Summary
Mesenchymal stromal cell-like (MSCl) cells generated from human embryonic stem cells are considered to be an eligible cell line to model the immunomodulatory behavior of mesenchymal stromal cells (MSCs) in vitro. Dendritic cells (DCs) are essential players in the maintenance and restoration of the sensitive balance between tolerance and immunity. Here, the effects of MSCl cells on the in vitro differentiation of human monocytes into DCs were investigated. MSCl cells promote the differentiation of CTLA-4 expressing DCs via the production of all-trans retinoic acid (ATRA) functioning as a ligand of RARα, a key nuclear receptor in DC development. These semi-matured DCs exhibit an ability to activate allogeneic, naive T cells and polarize them into IL-10 + IL-17 + double-positive T helper cells in a CTLA-4-dependent manner. Mapping the molecular mechanisms of MSC-mediated indirect modulation of DC differentiation may help to expand MSCs' clinical application in cell-free therapies.
Subject areas: Molecular Biology, Immunology, Components of the Immune System, Stem Cells Research
Graphical abstract

Highlights
-
•
Mesenchymal stromal cell-like cells alter moDC differentiation via RARα activation
-
•
Mesenchymal stromal cell-like cells express genes known to play role in ATRA synthesis
-
•
MoDCs, differentiated in the presence of MSCl-derived factors, express CTLA-4
-
•
CTLA-4+ moDCs are able to induce polarization of IL-10- and IL-17-producing helper T cells
Molecular Biology; Immunology; Components of the Immune System; Stem Cells Research
Introduction
Due to their multipotent differentiation ability and strong immunomodulatory potential, mesenchymal stromal cells (MSCs) are promising candidates for cell-based therapy of several inflammatory, immune-mediated, and degenerative diseases (Saeedi et al., 2019). There are a number of ongoing clinical trials related to the immunomodulatory effects of MSCs or linked to graft enhancement utilizing their immunosuppressive functions (Wang et al., 2018). However, the exact cellular and molecular mechanisms underlying the MSC-mediated immunomodulation have yet to be revealed. Despite a large number of studies focusing on changes in cells and tissues following MSCs administration, several pieces of the puzzle are still missing and results are often inconsistent. Possible reasons include that MSCs from different sources and under different culture conditions may have diverse phenotype and epigenetic background, secrete distinct patterns of soluble factors and possess different capacities to proliferate (Weiss and Dahlke, 2019). One of the strategies to overcome these limitations is the use of MSC-like (MSCl) cell lines derived from human pluripotent stem cells, generating unlimited numbers of early passage MSCs with consistent quality and immune suppressive properties (Kimbrel et al., 2014).
We have earlier demonstrated in a collaborative study that based on their phenotypic and functional properties such as differentiation potential and immunomodulatory activity, human MSCl cells generated from pluripotent HUES9 embryonic stem cells are eligible to model the behavior of bone marrow-derived MSCs in vitro (Varga et al., 2011). In a recent study, we have provided a body of evidence that these MSCl cells are able to modulate the responses of monocyte-derived dendritic cells (moDCs) to retinoic acid-inducible gene I receptor-mediated activation (Bacskai et al., 2015). Human moDCs stimulated in the presence of MSCl cells exhibited reduced expression of phenotypic indicators of DC activation, lower production of TNF-α, CXCL10, IL-12 and IFNγ, as well as a decreased migration and T cell polarization as compared to moDCs activated in the absence of MSCl cells (Bacskai et al., 2015). These prior observations indicate that activation of mature moDCs can be efficiently suppressed by MSCl cell-derived signals and mechanisms. In this study, we investigated whether and how the in vitro differentiation of human monocytes into DCs is altered by MSCl cells.
Results
The cytokine and chemokine production of moDCs is modulated by MSCl cells
To analyze the direct and indirect immunomodulatory effects of MSCl cells, moDCs were differentiated in the presence of MSCl-CM or MSCl cells and relative cytokine, chemokine and other soluble protein levels in the cell culture supernatants were investigated using a protein array (Figure S1). It was observed that soluble factors released by MSCl cells remarkably altered the protein secretion profile of moDCs. Elevated levels of BAFF, complement factor D, EGF, IL-2, IL-22, lipocalin-2, TGFα, TIM3, myeloperoxidase, C-reactive protein, and Dkk-1, while decreased productions of ICAM-1 and EMMPRIN were detected in the supernatant of moDCs differentiated in the presence of MSCl-CM (Figure 1A). However, increased levels of Vitamin D BP, Endoglin, ENA78, GDF-15, GRO-α, IL-24, MCP-3, VEGF, IL-8, IL-10, and IFNγ, but reduced amounts of FGF-19, Osteopontin, CD31, and IL-18 Bpa were found in the supernatant of moDC-MSCl cell co-cultures (Figure 1A). Both exposure to MSCl-CM and direct moDC-MSCl cell contact raised the secretion of IL-19, VCAM-1, leptin, IL-6, CD14, FGF basic, IGFBP2, TFF3 and TfR, while the production of IL-27, Cystatin C, chitinase 3-like 1, MMP-9, and PDGF-AB/BB was diminished in the presence of either MSCl-CM or MSCl cells (Figure 1A). To validate the protein array results, concentrations of some inflammatory (IL-6) and anti-inflammatory (IL-10 and TGFβ) mediators were determined by ELISA (Figure 1B). In the supernatant of moDC-MSCl cell co-cultures significantly enhanced IL-6 and IL-10 productions were detected, while in the presence of MSCl-CM moDCs secreted significantly more IL-6, IL-10 and TGFβ than control cells (Figure 1B). These results demonstrate that the baseline levels of both inflammatory and anti-inflammatory mediators in moDCs cultures can be modulated by MSCl cells through the action of soluble or membrane-bound molecules.
Figure 1.
The cytokine and chemokine production of moDCs is modulated by MSCl cells
To examine the regulatory effects of MSCl cells or the MSCl-CM on the cytokine and chemokine production of moDCs, on day 4 of moDC differentiation the integrated density of soluble mediators was measured by Human XL Cytokine Array Kit (A) and the concentration of secreted cytokine IL-6, IL-10, TGFβ was detected by ELISA (B).
In case of co-cultures, the diagrams represent the secretion of cytokines released by moDCs and MSCl cells. Mean values of relative cytokine levels and concentrations were calculated from 4 or more independent experiments. Data are represented as individual data points with the mean ± standard deviation. In the statistical analysis, one-way ANOVA followed by Bonferroni's multiple comparison test was used with significance defined as ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗∗p < 0.0001.
See also Figure S1.
MSCl cells modify the phenotype of monocyte-derived cells
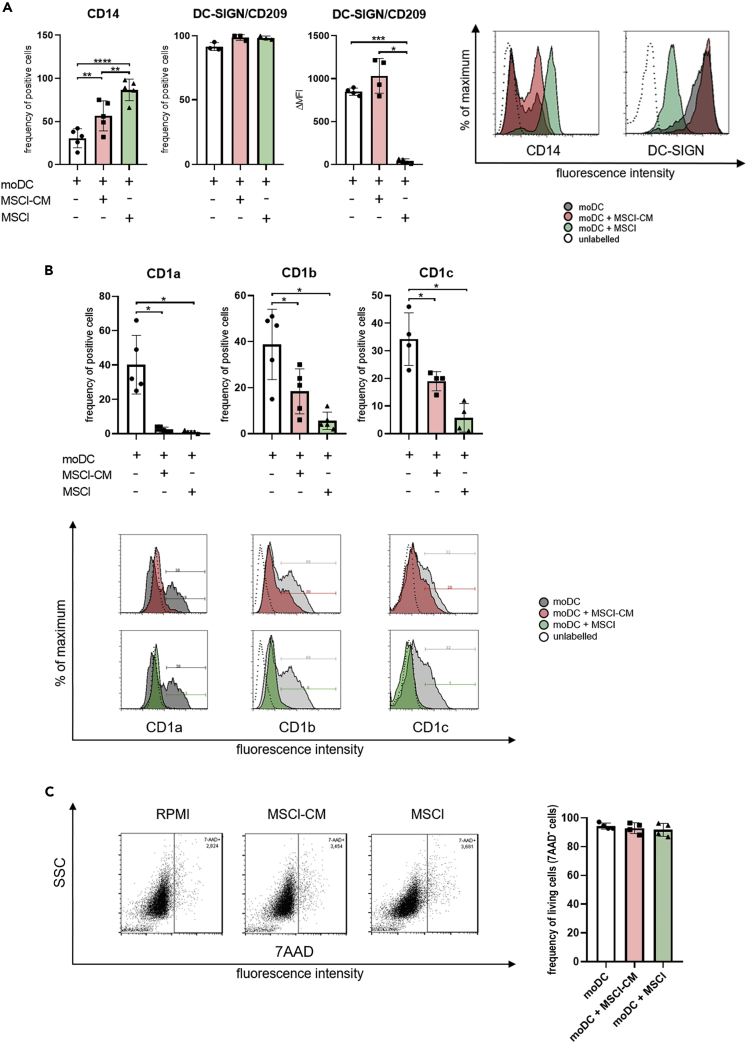
To get insight into how the presence of MSCl-CM or MSCl cells affects the phenotypic characteristics of moDC differentiated in vitro, we monitored the expression of cell surface molecules by flow cytometry. During the differentiation of moDCs in the presence of GM-CSF and IL-4, the expression of CD14 is downregulated (Sallusto and Lanzavecchia, 1994), while that of DC-SIGN (Geijtenbeek et al., 2000) and group 1 CD1 family members (Sallusto and Lanzavecchia, 1994) is upregulated. In our experiments, the percentage of CD14+ cells was higher in the presence of MSCl-CM or MSCl cells than in control cell cultures (Figure 2A). While the ratio of DC-SIGN+ cells was similar in treated and control cell cultures, the cell surface expression of DC-SIGN molecule was significantly downregulated on MSCl-exposed cells (Figure 2A). We found that under our experimental conditions, approx. 40% of untreated cells expressed group 1 CD1 family members including CD1a, CD1b, and CD1c (Figure 2B). Exposure to either MSCl-CM or MSCl cells significantly reduced the cell surface expression of these glycolipid receptors on monocyte-derived cells, while these treatments did not modify their viability (Figure 2C). This observation suggests that the presentation of lipid and glycolipid antigens by monocyte-derived cells through group 1 CD1 proteins may be negatively modulated by MSCl cells either in a direct or an indirect manner. For further characterization of phenotypic changes of MSCl-CM- and MSCl cell-treated monocyte-derived cells, expression of CD163, a macrophage scavenger receptor (Skytthe et al., 2020), was investigated. Interestingly, treatment with MSCl-CM induced only a slight increase in the frequency of CD163+ cells, whereas exposure to MSCl cells triggered a significant rise in the ratio of the macrophage marker-expressing cells (Figure S2A).
Figure 2.
MSCl cells and MSCl-CM modify the cell surface expression of CD14, DC-SIGN/CD209 and group1 CD1 family members
CD14+ monocytes were cultured with recombinant IL-4 and GM-CSF ± MSCl cells or MSCl-CM for 4 days. On day 4, the cell surface expression of CD14 and DC-SIGN (A), and that of CD1a, CD1b, and CD1c (B) were analyzed by flow cytometry on monocyte-derived cells.
To exclude the possibility of unspecific staining because of the presence of dead cells, the viability of cells was measured by 7-aminoactinomycin D (7-AAD) staining using flow cytometry (C). The MFI (median fluorescence intensity) and the mean values of the ratio of cells positive for the measured surface molecules were calculated from at least three independent experiments. Data are represented as individual data points with the mean ± standard deviation. Histograms show one of at least four independent experiments. In the statistical analysis, one-way ANOVA followed by Bonferroni's multiple comparison test was used with significance defined as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
See also Figure S2.
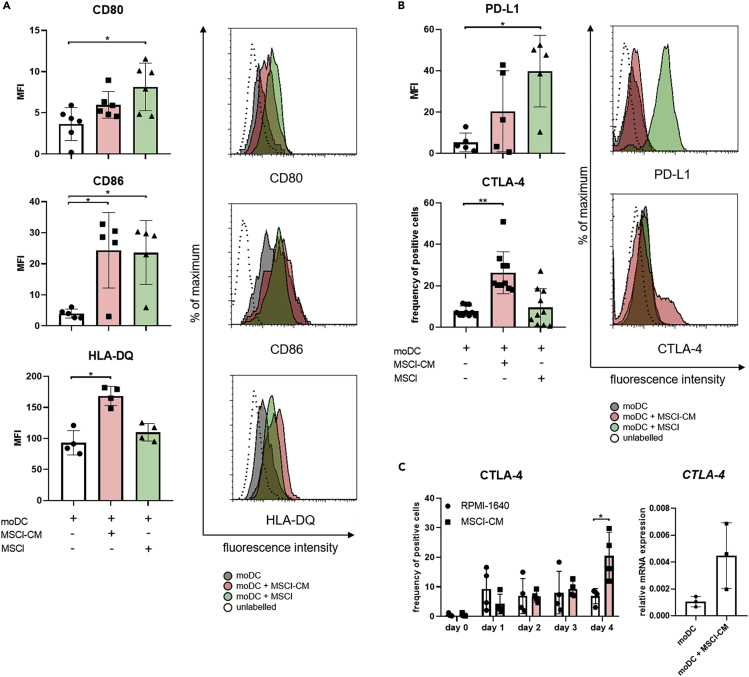
We also found that MSCl-CM significantly increased the cell surface levels of the peptide antigen-presenting HLA-DQ and the co-stimulatory molecule CD86, whereas contact with MSCl cells significantly enhanced the expression of both CD80 and CD86 on monocyte-derived cells (Figure 3A). Augmented expression of PD-L1, a co-inhibitory molecule, could also be detected on monocyte-derived cells in the presence of MSCl cells (Figure 3B). Importantly, an increased expression of another co-inhibitory molecule CTLA-4 on monocyte-derived cells was induced exclusively by MSCl-CM (Figure 3B). Elevated expression of CTLA-4 was induced by MSCl-CM in a time-dependent manner and a statistically significant difference in CTLA-4 levels on treated and control cells was found on day 4 of treatment (Figure 3C, left panel). A four-fold increase in the expression of CTLA-4 at mRNA level was also detected in four-day-old monocyte-derived cells differentiated in the presence of MSCl-CM compared to control moDCs (Figure 3C, right panel). When four-day-old immature moDCs were co-cultured with MSCl cells for 48 hr a remarkable increase in the expression of CD80, CD86, and PD-L1 on moDCs was observed (Figure S3). However, when immature moDCs were treated with MSCl-CM for a same period of time no or minor changes in the levels of co-stimulatory and co-inhibitory molecules on moDCs could be detected, suggesting that MSCl-derived soluble factors are able to modify the immune regulatory phenotype of moDCs only at the early stage of their development (Figure S3). These observations together with microscopic morphology of the cells (Figure S2B) indicate that MSCl-CM has a potential to regulate the differentiation of monocytes toward CD1a−DC-SIGN+CD163low semi-matured moDCs, whereas MSCl cells promote the differentiation of CD1a−DC-SIGNlowCD163high M2 macrophage-like cells.
Figure 3.
MSCl cells and MSCl-CM alter the cell surface expression of molecules involved in T cell activation
CD14+ monocytes were cultured with recombinant IL-4 and GM-CSF ± MSCl cells or MSCl-CM for 4 days. On day 4, monocyte-derived cells were analyzed for the cell surface expression of the T cell stimulatory CD80, CD86, HLA-DQ (A) and the regulatory CTLA-4 and PD-L1 (B) molecules by flow cytometry. The frequency of CTLA-4-expressing cells was monitored on each day during the differentiation process (C).
The gene expression level of CTLA-4 was measured by qPCR on day 4 (C). The MFI and the mean values of the ratio of cells positive for the measured cell surface molecules were calculated from at least four independent experiments. Mean values of relative mRNA levels were calculated from three independent experiments. Data are represented as individual data points with the mean ± standard deviation. Histograms show one of at least four independent experiments. In the statistical analysis, one-way ANOVA followed by Bonferroni's multiple comparison test (A and B), as well as Student's t-test and Mann-Whitney rank-sum test (C) were used with significance defined as ∗p < 0.05, ∗∗p < 0.01.
See also Figure S3.
MSCl cells change the phenotypic characteristics of moDCs at least partially via ATRA production
It has recently been reported that RARα is able to regulate the differentiation of specialized DCs from human blood monocytes (Hashimoto-Hill et al., 2018). Therefore, to investigate the molecular mechanisms guiding the phenotypic changes of moDCs in the presence of MSCl-CM, we analyzed the role of RARα in this process using all-trans retinoic acid (ATRA) as an agonist and BMS614 as a selective antagonist of this nuclear receptor.
The expression level of CD1a on moDCs was significantly lowered by soluble factors in MSCl-CM (Figures 2C and 4A) and when cells were treated with ATRA (Figure 4A). As expected, BMS614 prevented the effect of ATRA on CD1a expression. The addition of BMS614 to MSCl-CM induced some but statistically insignificant increase in the expression of CD1a (Figure 4A). Inhibition of RARα blocked the effect of ATRA on HLA-DQ expression and also decreased the ability of MSCl-CM to intensify peptide antigen presentation; however, the observed differences were statistically non-significant (Figure 4A). The enhancement in the expression of T cell co-stimulatory and co-inhibitory molecules on moDCs by ATRA or also by MSCl-CM was largely dependent on functional RARα receptor (Figure 4A). Indeed, the upregulation of CD86 expression on moDCs by ATRA or MSCl-CM was significantly reduced when the function of RARα was inhibited. Furthermore, enhanced ratio of CTLA-4+ moDCs induced by ATRA or MSCl-CM was also significantly diminished by the selective blockade of RARα (Figure 4A).
Figure 4.
MSCl cells change the immune regulatory potential of moDCs at least partially via nuclear factor RARα and ATRA
To investigate how MSCl-CM affects the differentiation of moDC, monocytes were differentiated in the presence or absence of 1 nM RARα activator, ATRA followed by a 75 min incubation period with or without 1 μM BMS614 (BMS) specific RARα-antagonist prior to exchange the cell culture medium to RPMI-1640 or MSCl-CM. Monocytes were differentiated into moDC in the presence or absence of MSCl-CM for 4 days. On day 4, moDCs were analyzed for the cell surface expression of CD1a, the T cell stimulatory HLA-DQ, CD86 and the T cell co-inhibitory CTLA-4 proteins by flow cytometry (A).
To test and compare the capability of freshly isolated monocytes, moDCs and MSCl to produce ATRA, mRNA was extracted from the different cell types. The relative mRNA expression level of target genes RDH10, ALDH1A1, ALDH1A2, and ALDH1A3 was measured by qRT-PCR (B).
To prove the effect of ATRA to the differentiation process of monocytes into moDCs, the RALDH enzymes essential for the synthesis of ATRA were inhibited specifically by 1μM DEAB in the MSCl cultures for 24 hr before the collection of MSCl-CM. CD14+ monocytes were cultured with recombinant IL-4 and GM-CSF and MSCl-CM with or without ATRA for 4 days. On day 4, moDCs were analyzed for the cell surface expression level of CD1a, HLA-DQ, CD86 and the regulatory CTLA-4 molecules by flow cytometry (C).
Mean values of MFI and moDCs positive for the measured cell surface antigen were calculated from at least four independent experiments. Mean values of relative mRNA levels were calculated from three independent experiments. Data are represented as individual data points with the mean ± standard deviation. In the statistical analysis, one-way ANOVA followed by Bonferroni's multiple comparison test was used with significance defined as ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Next, it was further analyzed whether MSCl cells modify the immune regulatory potential of moDCs via ATRA production. First, expression of genes known to play a role in ATRA synthesis was investigated in MSCl cells and found that these cells express retinol dehydrogenase 10 (RDH10), and aldehyde dehydrogenase 1 family members ALDH1A1 and ALDH1A3, but not ALDH1A2 (Figure 4B). In next series of experiments, the activity of aldehyde dehydrogenase isoenzymes was inhibited using a highly selective inhibitor DEAB (Morgan et al., 2015) in MSCl cells and the differentiation of moDCs in the presence of ATRA deficient MSCl-CM was monitored. MSCl-CM collected from cultures of MSCl cells with inhibited ATRA synthesis was still able to significantly enhance the cell surface expression of HLA-DQ molecule on moDCs (Figure 4C). In contrast to HLA-DQ, changes in the expression of CD1a, CD86 and CTLA-4 on moDCs exposed to MSCl-CM was dependent on ATRA production by MSCl cell (Figure 4C).
Taken together, these results suggest that MSCl cells bring about the phenotypic changes of moDCs at least partially via ATRA production.
CTLA-4 expression on moDCs differentiated in the presence of MSCl-CM is essential to drive the development of IL-17- and IL-10-producing T cells
To further dissect the immune regulatory capacity of moDCs exposed to MSCl-CM, ELISPOT assays appropriate to monitor the moDC-mediated allogeneic T-lymphocyte activation were used. It was found that moDCs treated with MSCl-CM have a potential to significantly increase the polarization of T cells producing IL-17 (Figure 5A) and IL-10 (Figure 5B). Moreover, flow cytometric analysis revealed that moDCs cultured in the presence of MSCl-CM triggered the polarization of IL-10+IL-17+ double-positive T helper cells (Figure 5C).
Figure 5.
CTLA-4 expression on moDCs differentiated in the presence of MSCl-CM is essential to drive the development of IL-10- and IL-17-producing T cells
CD14+ monocytes were differentiated into moDC in the presence or absence of MSCl-CM for 4 days. On day 4, the moDCs were treated with anti-CTLA-4 blocking antibody and were co-cultured with allogeneic T lymphocytes for further 3, 5 or 9 days. ELISPOT assays were used to determine the number of IL-17- (A) and IL-10- (B) producing T cells. The average values of spot numbers indicating T-lymphocyte responses were counted from 3 micro-wells. Mean values of spot numbers were calculated from 3 independent experiments. IL-10- and IL-17-producing CD4+ T cells were detected by flow cytometry (C).
Mean values of the ratio of T cells positive for the measured cell intracellular cytokines were calculated from four independent experiments. Data are represented as individual data points with the mean ± standard deviation. Contour plots show one of the three independent experiments. In the statistical analysis, one-way ANOVA followed by Bonferroni's multiple comparison test was used with significance defined as ∗p < 0.05.
See also Figures S4–S6.
To analyze the role of CTLA-4 in moDC-mediated T cell polarization, the CTLA-4-mediated signaling pathway was inhibited using specific antibodies. Unexpectedly, a blockade of CTLA-4 signaling in MSCl-CM-treated moDCs significantly reduced their ability to trigger the development of IL-17- and IL-10-producing T cells (Figures 5A–5C). Importantly, isotype control antibodies did not affect the IL-10 and IL-17 production of T cells primed by MSCl-CM-treated moDCs (Figure S4). In parallel experiments, a CTLA-4 blockade did not modify significantly the Th1- and Th2-polarizing capacity of MSCl-CM-exposed moDCs (Figure S5). When moDCs were treated with MSCl-CM in the presence of the specific RARα antagonist (BMS614), which partially prevented the induction of CTLA-4 expression on moDCs by MSCl-CM (Figure 4A), they also displayed a reduced capability to bring about the polarization of IL-17- and IL-10-producing T cells (Figure S6). Although the observed decreases in the number of polarized T cells in the presence of the RARα inhibitor were not statistically significant (Figure S6).
These results clearly demonstrate that the T cell-polarizing capacity of moDCs can be modulated by soluble factors derived from the MSCl cells. Furthermore, the ability of MSCl-CM-treated moDCs to induce polarization of IL-17- and IL-10-producing T cells is dependent on the level of functional CTLA-4 molecules on their surface.
Discussion
Human DCs represent a very heterogeneous cell population, including distinct subsets associated with both inflammatory and regulatory functions, and with remarkable phenotypic and functional plasticity (Qian and Cao, 2018). The interplays between DCs and other cell types depend on the developmental status and the functional activities of DCs determined by current humoral and cellular components of the given tissue milieu (Dong et al., 2015). MSCs have the potential to modulate the differentiation and the functions of T and B lymphocytes as well as innate immune cells including granulocytes (Park et al., 2012), natural killer (NK) cells (Abumaree et al., 2018), monocytes (Chen et al., 2014), macrophages (Vasandan et al., 2016), and DCs (Jiang et al., 2005). However, the capacity of MSCl cells and MSCl-CM to alter the initial differentiation of human DCs from monocytes has not yet been investigated.
During both homeostasis and inflammation, circulating monocytes leave the blood circulation and migrate into tissues where they are able to differentiate into DCs (Shi and Pamer, 2011). Several previous studies have examined the effects of MSCs on the differentiation of human moDCs. When irradiated (30 Gy) bone marrow-derived MSCs were co-cultured with freshly isolated monocytes (1:5) together with GM-CSF and IL-4, MSCs greatly inhibited monocyte differentiation into immature DCs (Zhang et al., 2004). Most of cultured cells in the presence of MSCs were negative for CD1a, indicating that the cells had not differentiated into immature DCs; however, MSCs did not affect the expression of CD14. Co-culture with MSCs substantially prevented the upregulation of the co-stimulatory molecules CD40, CD86, and CD80, as well as down-regulated HLA-DR expression. On the contrary, MSCs' supernatants had no effects on DC differentiation (Zhang et al., 2004). In another study, the transwell chamber system was used to separate monocytes from irradiated (15 Gy) bone marrow-derived MSCs during differentiation (Jiang et al., 2005). The MSC/monocyte ratio of 1:10 could completely prevent monocyte differentiation to DCs. Monocytes in the presence of MSC-released factors retained CD14-positive cells without acquisition of CD1a and displayed no up-regulation of CD83 and CD80 (Jiang et al., 2005). In a latter study, in the presence of GM-CSF, IL-4 and non-irradiated bone marrow-derived MSCs, monocytes also did not acquire the surface phenotype typical of immature (CD14-, CD1a+) DCs (Spaggiari et al., 2009). In a recent publication, it has been reported that after co-culture with MSCs from human induced pluripotent stem cells, monocytes displayed a low expression of CD1a, but a high expression of CD14, while exhibiting low expression of CD40, CD80, CD83, and HLA-DR (Gao et al., 2017).
In agreement with these previous findings both MSCl cells and MSCl-CM have the potential to regulate the differentiation of monocytes toward CD14+CD1a− cells. In our experimental setup MSCl-CM had only a minor effect on DC-SIGN levels, whereas exposure to MSCl cells significantly reduced its expression. Furthermore, MSCl cells, but not MSCl-CM, triggered a significant increase in the frequency of cells expressing CD163, an M2 macrophage marker (Hu et al., 2017). These findings indicate that MSCl-CM regulates the differentiation of monocytes toward a DC-like phenotype, while MSCl cells switch monocyte differentiation to M2 macrophage rather than DC direction. In line with our observations, it was reported in a prior study that bone marrow-derived MSCs drive the differentiation of monocytes to macrophages (Jiang et al., 2005). We have found that MSCl cells produce several inflammatory and anti-inflammatory factors, among them IL-6 and IL-10. It comes as no surprise because several soluble factors have been reported to contribute to the immunomodulatory effects of MSCs, and the roles of IL-6 and IL-10 along with PGE2 and TSG-6 have been especially well established (Weiss and Dahlke, 2019). It is important to mention that anti-inflammatory mediators such as IL-6 and IL-10 induce the expression of CD163 (Etzerodt and Moestrup, 2013); therefore, these cytokines may have a pivotal role in the induction of the differentiation of M2 macrophage-like cells in our experiments. On the other hand, cytokine-independent pathways may also be involved in the MSC-induced polarization of monocytes/macrophages. In a mouse model of asthma, the phagocytosis of MSCs caused lung macrophages to turn into a type 2 immunosuppressive phenotype (Braza et al., 2016). In a recent study, it has been found that the phagocytosis of MSCs caused CD14++CD16− classical monocytes to polarize toward a CD14++CD16+CD206+ immune regulatory intermediate subtype with anti-inflammatory properties, increased expression of PD-L1 and production of IL-10 (De Witte et al., 2018). After phagocytosis of MSCs, these primed monocytes were able to induce CD4+CD25hi Treg formation in vitro to a significantly higher extent than un-primed monocytes (De Witte et al., 2018). Furthermore, different Notch ligands could modulate monocyte/macrophage function and determine the M1 versus M2 macrophage polarization (Wang et al., 2010). Since Notch-mediated signals play a role in the protection against inflammation by MSCs (Li et al., 2008; Zhang et al., 2009; Lu et al., 2020), it is possible that the Notch pathway becomes activated during the direct contact between MSCl cells and monocytes. Further studies might reveal the exact mechanisms contributing to the different effects of MSCl cells and MSCl-CM on the monocyte differentiation.
Semi-mature moDCs differentiated in the presence of MSCl-CM express a low level of group 1 CD1 proteins responsible for the presentation of lipid and glycolipid antigens, while expressing increased levels of class II MHC molecules and co-stimulatory B7 family members that are essential for the activation of naive CD4+ T cells. Elevated expression of CTLA-4 on semi-mature moDCs was also induced by MSCl-CM. This co-inhibitory molecule can be expressed or produced by cells with either myeloid or lymphoid origin and competes with CD28 on T lymphocytes for binding to co-stimulatory B7 family members (Halpert et al., 2016; Wang et al., 2002). CTLA-4-expressing DCs were detected in patients suffering from certain carcinomas, highlighting the importance of this molecule during tumor progression (Han et al., 2014). Furthermore, genetically modified DCs expressing CTLA-4-Ig fusion protein prevented alloimmune activity in inflammatory conditions and ensured the survival of allografts through the induction of IL-10 production by Th17 cells, indicating a tolerogenic role of CTLA-4-expressing DCs in chronic inflammation (Watanabe et al., 2016). In addition to DCs, monocytes are also able to express CTLA-4, which can be downmodulated during the differentiation process into moDCs (Laurent et al., 2010). However, our results demonstrate that the ratio of CTLA-4-expressing cells was very low in the freshly isolated monocyte population and it was enhanced from the first day of moDC differentiation and further increased in the presence of MSCl cell-derived factors on day 4. There are no available data in the literature about CTLA-4 expression on M2 macrophages that further confirms the observed distinct effects of MSCl cells and MSC-CM on the differentiation of monocyte-derived cells.
According to our observations, MSCl-CM changed the expression levels of CD1a, CD86, and CTLA-4 on semi-mature moDCs at least partially via ATRA production. We have demonstrated that MSCl cells express genes essential for ATRA synthesis. The treatment of moDCs with ATRA mimicked the effects of MSCl-CM on the expression of CD1a, CD86, and CTLA-4 molecules. ATRA is derived from retinol and acts as a ligand of nuclear hormone receptors to drive the differentiation program of moDCs (Henning et al., 2015; Nagy et al., 2012; Bene et al., 2017). The lipid-ligation of nuclear hormone receptors such as PPARγ and RARα results in altered transcriptional regulation of moDC development, metabolism, and T cell polarizing capacity (Szatmari et al., 2004). Indeed, the ability of MSCl-CM to trigger phenotypic alterations was significantly diminished by either selective blockade of RARα in moDCs or by that of ATRA synthesis in MSCl cells. The fact that in the lamina propria of gut, DC subpopulations and MSCs constitutively express the enzymatic machinery of ATRA production even in the absence of dietary vitamin A (Vicente-Suarez et al., 2015) further supports our in vitro observations.
We have found that moDCs treated with MSCl-CM have the potential to trigger the polarization of IL-10+IL-17+ double-positive T helper cells in a CTLA-4-dependent manner. Blocking of CTLA-4 signaling by specific antibodies significantly reduced the ability of MSCl-CM-treated moDCs to trigger the development of IL-17- and IL-10-producing T cells, whereas inhibition of CTLA-4 expression in MSCl-CM-primed moDCs by applying BMS614, the RARα antagonist, induced only a slight, statistically insignificant decrease in the number of polarized T cells. This phenomenon can be explained by the fact that BMS614 only partially prevented the induction of CTLA-4 expression on moDCs by MSCl-CM (Figure 4A).
An increased secretion of IL-6 and TGFβ by moDCs differentiated in the presence of MSCl-CM was detected, and these cytokines are known to be crucial regulators of IL-10 production by Th17 cells (Mcgeachy et al., 2007). We presume that CTLA-4 expressed by semi-matured DCs decreases the levels of available co-stimulatory molecules for CD28 receptors on the surface of interacting T cells. Our hypothesis is supported by a recent study showing that myeloid DCs constitutively secrete CTLA-4 in microvesicular structures (Halpert et al., 2016). CTLA-4+ microvesicles can competitively bind B7 co-stimulatory molecules on bystander DC, resulting in the downregulation of B7 surface expression with significant functional consequences for T cell responses (Halpert et al., 2016). On the other hand, CTLA-4-mediated signals can also modulate cytokine secretion, as CTLA-4 triggering on mDCs increases IL-10 and reduces IL-8 and IL-12p70 production (Laurent et al., 2010). IL-17/IL-10 double-producing Th cells are indicative of a nonpathogenic Th17 cell population that has been shown to have a critical role in restraining Th17 cell-mediated inflammatory and autoimmune diseases (Mcgeachy et al., 2007; Gagliani et al., 2015). Furthermore, a significantly higher frequency of IL-17/IL-10 double-producing Th cells was found in acute myeloid leukemia patients than in healthy controls (Musuraca et al., 2015). The substantial imbalance between IL-17-/IL-10-producing cells and IL-17/IFN-γ-producing cells, together with a reduced frequency in Th1 and Th2 cells, may act as an immunosuppressive factor in these patients, altering the physiological role of Th17, contributing to the infections and probably promoting leukemia escape (Musuraca et al., 2015).
In summary, in this work we identified a previously undescribed mechanism for the immunomodulatory effects of MSCl cells on human DCs. In particular, we showed that monocyte-derived cells differentiated in the presence of MSCl cells or MSCl-CM could be characterized by a unique phenotype and functional profile. We demonstrated that despite the increased levels of class II MHC and co-stimulatory molecules on MSCl-CM-treated moDCs, these cells have the ability to induce immunosuppression via promoting the development of IL-17/IL-10 double-producing Th cells in a CTLA-4-dependent manner.
Limitations of the study
Human MSCs with different origins and under diverse culture conditions may exhibit different capacities to proliferate and may have diverse phenotypic and functional properties. To overcome these limitations, human MSC-like cells generated from pluripotent HUES9 embryonic stem cells were used in this study. These cells proved to be eligible to model the behavior of bone marrow-derived MSCs in vitro (Varga et al., 2011). In our experimental conditions, MSCl cells promote the differentiation of CTLA-4 expressing DCs by production of ATRA, functioning as a ligand of nuclear receptor RARα. These semi-matured DCs activate allogeneic, naive T cells and polarize them into IL-10- and IL-17-producing T helper cells in a CTLA-4-dependent manner. Further work is required to confirm these findings in primary human MSCs from different sources and also to reveal the in vivo relevance of this regulatory mechanism.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Attila Bácsi (etele@med.unideb.hu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Data and codes are available from the corresponding author upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The work was supported by TÁMOP-4.2.2.A-11/1/KONV-2012 0023 project implemented through the New Hungary Development Plan, and the National Research, Development and Innovation Office (NKFIH PD 132570 to ZV, NK 101538 and NN 114423 to ÉR and K 125337 to AB). The work was also supported by GINOP-2.3.2-15-2016-00050 project (TB and AB). The project is co-financed by the European Union and the European Regional Development Fund. The work was also supported by the UNKP-17-1, UNKP-17-3, UNKP-17-4, and UNKP-18-3 “New National Excellence Program” of the Ministry of Human Capacities. The project is co-financed by the European Union, by the State of Hungary, by the European Social Fund and the European Regional Development Fund. K.P. and Z.V. were supported by János Bolyai Research Scholarship from the Hungarian Academy of Sciences and by the UNKP-20-5 “New National Excellence Program” of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. The work of Z.V. was supported by the EFOP-3.6.1-16-2016-00008 project (co-financed by the European Union and the European Regional Development Fund). We thank Dr. Péter Gogolák and Erzsébet Magi for their help in measurements and Erzsébet Nagyné Kovács for her excellent technical assistance. We greatly appreciate Dr. Brahma V. Kumar's kind help in reviewing the manuscript.
Author contributions
Conceptualization, A.M., Z.V., K.B., and É.R.; formal analysis, A.M., K.B., and A.B.; funding acquisition, É.R., T.B., and A.B.; investigation, A.M., K.B., R.K., M.T., M.N., I.B., and K.P.; methodology, A.M., M.T., and I.B.; resources, Z.V. and Á.A.; supervision, É.R., T.B., and A.B.; validation, A.M., K.B., M.T., and I.B.; visualization, K.Sz.; writing – original draft preparation, A.M., K.B., and A.B.; writing – review & editing, A.M., K.B., and A.B.
Declaration of interests
The authors declare no conflicts of interest related to this research.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102312.
Supplemental information
References
- Abumaree M.H., Bahattab E., Alsadoun A., Al Dosaimani A., Abomaray F.M., Khatlani T., Kalionis B., El-Muzaini M.F., Alawad A.O., Alaskar A.S. Characterization of the interaction between human decidua parietalis mesenchymal stem/stromal cells and natural killer cells. Stem Cell Res. Ther. 2018;9:102. doi: 10.1186/s13287-018-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai I., Mazlo A., Kis-Toth K., Szabo A., Panyi G., Sarkadi B., Apati A., Rajnavolgyi E. Mesenchymal stromal cell-like cells set the balance of stimulatory and inhibitory signals in monocyte-derived dendritic cells. Stem Cells Dev. 2015;24:1805–1816. doi: 10.1089/scd.2014.0509. [DOI] [PubMed] [Google Scholar]
- Bene K., Varga Z., Petrov V.O., Boyko N., Rajnavolgyi E. Gut microbiota species can provoke both inflammatory and tolerogenic immune responses in human dendritic cells mediated by retinoic acid receptor alpha ligation. Front. Immunol. 2017;8:427. doi: 10.3389/fimmu.2017.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braza F., Dirou S., Forest V., Sauzeau V., Hassoun D., Chesne J., Cheminant-Muller M.A., Sagan C., Magnan A., Lemarchand P. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016;34:1836–1845. doi: 10.1002/stem.2344. [DOI] [PubMed] [Google Scholar]
- Chen P.M., Liu K.J., Hsu P.J., Wei C.F., Bai C.H., Ho L.J., Sytwu H.K., Yen B.L. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J. Leukoc. Biol. 2014;96:295–303. doi: 10.1189/jlb.3A0513-242R. [DOI] [PubMed] [Google Scholar]
- De Witte S.F.H., Luk F., Sierra Parraga J.M., Gargesha M., Merino A., Korevaar S.S., Shankar A.S., O'flynn L., Elliman S.J., Roy D. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018;36:602–615. doi: 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- Dong X., Shen K., Bulow H.E. Intrinsic and extrinsic mechanisms of dendritic morphogenesis. Annu. Rev. Physiol. 2015;77:271–300. doi: 10.1146/annurev-physiol-021014-071746. [DOI] [PubMed] [Google Scholar]
- Etzerodt A., Moestrup S.K. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid. Redox Signal. 2013;18:2352–2363. doi: 10.1089/ars.2012.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N., Amezcua Vesely M.C., Iseppon A., Brockmann L., Xu H., Palm N.W., De Zoete M.R., Licona-Limon P., Paiva R.S., Ching T. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W.X., Sun Y.Q., Shi J., Li C.L., Fang S.B., Wang D., Deng X.Q., Wen W., Fu Q.L. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res. Ther. 2017;8:48. doi: 10.1186/s13287-017-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Torensma R., Van Vliet S.J., Van Duijnhoven G.C., Adema G.J., Van Kooyk Y., Figdor C.G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Halpert M.M., Konduri V., Liang D., Chen Y., Wing J.B., Paust S., Levitt J.M., Decker W.K. Dendritic cell-secreted cytotoxic T-lymphocyte-associated protein-4 regulates the T-cell response by downmodulating bystander surface B7. Stem Cells Dev. 2016;25:774–787. doi: 10.1089/scd.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Chen Z., Yang Y., Jiang Z., Gu Y., Liu Y., Lin C., Pan Z., Yu Y., Jiang M. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59:567–579. doi: 10.1002/hep.26694. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Hill S., Friesen L., Park S., Im S., Kaplan M.H., Kim C.H. RARalpha supports the development of Langerhans cells and langerin-expressing conventional dendritic cells. Nat. Commun. 2018;9:3896. doi: 10.1038/s41467-018-06341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning P., Conaway H.H., Lerner U.H. Retinoid receptors in bone and their role in bone remodeling. Front. Endocrinol. (Lausanne) 2015;6:31. doi: 10.3389/fendo.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.M., Liu K., Liu J.H., Jiang X.L., Wang X.L., Chen Y.Z., Li S.G., Zou H., Pang L.J., Liu C.X. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget. 2017;8:21526–21538. doi: 10.18632/oncotarget.15630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.X., Zhang Y., Liu B., Zhang S.X., Wu Y., Yu X.D., Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- Kimbrel E.A., Kouris N.A., Yavanian G.J., Chu J., Qin Y., Chan A., Singh R.P., Mccurdy D., Gordon L., Levinson R.D., Lanza R. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 2014;23:1611–1624. doi: 10.1089/scd.2013.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S., Carrega P., Saverino D., Piccioli P., Camoriano M., Morabito A., Dozin B., Fontana V., Simone R., Mortara L. CTLA-4 is expressed by human monocyte-derived dendritic cells and regulates their functions. Hum. Immunol. 2010;71:934–941. doi: 10.1016/j.humimm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Li Y.P., Paczesny S., Lauret E., Poirault S., Bordigoni P., Mekhloufi F., Hequet O., Bertrand Y., Ou-Yang J.P., Stoltz J.F. Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J. Immunol. 2008;180:1598–1608. doi: 10.4049/jimmunol.180.3.1598. [DOI] [PubMed] [Google Scholar]
- Lu Z., Meng S., Chang W., Fan S., Xie J., Guo F., Yang Y., Qiu H., Liu L. Mesenchymal stem cells activate Notch signaling to induce regulatory dendritic cells in LPS-induced acute lung injury. J. Transl. Med. 2020;18:241. doi: 10.1186/s12967-020-02410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., Mcclanahan T., Cua D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Morgan C.A., Parajuli B., Buchman C.D., Dria K., Hurley T.D. N,N-diethylaminobenzaldehyde (DEAB) as a substrate and mechanism-based inhibitor for human ALDH isoenzymes. Chem. Biol. Interact. 2015;234:18–28. doi: 10.1016/j.cbi.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musuraca G., De Matteis S., Napolitano R., Papayannidis C., Guadagnuolo V., Fabbri F., Cangini D., Ceccolini M., Giannini M.B., Lucchesi A. IL-17/IL-10 double-producing T cells: new link between infections, immunosuppression and acute myeloid leukemia. J. Transl. Med. 2015;13:229. doi: 10.1186/s12967-015-0590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L., Szanto A., Szatmari I., Szeles L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol. Rev. 2012;92:739–789. doi: 10.1152/physrev.00004.2011. [DOI] [PubMed] [Google Scholar]
- Park Y.S., Lim G.W., Cho K.A., Woo S.Y., Shin M., Yoo E.S., Chan Ra J., Ryu K.H. Improved viability and activity of neutrophils differentiated from HL-60 cells by co-culture with adipose tissue-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012;423:19–25. doi: 10.1016/j.bbrc.2012.05.049. [DOI] [PubMed] [Google Scholar]
- Qian C., Cao X. Dendritic cells in the regulation of immunity and inflammation. Semin. Immunol. 2018;35:3–11. doi: 10.1016/j.smim.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Saeedi P., Halabian R., Imani Fooladi A.A. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Invest. 2019;6:34. doi: 10.21037/sci.2019.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skytthe M.K., Graversen J.H., Moestrup S.K. Targeting of CD163(+) macrophages in inflammatory and malignant diseases. Int. J. Mol. Sci. 2020;21:5497. doi: 10.3390/ijms21155497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaggiari G.M., Abdelrazik H., Becchetti F., Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- Szatmari I., Gogolak P., Im J.S., Dezso B., Rajnavolgyi E., Nagy L. Activation of PPARgamma specifies a dendritic cell subtype capable of enhanced induction of iNKT cell expansion. Immunity. 2004;21:95–106. doi: 10.1016/j.immuni.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Varga N., Vereb Z., Rajnavolgyi E., Nemet K., Uher F., Sarkadi B., Apati A. Mesenchymal stem cell like (MSCl) cells generated from human embryonic stem cells support pluripotent cell growth. Biochem. Biophys. Res. Commun. 2011;414:474–480. doi: 10.1016/j.bbrc.2011.09.089. [DOI] [PubMed] [Google Scholar]
- Vasandan A.B., Jahnavi S., Shashank C., Prasad P., Kumar A., Prasanna S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016;6:38308. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Suarez I., Larange A., Reardon C., Matho M., Feau S., Chodaczek G., Park Y., Obata Y., Gold R., Wang-Zhu Y. Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol. 2015;8:141–151. doi: 10.1038/mi.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Yuan Q., Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int. 2018;2018:3057624. doi: 10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.B., Giscombe R., Yan Z., Heiden T., Xu D., Lefvert A.K. Expression of CTLA-4 by human monocytes. Scand. J. Immunol. 2002;55:53–60. doi: 10.1046/j.0300-9475.2001.01019.x. [DOI] [PubMed] [Google Scholar]
- Wang Y.C., He F., Feng F., Liu X.W., Dong G.Y., Qin H.Y., Hu X.B., Zheng M.H., Liang L., Feng L. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70:4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Kaminuma O., Kitamura N., Hiroi T. Induced Treg cells augment the Th17-mediated intestinal inflammatory response in a CTLA4-dependent manner. PLoS One. 2016;11:e0150244. doi: 10.1371/journal.pone.0150244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A.R.R., Dahlke M.H. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019;10:1191. doi: 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Liu R., Shi D., Liu X., Chen Y., Dou X., Zhu X., Lu C., Liang W., Liao L. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113:46–57. doi: 10.1182/blood-2008-04-154138. [DOI] [PubMed] [Google Scholar]
- Zhang W., Ge W., Li C., You S., Liao L., Han Q., Deng W., Zhao R.C. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and codes are available from the corresponding author upon request.