Abstract
Silent information regulator 1 (Sirt1) is a deacetylase, which plays an important role in the occurrence and development of diabetic nephropathy (DN). Our previous study shows that Yin yang 1 (YY1), a widely expressed zinc finger DNA/RNA-binding transcription factor, is a novel regulator of renal fibrosis in diabetic nephropathy. Since the activity of YY1 is regulated via acetylation and deacetylation modification, this study aimed to explore whether Sirt1-induced deacetylation of YY1 mediated high glucose (HG)-induced renal tubular epithelial–mesenchymal transition (EMT) and renal fibrosis in vivo and in vitro. We first confirmed that Sirt1 expression level was significantly decreased in the kidney of db/db mice and in HG-treated HK-2 cells. Diabetes-induced Sirt1 reduction enhanced the level of YY1 acetylation and renal tubular EMT. Then, we manipulated Sirt1 expression in vivo and in vitro by injecting resveratrol (50 mg·kg−1·d−1. ip) to db/db mice for 2 weeks or application of SRT1720 (2.5 μM) in HG-treated HK-2 cells, we found that activation of Sirt1 reversed the renal tubular EMT and YY1 acetylation induced by HG condition. On the contrary, Sirt1 was knocked down in db/m mice or EX527 (1 μM) was added in HK-2 cells, we found that inhibition of Sirt1 exacerbated renal fibrosis in diabetic mice and enhanced level of YY1 acetylation in HK-2 cells. Furthermore, knockdown of YY1 inhibited the ameliorating effect of resveratrol on renal tubular EMT and renal fibrosis in db/db mice. In conclusion, this study demonstrates that Sirt1 plays an important role in renal tubular EMT of DN through mediating deacetylation of YY1.
Keywords: diabetic nephropathy, Sirt1, Yin yang 1, acetylation, epithelial–mesenchymal transition, db/db mice, human proximal tubular epithelial cell line HK-2, resveratrol, SRT1720, EX527
Introduction
Diabetic nephropathy (DN) is one of the most common microvascular complications of diabetes, and its incidence has been increasing over the past decades; DN has become the main cause of end-stage renal disease in most developed countries [1]. Many factors are involved in the initiation and development of DN, and the mechanism of its occurrence is complex. Among them, progressive renal interstitial fibrosis has been recognized as the landmark pathological feature of DN [2, 3]. The epithelial–mesenchymal transition (EMT) is one of the potential mechanisms of renal fibrosis in which polarized immotile epithelial cells are converted into motile mesenchymal cells [4]. Epithelial cells lose several epithelial characteristics, such as E-cadherin (E-Ca) expression and apical–basal polarity, enabling the cells to differentiate into mesenchymal cells during EMT [5, 6]. EMT is generally considered to be an important step in malignant tumor metastasis and fibrotic diseases. Growing evidence shows that EMT is crucial to initiate and promote renal fibrosis in DN. However, the pathogenesis of EMT in DN is yet to be fully elucidated.
Silent information regulator 1 (Sirt1), a member of the mammalian sirtuin family (Sirt1–Sirt7), plays an important role in many biological processes, such as drug resistance, apoptosis, tumorigenesis, and development [7–9]. Sirt1 overexpression and deletion experiments in mouse and cell models uncovered its protective effect on the kidney through regulating metabolism, inflammation, oxidative stress, and other pathways [10–14]. In podocytes, Hong et al. confirmed that overexpression of Sirt1 can promote PGC activation and can protect cells from high glucose (HG)-mediated mitochondrial damage, reducing glomerular podocyte loss and oxidative stress in OVE26 mice induced by diabetes [15]. SRT1720, a Sirt1 agonist, inhibits fibrotic responses in HG-cultured HK-2 cells via the Sirt1/Hif1α/GLUT1/Snail pathway [16]. In addition, studies have shown that Sirt1 plays an important role in the regulation of autophagy and EMT by deacetylating many functional proteins, such as p53, claudin 1, TGF-β, and the FoxOs [17–19]. These results indicate that Sirt1 is vital for renal protection and fibrosis recovery. However, the role of Sirt1 in EMT in DN has not been reported, and its mechanism has not yet been clarified.
Various transcription factors (TFs) that are activated under diabetic conditions are known to mediate hyperglycemia and other changes in DN. Yin yang 1 (YY1) is a universally expressed and multifunctional zinc finger DNA/RNA-binding transcription factor, and its activity can be regulated through acetylation and deacetylation modification; further, it plays an important role in many biological and pathological processes [20–22]. Previous studies have shown that YY1 is involved in metabolic diseases such as nonalcoholic fatty liver and diabetes [23, 24]. Our previous studies have shown that YY1 is an important regulator of renal fibrosis in DN [25]. The activation of TFs is generally determined by their phosphorylation state, and there is increasing evidence that protein acetylation is crucial to the activation of TFs. Yao et al. confirmed that the interaction between histone deacetylases (HDACs) and two different regions of YY1 had different effects on the transcriptional regulation of YY1 more than a decade ago [26]. A recent study showed that the 170–200 residue region of YY1 was deacetylated by HDAC8, thus mediating the downregulation of mtp53 transcription in TNBC cells [27]. At present, there are few studies on the regulation of acetylation and deacetylation of YY1, and its specific mechanism and main function are still unclear. Sirt1 is an enzyme that mediates NAD+-dependent deacetylation of target substrates, which regulates the activity of multiple TFs by deacetylation. Sirt1 regulates the acetylation status of the FoxO family and affects the expression of downstream genes [28]. A recent study showed that knocking out Sirt1 in podocytes probably aggravates HG-induced kidney injury by promoting TF acetylation in podocytes [29]. In summary, we speculate that Sirt1 regulates the deacetylation of YY1 and plays an important role in DN.
The present study explored the underlying mechanisms that regulate the initiation of EMT within the kidney tubules in diabetes. By verifying this hypothesis, it was discovered that Sirt1 plays a pivotal role in the deacetylation of YY1 in improving EMT in DN. These findings suggest a novel mechanism for Sirt1 in suppressing EMT in DN and uncover the potential for Sirt1 as a therapeutic target in the treatment of DN with renal fibrosis.
Materials and methods
Animals
Male db/db mice (7 weeks old, 30–35 g) and db/m mice (7 weeks old, 15–20 g) were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). All animals were housed in the same cages before and during the experiment, allowing them free access to water and food. All experimental procedures were approved by the Animal Ethics Committee of Xuzhou Medical University, following the Guiding Principles for Care and Use of Laboratory Animals of Xuzhou Medical University. The db/m mice were randomly divided into three groups with at least six mice in each group: the control group (db/m), the empty lentivirus injection group (db/m + LV-NC), and the Sirt1-shRNA lentivirus-injected group (db/m + LV-Sirt1-shRNA). The db/db mice were randomly divided into five groups with at least six mice in each group: the model group (db/db), the solvent-injected control group (db/db + vehicle), the empty lentivirus-injected group (db/db + LV-NC), the resveratrol treatment group (db/db + resveratrol), and the YY1-shRNA lentivirus-injected and resveratrol treatment group (db/db + LV-YY1-shRNA+resveratrol). At the age of 12 weeks, db/m mice were injected with empty lentivirus (db/m + LV-NC) or Sirt1-shRNA lentivirus (db/m + LV-Sirt1-shRNA), and db/db mice were injected with empty lentivirus (db/db + LV-NC) or YY1-shRNA lentivirus (db/db+ LV-YY1-shRNA+ resveratrol). Mice were treated with lentivirus (6 × 107 TU/mouse) via tail vein injection. Lentiviruses expressing Sirt1-shRNA (LV-Sirt1-shRNA) (5′-CCAUCUCUCUGUCACAAAUTT-3′) and YY1-shRNA (LV-YY1-shRNA) (5′-CCTCCTGATTATTCAGAATAT-3′) were constructed by GenePharma (Suzhou, China). At the age of 14 weeks, db/db mice were intraperitoneally injected with 1% DMSO solution (diluted with normal saline) and resveratrol solution (dissolved with DMSO). Resveratrol (HY-16561, MedChemExpress, Shanghai, China) was continuously injected intraperitoneally for 2 weeks at a dose of 50 mg/kg. After that, the mice were placed in metabolic cages, and blood samples were also collected. Part of the kidney tissue was fixed in 4% paraformaldehyde, and the rest was stored at −80 °C for biochemical analysis.
Cell culture
The human proximal tubular epithelial cell line HK-2 was a kindly gift from Nanjing University. HK-2 cells were cultured in serum-free DMEM medium containing 5.56 mmol/L D-glucose (31600034, GIBCO by Life Technologies, USA) and 1% penicillin and streptomycin (100×; P11–010, Beyotime Institute of Biotechnology, Nantong, China); cells were maintained in a humidified incubator at 37 °C with 5% CO2. The cells were divided into the normal glucose (NG) group, HG group, NG with EX527 group (NG + EX527), and HG with SRT1720 group (HG + SRT1720). Confluent cells were cultured for 24 h in synchronization, cultured in DMEM medium containing 60 mmol/L D-glucose for 48 h, and then 1 μL of DMSO-dissolved SRT1720 solution (2.5 μmol/L, S1129, Selleck, Shanghai, China) was added per 1 mL of the medium. After the fused cells were cultured for 24 h, 1 μL of EX527 solution (1 μmol/L, S1541, Selleck, Shanghai, China) dissolved in DMSO was added per 1 mL of the medium. The medium was changed every 36 h, and the cells were harvested after 76 h.
Renal function assessment
Fasting blood glucose (FBG) was measured with a glucose assay kit (Jiancheng Bioengineering Institute, Nanjing, China). The kidney index was calculated as the ratio of the weight of the two kidneys to the total body weight. Blood urea nitrogen (BUN) was sent to the east hospital of the affiliated hospital of Xuzhou Medical University for testing. Creatinine (CRE) was measured with a kit purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. The detection of these biochemical indicators was used to assess the DN progression.
Renal morphological assessment
Tissue sections of 4-μm thickness were prepared from paraffin-embedded kidney tissue and then were stained with periodic acid Schiff (PAS), H&E, Masson, and Sirius red after deparaffinization to assess kidney morphology, glycogen deposition, and collagen accumulation. These kits were purchased from Solarbio, Beijing, China. Photographs were taken randomly and blindly under a microscope (OLYMPUS, Tokyo, Japan).
Immunofluorescence analysis
Kidney samples were fixed with 4% paraformaldehyde and then were embedded in paraffin. Sections of 4-μm thickness were cut perpendicularly along the long axis of the kidney, and they were used for immunofluorescence analysis. The sections were deparaffinized in xylene, hydrated in graded alcohol and water, and then placed in 3% H2O2 for 10 min to eliminate endogenous peroxidase activity. After incubating the sections for 30 min with pepsin, they were washed three times in PBS, and then the samples were blocked at room temperature with 2% BSA for 0.5 h. The sections were incubated with a primary antibody against Sirt1 at 4 °C overnight and then incubated with a secondary antibody conjugated with DyLight 488 or DyLight 594 (Earthox, Millbrae, CA, USA) at 37 °C for 1 h. Then, the sections were stained with DAPI (Beyotime, Nantong, China) and were observed with an Olympus BX43F fluorescence microscope (Tokyo, Japan).
HK-2 cells that were in a glass petri dish were washed three times with cold PBS and fixed at −20 °C for 20 min with cold methanol. After washing with PBS three times, the cells were sealed with 2% BSA at room temperature for 1 h, and then the same operation was performed as described above.
Real-time quantitative RT-PCR
Total RNA in the renal cortex and HK-2 cells was isolated using TRIzol reagent according to the manufacturer’s instructions, and the steps of RT-PCR were carried out as described previously [30, 31]. Primers were designed and synthesized by Sangon Biotech (Shanghai, China). The gene sequences are shown in Table 1.
Table 1.
Primer sequence.
| Gene | Species | Primer sequence (5′ to 3′) | Products length |
|---|---|---|---|
| Snail | Homo sapiens | Forward: CCCAATCGGAAGCCTAACTA | 65 bp |
| Reverse: GGCTGCTGGAAGGTAAACTCTC | |||
| Snail | Mus musculus | Forward: TGGCTGATGGAGTGCCTTTG | 115 bp |
| Reverse: AGCCAGTGGGTTGGCTTTAG | |||
| Twist | Homo sapiens | Forward: GGAGTCCGCAGTCTTACGAG | 201 bp |
| Reverse: TCTGGAGGACCTGGTAGAGG | |||
| Twist | Mus musculus | Forward: CTTGCCAATCAGCCACTGAC | 216 bp |
| Reverse: CCAGTTTGATCCCAGCGTTT | |||
| β-actin | Homo sapiens | Forward: GCAAAGACCTGTACGCCAAC | 144 bp |
| Reverse: AGTACTTGCGCTCAGGAGGA | |||
| β-actin | Mus musculus | Forward: AGAGGGAAATCGTGCGTGAC | 138 bp |
| Reverse: CAATAGTGATGACCTGGCCGT |
Western blotting
Protein analysis was performed on mouse renal cortex tissues and cultured HK-2 cells as described previously [30, 32]. ZO-1, E-Ca, vimentin, snail, YY1, and acetylated-lysine antibodies were purchased from Cell Signaling (Beverly, MA, USA), α-smooth muscle actin (α-SMA), twist, and Sirt1 antibodies were purchased from Abcam (Cambridge, UK), and a β-actin antibody was purchased from Bioworld Technology (St. Louis, MO, USA).
Coimmunoprecipitation assay
HK-2 cells and tissues were lysed at 4 °C with RIPA buffer containing protease inhibitor. The lysate concentration was adjusted to 1 mg/mL and then was precleared with an IP grade anti-acetyl lysine antibody (1:1000, 9441S, CST) and a YY1 antibody. Protein A/G PLUS Agarose (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added to the lysate/antibody mixture and was gently mixed at 4 °C overnight. The immunoprecipitants were collected by centrifugation, washed five times with RIPA lysis buffer, and then incubated with 2× loading buffer (62.5 mM Tris-HCl, pH 6.8, 2% w/v SDS, 10% glycerol, 50 mM DTT, and 0.01% w/v bromophenol blue). Primary antibodies against Sirt1 and YY1 were used for subsequent Western blotting analysis.
Transmission electron microscopy
For transmission electron microscopy, pretreatment methods for the kidney samples were performed as described in a previous study. A transmission electron microscope Tecnai G2 (FEI, Hillsboro, OR, USA) was used for viewing and photographing the samples.
Luciferase assay
HK-2 cells at 30% confluence were transfected with a mixture of a YY1-dependent luciferase reporter and a Renilla vector (Promega, Madison, WI, USA) using PolyFect. The HK-2 cells were lysed 72 h after transfection, and luciferase activity was measured using a dual-luciferase reporter assay system (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). Intergroup differences were compared using one-way analysis of variance followed by Dunnett’s test. Statistical significance was defined as P < 0.05.
Results
The expression of Sirt1 decreases in HG-induced renal tubular epithelial cell EMT in vivo and in vitro
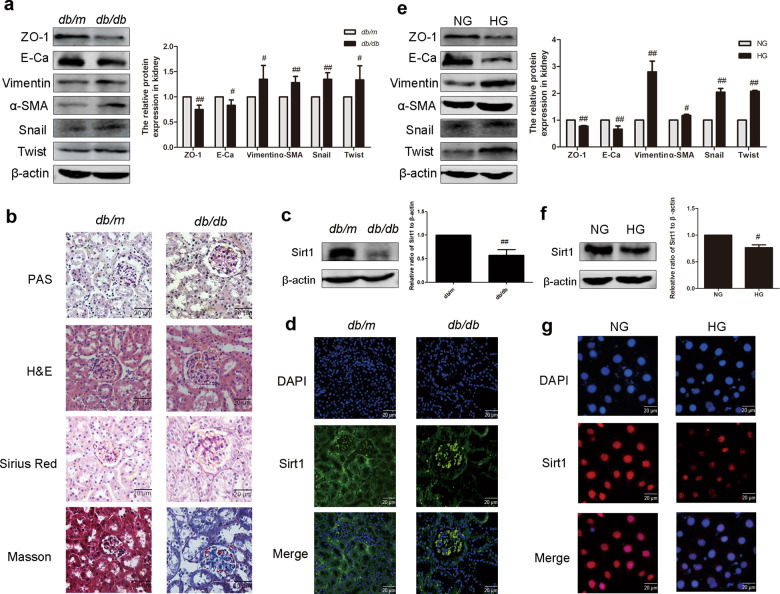
As shown in Table 2, the body weight, FBG levels, BUN, and CRE of db/db mice were markedly elevated compared with the levels in db/m mice while the kidney index was markedly downregulated. The expression of E-Ca, ZO-1, α-SMA, vimentin, snail, and twist was analyzed by Western blotting. Compared with the db/m group, the expression of E-Ca and ZO-1 in the kidneys of db/db mice was significantly downregulated, while α-SMA, vimentin, snail, and twist protein levels were significantly increased (Fig. 1a). Through PAS staining, more glycogen deposition was shown in the renal cortex of db/db mice than in the db/m group. H&E staining showed abnormal kidney glomerular volume and lumen expansion in db/db mice. Masson and Sirius red staining were used to assess the extent of collagen accumulation. The positively stained area in db/db mice increased significantly, indicating an increase in renal collagen accumulation in db/db mice (Fig. 1b). These experimental results indicate that HG induces the occurrence of renal EMT in vivo. To investigate whether Sirt1 is involved in EMT, we analyzed the expression of Sirt1 in db/db mice by Western blotting and immunofluorescence. Sirt1 was significantly reduced in db/db mouse kidney tissue compared with that of db/m mouse kidney tissue (Fig. 1c). Immunofluorescence also confirmed this result (Fig. 1d). In addition, EMT-related proteins were also detected in HG-induced HK-2 cells, and the results were the same as the results of the in vivo experiments (Fig. 1e). HK-2 cells cultured with HG showed a significant decrease in Sirt1 expression compared with cells cultured in low glucose (Fig. 1f). Consistent with Western blotting, immunofluorescence also confirmed that the expression of Sirt1 was reduced in HG-cultured HK-2 cells versus untreated cells (Fig. 1g). Our data showed that HG induces EMT in renal tubules and reduces Sirt1 expression in vitro and in vivo.
Table 2.
The body weight, kidney index, FBG, BUN, and CRE levels of mice.
| Group | BW (g) | Kidney index (mg/kg) | FBG (mmol/L) | BUN (mmol/L) | CRE (µmol/L) |
|---|---|---|---|---|---|
| db/m | 27.15 ± 1.13 | 13.69 ± 2.43 | 5.48 ± 0.79 | 7.15 ± 1.12 | 55.65 ± 20.29 |
| db/db | 52.25 ± 5.12## | 8.01 ± 0.45## | 31.18 ± 3.41## | 10.78 ± 1.14## | 157.14 ± 56.59## |
Data are presented as the mean ± SD
BW body weight, CRE creatinine, BUN blood urea nitrogen
##P < 0.01, means db/db vs. db/m
Fig. 1. The expression and activity of Sirt1 decreased in high glucose-induced renal tubular epithelial cell EMT in vivo and in vitro.
a The relative protein levels of EMT-associated proteins in mice. Data are expressed as the mean ± SD, n = 6. #P < 0.05, ##P < 0.01 vs. db/m. b PAS, H&E, Sirius red, and Masson staining of renal cortex sections of mice. Scale bar = 20 μm. c The relative protein levels of Sirt1 in mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/m. d The expression of Sirt1 in mice, as shown by immunohistochemistry. Scale bar = 20 μm. e The relative protein levels of EMT-associated proteins in HK-2 cells. Data are expressed as the mean ± SD, n = 3. #P < 0.05, ##P < 0.01 vs. NG. f The relative protein levels of Sirt1 in HK-2 cells. Data are expressed as the mean ± SD, n = 3. #P < 0.05 vs. NG. g The expression of Sirt1 in HK-2 cells, as shown by immunohistochemistry. Scale bar = 20 μm.
Sirt1 knockdown induces renal tubular EMT in db/m mice and HG-induced HK-2 cells
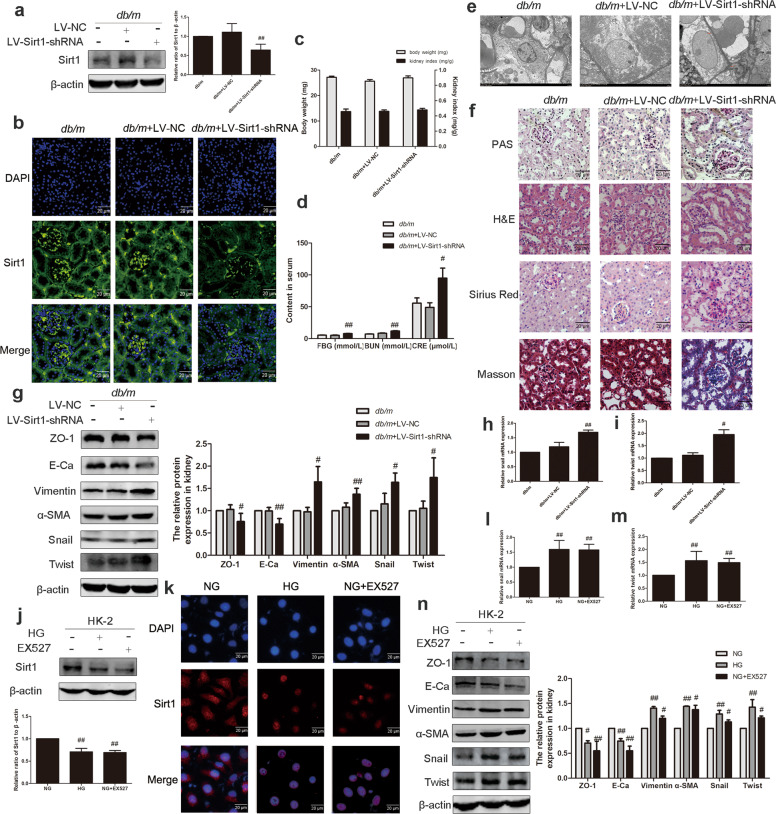
To elucidate the contribution of Sirt1 to the underlying effect on EMT, we used lentivirus encoding an shRNA that silences Sirt1 in db/m mice. Four weeks later, infection efficiency was assessed by the expression of Sirt1 through Western blotting and immunofluorescence. The expression of Sirt1 was significantly reduced, which proved that we successfully established a mouse model (Fig. 2a, b). Our data indicated that Sirt1 knockdown had no effect on mouse body weight and kidney index, but it significantly increased FBG, BUN, and CRE compared with the db/m group (Fig. 2c, d). Transmission electron microscopy showed that db/m mice with reduced Sirt1 expression had abnormal subcellular structure and foot process fusion (Fig. 2e). PAS staining indicated that renal glycogen deposition increased in the Sirt1 knockdown group compared with the normal group. H&E staining indicated that the kidney tissue of the model group with Sirt1 knocked down had abnormal changes in glomerular enlargement, mesangial hyperplasia, and basement membrane thickening. Compared with db/m mice, the distribution of positive regions of Masson and Sirius red staining was significantly increased after Sirt1 knockdown, which indicated collagen accumulation in the renal cortex (Fig. 2f). In short, Sirt1 knockdown decreased renal function in db/m mice. The expression of epithelial cell markers (E-Ca and ZO-1) was significantly decreased in lentivirus-treated db/m mice, while the expression of interstitial cell markers (vimentin and α-SMA) and TFs (snail and twist) was significantly increased (Fig. 2g). Remarkably, TFs were also observed at the mRNA level (Fig. 2h, i). Moreover, EX527 significantly reduced the expression of Sirt1 in HK-2 cells in vitro (Fig. 2j, k). Changes in EMT-related protein expression and mRNA levels of TFs were consistent with the results of the in vivo experiments (Fig. 2l–n). These results demonstrated that knocking down Sirt1 in vivo and in vitro induced renal EMT.
Fig. 2. Inhibition of Sirt1 promoted EMT in db/m mice and HK-2 cells.
a The relative protein levels of Sirt1 in db/m mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/m. b Distribution and expression of Sirt1 in db/m mice, as shown by immunofluorescence. Scale bar = 20 μm. c Effects of Sirt1 on the body weight and kidney index levels of db/m mice. d Effects of Sirt1 on the FBG, CRE, and BUN levels of db/m mice. Data are expressed as the mean ± SD, n = 6. #P < 0.05, ##P < 0.01 vs. db/m. e Transmission electron microscopy of renal tubular epithelial cells in db/m mice. f PAS, H&E, Sirius red, and Masson staining of renal cortex sections of mice. Scale bar = 20 μm. g The relative protein levels of EMT-associated proteins in mice. Data are expressed as the mean ± SD, n = 6. #P < 0.05, ##P < 0.01 vs. db/m. h, i Effects of Sirt1 on the mRNA levels of snail and twist in mice. Data are expressed as the mean ± SD, n = 6. #P < 0.05, ##P < 0.01 vs. db/m. j The relative protein levels of Sirt1 in HK-2 cells. Data are expressed as the mean ± SD, n = 3. ##P < 0.01 vs. NG. k Distribution and expression of Sirt1 in HK-2 cells, as shown by immunofluorescence. Scale bar = 20 μm. l, m Effects of Sirt1 on the mRNA levels of snail and twist in HK-2 cells. Data are expressed as the mean ± SD, n = 3. ##P < 0.01 vs. NG. n The relative protein levels of EMT-associated proteins in HK-2 cells. Data are expressed as the mean ± SD, n = 3. #P < 0.05, ##P < 0.01 vs. NG.
Sirt1 activation reverses renal tubular EMT in vivo and in vitro
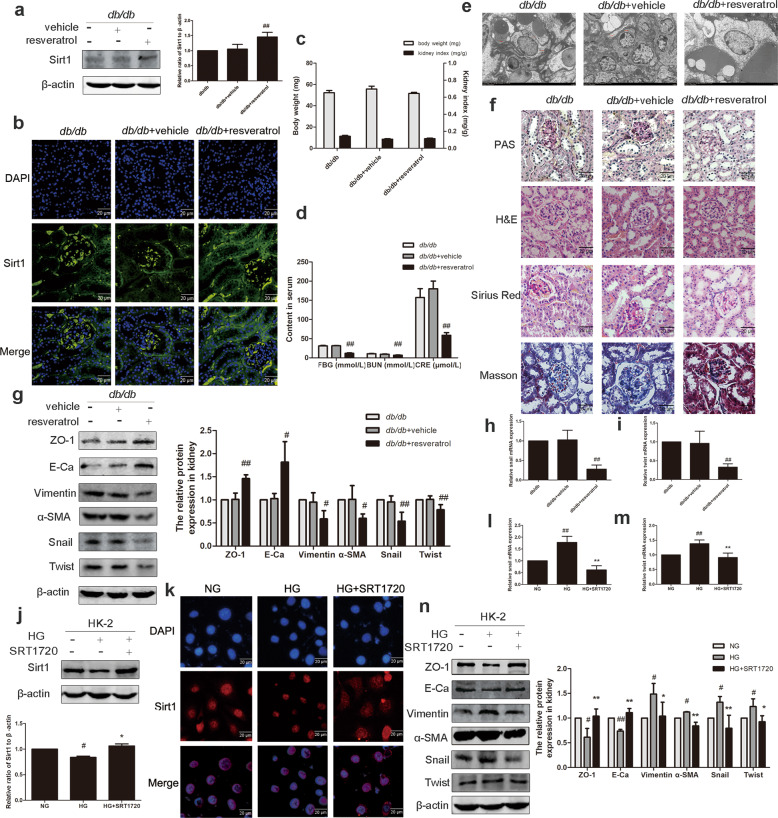
After confirming the important role of Sirt1 expression in the progression of EMT, we explored the role of Sirt1 activation in the alleviation of DN. The expression of Sirt1 in the renal cortex of db/db mice treated with resveratrol was significantly increased (Fig. 3a). Immunofluorescence data also supported this result (Fig. 3b). Resveratrol had no effect on body weight and kidney index in mice, but it significantly reduced FBG, BUN, and CRE (Fig. 3c, d). Transmission electron microscopy showed that the abnormal ultrastructure of kidneys from db/db mice treated with resveratrol was improved (Fig. 3e). Through PAS staining, it was observed that the kidney glycogen deposition in db/db mice in the resveratrol group was improved compared with that of the db/db group. H&E staining of db/db mice showed abnormal morphological structure of the kidney, and resveratrol improved it. In addition, Masson and Sirius red staining were used to assess the degree of collagen accumulation, showing an improvement in the degree of renal fibrosis in resveratrol-treated mice compared with db/db mice (Fig. 3f). Western blotting demonstrated that resveratrol increased the expression of E-Ca and ZO-1 and decreased the expression of α-SMA, vimentin, snail, and twist (Fig. 3g). The mRNA levels of snail and twist were also reduced compared with the db/db group (Fig. 3h, i). The above results demonstrate that resveratrol activates Sirt1 in the body and acts as a kidney protector. We performed in vitro experiments using a small molecule agonist of Sirt1, SRT1720. Western blotting and immunofluorescence results confirmed that HG downregulated Sirt1 expression (Fig. 3j, k), and SRT1720 stimulated Sirt1 to restore its expression to normal levels. The phenomenon that HG promoted EMT in HK-2 cells was reversed by SRT1720 (Fig. 3l–n). These results demonstrated that activation of Sirt1 expression in vivo and in vitro reverses the renal EMT induced by HG.
Fig. 3. Activation of Sirt1 promoted EMT in db/db mice and in HK-2 cells.
a The relative protein levels of Sirt1 in db/db mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/db. b Distribution and expression of Sirt1 in db/db mice, as shown by immunofluorescence. Scale bar = 20 μm. c Effects of Sirt1 on the body weight and kidney index levels of db/db mice. d Effects of Sirt1 on the FBG, CRE, and BUN levels of db/db mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/db. e Transmission electron microscopy of renal tubular epithelial cells in db/db mice. f PAS, H&E, Sirius red, and Masson staining of renal cortex sections of mice. Scale bar = 20 μm. g The relative protein levels of EMT-associated proteins in mice. Data are expressed as the mean ± SD, n = 6. #P < 0.05, ##P < 0.01 vs. db/db. h, i Effects of Sirt1 on the mRNA levels of snail and twist in mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/db. j The relative protein levels of Sirt1 in HK-2 cells. Data are expressed as the mean ± SD, n = 3. #P < 0.05 vs. NG, and *P < 0.05 vs. HG. k Distribution and expression of Sirt1 in HK-2 cells, as shown by immunofluorescence. Scale bar = 20 μm. l, m Effects of Sirt1 on the mRNA levels of snail and twist in HK-2 cells. Data are expressed as the mean ± SD, n = 3. ##P < 0.01 vs. NG. **P < 0.01 vs HG. n The relative protein levels of EMT-associated proteins in HK-2 cells. Data are expressed as the mean ± SD, n = 3. #P < 0.05, ##P < 0.01 vs. NG, *P < 0.05, **P < 0.01 vs. HG.
Sirt1 mediates deacetylation of YY1 in db/db mice and HG-induced HK-2 cells
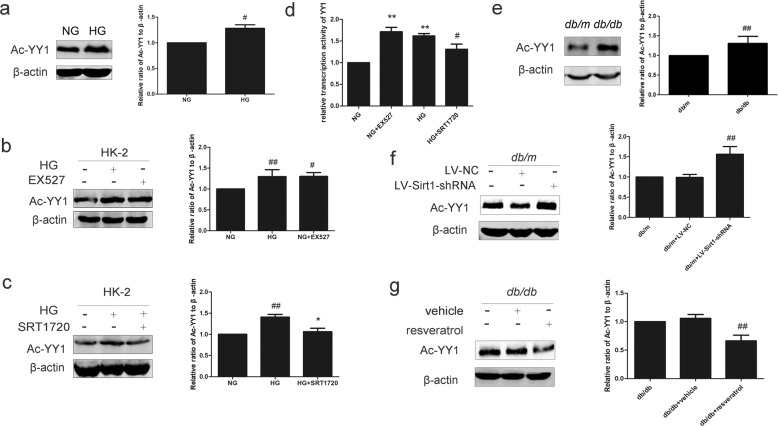
To test whether the difference in expression patterns of YY1 in HG conditions was dependent on acetylation, we performed immunoprecipitation experiments with an antibody recognizing acetyllysine, which was followed by Western blotting with an antibody against YY1. A significantly greater amount of Ac-YY1 protein was immunoprecipitated from the HG group that was isolated from the NG group (Fig. 4a). We next tried to explore whether the change in the acetylation state of YY1 was related to Sirt1. HK-2 cells treated with EX527 showed significantly more immunoprecipitated Ac-YY1 expression than the no-treatment group (Fig. 4b). The presence of SRT1720 in HG-cultured HK-2 cells markedly reduced Ac-YY1 expression to the levels observed in NG-cultured HK-2 cells (Fig. 4c). We confirmed that SRT1720 reversed YY1 activation induced by HG in HK-2 cells, while EX527 increased the transcriptional level of YY1 in HK-2 cells (Fig. 4d). HG conditions induced an increase in total YY1 expression together with a decrease in E-Ca expression, and they promoted the translocation of YY1 from the cytoplasm to the nucleus (Supplementary Fig. S1). Further study in coimmunoprecipitation assays found that Sirt1 and YY1 were coimmunoprecipitated with each other in the four groups (Supplementary Fig. S2). Consistent with the in vitro cell culture results, significant acetylation of YY1 was observed in db/db mice compared with db/m mice (Fig. 4e). YY1 was hyperacetylated in response to Sirt1-siRNA (Fig. 4f), while resveratrol reduced the expression of Ac-YY1 (Fig. 4g). Thus, to our knowledge, these are the first results that show that Sirt1 negatively regulates YY1 acetylation.
Fig. 4. Effect of Sirt1 on the acetylation of YY1 in HK-2 cells and mice.
a The relative protein levels of Ac-YY1 in HK-2 cells. Data are expressed as the mean ± SD, n = 3. #P < 0.05 vs. NG. b Effect of Sirt1 inhibition on the relative protein levels of Ac-YY1 in HK-2 cells. Data are expressed as the mean ± SD, n = 3. #P < 0.05, ##P < 0.01 vs. NG. c Effect of Sirt1 activation on the relative protein levels of Ac-YY1 in HK-2 cells. Data are expressed as the mean ± SD, n = 3. ##P < 0.01 vs. NG, and *P < 0.05 vs. HG. d The relative activity of YY1 in HK-2 cells by luciferase. Data are expressed as the mean ± SD, n = 3. **P < 0.01 vs. NG, and #P < 0.05 vs. HG. e The relative protein levels of Ac-YY1 in mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/m. f Effect of Sirt1 inhibition on the relative protein levels of Ac-YY1 in db/m mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/m. g Effect of Sirt1 activation on the relative protein levels of Ac-YY1 in db/db mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/db.
Sirt1-mediated YY1 deacetylation is involved in EMT in renal tubules in db/db mice
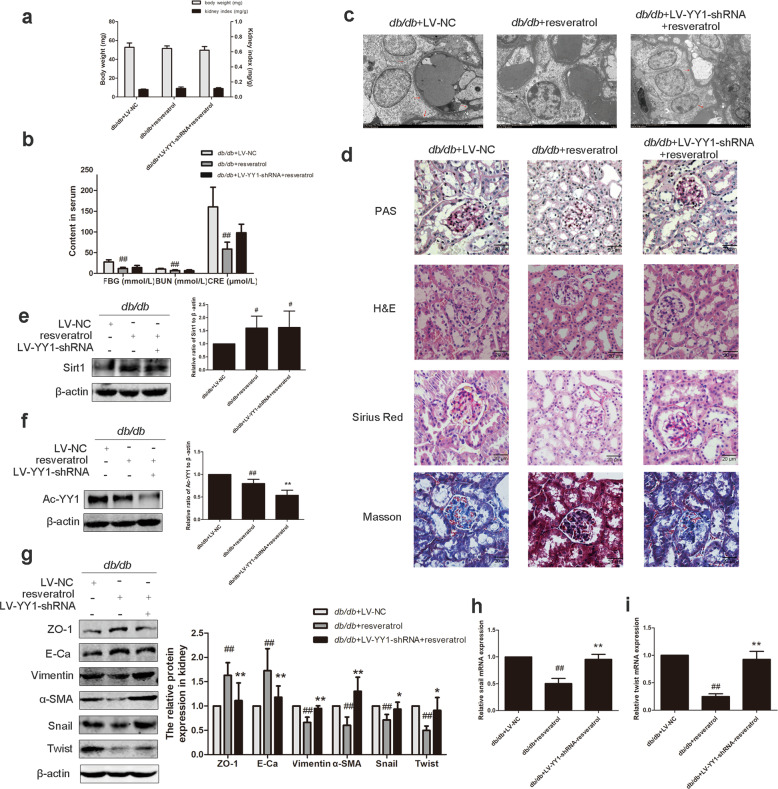
To explore the role of the Sirt1/Ac-YY1 pathway in the actions of EMT in DN, we injected YY1-shRNA via the tail vein together with intraperitoneally injected resveratrol. Body weight and kidney index did not show significant differences among these groups (Fig. 5a). Treatment with resveratrol significantly reduced FBG, BUN, and CRE levels, and while YY1 was downregulated, this effect was still persistent, but it was not as significant as resveratrol group (Fig. 5b). Transmission electron microscopy showed that abnormal changes in renal ultrastructure were reversed by resveratrol treatment (Fig. 5c). Kidney tissue section staining confirmed that resveratrol could improve glomerular enlargement, basement membrane thickening and other abnormal structural changes, and collagen accumulation in the renal cortex (Fig. 5d). Notably, all these resveratrol-induced effects were blunted after administration of a YY1-siRNA. To determine the effect of Ac-YY1 on Sirt1-mediated EMT, we detected the expression of Sirt1, Ac-YY1 and EMT-related proteins. The results revealed that the YY1-siRNA had no effect on Sirt1 expression (Fig. 5e), and the expression of Ac-YY1 in the resveratrol and YY1 knockdown group was significantly lower than it was in the resveratrol group (Fig. 5f). Resveratrol significantly improved renal EMT in db/db mice. After YY1 knockdown, the resveratrol group could not reverse the EMT status of db/db mice (Fig. 5g–i). The results show that Sirt1 promotes the deacetylation of YY1, which is an effect that, together with Sirt1, inhibits EMT in DN.
Fig. 5. Sirt1-mediated YY1 deacetylation is involved in EMT in renal tubules in db/db mice.
a Effects of Sirt1 and YY1 on the body weight and kidney index levels of mice. b Effects of Sirt1 and YY1 on the FBG, CRE, and BUN levels of mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/db + LV-NC. c Transmission electron microscopy of renal tubular epithelial cells in mice. d PAS, H&E, Sirius red and Masson staining of renal cortex sections of mice. Scale bar = 20 μm. e The relative protein levels of Sirt1 in mice. Data are expressed as the mean ± SD, n = 6. #P < 0.05 vs. db/db + LV-NC. f The relative protein levels of Ac-YY1 in mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/db + LV-NC, and **P < 0.01 vs. db/db + resveratrol. g The relative protein levels of EMT-associated proteins in mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/db + LV-NC, *P < 0.05, **P < 0.01 vs. db/db + resveratrol. h, i The mRNA levels of snail and twist in mice. Data are expressed as the mean ± SD, n = 6. ##P < 0.01 vs. db/db + LV-NC, and **P < 0.01 vs. db/db + resveratrol.
Discussion
Because of the complex pathological mechanism of DN, many studies have been undertaken through the years to try and clarify the molecular mechanism of DN and identify new effective molecular therapeutic targets. Currently, the understanding of Sirt1 in renal biological function is limited, and its role in the kidney remains to be clarified. In this study, we found that Sirt1 expression decreased in both the kidneys of DN mice and HK-2 cells induced by HG, and then we sought to identify the molecular mechanisms driving the impact of Sirt1 in DN. Our data showed for the first time that Sirt1 is an important regulator of EMT-related proteins in DN, and stimulation of Sirt1 can significantly improve HG-induced renal EMT. One of the important findings of this study was that the decrease in Sirt1 expression induced by HG significantly changed the acetylation level of the transcriptional regulator YY1. We hypothesized that YY1 acetylation was increased because Sirt1 expression was inhibited by HG in DN. We confirmed that increasing Sirt1 expression in vivo or in vitro could regulate the state of YY1 acetylation and inhibit the occurrence of renal EMT. Overall, this is the first study to identify the role of Sirt1 and the acetylation of YY1 as molecular mechanisms involved in DN. Our results strongly support a crucial role for the downregulation of Sirt1 and hyperacetylation of YY1 in the pathogenesis of DN, further highlighting their potential as targets for the treatment of kidney fibrosis caused by diabetes, with broader application in DN.
EMT is a process in which endothelial cells acquire mesenchymal properties and lose the characteristics of epithelial cells. It is an important process in diabetic renal fibrosis and is considered to be a driving factor in the development of DN. EMT is characterized by decreased expression of endothelial-specific markers, such as CD31 and E-Ca, and increased expression of mesenchymal-specific markers, such as α-SMA and vimentin. During this process, myofibroblasts migrate to interstitial tissues, and an extracellular matrix is produced and deposited, resulting in fibrosis. We found that the expression of Sirt1 was significantly reduced in diabetic animals compared with nondiabetic animals, promoting the expression of EMT-related proteins. The results of experiments in HG-induced HK-2 cells also confirmed this observation. These results suggest that the occurrence of tubular EMT may be associated with the downregulation of Sirt1. To verify the role of Sirt1 in DN, we modulated Sirt1 expression in mouse kidneys by injecting drugs or viral vectors. In this study, resveratrol and SRT1720 were used to activate Sirt1. Previous studies have confirmed that resveratrol is an effective agonist of Sirt1 that can significantly upregulate Sirt1 and delay the occurrence and development of many diseases [33, 34]. In addition, SRT1720, which is a synthesized small molecule Sirt1 agonist, has been reported to increase the expression of Sirt1 and inhibit the fibrosis reaction in HG-induced HK-2 cells [16]. We found that intraperitoneal injection of resveratrol substantially improved kidney EMT in db/db mice. Staining techniques (Masson and Sirius red) indicate that collagen is deposited in the renal cortex, while the upregulation of Sirt1 reduces the degree of accumulation. In addition, tail vein injection of Sirt1-shRNA can induce renal EMT, which is characterized by decreased E-Ca and ZO-1 of epithelial cell markers, increased vimentin and α-SMA of interstitial cell markers, and increased transcription factor snail and twist. Therefore, this study suggests that Sirt1 may be a potential target for the reduction of HG-induced tubular EMT. Furthermore, SRT1720 significantly inhibited the decrease in E-Ca and ZO-1 expression and the increase in vimentin and snail expression in HG-induced HK-2 cells. EX527 reduced the expression of E-Ca and ZO-1 in HK-2 cells cultured in normal sugar and increased the expression of vimentin and snail. These results further support the idea that Sirt1 is involved in renal tubular EMT in DN. It is worth noting that knockdown of Sirt1 significantly increased FBG in mice, which may be related to its regulation of insulin sensitivity. Previous studies confirmed that a Sirt1 agonist could significantly improve the insulin sensitivity of the liver in Zucker fa/fa rats, and the sensitivity of insulin secretion to blood glucose decreases after Sirt1 gene knockout. Sirt1 is a key factor in metabolic regulation, but its specific mechanism of action is unknown [27, 35].
Increasing evidence has shown that epigenetics play an important role in regulating renal function [36, 37]. Acetylation is a posttranslational modification of YY1 that plays a key role in regulating protein stability, DNA-binding ability and transcriptional activity. YY1 has two regions that are acetylated. The change in the acetylation status for the inhibiting domain (where residues 170–200 are located) can affect the transcriptional activity of YY1, while acetylation of the partial zinc finger domain (where residues 261–414 are located) can significantly reduce its DNA-binding activity [38]. We found that lysine acetylation is one of the important mechanisms by which YY1 participates in EMT in DN, at least in part. Other studies suggest that acetylation of TFs may play an important role in DN. Liu et al. confirmed that restoring Sirt1 expression and reducing the acetylation of STAT3 and NF-κB improved renal injury in diabetic mice [29]. There is also a study demonstrating that resveratrol stimulates Sirt1 to deacetylate Smad3, which reverses TGF-β and induces increased expression of type IV collagen and fibronectin to improve renal fibrosis [33]. The pivotal role of FoxO4 acetylation in DN podocyte injury has been demonstrated [39]. These studies suggest that acetylation of TFs could be a key event leading to DN.
Among seven variants of the sirtuin gene in mammals, Sirt1 is the histone deacetylase with the most stable deacetylase activity [40, 41]. It regulates the balance between acetylation and deacetylation in posttranslational modification and plays an important role in the regulation of sugar and lipid metabolism. Previous studies have shown that the substrates of HDACs not only are histones but also are p53, YY1, STAT3, and other proteins; however, the specific interaction relationship is still unclear [42]. Our study suggests that the beneficial effects of Sirt1 likely occur through deacetylation of YY1. In our in vitro model of HK-2 cells exposed to HG, we rescued Sirt1 expression, thereby reducing the acetylation of YY1. Further confirming that YY1 is one of the targets of Sirt1, treatment of HK-2 cells with the Sirt1 inhibitor EX527 increased the acetylation of YY1. Furthermore, pharmacological activation of Sirt1 with resveratrol or SRT1720 reduced the expression of Ac-YY1 in db/db mice and in HG-cultured HK-2 cells. A recent study reported that Sirt1 was recruited to the DNA-binding elements of YY1, synergistically inhibiting the expression of miR134, regulating the expression of CERB and brain-derived neurotrophic factor, and participating in the regulation of memory and plasticity [38]. Our data indicate that treatment with a YY1-siRNA can significantly reduce the resveratrol-induced decrease in Ac-YY1 expression and reverse the effect of resveratrol on EMT. Since there are currently no specific Sirt1 agonists, it is important to develop methods to modulate Sirt1 or acetylation of TFs. These findings confirm that YY1 is a potential target for Sirt1. Inhibition of Sirt1 expression results in the maintenance of YY1 in an acetylated state that signals fibrosis and EMT in the kidney tubules. Taken together, the development of epigenetic tools for specific Sirt1 agonists or protein interactions may produce potential new treatments for DN.
In conclusion, our results demonstrate that HG-induced downregulation of Sirt1 leads to reduced deacetylation of YY1, which is responsible for HG-induced EMT-related protein expression disorders. The stimulation of Sirt1-mediated YY1 deacetylation significantly reduced HG-induced EMT in DN. Overall, our study provides new insights into the pathogenesis of HG-induced DN and provides new insights into effective therapeutic targets for DN and other kidney diseases associated with renal fibrosis.
Supplementary information
Acknowledgements
The work was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 15KJA310005, No. 19KJA460008), the National Natural Science Foundation of China (No. 81973377), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Science and Technology Project of Xuzhou (No. KC18202).
Author contributions
LD, QL, and XXY participated in the research design. LD, XQ, YL, CCL, XZL, LX, and YQL conducted experiments. XQ, YL, LLH, CCL, PM, and FLS performed the data analysis. LD, XQ, YL, QL, and XXY wrote or contributed to the writing of the paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Lei Du, Xuan Qian, Yuan Li
Supplementary information
The online version of this article (10.1038/s41401-020-0450-2) contains supplementary material, which is available to authorized users.
References
- 1.Rossing P. Diabetic nephropathy: worldwide epidemic and effects of current treatment on natural history. Curr Diabetes Rep. 2006;6:479–83. doi: 10.1007/s11892-006-0083-y. [DOI] [PubMed] [Google Scholar]
- 2.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziyadeh FN. The extracellular matrix in diabetic nephropathy. Am J Kidney Dis Off J Natl Kidney Found. 1993;22:736–44. doi: 10.1016/S0272-6386(12)80440-9. [DOI] [PubMed] [Google Scholar]
- 4.Xuan YW, Liao M, Zhai WL, Peng LJ, Tang Y. MicroRNA-381 inhibits lung adenocarcinoma cell biological progression by directly targeting LMO3 through regulation of the PI3K/Akt signaling pathway and epithelial-to-mesenchymal transition. Eur Rev Med Pharmacol Sci. 2019;23:8411–21.. doi: 10.26355/eurrev_201910_19152. [DOI] [PubMed] [Google Scholar]
- 5.Gurzu S, Turdean S, Kovecsi A, Contac AO, Jung I. Epithelial–mesenchymal, mesenchymal-epithelial, and endothelial-mesenchymal transitions in malignant tumors: an update. World J Clin Cases. 2015;3:393–404. doi: 10.12998/wjcc.v3.i5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vu T, Datta PK. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers. 2017;9:171–193. doi: 10.3390/cancers9120171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitada M, Ogura Y, Monno I, Koya D. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol. 2019;10:187. doi: 10.3389/fendo.2019.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang XJ, Finkel T, Shen DW, Yin JJ, Aszalos A, Gottesman MM. SIRT1 contributes in part to cisplatin resistance in cancer cells by altering mitochondrial metabolism. Mol Cancer Res MCR. 2008;6:1499–506. doi: 10.1158/1541-7786.MCR-07-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan YY, Kohno M, Hitomi H, Kitada K, Fujisawa Y, Yatabe J, et al. Aldosterone/Mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology. 2011;152:680–8. doi: 10.1210/en.2010-0829. [DOI] [PubMed] [Google Scholar]
- 11.Huang K, Huang J, Xie X, Wang S, Chen C, Shen X, et al. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-beta1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2013;65:528–40.. doi: 10.1016/j.freeradbiomed.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–55. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu HW, Kao HH, Wu CH. Exercise training upregulates SIRT1 to attenuate inflammation and metabolic dysfunction in kidney and liver of diabetic db/db mice. Nutr Metab. 2019;16:22. doi: 10.1186/s12986-019-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Chen Z, Gong W, Zou Y, Xu F, Chen L, et al. Paeonol ameliorates diabetic renal fibrosis through promoting the activation of the Nrf2/ARE pathway via up-regulating Sirt1. Front Pharmacol. 2018;9:512. doi: 10.3389/fphar.2018.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong Q, Zhang L, Das B, Li Z, Liu B, Cai G, et al. Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 2018;93:1330–43. doi: 10.1016/j.kint.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han W, Wang C, Yang Z, Mu L, Wu M, Chen N, et al. SRT1720 retards renal fibrosis via inhibition of HIF1alpha /GLUT1 in diabetic nephropathy. J Endocrinol. 2019;241:85–98. doi: 10.1530/JOE-18-0536. [DOI] [PubMed] [Google Scholar]
- 17.Byles V, Zhu L, Lovaas JD, Chmilewski LK, Wang J, Faller DV, et al. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012;31:4619–29. doi: 10.1038/onc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nihalani D, Susztak K. Sirt1-Claudin-1 crosstalk regulates renal function. Nat Med. 2013;19:1371–2. doi: 10.1038/nm.3386. [DOI] [PubMed] [Google Scholar]
- 19.Preyat N, Leo O. Sirtuin deacylases: a molecular link between metabolism and immunity. J Leukoc Biol. 2013;93:669–80. doi: 10.1189/jlb.1112557. [DOI] [PubMed] [Google Scholar]
- 20.Figiel M, Gorecki A. Physical interaction of human Yin Yang 1 protein with DNA. Crit Rev Oncog. 2017;22:75–97. doi: 10.1615/CritRevOncog.2017020976. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–42. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 22.Kim JD, Hinz AK, Bergmann A, Huang JM, Ovcharenko I, Stubbs L, et al. Identification of clustered YY1 binding sites in imprinting control regions. Genome Res. 2006;16:901–11.. doi: 10.1101/gr.5091406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Xiong X, Wang X, Zhang Z, Li J, Shi G, et al. Yin Yang 1 promotes hepatic gluconeogenesis through upregulation of glucocorticoid receptor. Diabetes. 2013;62:1064–73. doi: 10.2337/db12-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu GY, Rui C, Chen JQ, Sho E, Zhan SS, Yuan XW, et al. MicroRNA-122 inhibits lipid droplet formation and hepatic triglyceride accumulation via Yin Yang 1. Cell Physiol Biochem. 2017;44:1651–64. doi: 10.1159/000485765. [DOI] [PubMed] [Google Scholar]
- 25.Yang T, Shu F, Yang H, Heng C, Zhou Y, Chen Y, et al. YY1: a novel therapeutic target for diabetic nephropathy orchestrated renal fibrosis. Metab Clin Exp. 2019;96:33–45. doi: 10.1016/j.metabol.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZT, Chen ZJ, Jiang GM, Wu YM, Liu T, Yi YM, et al. Histone deacetylase inhibitors suppress mutant p53 transcription via HDAC8/YY1 signals in triple negative breast cancer cells. Cell Signal. 2016;28:506–15.. doi: 10.1016/j.cellsig.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Li F, Li H, Jin X, Zhang Y, Kang X, Zhang Z, et al. Adipose-specific knockdown of Sirt1 results in obesity and insulin resistance by promoting exosomes release. Cell Cycle. 2019;18:2067–82. doi: 10.1080/15384101.2019.1638694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 29.Liu R, Zhong Y, Li X, Chen H, Jim B, Zhou MM, et al. Role of transcription factor acetylation in diabetic kidney disease. Diabetes. 2014;63:2440–53. doi: 10.2337/db13-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang MY, Li Y, Yin SY, Kong L, Liu XL, Yin XX, et al. Sarsasapogenin suppresses Abeta overproduction induced by high glucose in HT-22 cells. Naunyn Schmiedeberg’s Arch Pharmacol. 2018;391:159–68.. doi: 10.1007/s00210-017-1445-5. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Cheng YQ, Du L, Li Y, Zhang F, Guo H, et al. Mangiferin attenuates renal fibrosis through down-regulation of osteopontin in diabetic rats. Phytother Res PTR. 2015;29:295–302. doi: 10.1002/ptr.5254. [DOI] [PubMed] [Google Scholar]
- 32.Liu YW, Hao YC, Chen YJ, Yin SY, Zhang MY, Kong L, et al. Protective effects of sarsasapogenin against early stage of diabetic nephropathy in rats. Phytother Res PTR. 2018;32:1574–82.. doi: 10.1002/ptr.6088. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177:1065–71. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raj P, Louis XL, Thandapilly SJ, Movahed A, Zieroth S, Netticadan T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014;95:63–71. doi: 10.1016/j.lfs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Sui M, Chen G, Mao X, Wei X, Chen Y, Liu C, et al. Gegen qinlian decoction ameliorates hepatic insulin resistance by silent information regulator1 (SIRT1)-dependent deacetylation of forkhead box O1 (FOXO1) Med Sci Monit. 2019;25:8544–53. doi: 10.12659/MSM.919498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranganathan P, Hamad R, Mohamed R, Jayakumar C, Muthusamy T, Ramesh G. Histone deacetylase-mediated silencing of AMWAP expression contributes to cisplatin nephrotoxicity. Kidney Int. 2016;89:317–26. doi: 10.1038/ki.2015.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Liu J, Zhen J, Zhang C, Wan Q, Liu G, et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014;86:712–25. doi: 10.1038/ki.2014.111. [DOI] [PubMed] [Google Scholar]
- 38.Yao YL, Yang WM, Seto E. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol Cell Biol. 2001;21:5979–91. doi: 10.1128/MCB.21.17.5979-5991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuang PY, Cai W, Li X, Fang L, Xu J, Yacoub R, et al. Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am J Physiol Renal Physiol. 2017;313:F621–f8.. doi: 10.1152/ajprenal.00255.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris BJ. Seven sirtuins for seven deadly diseases of aging. Free Radic Biol Med. 2013;56:133–71. doi: 10.1016/j.freeradbiomed.2012.10.525. [DOI] [PubMed] [Google Scholar]
- 41.Oon CE, Strell C, Yeong KY, Ostman A, Prakash J. SIRT1 inhibition in pancreatic cancer models: contrasting effects in vitro and in vivo. Eur J Pharmacol. 2015;757:59–67. doi: 10.1016/j.ejphar.2015.03.064. [DOI] [PubMed] [Google Scholar]
- 42.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–9. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.