Abstract
Soil bacteria and fungi are key drivers of carbon released from soils to the atmosphere through decomposition of plant-derived organic carbon sources. This process has important consequences for the global climate. While global change factors, such as increased temperature, are known to affect bacterial- and fungal-mediated decomposition rates, the role of trophic interactions in affecting decomposition remains largely unknown. We designed synthetic microbial communities consisting of eight bacterial and eight fungal species and tested the influence of predation by a model protist, Physarum polycephalum, on litter breakdown at 17 and 21 °C. Protists increased CO2 release and litter mass loss by ~35% at 17 °C lower temperatures, while they only had minor effects on microbial-driven CO2 release and mass loss at 21 °C. We found species-specific differences in predator–prey interactions, which may affect microbial community composition and functioning and thus underlie the impact of protists on litter breakdown. Our findings suggest that microbial predation by fast-growing protists is of under-appreciated functional importance, as it affects decomposition and, as such, may influence global carbon dynamics. Our results indicate that we need to better understand the role of trophic interactions within the microbiome in controlling decomposition processes and carbon cycling.
Subject terms: Climate-change impacts, Soil microbiology, Microbial ecology
Soil microorganisms, mainly bacteria and fungi, are major drivers of soil carbon cycling through their decomposing activity of plant-derived carbon [1, 2] and their role in soil carbon stabilization [3, 4]. This has important consequences for atmospheric carbon concentrations and thereby, for ongoing climate change [5, 6]. It is well established that large-scale abiotic factors, such as climate, affect microbial activity and thereby, decomposition rates [7]. More recently it was shown that climate-independent variation in local-scale factors can drive broad-scale variation in decomposition rates [8]. Among these might be microbial predators that vary and affect microbial community composition and functioning at the local scale [9]. However, how microbial predators alter litter breakdown remains largely unknown.
Protists are major microbial predators of soil bacteria and to some extent fungi [10]. Protists are the taxonomically most diverse eukaryotes and occupy all key functional roles in soil food webs [10]. Most soil protists are phagotrophic [11] and prey on bacteria and fungi, which leads to changes in microbial biomass, activity, and community structure [10]. This is likely to have important functional consequences, including impacts on litter decomposition processes and thereby, the global carbon cycle. However, there is little experimental evidence underpinning how protists impact decomposition. Moreover, both protist and microbial activity are affected by temperature [9, 12], but whether temperature also modifies protist-induced changes in microbial functioning remains unknown.
To test the role of protist predation on microbial-driven decomposition we inoculated microcosms of synthetic microbial communities consisting of sixteen bacterial and fungal species (Tables S1 and S2) to sterilized oak litter (Quercus robur) at both 17 and 21 °C. After one week we added protists of the model species Physarum polycephalum at three different concentrations (no protists, and low, medium, and high concentration). This resulted in a full-factorial design with 16 treatments: 2 microbial inocula (yes/no) × 2 temperatures (17/21 °C) × 4 protist concentrations (Table S3) and we used six replicates per treatment. Microcosms without microbial inocula were established to test for successful establishment of the synthetic microbial community and were not used for further analyses as they did not remain sterile. For each microcosm, we measured CO2 production, litter mass loss and litter nitrogen and carbon content of the remaining litter. See supplementary methods for further details.
Before the addition of protists, microcosms with bacteria and fungi produced more CO2 than microbial-free ones (F1,92 = 431.16, p < 0.001), and this effect was not different between temperatures (F1,92 = 0.04, p = 0.846; Fig. S1), indicating successful establishment of a synthetic microbial community after inoculation. After protistan addition, there was no interactive effect of protists and temperature on CO2 production (F3,40 = 1.48, p = 0.234). However, both increased temperature (F1,40 = 14.96, p < 0.001) and presence of protists irrespective of their concentration (F3,40 = 3.24, p = 0.032) increased CO2 production (Fig. 1a). A posthoc analysis indicated that protist addition effects appeared stronger at lower than at higher temperatures (Fig. 1; please note that boxplots highlight medians while posthoc tests compare means). An interaction between the protist and temperature treatment affected litter mass loss (F3,40 = 10.50, p < 0.001; Fig. 1b), indicating that the addition of protists at all concentrations increased litter mass loss at 17 °C by more than 35% on average, but not at 21 °C (Fig. 1b). The addition of protists did not affect litter carbon (C) (F3,40 = 0.55, p = 0.653) and nitrogen (N) content (F3,40 = 0.03, p = 0.993) and the litter C:N ratio (F3,40 = 0.04, p = 0.990) at the end of the experiment (Fig. S2). Litter N content was higher at 21 than at 17 °C, indicating higher N loss during decomposition at lower temperatures (F1,30 = 7.42, p = 0.010; Fig. S2b), resulting in higher C:N ratios at 17 °C than at 21 °C (F1,40 = 8.08, p = 0.007).
Fig. 1. Changes in microbial CO2 production and litter decomposition rates as induced by protist predators.
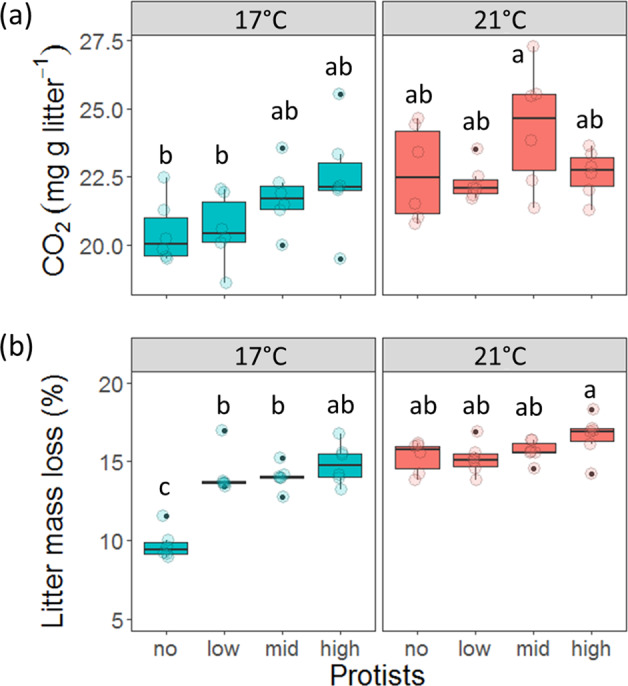
Boxplots showing (a) cumulative CO2 respiration (measured from the addition of protists until the end of the experiment) and (b) litter mass loss for microcosms with no protists or low, medium (mid) or high concentrations of protists (x-axis) at 17° and 21 °C. Different letters above the boxes indicate significant differences (p < 0.05) between treatments, as was indicated in a Tukey HSD posthoc test. Tukey tests were carried out across the protists × temperature interactions, so letters can be compared across facets.
Interaction-assays in split-petri dishes to test for volatile-induced microbial effects (Fig. S3) showed that protist growth (plasmodial length) was affected by bacterial (F5,23 = 63.22, p < 0.001) and fungal volatiles (F5,24 = 12.29, p < 0.001; Fig. 2). Presence of Collimonas pratensis T91, Pseudomonas sp. AD21 and Trichoderma citrinoviride reduced protist growth most strongly (Fig. 2). The overall negative effects of bacteria and fungi on protists likely through volatiles contradict with the variable effects of volatiles on other protist species which ranged from stimulation to inhibition [13]. But as inhibition differed between microbial species, some potentially efficient decomposers might benefit through a reduction of competition from more easily preyed microbes, which could explain the observed increased decomposition rates. Yet, other mechanisms are likely to contribute to increased decomposition in presence of predators, such as predation-induced increased microbial activity or alternative enzyme production- details to be explored in future studies.
Fig. 2. Bacterial and fungal long-distance effects on protist growth.
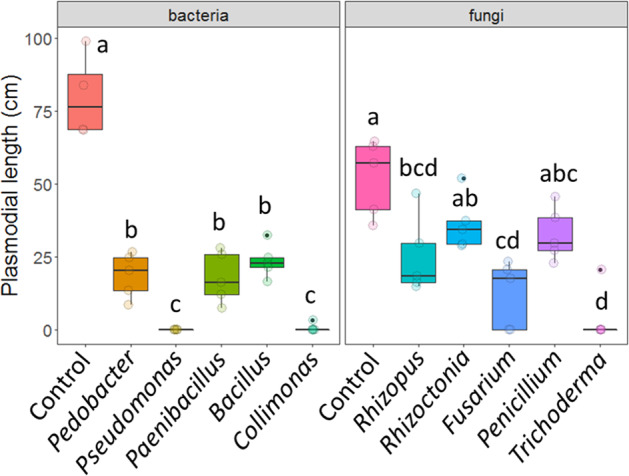
Boxplots showing plasmodial length of the model protist Physarum polycephalum in response to different (a) bacterial and (b) fungal taxa (x-axis) that were part of the microbial decomposer communities (Tables S1 and S2). C is the control with only nutrient agar without bacteria (left) or potato dextrose agar without fungi (right). Different letters above the bars indicate that protist responses differed significantly (p < 0.05) between the microbial species in a Tukey HSD test. Tukey HSD tests were carried out for bacteria and fungi separately, therefore letters should be compared within panels only.
Our results support previous findings showing that predator–prey interactions within the microbiome affect microbial-derived CO2 production [14], but we extend this knowledge and show that this effect tends to of lower importance at higher temperature. Furthermore, we now show that microbial predators alter litter decomposition in a temperature-dependent manner, with an increased importance at lower temperature. This result extends the known importance of larger-sized soil animals in increasing litter decomposition [15, 16] and contrasts previous findings that microscopic predators (mostly protists and nematodes) have a limited effect on litter breakdown [16]. Mechanistically, protists might increase decomposition via microbe-specific predator–prey interactions [10] that change microbial community composition and functioning [17]. Our interaction-assays suggests that microbial predator–prey interactions mediated by volatiles could differ, which might benefit some efficient microbial decomposers.
The effect of protists on litter decomposition was strongest at lower temperatures, contradicting previous findings that larger soil animals have increased effects on decomposition at higher temperatures [18]. This discrepancy might be explained by the higher microbial diversity in our model communities compared to often single-decomposer model species used before, in which predation might favor metabolically active microorganisms [10]. The effect of predation on microbial-driven decomposition seems to differ between protists and soil animals, as soil animals were shown to have limited effects on decomposition rates [16]. The increased importance of protist predation on microbial decomposition at lower temperatures suggest a more profound role of predation on carbon cycling in colder, non-tropical climates that host most microbial biomass [19] and store most carbon [20]. If this pattern can be confirmed with a wider range of protists, and in natural soils rather than this simplified laboratory assay, these microbial predators may play a key role in accelerating the global carbon cycle. Further studies should test exactly those by using realistic climate scenarios, more diverse protists and microbial decomposers, and in natural settings to untangle the importance of protists on decomposition and the carbon cycle. In turn, even more detailed laboratory analyses are needed to unreliably determine the exact mechanisms of how protists affect decomposition.
In summary, we reveal microbiome predation by protists as a key driver of microbial-driven decomposition with potential impacts on the global carbon cycle. Further integrated microbiome analyses are needed to investigate how and under which conditions microbial predation affects litter decomposition and if and how protists contribute to the global carbon cycle.
Supplementary information
Acknowledgements
We thank Freddy ten Hooven and Paolina Garbeva for providing fungal and bacterial cultures and Audrey Dussutour, Universite Paul Sabatier, for the Physarum polycephalum culture. Hans Zweers, Ciska Raaijmakers, and Femke Beersma assisted with chemical analyses. SG was supported by an NWO-Veni grant (016.Veni.181.078). SG and TEDC acknowledge the Royal Netherlands Academy of Arts and Sciences—Visiting Professors Programme (KNAW-VPP) for the research fellowship and grant. We acknowledge constructive comments of three unknown reviewers that helped improving our manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Stefan Geisen, Shunran Hu
Supplementary information
The online version of this article (10.1038/s41396-020-00792-y) contains supplementary material, which is available to authorized users.
References
- 1.Singh BK, Bardgett RD, Smith P, Reay DS. Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Rev Microbiol. 2010;8:779–90. doi: 10.1038/nrmicro2439. [DOI] [PubMed] [Google Scholar]
- 2.Schlesinger WH, Andrews JA. Soil respiration and the global carbon cycle. Biogeochemistry. 2000;48:7–20. doi: 10.1023/A:1006247623877. [DOI] [Google Scholar]
- 3.Kallenbach CM, Frey SD, Grandy AS. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun. 2016;7:13630. doi: 10.1038/ncomms13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Six J, Frey SD, Thiet RK, Batten KM. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J. 2006;70:555–69. doi: 10.2136/sssaj2004.0347. [DOI] [Google Scholar]
- 5.Cavicchioli R, Ripple WJ, Timmis KN, Azam F, Bakken LR, Baylis M, et al. Scientists’ warning to humanity: microorganisms and climate change. Nat Rev Microbiol. 2019;17:569–86. doi: 10.1038/s41579-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Xue K, Xie J, Deng Y, Wu L, Cheng X, et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat Clim Change. 2012;2:106–10. doi: 10.1038/nclimate1331. [DOI] [Google Scholar]
- 7.Aerts R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos. 1997;79:439–49. doi: 10.2307/3546886. [DOI] [Google Scholar]
- 8.Bradford MA, Veen GFC, Bonis A, Bradford EM, Classen AT, Cornelissen JHC, et al. A test of the hierarchical model of litter decomposition. Nat Ecol Evol. 2017;1:1836–45. doi: 10.1038/s41559-017-0367-4. [DOI] [PubMed] [Google Scholar]
- 9.Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol. 2017;15:579–90. doi: 10.1038/nrmicro.2017.87. [DOI] [PubMed] [Google Scholar]
- 10.Geisen S, Mitchell EAD, Adl S, Bonkowski M, Dunthorn M, Ekelund F, et al. Soil protists: a fertile frontier in soil biology research. FEMS Microbiol Rev. 2018;42:293–323. doi: 10.1093/femsre/fuy006. [DOI] [PubMed] [Google Scholar]
- 11.Oliverio AM, Geisen S, Delgado-Baquerizo M, Maestre FT, Turner BL, Fierer N. The global-scale distributions of soil protists and their contributions to belowground systems. Sci Adv. 2020;6:eaax8787. doi: 10.1126/sciadv.aax8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose JM, Vora NM, Countway PD, Gast RJ, Caron DA. Effects of temperature on growth rate and gross growth efficiency of an Antarctic bacterivorous protist. ISME J. 2009;3:252–60. doi: 10.1038/ismej.2008.96. [DOI] [PubMed] [Google Scholar]
- 13.Schulz-Bohm K, Geisen S, Wubs ERJ, Song C, de Boer W, Garbeva P. The prey’s scent—volatile organic compound mediated interactions between soil bacteria and their protist predators. ISME J. 2017;11:817–20. doi: 10.1038/ismej.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuikman PJ, Jansen AG, van Veen JA, Zehnder AJB. Protozoan predation and the turnover of soil organic carbon and nitrogen in the presence of plants. Biol Fertil Soils. 1990;10:22–28. doi: 10.1007/BF00336120. [DOI] [Google Scholar]
- 15.Crowther TW, Boddy L, Hefin Jones T. Functional and ecological consequences of saprotrophic fungus–grazer interactions. ISME J. 2012;6:1992–2001. doi: 10.1038/ismej.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE. Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos. 2002;99:317–23. doi: 10.1034/j.1600-0706.2002.990212.x. [DOI] [Google Scholar]
- 17.Jousset A, Rochat L, Pechy-Tarr M, Keel C, Scheu S, Bonkowski M. Predators promote defence of rhizosphere bacterial populations by selective feeding on non-toxic cheaters. ISME J. 2009;3:666–74. doi: 10.1038/ismej.2009.26. [DOI] [PubMed] [Google Scholar]
- 18.Crowther TW, Thomas SM, Maynard DS, Baldrian P, Covey K, Frey SD, et al. Biotic interactions mediate soil microbial feedbacks to climate change. Proc Natl Acad Sci. 2015;112:7033. doi: 10.1073/pnas.1502956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serna-Chavez HM, Fierer N, van Bodegom PM. Global drivers and patterns of microbial abundance in soil. Glob Ecol Biogeogr. 2013;22:1162–72. doi: 10.1111/geb.12070. [DOI] [Google Scholar]
- 20.Scharlemann JPW, Tanner EVJ, Hiederer R, Kapos V. Global soil carbon: understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014;5:81–91. doi: 10.4155/cmt.13.77. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


