Abstract
Psychiatric diseases characterized by dysregulated risky decision making are differentially represented in males and females. The factors that govern such sex differences, however, remain poorly understood. Using a task in which rats make discrete trial choices between a small, “safe” food reward and a large food reward accompanied by varying probabilities of footshock punishment, we recently showed that females are more risk averse than males. The objective of the current experiments was to test the extent to which these sex differences in risky decision making are mediated by gonadal hormones. Male and female rats were trained in the risky decision-making task, followed by ovariectomy (OVX), orchiectomy (ORX), or sham surgery. Rats were then retested in the task, under both baseline conditions and following administration of estradiol and/or testosterone. OVX increased choice of the large, risky reward (increased risky choice), an effect that was attenuated by estradiol administration. In contrast, ORX decreased risky choice, but testosterone administration was without effect in either ORX or sham males. Estradiol, however, decreased risky choice in both groups of males. Importantly, none of the effects of hormonal manipulation on risky choice were due to altered shock sensitivity or food motivation. These data show that gonadal hormones are required for maintaining sex-typical profiles of risk-taking behavior in both males and females, and that estradiol is sufficient to promote risk aversion in both sexes. The findings provide novel information about the mechanisms supporting sex differences in risk taking and may prove useful in understanding sex differences in the prevalence of psychiatric diseases associated with altered risk taking.
Subject terms: Reward, Motivation
Introduction
Cognitive deficits, including impaired decision making, are associated with several psychiatric diseases such as substance use disorders (SUDs) and attention deficit hyperactivity disorder [1–3]. Consequently, research has focused on understanding how such impairments arise in pathological conditions. While great strides have been made through the use of animal models to understand decision making and its neural substrates, the majority of this research has been conducted exclusively in male subjects. Consideration of both sexes in these research questions is critical, as many of the diseases characterized by impaired decision making are disproportionately represented in males and females [4]. For example, in SUDs, males have overall higher rates of substance use, but females develop dependence more rapidly and are at greater risk for relapse [5, 6]. Thus, although previous animal studies have been invaluable in laying the foundation for understanding how decision making becomes compromised in psychiatric diseases, they are not representative of the entire population affected by these diseases. In addition, it is conceivable that potential sex differences in decision making and the mechanisms therein could account at least in part for sex differences in manifestation of psychiatric diseases such as SUDs. Thus, better understanding of sex differences in decision making linked to psychiatric disorders could enable development of tailored treatments for males and females.
To begin to address this gap in knowledge, considerable effort has been recently directed toward understanding how males and females differ in decision making using preclinical models [7]. For example, in a rodent task modeled after the Iowa Gambling Task, female rats make more disadvantageous choices and take longer to learn the optimal decision-making strategy compared to male rats [8, 9]. Using a “Risky Decision-making Task” (RDT), in which rats choose between a small, “safe” food reward and a large, “risky” food reward associated with variable probabilities of footshock punishment, our laboratory reported that females choose the large, risky option less than males, exhibiting a risk-averse phenotype [10]. This is consistent with recent work showing that males and females utilize distinct decision-making strategies when faced with the possibility of punishment in other settings [11, 12], such as foraging for food [13]. Collectively, these data suggest that relative to males, females are more risk averse in their choice behavior. The mechanisms underlying this phenotype, however, are unknown.
One possible explanation for sex differences in decision making is the different predominant gonadal hormones regulating behavior. Indeed, there is precedence for gonadal hormones modulating decision making in both sexes. For instance, prior work reported that administration of supraphysiological doses of testosterone to gonadally intact males modulated decision making differently depending on the cost associated with the decision: testosterone increased choice of larger rewards associated with greater risk of punishment or that require greater physical effort, but decreased choice of larger rewards associated with uncertainty of reward delivery [14–16]. Another study showed that in females, removal of ovarian hormones increased choice of large, high-effort options, and acute administration of estradiol reversed this effect [17], suggesting that estradiol is responsible for female phenotypic choice behavior. As additional support for this hypothesis, others have shown that greater preference for cocaine over a concurrent food option in females (compared to males) was abolished by ovariectomies, but could be restored to levels similar to that of intact females after estradiol benzoate administration [18]. Furthermore, orchiectomized males treated with estradiol benzoate exhibited this phenotypic female preference for cocaine over food [19]. Considered together, both testicular and ovarian hormones may contribute to decision-making behavior in males and females, respectively, and therefore could promote distinct sex-typical decision-making phenotypes (i.e., risk aversion in females and risk-seeking in males). To directly test this hypothesis, we examined the effects of testosterone and estradiol administration in gonadectomized (GDX) male and female rats on decision making involving risk of explicit punishment, with the prediction that removal of gonadal hormones would disrupt choice behavior and hormone administration would restore it.
Materials and methods
Subjects
Male (n = 27) and female (n = 31) Long-Evans rats were individually housed and maintained on a 12-h light/dark cycle. Rats were given ad libitum access to water, but were food restricted to 85% of their free feeding weight during testing. All procedures were conducted in accordance with the University of Florida Institutional Animal Care and Use Committee and adhered to NIH guidelines.
Overview of experimental design
In all experiments, rats underwent surgery (GDX or sham) after reaching stable baseline performance in the RDT.
Experiment 1 determined how gonadal hormones modulate risky decision making in female rats. Females were trained in the RDT and the effects of ovariectomy (OVX) on choice behavior were evaluated. After 3 weeks, they received 7 days of estradiol benzoate (EB) treatment to assess the impact of this gonadal hormone on choice behavior. Upon conclusion of RDT testing, rats were tested on a progressive ratio (PR) schedule of reinforcement for 6 days, after which 7 days of EB treatment began again while PR testing continued. On the final day of the EB dose regimen during PR testing, they were tested for shock reactivity.
Experiment 2 determined how gonadal hormones modulate risky decision making in male rats. In the first cohort, males (sham, n = 7; ORX, n = 6) were trained in the RDT and the effects of orchiectomy (ORX) on choice behavior were evaluated. They subsequently received 7 days of testosterone (T) treatment beginning 2.5 weeks after surgery to assess the impact of this gonadal hormone on choice behavior. A second cohort of males (sham, n = 7; ORX, n = 7) underwent the same procedures as the first cohort, but after T administration, they were treated with EB (for the same duration as that used for other hormone manipulations) to assess its impact on risky choice. Subsequently, males were tested on a PR schedule of reinforcement for 6 days, after which they were tested for shock reactivity. This was followed by another 7 days of EB treatment during PR testing.
Surgery
Detailed descriptions of surgical procedures are found in Supplementary information. Briefly, rats were anesthetized with isoflurane and underwent either OVX, ORX, or sham surgeries. Rats recovered for one week before food restriction in preparation for behavioral testing. Because ORX and OVX can affect food intake and body weight, weights were recorded throughout the duration of the experiments.
Risky decision-making task (RDT)
Testing was conducted in operant chambers equipped with two retractable levers flanking a centrally located food trough. Rats were initially shaped to perform the basic task components as described previously [10, 20, 21], followed by training in the RDT. In this task, rats were presented with either one lever (forced choice trials) or two levers (free choice trials). A press on one of the levers yielded a small, safe food reward (one pellet) whereas a press on the other yielded a large food reward (two pellets) accompanied by a possible 1 s footshock. The probability of footshock delivery (0, 25, 50, 75, 100%) was specific to each of the five blocks of trials in each session. Previous work shows that performance in the RDT recapitulates many aspects of human risk-taking behavior, such as individual differences in choice preference [20, 21]. Corroborating findings in human studies [22–24], there are also robust sex differences in the RDT, with females exhibiting more risk-averse behavior than males [10]. Importantly, these sex differences are not due to differences in footshock perception resulting from different body weights or due to differences in food motivation levels during decision making. Hence, the RDT is a well-validated model in which to investigate how gonadal hormones modulate male and female phenotypic risk taking.
Progressive ratio (PR) schedule of reinforcement
To assess motivation to work for food, rats were tested on a PR schedule of reinforcement in which the number of lever presses required to earn a food pellet increased across a test session. The number of lever presses at which rats ceased lever pressing (i.e., their breakpoint) was the primary outcome measure, serving as a measure of motivation to work for food.
Determination of shock reactivity thresholds
Shock sensitivity in females and males was assessed using an “up-and-down method” wherein shocks were systematically increased and decreased to identify the threshold at which a flinch was elicited [25, 26].
Hormone administration
Estradiol benzoate [β-estradiol 3-benzoate; Sigma-Aldrich; 20 µg) and testosterone propionate (Sigma Aldrich; 125 µg, 500 µg) were dissolved in sesame oil and administered subcutaneously (0.1 mL) ~30 min following behavioral testing every day for 7 days. The dose of EB was chosen because it is within the range of doses that produce levels of estradiol equal to those observed in the late stage of proestrus in females [27, 28]. Further, a similar dose of EB is effective in decreasing cocaine-seeking behavior in OVX females (compared to OVX females without EB) [29]. Note that only one dose was used for EB to ensure that hormone treatment did not extend far beyond the period in which OVX rats are sensitive to EB administration [30]. Because prior studies show that the same dose of EB can result in equivalent effects on behavior in females and males [19, 31, 32], the same dose of EB used for females was used for males. The doses of T were chosen based on previous studies showing that T administration at these doses results in circulating T levels within the physiological range [33–35] and is effective in improving working memory in ORX males [33, 34]. Hormones were administered in a randomized, counterbalanced order such that each rat underwent a 7-day regimen of vehicle and the hormone doses, with 7 days between successive injection regimens.
In females, vaginal lavages were conducted daily throughout each hormone injection regimen as well as during each intervening washout session to confirm that their hormonal status matched their hormone treatment condition [29, 36]. In males, the presence (or absence during washout sessions) of circulating estradiol was verified by collecting venous tail blood on the third day of each hormone injection regimen and on the fourth day of each washout session. Venous tail blood was also sampled during each regimen of T injections and during intervening washout sessions. See Supplementary information for additional details and results of hormone assays.
Data analyses
Detailed descriptions of analyses for each experiment are in Supplementary information. In all experiments involving the RDT, the primary variable of interest was choice of the large, risky reward (risk taking or risky choice). A p value ≤ 0.05 was considered statistically significant. Repeated-measures analyses of variance (ANOVA) were used to determine the impact of GDX on risky choice. In the case of significant changes in risky choice, additional trial-by-trial analyses were conducted to assess whether such changes were due to alterations in the degree to which feedback from prior trials affected future choices (win-stay or lose-shift behavior). If parent ANOVAs revealed significant main effects or interactions, additional repeated-measures ANOVAs were conducted to identify the sources of significance. Independent samples t-tests were used to analyze group differences in win-stay or lose-shift behavior. Similar analyses were used to detect baseline group differences in lever pressing on the PR and shock reactivity thresholds.
To analyze the effects of hormone administration, choice performance was averaged across 6 days, starting with the first day on which physiological measures of exogenous hormones were detected (day 3; via vaginal lavages in females and ELISA assays in males) and ending with the day following the last hormone injection. This ensured that the analysis was restricted to the test days on which hormone status was congruent with treatment group. The effects of hormone administration were analyzed with a three-factor repeated-measures ANOVA, with dose and block as the within-subjects factors and group as the between-subjects factor. If this parent ANOVA revealed a significant effect of dose or any significant interactions, separate, independent repeated-measures ANOVAs were conducted to identify which hormone dose differed from vehicle and whether these effects differed between groups. Another three-factor repeated-measures ANOVA was used to analyze the effects of hormone administration on win-stay or lose-shift behavior, with dose and trial type (e.g., win-stay, lose-shift) as within-subjects factors and group as the between-subjects factor. Paired samples t-tests were used to analyze the effects of EB on PR responding and shock reactivity thresholds within each group (i.e., Sham, ORX, OVX).
Results
As expected based on previous work [10], comparison of male and female rats’ performance prior to surgery revealed greater risky choice in males than females [main effect of sex, F(1,57) = 9.0, p < 0.01, ηp2 = 0.14; sex X block interaction, F(1,57) = 5.20. p = 0.03, ηp2 = 0.08; Fig. 1].
Fig. 1. Sex differences in risky decision making.
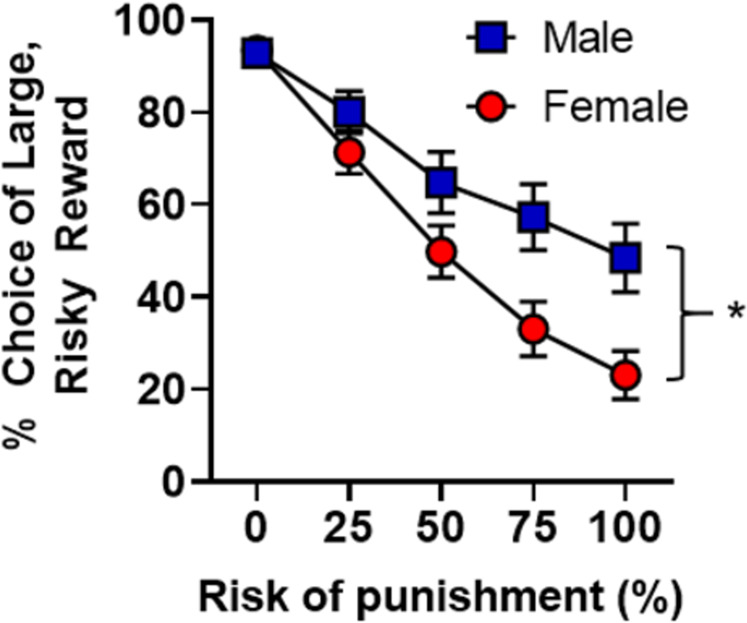
When pre-surgery stable behavior was compared between males (n = 27) and females (n = 31), significant sex differences emerged whereby males chose the large, risky reward significantly more than females. Data are represented as the mean ± SEM percent choice of the large, risky reward. An asterisk indicates statistical significance.
Effects of gonadectomies in males and females on risky choice
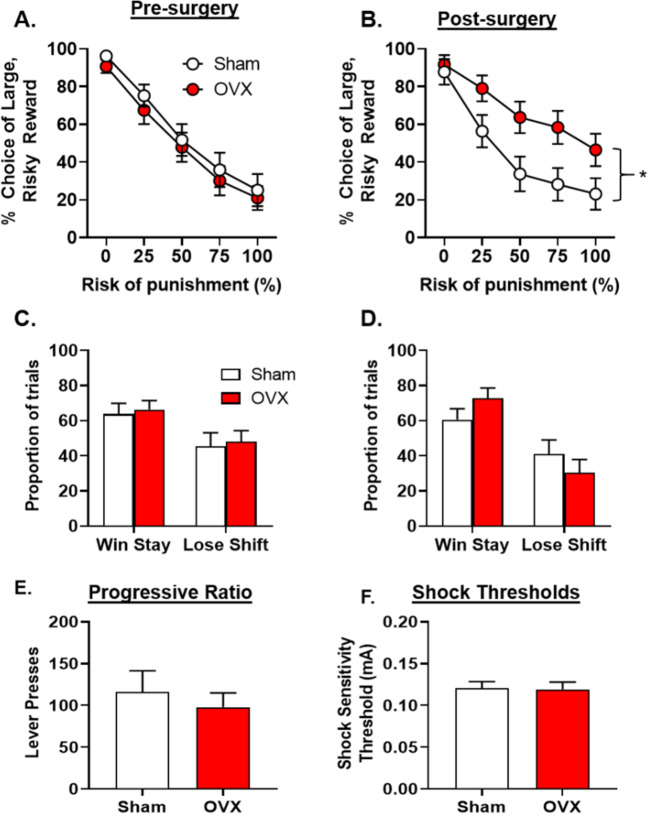
OVX (n = 16), but not sham females (n = 15), displayed a significant increase in risky choice compared to their pre-surgery performance [Fig. 2a, b; time X group, F(1,29) = 5.62, p = 0.03, ηp2 = 0.16; time, F(1,29) = 0.48, p = 0.50, ηp2 = 0.02; group, F(1,29) = 1.66, p = 0.21, ηp2 = 0.05; time × group X block, F(4,116)=1.54, p = 0.20, ηp2 = 0.05]. Follow-up analyses revealed that after surgery, OVX females chose the large, risky reward significantly more than sham females [group, F(1,29) = 5.13, p = 0.03, ηp2 = 0.15; group X block, F(4,116) = 2.76, p = 0.03, ηp2 = 0.09].
Fig. 2. Effects of ovariectomies on risky decision making in females.
a Prior to surgery, there were no differences in choice of the large, risky reward between female rats that would go on to receive sham (n = 15) or ovariectomy (OVX; n = 16) surgeries. b After surgery, there was a significant increase in choice of the large, risky reward in OVX females relative to sham females. c Prior to surgery, there were no differences in win-stay or lose-shift performance between rats that would go on to receive sham or OVX surgeries. d After surgery, there were no differences in win-stay or lose-shift performance between sham and OVX females. e There were no differences in the number of lever presses between OVX and sham females on a progressive ratio (PR) schedule of reinforcement. f There were no differences in shock sensitivity between OVX and sham females. Data are represented as the mean ± SEM percent choice of the large, risky reward (a, b), proportion of trials (c, d), number of lever presses (e) or shock sensitivity thresholds (f). An asterisk indicates statistical significance.
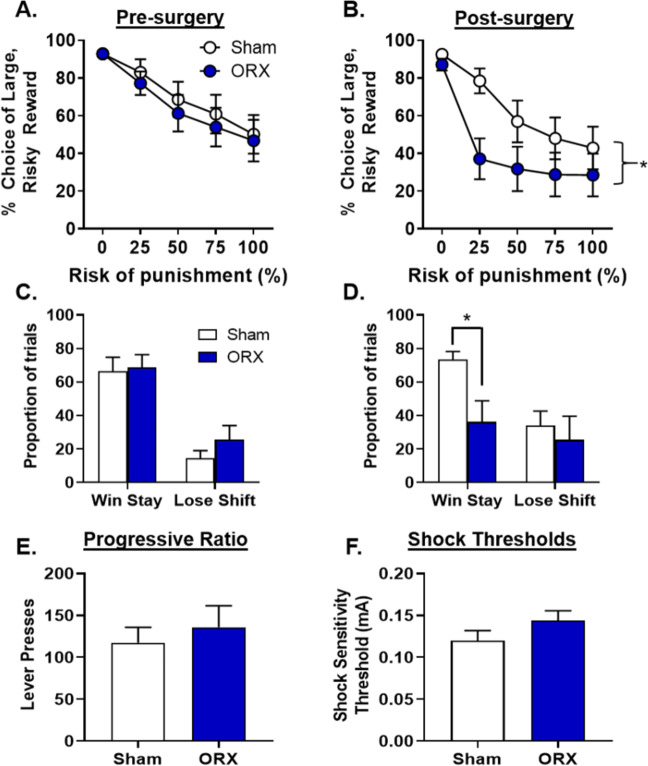
In males, relative to pre-surgery baseline, there was a significant decrease in risky choice in both ORX (n = 14) and sham [n = 13; Fig. 3a, b; time, F(1,25) = 4.53, p = 0.05, ηp2 = 0.15; group, F(1,25) = 2.25, p = 0.15, ηp2 = 0.08; time X group, F(1, 25) = 1.21, p = 0.28, ηp2 = 0.05; time X group X block, F(4,100) = 1.62, p = 0.18, ηp2 = 0.06] groups. Despite the overall change in baseline risky choice in both groups, ORX males chose the large, risky reward significantly less than sham males during post-surgery performance [group, F(1,25) = 2.88, p = 0.10, ηp2 = 0.10; group X block, F(4,100) = 3.74, p < 0.01 ηp2 = 0.13]. Consistent with this, ORX males were less likely than sham males to continue to choose the large, risky reward after receiving a large, unpunished reward in the previous trial [i.e., less win-stay behavior; t(22) = −2.91, p < 0.01; Fig. 3d].
Fig. 3. Effects of orchiectomies on risky decision making in males.
a Prior to surgery, there were no differences in choice of the large, risky reward between male rats that would go on to receive sham (n = 14) or orchiectomy (ORX; n = 13) surgeries. b After surgery, there was a significant decrease in choice of the large, risky reward in ORX males relative to sham males. c Prior to surgery, there were no differences in win-stay or lose-shift performance between rats that would go on to receive sham or ORX surgeries. d After surgery, there was a significant reduction in win-stay behavior in ORX rats compared to sham rats. e There were no differences in the number of lever presses between ORX and sham males on a PR schedule of reinforcement. f There were no differences in shock sensitivity between ORX and sham males. Data are represented as the mean ± SEM percent choice of the large, risky reward (a, b), proportion of trials (c, d), number of lever presses (e), or shock sensitivity thresholds (f). An asterisk indicates statistical significance.
To address the possibility that GDX-induced changes in risky choice were due to alterations in shock reactivity or food motivation, shock reactivity thresholds and performance on a PR schedule of reinforcement were tested in subsets of rats. There were no effects of GDX on PR in female [t(21) = −0.55, p = 0.58; Fig. 2e] or male [t(12) = 0.59, p = 0.57] rats (Fig. 3e). Similarly. GDX had no effect on the shock intensity at which a flinch response was elicited in either female [t(29) = −0.12, p = 0.91; Fig. 2f] or male [t(12) = 1.54, p = 0.15; Fig. 3f] rats.
Effects of estradiol administration on risk taking in females
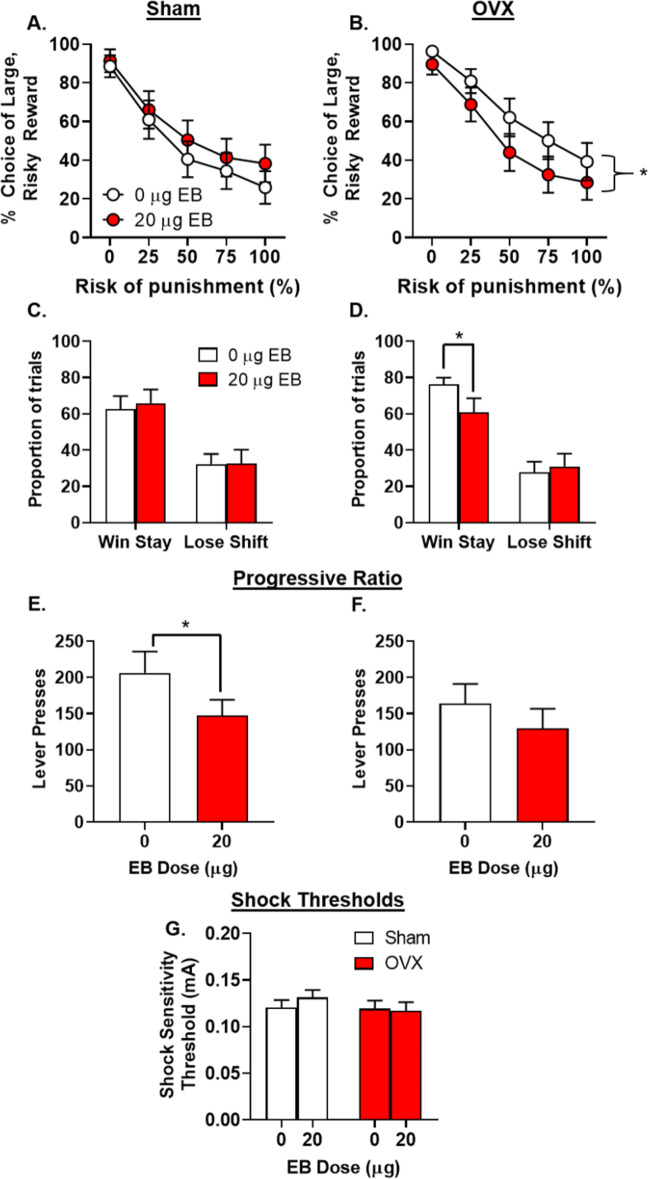
Previous work showed that OVX-induced changes in effort-based decision making are attenuated by EB [17], suggesting that EB might also mitigate the effects of OVX on risky choice. Females were given both EB and vehicle for 7 consecutive days, using a within-subjects design. While there was neither a main effect of dose [F(1,29) = 0.52, p = 0.18, ηp2 = 0.02] nor a dose X group X block interaction [F(4,116) = 1.03, p = 0.39, ηp2 = 0.03], there was a significant dose X group interaction [F(1,29) = 5.24, p = 0.03, ηp2 = 0.15; Fig. 4]. Additional analyses revealed that EB decreased risky choice in OVX [F(1,15) = 5.81, p = 0.03, ηp2 = 0.28; Fig. 4b], but not sham [F(1,14) = 0.98, p = 0.34, ηp2 = 0.07; Fig. 4a], females. Analyses of win-stay behavior revealed a significant interaction between dose and group [F (1, 26) = 4.17, p = 0.05, ηp2 = 0.14], which was driven by an EB-induced decrease in win-stay behavior in OVX [t(14) = 2.36, p = 0.03; Fig. 4d], but not sham [t(12) = −0.35, p = 0.74; Fig. 4c], females.
Fig. 4. Effects of estradiol benzoate administration on risky decision making in sham and ovariectomized females.
a There were no effects of estradiol benzoate (EB) administration on choice of the large, risky reward in sham females (n = 15). b EB administration decreased choice of the large, risky reward in ovariectomized (OVX) females (n = 16). c There were no effects of EB administration on win-stay or lose-shift performance in sham females. d EB administration reduced win-stay behavior in OVX females, but had no effect on lose-shift behavior. EB administration reduced lever pressing for food in sham (e) but not OVX (f) females. g There were no effects of EB in either OVX or sham female rats. Data are represented as the mean ± SEM percent choice of the large, risky reward (a, b), proportion of trials (c, d), number of lever presses (e, f), or shock sensitivity thresholds (g). An asterisk indicates statistical significance.
Effects of testosterone administration on risk taking in males
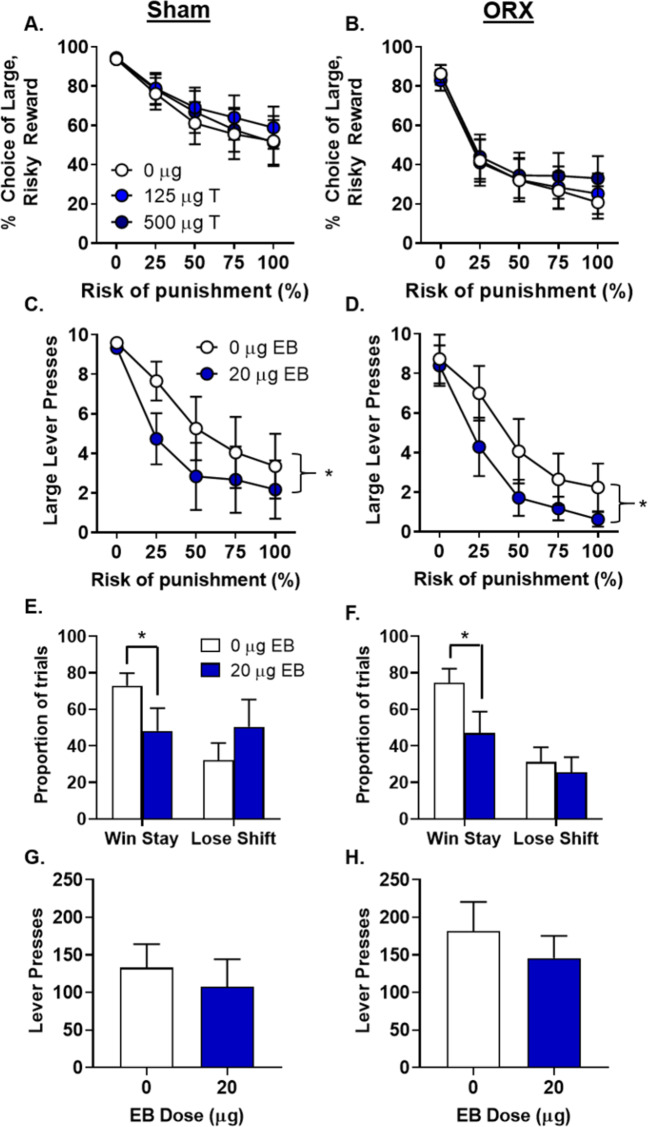
Previous work showed that chronic administration of a high dose of T to intact males increases risk taking in the RDT [14]. Although the dose used in this previous study resulted in supraphysiological levels of T, it does suggest that, in combination with the fact that removal of endogenous T affected RDT performance, exogenous T administration could attenuate ORX-induced decreases in risky choice and potentially further increase risky choice in sham males. Consequently, males were treated with T or vehicle for 7 consecutive days. Surprisingly, there was no effect of T in either the ORX or sham males [dose, F(2,50) = 0.42, p = 0.66, ηp2 = 0.02; dose X group, F(2,50) = 0.51, p = 0.66, ηp2 = 0.02; dose X group X block, F(8,200) = 0.66, p = 0.72, ηp2 = 0.03; Fig. 5a, b].
Fig. 5. Effects of testosterone and estradiol on risky decision making in sham and orchiectomized males.
a There were no effects of testosterone (T) on choice of the large, risky reward in sham males (n = 14). b There were no effects of T on choice of the large, risky reward in orchiectomized (ORX) males (n = 13). c Estradiol benzoate (EB) administration caused a significant reduction in lever presses to obtain the large, risky reward in sham males (n = 7). d EB administration caused a significant decrease in lever presses for the large, risky reward in ORX males (n = 7). e EB administration reduced win-stay behavior in sham males, but had no effect on lose-shift behavior. f EB administration reduced win-stay behavior in ORX males, but had no effect on lose-shift behavior. g, h There was no effect of EB administration on lever pressing in either sham (g; n = 7) or ORX (h; n = 7) males. Data are represented as the mean ± SEM percent choice of the large, risky reward (a–d), proportion of trials (e, f), or number of lever presses (g, h). An asterisk indicates statistical significance.
Effects of estradiol administration on risk taking in males
Although risky choice in male rats was not responsive to exogenous T, it is possible that it could be responsive to EB. Males are sensitive to estradiol’s effects on memory independent of gonadal status [37–40], and EB administration to ORX males alters cocaine-seeking behavior [19]. Consequently, a subset of males (ORX, n = 7; sham, n = 7) was given EB or vehicle for 7 consecutive days. Initial analyses did not reveal a main effect of EB [F(1,12) = 2.55, p = 0.14, ηp2 = 0.18] nor interactions between EB and group [F(1,12) = 0.09, p = 0.77, ηp2 < 0.01] or between EB, group and block [F(4,48) = 0.12, p = 0.98, ηp2 = 0.01]. Administration of EB did, however, cause a significant increase in omissions during free choice trials [F(1,12) = 4.93, p = 0.05, ηp2 = 0.29], suggesting that the percent choice of large, risky rewards may not be the optimal measure to assess effects of EB on choice performance. When the number of lever presses for the large, risky reward was used in the analysis, a trend toward a main effect of EB [F(1,12) = 3.29, p = 0.09, ηp2 = 0.22] and a significant EB X block interaction [F(4,48) = 2.80, p = 0.03, ηp2 = 0.19] emerged, which behaviorally manifested as a significant decrease in large, risky lever presses in both ORX and sham males [i.e., no main effect of group, F(1,12) < 0.01, p = 0.97, ηp2 < 0.01; Fig. 5c, d]. Similar to the effects of EB in OVX females, there was a significant decrease in win-stay behavior in both ORX and sham males [dose, F(1,11) = 6.01, p = 0.03, ηp2 = 0.35; group, F(1,11) < 0.01, p = 0.98, ηp2 < 0.01; dose X group, F(1,11) = 0.02, p = 0.89, ηp2 < 0.01; Fig. 5e, f].
Effects of estradiol administration on shock sensitivity and food motivation
One interpretation of the EB-induced decreases in risky choice is that EB decreased motivation for the large reward, irrespective of the associated risk. Alternatively, EB may render rats more sensitive to footshock, causing them to shift their preference to the small, safe reward. To address these possibilities, differences in lever pressing in the PR (GDX and sham male and female rats) and shock reactivity thresholds (females only) were assessed under EB conditions. While there was no effect of EB on lever pressing for food in OVX females [t(10) = 1.14, p = 0.30, ηp2 = 0.22; Fig. 4f], EB significantly reduced lever pressing for food in sham females [t(11) = 2.67, p = 0.02; Fig. 4e]. This indicates that, although EB may reduce motivation to work for food (as evidenced in sham females), such changes in motivation likely do not explain the EB-induced decreases in risky choice in OVX females as EB had no effect on PR responding in this group. There was no effect of EB on shock reactivity in either OVX [t(15) = 0.17, p = 0.87; Fig. 4g] or sham females [t(14) = −0.80, p = 0.44]. In males, there was no effect of EB on lever pressing for food in either sham [t(6) = 0.96, p = 0.37; Fig. 5g] or ORX [t(6) = 0.78, p = 0.46; Fig. 5h] males.
Discussion
Previous work in both rodents and humans suggests that females are more risk-averse than males [10, 11, 41, 42]. The results of the current study show that these sex-typical patterns of risky decision making are mediated at least in part by gonadal hormones in both sexes, as OVX increased risk taking in females and ORX decreased risk taking in males. Critically, neither of these effects were due to differences in shock reactivity or food motivation. EB attenuated the effects of OVX on choice behavior in females, further supporting a role for ovarian hormones in female-typical risk aversion. In contrast, T at physiological doses did not affect choice behavior in either ORX or sham males. EB administration did significantly reduce risky choice in males, however, irrespective of gonadal status. Collectively, these data establish a causal role for gonadal hormones in regulating risk taking and, in particular, highlight estradiol’s ability to promote risk aversion in both sexes.
Role of ovarian hormones in female-typical risk taking
Consistent with our predictions, OVX significantly increased risky choice, an effect that was attenuated by EB administration. Interestingly, the EB-induced decrease in risky choice in OVX females was accompanied by a decrease in win-stay behavior, a measure sometimes used as a proxy for reward sensitivity [43]. It is important to note, however, that this decrease in win-stay behavior is not equivalent to a decrease in food motivation, as EB did not impact PR performance in OVX females. The finding that ovarian hormones modulate choice behavior is consistent with previous work showing that OVX increases preference for large, high effort, over small, low effort rewards and that EB attenuates this effect [17]. In further support of estradiol contributions to female risk aversion, a neuroimaging study in human subjects demonstrated an indirect relationship between estradiol levels and risk taking insofar as decreased risk taking was associated with reduced nucleus accumbens activity, but only in females with high levels of circulating estradiol [44]. Collectively, these findings indicate that estradiol plays an important role in modulating choice behavior in females.
There are, however, studies that report conflicting results. For example, a study in rats reported ovarian hormones do not mediate risk aversion in females, although this was in the context of foraging behavior and did not involve explicit choices between safe and potentially harmful options [13]. Additionally, a more recent study showed that, compared to OVX females treated with EB, OVX females decreased their risky choice in a probability discounting task [45]. This discrepancy with the current findings, however, is likely due to differences in the nature of the risks involved in the decision-making tasks. This is a critical distinction given that the neuropharmacology of decisions involving risk of explicit punishment (current study) differs substantially from that of decisions involving risk of reward omission (probability discounting) [46–48]. Considered together, these findings suggest that ovarian hormones modulate female decision making, but their role may differ depending on the type of decision costs involved.
It is important to note that OVX not only removes estradiol, but also eliminates progesterone (P). This may explain why exogenous EB did not completely rescue the effects of OVX. Indeed, exogenously administration P decreases impulsivity in both rodents [49–51] and humans [52], and risky choice behavior varies with fluctuations of P in humans [53]. Interestingly, however, several studies show that P has effects opposite those of estradiol on drug-seeking behavior [54–56], suggesting that if P were administered either with or without EB, OVX-induced elevations in risk taking could be exacerbated. Given that very little is known about the explicit role of P in risky decision making, future experiments are necessary to determine how both ovarian hormones, either individually or in combination, modulate female-specific risky choice.
Role of testicular hormones in male-typical risk taking
ORX also altered decision making, decreasing risky choice relative to sham males. This is consistent with a prior study showing that ORX males exhibited decreased preference for larger, uncertain rewards compared to ORX males treated with T in a probability discounting task [31]. Surprisingly, T did not affect risky choice in either the ORX or sham males in the current study. This finding is at odds with studies showing that exogenous T can modulate decision making in rodents. For example, T administration to intact rats increases risk taking in the RDT [14] and decreases choice of larger, uncertain rewards in favor of smaller, certain rewards in a probability discounting task [15]. In light of this previous work, one would expect that T would, at least, alter risky choice in sham males; however, these prior studies used a higher dose of T (7.5 mg/kg) than those used in the current study (roughly 0.31 mg/kg and 1.25 mg/kg) for a longer duration of T exposure, with the goal of modeling anabolic steroid abuse [14, 15, 45]. Hence, if a higher dose of T had been used and/or if T administration had been extended for a longer duration, there may have indeed been increases in risky choice in sham, and possibly ORX, males. The doses in the current study were chosen to produce physiological T levels rather than model anabolic steroid abuse levels. Notwithstanding, it is still unclear why, given the effects of ORX on risky choice, T had no impact on choice behavior in ORX males. One possibility is that the duration of hormone deprivation preceding hormone treatment may have been related to the inability of T to attenuate ORX-induced decreases in risky choice. Although there is little evidence for such a critical post-ORX window in which T must be administered to influence cognition and behavior, this has been shown to be the case with some effects of EB on behavior in females [30] and on changes in receptor expression and hormone levels in the brain in both males and females [30, 57]. Another possibility is that T is not the critical androgen responsible for modulation of risk taking; rather, it may be the T metabolite dihydrotestosterone (DHT), which is considered to be a more potent androgen than T [58, 59]. Indeed, acute administration of this nonaromatizable androgen can improve performance of avoidance-related behavior in ORX males [60]. Because DHT is a metabolite of T, one might expect that exogenous T, when metabolized to DHT, would have still attenuated ORX-induced decreases in risky choice. On the other hand, this may not be the case for chronic administration in ORX males as levels of androgenic enzyme mRNA are reduced following prolonged absence of circulating T, leading to a reduction in T metabolites [57, 60, 61]. Clearly, understanding ORX-induced decreases in risky choice and resolving questions regarding T’s role in risky choice will require experiments focused on the timing of T administration and/or androgenic metabolic pathways.
It is worth noting that the differences in the restorative effects of EB and T on GDX female and male behavior, respectively, may be due to inequity in the strength of the chosen doses. For instance, female sexual behavior (e.g., lordosis) is restored in OVX females with 1–10 µg of EB [62–66], a range of doses significantly lower than that used in the current study. In contrast, male sexual behavior (e.g., mounting) is restored with 500 µg of T [67, 68], the same dose used in this study but that was without effect in ORX males. Hence, while the dose of T matches the dose used to restore other ORX-induced deficits, the dose of EB (20 µg) used to attenuate the effects of OVX on risk taking is higher than EB doses used to restore other OVX-induced deficits. This raises the possibility that lower doses of EB used to restore other behavior in OVX females may have no effect on risk taking in OVX females. While this is certainly conceivable, manipulations that affect sexual behavior (e.g., GDX, hormone manipulations) do not necessarily result in effects in the same direction in other behavioral contexts [54, 69]. For example, while ORX abolishes male sexual behavior, it has no effect on behavior related to cocaine self-administration [54]. Finally, the dose of EB was carefully chosen based on evidence showing it produces physiological levels of circulating estradiol [27, 28] and is effective in restoring female drug-seeking and choice behavior [18, 56]. It is nevertheless important to consider these differences when constructing a meaningful framework with which to understand how hormones contribute to sex-typical behavior.
Estradiol regulation of risk aversion
Although males were insensitive to exogenous T administration, both ORX and sham males responded to EB administration. Given the increase in omissions on free choice trials, this effect was observed only when analyzing the number of large lever presses, initially suggesting that EB’s effect on risky choice may be primarily due to its effect on motivation to work for food rather than on risk taking per se. This explanation, however, is incongruent with the fact that EB had no effect on lever pressing for food in the PR test in males. It is more likely that the increase in omitted choice trials is another manifestation of risk aversion, a notion that has been explored in the context of female risk-taking strategies [7, 10, 23]. While the EB-induced decrease in risky choice in males was initially unexpected, there is precedent for EB inducing female-typical patterns of choice behavior in males. When given a choice between food and cocaine, females will largely choose cocaine [18, 70], a preference that is abolished by OVX and reinstated by EB administration [18]. In contrast, males generally prefer food over cocaine, but when ORX males are treated with EB, they predominantly choose cocaine [19]. Together with the current findings, this suggests that males and females are equally sensitive to estradiol’s ability to modulate risk taking.
In addition to effects on choice performance, EB also selectively reduced win-stay behavior in both ORX (and sham) and OVX rats. Although increased risk aversion is more often accompanied by an increase in lose-shift behavior [26], indicative of enhanced sensitivity to negative feedback, a decrease in win-stay performance may also reflect elements of risk aversion. Given the probabilistic nature of punishment in the RDT, a more risk-averse strategy, particularly at punishment probabilities of 50–75%, would be to shift choices to the safer option immediately after receiving a large, unpunished reward, as the same choice on the next trial may not be so favorable. Finally, because EB caused a further decrease in risky choice in ORX males (as opposed to an increase, which could be considered an attenuation of ORX effects), these data also suggest that hormonal regulation of risky choice in males is not mediated through aromatization of T to estradiol in the brain, consistent with the aforementioned potential role of DHT in risky choice in males. Interestingly, male sensitivity to EB may be specific to choice settings, as EB administration to ORX males, in contrast to OVX females, does not affect cocaine self-administration in the absence of a non-drug option [6, 54]. Future experiments are therefore warranted to understand how and under what conditions EB is able to modulate male choice behavior.
Broadly, these findings suggest that, irrespective of sex and/or gonadal status, estradiol promotes risk aversion and, therefore, will decrease risk taking when administered to any system low in estradiol. Based on this hypothesis, EB would decrease risk taking in females without circulating estradiol (e.g., OVX), but would not affect intact females (e.g., sham). Similarly, EB would decrease risk taking in males regardless of gonadal status, due to their normally low levels of circulating estradiol. All of these predictions were borne out in the findings presented here. Further support for the hypothesis that estradiol has a unidirectional role in choice behavior irrespective of sex derives from previous studies in which EB administration to either OVX or ORX rats resulted in an identical shift in choice behavior and one that mimicked a pattern of choice behavior specific to females [18, 19]. Notably, in contrast to the current study, this previous study did not include male sham controls [19] and therefore it is unknown whether, as observed here, EB administration would have shifted choice behavior in a similar direction as that observed in OVX and ORX rats.
One mechanism by which estradiol may promote risk aversion in males and females is through modulation of dopamine (DA) signaling. Estradiol mediates the enhanced sensitivity to DA-induced changes in behavior in females [71, 72] and contributes to the motivational and reinforcing properties of cocaine and amphetamine [54, 73]. Additionally, OVX decreases, while EB administration increases, amphetamine-induced striatal DA release [71]. In the context of the RDT, administration of both EB (current study) and the indirect DA agonist amphetamine [10, 46] decrease risky choice, suggesting greater risk aversion in intact females could be mediated by EB-induced increases in striatal DA release. Clues to the mechanisms by which this might occur come from evidence that EB administration to OVX females downregulates striatal DA D2R binding [74]. Although limited, there is also evidence that estradiol can modulate DA signaling in males, albeit via different mechanisms than in females. For example, in contrast to females, ORX and subsequent EB administration does not affect amphetamine-induced striatal DA release in males [75]. EB administration to ORX males, however, increases striatal DA D2R binding [74], and high levels of D2R mRNA in both dorsal and ventral striatum are associated with less risky choice in the RDT in intact males [46, 76], suggesting the EB-induced decrease in risky choice in at least ORX males is due to coincident increases in striatal D2R availability. The fact that EB administration has the same effect on risk taking in both sexes despite opposite effects on striatal D2R availability could suggest that DA regulation of risky choice is sexually dimorphic. In support of this idea, males and females are differentially sensitive to the effects of manipulations of D2R activity on risky choice in a rodent gambling task, with male choice behavior selectively affected by D2R antagonists and female choice behavior selectively affected by D2R agonists [77]. Future experiments will test whether this also extends to decision making involving risk of punishment (RDT).
Conclusion
In conclusion, this study demonstrated that risky decision making is regulated by gonadal hormones in females and males. Removal of ovarian hormones increased risky choice in females, an effect that was ameliorated with exogenous EB administration, while removal of testicular hormones decreased risky choice in males. This latter effect was not attenuated by T, indicating that risky choice in males has limited sensitivity to exogenous T administration. Surprisingly, however, males, irrespective of gonadal status, were sensitive to EB, with all males displaying a reduction in risky choice. These findings are significant because they highlight the necessity of including sex (and the associated hormonal milieu) as a biological variable in the study of behavior vulnerable to pathological conditions. Moreover, they begin to reveal the poorly understood mechanisms underlying sex differences in risky choice. Because many psychiatric diseases associated with impaired decision making manifest differently between sexes and are subject to hormonal influences (e.g., substance use disorder) [7], understanding how such mechanisms contribute to sex differences in decision making could provide additional knowledge about the etiology of certain sex-biased pathological conditions.
Funding and disclosure
This work was supported by: NIH R01DA036534 to BS; NIH K99DA041493 and a Thomas H. Maren Fellowship to CAO; a McKnight Brain Institute Fellowship to SLB.; the McKnight Foundation and Pat Tillman Foundation to CMH.; the McKnight Brain Research Foundation to JLB; University Scholars Program to ARW. The authors do not have any financial disclosures or conflicts of interest to report.
Supplementary information
Acknowledgements
We thank Ms. Bonnie McLaurin for her role in gonadectomy surgeries. We are additionally grateful to Mr Matt Bruner, Ms Shannon Wall, and Ms Vicky Kelley for their assistance with behavioral testing.
Author contributions
CAO and BS designed the experiments. CAO, SLB, CMH, SMB, HP, ARW, TWT, TSG conducted experiments. CAO, SMB, CMH, and TSG performed data analyses. CAO, BS, and JLB wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00827-0).
References
- 1.Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–89. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 2.Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: Imbalance of pain and gain. Drug alcohol Depend. 2013;132:13–21. doi: 10.1016/j.drugalcdep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005;13:311–8. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- 4.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 5.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–9. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orsini CA, Setlow B. Sex differences in animal models of decision making. J Neurosci Res. 2017;95:260–9. doi: 10.1002/jnr.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Bos R, Jolles J, van der Knaap L, Baars A, de Visser L. Male and female Wistar rats differ in decision-making performance in a rodent version of the Iowa Gambling Task. Behav Brain Res. 2012;234:375–9. doi: 10.1016/j.bbr.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Georgiou P, Zanos P, Bhat S, Tracy JK, Merchenthaler IJ, McCarthy MM, et al. Dopamine and stress system modulation of sex differences in decision making. Neuropsychopharmacology. Neuropsychopharmacology. 2018;43:313–24. doi: 10.1038/npp.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orsini CA, Willis ML, Gilbert RJ, Bizon JL, Setlow B. Sex differences in a rat model of risky decision making. Behav Neurosci. 2016;130:50–61. doi: 10.1037/bne0000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liley AE, Gabriel DBK, Sable HJ, Simon NW. Sex differences and effects of predictive cues on delayed punishment discounting. eNeuro. 2019;6:ENEURO.0225-19.2019. [DOI] [PMC free article] [PubMed]
- 12.Chowdhury TG, Wallin-Miller KG, Rear AA, Park J, Diaz V, Simon NW, et al. Sex differences in reward- and punishment-guided actions. Cogn Affect Behav Neurosci. 2019;19:1404–17. doi: 10.3758/s13415-019-00736-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellman BA, Schuessler BP, Tellakat M, Kim JJ. Sexually dimorphic risk mitigation strategies in rats. eNeuro. 2017;4:ENEURO.0288-16.2017. [DOI] [PMC free article] [PubMed]
- 14.Cooper SE, Goings SP, Kim JY, Wood RI. Testosterone enhances risk tolerance without altering motor impulsivity in male rats. Psychoneuroendocrinology. 2014;40:201–12. doi: 10.1016/j.psyneuen.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallin KG, Alves JM, Wood RI. Anabolic-androgenic steroids and decision making: probability and effort discounting in male rats. Psychoneuroendocrinology. 2015;57:84–92. doi: 10.1016/j.psyneuen.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood RI, Armstrong A, Fridkin V, Shah V, Najafi A, Jakowec M. ‘Roid rage in rats? Testosterone effects on aggressive motivation, impulsivity and tyrosine hydroxylase. Physiol Behav. 2013;110-111:6–12. doi: 10.1016/j.physbeh.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uban KA, Rummel J, Floresco SB, Galea LA. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacology. 2012;37:390–401. doi: 10.1038/npp.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. 2012;37:2605–14. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagley JR, Adams J, Bozadjian RV, Bubalo L, Ploense KL, Kippin TE. Estradiol increases choice of cocaine over food in male rats. Physiol Behav. 2019;203:18–24. doi: 10.1016/j.physbeh.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orsini CA, Blaes SL, Setlow B, Simon NW. Recent updates in modeling risky decision making in rodents. Methods Mol Biol. 2019;2011:79–92. doi: 10.1007/978-1-4939-9554-7_5. [DOI] [PubMed] [Google Scholar]
- 21.Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–17. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137:97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- 23.Grissom NM, Reyes TM. Let’s call the whole thing off: evaluating gender and sex differences in executive function. Neuropsychopharmacology. 2019;44:86–96. doi: 10.1038/s41386-018-0179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Bos R, Homberg J, de Visser L. A critical review of sex differences in decision-making tasks: focus on the Iowa Gambling Task. Behav Brain Res. 2013;238:95–108. doi: 10.1016/j.bbr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Crocker AD, Russell RW. The up-and-down method for the determination of nociceptive thresholds in rats. Pharmacol, Biochem, Behav. 1984;21:133–6. doi: 10.1016/0091-3057(84)90142-4. [DOI] [PubMed] [Google Scholar]
- 26.Orsini CA, Hernandez CM, Singhal S, Kelly KB, Frazier CJ, Bizon JL, et al. Optogenetic inhibition reveals distinct roles for basolateral amygdala activity at discrete time points during risky decision making. J Neurosci. 2017;37:11537–48. doi: 10.1523/JNEUROSCI.2344-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbs RB. Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: effects of estrogen and progesterone. J Neurosci. 1996;16:1049–55. doi: 10.1523/JNEUROSCI.16-03-01049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benmansour S, Piotrowski JP, Altamirano AV, Frazer A. Impact of ovarian hormones on the modulation of the serotonin transporter by fluvoxamine. Neuropsychopharmacology. 2009;34:555–64. doi: 10.1038/npp.2008.23. [DOI] [PubMed] [Google Scholar]
- 29.Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol, Biochem, Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta. 2010;1800:1068–76. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Hormones Behav. 2005;48:268–77. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Bruijn M, Broekman M, van der Schoot P. Sexual interactions between estrous female rats and castrated male rats treated with testosterone propionate or estradiol benzoate. Physiol Behav. 1987;43:35–39. doi: 10.1016/0031-9384(88)90095-9. [DOI] [PubMed] [Google Scholar]
- 33.Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Hormones Behav. 2011;59:484–96. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner BA, Braddick VC, Batson CG, Cullen BH, Miller LE, Spritzer MD. Effects of testosterone dose on spatial memory among castrated adult male rats. Psychoneuroendocrinology. 2018;89:120–30. doi: 10.1016/j.psyneuen.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson MJ, McMillin JM, Seal US, Ahmed K. Circadian variation of serum testosterone in the adult male rat with a late morning acrophase. Experientia. 1976;32:944–5. doi: 10.1007/BF02003784. [DOI] [PubMed] [Google Scholar]
- 36.Roth ME, Casimir AG, Carroll ME. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav. 2002;72:313–8. doi: 10.1016/s0091-3057(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 37.Koss WA, Haertel JM, Philippi SM, Frick KM. Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17beta-estradiol. eNeuro. 2018;5:ENEURO.0267-18.2018. [DOI] [PMC free article] [PubMed]
- 38.Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN. Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology. 2016;157:1357–62. doi: 10.1210/en.2015-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–6. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- 40.Locklear MN, Kritzer MF. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Hormones Behav. 2014;66:298–308. doi: 10.1016/j.yhbeh.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Killgore WD, Grugle NL, Killgore DB, Balkin TJ. Sex differences in self-reported risk-taking propensity on the Evaluation of Risks scale. Psychol Rep. 2010;106:693–700. doi: 10.2466/pr0.106.3.693-700. [DOI] [PubMed] [Google Scholar]
- 42.Rosenblitt JC, Soler H, Johnson SE, Quadagno DM. Sensation seeking and hormones in men and women: exploring the link. Hormones Behav. 2001;40:396–402. doi: 10.1006/hbeh.2001.1704. [DOI] [PubMed] [Google Scholar]
- 43.St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–33.. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Op de Macks ZA, Bunge SA, Bell ON, Wilbrecht L, Kriegsfeld LJ, Kayser AS, et al. Risky decision-making in adolescent girls: the role of pubertal hormones and reward circuitry. Psychoneuroendocrinology. 2016;74:77–91. doi: 10.1016/j.psyneuen.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Wallin-Miller KG, Chesley J, Castrillon J, Wood RI. Sex differences and hormonal modulation of ethanol-enhanced risk taking in rats. Drug Alcohol Depend. 2017;174:137–44. doi: 10.1016/j.drugalcdep.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, et al. Dopaminergic modulation of risky decision-making. J Neurosci. 2011;31:17460–70. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–97. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- 48.Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci Biobehav Rev. 2015;58:147–67. doi: 10.1016/j.neubiorev.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smethells JR, Swalve NL, Eberly LE, Carroll ME. Sex differences in the reduction of impulsive choice (delay discounting) for cocaine in rats with atomoxetine and progesterone. Psychopharmacology. 2016;233:2999–3008. doi: 10.1007/s00213-016-4345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swalve N, Smethells JR, Carroll ME. Progesterone attenuates impulsive action in a Go/No-Go task for sucrose pellets in female and male rats. Hormones Behav. 2016;85:43–7. doi: 10.1016/j.yhbeh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swalve N, Smethells JR, Younk R, Mitchell J, Dougen B, Carroll ME. Sex-specific attenuation of impulsive action by progesterone in a go/no-go task for cocaine in rats. Psychopharmacology. 2018;235:135–43. doi: 10.1007/s00213-017-4750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milivojevic V, Fox HC, Sofuoglu M, Covault J, Sinha R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology. 2016;65:44–53. doi: 10.1016/j.psyneuen.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derntl B, Pintzinger N, Kryspin-Exner I, Schopf V. The impact of sex hormone concentrations on decision-making in females and males. Front Neurosci. 2014;8:352. doi: 10.3389/fnins.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–38. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 55.Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–80. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- 56.Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–71. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- 57.Tobiansky DJ, Korol AM, Ma C, Hamden JE, Jalabert C, Tomm RJ, et al. Testosterone and corticosterone in the mesocorticolimbic system of male rats: effects of gonadectomy and caloric restriction. Endocrinology. 2018;159:450–64. doi: 10.1210/en.2017-00704. [DOI] [PubMed] [Google Scholar]
- 58.Tobiansky DJ, Wallin-Miller KG, Floresco SB, Wood RI, Soma KK. Androgen regulation of the mesocorticolimbic system and executive function. Front Endocrinol. 2018;9:279. doi: 10.3389/fendo.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Celec P, Ostatnikova D, Hodosy J. On the effects of testosterone on brain behavioral functions. Front Neurosci. 2015;9:12. doi: 10.3389/fnins.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edinger KL, Lee B, Frye CA. Mnemonic effects of testosterone and its 5alpha-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78:559–68. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 61.Frye CA, Edinger KL, Seliga AM, Wawrzycki JM. 5alpha-reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendocrinology. 2004;29:1019–27. doi: 10.1016/j.psyneuen.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Dominguez-Ordonez R, Garcia-Juarez M, Lima-Hernandez FJ, Gomora-Arrati P, Blaustein JD, Etgen AM, et al. Estrogen receptor alpha and beta are involved in the activation of lordosis behavior in estradiol-primed rats. Hormones Behav. 2016;86:1–7. doi: 10.1016/j.yhbeh.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Dominguez-Ordonez R, Garcia-Juarez M, Lima-Hernandez FJ, Gomora-Arrati P, Blaustein JD, Gonzalez-Flores O. Sexual receptivity facilitated by unesterified estradiol: dependence on estrogen and progestin receptors and priming dose of estradiol benzoate. Behav Neurosci. 2015;129:777–88. doi: 10.1037/bne0000103. [DOI] [PubMed] [Google Scholar]
- 64.Olster DH, Blaustein JD. Progesterone facilitation of lordosis in male and female Sprague-Dawley rats following priming with estradiol pulses. Hormones Behav. 1988;22:294–304. doi: 10.1016/0018-506x(88)90002-5. [DOI] [PubMed] [Google Scholar]
- 65.Mazzucco CA, Walker HA, Pawluski JL, Lieblich SE, Galea LA. ERalpha, but not ERbeta, mediates the expression of sexual behavior in the female rat. Behav Brain Res. 2008;191:111–7. doi: 10.1016/j.bbr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 66.Beach FA, Orndoff RK. Variation in the responsiveness of female rats to ovarian hormones as a function of preceding hormonal deprivation. Hormones Behav. 1974;5:201–5. doi: 10.1016/0018-506x(74)90028-2. [DOI] [PubMed] [Google Scholar]
- 67.Whalen RE, DeBold JF. Comparative effectiveness of testosterone, androstenedione and dihydrotestosterone in maintaining mating behavior in the castrated male hamster. Endocrinology. 1974;95:1674–9. doi: 10.1210/endo-95-6-1674. [DOI] [PubMed] [Google Scholar]
- 68.Beach FA, Holz-Tucker AM. Effects of different concentrations of androgen upon sexual behavior in castrated male rats. J Comp Physiol Psychol. 1949;42:433–53. doi: 10.1037/h0059086. [DOI] [PubMed] [Google Scholar]
- 69.Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 70.Perry AN, Westenbroek C, Becker JB. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PloS ONE. 2013;8:e79465. doi: 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118:169–71. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- 72.Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566:255–64. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- 73.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 74.Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–72. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- 75.Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–34. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, Setlow B. Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacology. 2014;39:955–62. doi: 10.1038/npp.2013.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Georgiou P, Zanos P, Bhat S, Tracy JK, Merchenthaler IJ, McCarthy MM, et al. Dopamine and stress system modulation of sex differences in decision making. Neuropsychopharmacology. 2018;43:313–24. doi: 10.1038/npp.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.