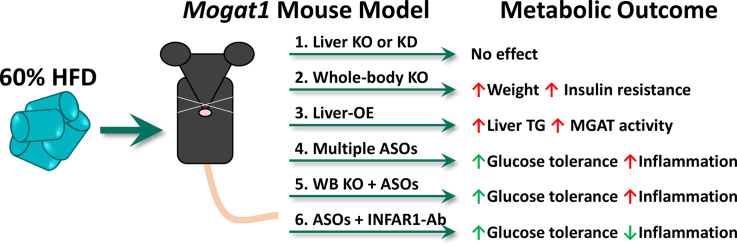
Abstract
Objective
Monoacylglycerol acyltransferase (MGAT) enzymes catalyze the synthesis of diacylglycerol from monoacylglycerol. Previous work has suggested the importance of MGAT activity in the development of obesity-related hepatic insulin resistance. Indeed, antisense oligonucleotide (ASO)-mediated knockdown of Mogat1 mRNA, which encodes MGAT1, reduced hepatic MGAT activity and improved glucose tolerance and insulin resistance in high-fat diet (HFD)-fed mice. However, recent work has suggested that some ASOs may have off-target effects on body weight and metabolic parameters via activation of the interferon alpha/beta receptor 1 (IFNAR-1) pathway.
Methods
Mice with whole-body Mogat1 knockout or a floxed allele for Mogat1 to allow for liver-specific Mogat1-knockout (by either a liver-specific transgenic or adeno-associated virus-driven Cre recombinase) were generated. These mice were placed on an HFD, and glucose metabolism and insulin sensitivity were assessed after 16 weeks on diet. In some experiments, mice were treated with control scramble or Mogat1 ASOs in the presence or absence of IFNAR-1 neutralizing antibody.
Results
Genetic deletion of hepatic Mogat1, either acutely or chronically, did not improve hepatic steatosis, glucose tolerance, or insulin sensitivity in HFD-fed mice. Furthermore, constitutive Mogat1 knockout in all tissues actually exacerbated HFD-induced obesity, insulin sensitivity, and glucose intolerance on an HFD. Despite markedly reduced Mogat1 expression, liver MGAT activity was unaffected in all knockout mouse models. Mogat1 overexpression in hepatocytes increased liver MGAT activity and TAG content in low-fat-fed mice but did not cause insulin resistance. Multiple Mogat1 ASO sequences improved glucose tolerance in both wild-type and Mogat1 null mice, suggesting an off-target effect. Hepatic IFNAR-1 signaling was activated by multiple Mogat1 ASOs, but its blockade did not prevent the effects of either Mogat1 ASO on glucose homeostasis.
Conclusion
These results indicate that genetic loss of Mogat1 does not affect hepatic MGAT activity or metabolic homeostasis on HFD and show that multiple Mogat1 ASOs improve glucose metabolism through effects independent of targeting Mogat1 or activation of IFNAR-1 signaling.
Keywords: Monoacylglycerol acyltransferase, Insulin resistance, Antisense oligonucleotides, Interferon alpha/beta receptor 1
Graphical abstract
Highlights
-
•
Mogat1 liver-specific KO or KD does not improve metabolism in HFD-fed mice.
-
•
Whole-body Mogat1 deletion impairs insulin tolerance in HFD-fed mice.
-
•
Mogat1 ASOs improve whole-body metabolism independently of gene knockdown.
-
•
Blockade of the INFAR1 response does not prevent off-target effects of Mogat1 ASOs.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) results from ectopic hepatic lipid accumulation (steatosis) and is believed to be a key driver of many metabolic abnormalities associated with obesity, including insulin resistance and diabetes [1,2]. In obesity, the liver is overloaded with fatty acids from dietary intake, increased adipose tissue lipolysis, and higher rates of de novo lipid synthesis [[3], [4], [5]]. The liver must efficiently store these lipids as neutral triacylglycerol (TAG) to prevent deleterious effects of toxic lipid intermediates, such as activation of inflammation, endoplasmic reticulum (ER) stress, and insulin resistance [6].
There are two pathways of TAG synthesis in the liver: the glycerol-3-phosphate (G-3-P) pathway and the monoacylglycerol O-acyltransferase (MGAT) pathway. These pathways converge at diacylglycerol (DAG); the sole precursor of TAG [[7], [8], [9]]. MGATs acylate monoacylglycerol to form DAG and this enzymatic activity is encoded by several genes including Mogat1, Mogat2, and Dgat1 in mice [[10], [11], [12], [13]]. The importance of MGAT activity in intestinal absorption of dietary fat has long been studied [8,14]. Generally, the G-3-P pathway is considered to be the primary route of TAG synthesis in tissues other than the intestine, and the MGAT pathway is believed to be an auxiliary pathway. We have recently demonstrated that Mogat1 is highly expressed in adipocytes and may function to suppress aberrant lipolysis [15]. In addition, the expression and activity of the MGATs are increased in humans and mouse models of NAFLD [[16], [17], [18], [19], [20]]. Suppression of hepatic and adipose tissue Mogat1 expression, through use of antisense oligonucleotides (ASO), reduces hepatic MGAT activity and improves hepatic insulin sensitivity and glucose metabolism without affecting hepatic DAG or TAG levels in obese mice [17,18].
Second-generation ASOs are known to target multiple tissues, including liver, adipose, and intestine, all of which have MGAT activity [21]. Moreover, off-target effects of ASO treatment have also been described [22]. McCabe et al. recently demonstrated weight loss, adipocyte browning, and improvement in adipose tissue metabolism by ASO treatment that was independent of target gene knockdown [22]. These effects were mediated through the activation of interferon alpha/beta receptor 1 (IFNAR-1) signaling in adipose-derived macrophages [22]. Thus, we sought to obtain rigorous and independent confirmation that hepatic Mogat1 plays an important role obesity-related hepatic insulin resistance and metabolic abnormalities using novel liver-specific and global Mogat1 knockout mice. Our data surprisingly suggest that multiple Mogat1 ASO sequences improve glucose metabolism through MGAT1-independent mechanisms and that the effects are not mediated through IFNAR-1 activation.
2. Methods
2.1. Generation of mouse models
All mouse studies were approved by the Institutional Animal Care and Use Committee of Washington University. Due to the resistance of female mice to the effects of high fat diet (HFD), male mice in the C57BL6/J background were used in all studies. Mice were group housed and maintained on standard laboratory chow on a 12 h light/dark cycle. At eight-weeks of age mice were placed on control low-fat diet (LFD, Research Diets, 10 kcal, % fat matched sucrose, D12450J) or HFD (Research Diets, 60 kcal % fat, D12492) for the durations indicated. ASO treatments were performed as previously described [17]. Briefly, starting at 16 weeks on diet, mice were given twice weekly intraperitoneal injections of ASO directed against Mogat1 (sequence #1: 5′-GATCTTGGCCACGTGGAGAT-3’ (20-mer), or sequence #2: 5′-TGGCCACGTGGAGATACGAT-3’ (20-mer), where indicated) or scramble control (Ionis, Pharmaceuticals, Inc., Carlsbad, CA; 25 mg/kg body weight) for three weeks.
Embryonic stem (ES) cells used to generate Mogat1 whole-body knockout and Mogat1 floxed mice were obtained from the Knockout Mouse Consortium (KOMP, project# CSD35789) [15]. Briefly, these ES cells contained a Mogat1 allele wherein a cassette containing cDNAs encoding LacZ and a neomycin-resistance locus was inserted just upstream of Mogat1 exon 4 that was flanked by LoxP sites. Mogat1 exon 4 encodes the HPHG catalytic site of MGAT1, and thus loss of this exon will result in loss of its enzymatic activity. Resulting chimeric offspring from ES cell injections were mated to C57BL6/J mice to establish a line of constitutive whole-body Mogat1 knockout mice. Other heterozygous whole-body knockout mice were crossed to transgenic mice expressing Flp recombinase to remove the LacZ/Neo cassette and generate the Mogat1 floxed line. These mice were then crossed with mice expressing albumin promoter-driven Cre recombinase (Jackson Laboratory, B6.Cg-Speer6-ps1Tg (Alb-cre)21Mgn/J). Control mice are considered Mogat1 floxed mice without the expression of the albumin promotor-driven Cre recombinase.
Acute liver-specific knockout mice were generated by retro-orbital injection of Mogat1 floxed mice with 2.0 × 1011 genomic copies (GC) of adeno-associated virus serotype 8 (AAV8) expressing Cre recombinase under the control of human thyroid hormone-binding globulin (TBG) promoter (Vector Biolabs, VB1724). Control mice received AAV8 expressing enhanced green fluorescent protein (eGFP) under control of the same promoter (Vector Biolabs, VB1743). For hepatic Mogat1 overexpression, eight-week-old male C57BL6/J mice were given LFD or HFD for six weeks, then administered AAV8-TBG-eGFP or AAV8-TBG-mouse-Mogat1 by retro-orbital injection (Vector Biolabs, VB1743, custom refseq# BC106135) and continued on diet for the times indicated.
In the IFNAR-1 inhibition study, HFD-fed male C57BL/6J mice were obtained from Jackson Laboratory after 12 weeks of HFD feeding. After acclimation and four additional weeks of HFD feeding, mice were weight matched into four treatment groups. Mice were given scramble ASO or ASOs targeting Mogat1 as described above. During each ASO treatment, mice were also given intraperitoneal (IP) injections of IgG control (BioCell InVivoIMAb, #BE0083, clone MOPC-21) or a monoclonal neutralizing antibody targeting interferon alpha/beta receptor 1 (IFNAR-1) (BioCell InVivoIMAb, #BE0241, clone MAR1-5A3) [23]. Antibody treatments were given as IP injections of 250 ug per mouse in 100 uL of dilution buffer (BioCell, InVivoPure Ph 6.5 Dilution Buffer, #IP0065) twice a week for the first two weeks of ASO treatment, followed by three separate injections of 500 ug per mouse the third week of ASO treatments.
Prior to sacrifice, mice were fasted for 4 h starting at 0900. Mice were euthanized via CO2 asphyxiation. Blood was collected from venipuncture of the inferior vena cava into EDTA-coated tubes, and plasma was removed by centrifugation. Liver and other tissues were immediately collected, flash-frozen in liquid nitrogen, and stored at −80 °C until further use.
2.2. Histology and NAFLD scoring
Liver tissue was harvested as described in section 2.1. Immediately following dissection, livers were fixed in 10% buffered formalin for 48 h. Liver tissue was rinsed and stored in 70% EtOH until paraffin embedding, sectioning, and staining with hematoxylin-eosin (H&E) in the Anatomic and Molecular Pathology Core Labs at Washington University School of Medicine. H&E sections were used to determine NAFLD activity score by a blinded independent clinical pathologist [24].
2.3. Metabolic phenotyping of mouse models
Glucose tolerance tests (GTTs) were performed in mice fasted for 5 h starting at 0900. Mice were given an IP injection of glucose (1 g/kg body weight dissolved in saline), and blood glucose was measured from the tail using a One Touch Ultra glucometer (Life Scan, Inc.) at the times indicated. For insulin tolerance tests (ITTs), mice were fasted for 4 h before IP injections of recombinant Humulin R® (0.75 U/kg body weight in saline). Tolerance tests were performed one week apart to allow the mice to recover starting at 15 and 16 weeks of diet or week two and three of acute treatments. Body composition was determined in fed mice using ECHO MRI. Because the whole-body null mice had differences in body weights compared to WT mice, the tolerance tests in these mice were dosed based on lean mass (1 g glucose/kg lean mass and 1.5 U insulin/kg lean mass).
2.4. Liver lipids and plasma metabolites
Frozen liver pieces were homogenized with bead disruption in phosphate-buffered saline (PBS, 100 mg/mL). Lipids were solubilized in 1% sodium deoxycholate via vortexing and heating at 37 °C for 5 min. Triglycerides and total cholesterol were measured enzymatically using the Infinity triglyceride and cholesterol colorimetric assay kits (Thermo Fisher, TR22421 and TR13421) and normalized to mg tissue (wet weight). Plasma insulin was determined by Singulex Immunoassay (Millipore Sigma) via the Washington University Core Laboratory for Clinical Studies. Plasma ALT and AST were measured using TECO Diagnostic liquid kinetic assays (A524 and A559).
2.5. mRNA isolation and quantitative polymerase chain reaction (PCR)
Total liver RNA was isolated from frozen liver samples using RNA STAT (Iso Tech, CS-502) according to the manufacturer's protocol. RNA was reverse transcribed into cDNA using Taqman high-capacity reverse transcriptase (Life Technologies, 43038228). Quantitative PCR was performed using Power SYBR green (Applied Biosystems, 4367659) and measured on an ABI PRISM 7500 or ABI QuantStudio 3 sequence detection system (Applied Biosystems). Results were quantified using the 2−ΔΔCt method and shown as arbitrary units relative to control groups. Primer sequences are listed in Supplemental Table 1.
2.6. Primary hepatocyte isolations
Primary hepatocytes were isolated as previously described [25]. Briefly, 10- to 16-week-old female mice were given an overdose of isoflurane prior to perfusions. The livers were perfused via catheterization of the hepatic portal vein and flushed with 30 mL of HBSS (Ca2+/Mg2+-free, 0.5 mM EGTA) prior to digestion with 20 mL of collagenase solution Type IV collagenase (sigma S5138) at 1 mg/mL in DMEM (serum free, 1 mM sodium pyruvate). Following perfusion, the livers were removed and disrupted in the collagenase solution. Cells were added to ice-cold complete DMEM (10% FBS, 1 mM sodium pyruvate, 100 U PenStrep, 0.25 μg/mL amphotericin b) and passed through a 50-μM filter prior to centrifugation at 50g × 2 min. The supernatant was removed, and hepatocytes were resuspended in complete DMEM and washed two more times. Five hundred thousand cells were then directly added to RNA STAT for RNA isolation and quantification.
2.7. MGAT enzymatic assay
MGAT activity was determined as previously described [26]. Liver tissue (50 mg/mL) was homogenized by probe sonicated in ice-cold membrane buffer (50 mM Tris–HCl pH 7.4, 1 mM EDTA, 250 mM sucrose, and complete protease inhibitors table (Roche Diagnostics, A32965)). Homogenates were clear of whole-cell debris by low-speed centrifugation at 500g × 10 min at 4 °C. The resulting supernatants were spun at 100,000 g × 60 min at 4 °C using a Beckman benchtop ultra-centrifuge. The cytosolic fractions (supernatant) were removed, and the membranes were reconstituted via pipetting in membrane buffer without protease inhibitors. Proteins were quantified using the bicinchoninic acid (BCA) assay according to the manufacturer's protocols (Thermo Fisher, 23225). Fifty micrograms of membrane were incubated in assay buffer (5 mM MgCl2, 1.25 mg/mL BSA, 200 mM sucrose, 100 mM Tris–HCl (pH 7.4)) containing 20 μM of [14C]oleoyl-CoA (American Radiolabeled Chemicals, ARC 0527), and 200 μM of sn-2-oleoylglycerol (Caymen Chemical, 16537) for 10 min. The reaction was stopped with 50 μL of 1% phosphoric acid. Lipids were extracted in 2:1 v/v % CHCl3:MeOH and separated by thin-layer chromatography in hexane/ethyl ether/acetic acid (80:20:1, v/v/v %). Samples were run against standards for oleic acid, 1, 3 diacylglycerol, and triglyceride, and corresponding spots were scraped from the plate and 14C-radioactivity was measured via scintillation counter. Backgrounds were calculated from reaction mixtures without membrane fractions.
2.8. Statistical analysis
Data were analyzed using GraphPad Prism software. Independent and paired T-tests, one-way analysis of variance (ANOVA), or factorial ANOVAs were performed where appropriate. Secondary post-hoc analysis found differences in groups using either Tukey or Sidek multiple comparisons where appropriate. P < 0.05 was considered significant.
3. Results
3.1. Liver-specific knockout of Mogat1
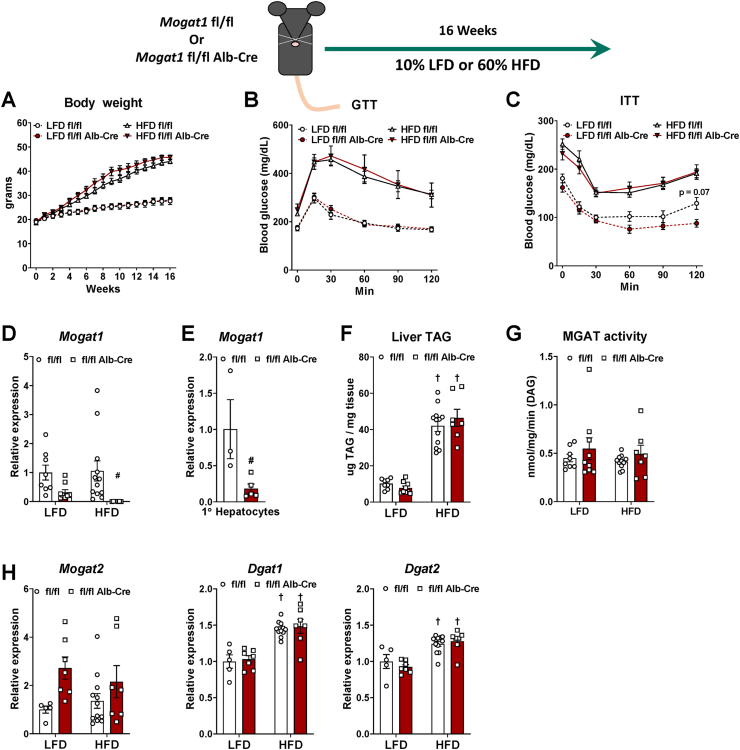
We generated mice with hepatocyte-specific deletion of Mogat1, and the resulting offspring were outwardly normal on a standard chow diet. When given ad libitum access to a 10% fat (low-fat diet, LFD) or 60% fat (HFD), the mice gained weight similar to littermate fl/fl controls (Figure 1A). Contrary to our previous studies showing a beneficial effect of Mogat1 ASOs on metabolic parameters in obese mice, glucose and insulin tolerance was not different between genotypes on LFD or HFD (Figure 1B,C). We confirmed that Mogat1 expression was markedly suppressed in Mogat1 fl/fl Alb-Cre + livers and freshly isolated primary hepatocytes (of note, Mogat1 expression drastically decreases after hepatocyte isolation and plating, data not shown) (Figure 1D,E). Hepatic TAG content increased with HFD but was similar between genotypes (Figure 1F). In contrast to previous work with Mogat1 ASO-treated mice, MGAT activity in isolated liver membranes was not affected by Mogat1 knockout (Figure 1G and ref. [17]). Therefore, gene expression of similar acyltransferases with known MGAT activity (Mogat2, Dgat1) and DGAT activity (Dgat2) were measured. Mogat2 was not significantly increased in knockout mice, while both Dgats increased with HFD but were unaffected by Mogat1 knockout (Figure 1H).
Figure 1.
Constitutive liver-specific Mogat1 deletion does not improve insulin sensitivity in mice. Male Mogat1 fl/fl mice and littermate Mogat1 fl/fl albumin Cre + mice were fed an LFD or an HFD starting at 8 weeks of age for 16 weeks. Mice were fasted for 4 h prior to sacrifice and tissue collection. A: HFD increased body weight in both groups. B,C: HFD-fed mice have impaired glucose and insulin tolerance compared to LFD groups. D,E: Mogat1 gene expression is reduced in knockout liver and primary hepatocytes. F: HFD increased liver TAG content in both genotypes. G: MGAT activity was not affected by diet or genotype. H: Gene expression of Mogat2, Dgat1, and Dgat2 were unaffected by Mogat1 knockout. Data are expressed as means ± S.E.M, and significance was assessed by two-way ANOVA with Sidak's multiple comparison test, #p < 0.05 gene effect, and †p < 0.05 diet effect; n = 5–10 for mouse studies. n = 3–5 female mice for primary hepatocyte isolations and two-tailed Student's t-test were performed, #p < 0.05 gene effect.
3.2. AAV8-mediated liver-specific knockout of Mogat1
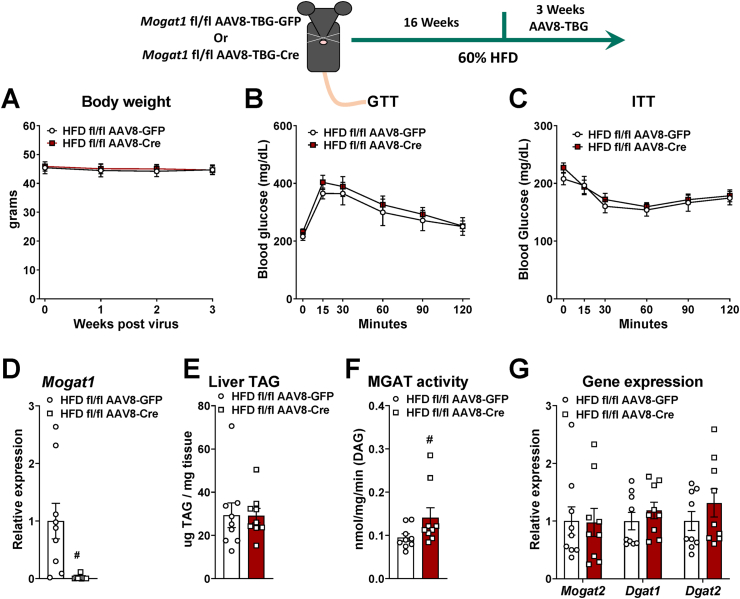
To avoid possible compensation from chronic Mogat1 knockout, we injected Mogat1 fl/fl mice with an AAV8-TBG-Cre or eGFP control after 16 weeks of HFD feeding and assessed glucose homeostasis three weeks later (Figure 2). This paradigm was selected because three weeks of Mogat1 ASO treatment after 16 weeks on diet is effective at improving insulin sensitivity and glucose homeostasis [16,17]. After three weeks, compared to GFP controls, AAV8-Cre knockout mice had similar body weight and no differences in glucose or insulin tolerance (Figure 2A–C). The AAV8-Cre reduced Mogat1 expression (Figure 2D). Similar to constitutive Mogat1 liver-specific knockout, acute deletion of hepatic Mogat1 did not alter liver TAG content and slightly increased MGAT activity (Figure 2E,F). Gene expression of similar acyltransferases (Mogat2, Dgat1, Dgat2) were unaffected by Mogat1 knockout (Figure 2G) [[10], [11], [12], [13]]. These data indicate that genetic deletion of Mogat1 in the livers of diet-induced obese mice for 3 weeks does not affect glucose or insulin tolerance, and may suggest compensation from other enzymes with MGAT activity [11,12,27].
Figure 2.
Acute liver-specific deletion of Mogat1 does not improve glucose or insulin tolerance in HFD-fed mice. Male Mogat1 fl/fl mice were fed an HFD starting at 8 weeks of age. After 16 weeks, mice were given a retro-orbital injection of AAV8-TBG-GFP or Cre recombinase (2 × 1011 GC per mouse) and remained on diet for an additional 3 weeks. Mice were fasted for 4 h prior to sacrifice and tissue collection. A: Acute liver-specific Mogat1 knockout did not affect body weight. B,C: Mogat1 knockout did not improve glucose or insulin tolerance. D: Mogat1 knockout reduced Mogat1 gene expression in liver. E: Liver TAG content was not different among groups. G: Hepatic MGAT activity was increased by acute Mogat1 knockdown in liver. Mogat1 knockout did not increase Mogat2, Dgat1, or Dgat2 gene expression in liver. Data are expressed as means ± S.E.M., and two-tailed Student's t-tests were performed, #p < 0.05 gene effect, n = 9.
3.3. Mogat1 whole-body deletion
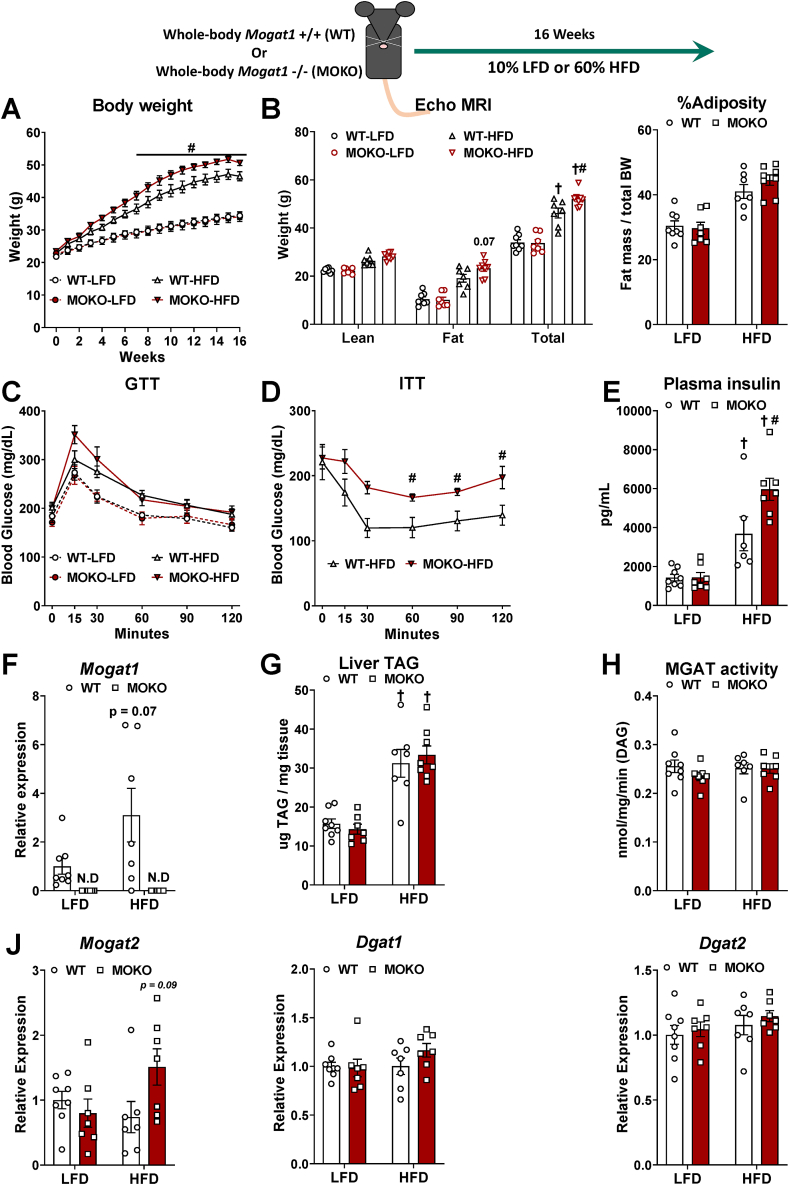
Mogat1 ASO treatment likely results in gene knockdown in multiple tissues [18,21]. Thus, we generated Mogat1 whole-body null mice (MOKO) to delete Mogat1 in all tissues (Figure 3). MOKO mice were outwardly normal on standard chow diet. Unexpectedly, when given an HFD, MOKO mice gained more total body weight than littermate wild-type (WT) control mice (Figure 3A,B). The heterozygous littermates had an intermediate phenotype (data not shown). The increase in weight gain was not attributed to increased adiposity as evidenced by fat mass and percent adiposity in ECHO MRI and individual fat pad weights and thus may be due to larger body size (Figure 3B and Supplemental Fig. 1). Glucose tolerance was not affected by Mogat1 knockout (Figure 3C). However, HFD-fed MOKO mice had reduced insulin tolerance and increased plasma insulin concentrations, which could be indicative of insulin resistance (Figure 3D,E). Mogat1 gene expression was undetectable in livers of MOKO mice (Figure 3F) or in any other tissue analyzed (data not shown). MOKO mice had similar TAG content and MGAT activity (Figure 3G,H). Similar to the liver-specific knockout models, MOKO mice had no increases in hepatic Mogat2, Dgat1, and Dgat2 (Figure 3J).
Figure 3.
Whole-body deletion of Mogat1 causes weight gain and insulin intolerance on an HFD. Male wild-type (WT) and littermate Mogat1 whole-body knockout (MOKO) mice were fed an LFD or an HFD starting at 8 weeks of age for 16 weeks. Mice were fasted for 4 h prior to sacrifice and tissue collection. A: Mogat1 knockout mice gain (MOKO) more weight on an HFD than littermate WT controls. B: ECHO MRI indicates MOKO mice have increased whole body mass, while compared to WT controls. C: Glucose tolerance (1 g/kg lean mass) was not significantly changed in MOKO mice. D,E: HFD-fed MOKO mice had significantly impaired insulin tolerance (1.5 U/kg lean mass) and plasma insulin levels compared to WT controls. F: Mogat1 gene expression was not detectable in MOKO mice on either diet. G: Liver TAG was increased by HFD but unaffected by genotype. H: MGAT activity was unaffected by either diet or genotype. Data are expressed as means ± S.E.M, and two-way ANOVA with Sidak's or Tukey's multiple comparison tests were performed where appropriate, #p < 0.05 gene effect, †p < 0.05 diet effect. For the ITT, two-tailed Student's t-test were performed, #p < 0.05 gene effect n = 6–7.
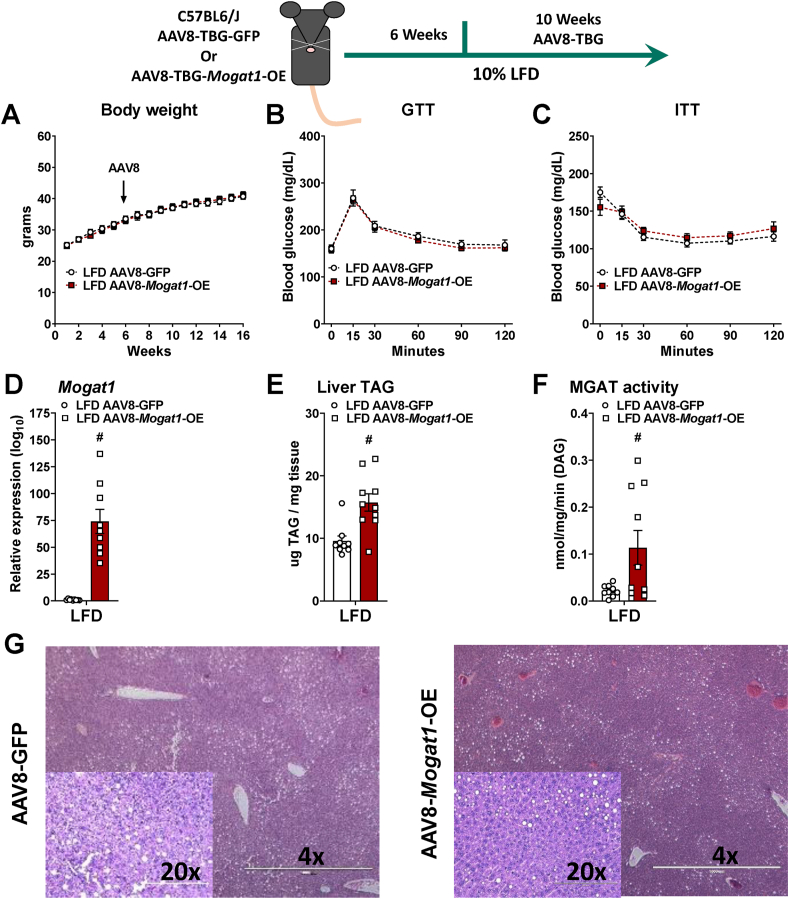
3.4. Hepatic Mogat1 overexpression
We next aimed to determine whether overexpression of hepatic Mogat1 was sufficient to promote hepatic steatosis, insulin resistance, and deleterious effects on systemic glucose metabolism. We fed C57BL6/J mice an LFD for six weeks then injected them with AAV8 expressing either GFP or mouse Mogat1 under control of the hepatocyte-specific TBG promoter and continued them on diet for an additional ten weeks (Figure 4). Mogat1 overexpressing (OE) mice gained similar weight as the GFP control-treated mice (Figure 4A). After 16 weeks (ten weeks of overexpression), there was no difference in glucose or insulin tolerance between groups (Figure 4B,C). Mogat1 gene expression was significantly increased by the administration the AAV8-Mogat1 (Figure 4D). MGAT1 overexpression increases liver TAG and MGAT activity. (Figure 4E,F). Despite the increases in MGAT activity and hepatic TAG content, Mogat1 overexpression did not alter liver histology or markers of NAFLD progression, including liver cholesterol, NAFLD score, or plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Figure 4G and Supplemental Fig. 2).
Figure 4.
Hepatic Mogat1 overexpression increases liver TAG and MGAT activity in mice fed an LFD. Male C57BL6/J mice were fed an LFD at 8 weeks of age. After 6 weeks of diet mice were injected (retro-orbital) with AAV8-TBG-GFP- or AAV8-TBG-Mogat1 (2 × 1011 GC per mouse) and remained on the diet for an additional 10 weeks. Mice were fasted for 4 h prior to sacrifice and tissue collection. A: AAV8-Mogat1-OE did not affect body weight. B,C: Mogat1 overexpression did not impair glucose or insulin tolerance. D: Mogat1 gene expression was significantly increased in AAV8-Mogat1-OE treated mice. E,F: Mogat1 overexpression increased both liver TAG and MGAT activity. G: H&E staining of liver sections reveals similar histology between groups. Data are expressed as means ± S.E.M., and two-tailed Student's t-test were performed, #p < 0.05 gene effect, n = 8–10.
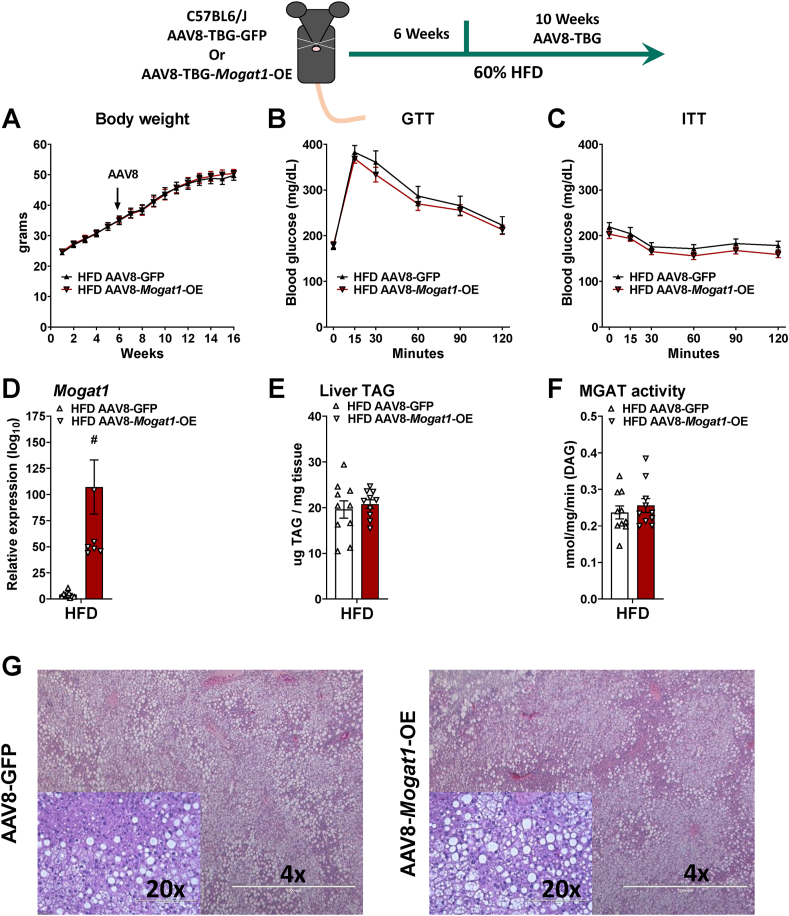
Utilizing the same overexpression regimen, we investigated whether Mogat1 overexpression exacerbates hepatic steatosis and insulin resistance during HFD feeding (Figure 5). Similar to the LFD-fed mice, Mogat1 overexpression did not affect body weight or glucose and insulin tolerance in HFD-fed mice (Figure 5A–C). Mogat1 gene expression was significantly increased by the AAV8-Mogat1-OE treatment (Figure 5D). Unlike the LFD-fed mice, hepatic TAG and MGAT activity were not different between groups (Figure 5E,F). Similarly, Mogat1 overexpression did not alter liver histology or markers of NAFLD progression in HFD-fed mice (Figure 5G and Supplemental Fig. 2). Thus, we concluded that hepatic Mogat1 overexpression is sufficient to increase hepatic triglyceride accumulation on an LFD, but not insulin resistance or systemic dysregulation of glucose metabolism.
Figure 5.
Hepatic Mogat1 overexpression increases liver TAG and MGAT activity in mice fed an HFD. Male C57BL6/J mice were fed HFD at 8 weeks of age. After 6 weeks of diet, mice were injected (retro-orbital) with AAV8-TBG-GFP- or AAV8-TBG-Mogat1 (2 × 1011 GC per mouse) and remained on the diet for an additional 10 weeks. Mice were fasted for 4 h prior to sacrifice and tissue collection. A: AAV8-Mogat1-OE did not affect body weight. B,C: Mogat1 overexpression did not impair glucose or insulin tolerance. D: Mogat1 gene expression was significantly increased in AAV8-Mogat1-OE treated mice. E,F: Mogat1 overexpression did not affect liver TAG and MGAT activity. G: H&E staining of liver sections reveals similar histology between groups. Data are expressed as means ± S.E.M. and two-tailed Student's t-test were performed, #p < 0.05 gene effect, n = 8–10.
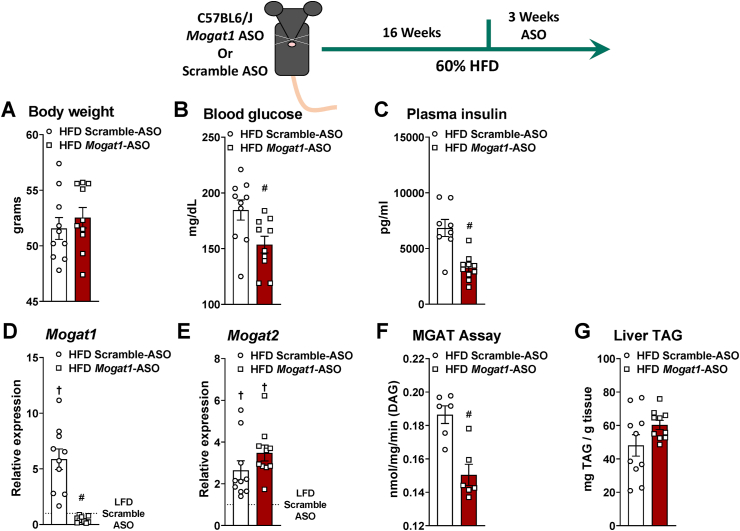
3.5. Mogat1 ASO treatment improves hepatic insulin sensitivity on HFD
To confirm our previous reports indicating that Mogat1 suppression by ASO improves hepatic insulin sensitivity [17,18], we fed C57BL6/J mice with an HFD. After 16 weeks, mice were weight-matched and randomized to receive ASOs targeting either Mogat1 or scramble control (Figure 6). As previously described [17], Mogat1 ASO treatment did not affect body weight but reduced blood glucose and plasma insulin levels on an HFD (Figure 6A–C). Mogat1 and Mogat2 expression was increased by the HFD and Mogat1 ASO treatments suppressed Mogat1 without affecting Mogat2 (Figure 6D,E). MGAT activity was reduced without affecting liver TAG content (Figure 6F,G). Moreover, glucose tolerance was improved in mice using two different Mogat1 targeting ASO sequences in HFD fed mice (Supplemental Fig. 3). Together, these data confirm our previous reports that multiple Mogat1 ASO sequences improve hepatic metabolism during an HFD challenge.
Figure 6.
Mogat1 antisense oligonucleotide (ASO) treatment improves insulin sensitivity in HFD fed mice. Male C57BL6/J mice were fed an HFD starting at 8 weeks of age. After 16 weeks of diet, mice were injected (intraperitoneally) twice weekly with ASOs targeted against Mogat1 or scramble control (25 mg/kg) for 3 weeks. Mice were fasted for 4 h prior to sacrifice and tissue collection. A: Body weight was not affected by Mogat1 ASO treatment. B,C: Mogat1 ASO treatment reduced blood glucose and plasma insulin levels in mice fed an HFD. Gene expression was normalized to LFD-fed mice given scramble control. D: Hepatic Mogat1 gene expression was increased in HFD-fed mice and significantly reduced by Mogat1 ASO treatment E: Mogat2 gene expression was increased in HFD-fed mice. F,G: Hepatic MGAT activity was significantly reduced by Mogat1 ASO treatment without affecting liver TAG. Data are expressed as means ± S.E.M., and two-tailed Student's t-test or one-way ANOVA with Tukey's multiple comparison test were performed where appropriate, #p < 0.05 ASO effect, †p < 0.05 diet effect; n = 5–10.
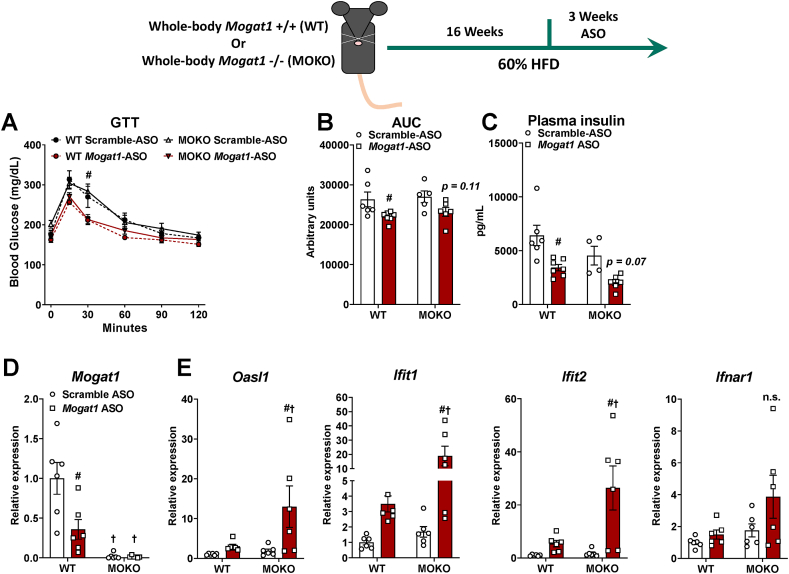
3.6. Mogat1 ASO treatment improves glucose tolerance in Mogat1 whole-body null mice
Given the inability to reproduce the insulin-sensitizing effects of Mogat1 ASOs in any of our genetic models, we tested whether Mogat1 ASO treatment improves glucose metabolism independent of Mogat1. We treated MOKO mice, fed an HFD for 16 weeks, with multiple ASOs targeting Mogat1 or scramble control (Figure 7 and Supplemental Fig. 4). Remarkably, Mogat1 ASO treatment improved glucose tolerance in both MOKO and WT mice as indicated by the area under the curve for WT and at 30 min post-injection for the MOKO mice (Figure 7A,B and Supplemental Figs. 4A and B). Mogat1 ASO treatment lowered plasma insulin concentrations in WT mice and caused insulin to trend lower in MOKO mice treated with Mogat1 ASO compared to control ASO (Figure 7C). Mogat1 expression was nearly undetectable in the livers of MOKO mice and was reduced by Mogat1 ASO only in WT mice (Figure 7D and Supplemental Fig. 4C).
Figure 7.
Mogat1 ASO treatment improves glucose tolerance in whole-body Mogat1 null mice on an HFD. Male wild-type (WT) and littermate Mogat1 whole-body knockout (MOKO) mice were fed an HFD starting at 8 weeks of age. After 16 weeks of diet, mice were injected (intraperitoneal) twice weekly with ASOs targeted against Mogat1 or scramble control (25 mg/kg) for 3 weeks. Mice were fasted for 4 h prior to sacrifice and tissue collection. A–C: Mogat1 ASO treatment improves glucose tolerance and AUC independently of Mogat1 expression and lowers plasma insulin in WT mice. D: Mogat1 gene expression was reduced by ASO treatment and nearly absent in MOKO livers. E: Gene expression markers of interferon type-1 alpha/beta signaling are increased in Mogat1 ASO treated MOKO mice on HFD. Data are expressed as means ± S.E.M, and two-way ANOVA with Sidak's or Tukey's multiple comparison test were performed where appropriate #p < 0.05 from scramble ASO †p < 0.05 from WT controls; n = 5–7.
Recently, McCabe et al. demonstrated that ASO targeting TTC39B non-specifically protects against diet-induced obesity and induces adipose tissue browning through activation of a type I interferon alpha/beta receptor 1 (IFNAR-1) in adipose tissue-derived macrophages even in TTC39B knockout mice [22]. Although we did not observe any effect on body weight associated with use of the Mogat1 ASO (Figure 6A), we found that expression of several key indicators of IFNAR-1 signaling (Oasl1, Ifit1, and IIfit2) were significantly increased in MOKO mice treated with Mogat1 ASOs (Figure 7E and Supplemental Fig. 4D). The Mogat1 ASO also tended to increase the expression of these genes in WT mice, while Ifnar1 gene expression was unchanged (Figure 7E and Supplemental Fig. 4D).
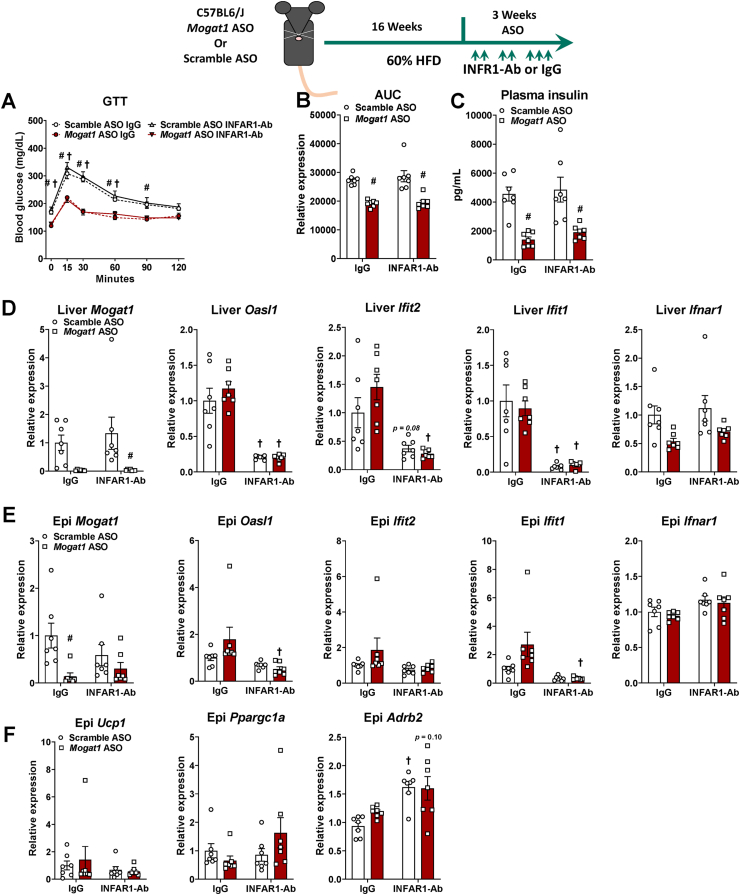
3.7. IFNAR-1 blockade does not prevent improvements in glucose homeostasis in Mogat1 ASO treated mice
To determine whether the increase in IFNAR-1 activation in response to Mogat1 ASO treatment drives the improvements in glucose metabolism, we injected ASO treated mice with a IFNAR-1 neutralizing antibody or IgG control during an HFD challenge (Figure 8) [22,23]. We repeated the previous observation that this Mogat1 ASO sequence improved glucose tolerance and lowered plasma insulin in HFD-fed mice (Figure 8A,B), but co-treatment with IFNAR-1 neutralizing antibody (INFAR1-Ab) did not block the improvements in glucose tolerance nor plasma insulin concentrations in Mogat1 ASO-treated mice (Figure 8A–C). Mogat1 ASO treatment suppressed liver Mogat1 gene expression in IFNAR-1 neutralizing antibody-treated mice (Figure 8D). Mogat1 ASO treatment did not significantly activate IFNAR-1-responsive genes in the livers of IgG-treated mice (Figure 8D) All of these genes were lowered by use of the INFAR1-Ab (Figure 8D). Mogat1 ASO treatment also suppressed Mogat1 gene expression in epididymal white adipose tissue (WAT) (Figure 8E) but did not increase most INFAR-1 responsive genes in adipose tissue (Figure 8E). Lastly, Mogat1 ASO treatment did not affect the expression of the WAT browning markers, Ucp1, Ppargc1a, or Arb2, which is in contrast to previous work (Figure 8F and ref [22]). In fact, Arb2 was increased in mice treated with INFAR1-Ab regardless of which ASO mice received (Figure 8F).
Figure 8.
Type I interferon alpha/beta receptor 1 neutralizing antibody (INFAR1-Ab) prevents inflammation without affecting glucose tolerance. Male C57BL6/J mice were fed an HFD starting at 8 weeks of age. After 16 weeks of diet, mice were injected (intraperitoneal) twice weekly with ASOs targeted against Mogat1 or scramble control (25 mg/kg) with an INFAR-1 neutralizing antibody (INFAR1-Ab) or IgG control (250 mg per mouse twice a week for weeks 1 and 2 and 500 mg per mouse 3 times for week 3). Mice were fasted for 4 h prior to sacrifice and tissue collection. A–C: Mogat1 ASO treatment improves glucose tolerance and lowers plasma insulin despite INFAR1-Ab treatment. D: Liver Mogat1 gene expression was reduced by Mogat1 ASO treatment in the INFAR1-Ab treated mice and liver gene expression markers of interferon signaling are lowered by INFAR1-Ab treatment. E: Epididymal Mogat1 gene expression was decreased by Mogat1 ASO treatment. Epididymal gene expression markers of interferon signaling were not increased by Mogat1 ASO treatment. F: Expression of adipose browning genes were not affected by Mogat1 ASO treatment. Data are expressed as means ± S.E.M and two-way ANOVA with Sidak's or Tukey's multiple comparison test where appropriate, #p < 0.05 from Scramble ASO †p < 0.05 from IgG controls; n = 7.
Alternatively, our second ASO sequence targeting Mogat1 had only modest effects on glucose tolerance and plasma insulin levels that were unaffected by the INFAR1-Ab treatment (Supplemental Figs. 5A–C). Similar reductions of Mogat1 expression were observed in both liver and adipose tissue. However, the second sequence induced IFNAR-1 signaling in both liver and adipose tissue of IgG-treated mice, and these affects were suppressed by INFAR1-Ab treatment (Supplemental Figs. 5D and E). Finally, the second sequence induced epididymal Upc1 expression (Supplemental Fig. 5F). These data indicate that multiple Mogat1 ASO treatments non-specifically improve glucose metabolism, and this effect is not mediated through the previously implicated mechanism of IFNAR-1 activation [22].
4. Discussion
Increased intrahepatic lipid content is tightly and mechanistically linked to development of insulin resistance and systemic metabolic abnormalities. Previous work by our group and others using RNA interference approaches has demonstrated that knocking down expression of Mogat1 in the livers of obese mice with hepatic steatosis dissociates liver fat from insulin resistance and improves glucose homeostasis [17,18,20,28]. In the present study, we knocked out Mogat1 specifically in hepatocytes, both chronically and acutely, and also generated a global Mogat1 knockout, but did not observe any improvements in glucose homeostasis or insulin sensitivity in diet-induced obese mice. Hepatic Mogat1 overexpression was sufficient to increase hepatic MGAT activity and TAG content, but only in LFD-fed mice, and did not result in insulin resistance or glucose intolerance. Although we reproduced our previous findings to demonstrate that multiple Mogat1 ASOs improve glucose tolerance in obese mice, we also conclusively demonstrate that these ASOs still improve metabolic homeostasis in mice with global Mogat1 deletion. Lastly, we confirm that these improvements were not due to increased activation of IFNAR-1 as recently reported for an ASO against TTC39B [22]. Collectively, these data suggest that Mogat1 ASO treatment improves glucose homeostasis and insulin sensitivity independently of knocking down Mogat1 expression and by mechanisms that remain to be elucidated.
The development of technologies for in vivo RNA interference through use of modified oligonucleotides has been a boon to hepatology and related disciplines. The ease of administration and liver-trophic nature of these designer oligonucleotides has allowed researchers to suppress the expression of a variety of genes in the liver and also led to clinically-approved approaches to treat metabolic disease [29,30]. Furthermore, in humans, unlike murine models, ASO treatment does not increase clinical indicators of liver and kidney injury [31] and next-generation ASO technology (GalNAc conjugation) has increased potency and reduced inflammation in the livers of mice, although their effects on off-target issues remains to be determined [21,32].
We were quite flummoxed when characterizing the phenotype of mice lacking Mogat1 in liver by the inability to phenocopy our previously reported phenotypes obtained using ASOs [17,18,26] or work by other groups using adenoviral-driven expression of shRNA [20,28]. We considered a number of possibilities to explain this. It is likely that genetic deletion of Mogat1 leads to compensatory changes in other enzymes that have MGAT activity, since ASO treatment leads to reduced MGAT activity in the liver [17], whereas MGAT knockout either acutely or chronically does not (Figure 1, Figure 2, Figure 3). Another possibility is that the Mogat1 ASO was mediating its effects on a tissue other than liver. Brandon et al. recently reported that that liver-specific KO of protein kinase C ε (PKCε) did not phenocopy previous ASO work conducted in rats and found instead that deletion of PKCε in adipose tissue, which is often targeted by ASOs as well, produced an insulin-sensitive phenotype [33,34]. However, our studies with adipocyte-specific Mogat1 KO mice (manuscript in preparation) or with the global MOKO mice did not reveal an insulin-sensitive phenotype. A caveat to this is that we did not assess the effects of a combined conditional Mogat1 knockout in adipocytes and liver, two tissues primarily targeted by the Mogat1 ASO, without deletion in other tissues that may have compensatory effects. This will require further consideration, but the observation that these ASOs have beneficial effects in MOKO mice suggests that the effects of the ASO is likely disconnected from the suppression of Mogat1 expression. Taken together with previous work, these studies demonstrate the importance of using rigorous and complementary controls when employing ASOs to study intermediary metabolism and insulin sensitivity.
It is now clear that Mogat1 ASOs elicit their effects even in the absence of Mogat1 expression. In this work and in previous studies, we have used multiple ASO sequences targeting Mogat1 with similar effects on glucose metabolism, and these effects do not appear to be due to silencing another gene from off-target interactions with RNA. The present results parallel another recent paper that showed that ASOs targeting TTC39B (T39) still elicited metabolic benefits in global T39 KO mice [22]. McCabe et al. [22] demonstrated in their model that ASO treatment activated an interferon signaling pathway as indicated by an induction of IFNAR-1 responsive genes (Oasl1, Ifit1, and Ifit2) that led to adipocyte browning and the mice to lose weight. Exactly how the interferon response is activated by ASO, how this occurs in the KO in the absence of target RNA, and why the scramble control ASO does not provoke the same response remains unclear. Although we did not observe weight loss in our studies, Mogat1 ASO treatment in MOKO mice stimulated the expression of Oasl1, Ifit1, and Ifit2 in liver. These responses were strikingly exaggerated in MOKO mice but in the WT mice, and we can only speculate that the lack of target RNA leads to higher non-hybridization dependent toxicity noted for many ASOs [35], while the scramble ASOs are designed to have minimal toxicities [21]. The activation of IFNAR-1 signaling may be consistent with our previous work demonstrating that Mogat1 ASO exacerbates hepatic inflammation on a diet that induces nonalcoholic steatohepatitis, including components of interferon signaling [18,22]. While IFNAR-1 signaling activates a multitude of signaling pathways that could plausibly affect glucose metabolism and insulin sensitivity [36], we were unable to prevent the improvements in glucose metabolism by blocking IFNAR-1 activation with an antibody. Thus, IFNAR-1 activation is not the mechanism for improved glucose and insulin tolerance in response to Mogat1 ASO. However, we should note that the effects of the neutralizing antibody were not assessed in the MOKO mice, which have a greater inflammatory response to Mogat1 ASO. It is possible that the antibody could have an effect in MOKO mice, but given the complete lack of effect in WT mice, we feel that it is unlikely that it will. The mechanism by which Mogat1 ASO elicits beneficial metabolic effects, even in the absence of Mogat1 RNA, will require further study.
Based on previous work with the Mogat1 ASOs [17,18,26], we were surprised to find that genetic deletion of Mogat1 in the liver did not affect hepatic MGAT activity. Our targeted allele deletes exon 4 of the Mogat1 gene, which encodes the catalytic domain (HPHG), and we have previously shown that this effectively deletes all MGAT1 protein [15]. Moreover, the lack of effect on hepatic MGAT activity is consistent with previous work by another group with an independently-generated KO mouse showing that global Mogat1 KO mice do not have deficits in hepatic MGAT activity [37]. Several acyltransferase enzymes exhibit MGAT activity, including MGAT2 and DGAT1, and the observation that genetic loss of MGAT1 in the liver did not affect MGAT activity could indicate that the other MGAT enzymes are the primary source of MGAT activity in the liver [[10], [11], [12], [13],15]. It is known that MGAT2 has a higher specific activity than MGAT1, and in many of the current studies, Mogat2 was upregulated by HFD feeding. Our previous work has shown that adipocyte-specific Mogat1 KO mice exhibit reduced adipose tissue MGAT activity [15], which may be consistent with the very low expression of Mogat2 in adipose tissue. While we did not detect increased expression of the known MGAT enzymes in any of our genetic models [[10], [11], [12], [13]], we cannot rule out post-transcriptional mechanisms, including post-translational modifications that enhance activity.
5. Conclusions
Here, we provide evidence that multiple Mogat1 ASOs improve whole-body metabolism through Mogat1-independent effects. Liver-specific Mogat1 ablation does not improve insulin resistance in HFD-fed mice, as observed with Mogat1 ASO treatments [17,18]. Moreover, we show novel evidence that whole-body Mogat1 deletion leads to insulin resistance in the context of diet-induced obesity. Finally, we provide preliminary evidence that Mogat1 ASOs improve glucose intolerance even in global Mogat1 KO mice. These findings also demonstrate that careful consideration should be given for using ASOs to target gene suppression, including use of genetic knockout models, in metabolic studies that could be affected by these off-target effects.
Conflict of interest
None declared.
Acknowledgments
The authors would like to thank Dr. Kyle McCommis at St. Louis University for his insight into off-target effects of ASO treatments, Dr. Eric Yen at the University of Wisconsin for his advice on MGAT biology, Valerie Blanc for assistance with membrane preparations, and Daniel Ferguson for editing the manuscript during quarantine. We also thank the Washington University School of Medicine Nutrition and Obesity Research Center for continued support in research. Work in the authors' lab was supported by grants from the NIH (R56 DK111735) and the American Diabetes Association (1-17-IBS-109) to BNF and core laboratories of Washington University School of Medicine Diabetes Research Center (P30 DK020579), Digestive Diseases Research Cores Center, (P30 DK052574), and the Nutrition Obesity Research Center (P30 DK056341).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101204.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Adams L.A., Lymp J.F., St Sauver J., Sanderson S.O., Lindor K.D., Feldstein A. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabbrini E., Yoshino J., Yoshino M., Magkos F., Luecking C.T., Samovski D. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125(2):787–795. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert J.E., Ramos–Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130(3):1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Coleman R.A., Lee D.P. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43(2):134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 8.Chon S.-H., Zhou Y.X., Dixon J.L., Storch J. Intestinal monoacylglycerol metabolism: developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. J Biol Chem. 2007;282(46):33346–33357. doi: 10.1074/jbc.M706994200. [DOI] [PubMed] [Google Scholar]
- 9.Han G.-S., Wu W.-I., Carman G.M. The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J Biol Chem. 2006;281(14):9210–9218. doi: 10.1074/jbc.M600425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J., Burn P., Shi Y. Properties of the mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J Biol Chem. 2003;278(28):25657–25663. doi: 10.1074/jbc.M302835200. [DOI] [PubMed] [Google Scholar]
- 11.Yen C.-L.E., Stone S.J., Cases S., Zhou P., Farese R.V. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8512–8517. doi: 10.1073/pnas.132274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen C.-L.E., Farese R.V. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J Biol Chem. 2003;278(20):18532–18537. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- 13.Cases S., Smith S.J., Zheng Y.W., Myers H.M., Lear S.R., Sande E. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(22):13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho S.-Y., Storch J. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am J Physiol Cell Physiol. 2001;281(4):C1106–C1117. doi: 10.1152/ajpcell.2001.281.4.C1106. [DOI] [PubMed] [Google Scholar]
- 15.Liss K.H.H., Lutkewitte A.J., Pietka T., Finck B.N., Franczyk M., Yoshino J. Metabolic importance of adipose tissue monoacylglycerol acyltransferase 1 in mice and humans. J Lipid Res. 2018 doi: 10.1194/jlr.M084947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall A.M., Kou K., Chen Z., Pietka T.A., Kumar M., Korenblat K.M. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J Lipid Res. 2012;53(5):990–999. doi: 10.1194/jlr.P025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall A.M., Soufi N., Chambers K.T., Chen Z., Schweitzer G.G., McCommis K.S. Abrogating monoacylglycerol acyltransferase activity in liver improves glucose tolerance and hepatic insulin signaling in obese mice. Diabetes. 2014;63(7):2284–2296. doi: 10.2337/db13-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soufi N., Hall A.M., Chen Z., Yoshino J., Collier S.L., Mathews J.C. Inhibiting monoacylglycerol acyltransferase 1 ameliorates hepatic metabolic abnormalities but not inflammation and injury in mice. J Biol Chem. 2014;289(43):30177–30188. doi: 10.1074/jbc.M114.595850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi Y., Suemitsu E., Kajimoto K., Sato Y., Akhter A., Sakurai Y. Hepatic monoacylglycerol O-acyltransferase 1 as a promising therapeutic target for steatosis, obesity, and type 2 diabetes. Mol Ther Nucleic Acids. 2014;3 doi: 10.1038/mtna.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J.H., Song S.J., Kim A., Choi Y., Seok J.W., Kim H.J. Suppression of PPARγ-mediated monoacylglycerol O-acyltransferase 1 expression ameliorates alcoholic hepatic steatosis. Scientific Reports. 2016;6:29352. doi: 10.1038/srep29352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geary R., Norris D., Bennett F., Rosie Y. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev. 2015;87:46–51. doi: 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 22.McCabe K.M., Hsieh J., Thomas D.G., Molusky M.M., Tascau L., Feranil J.B. Antisense oligonucleotide treatment produces a type I interferon response that protects against diet-induced obesity. Mol Metabol. 2020 doi: 10.1016/j.molmet.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson E.B., Yamada D.H., Elsaesser H., Herskovitz J., Deng J., Cheng G. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340(6129):202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liss K.H.H., Ek S.E., Lutkewitte A.J., Pietka T.A., He M., Skaria P. Monoacylglycerol acyltransferase 1 knockdown exacerbates hepatic ischemia-reperfusion injury in mice with hepatic steatosis. Liver Transplantation. 2021 doi: 10.1002/lt.25886. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCommis K.S., Chen Z., Fu X., McDonald W.G., Colca J.R., Kletzien R.F. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metabolism. 2015;22(4):682–694. doi: 10.1016/j.cmet.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutkewitte A.J., McCommis K.S., Schweitzer G.G., Chambers K.T., Graham M.J., Wang L. Hepatic monoacylglycerol acyltransferase 1 is induced by prolonged food deprivation to modulate the hepatic fasting response. J Lipid Res. 2019 doi: 10.1194/jlr.M089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao J., Cheng L., Shi Y. Catalytic properties of MGAT3, a putative triacylgycerol synthase. J Lipid Res. 2007;48(3):583–591. doi: 10.1194/jlr.M600331-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y.J., Ko E.H., Kim J.E., Kim E., Lee H., Choi H. Nuclear receptor PPARγ-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(34):13656–13661. doi: 10.1073/pnas.1203218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khvorova A., Watts J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nature Biotechnology. 2017;35(3):238–248. doi: 10.1038/nbt.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossor A.M., Reilly M.M., Sleigh J.N. Antisense oligonucleotides and other genetic therapies made simple. Practical Neurology. 2018;18(2):126–131. doi: 10.1136/practneurol-2017-001764. [DOI] [PubMed] [Google Scholar]
- 31.Crooke S.T., Baker B.F., Kwoh T.J., Cheng W., Schulz D.J., Xia S. Integrated safety assessment of 2’-O-methoxyethyl chimeric antisense oligonucleotides in NonHuman primates and healthy human volunteers. Molecular Therapy: J American Society of Gene Therapy. 2016;24(10):1771–1782. doi: 10.1038/mt.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y. Preclinical and clinical advances of GalNAc-decorated nucleic acid therapeutics. Molecular therapy. Nucleic Acids. 2017;6:116–132. doi: 10.1016/j.omtn.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel V.T., Liu Z.-X., Wang A., Beddow S.A., Geisler J.G., Kahn M. Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117(3):739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandon A.E., Liao B.M., Diakanastasis B., Parker B.L., Raddatz K., McManus S.A. Protein kinase C epsilon deletion in adipose tissue, but not in liver, improves glucose tolerance. Cell Metabolism. 2019;29(1):183–191. doi: 10.1016/j.cmet.2018.09.013. e7. [DOI] [PubMed] [Google Scholar]
- 35.Frazier K.S. Antisense oligonucleotide therapies: the promise and the challenges from a toxicologic pathologist's perspective. Toxicologic Pathology. 2015;43(1):78–89. doi: 10.1177/0192623314551840. [DOI] [PubMed] [Google Scholar]
- 36.Dodington D.W., Desai H.R., Woo M. JAK/STAT – emerging players in metabolism. Trends Endocrinol Metabol. 2018;29(1):55–65. doi: 10.1016/j.tem.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal A.K., Tunison K., Dalal J.S., Yen C.-L.E., Farese R.V., Horton J.D. Mogat1 deletion does not ameliorate hepatic steatosis in lipodystrophic (Agpat2−/−) or obese (ob/ob) mice. J Lipid Res. 2016;57(4):616–630. doi: 10.1194/jlr.M065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.