the epitopes ex vivo. These responses are highly noteworthy, as neoantigen-specific immune responses are only very rarely detected using ELISPOT or tetramer staining without prior in vitro expansion.35 The detection of ex vivo responses to a nonmutant self-antigen show that the immune system constantly maintains a pool of primed TGFβ-specific T cells that are ready to dampen local TGFβ-mediated immune suppression. We speculate that the high immunogenic potential of several of the identified TGFβ epitopes could reflect that the immune system must control the number of TGFβ-expressing cells in a very strict manner to curtail deleterious local immune suppression.
We chose to continue our experiments with two different epitopes—one 9-mer epitope derived from the signal sequence of TGFβ (TGFβ-A2-01) and one 20-mer epitope (TGFβ-15) derived from the LAP sequence of TGFβ. Responses to both epitopes were HLA-A2-restricted, as the peptide-pulsed HLA-A2+ K562 cells were readily lysed by T cells, and we identified the minimal HLA-A2-restricted epitope in the 20-mer TGFβ-15 epitope. Next, we revealed that the specific T cells recognized and killed HLA-A2+ TGFβ-expressing cancer cell lines. First, we showed that all investigated cancer cell lines express TGFβ. Using Cr51 release assays, we showed that TGFβ-A2-01-specific T cells were able to kill THP-1, UKE-1 and SET-2 cells, whereas TGFβ-15-specific T cells killed only THP-1 and UKE-1 cells. Of note, neither of the T-cell lines killed WM-852 cells, which are HLA-A2+, and only the TGFβ-A2-01-specific cells killed SET-2 cells. This could be due to the inability of the target cells to process and present the epitope of interest for the specific T cells, whereas another explanation could be that the cancer cell lines employ several immunosuppressive mechanisms that inactivate the TGFβ-specific T cells. Cancer cells may also express different amounts of TGFβ, and the lack of killing may simply be due to the low expression of TGFβ.
This is supported by our experiments showing that the killing of TGFβ-expressing cancer cells depends on the amount of expressed TGFβ. It is well known that the cytokine expression profile of THP-1 cells can be modulated through stimulation with different cytokines. We used IL-4 and the active form of βTGFβ, which did not contain the TGFβ-15 epitope, to modulate TGFβ expression. Importantly, the cytokine-induced increase in intracellular expression of TGFβ resulted in increased recognition by TGFβ-specific T cells. We were, however, puzzled by the fact that IL-4-treated cells were more readily killed than TGFβ-treated cells, even though IL-4 did not increase intracellular TGFβ levels as much as TGFβ. This was explained by the fact that TGFβ-treated cells displayed markedly lower levels of HLA-I than IL-4-stimulated cells. Interestingly, we showed that even though HLA-I expression was attenuated by cytokine stimulation, we identified an increase in the fraction of killed cells. This is explained by the fact that cytokine stimulation increased the levels of intracellular TGFβ, thus leading to an increased presentation of TGFβ-derived epitopes by HLA-I molecules on the cell surface. In addition, it is important to underscore that the peptides TGFβ-15 and TGFb-A2-01 are not part of the active recombinant form of TGFβ used to treat THP-1 cells. These peptides are found in the LAP sequence of TGFβ and the signal peptide, respectively. As such, the increased recognition of TGFβ-stimulated THP-1 cells cannot be attributed to the cross-presentation of recombinant TGFβ. Finally, partial inhibition of TGFβ expression through transfection of THP-1 cells with TGFβ siRNA resulted in a significant reduction in the fraction of killed THP-1 cells.
As described above, TGFβ remains a highly immunosuppressive cytokine, and in the setting of advanced cancer, TGFβ has potent tumor-promoting effects. Different strategies targeting TGFβ have been investigated in the setting of malignant disease, and several of these have shown promising results. The TGFβ-receptor inhibitor galunisertib has been tested in clinical trials in patients with glioma and pancreatic cancer, and the compound showed some clinical activity in both diseases.36,37 Another interesting method involving TGFβ signaling is the use of belagenpumatucel-L, a vaccine consisting of irradiated lung cancer cell lines transfected with a TGFβ2 antisense plasmid to attenuate the expression of TGFβ2 and thus decrease the immunosuppressive properties of the injected tumor cells, which would then induce a robust tumor-specific immune response. This drug reached phase III in clinical trials but failed to reach the primary endpoint.38 The TGFβ1/2/3 blocking antibody fresulimumab has shown some effect in both breast cancer and primary myelofibrosis.39,40 In addition, several in vivo studies have tested the ability of TGFβ-specific antibodies to enhance the effect of cancer-targeting vaccines. In one murine CT26 colon cancer model, TGFβ-specific antibodies were administered in addition to irradiated CT26 tumor cells in a prophylactic setting, showing a benefit in mice that received both antibody and vaccination therapy.41 In another murine model of TC1 cells that expressed the human papillomavirus E6 and E7 antigens, mice that received both antibody- and E6/E7-directed vaccines showed a better tumor response and immune response to the vaccine antigens than mice that received only the vaccine.42 Furthermore, in a murine glioma model, mice that received both antibodies and vaccines directed against glioma-associated antigens displayed an enhanced immune response to the vaccination antigens.43 Only two in vivo studies have employed anti-TGFβ-directed vaccines. One study aimed to decrease the fibrogenic effects of TGFβ. Vaccinated mice displayed increased body weight, decreased TGFβ activity, decreased amounts of collagen, and reduced pSMAD3 levels.44 In another setting, a murine HPV model was treated with vaccines with both the HPV antigen and the TGFβ-derived peptide used in the colitis model. Mice that received both vaccines showed reduced tumor weights, and decreased numbers of Tregs and splenocytes showed reactivity toward the vaccination antigen.45 Hence, these studies showed that induction of a TGFβ-specific immune response would decrease the activity of TGFβ. However, none of the studies investigated the occurrence of immune responses against the TGFβ epitopes in splenocytes.
We believe that our results showing that both healthy donors and cancer patients harbor a high number of T cells that are able to recognize and kill TGFβ-expressing cells warrant further investigation. Our findings provide evidence for the existence of anti-regulatory T cells, which are naturally occurring T cells that may recognize and kill immunosuppressive cells to dampen anti-inflammation promoted by regulatory cells.9,46 As the tumor microenvironment is highly immunosuppressive, the clinical relevance of these anti-regulatory T cells is to enhance the anti-regulatory T-cell response in cancer patients to increase the levels of inflammation in the tumor, which would allow for the improved immune-mediated killing of tumor cells.15 In fact, two clinical trials aiming at inducing/enhancing the anti-regulatory T-cell response in cancer patients have shown promising results.16,17 To the best of our knowledge, the identified TGFβ-specific T cells represent another class of anti-regulatory T cells that target and kill immunosuppressive cells, and we believe that the identified T-cell responses could be used in the setting of therapeutic cancer vaccination with TGFβ-derived epitopes. Enhancement/induction of a TGFβ-specific immune response has the potential to enhance the tumor-specific immune response in several cancers, just as induction of the immune response against other immunoregulatory mechanisms has been shown to enhance the immunogenicity of dendritic cell-based vaccines.47 Generally, vaccines show a robust safety profile48 and could be relatively easily incorporated into existing anti-neoplastic treatment regimens. However, before targeting TGFβ, one needs to take the antitumorigenic properties of TGFβ into account. In the early stage of tumors and premalignant lesions, TGFβ restrains the growth of transformed cells and instead induces the apoptosis of these cells. Therefore, TGFβ is a potent tumor suppressor at the early/premalignant stage, and TGFβ-directed therapy could result in deleterious tumor escape. However, in the murine vaccination study discussed above, vaccination against TGFβ alone did not result in increased tumor cell growth.45 This finding needs to be confirmed in additional studies, in which animal models with either inducible or spontaneous tumors would receive a TGFβ-directed vaccine. An increase in the frequency of tumors in animals with a TGFβ-specific immune response would suggest that TGFβ-specific immune responses are deleterious in the very early or premalignant setting. Therefore, it would be of relevance to identify any differences in the levels of TGFβ in the malignant and premalignant setting, as we have shown that recognition of target cells depends on the intracellular levels of TGFβ. This recognition may be suppressed by both active extracellular TGFβ and by other immunosuppressive proteins, such as IDO and PD-L1. Thus, the regulation of TGFβ-specific T cells must be very complex, as the levels of intracellular and extracellular TGFβ in concert with the amounts of additional immunosuppressive proteins will influence the activation of TGFβ-specific T cells.
TGFβ-specific immune therapy could have great potential in the adjuvant setting. Induction of TGFβ-specific T-cell responses may not only abrogate local TGFβ-mediated immune suppression, thus facilitating the entry of neoantigen-specific T cells into the TME, but may also reduce the metastatic potential of transformed cells by inhibiting the process of EMT and prevent the formation of the metastatic niche, which has been shown to partially rely on TGFβ signaling.33 Hence, traditional beliefs regarding the beneficial effects of vaccination in the early disease stages may not apply to TGFβ-directed cancer vaccines. Instead, we speculate that the vaccines could be used in the adjuvant setting in concert with other immune-therapeutic modalities, such as immune checkpoint inhibitors or neoantigen-specific cancer vaccines.
In conclusion, the highly immunosuppressive cytokine TGFβ is a target for specific T cells, and TGFβ contains several highly immunogenic epitopes, which are found in all parts of the TGFβ protein. TGFβ-specific T cells are both CD4+ and CD8+, and CD8+ TGFβ-specific T cells can kill several tumor cell lines expressing TGFβ. The fraction of killed cancer cells depends upon the level of intracellular TGFβ, as increased levels of intracellular TGFβ result in an increase in the killed cell fraction, whereas abrogation of TGFβ production by siRNA transfection results in a reduced fraction of killed cells.
Supplementary information
Acknowledgements
This project was supported by the Danish Health Authority grant “Empowering Cancer Immunotherapy in Denmark”, grant number 4-1612-236/8, the Copenhagen University Hospital, Herlev, and Gentofte, and it was also supported through a research funding agreement between IO Biotech ApS and the National Center for Cancer Immune Therapy (CCIT-DK). We thank Anne Rahbech and Sara Ram Petersen for assistance to MOH in performing western blotting. We greatly appreciate the tremendous technical support from Merete Jonassen.
Author contributions
M.O.H. performed experiments, interpreted the data, and wrote the manuscript, R.E.J.M. performed experiments and interpreted the data, M.A.P. performed experiments and interpreted the data, E.M. performed experiments and interpreted the data, S.E.W.-B. performed experiments and interpreted the data, M.A.-J. performed experiments and interpreted the data, S.K.B. performed experiments and interpreted the data, Ö.M. provided critical reagents, interpreted data, and wrote the manuscript, A.W.P. interpreted the data, M.D. interpreted the data, I.M.S. interpreted the data, and M.H.A. conceived the project, interpreted the data, and wrote the manuscript.
Competing interests
It should be noted, however, that Mads Hald Andersen has generated an invention based on the use of TGFβ for vaccinations. The rights to the invention have been transferred to Copenhagen University Hospital Herlev according to the Danish Law of Public Inventions at Public Research Institutions. The capital region has licensed the rights to the company IO Biotech ApS. The patent application was filed by IO Biotech ApS. Mads Hald Andersen is a board member, consultant, and shareholder in IO Biotech. Evelina Martinenaite and Ayako Wakatsuki Pedersen are employees at IO Biotech ApS. Inge Marie Svane is a consultant and shareholder in IO Biotech. The remaining authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-020-00593-5) contains supplementary material.
References
- 1.Mullen, A. C. & Wrana, J. L. TGF-β family signaling in embryonic and somatic stem-cell renewal and differentiation. Cold Spring Harb. Perspect. Biol. 9, a027987 (2017). [DOI] [PMC free article] [PubMed]
- 2.Batlle E, Massagué J. Transforming grown factor-β signaling in immunity and cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFβ. Nat. Rev. Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massagué J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott RL, Blobe GC. Role of transforming growth factor beta in human cancer. J. Clin. Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Hao, Y., Baker, D. & Dijke, P. T. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. Int. J. Mol. Sci. 20, 2767 (2019). [DOI] [PMC free article] [PubMed]
- 7.Kobayashi H, et al. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2019;16:282–295. doi: 10.1038/s41575-019-0115-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 9.Andersen MH. Anti-regulatory T cells. Semin. Immunopathol. 2017;39:317–326. doi: 10.1007/s00281-016-0593-x. [DOI] [PubMed] [Google Scholar]
- 10.Munir S, et al. HLA-restricted CTL that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res. 2013;73:1764–1776. doi: 10.1158/0008-5472.CAN-12-3507. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad, S. M. et al. The inhibitory checkpoint, PD-L2, is a target for effector T cells: Novel possibilities for immune therapy. Oncoimmunology10.1080/2162402X.2017.1390641 (2017). [DOI] [PMC free article] [PubMed]
- 12.Sørensen RB, et al. Spontaneous cytotoxic T-cell reactivity against indoleamine 2,3-dioxygenase-2. Cancer Res. 2011;71:2038–2044. doi: 10.1158/0008-5472.CAN-10-3403. [DOI] [PubMed] [Google Scholar]
- 13.Martinenaite E, et al. Frequent adaptive immune responses against arginase-1. Oncoimmunology. 2017;7:e1404215. doi: 10.1080/2162402X.2017.1404215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weis-Banke, S. E. et al. The metabolic enzyme arginase-2 is a potential target for novel immune modulatory vaccines. Oncoimmunology9, 1771142 (2020). [DOI] [PMC free article] [PubMed]
- 15.Andersen MH. The T-win® technology: immune-modulating vaccines. Semin. Immunopathol. 2019;41:87–95. doi: 10.1007/s00281-018-0695-8. [DOI] [PubMed] [Google Scholar]
- 16.Iversen TZ, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin. Cancer Res. 2014;20:221–232. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
- 17.Svane I-M, Kjeldsen JW, Lorentzen CL, Martinenaite E, Andersen MH. Clinical efficacy and immunity of combination therapy with nivolumab and IDO/PD-L1 peptide vaccine in patients with metastatic melanoma: a phase I/II trial. Ann. Oncol. 2020;31:S1176. [Google Scholar]
- 18.Moodie Z, et al. Response definition criteria for ELISPOT assays revisited. Cancer Immunol. Immunother. 2010;59:1489–1501. doi: 10.1007/s00262-010-0875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmstrom MO, et al. The calreticulin (CALR) exon 9 mutations are promising targets for cancer immune therapy. Leukemia. 2018;32:429–437. doi: 10.1038/leu.2017.214. [DOI] [PubMed] [Google Scholar]
- 20.Andersen MH, et al. Phosphorylated peptides can be transported by TAP molecules, presented by class I MHC molecules, and recognized by phosphopeptide-specific CTL. J. Immunol. 1999;163:3812–3818. [PubMed] [Google Scholar]
- 21.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinenaite, E. et al. CCL22-specific T cells: modulating the immunosuppressive tumor microenvironment. Oncoimmunology5, e1238541 (2016). [DOI] [PMC free article] [PubMed]
- 23.Met Ö, Balslev E, Flyger H, Svane IM. High immunogenic potential of p53 mRNA-transfected dendritic cells in patients with primary breast cancer. Breast Cancer Res. Treat. 2011;125:395–406. doi: 10.1007/s10549-010-0844-9. [DOI] [PubMed] [Google Scholar]
- 24.Hjortsø MD, et al. Tryptophan 2,3-dioxygenase (TDO)-reactive T cells differ in their functional characteristics in health and cancer. Oncoimmunology. 2015;4:968480. doi: 10.4161/21624011.2014.968480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gnjatic S, et al. Cross-presentation of HLA class I epitopes from exogenous NY-ESO-1 polypeptides by nonprofessional APCs. J. Immunol. 2003;170:1191–1196. doi: 10.4049/jimmunol.170.3.1191. [DOI] [PubMed] [Google Scholar]
- 26.Clark, R. E. et al. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood10.1182/blood.V98.10.2887 (2001). [DOI] [PubMed]
- 27.Wang XF, et al. The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: Focus on macrophage polarization of THP-1 cells. Cell. Immunol. 2014;289:42–48. doi: 10.1016/j.cellimm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Rammensee, H.-G., Bachmann, J., Emmerich, N. N., Bachor, O. A. & Stevanovic, S. SYFPEITHI: database for MHC ligands and peptide motifs. www.syfpeithi.de. Accessed 30 Aug 2019. [DOI] [PubMed]
- 29.Thomas DA, Massagué J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Rook AH, et al. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J. Immunol. 1986;136:3916–3920. [PubMed] [Google Scholar]
- 31.Torroella-Kouri M, et al. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 2009;69:4800–4809. doi: 10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang Y, et al. TGF-β Signaling in myeloid cells is required for tumor metastasis. Cancer Discov. 2013;3:936–951. doi: 10.1158/2159-8290.CD-12-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, et al. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keilholz U, et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J. Immunother. 2002;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Rodon J, et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin. Cancer Res. 2015;21:553–560. doi: 10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melisi D, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer. 2018;119:1208–1214. doi: 10.1038/s41416-018-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giaccone G, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur. J. Cancer. 2015;51:2321–2329. doi: 10.1016/j.ejca.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 39.Formenti SC, et al. Focal irradiation and systemic TGFb blockade in metastatic breast cancer. Clin. Cancer Res. 2018;24:2493–2504. doi: 10.1158/1078-0432.CCR-17-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mascarenhas J, et al. Anti-transforming growth factor-β therapy in patients with myelofibrosis. Leuk. Lymphoma. 2014;55:450–452. doi: 10.3109/10428194.2013.805329. [DOI] [PubMed] [Google Scholar]
- 41.Takaku S, et al. Blockade of TGF-β enhances tumor vaccine efficacy mediated by CD8 + T cells. Int. J. Cancer. 2010;126:1666–1674. doi: 10.1002/ijc.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terabe M, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-β monoclonal antibody. Clin. Cancer Res. 2009;15:6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueda R, et al. Systemic inhibition of transforming growth factor-β in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Clin. Cancer Res. 2009;15:6551–6559. doi: 10.1158/1078-0432.CCR-09-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Y, et al. Targeting TGF-β1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm. Bowel Dis. 2010;16:1040–1050. doi: 10.1002/ibd.21167. [DOI] [PubMed] [Google Scholar]
- 45.Chu X, et al. Combined immunization against TGF-β1 enhances HPV16 E7-specific vaccine-elicited antitumour immunity in mice with grafted TC-1 tumours. Artif. Cells Nanomed. Biotechnol. 2018;46:1199–1209. doi: 10.1080/21691401.2018.1482306. [DOI] [PubMed] [Google Scholar]
- 46.Ødum N. Anti-regulatory T cells are natural regulatory effector T cells. Cell Stress. 2019;3:310–311. doi: 10.15698/cst2019.10.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munir Ahmad S, et al. PD-L1 peptide co-stimulation increases immunogenicity of a dendritic cell-based cancer vaccine. Oncoimmunology. 2016;5:e1202391. doi: 10.1080/2162402X.2016.1202391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahma OE, Gammoh E, Simon RM, Khleif SN. Is the ‘3+3’ dose-escalation Phase i Clinical trial design suitable for therapeutic cancer vaccine development? A recommendation for alternative design. Clin. Cancer Res. 2014;20:4758–4767. doi: 10.1158/1078-0432.CCR-13-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.