Class switch recombination (CSR) enables activated mature B cells to change immunoglobulin (Ig) isotype expression from IgM to IgG, IgE, or IgA. The process requires activation-induced cytidine deaminase (AID) expression and transcription of the target sequences at the Ig heavy chain (IgH) constant locus, which is composed of multiple constant (CH) genes, each specifying an Ig isotype.1 The CH genes are organized in transcription units made up of I promoters/exons upstream of highly repetitive sequences called switch (S) sequences and the CH exons. Signal-dependent induction of switch transcription at downstream S sequences is required for recombination with the constitutively transcribed Sµ sequence, the universal switch donor site.1 CSR can also occur in developing B cells, albeit at a lower frequency than in mature cells. The signaling pathways and transcriptional regulatory elements that control CSR in developing B cells are still ill-known. There is evidence that signaling through Toll-like receptors induces AID expression and CSR at early developmental stages,2–4 and some cis-acting elements are involved in preventing switch transcription in developing cells.5
Interleukin 7 (IL7) is a nonredundant cytokine that plays an important role in early B-cell development.6,7 IL7, via signaling through its receptor (IL7R), regulates multiple fundamental and pathological processes, including proliferation, survival, V(D)J recombination, gene expression, autoimmunity, and leukemia, e.g., refs. 8–12 However, its role in the regulation of switch transcription is unknown.
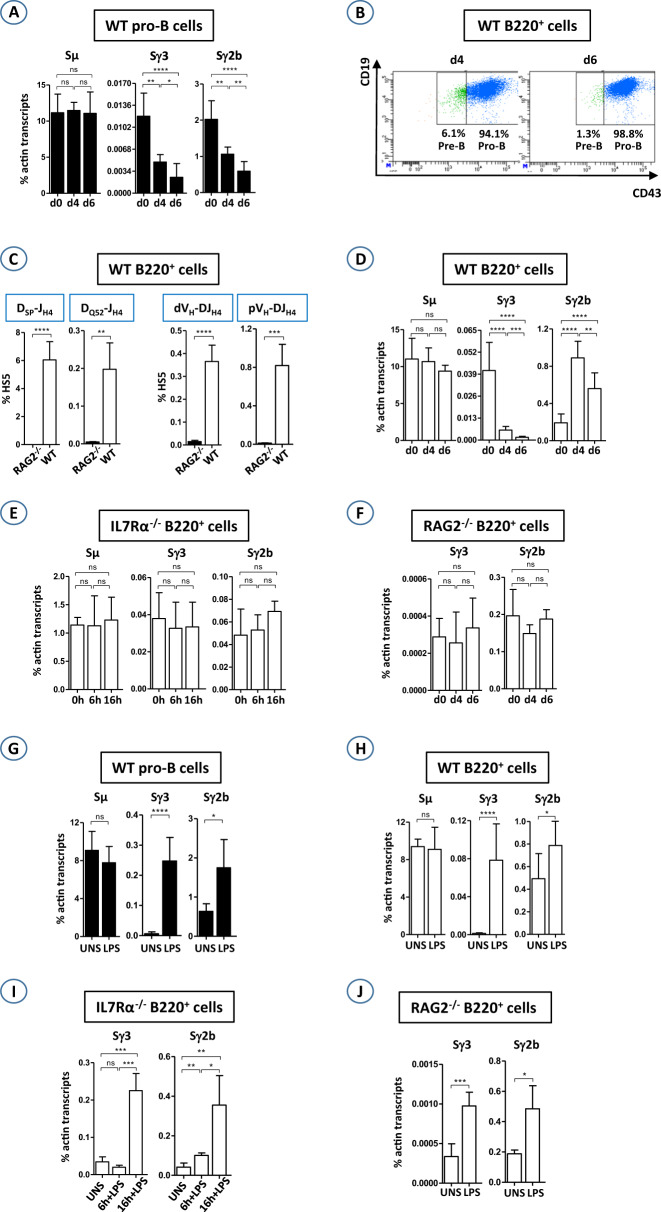
To investigate the role of IL7 in switch transcription, we set up a stromal cell-free culture system that contains IL7 only (see Additional Information). IL7R is expressed in pro-B cells and is downregulated in pre-B cells.6,7 Consequently, by day 2, all sorted pre-B cells were dead, in stark contrast to the sorted pro-B cells, which proliferated in the IL7 medium (data not shown). In pro-B cells, the transcript levels of Sγ1, Sγ2a, Sε, and Sα were extremely low to undetectable (data not shown). We therefore focused on Sγ3 and Sγ2b transcripts. Unexpectedly, the Sγ3 and Sγ2b transcript levels steadily decreased with time, while the Sµ transcript levels did not vary (Fig. 1a). We also adapted the IL7 culture system to the whole medullar B220+ population, a mixture of B-cell precursors, immature cells, and circulating cells. We reasoned that IL7R-expressing B cells (pre-pro-B and pro-B cells), though representing only a small fraction (8–15%) of B220+ cells, would proliferate, whereas IL7R-negative cells would die. Indeed, pro-B cells proliferated vigorously (Fig. S1A) and represented almost the whole population (>98%) on day 6 (Fig. 1b and Fig. S1B). The propagated cells underwent V(D)J recombination at the IgH locus (Fig. 1c and Fig. S1C). Again, the Sµ transcript levels did not vary, but the Sγ3 levels steadily dropped until they reached background levels on day 6. The levels of Sγ2b transcripts increased until day 4 and then significantly decreased (Fig. 1d). Our interpretation is that when starting with a B220+ population, the actual effect of IL7 on pro-B cells becomes obvious only after 4 days of culture (see Supplementary discussion). Altogether, the above data reveal that IL7 inhibits Sγ3 and Sγ2b transcription in ex vivo-propagated pro-B cells.
Fig. 1.
a Sorted WT pro-B cells were cultured for 6 days in IL7 medium. On days 0, 4, and 6, total RNA was extracted, reverse transcribed, and subjected to qPCR for the indicated switch transcripts. The histograms show the standard deviations. b WT B220+ cells were sorted and cultured for 6 days in IL7 medium. On days 4 and 6, cells were stained with anti-CD19, anti-CD43, and anti-IgM antibodies and gated on the IgM− population. c Genomic DNA was prepared on day 6 and was assayed for DJH4 rearrangements and for VH-DJH4 rearrangements. WT (d), IL7Rα−/− (e), and RAG2−/− (f) B220+ cells were sorted and cultured in IL7 medium. At the indicated time points, total RNA was extracted and assayed for switch transcripts as in a. Sorted WT pro-B (g) or sorted B220+ cells from WT (h), IL7Rα−/− (i), and RAG2−/− (j) BMs were cultured for 4 days in IL7 medium and then in the presence (LPS) or absence (UNS) of LPS for an additional 2 days, except for IL7Rα−/− B220+ cells (6 h and 16 h in IL7+LPS medium). The switch transcripts were quantified as in a. (n ≥ 4 for a and g, n ≥ 8 for b–f, n > 8 for h, n = 4 for i, and n > 4 for j) (****p < 0.0001, ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant)
Since IL7 conveys its effects through IL7R, switch transcript levels should not vary in the absence of the receptor. IL7R is composed of a common γ chain and a α chain.6,7 Mice devoid of the IL7Rα chain display severe blockade at the early pro-B-cell stage.13 B220+ cells, containing mainly pre–pro-B cells and potentially some early pro-B cells, were sorted from IL7Rα−/− BMs. Preliminary experiments revealed that cultured IL7Rα-deficient B220+ cells died after 24 h (data not shown). Therefore, we were compelled to quantify Sγ3 and Sγ2b transcripts at earlier time points. Interestingly, Sγ3 and Sγ2b transcript levels did not significantly vary after 6 h of culture (Fig. 1e), suggesting that IL7R was required to inhibit Sγ3 and Sγ2b transcription.
IL7-mediated inhibition of Sγ3 and Sγ2b transcription was seen in pro-B cells that had rearranged their IgH loci (Fig. 1c). To explore whether the effect of IL7 on switch transcription required V(D)J recombination, we quantified Sγ3 and Sγ2b transcript levels in cultured RAG2-deficient pro-B cells. We found no obvious effects on Sγ3 and Sγ2b transcription (Fig. 1f). We conclude that IL7 inhibits switch transcription in cultured pro-B cells that have undergone V(D)J recombination.
Sγ3 and Sγ2b transcription is induced by LPS. To explore the interplay between LPS and IL7 with regard to switch transcription, sorted pro-B cells or BM B220+ cells were cultured in the presence or absence of IL7. LPS readily induced switch transcription, although the effect on Sγ3 transcript levels was stronger than that on Sγ2b transcription (Fig. 1g, h). Induction of Sγ3 and Sγ2b transcription led to CSR to Sγ3 and Sγ2b, respectively, (Fig. S2A). Transcription of the Aicda gene (encoding AID) was also induced by LPS (Fig. S2B). Induction of switch transcription was not restricted to Sγ3 and Sγ2b, as Sγ1 and Sε transcript levels, which were undetectable in unstimulated pro-B cells after 6 days of IL7 culture, were readily increased by LPS + IL4 (Fig. S2C).
Sγ3 transcript levels were increased in LPS-activated IL7Rα-deficient B220+ cells, though only after 16 h, whereas Sγ2b transcript levels were already significantly increased after 6 h (Fig. 1i). In activated RAG2-deficient pro-B cells, Sγ3 and Sγ2b transcription was moderately induced (Fig. 1j), while the induction was stronger for Sγ1 and Sε transcription (Fig. S2D).
Overall, activation of cultured pro-B cells induced switch transcription, strongly suggesting that the inhibitory effect conveyed by IL7R signaling is overridden by the pathways that induce CSR.
Supplementary information
Acknowledgements
We thank Amy L. Kenter and Cornelis Murre for their help with the RAG-deficient pro-B cell cultures and the IPBS animal facility staff and the Imaging Core Facility TRI-IPBS, in particular Emmanuelle Näser, for their excellent work. This work was supported by the Agence Nationale de la Recherche (ANR-16-CE12-0017), the Institut National du Cancer (INCA_9363, PLBIO15-134), the Fondation ARC pour la Recherche sur le Cancer (PJA 20191209515), and the Ligue Contre le Cancer (Ligue Régionale: Comités de l’Ex Région Midi-Pyrénées). F.-Z.B. was supported by a fellowship from the INCa. C.O. is a fellow of the Ministry of Higher Education & Research and is the recipient of a fellowship from the Fondation pour la Recherche Médicale. Tri-IPBS has financial support from ITMO Cancer Aviesan (the National Alliance for Life Science and Health) within the framework of the Cancer Plan.
Author contributions
Conceptualization: A.A.K. Methodology: A.D., F.-Z.B., C.O., and A.A.K. Investigation: A.D., F.-Z.B., and C.O. Handling of mouse lines: A.D. Writing—original draft: A.A.K. Writing—review and editing: A.D., F.-Z.B., C.O., and A.A.K. Supervision: A.A.K. Funding acquisition: A.A.K.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-020-0430-y) contains supplementary material.
References
- 1.Yu K, Lieber MR. Current insights into the mechanism of mammalian immunoglobulin class switch recombination. Crit. Rev. Biochem. Mol. Biol. 2019;54:333–351. doi: 10.1080/10409238.2019.1659227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han JH, et al. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edry E, Azulay-Debby H, Melamed D. TOLL-like receptor ligands stimulate aberrant class switch recombination in early B cell precursors. Int. Immunol. 2008;20:1575–1585. doi: 10.1093/intimm/dxn117. [DOI] [PubMed] [Google Scholar]
- 4.Swaminathan S, et al. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nat. Immunol. 2015;16:766–774. doi: 10.1038/ni.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braikia FZ, et al. Inducible CTCF insulator delays the IgH 3’ regulatory region-mediated activation of germline promoters and alters class switching. Proc. Natl Acad. Sci. USA. 2017;114:6092–6097. doi: 10.1073/pnas.1701631114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceredig R, Rolink AG. The key role of IL-7 in lymphopoiesis. Semin. Immunol. 2012;24:159–164. doi: 10.1016/j.smim.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat. Rev. Immunol. 2014;14:69–80. doi: 10.1038/nri3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran AE, et al. The interleukin-7 receptor alpha chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. EMBO J. 1996;15:1924–1932. doi: 10.1002/j.1460-2075.1996.tb00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury D, Sen R. Transient IL-7/IL-7R signaling provides a mechanism for feedback inhibition of immunoglobulin heavy chain gene rearrangements. Immunity. 2003;18:229–241. doi: 10.1016/S1074-7613(03)00030-X. [DOI] [PubMed] [Google Scholar]
- 10.Malin S, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat. Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heltemes-Harris LM, Farrar MA. The role of STAT5 in lymphocyte development and transformation. Curr. Opin. Immunol. 2012;24:146–152. doi: 10.1016/j.coi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dooms H. Interleukin-7: fuel for the autoimmune attack. J. Autoimmun. 2013;45:40–48. doi: 10.1016/j.jaut.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Peschon JJ, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.