Abstract
Tyrosine kinase inhibitors (TKIs), VEGF/VEGF receptor inhibitors (VEGFIs) and immune checkpoint inhibitors (ICIs) have revolutionized the treatment of advanced cancers including non-small-cell lung cancer (NSCLC). This study aims to evaluate the utility of plasma cell-free DNA (cfDNA) as a prognostic biomarker and efficacy predictor of chemotherapy (CT) with or without these precision therapies in NSCLC patients. Peripheral cfDNA levels in 154 NSCLC patients were quantified before and after the first target cycle of chemotherapy. The correlations of cfDNA with tumor burden, clinical characteristics, progression-free survival (PFS)/disease-free survival (DFS), objective response ratio (ORR), and therapy regimens were analyzed respectively. Baseline cfDNA, but not post-chemotherapeutic cfDNA, positively correlates with tumor burden. Notably, cfDNA kinetics (cfDNA Ratio, the ratio of post-chemotherapeutic cfDNA to baseline cfDNA) well distinguished responsive individuals (CR/PR) from the non-responsive (PD/SD). Additionally, cfDNA Ratio was found negatively correlated with PFS in lung adenocarcinoma (LUAD), but not lung squamous-cell carcinoma (LUSC) which may be due to a limited number of LUSC patients in this cohort. LUAD patients with low cfDNA Ratio have prolonged PFS and improved ORR, compared to those with high cfDNA Ratio. When stratified by therapy regimen, the predictive value of cfDNA Ratio is significant in patients with chemotherapy plus VEGFIs, while more patients need be included to validate the value of cfDNA Ratio in other regimens. Thus, the kinetics of plasma cfDNA during chemotherapy may function as a prognostic biomarker and efficacy predictor for NSCLC patients.
Subject terms: Non-small-cell lung cancer, Predictive markers, Prognostic markers
Introduction
Lung cancer is the most common cancer worldwide with a high morbidity (11.6% of the total cases) and mortality (18.4% of the total cancer deaths)1. In 2018 there was an estimated 2.1 million new cases and 1.8 million deaths, representing 1 in 5 cancer deaths1. The main histological categories of lung cancer are non–small cell lung cancer (NSCLC, 85% of patients) and small cell lung cancer (SCLC,15%)2. NSCLC consists of several subtypes, predominantly lung adenocarcinoma (LUAD, 40%), lung squamous-cell carcinoma (LUSC, 25–30%), and large-cell carcinoma (LULC, 5–10%)3. The 3-year or 5-year overall survival (OS) of early stage (I and II) NSCLC patients undergoing resection has reached to 83% and 76% respectively4. Despite multiple treatment options, the 5-year OS of late stage NSCLC remains extremely low5, with over 50% die within one year following diagnosis6. Unfortunately, over one third of NSCLC cases are diagnosed at late stage (III and IV)2. Advanced NSCLC patients are increasingly benefitting from targeted therapies and immunotherapies7, 8. These therapies seem to produce some synergistic effects when combined with chemotherapy9. Thus, there is an urgent need to discover biomarkers that can assist in selecting optimal treatment, predicting response and prognostics to improve the clinical outcome of NSCLC patients.
Genotyping tumor tissue with next generation sequencing (NGS) represents an effective way to capture actionable genetic alterations as potential biomarkers in clinical oncology10. However, tissue biopsy may be limited due to insufficiency of sampling or inaccessibility for biopsy and only 25–50% of lung cancer patients have sufficient tissues for genotyping11. Complicating biopsy availability, the biopsy represents a single snapshot in time and is often a sample from a heterogenous location within the tumor12, obtaining repeat specimens for genetic analysis before and after treatment is logistically difficult13. Therefore, liquid biopsy or blood sample becomes an alternative source and promising technology for genotyping. Increasingly, concordance has been established between liquid- and tissue-based genomic screenings14. Of note, some studies have suggested that liquid biopsy, specifically cell-free DNA (cfDNA), may better capture the heterogeneity of certain cancer features such as acquired resistance15–17, and could be useful to monitor tumor burden and metastasis18.
Emerging data have demonstrated that liquid biopsy-based biomarkers may serve as indirect indicators for NSCLC diagnosis and treatment monitoring, including circulating tumor cells (CTCs)19, 20, circulating free tumor DNA (ctDNA)21, 22, exosomes23 and tumor-educated platelets (TEP)24. However, none of these platforms are perfect. All the above methods still have issues, making none fully satisfactory. For instance, limited CTCs detection efficiency is low, with only 32% of NSCLC patients having ≥ 2 CTCs using CellSearch (the only approved methodology by the U.S. Food and Drug Administration)25. Low quantities of ctDNA in blood and sequencing artifacts may debilitate the confidence of NGS applications in detecting the actionable mutations26. Both CT and ctDNA are relatively time consuming and not cost-friendly for daily clinical practice. cfDNA, on the other hand, is relatively abundant and easier to quantify in circulating blood. Though the majority of cfDNA is often not of cancerous origin, preliminary studies suggest that cfDNA level and kinetics may still be used to assist in cancer diagnosis, treatment response or prognostic prediction27–32.
However, the clinical value of cfDNA application in NSCLC has not been well-established due to inconsistent reports33–36. Our recent study confirmed that plasma cfDNA concentration was significantly increased in patients with advanced gastric cancer and can serve as a potential biomarker for chemotherapy monitoring37. Here we sought to investigate the predictive value of cfDNA in efficacy of treatment and prognosis for NSCLC patients with chemotherapy, targeted therapy, immunotherapy or combined treatment.
Results
Pathological and demographic characteristics
The pathological and demographic characteristics of the 154 patients were summarized (Table 1). The median age was 62 years (34–79); 107 (69%) of participants were male and 47 (31%) were female. Since our aim is evaluating cfDNA clinical utilization, the most typical patients (LUAD in late stage) were selected. For example, 128 (83%) of patients were LUAD, and 26 (17%) were LUSC. 126 (82%) were in stage IV, 19 (12%) were in stage III and 9 (6%) were in stage I/II (all these early-stage patients received adjuvant/neoadjuvant chemotherapy and until the last follow-up we have not observed recurrences in them).
Table 1.
Baseline characteristics of patients.
| Characteristics | Number (N = 154) | Proportion (%) |
|---|---|---|
| Sex and age | ||
| Male | 107 | 69.5 |
| Female | 47 | 30.5 |
| Median age (years) | 62 (34–79) | |
| Histology | ||
| LUAD | 128 | 83.1 |
| LUSC | 26 | 16.9 |
| Clinical stage | ||
| I | 2 | 1.3 |
| II | 4 | 2.6 |
| III | 19 | 12.3 |
| IV | 126 | 81.8 |
| ECOG (Eastern Cooperative Oncology Group, baseline) | ||
| 0 | 6 | 3.9 |
| 1 | 72 | 46.8 |
| 2 | 1 | 0.7 |
| NA | 75 | 48.7 |
Peripheral cfDNA baseline correlates with tumor burden
To assess the relationship between cfDNA and TB, we defined TB_baseline as the pre-treatment TB, and only selected those whose interval between cfDNA test and TB evaluation was within 7 days (N = 80). We defined TB_post-chemotherapy as the post-chemotherapeutic TB and restricted the interval between cfDNA test and TB evaluation to no more than 7 days (N = 47). Overall, a weakly positive correlation between TB and cfDNA was observed at baseline (N = 80, Pearson’s coefficient = 0.24; 95% confidence interval (CI) 0.017–0.433; P = 0.03, Fig. 1A), while no significant correlation was found for post-chemotherapy (N = 47, Pearson’s coefficient = 0.124; 95% CI − 0.169 to 0.397; P = 0.4, Fig. 1B). We also analyzed the correlation in total population, and the results were similar: a weakly positive correlation between TB and cfDNA was observed both at baseline (N = 154, Pearson’s coefficient = 0.16; 95% confidence interval (CI) 0.003–0.312; P = 0.046) and post-chemotherapy (N = 154, Pearson’s coefficient = 0.16; 95% CI 0.0002–0.3097; P = 0.049).
Figure 1.
Scatter plot showing a weakly positive correlation of baseline cfDNA with baseline tumor burden. Tumor burden was evaluated by response evaluation criteria in solid tumors, version 1.1. cfDNA was quantified by QuantiDNA Direct cfDNA Test Kit (Diacarta. Inc., CA, USA) according to the manual both (A) at baseline and (B) post-chemotherapy. We selected those whose interval between cfDNA test and TB evaluation was within 7 days, so 80 cases were qualified (A) at baseline, and 47 cases were qualified (B) post-chemotherapy. A weakly positive correlation between TB and cfDNA was observed at baseline (N = 80, Pearson’s coefficient = 0.24; 95% CI 0.017–0.433; P = 0.03), while no significant correlation was found for post-chemotherapy (N = 47, Pearson’s coefficient = 0.124; 95% CI − 0.169 to 0.397; P = 0.4).
In addition, we also assessed other clinical factors which may be correlated with cfDNA. No significant correlations were found between age and cfDNA either at baseline (P = 0.1) or post-chemotherapy (P = 0.4), stage at baseline (P = 0.9) or post-chemotherapy (P = 0.4), ECOG score at baseline (P = 0.8) or post-chemotherapy (P = 0.8), gender at baseline (Wilcoxon rank sum test, P = 0.5) or post-chemotherapy (Wilcoxon rank sum test, P = 0.4). We also found no significant difference of cfDNA between LUAD and LUSC at baseline (Wilcoxon rank sum test, P = 0.16) or post-chemotherapy (Wilcoxon rank sum test, P = 0.4), or among different therapy regimens (Supplementary Table S1).
Plasma cfDNA relates to objective response rate (ORR) and progression-free survival (PFS)/ disease-free survival (DFS)
Since tumor burden usually correlates with clinical outcomes, we then investigated the relationship between clinical outcomes and peripheral cfDNA, we monitored peripheral cfDNA of all available patients (N = 154) at baseline (79% of which were tested before chemotherapy by 0–7 days), post-chemotherapy (89% of which were tested after chemotherapy by 20–30 days) and derived cfDNA Ratio (the ratio of post-chemotherapeutic cfDNA to baseline cfDNA) for each patient.
Firstly, we compared the baseline cfDNA and post-chemotherapeutic cfDNA between responsive group (PR/CR, N = 56) and non-responsive group (SD/PD, N = 80). Overall, the responsive group trended toward higher baseline cfDNA (median 17.68 ng/mL) than the non-responsive (median 13.70 ng/mL) (P = 0.058, Wilcoxon rank-sum test, Fig. 2A). However, we found no significant difference in post-chemotherapeutic cfDNA between the two (P = 0.6, Wilcoxon rank-sum test, Fig. 2B), although the median post-chemotherapeutic cfDNA in the responsive (17.18 ng/mL) was modestly lower than that of the non-responsive (19.15 ng/mL). Notably we found a significantly lower ratio in the responsive group (median 0.87) than that of the non-responsive (median 1.21) (P = 0.012, Wilcoxon rank-sum test, Fig. 2C). These data suggested that cfDNA can be used to discriminate responsive patients from the non-responsive well, especially with cfDNA ratio which reflected the dynamic change of plasma cfDNA.
Figure 2.
Comparison of cfDNA levels and cfDNA ratio between the responsive group and non-responsive group. Boxplots from top to bottom showed the baseline value (A), post-therapy value (B), and ratio value (C) of cfDNA respectively in both the responsive (PR/CR, N = 56) group and non-responsive (PD/SD, N = 80) group, the significance of difference between the two was estimated by Wilcoxon test.
To better evaluate the utility of cfDNA as a predictive tool, we divided this cohort into Ratio_low and Ratio_high group by the median of cfDNA Ratio (1.0271). Comparative analysis was then carried out between these two groups. Similar procedure was also performed between Baseline_low and Baseline_high group (cut-value: the median of cfDNA baseline, 15.43 ng/mL) and between Post-chemotherapy_low and Post-chemotherapy_high group (cut-value: the median of post-chemotherapeutic cfDNA, 18.42 ng/mL), respectively.
Significantly improved PFS/DFS benefit was observed for Ratio_low (HR: 0.54 (95% CI 0.29–1.01); Log-rank test, P = 0.05, Fig. 3A) compared with Ratio_high, while no significant difference was found between Baseline_low and Baseline_high group (Log-rank test, P = 0.86, Fig. 3B) and between Post-chemotherapy_low and Post-chemotherapy_high group (Log-rank test, P = 0.57, Fig. 3C). After a median follow-up of 6.4 months, the median PFS/DFS of Ratio_low group was 6.1 months which was 2 months longer than that of Ratio_high group (4.1 months). The objective response ratio (ORR) of the Ratio_low group (33/77, 42.8%) was also 1.5 times higher than that of the Ratio_high group (22/77, 28.5%).
Figure 3.
Progression-free survival (PFS)/disease free survival (DFS) in the overall cohort (N = 154). Kaplan–Meier curves for comparisons of progression-free survival between (A) high cfDNA Ratio and low cfDNA Ratio groups, (B) high cfDNA baseline and low cfDNA baseline groups, (C) high post-therapy cfDNA and low post-therapy cfDNA groups (cut-values were set as median value), respectively. (D) The hazard ratios of cfDNA ratio and other important clinical factors by multivariate Cox model. Cut-values were set as the median value of the overall cohort, respectively. PFS/DFS was assessed according to Response Evaluation Criteria in Solid Tumors, version 1.1 through investigators’ review, and tick marks represent data censored at the last time the patient was known to be alive and without disease progression.
Other factors which may impact PFS/DFS were evaluated by univariate Cox model such as age (HR:1.00 (95% CI 0.96–1.03); P = 0.8), gender (HR:1.01 (95% CI: 0.51–2.00); P = 1.0), subtype (LUSC v.s. LUAD, HR:1.36 (95% CI 0.64–2.86); P = 0.4), ECOG (HR:0.29 (95% CI 0.08–1.00); P = 0.05), stage (HR:0.86 (95% CI 0.42–1.76); P = 0.7), therapy regimen (chemotherapy + ICIs v.s. chemotherapy, HR:0.77 (95% CI 0.33–1.78); P = 0.5), chemotherapy + TKIs v.s. chemotherapy, HR:0.69 (95% CI 0.23–1.97); P = 0.48), chemotherapy + VEGFIs v.s. chemotherapy, HR:0.68 (95% CI 0.30–1.52); P = 0.35).
To exclude potential effects of predefined low/high groups, we also evaluated the correlation between cfDNA and PFS by both univariate Cox model and multivariate Cox model. Similar log Log-rank test results, only cfDNA ratio (HR: 1.55 (95% CI 1.11–2.18); P = 0.01) was significantly negatively related with PFS in univariate Cox model, but not cfDNA baseline (HR: 0.99 (95% CI 0.97–1.01); P = 0.4) and post-therapy cfDNA (HR: 1.01 (95% CI 0.99–1.03); P = 0.4). While in multivariate Cox model (taking into account age, gender, subtype, ECOG, stage, and therapy regimen as Fig. 3D illustrated), both cfDNA baseline (HR:0.95 (95% CI 0.91–1.00); P = 0.03) and cfDNA ratio (HR:1.90 (95% CI 1.2–2.95); P = 0.004) were shown to be significantly related with PFS/DFS, but not post-therapy cfDNA (HR:1.02 (95% CI 0.99–1.05); P = 0.28).
Furthermore, we compared the demographic (age and gender), pathological (subtype, stage, and ECOG scores), and therapeutic (therapy regimens) characteristics between Ratio_low and Ratio_high group, and found no significant difference (chi-square test) in all these factors (Table 2), which indicated the evenly distributed patients between these two groups.
Table 2.
Comparisons between Ratio_high group and Ratio_low group.
| Ratio | High | Low | P |
|---|---|---|---|
| N | 77 | 77 | |
| Age (mean (SD)) | 60.30 (8.68) | 61.65 (9.16) | 0.349 |
| Gender = MALE (%) | 53 (68.8) | 54 (70.1) | 1 |
| Subtype = LUSC (%) | 9 (11.7) | 17 (22.1) | 0.132 |
| ECOG (mean (SD)) | 0.95 (0.32) | 0.93 (0.27) | 0.721 |
| Stage (mean (SD)) | 3.75 (0.59) | 3.81 (0.51) | 0.483 |
| Response (%) | 0.157 | ||
| PD | 17 (22.4) | 11 (14.3) | |
| PR | 22 (28.9) | 33 (42.9) | |
| SD | 37 (48.7) | 33 (42.9) | |
| PFS/days (mean (SD)) | 139.59 (76.16) | 179.08 (83.75) | 0.003 |
| Regimen (%) | 0.227 | ||
| CT-only | 25 (32.5) | 20 (26.0) | |
| CT + ICIs | 17 (22.1) | 24 (31.2) | |
| CT + TKIs | 5 (6.5) | 10 (13.0) | |
| CT + VEGFIs | 30 (39.0) | 23 (29.9) |
Stratification analysis by subtype
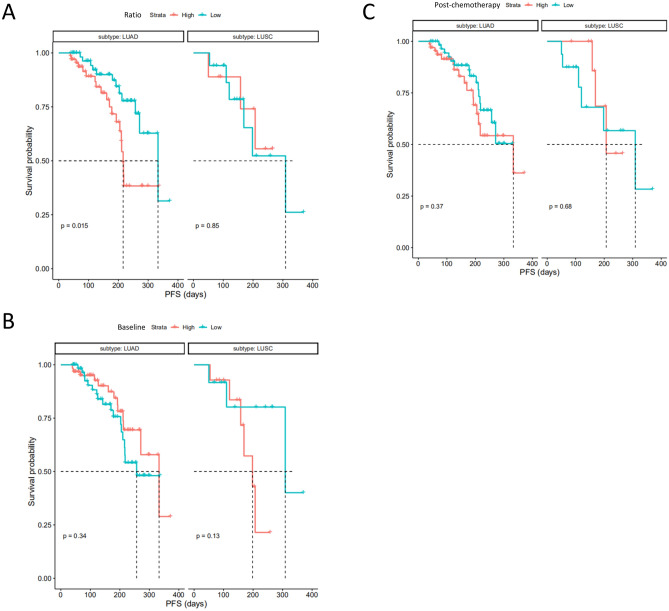
Since LUAD and LUSC are two main pathologic subtypes of NSCLC with different clinical managements and prognostics, we further analyzed the prognostic significance of the cfDNA baseline, cfDNA post-therapy, and cfDNA ratio in these two subgroups, respectively.
For the LUAD group (N = 128), we found a significantly improved PFS/DFS benefit for the Ratio_low group (HR: 0.42 (95% CI 0.20–0.86); P = 0.015, Fig. 4A) compared with Ratio_high group. The median PFS/DFS of Ratio_low group was 6.3 months which was 2.1 months longer than that of Ratio_high group (4.2 months). Additionally, ORR of Ratio_low group (43.3%) was also higher than that of the Ratio_high group (29.4%).
Figure 4.
Progression-free survival (PFS)/disease free survival (DFS) analysis by pathological subtype. Stratification analysis of PFS/DFS by pathological subtype (LUAD, N = 128 and LUSC, N = 26) (A) high cfDNA Ratio and low cfDNA Ratio groups, (B) high cfDNA baseline and low cfDNA baseline groups, (C) high post-therapy cfDNA and low post-therapy cfDNA groups (cut-values were set as median value), respectively. PFS/DFS was assessed according to Response Evaluation Criteria in Solid Tumors, version 1.1 through investigators’ review, and tick marks represent data censored at the last time the patient was known to be alive and without disease progression.
The LUSC group, as a minority of our cohort (N = 26), no significantly improved PFS/DFS benefit was found for the Ratio_low group (HR: 1.12 (95% CI 0.27–4.91); P = 0.85, Fig. 4A) compared with Ratio_high group. With only 2 patients followed past 300 days, both in the Ratio low group, the median PFS/DFS was 4.9 months in the Ratio low compared to 6.8 months in the Ratio_high group. However, ORR of Ratio_low group (41.1%) was trended higher than that of the Ratio_high group (22.2%).
Similar to the whole cohort, no significant difference of PFS/DFS was found between Baseline_low and Baseline_high group (Fig. 4B) or between Post-chemotherapy_low and Post-chemotherapy_high group (Fig. 4C) when stratified by LUAD and LUSC, respectively.
Stratification analysis by treatment
Since the change of cfDNA (Ratio) during treatment strongly correlated with PFS/DFS and objective response as shown above, we further analyzed in 4 subgroups stratified by therapy regimen: (1) chemotherapy only; (2) chemotherapy plus VEGF/VEGF receptor inhibitors (VEGFIs); (3) chemotherapy plus TKIs; (4) chemotherapy plus ICIs.
Only the Ratio_low group of patients received chemotherapy plus VEGFIs treatment (Supplementary Table S2) showed significantly prolonged PFS/DFS compared to those in Ratio_high group (HR: 0.23, 95% CI 0.06–0.88; P = 0.02, Fig. 5A). Additionally, ORR of Ratio_low group (9/23, 39%) was numerically higher than that of Ratio_high group (8/30, 27%). Importantly, only 2 (8.6%) of Ratio_low had Progressive Disease (PD), while 7 (23.3%) of Ratio_high had PD. In other therapy groups, no significant difference of PFS/DFS was found, e.g., chemotherapy-only group (HR = 0.82, P = 0.7), chemotherapy with TKIs group (HR = 0.68, P = 0.7), chemotherapy with ICIs group (HR = 0.72, P = 0.6). Whereas, ORR of Ratio_low group with all these three regimens are numerically higher than that of Ratio_high group (40% vs 28% for chemotherapy-only, 50% vs 20% for chemotherapy with TKIs, 46% vs 35% for chemotherapy with ICIs).
Figure 5.
Progression-free survival (PFS)/disease free survival (DFS) in subgroups by therapy regimen. Stratified analysis of Kaplan–Meier curves by therapy regimen (chemotherapy alone (N = 45), chemotherapy plus TKIs (N = 15), chemotherapy plus VEGFIs (N = 53), and chemotherapy plus ICIs (N = 41)) for comparisons of PFS/DFS between (A) high cfDNA Ratio and low cfDNA Ratio groups, (B) high cfDNA baseline and low cfDNA baseline groups, (C) high post-therapy cfDNA and low post-therapy cfDNA groups (cut-values were set as median value), respectively. PFS/DFS was assessed according to Response Evaluation Criteria in Solid Tumors, version 1.1 through investigators’ review, and tick marks represent data censored at the last time the patient was known to be alive and without disease progression.
In addition, no significant difference of PFS/DFS was found between Baseline_low and Baseline_high group (Fig. 5B) or Post-chemotherapy_low and Post-chemotherapy_high group (Fig. 5C) when stratified by these therapy regimens, respectively.
Discussion
Over the past two decades, important advancements have been achieved in the treatment of advanced NSCLC with our increasing understanding of the disease biology, tumorigenesis, early detection and multimodal care2. Notably, the utilization of targeted therapy and immunotherapy has brought about remarkable survival benefits in selected patients7, 8. However, there is a lack of universal and reliable biomarkers to predict or evaluate the treatment response and prognosis of different managements. In this study, we confirmed that the kinetics of plasma cfDNA (Ratio, post-/pre-) is well correlated with clinical response (ORR) and progression free survival (PFS)/disease free survival (DFS) at least in chemotherapy with VEGF inhibitor targeted therapy.
Circulating cfDNA is derived from a combination of apoptosis, necrosis and active secretion from both cancer cells and normal cells which are subjected to harsh stimuli such as chemotherapy38 or driven by inflammatory process39. It was found at higher levels in patients with advanced cancer than in early stage disease or healthy individuals40, 41. In the present study, we found a positive correlation between tumor burden and cfDNA baseline in NSCLC (Fig. 1). Although the tumor-derived fraction of these total cfDNA (ctDNA) has been widely investigated as a prognostic biomarker in various cancer types including breast, colon and lung cancer18, 42–44, the main challenges are low amount of ctDNA, detection cost and reproducibility limitations. For example, some typical difficulties of NGS application in this scenario include inadequate analytical sensitivity and specificity, such as detection limit of low allelic frequencies, and sequencing false positive41, 45. The total cfDNA with its higher feasibility has become an attractive alternative biomarker15–17. Our study utilized proven fluorescent probes and quick turnaround time of the SuperbDNA technology to measure plasma total cfDNA37, 46. This technology enables the cfDNA in patients’ plasma to be detected directly without any isolation procedures, which avoids any cfDNA loss during conventional isolation process and make the assay more accurate. Indeed, the Ratio (post-chemotherapy/pre-chemotherapy) of cfDNA shows a correlation with clinical response. The responsive patients obviously have a much lower Ratio than those with no response (Fig. 2). In addition, the Ratio, but not baseline or post-chemotherapy level of cfDNA, has a reversed correlation with PFS/DFS evaluated by RECIST1.1 (p = 0.05), combining all cases regardless of therapy regimens they received (Fig. 3). With Ratio cutoff-value set at the median (1.03), Ratio_low group has a significantly improved PFS with 2 months longer than that of Ratio_high group (4.1 months) (Fig. 3). Unlike other studies showing a correlation between a single snapshot of elevated cfDNA concentration and poor survival33, 36, 47, our data revealed that the response of cfDNA is an effective treatment efficacy indicator. To avoid potential effects of predefined cutoff-value, we also performed the correlations of PFS/DFS with each individual cfDNA baseline, post-chemotherapy and Ratio. Similarly, cfDNA Ratio, but not baseline or post-chemotherapy, was significantly negatively related with PFS/DFS (P = 0.01) in univariate analysis. Interestingly, when stratified by pathohistology, the predicted value of the cfDNA Ratio was only significant in the LUAD group. While the LUSC group was much smaller with only 2 patients exhibiting tumor progression, larger studies are needed to determine the utility of the cfDNA Ratio in LUSC patients. Among different therapy regimens, the strong negative correlation between PFS/DFS and Ratio was reproduced in patients with chemotherapy plus VEGFIs (P = 0.02), but not chemotherapy only (P = 0.74), chemotherapy plus TKIs (P = 0.68) or ICIs (P = 0.59). It may be attributed to a relative short term of follow-up, insufficient case number or non-molecular preselection, since reports have shown that TKIs mostly benefit NSCLC patients with driver (such as EGFR) mutations48, 49 and ICIs usually take a longer time to be clinically effective50. Further study targeting molecularly selected patients with a larger scale and longer follow-up is needed for validations.
In terms of clinical treatment response, objective response rate (ORR) showed a similar pattern as PFS/DFS. Only cfDNA Ratio, not baseline or post-chemotherapy, distinguished the subgroups who had a better clinical response and beneficial outcomes. Our results are consistent with a previous report that monitoring plasma DNA during chemotherapy can identify patients who are likely to exhibit a therapeutic response51. Other studies, however, suggested that cfDNA concentration is not reliable enough to predict treatment response in NSCLC when treatment is chemotherapy36, 52. One possible explanation is that sensitivity to chemotherapy and cfDNA levels during treatment may vary among individuals, or depend on timing of the sample acquisition34. We selected evaluating cfDNA level after one cycle of chemotherapy (post-treatment 20–30 days) based on the consideration that cfDNA would remain relatively stable during cycles and early evaluation could allow for therapeutic adjustment if needed. PFS/DFS can be measured but the results are too late to allow for therapeutic modification. ORR is a quicker index but still requires an imaging cycle and detailed image evaluation. The cfDNA Ratio is measured after the first cycle and is immediately interpretable, allowing for real time treatment adjustments. The Ratio-low group enjoyed an ORR more than 1.5 times higher than that of Ratio-high group (42.8% vs 28.5%) regardless of treatment regimen. The effect was most pronounced in the chemotherapy plus VEGFIs group, only 8.6% of patients in Ratio-low group had disease progressed (PD), while in Ratio-high group the proportion increased to 23.3%. These data support the predictive role of cfDNA Ratio in efficacy of chemotherapy.
To our knowledge, this is the first study to utilize the concept of cfDNA Ratio to better aid personalized medicine management. The concentration of cfDNA varies among individuals based on personalized nuances of the physiology and tumor characteristics53. Using a cfDNA ratio, captured in an appropriate time interval, normalizes the physiological effects leading to an estimate of tumor response. Indeed, from our data, the snapshot of baseline cfDNA did correlate with some clinical parameters like tumor burden. Yet for clinical response (ORR) and prognostic prediction, the cfDNA ratio seems to provide a better measure of tumor response.
This pioneer study has several limitations. (1) Neither ORR nor PFS/DFS are fully predictive of overall survival (OS). However, both ORR and PFS/DFS are used clinically to alter therapy, and an even earlier measure of tumor response would be advantageous. (2) Basal release and accumulation of cfDNA in the plasma, as mentioned above, is not an identical for every tumor or every patient. We have provided data supporting its imperfect potential for measuring basal TB in gastric cancer37 and now in NSCLC. As other common tumor markers (ex. CEA, PSA), more tumor subtypes should be screened for further validations. (3) cfDNA quantification is quick, accurate, and inexpensive, but it is not specific for cancer. Other pathological conditions such as inflammation and tissue necrosis can also affect cfDNA level. Logically ctDNA or other tests, if they can be made quantitative and reliable would be useful as adjuncts to calibrate the cfDNA test. (4) Small sample size in some groups (e.g. females and LUSC) could be a potential limitation for not observing significant differences. More female and/or LUSC patients are needed to validate the significance of cfDNA kinetics in different clinical settings. (5) Genetic variability could limit the such cfDNA based measurements. Nevertheless, in clinical settings, the genetic variability measurement currently relies on either NGS panel or specific mutation quantitation (qPCR), which requires prior knowledge of the disease or/and individual status. The cfDNA measurement in current study aims to overcome these inconvenience and provide an alternative way to predict clinical response and prognostics.
As with most initial discoveries, this is a single institution study that promises to advance a simple test that can provide an early indicator of NSCLC response to a number of different systemic therapies. We believe it should advance to a larger, multi-center trial.
Materials and methods
Study design and patient selection
This study is a single-institution protocol to evaluate peripheral cfDNA as a potential prognostic biomarker and efficacy predictor in NSCLC patients with chemotherapy or combination therapy. A total of 154 NSCLC patients who received chemotherapy or combined treatment in Jiangsu Cancer Hospital from December 2018 to February 2020 were enrolled. The clinical characteristics are shown in Table 1. Inclusion criteria include: (1) confirmed NSCLC diagnosis by pathohistology; (2) complete case data record. Exclusion criteria include: (1) patients with other malignant tumors; (2) patients with significant pre-existing cardiac, hepatic or renal disease; (3) patients with acute or chronic infectious disease; and (4) patients with mental illness prohibiting informed consent. All participants signed the informed consent agreement. The study was approved by the clinical research ethics committee of the Jiangsu Cancer Hospital and was conducted following the Declaration of Helsinki.
Assessment of peripheral cfDNA
All patients were subjected to peripheral blood samples collection before (baseline) and after (post-therapy) the first target cycle of chemotherapy. The cfDNA concentration was determined by QuantiDNA Direct cfDNA Test Kit (Diacarta. Inc., CA, USA) according to the manual and our previous publication37. The method is based on a patent technology with convenience and cost-effective. In brief, 2–3 ml peripheral blood was drawn and subjected to 10 min centrifugation in 1900×g for plasma isolation. The plasma sample were centrifuged 10 min at 13,000×g in 4C. Plasma samples were first diluted at tenfold by adding 10 µL of plasma into 90 µL of 1 × PBS (pH7.4). Diluted plasma samples were heated at 95 °C for 5 min for DNA denaturation and then immediately chilled on ice. Next, 20 µL of prepared plasma samples were loaded to a 96-well microplate (Greiner Bio-One, USA) together with 80 µL of Working Probe Solution containing Lysis buffer, DNA probe set, Blocking reagent, and Proteinase K. The microplate was incubated at 55 °C overnight (15–18 h) with shaking at 600 rpm followed by sequential hybridization with Pre-amplifier probe (55 °C 40 min), Amplifier probe (55 °C 40 min), Label probe (50 °C 40 min), and SAPE (Streptavidin, R-Phycoerythrin Conjugate) (37 °C 30 min). All of the probes were manufactured by DiaCarta Inc (Richmond, USA). Lastly, plate reading and data acquisition were performed on Luminex MAGPIX instrument with xPONENT software (Luminex, USA).
Efficacy and prognosis evaluation
The efficacy of treatment and prognosis were evaluated based on RECIST1.1 (Response Evaluation Criteria in Solid Tumors, Version 1.1)54. The criteria were as follows: complete Response (CR): absence of all measurable lesions, or all residual lesions lower than diagnostic threshold (10 mm for the longer diameter of tumors and 15 mm for the shorter diameter of lymph nodes); Partial Response (PR): tumor burden (TB) reduced by > 30% compared with baseline and the overall decrement ≥ 5 mm; Progressive Disease (PD): new measurable lesions or initial lesions increased by ≥ 20%; Stable Disease (SD): all which cannot be classified as CR, PR, or PD. Progression-free survival (PFS)/disease-free survival (DFS)55 was the primary outcome that was defined as the days from the date of initial chemotherapy until the date of progressive disease, recurrence, death, or the last follow up if progression or death had not occurred.
Statistical analysis
We stratified the treatment evaluation by dug combination regiment which consisted of four groups: (1) chemotherapy only; (2) chemotherapy plus VEGF/VEGF receptor inhibitors (VEGFIs); (3) chemotherapy plus tyrosine kinase inhibitors (TKIs); and (4) chemotherapy plus immune checkpoint inhibitors (ICIs). The primary outcome was (1) progression-free survival (PFS)/disease-free survival (DFS); and secondary outcomes was (2) objective response ratio (ORR), defined as the proportion of CR and PR in all subjects. An initial model without interactions was used to identify the prognostic impact of baseline cfDNA, post-therapy cfDNA, and the cfDNA ratio respectively. Other demographic or clinical factors which may be associated with PFS/DFS were also evaluated via univariate Cox model separately and multivariate Cox model together. Survival curves were plotted by the Kaplan–Meier method with R package ‘survival’ and ‘survminer’.
Supplementary Information
Abbreviations
- cfDNA
Cell-free DNA
- NSCLC
Non-small cell lung cancer
- LUAD
Lung adenocarcinoma
- LUSC
Lung squamous-cell carcinoma
- RECIST
Response evaluation criteria in solid tumors
- PFS
Progression-free survival
- DFS
Disease-free survival
- TKIs
Tyrosine kinases inhibitors
- VEGFIs
Vascular endothelial growth factor inhibitors
- ORR
Objective response ratio
- CR
Complete response
- PR
Partial response
- PD
Progressive
- SD
Stable disease
Author contributions
(I) Conception and design: D.L., M.S., Z.Z.; (II) administrative support: J.D., R.L.; (III) provision of study materials or patients: E.H., A.Z., P.O., J.L.; (IV) collection and assembly of data: X.Z., C.L., Z.Z., P.D., H.M., H.X.; (V) data analysis and interpretation: D.L., Z.Z., M.S.; (VI) manuscript writing: all authors; (VII) final approval of manuscript: all authors. (VIII) X.Z., C.L., Z.Z., D.L. contributed equally.
Data availability
The datasets and materials used during the present study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaorong Zhou, Chenchen Li, Zhao Zhang and Daniel Y. Li.
Contributor Information
Jianwei Lu, Email: lujw@medmail.com.cn.
Michael Y. Sha, Email: msha@diacarta.com
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-85797-z.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 3.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N. Engl. J. Med. 2004;350:379–392. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 4.Shewale JB, et al. Time trends and predictors of survival in surgically resected early-stage non-small cell lung cancer patients. J. Surg. Oncol. 2020 doi: 10.1002/jso.25966. [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Nelson RA, Bogardus A, Grannis FW., Jr Five-year lung cancer survival: Which advanced stage nonsmall cell lung cancer patients attain long-term survival? Cancer. 2010;116:1518–1525. doi: 10.1002/cncr.24871. [DOI] [PubMed] [Google Scholar]
- 6.Boloker G, Wang C, Zhang J. Updated statistics of lung and bronchus cancer in United States (2018) J. Thorac. Dis. 2018;10:1158–1161. doi: 10.21037/jtd.2018.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch FR, et al. Lung cancer: Current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 8.Borghaei H, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan M, Huang LL, Chen JH, Wu J, Xu Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target Ther. 2019;4:61. doi: 10.1038/s41392-019-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagan J, Van Allen EM. Next-generation sequencing to guide cancer therapy. Genome Med. 2015;7:80. doi: 10.1186/s13073-015-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrock AB, et al. Comprehensive genomic profiling identifies frequent drug-sensitive EGFR Exon 19 deletions in NSCLC not identified by prior molecular testing. Clin. Cancer Res. 2016;22:3281–3285. doi: 10.1158/1078-0432.CCR-15-1668. [DOI] [PubMed] [Google Scholar]
- 12.Thompson JC, et al. Detection of therapeutically targetable driver and resistance mutations in lung cancer patients by next-generation sequencing of cell-free circulating tumor DNA. Clin. Cancer Res. 2016;22:5772–5782. doi: 10.1158/1078-0432.CCR-16-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overman MJ, et al. Use of research biopsies in clinical trials: Are risks and benefits adequately discussed? J. Clin. Oncol. 2013;31:17–22. doi: 10.1200/JCO.2012.43.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastrisiou, M., Zarkavelis, G., Pentheroudakis, G. & Magklara, A. Clinical application of next-generation sequencing as a liquid biopsy technique in advanced colorectal cancer: A trick or a treat? Cancers (Basel)11. 10.3390/cancers11101573 (2019). [DOI] [PMC free article] [PubMed]
- 15.Goyal L, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7:252–263. doi: 10.1158/2159-8290.CD-16-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siravegna G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015;21:795–801. doi: 10.1038/nm.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh AR, et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 2019;25:1415–1421. doi: 10.1038/s41591-019-0561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson SJ, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 2013;368:1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, et al. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl. Oncol. 2013;6:697–702. doi: 10.1593/tlo.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorsey JF, et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: Pilot study results. Cancer. 2015;121:139–149. doi: 10.1002/cncr.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura H, et al. Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin. Cancer Res. 2006;12:3915–3921. doi: 10.1158/1078-0432.CCR-05-2324. [DOI] [PubMed] [Google Scholar]
- 22.Mok T, et al. Detection and dynamic changes of EGFR mutations from circulating tumor DNA as a predictor of survival outcomes in NSCLC patients treated with first-line intercalated erlotinib and chemotherapy. Clin. Cancer Res. 2015;21:3196–3203. doi: 10.1158/1078-0432.CCR-14-2594. [DOI] [PubMed] [Google Scholar]
- 23.Rosell R, Wei J, Taron M. Circulating MicroRNA signatures of tumor-derived exosomes for early diagnosis of non-small-cell lung cancer. Clin. Lung Cancer. 2009;10:8–9. doi: 10.3816/CLC.2009.n.001. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson RJ, et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget. 2016;7:1066–1075. doi: 10.18632/oncotarget.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs MG, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 26.Thress KS, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 2015;90:509–515. doi: 10.1016/j.lungcan.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Danese E, et al. Real-time polymerase chain reaction quantification of free DNA in serum of patients with polyps and colorectal cancers. Clin. Chem. Lab. Med. 2010;48:1665–1668. doi: 10.1515/CCLM.2010.301. [DOI] [PubMed] [Google Scholar]
- 28.Flamini E, et al. Free DNA and carcinoembryonic antigen serum levels: An important combination for diagnosis of colorectal cancer. Clin. Cancer Res. 2006;12:6985–6988. doi: 10.1158/1078-0432.CCR-06-1931. [DOI] [PubMed] [Google Scholar]
- 29.Sefrioui D, et al. Clinical value of chip-based digital-PCR platform for the detection of circulating DNA in metastatic colorectal cancer. Dig. Liver Dis. 2015;47:884–890. doi: 10.1016/j.dld.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Atamaniuk J, et al. Increased concentrations of cell-free plasma DNA after exhaustive exercise. Clin. Chem. 2004;50:1668–1670. doi: 10.1373/clinchem.2004.034553. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Garcia D, et al. Plasma cell-free DNA (cfDNA) as a predictive and prognostic marker in patients with metastatic breast cancer. Breast Cancer Res. 2019;21:149. doi: 10.1186/s13058-019-1235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patsch K, et al. Monitoring dynamic cytotoxic chemotherapy response in castration-resistant prostate cancer using plasma cell-free DNA (cfDNA) BMC Res. Notes. 2019;12:275. doi: 10.1186/s13104-019-4312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirera R, et al. Circulating DNA is a useful prognostic factor in patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 2011;6:286–290. doi: 10.1097/JTO.0b013e31820189a5. [DOI] [PubMed] [Google Scholar]
- 34.Gautschi O, et al. Circulating deoxyribonucleic Acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J. Clin. Oncol. 2004;22:4157–4164. doi: 10.1200/JCO.2004.11.123. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, et al. Efficacy of circulating plasma DNA as a diagnostic tool for advanced non-small cell lung cancer and its predictive utility for survival and response to chemotherapy. Lung Cancer. 2010;70:211–217. doi: 10.1016/j.lungcan.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Tissot C, et al. Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur. Respir. J. 2015;46:1773–1780. doi: 10.1183/13993003.00676-2015. [DOI] [PubMed] [Google Scholar]
- 37.Zhong Y, et al. Plasma cfDNA as a potential biomarker to evaluate the efficacy of chemotherapy in gastric cancer. Cancer Manag. Res. 2020;12:3099–3106. doi: 10.2147/CMAR.S243320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamfjord J, et al. Total circulating cell-free DNA as a prognostic biomarker in metastatic colorectal cancer before first-line oxaliplatin-based chemotherapy. Ann. Oncol. 2019;30:1088–1095. doi: 10.1093/annonc/mdz139. [DOI] [PubMed] [Google Scholar]
- 39.Nishimoto S, et al. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci. Adv. 2016 doi: 10.1126/sciadv.1501332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. [PubMed] [Google Scholar]
- 41.Newman AM, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med.6, 224ra224. 10.1126/scitranslmed.3007094 (2014). [DOI] [PMC free article] [PubMed]
- 43.Spellman PT, Gray JW. Detecting cancer by monitoring circulating tumor DNA. Nat. Med. 2014;20:474–475. doi: 10.1038/nm.3564. [DOI] [PubMed] [Google Scholar]
- 44.Ng SB, et al. Individualised multiplexed circulating tumour DNA assays for monitoring of tumour presence in patients after colorectal cancer surgery. Sci. Rep. 2017;7:40737. doi: 10.1038/srep40737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitt MW, et al. Detection of ultra-rare mutations by next-generation sequencing. Proc. Natl. Acad. Sci. USA. 2012;109:14508–14513. doi: 10.1073/pnas.1208715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou YQ, et al. Branched DNA-based Alu quantitative assay for cell-free plasma DNA levels in patients with sepsis or systemic inflammatory response syndrome. J. Crit. Care. 2016;31:90–95. doi: 10.1016/j.jcrc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 47.van der Drift MA, et al. Circulating DNA is a non-invasive prognostic factor for survival in non-small cell lung cancer. Lung Cancer. 2010;68:283–287. doi: 10.1016/j.lungcan.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Seto T, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): An open-label, randomised, multicentre, phase 2 study. Lancet. Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 49.Saito H, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 50.Dong J, Li B, Lin D, Zhou Q, Huang D. Advances in targeted therapy and immunotherapy for non-small cell lung cancer based on accurate molecular typing. Front. Pharmacol. 2019;10:230. doi: 10.3389/fphar.2019.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar S, et al. Plasma DNA level in predicting therapeutic efficacy in advanced nonsmall cell lung cancer. Eur. Respir. J. 2010;36:885–892. doi: 10.1183/09031936.00187909. [DOI] [PubMed] [Google Scholar]
- 52.Lee YJ, et al. Circulating cell-free DNA in plasma of never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. Clin. Cancer Res. 2011;17:5179–5187. doi: 10.1158/1078-0432.CCR-11-0400. [DOI] [PubMed] [Google Scholar]
- 53.Huang, C. C., Du, M. & Wang, L. Bioinformatics analysis for circulating cell-free DNA in cancer. Cancers (Basel)11. 10.3390/cancers11060805 (2019). [DOI] [PMC free article] [PubMed]
- 54.Nishino M, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am. J. Roentgenol. 2010;195:W221–228. doi: 10.2214/AJR.09.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Korn RL, Crowley JJ. Overview: progression-free survival as an endpoint in clinical trials with solid tumors. Clin. Cancer Res. 2013;19:2607–2612. doi: 10.1158/1078-0432.CCR-12-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and materials used during the present study are available from the corresponding author on reasonable request.