Abstract
Autoimmune neurological disorders, including neuromyelitis optica spectrum disorder, anti-N-methyl-D-aspartate receptor encephalitis, anti-MOG antibody-associated disorders, and myasthenia gravis, are clearly defined by the presence of autoantibodies against neurological antigens. Although these autoantibodies have been heavily studied for their biological activities, given the heterogeneity of polyclonal patient samples, the characteristics of a single antibody cannot be definitively assigned. This review details the findings of polyclonal serum and CSF studies and then explores the advances made by single-cell technologies to the field of antibody-mediated neurological disorders. High-resolution single-cell methods have revealed abnormalities in the tolerance mechanisms of several disorders and provided further insight into the B cells responsible for autoantibody production. Ultimately, several factors, including epitope specificity and binding affinity, finely regulate the pathogenic potential of an autoantibody, and a deeper appreciation of these factors may progress the development of targeted immunotherapies for patients.
Keywords: B cells, Recombinant antibodies, Autoimmunity, Central nervous system, Neurological disorders, Monoclonal, Single cell, Sequencing, Pathogenicity, Epitope, Affinity, B cell tolerance
Subject terms: Diagnostic markers, Neuroimmunology
Introduction
The humoral immune response mediated by B cells and antibodies is critical in the body’s defense against pathogens. A diverse B cell repertoire, which is essentialfor a robust immune response, is primarily generated through somatic recombination. As the human genome is limited, B cell diversity is created by the rearrangement of a discrete set of germline gene segments.1,2 This process occurs in each individual B cell to stochastically recombine V, D, and J gene segments at variable heavy (VH) and light (VL) chain immunoglobulin loci. Since the possibilities of V(D)J recombination are combinatorial, the production of self-reactive B cells and autoantibodies is inevitable. Recent paradigms in the field have shown that pathogenic autoantibodies tend to bind epitopes of functionally important cell surface proteins, such as receptors; therefore, removing them or inhibiting their production is essential in improving patient outcomes. Indeed, an exclusive subset of neurological disorders has been clinically and diagnostically associated with the presence of disease-specific autoantibodies against nervous system antigens, including antibodies identified against numerous neuronal cell surface-derived antigens, such as the N-methyl-D-aspartate receptor (NMDAR) and leucine-rich glioma-inactivated 1 (LGI1) in autoimmune encephalitis; the aquaporin-4 (AQP4) water channel or myelin oligodendocyte glycoprotein (MOG) in patients with neuromyelitis optica spectrum disorders (NMOSDs) and MOG antibody-associated disorders (MOGADs), respectively; and the acetylcholine receptor (AChR) and muscle-specific tyrosine kinase (MuSK) in patients with myasthenia gravis (MG).3–6 By defining the B cell populations responsible for disease-specific antibody production, clinicians may be able to provide patients with more directed immunotherapies.
Given the polyclonality of patient samples, it can be difficult to interpret the exact role of an autoantibody in disease. To resolve this, disease-specific antibodies must be isolated from clinically irrelevant antibodies also present in the sample. To eliminate the noise from background antibodies in the serum, Spadaro et al.7 affinity-purified anti-MOG antibodies (Table 1) from seropositive patients using a soluble form of the extracellular domain of MOG. However, the use of conformational epitopes in pathogenic cell surface antibody detection is now a widely accepted paradigm in neuroimmunology. The best illustration of this concept is MOG, as downstream detection of anti-MOG antibodies was recently shown to be more accurate when the native conformation of the protein was respected.8,9 Accordingly, although the extracellular domain of MOG used for affinity purification retained the characteristic beta-sheet folding of Ig-like domains, this construct was unable to bind all serum anti-MOG antibodies detectable by cell-based assay (CBA).7 As the extracellular domain lacks the anchoring transmembrane domain, there may still be small but significant modifications to the protein structure that inhibit the binding of some anti-MOG antibodies. Therefore, the use of native, conformationally intact protein for accurate autoantibody detection, a feature inherent to CBAs, should be emphasized. Several epitopes have been identified in both adult and pediatric patients seropositive for anti-MOG antibodies;9,10 however, in vitro differentiation of B cells into antibody-secreting cells (ASCs) suggests that more epitopes than those detected routinely in serum exist in patients.11 Each epitope recognized may also contribute to a distinct pathogenic response, emphasizing the need to separate individual responses to a particular antigen. This is an example of the many unanswered questions regarding antibody-mediated neurological disorders that may benefit from the resolution provided by single-cell technologies. This review discusses the contribution of B cells to autoimmune disorders of the nervous system, with a focus on more common syndromes mediated by autoantibodies against cell surface antigens, and how single-cell sequencing data and the generation of recombinant antibodies can be used to fill gaps in knowledge that exist within the field.
Table 1.
Autoimmune neurological disorders
| NMOSD recently reviewed in4,107 |
• NMOSDs, including optic neuritis and transverse myelitis, are characterized by isolated or recurrent episodes of immune-mediated demyelination of the CNS. • NMOSD can be distinguished from multiple sclerosis by the presence of autoantibodies against the astrocytic water channel AQP4,108,109 which has been implicated in the pathogenesis of NMOSD.25,110 |
| Anti-NMDAR encephalitis111 |
• Patients with anti-NMDAR encephalitis112,113 typically present with short-term memory loss; psychiatric symptoms such as agitation and paranoia; and physical manifestations of dyskinesia, dystonia, and epilepsy. • Antibodies against the extracellular NR1 subunit of the neuronal cell surface marker NMDAR are directly pathogenic and capable of downregulating NMDAR expression in vivo and in vitro,48,87,99,114–117 disturbing synaptic NMDAR-mediated currents,48,86 and led to behavioral changes when intrathecally injected into mice.117 • Patients typically respond positively to immunotherapy, with the majority of patients experiencing complete recovery.118,119 |
| AChR/MuSK MG120 |
• MG is a chronic peripheral disorder that presents with either local or general muscle weakness. • Approximately 85% of patients with MG harbor antibodies against neuromuscular junction-specific AChR,121 while a large proportion of the remaining 15% of patients have detectable antibodies against the cell surface enzyme MuSK.122 • AChR MG and MuSK MG are predominantly mediated by IgG1 and IgG4 antibodies, respectively. • B cell depletion therapies effectively treat both subgroups of MG patients, but in particular, MuSK MG patients display diminished anti-MuSK antibody titers coinciding with long-lasting clinical improvement, even upon the cessation of other immune suppressants.43,123 |
| Anti-MOG antibody-mediated disorders5,73 |
• MOG is expressed on the surface of oligodendrocytes and a putative autoantigen in human demyelination. Autoantibodies that recognize conformational MOG are biologically relevant in demyelinating phenotypes such as optic neuritis, transverse myelitis, and acute disseminated encephalomyelitis.124–126 • The presence of anti-MOG antibodies typically excludes the diagnosis of multiple sclerosis, which shares multiple clinical and radiological features with the aforementioned disorders. The pathogenic status of anti-MOG antibodies in human models has yet to be assigned. |
| Anti-LGI1 encephalitis127 |
• Anti-LGI1 encephalitis is another autoimmune encephalitis that, unlike anti-NMDAR encephalitis, primarily affects elderly males.128,129 • Clinically, this disorder heavily features the symptoms of seizure and cognitive impairment. Patients often respond well to immunotherapies with no lasting cognitive symptoms.130 |
Single-cell technologies
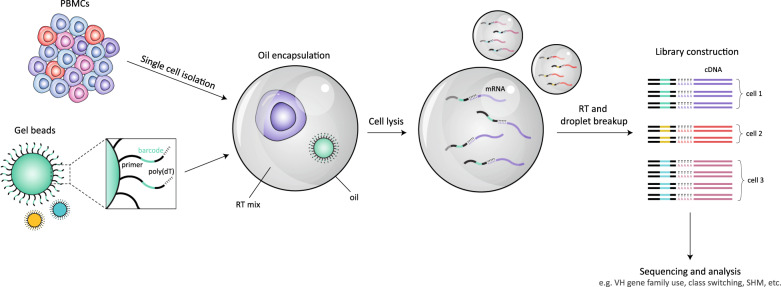
The study of autoantibodies is complicated, as the results of any characterization or functional study cannot be attributed to antigenic specificity due to the polyclonality of serum and cerebrospinal fluid (CSF). Over the past two decades, several technologies advertising high-resolution assessment of B cell and antibody repertories have emerged. The development of breakthrough microfluidic systems that barcode transcriptomic data derived from each single cell,12,13 herein referred to as single-cell RNA sequencing, allows for cognate VH-VL pairings that are otherwise lost in bulk analyses to be preserved. Due to its ability to process tens of thousands of cells, 10X Genomics Chromium, the most commonly used microfluidics-based platform, has an improved ability to detect rare cells over non-microfluidic single-cell sequencing approaches, which are limited by their manual nature. Briefly, microfluidics-based approaches involve single cells that are isolated and oil encapsulated in aqueous droplets along with a barcoded gel bead (Fig. 1). After the cells are lysed, mRNA is captured by poly(dT) sequences on each barcoded primer for reverse transcription (RT). cDNA from all droplets is then pooled into library preparations and sequenced. As each gel bead is uniquely barcoded, transcripts derived from each cell can be reunified during postsequencing analysis, automatically conserving original VH-VL pairings. Downstream analyses can then be conducted to characterize the intensity of the immune response by calculating clonal diversity within and between patient samples and ascertaining the extent of affinity maturation by quantifying the rate of somatic hypermutation (SHM) within the antibody sequence and the length of hypervariable CDR3 regions. As single-cell sequencing is a relatively new technology, it remains to be extensively applied in the setting of antibody-mediated neurological disorders.14 However, considering its potential, its use in these disorders is expected to increase in the near future.
Fig. 1.
Single-cell RNA sequencing. Single B cells and individual barcoded gel beads are oil encapsulated in droplets containing the products required for RT. Each gel bead is labeled with a unique barcode that is attached to a poly(dT) tail to capture mRNA upon cell lysis, as well as an RNA primer to start the RT process. This ensures that when droplets are combined after RT and the cDNA has been sequenced, each sequence can be traced back to the original B cell, allowing native VH-VL pairings to be conserved
Compared to other widely used single-cell RNA sequencing platforms, such as Smart-seq2, 10X Genomics Chromium is a more cost-effective and time-efficient system. Its ability to process large cell numbers enables the detection of rare cell types15. On the other hand, the quantity of genes captured per cell is not as robust as that captured with the plate-based Smart-seq2 platform.16 The 10X Genomics Chromium platform, in particular, also requires cells to be of high quality and high viability to generate optimal sequence data, but quality and viability may vary depending on the cell type and isolation method used.16
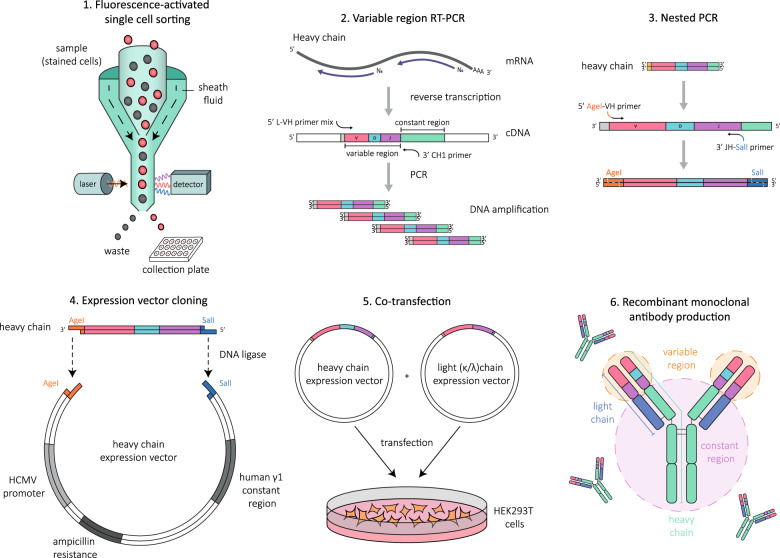
Although sequence analysis can aid in defining B cell development and the contribution of certain B cells to disease, it does not reveal the specificity or functional capacity of the secreted antibodies. Several studies have chosen to supplement BCR sequencing with the cloning of recombinant monoclonal antibodies. This process typically begins by RT-PCR amplification of VH and VL sequences from single B cells (Fig. 2).17,18 In summary, single B cells isolated by a fluorescence-activated cell sorter are deposited into wells containing lysis buffer. A preliminary RT step is followed by two stages of PCR using degenerated primers that allow the amplification of VH and VL regions. Cells with functional VH-VL are sequenced and superficially analyzed for variable gene usage and productive rearrangements. A final nested PCR step using gene-specific primers prepares the amplicons for subcloning by the addition of terminal restriction enzyme sites. VH and VL sequences are digested with the appropriate restriction enzymes and ligated into similarly restricted VH and VL expression vectors. Vectors are amplified in E. coli, purified, and sequenced to confirm homology to the original PCR sequence. Matching VH and VL sequences are finally cotransfected into human embryonic kidney (HEK) 293T cells for recombinant antibody production and purification. The RT-PCR amplification of variable genes is considerably more labor intensive than single-cell RNA sequencing methods, and the efficiency of amplification is also compromised at the expense of cost. Recombinant antibodies can also be generated from paired VH-VL sequences obtained by single-cell RNA sequencing by artificially synthesizing vectors containing selected sequences and cloning as described for RT-PCR amplification.
Fig. 2.
Cloning recombinant antibodies from single B cells. Peripheral blood mononuclear cells are stained with the appropriate markers to allow B cells of the desired phenotype to be individually sorted into each well of a collection plate containing lysis buffer. The variable region of both the heavy (VH) and light (VL) chains of immunoglobulin are RT-PCR amplified from each cell, sequenced and assessed for productive rearrangements. If both the VH and VL chains were successfully rearranged, they are further amplified by nested PCR such that terminal restriction enzyme sites are attached to prepare the sequences for cloning. The amplified sequences are digested and ligated into their respective expression vectors containing the human IgG1 constant region. Natively paired VH and VL vectors are then cotransfected into mammalian cells for recombinant antibody production and purification
The advantages of cloning recombinant antibodies are numerous. Notably, recombinant antibodies can be manufactured in bulk, producing unlimited source material for functional assays and characterization without the need to acquire more patient samples and removing the issues posed by polyclonality. Ultimately, cell type and sequence data can be linked with antigenic specificity and effector capabilities. However, it is important to highlight that given the rarity of disease-specific B cells in circulation, the generation of recombinant antibodies from single B cells sorted using flow cytometry does not guarantee that any or all of the resulting antibodies will possess the desired antigen specificity, especially given the manual limitations of the technique. In general, the odds of isolating an antigen-specific B cell can be increased by sampling during acute relapse or using CSF rather than peripheral blood as source material. Beyond this, the literature presents two main avenues to approach recombinant antibody generation: initial pre-enrichment or preselection of antigen-specific B cells prior to cloning to guarantee the generation of a disease-specific antibody or the unbiased cloning of all sorted B cells. Each method has its advantages, as the latter allows global and impartial sampling of the B cell and antibody repertoire, while the former serves to statistically increase the chance of obtaining a disease-specific recombinant antibody while reducing the workload. Common pre-enrichment techniques include the isolation of desired B cells with antigenic tetramers19,20 and the in vitro culture of single B cells into ASCs to detect antigen specificity in the secreted antibodies.21,22 The cells confirmed to harbor immunoreactivity to the target antigen are then exclusively cloned into recombinant antibodies. However, it is critical to acknowledge that these enrichment methods, particularly the use of antigenic tetramers, may impose a selection bias on the B cells isolated,20 as soluble forms of the antigen or antigenic domain required for tetramer formation may not retain the conformation of the native protein. Although limitations do exist, these single-cell technologies possess the ability to overcome several shortcomings posed by serum and CSF polyclonality and the inconclusiveness of subsequent findings.
B cells in antibody-mediated neurological disorders
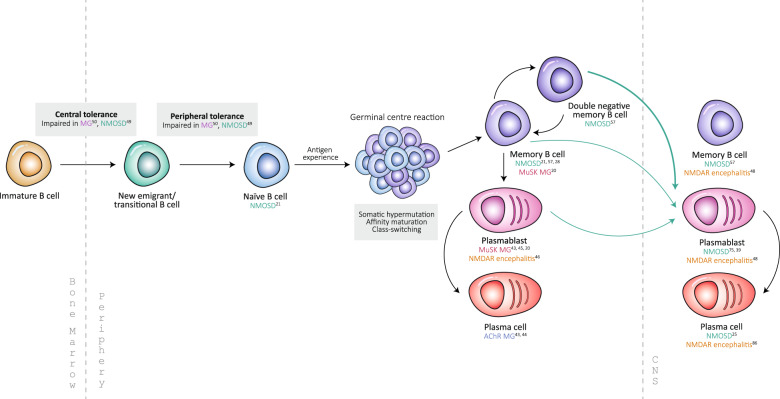
In addition to antibody production, B cells contribute to the autoimmune response by mediating the effects of other immune cells and secreting inflammatory cytokines. By pinpointing the B cell subsets responsible for disease-specific antibody production, clinicians may be able to target the problematic population with directed immunotherapies. Based on the literature, a number of B cell populations have been implicated in the pathogenesis of several antibody-mediated neurological disorders (Fig. 3 and Table 2).23
Fig. 3.
B cells in autoimmune neurological disorders B cell development and the peripheral and central populations implicated in antibody-mediated neurological disorders, summarized from both single-cell and polyclonal studies
Table 2.
Relevance of B cell subsets in autoimmune neurological diseases and future directions
| B cell subseta | Phenotype | Relevance in disease | Future directions |
|---|---|---|---|
| Transitional/ new emigrant B cells | CD19+CD20+CD21loCD10+IgMhi |
Periphery MG and NMOSD: Increased autoreactivity and polyreactivity due to impaired central tolerance49,50 |
MG and NMOSD: - Investigate whether polyreactive and autoreactive naïve B cells differentiate into disease-specific memory and antibody-secreting cells, which contribute to disease. |
| Naïve B cells | CD19+CD20+CD27−IgD+ |
Periphery NMOSD: Secretion of AQP4-IgG when cultured with ASCs in vitro21 MG and NMOSD: Increased autoreactivity and polyreactivity due to impaired peripheral tolerance49,50 |
|
| Memory B cells |
Traditional: CD19+CD20+CD27+IgD− |
Periphery NMOSD: Secretion of AQP4-IgG in culture,21 relation to CSF plasmablasts,57 reduction in relapse when treated upon re-emergence after rituximab28 MuSK MG: MuSK specificity identified20 CSF NMOSD: Clonally related to peripheral B cells57 NMDAR: NR1 specificity identified48 |
MuSK MG: - Elucidate potential selection bias of MuSK tetramers on the isolation MuSK-specific B cells. NMDAR: - Investigate the presence of additional pathogenic epitopes, as many memory B cell- and ASC-derived rAbs bound strongly to epitopes outside of NR1. - Determine the relationship between antibody titer and affinity with clinical disease progression. |
| Double-negative: CD19+CD20+CD27+IgD− |
Periphery NMOSD: Closest relation to CSF plasmablasts57 |
||
| Plasmablasts | CD19+CD20+CD27+CD38+ |
Periphery MuSK MG: Rituximab-induced depletion of MuSK antibody titers,43 MuSK specificity identified20,45 NMDAR: Rituximab-induced incomplete depletion of plasmablasts46 CSF NMOSD: AQP4 specificity identified,75 decreased risk of relapse on satralizumab39, may have peripheral57 or CNS origins75 NMDAR: NR1 specificity identified48 |
MuSK MG: - Study the relevance of IgG subgroups and epitopes in clinical disease manifestations. NMDAR: - Compare recombinant antibodies cloned from various neurological disorders with anti-NMDAR antibodies to find distinct features to characterize each disease phenotype. |
| Plasma cells | CD27+CD38+CD138+ |
Periphery AChR MG: AChR antibody secretion unaffected by rituximab,43 spontaneous production of AChR antibodies44 CSF NMOSD: AQP4 specificity and pathogenicity identified25 NMDAR: NR1 specificity and pathogenicity identified86 |
NMDAR: - Investigate the compartment in which affinity maturation of autoreactive B cells occurs. |
aStill largely unexplored in MOGAD
Often, patient responses to treatment are extremely telling of the mechanisms involved in disease. For example, a positive response to rituximab may indicate that a proportion of B cells involved in disease pathogenesis express CD20. Interestingly, most NMOSD (Table 1) patients respond well to anti-CD20 therapies,24 despite elevated numbers of CSF aquaporin-4 (AQP4)-specific plasmablasts25,26 and circulating ASCs26,27, which downregulate CD20 and are not significantly affected by rituximab. These findings suggest that other CD20-expressing B cell subsets, including CD27+ memory B cells, may be involved in disease pathogenicity. When treated in response to the frequency of re-emerging CD27+ memory B cells after rituximab therapy, NMOSD patients experienced a pronounced decrease in annual relapse rate, with 62% of patients entering remission upon treatment cessation,28 highlighting the notion that memory B cells may drive disease progression in this condition. This was not the case for a subgroup of anti-MOG antibody-positive patients, however, who exhibited relapse despite successful memory B cell depletion,29 suggesting that either the patients were not adequately treated or non-CD20-expressing B cells, such as circulating plasmablasts and plasma cells in bone marrow niches, may be the driving force of relapse in this subgroup of MOGAD patients. An anti-CD19 monoclonal antibody, inebilizumab, which has been proposed to target CD20- plasmablasts and plasma cells with greater efficacy, is currently completing phase 3 trials for NMOSD.30 The IL-6 signaling pathway is also heavily involved in the pathogenesis of NMOSD and has a role in promoting the survival of AQP4-IgG-secreting plasmablasts and plasma cells.27 As a result, both intravenous and subcutaneous formulations of the IL-6R-blocking antibody tocilizumab have demonstrated strong therapeutic benefit.31–37 In recent years, a new IgG2 IL-6R-blocking monoclonal antibody named satralizumab has emerged, with the advantage of antibody recycling technology38 to improve drug efficacy. As a monotherapy, compared to a placebo, satralizumab was shown to decrease the risk of protocol-defined relapse by 55%, with little adverse effect.39 The results of these clinical studies further support the contribution of antibody-secreting cells to NMOSD pathogenesis. Interestingly, when differentiated into ASCs, both naïve B cells and memory B cells secrete anti-AQP4 antibodies in vitro,21 supporting observations from patients treated with rituximab and antiproliferative therapies, which are primarily used to target B cells early in development (Fig. 3).24,40–42
Rituximab has also shown great therapeutic potential in both AChR and MuSK MG (Table 1), although MuSK MG patients exhibit longer periods of clinical remission than AChR patients. Clinical improvement in AChR patients following rituximab treatment was not accompanied by diminished anti-AChR antibody titers,43 suggesting that disease-producing B cells may not express CD20. Indeed, CD20-negative plasma cells have been implicated in anti-AChR antibody production, even in the absence of antigen stimulation.44 Conversely, anti-MuSK antibody titers were markedly reduced following B cell depletion, suggesting the presence of a CD20-expressing, MuSK-specific plasmablast population susceptible to immunotherapy.43 It was later confirmed that anti-MuSK antibodies were mostly secreted by CD20-expressing plasmablasts following rituximab treatment.20,45 Notably, both AChR MG and MuSK MG show distinct clinical features and are mediated by IgG1 and IgG4 antibodies, respectively, potentially accounting for their dichotomous responses to immunotherapy.
Jiang et al.14 were the first to use single-cell RNA sequencing methods, in combination with bulk-BCR repertoire sequencing and recombinant antibody generation, in the context of MG to identify that many of the clones that emerged in the post-rituximab repertoire were antigen-driven B cells that were unaffected by B cell depletion, as they were also present prior to treatment. Most of these persistent cells were recognized as CD20low memory B cells and ASCs.14
Anti-NMDAR encephalitis patients treated with rituximab reported a decline in both short-lived plasmablasts and autoantibody titer.46 Intriguingly, IgGs against the NR1 subunit of NMDAR (Table 1) in anti-NMDAR encephalitis were secreted by peripheral blood mononuclear cells (PBMCs) cultured into ASCs in vitro in the absence of ASC-sustaining factors.47 This implies that NR1-specific B cells arise from a pool of pre-ASCs primed to produce autoantibodies. Germinal center experience may also contribute to the development of NMDAR-specific B cells,47 which is supported by evidence of affinity maturation and class switching in ASCs and memory B cells.48 Multiple B cell subsets, particularly antigen-experienced populations, may also be involved in anti-NMDAR encephalitis pathogenesis, but further studies are needed to elucidate the key cellular players in disease.
Characterizing antibody-mediated neurological disorders using single-cell technologies
Recombinant antibody production and analysis of the accompanying sequencing data has led to advances in several key aspects of the humoral autoimmune response (Table 3). For example, the highly diverse humoral repertoire necessary to support a robust immune response inevitably involves the generation of self-reactive antibodies. This occurrence is usually negated by several tolerance checkpoints that remove autoreactive B cells throughout the bone marrow and periphery. Central tolerance and peripheral tolerance are two early mechanisms by which the frequency of autoreactive B cells is sequentially reduced during development.18 Analyzing the reactivity of B cells at different developmental stages through recombinant antibody cloning has highlighted the importance of tolerance in eliminating autoreactive B cells49–51 and has allowed tolerance defects to be identified in systemic lupus erythematosus (SLE),52 and rheumatoid arthritis (RA).53 The increasing use of checkpoint inhibitors in cancer treatment has also brought recognition of immune-related adverse effects due to the presumed expansion of autoreactive cells and/or autoantibody production.54–56
Table 3.
Summary of studies involving single-cell technologies in autoimmune neurological disorders
| Disease | Antigen | Methodology | Source | B cells sorted | Main findings |
|---|---|---|---|---|---|
| NMOSD49 | AQP4 | Recombinant antibody (rAb) generation by RT-PCR | Blood | New emigrant/transitional B cells (CD19+CD21loCD10+IgMhiCD27−), mature naïve B cells (CD19+CD21+CD10-IgMhiCD27-) |
• Defective central and peripheral B cell tolerance in NMOSD patients. • SHM may be involved in anti-AQP4 antibody formation. |
| NMOSD25 | AQP4 | rAb generation by RT-PCR | CSF | Plasma cells (CD138+) | • AQP4-specific rAbs can evoke pathogenic effects in vitro and in vivo. |
| NMOSD75 | AQP4 | rAb generation by RT-PCR | CSF | Plasmablasts (CD19−CD138+) | • Intrathecal Ig is produced by CSF B cells of varying origin and passively transferred from the periphery. |
| NMOSD57 | AQP4 |
1. Deep BCR sequencing 2. rAb generation by RT-PCR |
1. Blood 2. CSF |
1. Naïve B cells (CD19+CD20+CD27-CD38+IgD+), memory B cells (CD19+CD20+CD27+CD38+), double-negative B cells (CD19+CD20−CD27−IgG−CD38+), and plasmablasts (CD19+CD20−CD27++CD38high) 2. Plasmablasts (CD19+CD138+) |
• Dynamic movement of AQP4-specific B cells from peripheral to central compartments during acute disease. • Evidence of B cell maturation into ASCs within the CSF. • Double-negative B cells are most closely related to CSF AQP4-specific B cells. |
| NMOSD94 | AQP4 | rAb generation by RT-PCR | CSF | Plasmablasts (CD19−CD138+) | • H101/L104 epitope enables the formation of stable multimeric anti-AQP4 antibody platforms that promote complement activation. |
| MG45 | MuSK |
1. In vitro B cell culture 2. rAb generation by RT-PCR |
Blood | Plasmablasts (CD3−CD14−CD19+CD27hiCD38hi) | • Affinity-matured MuSK-specific plasmablasts re-emerge during post-rituximab relapse. |
| MG20 | MuSK |
1. Tetrameric selection of MuSK-specific B cells 2. rAb generation by RT-PCR |
Blood | MuSK-specific memory B cells (CD3−CD14−CD19+CD27+CD38−) and plasmablasts (CD3−CD14−CD19+CD27+CD38hi) |
• MuSK-specific rAbs enhance MuSK phosphorylation while still inhibiting AChR clustering. • MuSK-specific rAbs recognize Ig-like domain 2 of MuSK. |
| MG50 | MuSK, AChR | rAb generation by RT-PCR | Blood | New emigrant/transitional B cells (CD19+CD21loCD10+IgMhiCD27−), mature naïve B cells (CD19+CD21+CD10−IgM+CD27−) | • Defective central and peripheral B cell tolerance present in both subtypes of MG. |
| NMOSD69 | AQP4 | rAb generation by RT-PCR | CSF | Plasma cells and plasmablasts (CD138+) | • CSF ASCs from NMOSD patients recognize multiple epitopes within the extracellular domains of AQP4. |
| Anti-NMDAR encephalitis48 | NR1 | rAb generation by RT-PCR | CSF | ASCs (CD27+CD38+), memory B cells (CD20+IgD-CD27+) |
• AQP4-specific B cells are rare in CSF • Sequence analysis reveals both unmutated (germline) and affinity-matured AQP4-specific B cells. • CSF NR1-specific rAbs can downregulate NMDAR expression and reduce NMDAR currents in vitro. |
| Anti-NMDAR encephalitis86 | NR1 | rAb generation by RT-PCR | CSF | Plasma cells and plasmablasts (CD138+) | • Clonally expanded NR1-specific rAb exhibits pathogenic effects in vitro and in vivo. |
| Anti-NMDAR encephalitis91 | NR1 | rAbs selected from panel described in48 | CSF | ASCs (CD27+CD38+), memory B cells (CD20+IgD−CD27+) | • NR1-specific rAbs vary in their affinity to NMDAR. |
| Anti-NMDAR encephalitis70 | NR1 | rAb generation by RT-PCR | CSF | Unspecified |
• NR1-specific rAbs were less mutated than non-NR1 rAbs. • Germline NR1-specific rAbs showed lower affinity to hippocampal sections than mutated rAbs but could still decrease NMDAR currents. |
| MG19 | MuSK |
1. Tetrameric selection of MuSK-specific B cells 2. rAb generation by RT-PCR |
Blood | MuSK-specific B cells |
• MuSK-specific rAbs recognized epitopes within the first Ig-like domain of MuSK. • Unlike native monovalent IgG4 MuSK antibodies that inhibit MuSK phosphorylation, bispecific MuSK-rAbs stimulated MuSK phosphorylation in vitro. |
| MG14 | MuSK |
1. Deep BCR sequencing 3. Single-cell RNA sequencing |
Blood | Unselected PBMCs |
• ASCs and memory B cells present prior to rituximab treatment were also found in the post-rituximab relapse repertoire. • Persistent clones expressed low levels of CD20 and reflected antigen-driven responses. |
| Anti-LGI1 encephalitis22 | LRR, EPTP |
1. In vitro B cell culture 2. LGI1-specific B cell selection by fluorescent foci 3. rAb generation by RT-PCR |
Blood | LGI1-specific B cells |
• LRR- and EPTP-directed rAbs mediated domain-specific effects in vivo and in vitro. • High-affinity LRR-specific rAbs bound strongly to hippocampal tissue to mediate cognitive impairment in mice. |
Similarly, the mechanisms behind primary immune initiation have been studied in CNS autoimmune disorders such as NMOSD and anti-NMDAR encephalitis in an attempt to delineate the location of its origin and perhaps prevent or treat its occurrence. Although these particular disorders are characterized largely by CNS-exclusive immune-mediated insult, disease-specific antibodies are detectable in both the serum and CSF. It may be that CSF autoantibodies are produced by CNS-resident B cells due to their recognition of brain autoantigens or that peripherally produced antibodies are passively transferred into the CNS to induce brain injury. In demyelinating disorders such as NMOSD and MOGAD, evidence points toward peripheral initiation with eventual transit into the CNS.9,57 Single-cell sequencing enables transcriptomic libraries of VH and VL sequences to be constructed, allowing the original location of disease-relevant autoantibodies to be identified by comparing sequence overlap between blood and CSF compartments.57
Recombinant antibody generation from single cells allows the autoimmune response to be defined by connecting the phenotype of the originating B cell with its VH-VL sequence data and functional antibody capabilities. The epitope of a pathogenic response is clinically relevant as it often provides insight into the pathogenic capabilities of the antibody and contributes to the development of targeted immunotherapies. The epitope of an antibody is often considered in conjunction with its affinity. While high-affinity antibodies may be the obvious culprit, low-affinity antibodies in great numbers may also inflict significant damage given the appropriate settings. Furthermore, although it is commonly acknowledged that many autoantibodies are diagnostically relevant to their respective disorders, the exact role they play in disease pathology remains largely unresolved. Ideally, to prove pathogenicity, autoantibodies should replicate prototypic human disease in both cell and animal models, and removal of these antibodies should improve clinical symptoms,58 as postulated by Koch.
Although this review focuses on the generation of sequence data related to the rearrangement of V(D)J gene segments within the immunoglobulin loci, single-cell RNA sequencing analysis traditionally revolves around the expression of disease-relevant genes and identification of unique transcriptomic signatures as potential disease biomarkers. Although this facet has not yet been as heavily explored in autoimmune neurological disorders, its benefits have been well demonstrated in systemic autoimmune disorders, aging, and cancer.59,60 As such, single-cell RNA sequencing also has the potential to unveil dysregulation in more functional aspects of B cell regulation, as well as differences in the cytokine profiles of individual B cell subsets, and introduce potential targets that could be explored therapeutically.61
Defining B cell tolerance checkpoint fidelity with recombinant antibodies
Several autoimmune disorders, including NMOSD and both AChR and MuSK MG, display evidence of combined central and peripheral tolerance defects. Robust B cell tolerance mechanisms function to reduce the likelihood of autoimmunity; however, it is important to consider the weight of these defects in the context of additional contributing factors to disease. Recombinant antibodies were generated to investigate the fidelity of tolerance in NMOSD and MG patients.49,50 Antibodies cloned from transitional B cells and naïve mature B cells were analyzed for autoreactivity and polyreactivity to assess the strength of the central and peripheral tolerance checkpoints in these disorders. Autoreactivity was defined as recombinant antibody binding to slides coated with human epithelial type 2 cell lysate, while polyreactivity was dependent on the recombinant antibody’s ability to recognize a combination of three structurally distinct antigens: lipopolysaccharide, recombinant human insulin, and dsDNA. These binding characteristics were assessed independently of their reactivity to the disease-specific antigen. The new emigrant/transitional population of B cells during early B cell development experiences negative selection against self-reactive clones. The integrity of this regulatory mechanism was evaluated by the frequency of polyreactive new emigrant/transitional B cells in patients compared to controls. The proportion of recombinant antibodies derived from this population that could collectively bind lipopolysaccharide, insulin, and dsDNA was significantly elevated in both NMOSD and AChR/MuSK MG patients compared to healthy controls,49,50 suggesting that central tolerance mechanisms are not fully established in patients with NMOSD or MG. As central tolerance is not absolute even in healthy individuals,18 the contribution of functional peripheral tolerance mechanisms is integral in preventing autoimmunity. The peripheral checkpoint eliminates self-reactive new emigrant/transitional B cells prior to their development into mature naïve B cells. The fraction of polyreactive mature naïve B cells in NMOSD and MG patients was significantly greater than that in their control counterparts, suggesting that the peripheral tolerance mechanisms in these patients may also be incomplete. Similarly, compared to control antibodies, the repertoire of naïve mature B cell recombinant antibodies displayed increased autoreactivity in NMOSD, AChR and MuSK MG patients, implying the presence of additional peripheral tolerance defects that may favor the development of autoimmunity in these disorders.
Interestingly, these polyreactive and autoreactive naïve B cells can be found in even healthy controls and are thought to be natural antibodies that contribute to the first line of defense.62,63 It is uncertain, however, whether these autoreactive cells eventually differentiate into antigen-specific memory B cells or ASCs that contribute to disease. The extensive defects in central and peripheral compartments responsible for maintaining tolerance may explain why 13–22% of MG patients also present with comorbid autoimmune disorders with known tolerance defects,64 including autoimmune thyroid disease, SLE, RA, and NMOSD.
As these defects are fundamental, current B cell depletion therapies that target circulating cells may serve as only a temporary remedy, as disease-specific B cells were found to repopulate the re-emerging repertoire after rituximab treatment in type 1 diabetes patients.65 Despite this, there is some evidence in NMOSD to suggest that long-term rituximab treatment may slowly alter repopulation dynamics with an increased proportion of naïve B cells to memory B cells with gradually delayed repopulation.28 Sustained immune reconstitution has also been demonstrated with autologous hematopoietic stem cell transplantation treatment in MS, which has reported B cell peripheral tolerance abnormalities,66 although the exact mechanisms behind this effect remain to be defined.67
Combined deficits in central and peripheral tolerance likely contribute to the accumulation of autoreactive B cells, leading to the initiation of disease, as shown in patients with primary immunodeficiency diseases;68 however, it is not known whether tolerance defects directly correlate with the production of pathogenic antibodies in these disorders. While mature naïve B cell-generated recombinant antibodies from NMOSD or MG patients did not bind AQP4 or AChR/MuSK, respectively, when AQP4-specific antibodies generated from CSF ASCs of NMOSD patients25,69 were reverted to their germline sequences to mimic the unmutated state of their naïve B cell precursors,49 many of the germline revertants still displayed polyreactivity and autoreactivity. This suggests that disease-relevant antibodies may develop from a pool of autoreactive mature naïve B cells that have evaded early tolerance mechanisms.49 Interestingly, all germline-reverted recombinant antibodies of the IgG1 isotype also lost their ability to recognize AQP4, highlighting the role of SHM and other germinal center (GC) reactions in producing AQP4-specific B cells.49 These findings contrast those of Wilson et al.,21 who reported that faults in pre-germinal center tolerance allow for the accumulation of an autoreactive naïve B cell repertoire with the potential to activate and differentiate into anti-AQP4 antibody-secreting B cells, suggesting that naïve B cells already bear antigen specificity in NMOSD. Additionally, germline antibodies with very few or no SHMs derived from anti-NMDAR encephalitis patient CSF B cells could harbor immunoreactivity to the NR1 subunit of NMDAR and mediate functional effects in vitro,48,70 which may also suggest that tolerance induction is incomplete in these patients.48
Determining the site of disease initiation with single-cell technologies
Spatially defining the origin of pathogenic antibodies in CNS disorders is particularly important for diagnostic purposes, especially if these antibodies function as disease biomarkers. It can also contribute to our understanding of the origin and etiology of autoimmune neurological diseases. The source of antibodies, whether systemic or intrathecal, must be carefully considered to allow for the most accurate and reliable detection of autoantibodies. The location of disease activation also has implications for immunotherapeutics, as the integrity of the BBB to certain drugs may impact therapeutic efficacy in CNS diseases.
In NMOSD, several findings support the initial activation of AQP4-specific B cells in the peripheral compartment. Elevated levels of anti-AQP4 antibodies in the CSF coincide with high serum titers,71 implying that pathogenic antibodies potentially permeate through the BBB from the periphery to mediate CNS pathology during inflammation.72 This suggests that the bulk of anti-AQP4 antibodies are either synthesized in the periphery or produced intrathecally and sequestered within CNS tissue, given the CNS expression of AQP4.25 These observations also appear to be valid in anti-MOG antibody-associated disorders.9,73 Interestingly, NMOSD disease activity is more closely associated with CSF than serum anti-AQP4 antibody titers.74 To determine whether intrathecal anti-AQP4 antibodies originate from peripheral or CNS-resident B cells in NMOSD patients, transcriptomic libraries of VH and VL chains that were RT-PCR amplified from CSF plasmablasts were generated.75 The CSF B cell transcriptome was then compared to CSF and blood proteomes to pinpoint the origin of intrathecal anti-AQP4 antibodies. Over 50% of the AQP4-specific transcriptomic sequences matched peptide sequences exclusive to the CSF proteome, suggesting that CSF anti-AQP4 antibodies are in part produced by intrathecal plasma cells. Furthermore, 28% of all AQP4-specific transcriptomic sequences were recovered in both CSF and blood proteomes, suggesting that a proportion of intrathecal plasma cells may be the progeny of founding peripheral AQP4-specific B cells. The similarity between blood and CSF proteomes was also calculated to assess the dynamics of antibody movement throughout compartments. Considering that 45% of peptides were shared between central and peripheral compartments,75 a fair proportion of intrathecal anti-AQP4 antibodies may be the product of passive transfer. The authors proposed a new model of intrathecal AQP4-IgG production involving the passive influx of peripheral anti-AQP4 antibodies as well as intrathecal AQP4-IgG production by autochthonal CNS-resident B cells.75 To elaborate on this paradigm, a subsequent study sought to investigate the subset of peripherally derived CNS-resident B cells mentioned previously57 with next-generation RNA sequencing. Through aligning peripheral and intrathecal VH sequences, it was revealed that CSF plasmablasts had the shortest mutational distance and were therefore most closely related to peripheral CD27-IgD- (double-negative) B cells, although clonal relationships were also identified in peripheral class-switched memory B cells and plasmablasts. Moreover, these double-negative B cells were elevated in NMOSD patients compared to both MS patients and healthy controls. This implicates CD20+ double-negative B cells in NMOSD disease initiation and suggests that they may be the main reservoir for CSF plasmablasts. Regardless, the patterns of SHM among clonally related blood and CSF B cells reflect the movement of affinity-matured B cells between compartments. Taken together, these findings show that B cell biology in autoimmune neurological disease is complex, as shown in NMOSD, highlighting the potential role of several B cell subsets of both central and peripheral origin in autoantibody production.
Binding specificity and affinity of recombinant antibodies
Depending on the disease, the epitope as well as affinity of the autoantibody is critical in defining its ability to evoke pathogenicity and ultimately inform the treatment options available to patients. There have been continued efforts in developing a peptide-blocking therapy for pathogenic anti-dsDNA antibodies in SLE. DWEYS (D/EWD/EYS/G) peptides mimic DNA to sequester disease-specific antibodies, which were proven to have nephritogenic and neurotoxic effects in mice.76–79 This five amino acid consensus sequence can also be found in the extracellular NR2A and NR2B subunits of NMDAR78 but not the NR1 subunit bound by autoantibodies in anti-NMDAR encephalitis. Cross-reactivity of anti-dsDNA antibodies to NMDAR may explain why several SLE patients also presented with neuropsychiatric disease.80 Alternatively, therapies can be designed to block the epitopes of pathogenic antibodies to prevent the initiation of effector functions. The high-affinity recombinant monoclonal anti-AQP4 antibody aquaporumab competes against pathogenic anti-AQP4 antibodies for binding to AQP4.81,82 The Fc region of aquaporumab has been altered to ablate the ability of anti-AQP4 antibodies to participate in complement and cell-mediated effector functions and is consequently nonpathogenic.82 This therapy has not yet been approved for the treatment of human NMOSD; however, studies to improve the effectiveness of this drug are still ongoing.83 Certain epitopes may also be clinically associated with disease course or activity. In MOGAD , the predominant epitope found in seropositive children and adults was located at proline 42 (P42) of the extracellular Ig-like domain.9,10 Critically, 75% of the adult anti-MOG antibody-positive patients whose anti-MOG antibodies bound the non-P42 epitope demonstrated a relapsing disease course.9 Considering that non-P42 immunoreactivity is stable in patients over time,9 diagnostic testing for non-P42 antibodies could potentially predict relapse at disease onset. The prompt treatment of patients with multiphasic disease is crucial, as disability accumulates with relapse.3,84,85
To confirm the epitopes discovered in serum and CSF, Kreye et al.48 generated a total of 170 recombinant antibodies by RT-PCR amplification of CSF plasmablasts and memory B cells from anti-NMDAR encephalitis patients. Nine (5.29%) of these antibodies displayed reactivity to the NR1 subunit by CBA. When preselected to represent clonally expanded CSF plasma cells, the fraction of NR1-reactive cells increased to 14.29%.86 All NR1-reactive recombinant antibodies exhibited strong reactivity to the hippocampal region of rodent brains, with staining typical of anti-NMDAR encephalitis patient sera or CSF.48,86 All NR1-reactive antibodies displayed abrogated binding to N368Q-expressing mutant cells (including germline clones) compared to wild-type NR1-expressing cells, which is consistent with the restricted epitope on the amino-terminal domain reported for disease-relevant antibodies.87 However, a large proportion of CSF-derived recombinant antibodies that were not immunoreactive toward NR1 depicted strong binding to other epitopes on brain sections, which may explain why some clinical features of anti-NMDAR encephalitis cannot be explained by anti-NR1 antibodies alone. For example, NMDAR is known to internalize upon autoantibody binding, but a link between epileptic seizures and increased or unchanged receptor expression was reported in anti-NMDAR encephalitis.88–90 These results highlight the need to define additional pathogenic epitopes that may be present in patients with anti-NMDAR encephalitis.
NR1-reactive antibodies recognize the same epitope on the amino-terminal domain of the receptor87, and antibody titers in anti-NMDAR encephalitis do not completely reflect the clinical course of disease. This implies that other molecular determinants, including antibody affinity, may be involved. The affinity of an antibody is defined by the strength with which it binds its epitope. At equilibrium, high-affinity antibodies bind their epitopes at lower concentrations and may have greater pathological capacity than their low-affinity counterparts. NR1-reactive antibodies derived from CSF plasma and memory B cells48 varied in their affinities to NR1,91 and the strength of binding was not paralleled by increased evidence of affinity maturation based on SHM of the antibody sequence, which is generally expected in a typical immune response. Intriguingly, the sequences of these recombinant antibodies revealed the presence of germline NR1-reactive B cells in the CSF of anti-NMDAR encephalitis patients,48 indicating that affinity maturation may not be required for autoimmune development. This is contrary to evidence gathered using heterogeneous patient material, suggesting that GC experience may be required for NR1-specific cells to be generated.47 In fact, germline NR1-reactive B cell sequences were less mutated on average than non-NR1-binding antibodies.70 When investigated further, germline anti-NR1 antibodies replicated standard anti-NR1 staining patterns and selectively diminished total NMDA currents compared to isotype controls.70 By exhibiting a lower affinity to NR1,70 unmutated NR1-reactive B cells may have been allowed to persist within the naïve B cell repertoire, while higher affinity NR1-specific B cells may have been mostly eliminated through negative selection.51,92
These disparate findings demonstrate that more research is needed to determine the relationship between antibody titers and affinity in the progression and outcome of anti-NMDAR encephalitis. Both low- and high-affinity antibodies may be involved in the pathogenic process. Polyclonal studies revealed that high-affinity anti-MOG antibodies could be detected in only a small proportion of anti-MOG antibody-positive patients,7,9 and only high-affinity affinity-purified antibodies were pathogenic in animal models.7 However, it would also be prudent to consider the impact of low-affinity antibodies in disease. If present at high concentrations, for example, in brain tissue, prolonged exposure may be sufficient to inflict pathology and other aspects of clinical disease.
Similarly, as serum anti-AQP4 antibody titers in NMOSD are not reflected by the extent of complement-dependent cytotoxicity,93 the epitopes recognized by anti-AQP4 antibodies may also be major contributors to pathology. AQP4 exists as two isoforms: full-length M1 and the shorter M23 isoform. The M23 isoform specifically promotes the assembly of large, lattice-like structures known as orthogonal arrays of particles (OAPs), to which NMOSD-IgG can strongly bind. Recombinant antibodies were cloned from 12 CSF-derived AQP4-specific plasma cells from 5 NMOSD patients and screened against a comprehensive panel of 37 mutant AQP4 constructs that contained serial or single point mutations in the three extracellular loops (A, C, E) of the M23 isoform of AQP4,69 where two main patterns of recognition were revealed. While pattern 1 antibodies relied on epitopes in loops C and E for binding, binding of the remaining recombinant antibodies was strongly influenced by mutations to loop A. Soltys et al.94 further investigated the epitopes responsible for AQP4-IgG-driven complement-dependent cytotoxicity, uncovering that recombinant antibodies that recognized H101/L104 on extracellular loop V of M23 OAPs were able to cluster and bind the C1q component of the classical complement pathway to induce complement-dependent cytotoxicity (CDC). Large immobile arrays of target antigens, such as those of M23 AQP4 OAPs, allow anti-AQP4 antibodies to form stable multimers, initiating C1q binding and activating the complement cascade. Interestingly, these antibodies could enhance CDC regardless of affinity. The authors also showed that IgG clustering could be destabilized by a small peptide with high affinity for the Fc region of human immunoglobulin, inhibiting C1q binding and CDC activation.94 This finding is supported by the results of recent trials using a terminal complement inhibitor, eculizumab, in which treated patients demonstrated a significantly reduced rate of relapse compared to that of placebo-treated patients.95
Most serum MuSK antibodies in MG have been reported to recognize epitopes within the physiologically relevant first Ig-like domain of MuSK, ultimately disrupting LRP4-MuSK interaction96–98 and inhibiting the AChR clustering pathway. Takata et al.20 sought to identify whether any other relevant epitopes exist in MuSK MG by establishing antibody clones from peripheral MuSK-reactive memory B cells and plasmablasts. Although 11/77 recombinant antibodies cloned from six MuSK MG patients were above the positivity threshold, only three were considered to show robust binding to MuSK and selected for further analysis. Epitope studies of the MuSK-reactive antibodies were conducted using a panel of plasmid constructs containing isolated subdomains of MuSK or full-length MuSK with individual subdomains removed.20 While patient serum bound full-length MuSK and all MuSK mutant constructs, demonstrating the polyclonal nature of MuSK MG patient serum, the three patient-derived recombinant antibodies were only able to recognize epitopes within Ig-like domain 2.
These studies highlight how the epitopes in autoimmune neurological disorders can aid in understanding how disease may be triggered and how to prevent this adverse reaction from occurring through the development of directed immunotherapies. Cloning recombinant antibodies allows the complex heterogeneity of the autoimmune response to be dissected and reveals the epitopes that are relevant to clinical disease. Studies by Soltys et al.94 demonstrated that a pathogenic epitope may enable the formation of stable antibody complexes that facilitate complement activation. This mechanism may also explain the differences in pathology between anti-NMDAR encephalitis and NMOSD, despite the fact that both are IgG1 antibody-mediated disorders. While AQP4-IgG forms stable clusters on AQP4 to induce CDC,94 the binding of anti-NMDAR antibody to its cognate receptor does not result in direct CNS injury. The fast internalization of NMDAR upon receptor engagement99 does not allow for anti-NMDAR antibody clustering or subsequent complement activation. On the other hand, complement activation is routinely reported in AChR MG, in which AChR is arranged in highly ordered lattices at neuromuscular junctions.100,101 Therefore, large ordered antigen platforms may help promote antibody-mediated effects, including complement activation, in autoimmune neurological disorders.
Although pathogenicity often appears to rely solely on either the epitope of an antibody or its affinity, evidence suggests that in certain neurological disorders, both the epitope and antibody affinity contribute to the determination of pathogenicity.22 While leucine-rich repeat (LRR)-specific recombinant antibodies induced LGI1-ADAM22/23 receptor complex internalization in vitro, antibodies against the epitempin repeat (EPTP) domain instead inhibited the binding of LGI1 to ADAM22/23. In mouse models, only high-affinity LRR-reactive recombinant antibodies adsorbed strongly onto CNS tissue and elicited novel object recognition impairment. EPTP-specific antibodies, on the other hand, were readily detected in serum, indicating that a considerable proportion were unable to bind hippocampal tissue, possibly explaining their limited impact on behavioral measures.
Additionally, the pathogenic capability of an antibody can vary depending on the posttranslational modifications carried out on the antibody during production. In a research setting, the standard protocol involves the transient expression of recombinant antibodies from HEK293 cell lines.102 Although glycosylation is largely conserved between the human body and in vitro cell lines, even minor differences have the potential to invoke changes to the effector functions of an antibody. For example, minor residue changes to the N-linked glycan on IgG-Fc have been shown to skew the response in an anti-inflammatory or proinflammatory direction by impairing or promoting CDC, respectively.103,104 Understandably, the subclass of the IgG backbone into which the recombined VDJ locus is cloned may also alter the effector mechanisms of the generated antibody. In the case of Takata et al.,20 all initial recombinant antibodies were of the IgG1 subclass to maintain consistency upon MuSK reactivity screening. When reproduced to reflect their native IgG3 and IgG4 subclasses, these antibodies maintained their strong reactivity to MuSK and amplified agrin-induced MuSK phosphorylation, regardless of their subclass.20 Further investigation of IgG subclass differences may be necessary to comprehensively characterize the mechanisms driving pathogenesis in MG and other autoimmune neurological disorders.
As a note of caution, it is critical to confirm that the epitope recognized in human disease is also present in animal models before engaging in in vivo pathogenicity studies. The predominant epitope of human adult and pediatric patient antibodies in MOGAD9,10 is not found in rodent MOG, and therefore the relevance of animal disease models may be difficult to extrapolate.
Pathogenicity of recombinant antibodies
Reverse genetics can be applied to confirm the in vitro and in vivo pathogenic capacity of disease-specific B cells in autoimmune neurological disorders. The intrathecal B cell response in NMOSD was explored to assess the ability of anti-AQP4 antibodies to induce pathogenesis in vitro as well as in a rodent experimental autoimmune encephalomyelitis (EAE) model.25 In the presence of serum complement, all AQP4-reactive antibodies were able to induce the specific death of AQP4-expressing cells. Similarly, the viability of AQP4-expressing cells was selectively reduced in comparison to that of a control cell line after prolonged incubation with AQP4-reactive antibodies combined with human natural killer cells. However, upregulation of the degranulation marker CD107a was significantly increased only after incubation with four of the six AQP4-reactive antibodies and human fetal astrocytes, suggesting that the epitope of each antibody likely influences its effector abilities. These four CSF-derived AQP4-reactive antibodies were consequently transfused into EAE rats to study their immunopathological effects in vivo. One quarter of the recombinant antibodies reproduced key pathogenic features of NMOSD, displaying evidence of significant astrocyte loss, complement, and human Ig deposition. Therefore, human AQP4-specific recombinant antibodies derived from clonally expanded intrathecal plasma cells were capable of directly mediating NMOSD-related immunopathology in both cell and animal models.25
Clonally expanded intrathecal plasma cells were assessed for NMDAR binding and pathogenic capacity86 in anti-NMDAR encephalitis. Only one of seven NMDAR-reactive recombinant antibodies isolated specifically recognized the GluN1 subunit of NMDAR and could decrease NMDAR expression in cultured neurons and NMDAR-mediated currents in GluN1/GluN2-expressing oocytes. NMDAR expression was also reduced following antibody transfer into mice, along with reversible memory defects in the absence of neuronal death.
In MG, MuSK-specific recombinant antibodies derived from peripheral memory B cells and plasmablasts were investigated for their pathogenic potential. The three MuSK-reactive recombinant antibodies identified interrupted agrin-induced AChR clustering, demonstrating their functional relevance in disease activity.20 However, while the same antibodies could amplify MuSK phosphorylation,19,20 a critical step in AChR clustering, previous findings have dictated that polyclonal patient MuSK antibodies inhibited MuSK phosphorylation.6,96,97,105 This difference can be attributed to the bivalency of cloned MuSK-reactive recombinant antibodies and their ability to crosslink MuSK to force phosphorylation,19 whereas patient IgG4 anti-MuSK antibodies are functionally monovalent due to a phenomenon known as Fab exchange.106 Indeed, monovalent Fab fragments cloned from patient MuSK-reactive B cells inhibited the requisite LDL receptor-related protein 4 (LRP4)-MuSK binding needed for AChR clustering.19
Conclusion
This review summarizes the use of technologies including single-cell sequencing and recombinant antibody generation to address questions that cannot be answered at the polyclonal level, as well as to provide a high-resolution view of the B cell response mounted in autoimmune neurological disorders. Collectively, these findings indicate that several B cell populations contribute to each of these diseases, which are often shaped by a fundamental loss in tolerance. The epitope and affinity of an antibody finely regulate its pathogenic potential and are key considerations in developing targeted therapeutic interventions for the afflicted. The application of single-cell technologies to antibody-mediated neurological disorders is still in its infancy; however, it is our opinion that these techniques are uniquely suited for the study of these disorders and have the potential to revolutionize the field.
Search strategy
Search terms in PubMed: Single cell sequencing, 10X genomics chromium, recombinant antibody, autoimmune, B cell, NMOSD, MOG antibody associated disorder, NMDAR encephalitis, MG, epitope, tolerance, rituximab, tocilizumab, satralizumab, pathogenicity.
Acknowledgements
This work was supported by the Australian National Health and Medical Research Council [APP1078643 and APP1183968] (NHRMC, Australia), Multiple Sclerosis Research Australia, and a Sydney Research Excellence Initiative grant (University of Sydney, Australia).
Author contributions
A.Z. and F.B. designed the study. A.Z. wrote the first draft of the manuscript and prepared the figures and tables. A.Z., S.R., R.C.D., and F.B. reviewed the draft before submission.
Competing interests
A.Z. reports funding from the University of Sydney Postgraduate Award (UPA, Australia) and declares no other competing interests. S.R. is a consultant on an advisory board for UCB on the treatment of MOG antibody-associated demyelination. R.C.D. and F.B. have received honoraria from Biogen Idec and Merck Serono as invited speakers.
References
- 1.Hozumi N, Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc. Natl Acad. Sci. USA. 1976;73:3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brack C, Hirama M, Lenhard-Schuller R, Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978;15:1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- 3.Ramanathan S, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J. Neurol. Neurosurg. Psychiatry. 2018;89:127–137. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huda S, et al. Neuromyelitis optica spectrum disorders. Clin. Med. 2019;19:169–176. doi: 10.7861/clinmedicine.19-2-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun. Rev. 2016;15:307–324. doi: 10.1016/j.autrev.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Gilhus NE. Myasthenia Gravis. N. Engl. J. Med. 2016;375:2570–2581. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 7.Spadaro M, et al. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann. Neurol. 2018;84:315–328. doi: 10.1002/ana.25291. [DOI] [PubMed] [Google Scholar]
- 8.Reindl, M. et al. International multicenter examination of MOG antibody assays. Neurol. Neuroimmunol. Neuroinflamm.7, e674 (2020). [DOI] [PMC free article] [PubMed]
- 9.Tea F, et al. Characterization of the human myelin oligodendrocyte glycoprotein antibody response in demyelination. Acta Neuropathol. Commun. 2019;7:145. doi: 10.1186/s40478-019-0786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer MC, et al. Distinction and temporal stability of conformational epitopes on myelin oligodendrocyte glycoprotein recognized by patients with different inflammatory central nervous system diseases. J. Immunol. 2013;191:3594–3604. doi: 10.4049/jimmunol.1301296. [DOI] [PubMed] [Google Scholar]
- 11.Winklmeier S, et al. Identification of circulating MOG-specific B cells in patients with MOG antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2019;6:625. doi: 10.1212/NXI.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein AM, et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 2015;161:1187–1201. doi: 10.1016/j.cell.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macosko EZ, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang, R. et al. Single-cell repertoire tracing identifies rituximab refractory B cells during myasthenia gravis relapses. Preprint at https://www.biorxiv.org/content/10.1101/840389v2.full (2019). [DOI] [PMC free article] [PubMed]
- 15.Wang, X., He, Y., Zhang, Q., Ren, X. & Zhang, Z. Direct comparative analysis of 10X genomics chromium and smart-seq2. Preprint at https://www.biorxiv.org/content/10.1101/615013v1 (2019). [DOI] [PMC free article] [PubMed]
- 16.Baran-Gale J, Chandra T, Kirschner K. Experimental design for single-cell RNA sequencing. Brief. Funct. Genomics. 2018;17:233–239. doi: 10.1093/bfgp/elx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiller T, et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 19.Huijbers MG, et al. MuSK myasthenia gravis monoclonal antibodies: valency dictates pathogenicity. Neurol. Neuroimmunol. Neuroinflamm. 2019;6:e547. doi: 10.1212/NXI.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takata, K. et al. Characterization of pathogenic monoclonal autoantibodies derived from muscle-specific kinase myasthenia gravis patients. JCI Insight4, e127167 (2019). [DOI] [PMC free article] [PubMed]
- 21.Wilson R, et al. Condition-dependent generation of aquaporin-4 antibodies from circulating B cells in neuromyelitis optica. Brain. 2018;141:1063–1074. doi: 10.1093/brain/awy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramberger, M. et al. Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain143, 1731–1744 (2020). [DOI] [PMC free article] [PubMed]
- 23.Sabatino JJ, Jr., Probstel AK, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat. Rev. Neurosci. 2019;20:728–745. doi: 10.1038/s41583-019-0233-2. [DOI] [PubMed] [Google Scholar]
- 24.Damato V, Evoli A, Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol. 2016;73:1342–1348. doi: 10.1001/jamaneurol.2016.1637. [DOI] [PubMed] [Google Scholar]
- 25.Bennett JL, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chihara N, et al. Plasmablasts as migratory IgG-producing cells in the pathogenesis of neuromyelitis optica. PLoS One. 2013;8:e83036. doi: 10.1371/journal.pone.0083036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chihara N, et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc. Natl Acad. Sci. USA. 2011;108:3701–3706. doi: 10.1073/pnas.1017385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SH, et al. Less frequent rituximab retreatment maintains remission of neuromyelitis optica spectrum disorder, following long-term rituximab treatment. J. Neurol. Neurosurg. Psychiatry. 2019;90:486–487. doi: 10.1136/jnnp-2018-318465. [DOI] [PubMed] [Google Scholar]
- 29.Durozard P, et al. Comparison of the response to rituximab between myelin oligodendrocyte glycoprotein and aquaporin-4 antibody diseases. Ann. Neurol. 2020;87:256–266. doi: 10.1002/ana.25648. [DOI] [PubMed] [Google Scholar]
- 30.Cree BAC, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394:1352–1363. doi: 10.1016/S0140-6736(19)31817-3. [DOI] [PubMed] [Google Scholar]
- 31.Araki M, et al. Clinical improvement in a patient with neuromyelitis optica following therapy with the anti-IL-6 receptor monoclonal antibody tocilizumab. Mod. Rheumatol. 2013;23:827–831. doi: 10.1007/s10165-012-0715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araki M, et al. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology. 2014;82:1302–1306. doi: 10.1212/WNL.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayzenberg I, et al. Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti-CD20 therapy. JAMA Neurol. 2013;70:394–397. doi: 10.1001/jamaneurol.2013.1246. [DOI] [PubMed] [Google Scholar]
- 34.Ringelstein M, et al. Long-term therapy with interleukin 6 receptor blockade in highly active neuromyelitis optica spectrum disorder. JAMA Neurol. 2015;72:756–763. doi: 10.1001/jamaneurol.2015.0533. [DOI] [PubMed] [Google Scholar]
- 35.Kieseier BC, et al. Disease amelioration with tocilizumab in a treatment-resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol. 2013;70:390–393. doi: 10.1001/jamaneurol.2013.668. [DOI] [PubMed] [Google Scholar]
- 36.Lauenstein, A. S., Stettner, M., Kieseier, B. C. & Lensch, E. Treating neuromyelitis optica with the interleukin-6 receptor antagonist tocilizumab. BMJ Case Rep2014, bcr2013202939 (2014). [DOI] [PMC free article] [PubMed]
- 37.Trebst C, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS) J. Neurol. 2014;261:1–16. doi: 10.1007/s00415-013-7169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Igawa T, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol. 2010;28:1203–1207. doi: 10.1038/nbt.1691. [DOI] [PubMed] [Google Scholar]
- 39.Traboulsee A, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19:402–412. doi: 10.1016/S1474-4422(20)30078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cree BA, et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology. 2005;64:1270–1272. doi: 10.1212/01.WNL.0000159399.81861.D5. [DOI] [PubMed] [Google Scholar]
- 41.Jacob A, et al. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch. Neurol. 2009;66:1128–1133. doi: 10.1001/archneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 42.Costanzi C, et al. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology. 2011;77:659–666. doi: 10.1212/WNL.0b013e31822a2780. [DOI] [PubMed] [Google Scholar]
- 43.Diaz-Manera J, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78:189–193. doi: 10.1212/WNL.0b013e3182407982. [DOI] [PubMed] [Google Scholar]
- 44.Willcox HN, Newsom-Davis J, Calder LR. Cell types required for anti-acetylcholine receptor antibody synthesis by cultured thymocytes and blood lymphocytes in myasthenia gravis. Clin. Exp. Immunol. 1984;58:97–106. [PMC free article] [PubMed] [Google Scholar]
- 45.Stathopoulos, P., Kumar, A., Nowak, R. J. & O’Connor, K. C. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight2, e94263 (2017). [DOI] [PMC free article] [PubMed]
- 46.Hachiya Y, et al. Rituximab ameliorates anti-N-methyl-D-aspartate receptor encephalitis by removal of short-lived plasmablasts. J. Neuroimmunol. 2013;265:128–130. doi: 10.1016/j.jneuroim.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Makuch M, et al. N-methyl-D-aspartate receptor antibody production from germinal center reactions: therapeutic implications. Ann. Neurol. 2018;83:553–561. doi: 10.1002/ana.25173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreye J, et al. Human cerebrospinal fluid monoclonal N-methyl-D-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain. 2016;139:2641–2652. doi: 10.1093/brain/aww208. [DOI] [PubMed] [Google Scholar]
- 49.Cotzomi E, et al. Early B cell tolerance defects in neuromyelitis optica favour anti-AQP4 autoantibody production. Brain. 2019;142:1598–1615. doi: 10.1093/brain/awz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JY, et al. Compromised fidelity of B-cell tolerance checkpoints in AChR and MuSK myasthenia gravis. Ann. Clin. Transl. Neurol. 2016;3:443–454. doi: 10.1002/acn3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr. Opin. Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Yurasov S, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stucci S, et al. Immune-related adverse events during anticancer immunotherapy: pathogenesis and management. Oncol. Lett. 2017;14:5671–5680. doi: 10.3892/ol.2017.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suarez-Almazor ME, Kim ST, Abdel-Wahab N, Diab A. Review: immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol. 2017;69:687–699. doi: 10.1002/art.40043. [DOI] [PubMed] [Google Scholar]
- 56.Sandigursky S, Mor A. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr. Rheumatol. Rep. 2018;20:65. doi: 10.1007/s11926-018-0770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kowarik MC, et al. CNS Aquaporin-4-specific B cells connect with multiple B-cell compartments in neuromyelitis optica spectrum disorder. Ann. Clin. Transl. Neurol. 2017;4:369–380. doi: 10.1002/acn3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sinmaz N, et al. Autoantibodies in movement and psychiatric disorders: updated concepts in detection methods, pathogenicity, and CNS entry. Ann. N. Y. Acad. Sci. 2015;1351:22–38. doi: 10.1111/nyas.12764. [DOI] [PubMed] [Google Scholar]
- 59.Tang X, Huang Y, Lei J, Luo H, Zhu X. The single-cell sequencing: new developments and medical applications. Cell Biosci. 2019;9:53. doi: 10.1186/s13578-019-0314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma S, Wang C, Mao X, Hao Y. B cell dysfunction associated with aging and autoimmune diseases. Front Immunol. 2019;10:318. doi: 10.3389/fimmu.2019.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubin SJS, Bloom MS, Robinson WH. B cell checkpoints in autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2019;15:303–315. doi: 10.1038/s41584-019-0211-0. [DOI] [PubMed] [Google Scholar]
- 62.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv. Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 63.Elluru SR, Kaveri SV, Bayry J. The protective role of immunoglobulins in fungal infections and inflammation. Semin Immunopathol. 2015;37:187–197. doi: 10.1007/s00281-014-0466-0. [DOI] [PubMed] [Google Scholar]
- 64.Nacu A, Andersen JB, Lisnic V, Owe JF, Gilhus NE. Complicating autoimmune diseases in myasthenia gravis: a review. Autoimmunity. 2015;48:362–368. doi: 10.3109/08916934.2015.1030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chamberlain N, et al. Rituximab does not reset defective early B cell tolerance checkpoints. J. Clin. Invest. 2016;126:282–287. doi: 10.1172/JCI83840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinnunen T, et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J. Clin. Invest. 2013;123:2737–2741. doi: 10.1172/JCI68775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Massey JC, Sutton IJ, Ma DDF, Moore JJ. Regenerating immunotolerance in multiple sclerosis with autologous hematopoietic stem cell transplant. Front. Immunol. 2018;9:410. doi: 10.3389/fimmu.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann. N. Y. Acad. Sci. 2011;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Owens GP, et al. Mutagenesis of the aquaporin 4 extracellular domains defines restricted binding patterns of pathogenic neuromyelitis optica IgG. J. Biol. Chem. 2015;290:12123–12134. doi: 10.1074/jbc.M115.647149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wenke NK, et al. N-methyl-D-aspartate receptor dysfunction by unmutated human antibodies against the NR1 subunit. Ann. Neurol. 2019;85:771–776. doi: 10.1002/ana.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi T, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 72.Kinzel, S. & Weber, M. S. The role of peripheral CNS-directed antibodies in promoting inflammatory CNS demyelination. Brain Sci.7, 70 (2017). [DOI] [PMC free article] [PubMed]
- 73.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 2019;15:89–102. doi: 10.1038/s41582-018-0112-x. [DOI] [PubMed] [Google Scholar]
- 74.Dujmovic I, et al. Temporal dynamics of cerebrospinal fluid anti-aquaporin-4 antibodies in patients with neuromyelitis optica spectrum disorders. J. Neuroimmunol. 2011;234:124–130. doi: 10.1016/j.jneuroim.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Kowarik MC, et al. The cerebrospinal fluid immunoglobulin transcriptome and proteome in neuromyelitis optica reveals central nervous system-specific B cell populations. J. Neuroinflammation. 2015;12:19. doi: 10.1186/s12974-015-0240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fenton K, et al. Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice. PLoS One. 2009;4:e8474. doi: 10.1371/journal.pone.0008474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yung S, Chan TM. Anti-DNA antibodies in the pathogenesis of lupus nephritis-the emerging mechanisms. Autoimmun. Rev. 2008;7:317–321. doi: 10.1016/j.autrev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 78.DeGiorgio LA, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 79.Kowal C, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl Acad. Sci. USA. 2006;103:19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen-Solal J, Diamond B. Lessons from an anti-DNA autoantibody. Mol. Immunol. 2011;48:1328–1331. doi: 10.1016/j.molimm.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tradtrantip L, et al. Small-molecule inhibitors of NMO-IgG binding to aquaporin-4 reduce astrocyte cytotoxicity in neuromyelitis optica. FASEB J. 2012;26:2197–2208. doi: 10.1096/fj.11-201608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tradtrantip L, et al. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann. Neurol. 2012;71:314–322. doi: 10.1002/ana.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duan T, Tradtrantip L, Phuan PW, Bennett JL, Verkman AS. Affinity-matured ‘aquaporumab’ anti-aquaporin-4 antibody for therapy of seropositive neuromyelitis optica spectrum disorders. Neuropharmacology. 2020;162:107827. doi: 10.1016/j.neuropharm.2019.107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cobo-Calvo A, et al. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J. Neuroinflammation. 2019;16:134. doi: 10.1186/s12974-019-1525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hacohen Y, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2018;75:478–487. doi: 10.1001/jamaneurol.2017.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malviya M, et al. NMDAR encephalitis: passive transfer from man to mouse by a recombinant antibody. Ann. Clin. Transl. Neurol. 2017;4:768–783. doi: 10.1002/acn3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J. Neurosci. 2012;32:11082–11094. doi: 10.1523/JNEUROSCI.0064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalev-Zylinska ML, Symes W, Young D, During MJ. Knockdown and overexpression of NR1 modulates NMDA receptor function. Mol. Cell Neurosci. 2009;41:383–396. doi: 10.1016/j.mcn.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wasterlain CG, Naylor DE, Liu H, Niquet J, Baldwin R. Trafficking of NMDA receptors during status epilepticus: therapeutic implications. Epilepsia. 2013;54:78–80. doi: 10.1111/epi.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wright S, et al. Epileptogenic effects of NMDAR antibodies in a passive transfer mouse model. Brain. 2015;138:3159–3167. doi: 10.1093/brain/awv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ly LT, et al. Affinities of human NMDA receptor autoantibodies: implications for disease mechanisms and clinical diagnostics. J. Neurol. 2018;265:2625–2632. doi: 10.1007/s00415-018-9042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Melchers F. Checkpoints that control B cell development. J. Clin. Invest. 2015;125:2203–2210. doi: 10.1172/JCI78083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hinson SR, et al. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch. Neurol. 2009;66:1164–1167. doi: 10.1001/archneurol.2009.188. [DOI] [PubMed] [Google Scholar]
- 94.Soltys J, et al. Membrane assembly of aquaporin-4 autoantibodies regulates classical complement activation in neuromyelitis optica. J. Clin. Invest. 2019;129:2000–2013. doi: 10.1172/JCI122942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pittock SJ, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 2019;381:614–625. doi: 10.1056/NEJMoa1900866. [DOI] [PubMed] [Google Scholar]
- 96.Huijbers MG, et al. MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proc. Natl Acad. Sci. USA. 2013;110:20783–20788. doi: 10.1073/pnas.1313944110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koneczny I, Cossins J, Waters P, Beeson D, Vincent A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters. PLoS One. 2013;8:e80695. doi: 10.1371/journal.pone.0080695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Otsuka K, et al. Collagen Q and anti-MuSK autoantibody competitively suppress agrin/LRP4/MuSK signaling. Sci. Rep. 2015;5:13928. doi: 10.1038/srep13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hughes EG, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tuzun E, Christadoss P. Complement associated pathogenic mechanisms in myasthenia gravis. Autoimmun. Rev. 2013;12:904–911. doi: 10.1016/j.autrev.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Engel, A. G. & Fumagalli, G. Mechanisms of acetylcholine receptor loss from the neuromuscular junction. Ciba Found Symp. 197–224 (1982). [DOI] [PubMed]
- 102.Frenzel A, Hust M, Schirrmann T. Expression of recombinant antibodies. Front Immunol. 2013;4:217. doi: 10.3389/fimmu.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peschke B, Keller CW, Weber P, Quast I, Lunemann JD. Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front. Immunol. 2017;8:646. doi: 10.3389/fimmu.2017.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quast I, et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Invest. 2015;125:4160–4170. doi: 10.1172/JCI82695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klooster R, et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain. 2012;135:1081–1101. doi: 10.1093/brain/aws025. [DOI] [PubMed] [Google Scholar]
- 106.van der Neut Kolfschoten M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 107.Fujihara K. Neuromyelitis optica spectrum disorders: still evolving and broadening. Curr. Opin. Neurol. 2019;32:385–394. doi: 10.1097/WCO.0000000000000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lennon VA, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 110.Saadoun S, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dalmau J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18:1045–1057. doi: 10.1016/S1474-4422(19)30244-3. [DOI] [PubMed] [Google Scholar]
- 112.Dalmau J, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dalmau J, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pruss H, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology. 2010;75:1735–1739. doi: 10.1212/WNL.0b013e3181fc2a06. [DOI] [PubMed] [Google Scholar]
- 115.Mikasova L, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–1621. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- 116.Moscato EH, et al. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 2014;76:108–119. doi: 10.1002/ana.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Planaguma J, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2015;138:94–109. doi: 10.1093/brain/awu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramanathan S, Mohammad SS, Brilot F, Dale RC. Autoimmune encephalitis: recent updates and emerging challenges. J. Clin. Neurosci. 2014;21:722–730. doi: 10.1016/j.jocn.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 119.Titulaer MJ, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vincent, A. ANTIBODIES AND RECEPTORS: from neuromuscular junction to central nervous system. Neuroscience439, 48–61 (2020). [DOI] [PubMed]
- 121.Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26:1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- 122.Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357:2122–2128. doi: 10.1016/S0140-6736(00)05186-2. [DOI] [PubMed] [Google Scholar]
- 123.Nowak RJ, Dicapua DB, Zebardast N, Goldstein JM. Response of patients with refractory myasthenia gravis to rituximab: a retrospective study. Ther. Adv. Neurol. Disord. 2011;4:259–266. doi: 10.1177/1756285611411503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McLaughlin KA, et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J. Immunol. 2009;183:4067–4076. doi: 10.4049/jimmunol.0801888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O’Connor KC, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat. Med. 2007;13:211–217. doi: 10.1038/nm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brilot F, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann. Neurol. 2009;66:833–842. doi: 10.1002/ana.21916. [DOI] [PubMed] [Google Scholar]
- 127.Ramanathan, S., Al-Diwani, A., Waters, P. & Irani, S. R. The autoantibody-mediated encephalitides: from clinical observations to molecular pathogenesis. J. Neurol. 1–9 (2019). [DOI] [PMC free article] [PubMed]
- 128.Lopez-Chiriboga AS, et al. LGI1 and CASPR2 neurological autoimmunity in children. Ann. Neurol. 2018;84:473–480. doi: 10.1002/ana.25310. [DOI] [PubMed] [Google Scholar]
- 129.van Sonderen A, Petit-Pedrol M, Dalmau J, Titulaer MJ. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat. Rev. Neurol. 2017;13:290–301. doi: 10.1038/nrneurol.2017.43. [DOI] [PubMed] [Google Scholar]
- 130.Thompson J, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain. 2018;141:348–356. doi: 10.1093/brain/awx323. [DOI] [PMC free article] [PubMed] [Google Scholar]