Abstract
Skeletal muscle is responsible for the majority of glucose disposal following meals, and this is achieved by insulin-mediated trafficking of glucose transporter type 4 (GLUT4) to the cell membrane. The eight-protein exocyst trafficking complex facilitates targeted docking of membrane-bound vesicles, a process underlying the regulated delivery of fuel transporters. We previously demonstrated the role of exocyst subunit EXOC5 in insulin-stimulated GLUT4 exocytosis and glucose uptake in cultured rat skeletal myoblasts. However, the in vivo role of EXOC5 in skeletal muscle remains unclear. Using mice with inducible, skeletal-muscle-specific knockout of exocyst subunit EXOC5 (Exoc5-SMKO), we examined how muscle-specific disruption of the exocyst would affect glucose homeostasis in vivo. We found that both male and female Exoc5-SMKO mice displayed elevated fasting glucose levels. Additionally, male Exoc5-SMKO mice had impaired glucose tolerance and lower serum insulin levels. Using indirect calorimetry, we observed that male Exoc5-SMKO mice have a reduced respiratory exchange ratio during the light period and lower energy expenditure. Using the hyperinsulinemic–euglycemic clamp method, we further showed that insulin-stimulated skeletal muscle glucose uptake is reduced in Exoc5-SMKO males compared with wild-type controls. Overall, our findings indicate that EXOC5 and the exocyst are necessary for insulin-stimulated glucose uptake in skeletal muscle and regulate glucose homeostasis in vivo.
Keywords: diabetes, exocyst complex, glucose transporter, insulin resistance, skeletal muscle
Abbreviations: EE, energy expenditure; GLUT4, glucose transporter type 4; GSV, GLUT4 storage vesicle; GTT, glucose tolerance test; HFD, high-fat diet; HGP, hepatic glucose production; HSA-MCM, human skeletal muscle actin Mer-Cre-Mer; IP, intraperitoneal; IRS-1, insulin receptor substrate 1; ITT, insulin tolerance test; Pdk1, protein-dependent kinase 1; RER, respiratory exchange ratio; T2D, type 2 diabetes
Skeletal muscle is responsible for 80 to 90% of insulin-stimulated glucose uptake in the body (1). Insulin triggers the targeted trafficking of GLUT4 to the cell membrane, enabling glucose uptake into cells, and insulin-resistant cells show defects in GLUT4 trafficking to the membrane (2). The “solute carrier 2A” (SLC2A or GLUT) family of glucose transporters allow glucose to cross the cell membrane by facilitated diffusion. GLUT1 is constitutively present on the plasma membrane of cells in most tissues, while GLUT4 is the principal insulin responsive glucose transporter (3). In basal state, GLUT4 is sequestered in GLUT4 storage vesicles (GSVs) in the cytosol (4). When stimulated by insulin, GSVs are translocated along the cytoskeleton toward the cell membrane (5), where they are “docked” to the plasma membrane by tethering protein complexes (6). It is only after docking, when the membrane of the GSVs can be fused to the plasma membrane with the assistance of SNAP Receptor (SNARE) proteins (7).
In 3T3-L1 cells, it has been demonstrated that the exocyst protein complex is essential for the delivery of GLUT4-containing vesicles to the plasma membrane in response to insulin (8). The exocyst complex is an eight-member holocomplex that ensures that certain exocytic vesicles are trafficked to the appropriate location on the plasma membrane (9). The exocyst complex member EXOC6 is necessary for GLUT4 exocytosis in adipocytes (10), and the overexpression of EXOC3 and EXOC4 increases the magnitude of insulin-stimulated glucose uptake in cultured adipocytes (11).
The vast majority of the previous research demonstrating the essentiality of the exocyst complex for insulin-stimulated GLUT4 exocytosis has been performed in vitro in cultured adipocytes (12, 13). We have recently demonstrated that the exocyst complex is necessary for GLUT4 exocytosis in cultured skeletal myoblasts and myotubes as well (14). In healthy individuals, skeletal muscle takes up 80 to 90% of glucose in response to insulin, and one of the earliest detectable signs of insulin resistance preceding type 2 diabetes (T2D) is reduced insulin-stimulated glucose uptake in skeletal muscle (1). In the skeletal muscle cells of people with T2D, the insulin signaling pathway and subsequent GLUT4 exocytosis are suppressed (15), impairing glucose uptake into cells. A detailed map of the processes between the activation of the insulin receptor and the exocytosis of GLUT4 in skeletal muscle is yet to be completed. The discovery of new effectors of the insulin signaling pathway could provide novel therapeutic approaches for the treatment of insulin resistance and T2D (16). In this article, we are the first to report that in vivo disruption of the exocyst complex affects skeletal muscle glucose uptake and homeostasis in a novel conditional knockout mouse model.
Results
Generation of skeletal-muscle-specific Exoc5 knockout mice
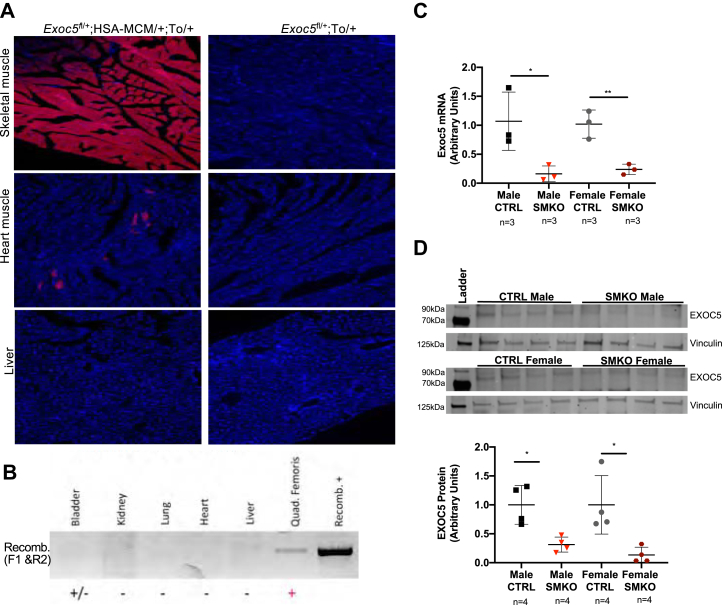
Previously, we demonstrated that the exocyst subunit EXOC5 is essential for insulin-stimulated GLUT4 exocytosis and glucose uptake in cultured rat skeletal myoblasts (17). To study the consequences of in vivo disruption of Exoc5 and the exocyst, we generated skeletal-muscle-specific Exoc5 conditional knockout (Exoc5-SMKO) mice. We crossed our “floxed” Exoc5 mouse strain with the human skeletal muscle actin Mer-Cre-Mer (HSA-MCM) mice strain and treated them with tamoxifen to induce Cre recombinase activation and thus inactivate Exoc5 in a skeletal-muscle-specific manner (Fig. 1A).
Figure 1.
Conditional knockouts of Exoc5 in mouse skeletal muscle were generated.A, fluorescent microscopy evaluating tissue-specific Cre expression using a mouse line with a tdTomato (To) reporter allele. The tissue sections are from female mice. B, recombination PCRs from genomic DNA of various tissues of tamoxifen-treated male mice to detect deletion of exons 7 to 10. C, real-time quantitative PCR measuring skeletal-muscle-specific EXOC5 mRNA expression (normalized to RPLP0) in Exoc5-SMKO and CTRL mice. D, representative image of a Western blot analysis (upper panel) used for the quantification (lower panel) of EXOC5 protein levels (normalized to Vinculin) in the soleus of Exoc5-SMKO and CTRL mice. Error bars = standard deviation, ∗p ≤ 0.05, ∗∗p ≤ 0.01.
We utilized a tdTomato reporter mouse strain to confirm skeletal-muscle-specific Cre activity. As anticipated, tamoxifen-treated Exoc5fl/+;HSA-MCM/+;To/+ female mice expressed tdTomato almost exclusively in skeletal muscle tissues (Fig. 1A, top panel). A few cardiomyocytes also expressed tdTomato (Fig. 1A, middle panel), but no tdTomato expression was detected in the liver (Fig. 1A, lower panel), lung, or kidney tissues (data not shown). We designed a PCR-based assay to test genomic DNA recombination, where a product is obtained only when exons 7 to 10 are removed from the Exoc5 gene. Recombination of genomic DNA was detected in male Exoc5-SMKO mice in skeletal muscle and bladder samples, but not in other tissues assayed (Fig. 1B).
Quantitative real-time PCR revealed that the knockout muscle tissue has significantly less Exoc5 mRNA expressed than controls (Fig. 1C) in both sexes. Western blot analysis demonstrated that Exoc5-SMKO male and female mice have decreased EXOC5 protein levels in soleus muscle tissue compared with littermate controls (Fig. 1D). Thus, in Exoc5-SMKO mice, following tamoxifen treatment, the Cre-recombinase was expressed in a skeletal-muscle-specific manner, and EXOC5 expression was markedly diminished in skeletal muscle tissue at both the RNA and protein levels, with similar efficiency in both male and female mice. Lastly, skeletal-muscle-specific ablation of Exoc5 did not impair neuromuscular muscle function in either sex, as Exoc5-SMKO performed similarly to control mice in open field and rotarod testing (Fig. S1).
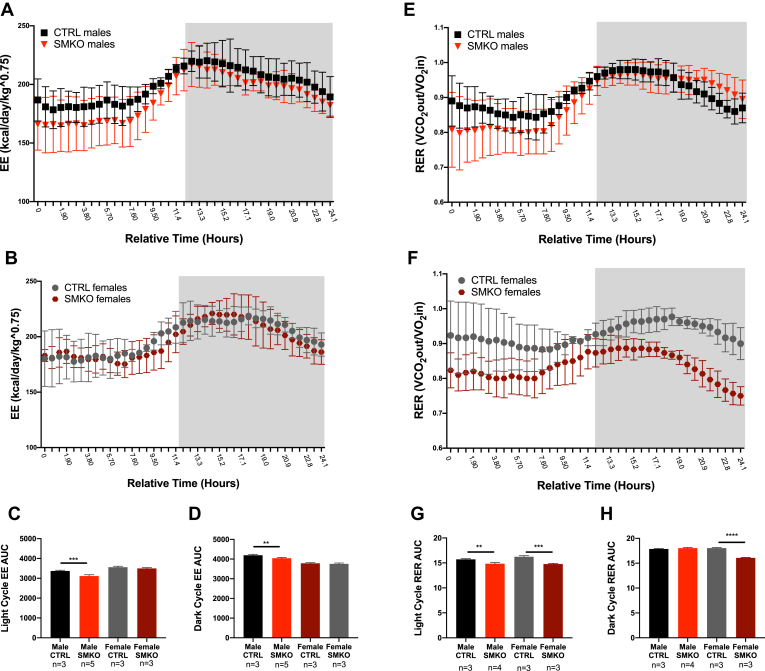
Altered energy expenditure and respiratory exchange ratio in Exoc5-SMKO mice
An indirect calorimetry study was conducted to assess whole-body energy balance in Exoc5-SMKO mice. The energy expenditure (EE) rate over a 24-h period was significantly reduced during both light and dark cycles in male Exoc5-SMKO mice, but not in female mice (Fig. 2, A–D). Additionally, respiratory exchange ratio (RER), measured over the course of a 24-h period, was significantly lower in male Exoc5-SMKO mice during light cycle and in female Exoc5-SMKO mice during both the light and dark cycle suggesting decreased glucose utilization in these mice compared with controls (Fig. 2, E–H). There were no significant differences in body weight, activity level (Fig. S2, A–C), and food or water intake (data not shown) between Exoc5-SMKO and control mice. These observations suggest that skeletal-muscle-specific disruption of Exoc5 affects whole-body energy substrate utilization.
Figure 2.
Indirect calorimetric analysis indicates a shift from glucose utilization in skeletal muscle specific Exoc5 knockout mice during daylight hours and a lower energy expenditure. Indirect calorimetry was performed on Exoc5-SMKO and CTRL mice by placing them in metabolic cages. Energy expenditure (EE) for male (A) and female (B) mice was calculated over a 24-h period, the dark cycle is symbolized by the grayed-out area. Area under the curve was calculated for the light period (C) and the dark period separately (D). The respiratory exchange ratio (RER) for male (E) and female (F) mice was calculated based on the ratio of O2 flowing into the cage and the CO2 flowing out of the cage over a 24-h period, the dark cycle is symbolized by the grayed-out area. Area under the curve was calculated for the light period (G) and the dark period separately (H). Error bars = standard deviation. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.
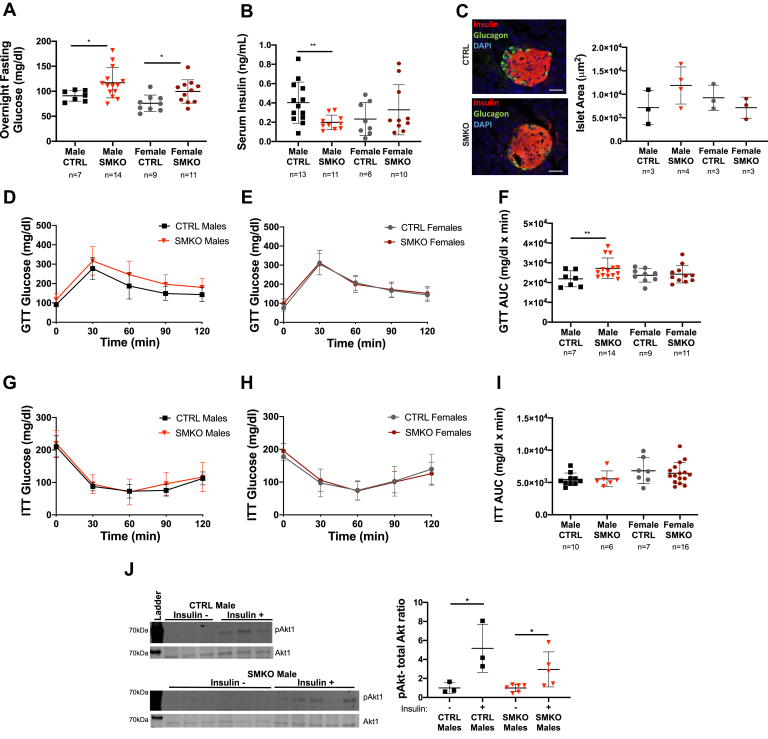
Altered glucose homeostasis in Exoc5-SMKO mice
In order to analyze the effect of the Exoc5 knockout in skeletal muscle on glucose homeostasis, we first measured fasting glucose and insulin levels. Blood glucose levels following overnight fast in male and female Exoc5-SMKO mice were significantly higher than in littermate controls (Fig. 3A), suggesting impaired glucose homeostasis and hepatic insulin resistance.
Figure 3.
Glucose homeostasis is perturbed in Exoc5 skeletal muscle knockout animals.A, Exoc5-SMKO and CTRL animals were fasted overnight, and fasting blood glucose concentrations were measured via tail bleed. B, six-hour fasting insulin levels were measured in Exoc5-SMKO and control littermate animals using serum samples and an ELISA assay. C, representative images of pancreatic islet immunofluorescent staining (left panel) and quantification of islet size in Exoc5-SMKO and CTRL mice (right panel). White scale bars, 20 μm. D and E, for the glucose tolerance testing, blood glucose concentrations were measured at 30-min intervals following an intraperitoneal bolus of glucose (1 mg/kg). F, quantification of area under the curve for data shown in D and E. G and H, prior to insulin tolerance testing, 4- to 5-month-old Exoc5-SMKO and CTRL mice were fasted for 6 h and blood glucose levels were measured by tail bleed. Following fasting glucose measurement, an intraperitoneal injection of insulin was administered (1 U/kg). Blood glucose was measured at baseline and every 30 min for 2 h by tail blood sampling. I, area under the curve calculations for data presented in G and H. J, Western blots (left panel) and quantification (right panel) of insulin signaling pathway component Akt in Exoc5-SMKO and CTRL following insulin injection. Error bars = standard deviation, ∗p ≤ 0.05; ∗∗p ≤ 0.01.
We also measured serum insulin levels by ELISA following a 6-h fast and found that male Exoc5-SMKO mice had significantly lower insulin levels than controls (Fig. 3B). This effect was selective to male mice because female Exoc5-SMKO mice had similar insulin levels to their littermate controls. The examination of pancreatic islets found no significant differences in islet size compared with controls in either sex (Fig. 3C).
During glucose tolerance test, blood glucose was measured every 30 min for 2 h following a glucose bolus. Male Exoc5-SMKO mice showed a slower clearance of glucose during GTT compared with male control mice, and area under the curve of GTT was significantly increased in male Exoc5-SMKO mice compared with controls (Fig. 3, D and F), suggesting impaired glucose tolerance in male Exoc5-SMKO mice. However, glucose tolerance and area under the curve of GTT were not significantly different in female Exoc5-SMKO mice compared with controls (Fig. 3, E and F).
We performed insulin tolerance test (ITT) in Exoc5-SMKO mice. The mice were fasted for 6 h and given an IP injection of insulin. Blood glucose was measured every 30 min for 2 h. Glucose clearance during ITT was similar between male and female Exoc5-SMKO mice and controls (Fig. 3, G and H). Area under the curve calculations depict a similar response to insulin in both male and female Exoc5-SMKO compared with controls (Fig. 3I). We also assessed insulin signaling pathway activation in Exoc5-SMKO mice compared with controls and found that the insulin signaling pathway component Akt1 is phosphorylated in both Exoc5-SMKO and control mice following insulin injection (Fig. 3J). Taken together, skeletal muscle knockout of Exoc5 resulted in increased fasting glycemia and impaired glucose tolerance, but did not impair a functional insulin signaling pathway activation. Of note, gender-specific differences in glucose tolerance were observed in these mice.
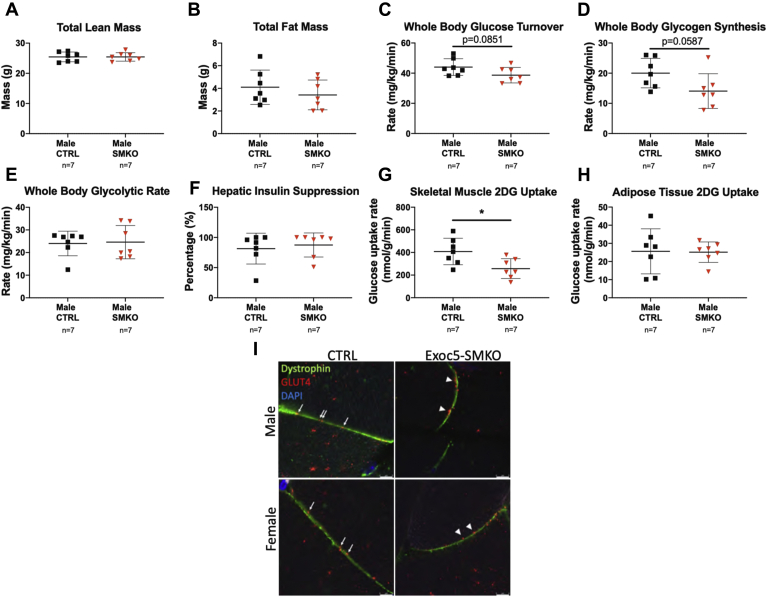
Male Exoc5-SMKO mice develop insulin resistance in skeletal muscle
Body composition analysis showed that Exoc5-SMKO and control male mice had no detectable differences in whole-body fat mass and lean mass (Fig. 4, A and B). We performed hyperinsulinemic–euglycemic clamp in awake mice to measure whole-body insulin sensitivity and glucose metabolism in individual organs. Whole-body glucose turnover during clamps tended to decrease by 10% in male Exoc5-SMKO mice compared with male controls, similarly to whole-body glycogen synthesis, although the differences did not reach statistical significance (p = 0.0851 and p = 0.0587, respectively) (Fig. 4, C and D). Whole-body glycolysis was not significantly different in male Exoc5-SMKO mice compared with male controls (Fig. 4E). Basal and clamp hepatic glucose production and hepatic insulin action were not altered in male Exoc5-SMKO mice (Fig. 4F).
Figure 4.
Exoc5-SMKO male mice have reduced skeletal muscle glucose uptake and whole-body glucose turnover during hyperinsulinemic–euglycemic clamp.A and B, proton magnetic resonance spectroscopy was applied to quantify whole-body lean and fat mass in male Exoc5-SMKO and control animals. C–F, hyperinsulinemic–euglycemic clamp was performed in awake mice to assess whole-body glucose turnover (C), whole-body glycogen synthesis (D), whole-body glycolysis (E), hepatic insulin action (insulin-mediated percent suppression of basal hepatic glucose production) (F), in Exoc5-SMKO and control males. G and H, insulin-stimulated glucose uptake in skeletal muscle (gastrocnemius) (G) and white adipose tissue (epididymal) (H) was determined following a bolus injection of 2-[14C] deoxy-glucose during clamp). I, immunofluorescent staining of skeletal muscle tissues of insulin-injected animals. GLUT4 vesicles are in red and dystrophin is labeled in green in Exoc5-SMKO male and female littermate controls. White scale bars, 5 μm. Error bars = standard deviation, ∗p ≤ 0.05.
Insulin-stimulated glucose uptake in individual organs was measured using a bolus injection of labeled 2-deoxyglucose during clamps. Skeletal muscle glucose uptake in response to insulin was significantly decreased in Exoc5-SMKO mice compared with controls (Fig. 4G). The insulin-stimulated glucose uptake in adipose tissue was not altered in Exoc5-SMKO mice (Fig. 4H). The impaired skeletal-muscle-specific glucose uptake was not due to defective insulin signaling or total GLUT4 expression because no differences were detected in the mRNA abundance of GLUT4, insulin receptor (InsR), insulin receptor substrate 1 (IRS-1), protein-dependent kinase 1(Pdk1), Rho-related GTP-binding protein (RhoQ), and RAC-beta serine threonine-protein kinase (Akt2) (Fig. 3J, Fig. S3). In support of decreased skeletal-muscle-specific glucose uptake, immunofluorescent staining of skeletal muscle tissues of insulin-injected animals revealed a disrupted GLUT4 membrane integration with membrane-proximal accumulation of GLUT4 vesicles in Exoc5-SMKO male and female mice compared with littermate controls (Fig. 4I) These results indicate that impaired glucose tolerance and insulin resistance in male Exoc5-SMKO mice were mostly due to decreases in insulin-stimulated glucose uptake in skeletal muscle. Based on these results, and our metabolic cage data demonstrating decreased glucose utilization, we expected a switch toward increased fatty acid metabolism in skeletal muscle tissues of our Exoc5-SMKO mice. Therefore, we quantified expression of proteins responsible for fatty acid flux as well as mitochondrial content in soleus muscles. Of note, we were not able to detect differences in fatty acid transporter/CD36, lipoprotein lipase (LPL), or mitochondrial components translocase of the outer mitochondrial membrane 20 (TOMM20), voltage-dependent anion-selective channel protein (VDAC1), and cytochrome c oxidase complex four (CoxIV) between groups (Fig. S4).
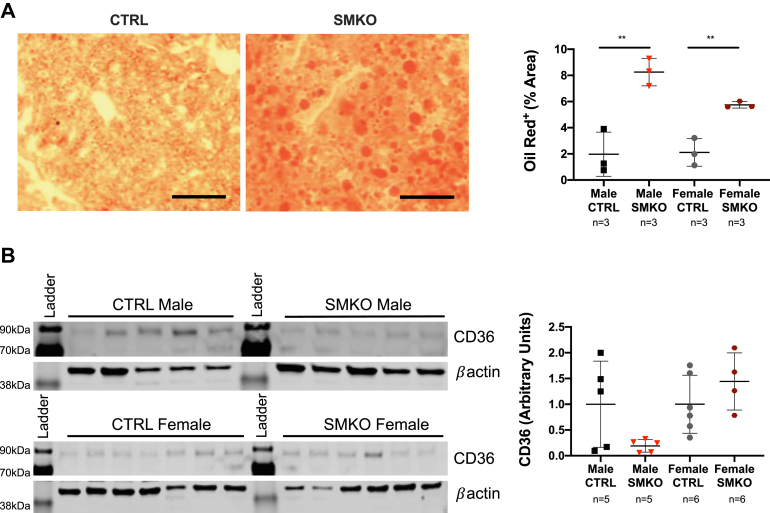
With reduced muscle glucose uptake but no differences in adiposity, we hypothesized a rerouting of increased glucose to the liver. Thus, we evaluated liver morphology and lipid content using Oil Red O staining. We have detected a significantly increased hepatic lipid accumulation in Exoc5-SMKO males and females compared with littermate controls (Fig. 5A). A significantly higher hepatic triglyceride content was indeed measured in the homogenate of Exoc5-SMKO male mice (Fig. S5A). We quantified the protein levels of CD36, LPL in the livers of Exoc5-SMKO and control mice to decipher if lipid accumulation was due to increased uptake (Fig. 4B, and Fig. S5B), but found no significant differences between groups. In support of these findings, we detected no differences in liver-specific uptake of the fluorescent lipid analog, BODIPY-palmitate in male or females Exoc5-SMKO mice compared with controls (Fig. S5C). To investigate if hepatic lipid accumulation was due to de novo lipogenesis, we measured protein levels of Fatty Acid Synthase (FASN), as well as mRNA levels of ChREBP, SREBP1, and Acetyl CoA Carboxylase 1 (ACACA), but detected no significant differences (Fig. S5, D–G). Moreover, there were no differences in serum triglycerides and markers of liver function Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) activity levels (Fig. S5, H–J).
Figure 5.
Exoc5-SMKO mice had increased hepatic lipid accumulation, yet no difference in insulin-sensitive fatty acid transporter CD36 protein levels was detected.A, representative images of control and Exoc5-SMKO liver samples stained with Oil Red O (left panels). Quantification of the ratio of Oil Red O-stained areas in liver tissues (right panel). B, images of Western blot analysis (left panel) and quantification (right panel) of CD36. CD36 levels in B as well as LPL and FASN levels in Fig. S5 were sequentially analyzed on a single Western blot, and their respective signal intensities were normalized using the same βactin loading control measurements. Thus we show the same βactin loading control images in Fig. S5,B and D as well. Black scale bars, 100 μm, Error bars = standard deviation, ∗∗p ≤ 0.01.
Chronic high-fat feeding exacerbates insulin resistance in male Exoc5-SMKO mice
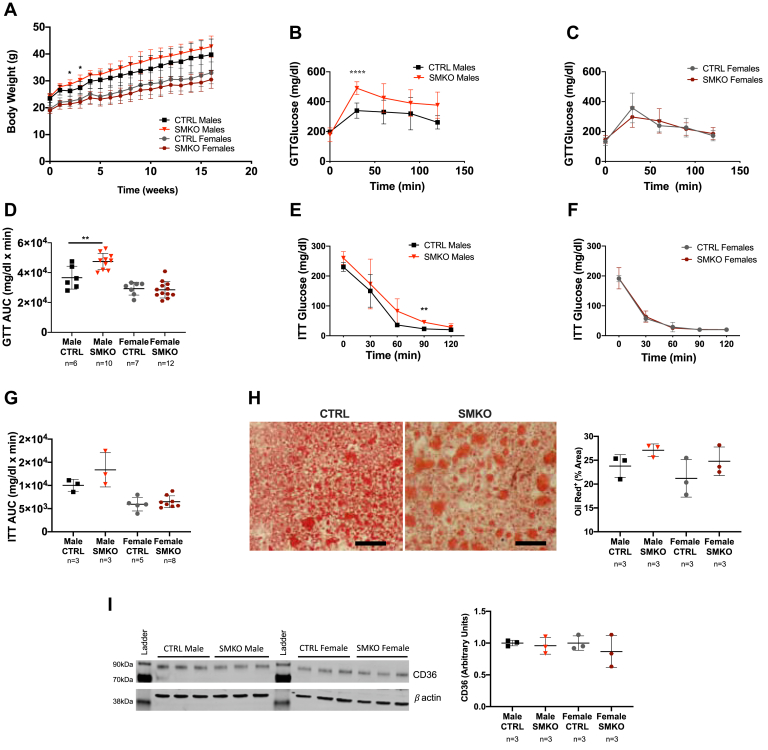
Male and female Exoc5-SMKO and control mice were challenged with a high-fat diet (HFD) for 16 weeks to assess if impaired exocyst function affects diet-induced changes in energy metabolism. Body weights were measured weekly, and after 16 weeks of HFD, we did not detect significant differences in body weights between knockout and control animals (Fig. 6A). GTT revealed significantly reduced glucose tolerance in HFD-fed male Exoc5-SMKO mice compared with controls, whereas no difference in glucose tolerance could be detected in HFD-fed Exoc5-SMKO females compared with the littermate controls (Fig. 6, B–D).
Figure 6.
High-fat diet challenge of skeletal-muscle-specific Exoc5-SMKO animals confirms disrupted glucose homeostasis.A, weekly body weight measurements of Exoc5-SMKO and control animals during a 16-week high-fat diet challenge. B and C, during the glucose tolerance testing, blood glucose concentrations were measured at 30-min intervals following an intraperitoneal bolus of glucose (1 mg/kg). D, quantification of area under the curve for data shown in B and C. E and F, during insulin tolerance testing, blood glucose was measured every 30 min for 2 h blood glucose measurement following an intraperitoneal injection of insulin (1 U/kg). G, area under the curve calculations for data presented in E and F. H, representative images of control and Exoc5-SMKO liver samples stained with Oil Red O (left panel) and quantification of the ratio of Oil Red O-stained areas in liver tissues (right panel) in Exoc5-SMKO and littermate control animals following. Black scale bars, 100 μm. Images of Western blot analysis (upper panel) used for the quantification of I, images of Western blot analysis (left panel) used for the quantification of CD36 protein levels (normalized to β-actin) in the livers of Exoc5-SMKO and control mice (right panel). Error bars = standard deviation, ∗p ≤ 0.05; ∗∗p ≤ 0.01, ∗∗∗∗p ≤ 0.0001.
ITT of the HFD-fed cohorts also showed reduced glucose clearance in male Exoc5-SMKO mice compared with controls, but no differences between female Exoc5-SMKO mice and controls after HFD (Fig. 6, E–G). While fasting serum insulin levels increased compared with regular diet-fed cohort, there were no significant differences in fasting serum insulin and islet size between high-fat diet-fed Exoc5-SMKO mice and littermate controls (Fig. S6, A and B). There were no differences in fasting serum triglyceride levels either (Fig. S6C). Of note, when we evaluated hepatic lipid accumulation in the HFD-fed animals, we could not detect a significant difference between Exoc5-SMKO and control animals in either sex (Fig. 6H). In addition, we did not detect significant differences in CD36 (Fig. 6I), LPL, and FASN (Fig. S6, D and E) protein levels in the livers of Exoc5-SMKO mice either.
Discussion
Insulin-stimulated glucose uptake by skeletal muscle is an essential part of glucose homeostasis (18). Insulin stimulation in a healthy individual results in the exocytosis of the GLUT4 and subsequent glucose uptake into major metabolic tissues such as skeletal muscle, fat, and heart (19). Discordance in the mechanisms that coordinate the GLUT4 exocytosis in skeletal muscle may contribute to the primary dysfunction in T2D (20).
Previous studies using gene knockout animals have demonstrated that GLUT4 and its function are essential for maintaining glucose homeostasis. The heterozygous whole-body knockout of GLUT4 in mice resulted in decreased glucose uptake in fat and muscle cells, increased adipocyte size, hypertension, and cardiac hypertrophy, as well as hepatic steatosis (21). Furthermore, knockout of GLUT4 in skeletal muscle alone resulted in reduced glucose tolerance, and the decreased serum lactate and β-hydroxybutyrate levels in skeletal muscle GLUT4-null mice indicated decreased glucose oxidation (22). However, GLUT4 overexpression in adipose tissue was able to rescue insulin resistance in mice lacking GLUT4 in skeletal muscle (23).
The eight-protein exocyst complex is involved in vesicle docking and has been evolutionarily conserved from yeast to humans (24). It has been extensively demonstrated that the exocyst complex is essential for insulin-stimulated GLUT4 exocytosis in cultured adipocytes (11, 12, 25). The siRNA knockdown of EXOC6/6b inhibited GLUT4 exocytosis in 3T3-L1 adipocytes (10). On the other hand, overexpression of EXOC3 and EXOC4 in 3T3-L1 adipocytes increased the magnitude of glucose transport in response to insulin (11). There have been very few studies directly examining the role of the exocyst complex in skeletal muscle tissues. We have previously demonstrated that chemical inhibition of the exocyst complex and the heterozygous knockout of Exoc5 lead to a reduction in insulin-stimulated GLUT4 membrane delivery and glucose uptake in rat skeletal myoblasts (17).
Here, we are the first to create an in vivo model of a skeletal-muscle-specific knockout of a component of the exocyst complex. Exoc5-SMKO mice appeared to have normal neuromuscular function as well as an intact insulin signaling pathway activation, and no differences in the expression levels of genes that are involved in insulin-stimulated glucose uptake were detected.
Food and water intake, activity level, body weight, and body composition were similar between Exoc5-SMKO and control animals as measured using metabolic cages and proton magnetic resonance spectroscopy. Our indirect calorimetry data suggest that the skeletal muscle depletion of EXOC5 results in a metabolic shift from glucose utilization toward increased amino acid and fatty acid metabolism in both sexes. A reduced carbohydrate utilization as an energy substrate is likely due to an impaired glucose uptake in skeletal muscle. In support of our findings, insulin-resistant mice were previously shown to have RER of approximately 0.8 and an overnight fasting glucose of 600 mg/dl starting at 15 weeks of age, while wild-type mice had an RER of 0.9 and a fasting glucose level around 100 mg/dl (26).
Hyperinsulinemic–euglycemic clamp demonstrated that insulin sensitivity was reduced selectively in skeletal muscle of male Exoc5-SMKO mice. Glucose uptake in adipose tissue was similar between Exoc5-SMKO mice and controls. No differences in whole-body glycogen synthesis were detected in Exoc5-SMKO mice. Although, 70 to 90% of glucose absorbed by skeletal muscle is converted into glycogen during a hyperinsulinemic–euglycemic clamp (27) and skeletal muscle stores 80% of the of the body’s glycogen (27), the effect of the impaired skeletal muscle glucose uptake was insufficient to alter these parameters. Overnight fasting glucose is higher in Exoc5-SMKO mice, and our findings indicate that the excess glucose may be routed to the liver, as suggested by hepatic lipid accumulation. Yet, we were not able to detect any significant differences in hepatic lipid uptake or de novo lipogenesis and further investigation is warranted. The hyperinsulinemic–euglycemic clamp data also showed no effects on hepatic insulin sensitivity as insulin maximally suppressed hepatic glucose production in Exoc5-SMKO and control mice. It should be noted, however, that since hepatic glucose production was maximally suppressed by insulin during clamp, potential effects on hepatic insulin action may have been masked in this study.
While body weights and body composition of Exoc5-SMKO and littermate control mice were similar for both sexes, glucose tolerance was significantly reduced in male Exoc5-SMKO mice only. The glucose tolerance of female Exoc5-SMKO mice was not altered. Differences in the glucose responsive tissues between male and female mice may protect female mice from dysregulation of glucose homeostasis. Studies on mice have shown that there are more than 300 differentially expressed genes in skeletal muscle between males and females (28). Thus, the gender-specific effects we describe in the glucose homeostasis of our Exoc5-SMKO animals warrant further investigation.
No differences in insulin tolerance were observed between Exoc5-SMKO and control mice. It has been documented that insulin tolerance test may lack the sensitivity necessary to resolve metabolic differences in mice, in particular when glucose metabolism is altered in selective metabolic organs (29). Male Exoc5-SMKO had lower insulin levels yet we did not find any significant differences in pancreatic islet size or insulin-positive cell ratios compared with controls.
The ad libitum HFD treatment closely mimics the effect of the nutrient-dense Western diet in humans. After 16 to 20 weeks, mice gain 20 to 30% more weight than mice fed normal chow and hyperglycemia usually begins after 4 weeks (30). After a 16-week HFD, the body weights of Exoc5-SMKO and littermate controls were similar. Male Exoc5-SMKO mice challenged with a HFD for 16 weeks had decreased glucose tolerance and reduced glucose clearance in response to exogenous insulin, and female Exoc5-SMKO mice were similar to controls. This may be due to estrogen’s role in female mice contributing to an increased reliance on lipid oxidation (31), masking any possible effects of altered skeletal muscle glucose uptake. The differences in male and female muscle and adipose tissue anatomy and physiology were mentioned earlier.
Lipid dysregulation, especially elevated serum triglycerides are commonly associated with T2D (32). Elevated serum triglycerides can be a potential sign of liver dysfunction (32). However, fasting serum insulin and serum triglycerides were not significantly different between Exoc5-SMKO and control mice challenged with a HFD. Of note, no significant impairment of liver function could be detected in our Exoc5-SMKO mice compared with controls.
Our previous study demonstrated that reducing the amount of EXOC5 impairs the ability of mouse skeletal muscle cells to uptake glucose in response to insulin (17). This was due to a failure to increase the amount of GLUT4 transporters at the plasma membrane. In this study, we highlight the central role of the exocyst complex in insulin-stimulated glucose uptake in skeletal muscle in vivo. We demonstrate that skeletal-muscle-specific knockout of Exoc5 causes insulin resistance in mice, and impaired skeletal-muscle-specific glucose uptake triggers a shift from carbohydrate metabolism toward lipid utilization and increases hepatic lipid accumulation as a possible substrate redistribution from skeletal muscle tissues to the liver.
Ongoing work will further investigate the molecular mechanism of exocyst-mediated GLUT4 trafficking in skeletal muscle.
Experimental procedures
Animals
The “floxed” Exoc5 (Exoc5fl/fl) mouse strain (kindly provided by Dr Ben Fogelgren, University of Hawaii) was generated and validated as previously described (33). To inactivate Exoc5 in a muscle-specific manner, we obtained HSA-MCM mice (Tg(ACTA1-cre/Esr1∗)2Kesr/J) from Dr Karyn Esser from the University of Kentucky Center for Muscle Biology. This HSA-MCM strain has a tamoxifen-inducible form of Cre recombinase (CreERT2) under the control of the human skeletal actin (HSA) promoter (34). The B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato) Hze/J reporter mouse strain was used to detect Cre recombinase activity through Cre-activated expression of the tdTomato red fluorescent protein (kindly provided by Dr Michelle Tallquist, University of Hawaii) (35).
All animal procedures and protocols were carried out in accordance with IACUC specifications approved by the University of Hawaii Animal and Veterinary Services and the University of Massachusetts Medical School.
Tamoxifen treatment
Mice at 6 to 8 weeks of age were given tamoxifen-containing chow (Envigo cat #TD. 130860) ad libitum for 2 weeks to induce skeletal-muscle-specific Cre recombinase activation and subsequent gene recombination. To assess tissue-specific Cre recombinase activation, heterozygous Exoc5fl/+; HSA-MCM/+;tdTomato/+ mice, age- and sex-matched littermate control animals of Exoc5fl/fl; +/+,tdTomato/+ genotype (both carrying the tdTomato reporter allele) were subjected to tamoxifen treatment. The Cre activation was assessed based on expression of the red fluorescent tdTomato in various tissues. To evaluate glucose homeostasis, Exoc5fl/fl;HSA-MCM/+ (Exoc5-SMKO) mice and age- and sex-matched littermate animals of Exoc5fl/fl; +/+ (control) genotype were used in all experiments.
High-fat diet and body composition analysis
Ten- to twelve-week-old Exoc5-SMKO and control mice were given a HFD (Envigo cat #TD.07011; containing 29% Fat, 30% Carbohydrate, 26% protein by weight) for 16 weeks. Body weight was measured every 7 days. Whole-body fat and lean mass of male 20-week-old male Exoc5-SMKO and control mice were noninvasively assessed using Proton magnetic resonance spectroscopy (1H-MRS, EchoMRI, EchoMedical Systems) (36).
Motor function testing
With assistance from the JABSOM Murine Behavior and Metabolic Research Support Facility (http://mbmc.jabsom.hawaii.edu), open-field and Rotarod tests were performed to assess locomotion and motor coordination as previously described (37).
Glucose and insulin tolerance test
For intraperitoneal glucose tolerance test (GTT), overnight fasted mice were injected intraperitoneally (IP) with glucose (1 mg/kg), and blood glucose levels were measured using a OneTouch Ultra handheld glucometer. For insulin tolerance test, mice were fated for 6 h, insulin was subsequently delivered via intraperitoneal (IP) injection at 0.75 IU/kg, and glycemia was measured using a OneTouch Ultra handheld glucometer.
Measurement of energy balance and indirect calorimetry
With assistance from the University of Hawaii Murine Behavior and Metabolic Research Support Facility (http://mbmc.jabsom.hawaii.edu), whole-body energy balance was assessed via indirect calorimetry using the Panlab OxyletPro System (Harvard Apparatus, Barcelona, Spain). Adult (16-week-old) male Exoc5-SMKO and control animals were weighed and individually housed in metabolic cages. Following a 24-h acclimation period, food and water intake, physical activity, and energy expenditure (O2 consumption, CO2 production) were measured over a 24-h period. Data collection and calculations were performed using the Panlab METABOLISM software.
Hyperinsulinemic–euglycemic clamp to assess insulin sensitivity in vivo
Hyperinsulinemic–euglycemic clamps were conducted in male 20-week-old Exoc5-SMKO and control mice as previously described (38). At the end of the clamps, mice were anesthetized, and tissues were taken for biochemical analysis.
Glucose concentrations during clamps were analyzed using 10 μl plasma by a glucose oxidase method on Analox GM9 Analyser (Analox Instruments Ltd). Plasma concentrations of [3-3H]glucose, 2-[14C]DG, and 3H2O were determined following deproteinization of plasma samples as previously described (29). For the determination of tissue 2-[14C]DG-6-Phosphate content, tissue samples were homogenized, and the supernatants were subjected to an ion-exchange column to separate 2-[14C]DG-6-P from 2-[14C]DG.
Rates of basal hepatic glucose production (HGP) and insulin-stimulated whole-body glucose turnover were determined as previously described (38).
Serum and hepatic triglyceride, serum insulin, and HbA1c analysis
Male and female Exoc5-SMKO and control mice were fasted for 6 h and upon euthanasia, blood was drawn by cardiac puncture. Plasma was separated using BD Microtainer Capillary Blood Collector and BD Microgard Closure tubes and stored at −20 °C. Serum triglyceride was measured using a Triglyceride Colorimetric Assay Kit (Cayman Chemical Catalog # 10010303) per the manufacturer’s recommendation with a SpectraMax M3 microplate reader (Molecular Devices, LLC). Serum insulin was measured using a Mouse Ultra Sensitive Insulin ELISA kit (ALPCO catalog #80-INSMSU-E01).
Histology and Immunofluorescence
All samples were imaged using an Olympus BX41 microscope. Image processing, quantification of pancreatic islet areas, and oil red staining were performed using ImageJ (39).
For immunofluorescence, deparaffinized and rehydrated sections were subjected to a citrate-based antigen retrieval. After permeabilization and blocking, sections were incubated with primary antibodies (anti-insulin (Cat #66198-1-Ig, Proteintech Group, Inc); anti-glucagon (Cat # 15954-1-AP,Proteintech Group, Inc)) overnight at 4 °C and incubated with appropriate fluorescent secondary antibodies (DyLight 488 Anti-Rabbit IgG and DyLight 594 Anti-Mouse, Vector Laboratories) for detection. Cell nuclei were stained with DAPI.
In the sections stained for insulin and glucagon, the borders of each pancreatic islet were identified morphologically and marked using the pen tool in ImageJ. The area of each islet was calculated with ImageJ software using the set scale function corresponding to the 20x scale bar. Insulin-positive nuclei were counted based on fluorescent signal. All islets with an area >566 μmˆ2 (corresponding to a diameter of >27 μm), approximately corresponding to islets containing a minimum of eight nuclei, were regarded for analysis.
Western blot analysis
Skeletal muscle proteins were extracted using an extraction buffer (20 mM Tris, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1% Triton X-100, 10% glycerol, 1 mM EDTA, 1 mM DTT) and a mechanical homogenizer. Lysates were separated on a gradient (4–15%) SDS-PAGE gel, and proteins were transferred onto a polyvinylidene fluoride membrane. The membrane was incubated with primary antibodies (AKT1, Santa Cruz, sc-5298; EXOC5, Proteintech, 17593-1-AP; GAPDH, Proteintech, 60004-1-Ig; FASN, Proteintech, 10624-2-AP; CD36, Novus, NB400-144; LPL, Novus, AF7197; pAKT (Ser 473), Cell Signaling, 4060T; TOMM20, Abcam, ab186735; VDAC1, Abcam, ab129002; Vinculin, Abcam, ab129002; CoxIV, Cell Signaling, 4850) diluted 1:1000 in 5% BSA in PBST at 4 °C overnight, and with fluorescent secondary antibodies (IRDye 800 conjugated goat anti-rabbit IgG, LI-COR Biosciences, 926-32211; IRDye 680RD donkey anti-mouse IgG, LI-COR Biosciences, 926-68072) diluted 1:15,000 in 5% BSA in PBST with 0.02% SDS. Blots were scanned with a LI-COR Odyssey Clx scanner, and densitometry was performed using the Image Studio software (LI-COR Biosciences).
Quantitative real-time PCR analysis
Total RNA from the quadriceps femoris muscle was collected using the RNeasy Fibrous Tissue Mini Kit (Qiagen Germany). The iScript reverse transcriptase (Bio-Rad) was used to generate cDNA from extracted RNA samples. Quantitative PCR was performed on a CFX96 Real Time System (Bio-Rad), using the iTaq Universal SYBR Green Supermix (Bio-Rad) using predesigned primer assays (PrimeTime, Integrated DNA Technologies). Gene expression was normalized to Rplp0. Quantitative PCR primer sequences are listed in Table S1.
BODIPY—palmitate uptake assay
In vivo hepatic uptake of lipid was measured by fasting Exoc5-SMKO and littermate controls overnight. A single IP injection of 50 μg BODIPY-conjugated palmitate diluted in 1% BSA was administered. Mice were sacrificed by isoflurane overdose 1 h after injection and tissues were harvested immediately. Livers were mechanically homogenized following the addition of 2 ml of radioimmunoprecipitation assay buffer. Lysates were centrifuged at 1000g and the fluorescence of the supernatant was measured ex/em 488/512. The fluorescence values were normalized by protein concentration determined by bicinchoninic acid assay.
Statistical methods
Graphs show mean ± standard deviation, unless otherwise indicated. Statistical comparisons between the subgroups were assessed by one-way analysis of variance (ANOVA). In all other cases, a Student’s t-test was performed to calculate p values and determine if there was a significant difference between two groups. p values of less than 0.05 were considered statistically significant. Area under the curve (positive incremental) values were calculated based on the trapezoid model, relative to the baseline, using the GraphPad Prism software (GraphPad, Inc) (40).
Data availability
All relevant data generated during this study are included in this published article (and its supplementary information files).
Supporting information
This article contains supporting information.
Conflict of interest
The authors disclose of no conflict of interest.
Acknowledgments
We would like to thank Dr Mariana Gerschenson of the Diabetes Research Center of the University of Hawaii for her support. At the University of Hawaii at Manoa, we express appreciation to the Health Sciences Microscopy and Imaging Core for their help with confocal microscopy; The Histopathology Core, Janna Zoll with the COBRE Diabetes Core, and Ann Hashimoto with the Murine Behavioral Core; Dr Michelle Tallquist for providing B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J mice; Dr Ben Fogelgren for the EXOC5fl/fl mice; and Dr Karyn Esser for the HSA-MCM mice. We would also like to acknowledge the Hawaii IDEA Networks of Biomedical Research Experience program for supporting M. Young and L. Carter in their research.
Author contributions
B.A.F. and N.P. conceived and designed the research and authored the paper. B.A.F., M.Y, N.N., H.H., L.C., M.W.P., D.T., H.L.N., S.S., and N.P. executed the experiments, including data collection and analysis. B.A.F., M.W.P., D.T., J.K.K., and N.P. interpreted the results and provided key insights. All the authors were involved in editing the article and approving the final submitted version.
Funding and additional information
This work was supported in part by NIH funding (P20GM113134 to Dr. Mariana Gerschenson/N. P., B. A. F. was supported by 3T32HL115505-05S2 awarded to R Shohet). The University of Hawaii Behavior & Metabolic Core was supported by grant number #U54MD007601 from the National Institute on Minority Health and Health Disparities. Part of this study was performed at the National Mouse Metabolic Phenotyping Center (MMPC) at UMass Medical School supported by an NIH grant 5U2C-DK093000 to J. K. K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Qi-Qun Tang
Supporting information
References
- 1.DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32:S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeFronzo R., Bonadonna R., Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 3.Jaldin-Fincati J.R., Pavarotti M., Frendo-Cumbo S., Bilan P.J., Klip A. Update on GLUT4 vesicle traffic: A cornerstone of insulin action. Trends Endocrinol. Metab. 2017;28:597–611. doi: 10.1016/j.tem.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Ploug T., van Deurs B., Ai H., Cushman S.W., Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: Identification of distinct storage compartments that are recruited by insulin and muscle contractions. J. Cell Biol. 1998;142:1429–1446. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tunduguru R., Thurmond D.C. Promoting glucose transporter-4 vesicle trafficking along cytoskeletal tracks: PAK-ing them out. Front. Endocrinol. (Lausanne) 2017;8:329. doi: 10.3389/fendo.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lizunov V.A., Matsumoto H., Zimmerberg J., Cushman S.W., Frolov V.A. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J. Cell Biol. 2005;169:481–489. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C., Coker K.J., Kim J.K., Mora S., Thurmond D.C., Davis A.C., Yang B., Williamson R.A., Shulman G.I., Pessin J.E. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J. Clin. Invest. 2001;107:1311–1318. doi: 10.1172/JCI12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X.W., Saltiel A.R. Ral’s engagement with the exocyst: Breaking up is hard to do. Cell Cycle. 2011;10:2299–2304. doi: 10.4161/cc.10.14.16524. [DOI] [PubMed] [Google Scholar]
- 9.Lürick A., Kümmel D., Ungermann C. Multisubunit tethers in membrane fusion. Curr. Biol. 2018;28:R417–R420. doi: 10.1016/j.cub.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Sano H., Peck G.R., Blachon S., Lienhard G.E. A potential link between insulin signaling and GLUT4 translocation: Association of Rab10-GTP with the exocyst subunit Exoc6/6b. Biochem. Biophys. Res. Commun. 2015;465:601–605. doi: 10.1016/j.bbrc.2015.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewart M.A., Clarke M., Kane S., Chamberlain L.H., Gould G.W. Evidence for a role of the exocyst in insulin-stimulated Glut4 trafficking in 3T3-L1 adipocytes. J. Biol. Chem. 2005;280:3812–3816. doi: 10.1074/jbc.M409928200. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M., Chang L., Hwang J., Chiang S.H., Saltiel A.R. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 13.Chen X.W., Leto D., Chiang S.H., Wang Q., Saltiel A.R. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Johnson T.L., Fujimoto B.A.S., Jiménez-Flores R., Peterson D.G. Growth hormone alters lipid composition and increases the abundance of casein and lactalbumin mRNA in the MAC-T cell line. J. Dairy Res. 2010;77:199–204. doi: 10.1017/S0022029910000087. [DOI] [PubMed] [Google Scholar]
- 15.Bouzakri K., Roques M., Gual P., Espinosa S., Guebre-Egziabher F., Riou J.P., Laville M., Le Marchand-Brustel Y., Tanti J.F., Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- 16.Barnard R.J., Youngren J.F. Regulation of glucose transport in skeletal muscle. FASEB J. 1992;6:3238–3244. doi: 10.1096/fasebj.6.14.1426762. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto B.A., Young M., Carter L., Pang A.P., Corley M.J., Folgelgren B., Polgar N. The exocyst complex regulates insulin-stimulated glucose uptake of skeletal muscle cells. Am. J. Physiol. Endocrinol. Metab. 2019;317:E957–E972. doi: 10.1152/ajpendo.00109.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFronzo R.A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 19.Huang S., Czech M.P. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Stockli J., Fazakerley D.J., James D.E. GLUT4 exocytosis. J. Cell Sci. 2011;124:4147–4159. doi: 10.1242/jcs.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenbit A.E., Tsao T.-S., Li J., Burcelin R., Geenen D.L., Factor S.M., Houseknecht K., Katz E.B., Charron M.J. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat. Med. 1997;3:1096–1101. doi: 10.1038/nm1097-1096. [DOI] [PubMed] [Google Scholar]
- 22.Zisman A., Peroni O.D., Abel E.D., Michael M.D., Mauvais-Jarvis F., Lowell B.B., Wojtaszewski J.F.P., Hirshman M.F., Virkamaki A., Goodyear L.J., Kahn C.R., Kahn B.B. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000;6:924–928. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho E., Kotani K., Peroni O.D., Kahn B.B. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am. J. Physiol. Metab. 2005;289:E551–E561. doi: 10.1152/ajpendo.00116.2005. [DOI] [PubMed] [Google Scholar]
- 24.Lepore D.M., Martínez-Núñez L., Munson M. Exposing the elusive exocyst structure. Trends Biochem. Sci. 2018;43:714–725. doi: 10.1016/j.tibs.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue M., Chiang S.H., Chang L., Chen X.W., Saltiel A.R. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol. Biol. Cell. 2006;17:2303–2311. doi: 10.1091/mbc.E06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi H.-M., Kim H.R., Kim E.-K., Byun Y.S., Won Y.-S., Yoon W.-K., Kim H.-C., Kang J.-G., Nam K.-H. An age-dependent alteration of the respiratory exchange ratio in the db/db mouse. Lab. Anim. Res. 2015;31:1. doi: 10.5625/lar.2015.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen J., Rustad P.I., Kolnes A.J., Lai Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011;2:1–11. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed D.R., Bachmanov A.A., Tordoff M.G. Forty mouse strain survey of body composition. Physiol. Behav. 2007;91:593–600. doi: 10.1016/j.physbeh.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alquier T., Poitout V. Considerations and guidelines for mouse metabolic phenotyping in diabetes research. Diabetologia. 2017;61:526–538. doi: 10.1007/s00125-017-4495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C.-Y., Liao J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012;821:421–433. doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsberg H.N., Zhang Y.L., Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch. Med. Res. 2005;36:232–240. doi: 10.1016/j.arcmed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Fogelgren B., Polgar N., Lui V.H., Lee A.J., Tamashiro K.K., Napoli J.A., Walton C.B., Zuo X., Lipschutz J.H. Urothelial defects from targeted inactivation of exocyst Sec10 in mice cause ureteropelvic junction obstructions. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarthy J.J., Srikuea R., Kirby T.J., Peterson C.A., Esser K.A. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet. Muscle. 2012;2:8. doi: 10.1186/2044-5040-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., Lein E.S., Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taicher G.Z., Tinsley F.C., Reiderman A., Heiman M.L. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal. Bioanal. Chem. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 37.Byrns C.N., Pitts M.W., Gilman C.A., Hashimoto A.C., Berry M.J. Mice lacking selenoprotein P and selenocysteine lyase exhibit severe neurological dysfunction, neurodegeneration, and audiogenic seizures. J. Biol. Chem. 2014;289:9662–9674. doi: 10.1074/jbc.M113.540682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.K. Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol. Biol. 2009;560:221–238. doi: 10.1007/978-1-59745-448-3_15. [DOI] [PubMed] [Google Scholar]
- 39.Hartig S.M. Basic image analysis and manipulation in ImageJ. Curr. Protoc. Mol. Biol. 2013 doi: 10.1002/0471142727.mb1415s102. Chapter 14:Unit14.15. [DOI] [PubMed] [Google Scholar]
- 40.Allison D.B., Paultre F., Maggio C., Mezzitis N., Pi-Sunyer F.X. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data generated during this study are included in this published article (and its supplementary information files).