Highlights
-
•
Allium cepa fortified feed protected against oxidative damage by potassium bromate.
-
•
Malondialdehyde (MDA) levels in the hepato-renal declined significantly in rat fed fortified feed.
-
•
Histo-architectural of hepato-renal cells were restored to near normal following the administration of fortified feed.
Keywords: Allium cepa, Antioxidant, Fortification, Potassium bromate, Histopathology, Haematology
Abstract
Allium cepa Linn (Onion) Organosulfuric compounds and phytonutrients have medicinal benefits. The study estimated the antioxidant effect of Allium cepa in fortified feed against oxidative damage caused by potassium bromate. Commercial feed was fortified by substituting 10 %, 20 %, and 30 % of rat's daily ration with the respective portion of pulverized Allium cepa. Potassium bromate was administered orally to the rats in all the groups except rats in the normal control. The rats in the test groups were allowed access to the fortified feed ad libitum. The animals were sacrificed; consequently, the serum, liver, and kidney were obtained for biochemical assay and histological assessment. The percentage composition of some amino acids and some proximate were higher in the fortified feed. Furthermore, Malondialdehyde (MDA) level in the liver, kidney, and serum decreased significantly (P ≤ 0.05) in rats fed with fortified feed compared to administered only Potassium bromate. Similarly, the concentration of total protein increased significantly (P ≤ 0.05) in the liver, kidney, and serum of the animals fed with fortified feed. The hematology result was normal in rats fed with fortified feed. The liver and kidney cell architecture was normal in animals fed with fortified feed. Allium cepa may have conferred protection and amelioration to oxidative damage by potassium bromate in rats.
1. Introduction
Allium cepa Linn (Onion) is a valuable vegetable/field crop cultivated for food, medicine, spices and condiments worldwide since times [1]. As a vegetable crop, it ranked the second most widely cultivated after tomato production [2]. Allium cepa is a vegetable consumed worldwide; it is also a rich source of phytochemicals and organosulphur dietary compounds that are considered to possess antioxidant properties and can also enhance systemic detoxification [3]. The prevalence of certain oxidative stress-related degenerative diseases [4] is troubling and most research focuses on seeking prevention or treatment of these diseases and other pathological conditions from natural sources such as plants used as spices or their isolated compounds [5]. Some of the health benefits reported of Allium cepa are demonstrated by its use as anti-tumour, anti-cancer, anti-inflammatory and antihypertensive [6]. Because of little side effect by plant-derived natural product attentions are drifting toward its utilization in chemo-prevention against degenerative diseases.
Previous studies in this direction have led to the identification of some phytochemicals that have medical advantages. Diallyl disulfide (DADS) is one of the health beneficial secondary metabolites founds mainly in garlic and onions which is responsible for the obvious anti-carcinogenic property of garlic and onions [7]. These compounds, as well as other phytochemicals found in onions, have the potential to protect body cells from free radicals linked to oxidative stress, which is characterized as an imbalance between the oxidant and antioxidant systems that favors the former, resulting in changes in redox signaling and control, as well as molecular damage [8,9]. Some researchers have also explored Allium cepa as a bioindicator of environmental pollution [10,11] and to estimate the genotoxic potential of medicinal plants [12,13]. Sulfur-containing compounds found in onions and garlic are attributed to their unique odour and flavour associated with Allium cepa and garlic, hence, constituting about 1 % and 0.5 % of the dry weight of garlic and onions respectively [14]. Allium cepa serves as a dietary source for carbohydrates, fibres, potassium, iron and vitamin C.
Potassium bromate (KBrO3) is extensively utilized in baking to improve and promote the rise of the dough [15]. Potassium bromate has been reported to have a severe and irreversible sensor neural hearing loss as well as renal failure [16]. From the previous study, bromate has been documented to produce oxygen species either directly or indirectly by intracellular reductant reaction [44]. With all this scientific report against bromate, it is still being used because of the quality of the results it produces and its affordability. Also, studies have shown that onions are abundant in complex sugars, compounds of sulphur, proteins, glycosides, flavonoids, saponins, and minerals. Golubev et al. [45]; therefore, this work investigated the onion's antioxidant potential when used as a food fortifier to ameliorate the oxidative stress due to potassium bromate.
2. Materials and methods
2.1. Preparation of Allium cepa fortified feed and authentication
Onion bulbs were purchased at a local market in Ilorin, Kwara State, Nigeria and authenticated at the Department of Plant Biology, the University of Ilorin with voucher number UILH/001/1332. The onion bulbs were peeled washed sliced and air-dried for a week. The dried onions were pulverized into powder. The commercial feed was fortified by substituting 10 % (10 % Allium cepa + 90 % normal feed), 20 % (20 % Allium cepa + 80 % normal feed) and 30 % (30 % Allium cepa + 70 % normal feed) of rat’s daily ration with respective portion of pulverized.
2.2. Evaluation of proximate and amino acids composition
Proximate quantification (ash content, protein, starch, moisture, fibre, sugar, calcium, phosphorus and fat) and amino acids profiling (leucine, arginine, cysteine, isoleucine, lysine, methionine, threonine, tryptophan and valine) of the fortified feed and normal feed was carried out using automated Perfect analyzer (Idexx, Sweden) and Amino acid analyzer (Jenway, Germany)
2.3. Animal grouping and treatments
Thirty male Wistar rats with an average weight of 200 ± 0.5 g were randomly distributed into five groups with five rats per group:
Negative control group: animals in this group were orally administered 0.2 mL distilled water
Positive control group: rats in this group were orally administered potassium bromate
10 %, 20 % and 30 % allium cepa groups: rat in these groups were orally administered potassium bromate and then fed commercial feed fortified with 10, 20 and 30 % allium cepa respectively. 0.2 mL 10 mg/kg body weight [17] potassium bromate was the dose administered orally to the rats.
2.4. Ethical approval, tissue collection and homogenization
The experimental animals were sacrificed 24 h after the 14-day treatment according to the ethical guideline of the American Veterinary Medical Association [18]. The ethical approval for the use and handling of the animal was obtained from Landmark University, Omu-Aran, Nigeria with an approval number LUAC-0030B. The experimental rats were anaesthetized after which they were sacrificed according to the standard method. The jugular vein was cut using a surgical blade and blood was collected in a sterile sample bottle; serum was extracted from the blood and stored frozen. In addition, the liver and kidneys of the sacrificed animals were extracted and homogenized in an ice-cold solution of 0.25 M sucrose [1:5 w/v] using a Teflon homogenizer. The homogenized tissue was centrifuged at 5000 rpm for 15 min; the supernatant was stored in a clean sample bottle and kept frozen until required for analysis.
2.5. Biochemical assay
Biochemical parameters in the tissue homogenate and serum of experimental rats were estimated using the Digital UV / VIS spectrometer (Single-cell holder Jenway visible spectrophotometer Model 6705 Series No 40143, England). Alanine transaminase activity, aspartate transaminase albumin and bilirubin concentrations were quantified using the respective assay kit (Randox Laboratories LTD, United Kingdom). Haematological parameters were estimated by automated Beckman Coulter (Beckman Coulter Inc. Brea CA, USA).
2.5.1. Organ-body weight ratio
The organ-body weight ratio is the organ weight to the total body weight according to the method of Gornall et al. [19]: Organ weight / total body weight x 100
2.5.2. Total protein concentration
Protein concentration was estimated following the method of Gornall et al. 13. 4 mL of biuret reagent was added to 1 mL of sample homogenate. The mixture was allowed to stand for 30 min and read at 340 nm. The Protein concentration was determined as follow: Cs × F (mg ml-1) where: Cs- corresponding protein concentration from the standard curve and F is the dilution factor
2.5.3. Reduced glutathione assay
The GSH level was estimated by the method estimated by Jollow et al. [20]. An aliquot of the homogenate/serum was deproteinized by the addition of an equal volume of 4 % sulfosalicylic acid and then centrifuged at 5000 r.p.m for 15 min at 2 °C. 0.5 mL of the supernatant was then added to the 4.5 mL Ellman reagent. A blank was prepared with 0.5 mL of sulfosalicylic acid in 0.1 mL phosphate buffer (twice dilution) and 4.5 mL of Ellman reagent. The calibration curve was also prepared: serial dilution of GSH solution was added different volume of phosphates buffer (pH 7.4) to make a total volume of 5 mL with 4.5 mL Ellman reagent. The mixture will turn a yellow colour and the absorbance take 412 nm.
2.5.4. Superoxide dismutase assay
The activity of SOD was estimated by the method described by Misra and Fridovich [21]. 0.2 mL of homogenate/serum was added to the mixture of 2.5 mL 0.05 M phosphate buffer (pH10) and 0.3 mL 0.3 mM adrenaline. The increase in absorbance at 480 nm was monitored every 30 s for a period of 150 s.
2.5.5. Lipid peroxidation
The level of lipid peroxidation in the tissue/serum was estimated by the thiobarbituric acid reaction method described by Kei [22]. 200 μL approximately diluted sample was added to the mixture of 250 μL 30 % trichloroacetic acid, 250 μL 0.07 % thiobarbituric acid and 800 μL Tris-KCl. The reaction was heated for 45 min at 800C for colour development. The reaction was then centrifuged at 5000 rpm for 4 min. The absorbance of the upper layer that is the organic phase was then taken at 532 nm.
2.5.6. Alkaline phosphatase assay
The assay was carried out on the tissue homogenate/serum by the method estimated by Wright et al. [23]. 2.2 mL concentrated sodium acetate buffer (pH 4.5) was mixed with an approximately diluted sample and then the mixture will be allowed to be incubated for 10 min. 0.5 mL 10 mM PNPP (substrate) was then added to the mixture and then allowed to incubate at 37ᵒC. The reaction was stopped by the addition of 2 mL 1 M sodium hydroxide and the absorbance was read at 400 nm.
2.6. Histological evaluation
The histological examination of the fixed tissue was carried out using the standard method [24]. The representative portions of the extracted liver and kidney of sacrificed rats were fixed in 10 % buffered formalin (pH 7.4) for 12 h and then embedded in paraffin. The tissue also was embedded in paraffin for some time and subsequently sectioned (5 mm) using a microtome. The tissue must then be stained with hematoxylin and eosin before being mounted in Canada balsam and then sectioned. The stained sections then were viewed under the light microscope and their histological features captured using Sony DSC-W35.
2.7. Statistical analyses
Biochemical data in this study were expressed as mean ± SEM. One- way analysis of variance was used followed by Turkey post-hoc mean comparison test for significant differences estimation of variables at P-value less than 0.05. Statistical Package for Social Science (SPSS version 22) was used for all the statistical evaluation of the data.
3. Results
The percentage content of moisture, fibre and ash of the fortified feed was higher compared to the commercial feed (Table 1A) Similarly, the percentage content of isoleucine (Ile), methionine (Met) and tryptophan (Trp) were higher in the fortified feed compared to commercial feed (Table 1B). Triterpene, Steroids, Flavonoids, Coumarins, Alkaloids, Terpenoids, Phenolics, Tannins, Saponins, and Glycosides were present in Allium cepa with the highest quantity of triterpene and the lowest quantity of glycoside (Table 2). White blood cell (WBC), haemoglobin (HGB) and haematocrit (HCT) increased significantly in animals feed fortified (Table 3).
Table 1.
Proximate Indices (A) and Amino acid content (B) of commercial rat’s feed and Allium cepa (Onion) supplemented feed.
| A | ||
|---|---|---|
| % Proximate Indices | Commercial feed | Allium cepa Compounded feed |
| Moisture | 10.33 | 13.1 |
| Protein | 13.73 | 10.75 |
| Fat | 5.86 | 4.05 |
| Fibre | 5.55 | 31.65 |
| Starch | 37.84 | 19.1 |
| Ash | 8.92 | 9.31 |
| Sugar | 4.67 | 3.71 |
| Calcium | 1.07 | 0.21 |
| Phosphorus | 0.66 | 0.19 |
| B | ||
|---|---|---|
| % Amino acids content | Commercial rat feed | Allium cepa compounded feed |
| Arginine | 2.21 | 1.78 |
| Cysteine | 0.49 | 0.43 |
| Isoleucine | 1.04 | 1.14 |
| leucine | 1.37 | 1.03 |
| Lysine | 3.82 | 2.31 |
| Methionine | 0.46 | 0.55 |
| Threonine | 0.74 | 0.61 |
| Tryptophan | 1.1 | 1.11 |
| Valine | 0.96 | 0.65 |
Table 2.
Phytochemical constituents of Allium cepa (Onion).
| S/N | Phytochemical | Quantity |
|---|---|---|
| 1. | Triterpene | 256.90 ± 0.01 |
| 2. | Steroids | 249.07 ± 0.15 |
| 3. | Flavonoids | 206. 27 ± 0.35 |
| 4. | Coumarins | 43.32 ± 0.15 |
| 5. | Alkaloids | 38.17 ± 0.01 |
| 6. | Terpenoids | 31.47 ± 0.02 |
| 7. | Phenolics | 15.51 ± 0.03 |
| 8. | Tannins | 7.46 ± 0.01 |
| 9. | Saponins | 5.06 ± 0.01 |
| 10. | Glycosides | 3.47 ± 0.02 |
Table 3.
Effect of supplemented feed on the haematological parameters of rats pre-fed with potassium bromate.
| Treatment/Parameters | RBC | WBC | HGB | HCT | MCH | MCHC | MCV | PLT |
|---|---|---|---|---|---|---|---|---|
| negative control | 5.59±0.59b | 10.17±1.55a | 10.6±1.06ab | 35.47±3.72ab | 19.30±1.51ab | 29.9±0.25a | 64.43±4.47b | 585.67±82.60a |
| Positive control | 4.74±0.93b | 3.39±0.74a | 4.85±1.21a | 3.85±0.32a | 34.00±6.35b | 32.67±6.94a | 33.04±1.12a | 504.67±63.46a |
| 10 % Allium cepa+feed | 1.47±1.30a | 1.01±0.58a | 11.73±1.22b | 9.80±8.70a | 11.11±10.45a | 16.42±15.69a | 22.7±22.15a | 511.33±43.51a |
| 20 % Allium cepa+feed | 3.43±1.54ab | 5.92±2.90a | 9.07±1.29ab | 22.90±9.67ab | 13.59±6.47ab | 21.07±10.41a | 80.3±16.56b | 667.33±135.02a |
| 30 % Allium cepa+feed | 2.56±0.85ab | 8.03±6.37a | 9.07±1.63ab | 14.93±6.53ab | 22.5±3.41ab | 32.17±11.42a | 60.3±2.74b | 761.00±272.40a |
Values are represented as mean ± SEM of Three replicates. abcStatistical difference relative to controls at P ≤ 0.05.
RBC: Red blood cell, HGB: Haemoglobin, HCT: Haematocrit, MCH: Mean Corpuscular Haemoglobin, MCHC: Mean Corpuscular Haemoglobin Concentration, WBC: White Blood Cell, PLT: Platelets Concentration MCV: and MCV: Mean Corpuscular Volume.
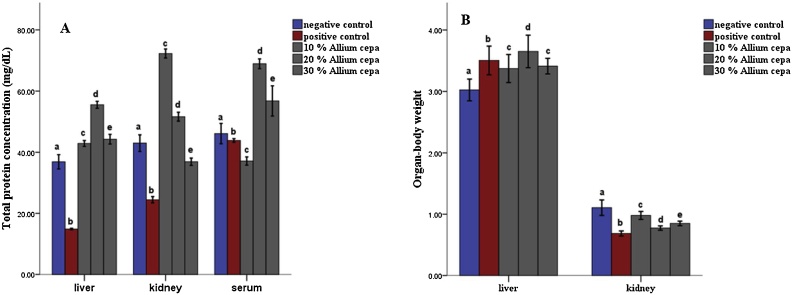
The total protein concentration showed a significant increase (P ≤ 0.05) across the groups that were fed fortified feed compared to the negative control while the positive control showed a significant decrease in total protein concentration compared to the negative (Fig. 1A). Also in the kidney, there was a significant increase (P ≤ 0.05) in the total protein concentration in the groups administered 10 and 20 % allium cepa fortified food compared to the negative control but the groups that were given 30 % Allium cepa fortified feed and the positive control showed a significant increase in total protein concentration (Fig. 1A). The total protein concentration in serum showed a significant increase (P ≤ 0.05) in the animals fed 20 and 30 % Allium cepa fortified feed while a significant decrease (P ≤ 0.05) was observed in the groups fed 10 % Allium cepa fortified feed and the positive control (Fig. 1A). The organ-body weight ratio of the liver showed a significant increase in the positive control and across the groups feed fortified feed compared to the negative control (Fig. 1B), while in the that of the kidney, there was a significant decrease across the treated group (Fig. 1B).
Fig. 1.
Total protein concentration (A) and Organ-body ratio (B) of tissues of rats pre-fed with potassium bromate and Allium cepa (Onion) supplemented rat’s feed ad libitum. Mean ± SEM of three variables. Bars with different superscripts are significantly different (P ≤ 0.05).
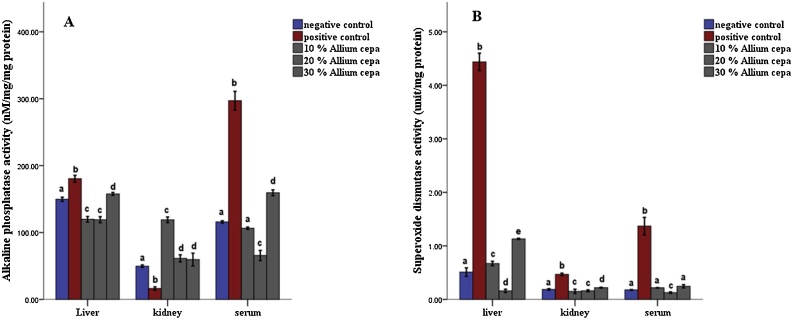
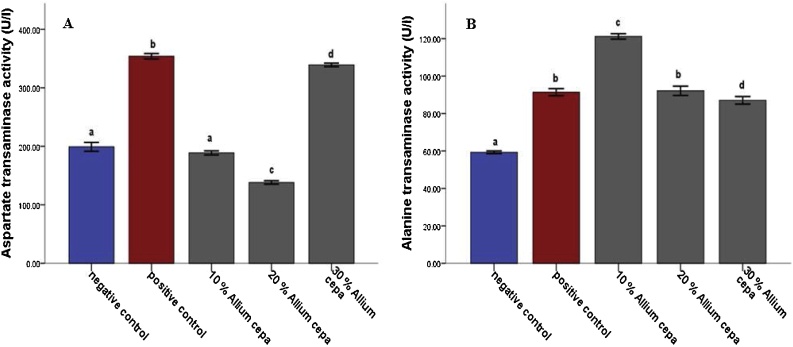
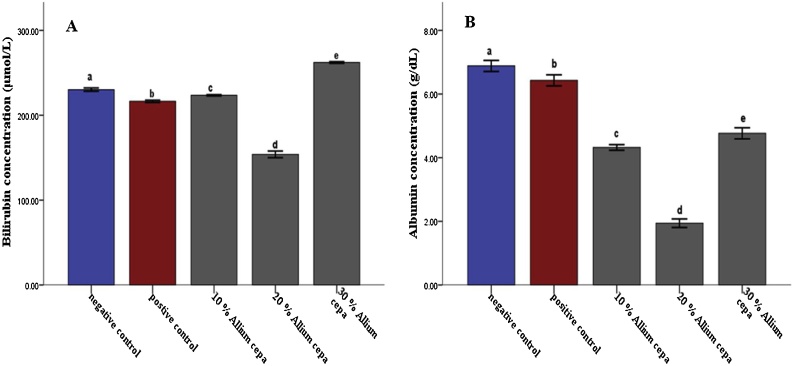
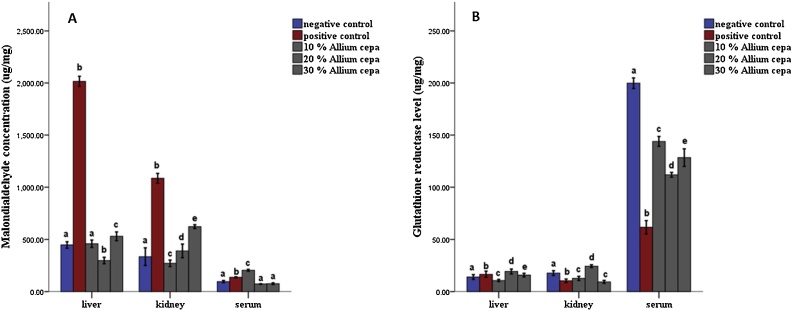
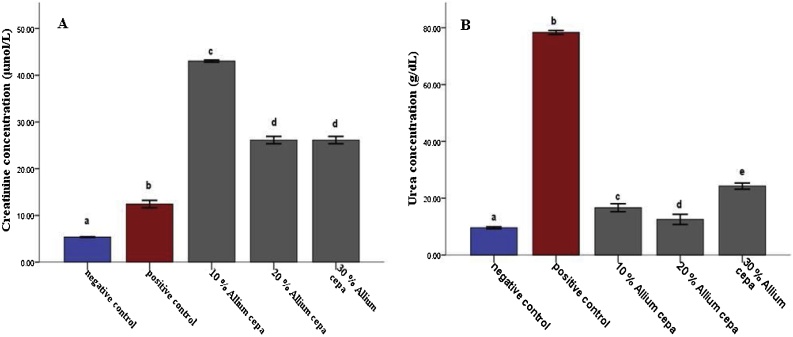
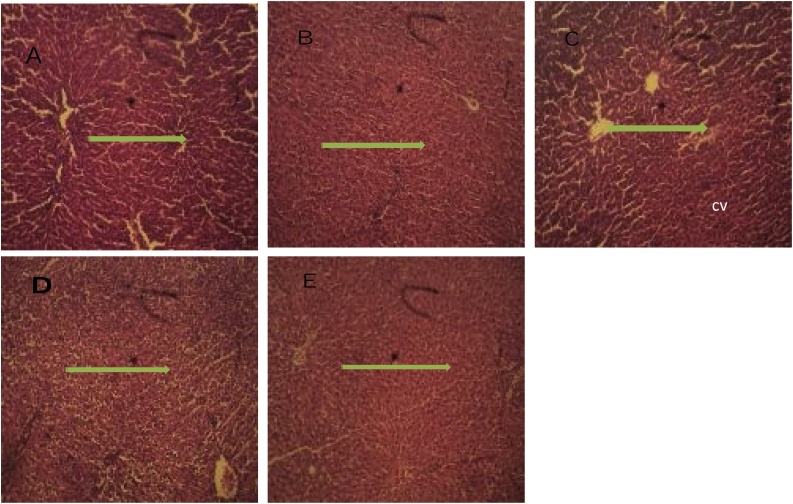
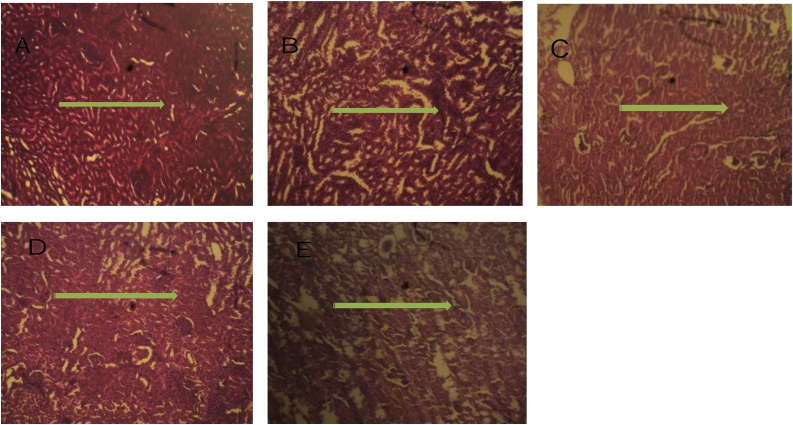
In the animals feed fortified feed, the Alkaline phosphatase showed decreased activity (P ≤ 0.05) in the liver and serum and increased activity in the kidney, conversely, the enzyme showed increased activity in the group administered potassium bromate only in liver and serum and decreased activity in the kidney compared to the normal control (Figs. 1 and 2A). Similarly, the serum alanine transaminase activity showed a significant increase (P ≤ 0.05) in anigmals in the potassium bromate group and test groups compared to the normal control (Figs. 2A and 4B), contrary, there was a significant increase in the serum aspartate transaminase activity in the animals administered potassium bromate only compared to the animals of the normal control and test groups (Figs. 2B and 4A). Furthermore, there was a significant increase in serum albumin (Figs. 2C and Fig. 5B) and a significant decrease (P ≤ 0.05) in serum bilirubin (Figs. 2D and 5A) in rats feed fortified feed compared to the animals in normal control and potassium bromate. Serum creatinine concentration was increased significantly (P ≤ 0.05) in the animals in all the treated groups compared to those in the normal control (Figs. 3A and 6A), and there was a significant reduction in urea concentration in the animals fed fortified feed. There was also a significant increase (P ≤ 0.05) in the serum urea concentration of the animals administered potassium bromate only (Figs. 3B and 6B). The SOD activities in the liver, kidney, and serum increased in the rat fed only potassium bromate compared to the rats' in the normal group and fortified feed groups (Figs. 2B and 4A). Similarly, there was a significant decrease (P ≤ 0.05) in GSH concentration in the kidney and serum in the potassium bromate group compared to normal control and those fed fortified feed (Figs. 3B and 4B). There was a significant increase (P ≤ 0.05) in the MDA concentration in the liver kidney and serum in the rats' given only potassium bromate (Figs. 3A and 4C) compared to the other groups. The photomicrograph of liver and kidney of rats administered potassium bromate only showed slight degeneration and distortion in the hepatic and nephrotic cells compared to the rats feed fortified feed which showed normal cell (Fig. 7, Fig. 8).
Fig. 2.
Alkaline phosphatase (A) and Superoxide dismutase activity (B) in tissues of rats pre-fed with potassium bromate and Allium cepa (Onion) supplemented rat’s feed ad libitum. Mean ± SEM of three variables. Bars with different superscripts are significantly different (P ≤ 0.05).
Fig. 4.
Effect of Allium cepa (Onion) fortified feed on Aspartate transaminase activity (A) and Alanine transaminase activity (B) and, in serum of rats administered potassium bromate. Mean ± SEM of three variables. Bars with different superscripts are significantly different (P ≤ 0.05).
Fig. 5.
Effect of Allium cepa (Onion) fortified feed on Bilirubin concentration (A) and Albumin concentration (B) in serum of rats administered potassium bromate. Mean ± SEM of three variables. Bars with different superscripts are significantly different (P ≤ 0.05).
Fig. 3.
Effect of Allium cepa (Onion) fortified feed on (A) Malondialdehyde and (B) Glutathione reductase concentration in tissues of rats administered potassium bromate. Mean ± SEM of three variables. Bars with different superscripts are significantly different (P ≤ 0.05).
Fig. 6.
Effect of Allium cepa (Onion) fortified feed on serum Urea (A) and Creatinine (B) concentrations in rats administered potassium bromate. Mean ± SEM of three variables. Bars with different superscripts are significantly different (P ≤ 0.05).
Fig. 7.
Photomicrograph of liver cross section (H&E X100): A (Negative control), Micrograph showed a grossly normal hepatic nodule. B (Positive control), Micrograph showed infiltrated hepatic tissue with cell proliferation. C (10 % Allium cepa in the feed), Micrograph showed an infiltrated hepatic tissue with slightly distorted central vein and cell proliferation. D (20 % Allium cepa in the feed), Micrograph showed a distorted and infiltrated hepatic tissue. E (30 % Allium cepa in the feed), Micrograph showed a fairly normal hepatic tissue with mild infiltration.
Fig. 8.
Photomicrograph of kidney cross section (H&E X100): A (Negative control), Micrograph showed a normal nephritic cell. B (Positive control), Micrograph showed a highly proliferated renal tissue with slightly distorted glomeruli. C (10 % Allium cepa in the feed), Micrograph shows a slightly distorted renal tissue with cell proliferation around the glomerulus, D (20 % Allium cepa in the feed), Micrograph showed a slightly distorted renal tissue with cell proliferation around the glomerulus E (30 % Allium cepa in the feed), Micrograph showed a grossly normal renal tissue.
4. Discussion
Feed fortification with Allium cepa improved the quality of feed which was shown by the differing percentage contents of amino acids and proximate compositions of the fortified feed compared to commercial feed. The high fibre content of the fortified feed will aid in the binding of potassium bromate through chelation or electrostatic force [25]. The ameliorating effect of the fortified feed against oxidative damage induced by potassium bromate was due to the antioxidant bioactive constituent of Allium cepa [26].
The ameliorating effect reported in the study could be due to the antioxidant phytochemical compound such as flavonoids and phenolics contained in the onions as was shown in this study. Previous studies reported that Allium cepa is rich in phenols and sulphur-containing compounds which were implicated in many health and beneficial effect [27,28]. The antioxidant feature of the onion, as well as intracellular GSH, may have provided protection against oxidative damage to blood cells and the haematopoietic system caused by potassium bromate [29]. Moreover, organ-body weight is a sensitive indicator of the effect of the test particle, as significant differences in organ weight between treated and untreated animals may occur in the absence of any morphological changes [30].
The decreased kidney organ-body weight could as a result of kidney cell death by potassium bromate; however, this effect was ameliorated in the rat’s fed fortified feed giving credence to the antioxidant principles in Allium cepa; but the increased metabolic functions of the liver due to assault by potassium bromate might be responsible for the increase in liver organ body ratio. Potassium bromate appeared to inhibit the protein synthesis but did not have the same effect in the animals fed fortified feed due to protection by Allium cepa. ALP is an intra-membrane enzyme, a biomarker of membrane integrity [31]. The decreased activity in the kidney of animal administered potassium bromate only could be due to the kidney cell membrane lipid peroxidation. Similarly, damage to the cell may have led to enzyme leakage in the serum which was responsible for the increased activity in the serum. Meanwhile, the increase in liver ALP activity may be due to adaptive de-novo synthesis. Aspartate and Alanine aminotransferases found in the cell cytoplasm are biomarkers of liver function; however, AST is not as specific as compared to ALT [32].
The effect of potassium bromate was abated in the animals fed fortified feed. Moreover, de-novo synthesis, an adaptive mechanism by cell due to assault to them KBrO3 explained the momentary increase in ALT and AST in rats’ fed fortified feed. The Cu2+ and Fe3+ which are pro-oxidative in free form, usually interact with hydroxyl ion to form oxygen radicals, similarly, the free thiol group in cyt 34 can scavenge hydroxyl radicals. The decreased level in serum albumin could be due to the increased breakdown of Albumin as a result of its oxidative function. Previous studies reported that the breakdown becomes rapid in a bound form and usually after oxidation, glycation and binding of pro-oxidative substances [33,34]. Bilirubin is the final product from the breakdown of heme mediated by heme oxygenase [35]. Decreased levels of serum bilirubin in rats in this study may suggest that potassium bromate oxidative damage to the red cell is not pronounced. The increased concentration of serum urea was due to the harmful effect of KBrO3, but the effect was decreased in rats fed with fortified feed. Malondialdehyde (MDA), is one of the most abundant carbonyl products of lipid peroxidation and biomarker of oxidative stress [36]. The free radical scavenging activity of the fortified feed was responsible for the reduced MDA level in all the tissues of rats fed with fortified feed; this aligns with the study of Nuutila et al. [37] which reported on high scavenging activities of onions.
Similarly, the increased oxidative damage by potassium bromated was responsible for the increased SOD activity in rats administered Potassium bromate only, however, Allium cepa proffers antioxidant first line of defence against ROS in the groups administered fortified feed. The interplay between SOD, catalase and glutathione have been reported in the previous studies to have a protective role on SOD against the overwhelming effect of high concentration of ROS in the cell, which inhibits the activity of SOD. The decreased level of glutathione reductase in the potassium bromate group in this study could be due to antioxidant activity on SOD and its direct mopping of ROS in the cell [38,39]. Furthermore, increased level of glutathione reductase, in the animals fed fortified feed, gave credence to the antioxidant property of Allium cepa. Creatinine, a metabolic intermediate of muscle metabolism that is cleared through glomeruli of the nephron [40]. The increased serum creatinine levels in the group fed fortified feed in this study might be as a result of a transient increase induced by Allium cepa [41]. Furthermore, the normal architectural cells of the liver and kidney gave credence to the protective potency of Allium cepa in the fortified feed. This may be due to the ameliorating activity of the Allium cepa against oxidative stress.
4.1. Conclusion
In rat kidney and liver cells, potassium bromate caused oxidative damage, as evidenced by increased MDA levels and other biochemical changes. The antioxidant and ameliorative effects of the fortified feed were also demonstrated in the study, which could be attributed to the antioxidant principles contained in Allium cepa.
Author statement
The authors declare that this manuscript is original and is not been considered for publication elsewhere.
Conflict of interest
The authors declare no conflict of interest.
Declaration of Competing Interest
The authors report no declarations of interest.
Edited by Dr. A.M. Tsatsaka
References
- 1.El-Aziz M.A., Morsi S.M.M., Salama D.M., Abdel-Aziz M.S., Elwahed M.S.A., Shaaban E.A., Youssef A.M. Preparation and characterization of chitosan/polyacrylic acid/copper nanocomposites and their impact on onion production. Int. J. Biol. Macromol. 2019;123:856–865. doi: 10.1016/j.ijbiomac.2018.11.155. [DOI] [PubMed] [Google Scholar]
- 2.Bahram‐Parvar M., Lim L.T. Fresh‐cut onion: a review on processing, health benefits, and shelf‐life. Compr. Rev. Food Sci. Food Saf. 2018;17(2):290–308. doi: 10.1111/1541-4337.12331. [DOI] [PubMed] [Google Scholar]
- 3.Jaiswal N., Kumar D., Rizvi S.I. Red onion extract (Allium cepa L.) supplementation improves redox balance in oxidatively stressed rats. Food Sci. Hum. Wellness. 2013;2(2):99–104. [Google Scholar]
- 4.Ojo O.A., Ajiboye B., Fadaka A., Taro P., Shariati M.A. Nrf2-Keap1 activation, a promising strategy in the prevention of cancer. Free Radic. Antioxid. 2017;7(1):1–7. [Google Scholar]
- 5.Sengupta A., Ghosh S., Das S. Modulatory influence of garlic and tomato on cyclooxygenase-2 activity, cell proliferation and apoptosis during azoxymethane induced colon carcinogenesis in rat. Cancer Lett. 2004;208(2):127–136. doi: 10.1016/j.canlet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang H., Hou K., Peng W., Liu Z., Deng H. Antioxidant and xanthine oxidase inhibitory activities of total polyphenols from onion. Saudi J. Biol. Sci. 2018;25(7):1509–1513. doi: 10.1016/j.sjbs.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somade O.T., Ugbaja R.N., Alli A.A., Odubote O.T., Yusuf T.S., Busari B.T. Diallyl disulfide, an organo-sulfur compound in garlic and onion attenuates trichloromethane-induced hepatic oxidative stress, activation of NFkB and apoptosis in rats. J. Nutr. Intermed. Metab. 2018;13:10–19. [Google Scholar]
- 8.Sies H., Jones D. Oxidative Stress. Encycl. Stress. 2007:45–48. doi: 10.1016/B978-012373947-6.00285-3. [DOI] [Google Scholar]
- 9.Kouka P., Tekos F., Papoutsaki Z., Stathopoulos P., Halabalaki M., Tsantarliotou M. Olive oil with high polyphenolic content induces both beneficial and harmful alterations on rat redox status depending on the tissue. Toxicol. Rep. 2020;7:421–432. doi: 10.1016/j.toxrep.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leme D.M., Marin-Morales M.A. Allium cepa test in environmental monitoring: a review on its application. Mutat. Res. Mutat. Res. 2009;682(1):71–81. doi: 10.1016/j.mrrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Camparoto M.L., Teixeira R.D.O., Mantovani M.S., Vicentini V.E.P. Effects of Maytenus ilicifolia Mart. and Bauhinia candicans Benth infusions on onion root-tip and rat bone-marrow cells. Genet. Mol. Biol. 2002;25(1):85–89. [Google Scholar]
- 12.Lubini G., Fachinetto J.M., Laughinghouse H.D., Paranhos J.T., Silva A.C., Tedesco S.B. Extracts affecting mitotic division in root-tip meristematic cells. Biologia. 2008;63(5):647–651. [Google Scholar]
- 13.Owolarafe T.A., Salawu K., Ihegboro G.O., Ononamadu C.J., Alhassan A.J., Wudil A.M. Investigation of cytotoxicity potential of different extracts of Ziziphus mauritiana (Lam) leaf Allium cepa model. Toxicol. Rep. 2020;7:816–821. doi: 10.1016/j.toxrep.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee E.J., Yoo K.S., Jifon J., Patil B.S. Characterization of shortday onion cultivars of 3 pungency levels with flavor precursor, free amino acid, sulfur, and sugar contents. J. Food Sci. 2009;74(6):C475–C480. doi: 10.1111/j.1750-3841.2009.01243.x. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad M.K., Naqshbandi A., Fareed M., Mahmood R. Oral administration of a nephrotoxic dose of potassium bromate, a food additive, alters renal redox and metabolic status and inhibits brush border membrane enzymes in rats. Food Chem. 2012;134(2):980–985. doi: 10.1016/j.foodchem.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Saad H.B., Driss D., Jaballi I., Ghozzi H., Boudawara O., Droguet M., Amara I.B. Potassium bromate-induced changes in the adult mouse cerebellum are ameliorated by vanillin. Biomed. Environ. Sci. 2018;31(2):115–125. doi: 10.3967/bes2018.014. [DOI] [PubMed] [Google Scholar]
- 17.Nwonuma C.O., Irokanulo E.O., Iji C.E., Alejolowo O.O., Adetunji C.O. Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity. Asian Pacific J. Reprod. 2016;5(6):500–505. [Google Scholar]
- 18.Underwood W., Anthony R., Cartner S., Corey D., Grandin T., Greenacre C.B. 2013 Edition. American Veterinary Medical Association; Schaumburg, IL: 2013. AVMA Guidelines for the Euthanasia of Animals. [Google Scholar]
- 19.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177(2):751–766. [PubMed] [Google Scholar]
- 20.Jollow D.J., Mitchell J.R., Zampaglione N.A., Gillette J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 21.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 22.Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 1978;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 23.Wright P.J., Leathwood P.D., Plummer D.T. Enzymes in rat urine. Alkaline phosphatase. Enzymologia. 1972;42:317–327. [PubMed] [Google Scholar]
- 24.Hewitson T.D., Darby I.A., editors. Histology Protocols. Humana Press; New York, USA: 2010. p. 229. [Google Scholar]
- 25.Champagne E.T. Effects of pH on mineral-phytate, protein-mineral-phytate, and mineral-fiber interactions. Possible consequences of atrophic gastritis on mineral bioavailability from high-fiber foods. J. Am. Coll. Nutr. 1988;7(6):499–508. doi: 10.1080/07315724.1988.10720266. [DOI] [PubMed] [Google Scholar]
- 26.El-Zainy A.R.M., El-Zamzamy F.M., Shalaby A.O.A., Mostafa M.Y.A. Protective effect of oat biscuits containing herbal oils on potassium bromate induced high oxidative stress rats. Res. J. Agric. Biol. Sci. 2014;10(2):93–108. [Google Scholar]
- 27.Bystrická J., Musilová J., Vollmannová A., Timoracká M., Kavalcová P. Bioactive components of onion (Allium cepa L.)—a review. Acta Aliment. 2013;42(1):11–22. [Google Scholar]
- 28.Gawlik-Dziki U., Świeca M., Dziki D., Baraniak B., Tomiło J., Czyż J. Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem. 2013;138(2–3):1621–1628. doi: 10.1016/j.foodchem.2012.09.151. [DOI] [PubMed] [Google Scholar]
- 29.Parsons J.L., Chipman J.K. The role of glutathione in DNA damage by potassium bromate in vitro. Mutagenesis. 2000;15(4):311–316. doi: 10.1093/mutage/15.4.311. [DOI] [PubMed] [Google Scholar]
- 30.Bailey S.A., Zidell R.H., Perry R.W. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol. Pathol. 2004;32(4):448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- 31.Amin K.A., Hameid H.A., II, Elsttar A.A. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem. Toxicol. 2010;48(10):2994–2999. doi: 10.1016/j.fct.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 32.Ozer J., Ratner M., Shaw M., Bailey W., Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Roche M., Rondeau P., Singh N.R., Tarnus E., Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 34.Soeters P.B., Wolfe R.R., Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. J. Parenter. Enter. Nutr. 2019;43(2):181–193. doi: 10.1002/jpen.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valášková P., Muchová L. Metabolism of bilirubin and its biological properties. Klinická biochemie a metabolismus. 2016;24(4) [Google Scholar]
- 36.Ahmad R., Tripathi A.K., Tripathi P., Singh S., Singh R., Singh R.K. Malondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemia. In Vivo. 2008;22(4):525–528. [PubMed] [Google Scholar]
- 37.Nuutila A.M., Puupponen-Pimiä R., Aarni M., Oksman-Caldentey K.M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003;81(4):485–493. [Google Scholar]
- 38.Flores-Mateo G., Carrillo-Santisteve P., Elosua R., Guallar E., Marrugat J., Bleys J., Covas M.I. Antioxidant enzyme activity and coronary heart disease: meta-analyses of observational studies. Am. J. Epidemiol. 2009;170(2):135–147. doi: 10.1093/aje/kwp112. [DOI] [PubMed] [Google Scholar]
- 39.Anwar F., Przybylski R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.) Acta Sci. Pol. Technol. Aliment. 2012;11(3):293–302. [PubMed] [Google Scholar]
- 40.Hall J.E. Elsevier Health Sciences; 2015. Guyton and Hall Textbook of Medical Physiology e-Book. [Google Scholar]
- 41.Payne R.B. Creatinine clearance: a redundant clinical investigation. Ann. Clin. Biochem. 1986;23(3):243–250. doi: 10.1177/000456328602300304. [DOI] [PubMed] [Google Scholar]
- 44.Chipman J.K., Parsons J.L., Beddowes E.J. The multiple influences of glutathione on bromate genotoxicity: implications for the dose–response relationship. Toxicology. 2006;221(2–3):187–189. doi: 10.1016/j.tox.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Golubev F.V., Golubkina N.A., Gorbunov Y.N. Mineral composition of wild onions and their nutritional value. Appl. Biochem. Microbiol. 2003;39(5):532–535. [Google Scholar]