Abstract
Psoriasis is a chronic inflammatory skin condition that has a fairly wide range of clinical presentations. Plaque psoriasis, which is the most common manifestation of psoriasis, is located on one end of the spectrum, dominated by adaptive immune responses, whereas the rarer pustular psoriasis lies on the opposite end, dominated by innate and autoinflammatory immune responses. In recent years, genetic studies have identified six genetic variants that predispose to pustular psoriasis, and these have highlighted the role of IL-36 cytokines as central to pustular psoriasis pathogenesis. In this review, we discuss the presentation and clinical subtypes of pustular psoriasis, contribution of genetic predisposing variants, critical role of the IL-36 family of cytokines in disease pathophysiology, and treatment perspectives for pustular psoriasis. We further outline the application of appropriate mouse models for the study of pustular psoriasis and address the outstanding questions and issues related to our understanding of the mechanisms involved in pustular psoriasis.
Keywords: Pustular psoriasis, Genetics, IL-36, Autoinflammation, Clinical features, Histology
Subject terms: Inflammation, Diagnostic markers
Introduction
Psoriasis is a chronic inflammatory skin disease that affects up to 3% of the world’s population and has a massive impact on quality of life among affected individuals. From a clinical standpoint, psoriasis can be broadly classified into pustular and nonpustular types,1 with a wide spectrum of clinical subtypes existing between these two clinical presentations. The most common form of psoriasis is plaque psoriasis, also known as psoriasis vulgaris (PV), which constitutes the great majority of cases, or up to 90% of the total2 and is characterized by sharply demarcated, elevated scaly plaques that are often distributed in a symmetric manner on the knees, elbows, scalp, and lumbosacral areas.3 Other subtypes of psoriasis that are frequently grouped with plaque psoriasis include inverse psoriasis, which involves the flexural areas of the skin; guttate psoriasis, a common form of eruptive psoriasis in young adults and children; and erythrodermic psoriasis, in which over 75% of the body surface area is affected by psoriasis.
The pustular form of psoriasis, as the name implies, is characterized by neutrophil-rich, sterile pustules and accounts for only a very small fraction of the total number of psoriasis cases. In contrast to plaque psoriasis, pustular psoriasis can at times be life-threatening.4,5 Pustular psoriasis can present as a localized disease or with generalized widespread skin lesions. Localized forms include palmoplantar pustulosis (PPP), in which pustules appear on the soles and palms, and acrodermatitis continua of Hallopeau (ACH), where pustules affect the nail apparatus.1 When PPP is present alongside plaque psoriasis, it is sometimes referred to as palmoplantar pustular psoriasis.6 Generalized pustular psoriasis (GPP) can present in several different forms. This includes the acute form, the von Zumbusch variant, which has an abrupt onset associated with systemic symptoms;7 impetigo herpetiformis, which is a GPP that occurs during pregnancy;8 and less common pustular subtypes such as annular pustular psoriasis9 and juvenile pustular psoriasis10 (Fig. 1). The classification of subcorneal pustular dermatosis, also known as Sneddon–Wilkinson disease, and its relationship to pustular psoriasis are still under debate.11 It should be noted that GPP has a high tendency to transition into erythrodermic psoriasis.1,12 This severe form of psoriasis is characterized by diffuse redness involving at least 75% of the body surface area, with finer scales typical of plaque psoriasis, and is also associated with high morbidity.13
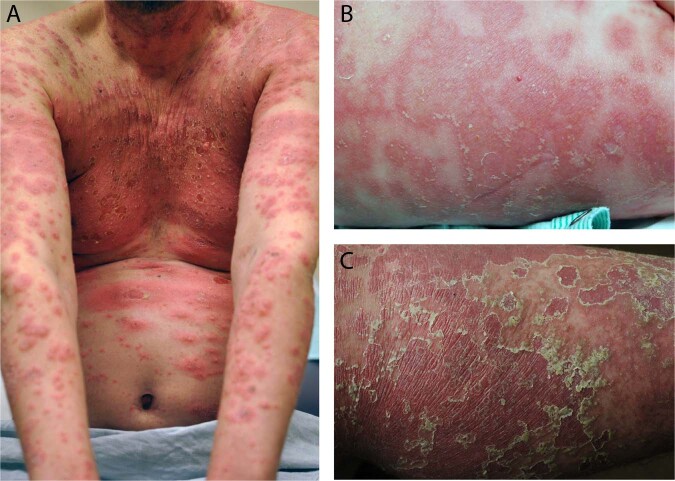
Fig. 1.
Clinical presentations of pustular psoriasis. Clinical photographs of patients with pustular forms of psoriasis. The patient in (a) has a robust and acute inflammatory response in the skin with marked erythema, edema, and central pustulation, with very limited amount of scaling, characteristic of acute pustular psoriasis. The patient in (b) has annular and serpiginous lesions with pustules located on the periphery of the inflamed areas, characteristic of annular pustular psoriasis. The patient in (c) has resolving pustular psoriasis. Often, in these patients, the pustules have a “dried up” appearance with slight brown discoloration. The stratum corneum often peels off with a characteristic trailing scale. “Resolved” pustular lesions often transition into erythematous patches and plaques with fine superficial scaling. When generalized, this would be consistent with erythrodermic psoriasis, a clinical state that frequently follows widespread pustular psoriasis
While the pathogenesis of plaque psoriasis has been well studied, much less is known about pustular forms of psoriasis. In terms of clinical presentation, there is a significant degree of overlap, and all forms of pustular psoriasis are also associated with plaque psoriasis, although they can exist independently.14 Immunologically, there appear to be differences, with IL-17 having a greater role in plaque psoriasis, whereas the IL-36 cytokine axis dominates in pustular forms of psoriasis.15,16 Lastly, genetic studies have shown that pustular forms of psoriasis may differ in their genetic etiology. Indeed, HLA-Cw6, a major risk factor for plaque psoriasis, is not associated with the pustular forms of psoriasis, and mutations in the gene encoding the IL-36 receptor antagonist (IL36RN) predispose patients to pustular psoriasis but not plaque psoriasis.17–20 In this review, we will discuss what is known about the clinical course, genetics, and immunology of the pustular forms of psoriasis.
Clinical and histological features
Diagnosis of both plaque and pustular psoriasis subtypes is made clinically. In the more severe GPP, clinical features such as conjunctivitis, liver abnormalities, leg swelling, and jaundice may be seen,21 and the onset is frequently accompanied by systemic symptoms such as fever and leukocytosis. In this acute phase, the presence of fever and leukocytosis may lead to a mistaken diagnosis of systemic infection, sometimes prompting a counterproductive discontinuation of immunosuppressive treatment.22 The condition most difficult to distinguish clinically from GPP is acute generalized exanthematous pustulosis, a pustular drug eruption frequently occurring in response to commonly used antibiotics.23 The clinical features of pustular psoriasis subtypes are shown in Table 1. Pustular psoriasis can arise superimposed on chronic plaque psoriasis or occur spontaneously on previously normal skin, and this is reflected in the histology of psoriasis (Fig. 2).
Table 1.
Clinical features of the pustular psoriasis subtypes
| Pustular psoriasis type | Clinical presentation | Diagnostic clues | Male:female ratio | Mean age of onset |
|---|---|---|---|---|
| Generalized pustular psoriasis (von Zumbusch) | Sterile subcorneal pustules that expand into lakes of pus and are associated with systemic symptoms | Macroscopically visible pustules with or without evidence of systemic inflammation (fever, increased inflammatory markers, leukocytosis) |
1:213 1:1.524 1:1.77 1:1.228 |
40.9 years13 (102 cases in Malaysia) 57 years21 (34 cases in Portugal) 41.7 years102 (7 cases in Japan) 31 ± 19.7 years7 (251 patients, Asian and European populations) 31.4 ± 19.6 years61 (191 cases, multiple ethnicities) |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Subcorneal pustules on erythematous patches |
Systemic symptoms including fever, malaise, nausea, and diarrhea Elevation in inflammatory markers; leukocytosis |
Occurs in the setting of pregnancy and can also have postpartum flareups |
Typical during the third trimester of pregnancy but eruptions as early as first trimester can occur120 |
| Annular pustular psoriasis | Polycyclic erythematous lesions with pustules on periphery of the lesions |
Milder systemic symptoms than those of GPP Often no history of psoriasis |
4:328 1:1123 |
43 years123 (2 patients in Singapore) 39 ± 13 years28 (7 patients in Korea) |
| Juvenile pustular psoriasis | Present as annular or circinate lesions with pustules similar to adult GPP | Affected children exhibit systemic findings similar to those of adult GPP patients | 2:1124 |
6.9 years125 (26 patients in China) 6.6 year126 (boys) and 5.5 years (girls) (7 patients in Brazil) 4.6 years123 (boys) and 9.6 years (girls) (6 patients in Singapore) |
| Palmoplantar pustular psoriasis | Sterile pustules on the palms and soles with yellow–brown discoloration | Yellow to brown macroscopic pustules on the hands and feet with or without concomitant classic plaque psoriasis | 1:3.57 |
42 years123 (5 patients in Singapore) 43.7 ± 14.4 years7 (560 patients across Asian and European population) |
| Acrodermatitis continua of Hallopeau | Sterile pustule formation affecting the tips of the fingers and toes |
Suppurative nail unit involvement Frequently unilateral |
1:1.77 |
49 years123 (4 patients in Singapore) 51.8 ± 20.4 years7 (28 patients across Asian and European populations) |
GPP generalized pustular psoriasis, APP annular pustular psoriasis, PPP palmoplantar pustular psoriasis, ACH acrodermatitis continua of Hallopeau
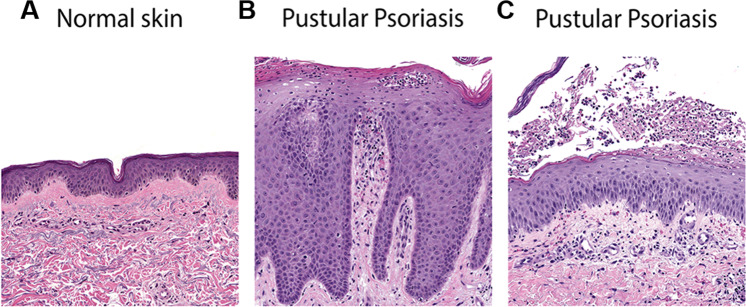
Fig. 2.
Histology of pustular psoriasis. H&E of (a) normal skin and skin from two generalized pustular psoriasis cases, one in the background of plaque psoriasis (b) and the other spontaneous (c). Thus, the section in (b) demonstrates neutrophil microabscesses in the stratum spinulosum and stratum corneum on a background of marked acanthosis and elongated rete ridges characteristic of plaque psoriasis. (c) Demonstrates a neutrophil-containing macroscopic pustule underneath the stratum corneum on a background of otherwise near normal skin
Clinical course
A major limitation to our understanding of the clinical course of pustular forms of psoriasis is that extensive data on its natural history do not exist. GPP, the best described subtype, is characterized by the development of innumerable, generalized, sterile pustules that may have an acute, subacute, or occasionally chronic presentation. Patients may have phases of typical plaque-type psoriasis before or after GPP.24 Approximately 65% of GPP cases occur in patients with a prior diagnosis of PV,13 yet despite this overlap, the pathogenic relationship between these conditions is still not fully clear. If the patient does not die of exhaustion, toxicity, or infection, remission may occur within days or weeks, with the psoriasis returning to its normal state. Alternatively, a nonpustular erythroderma may develop. The rate of relapse is unknown but may be frequent, and similarly, there is a paucity of data on the long-term prognosis of GPP. In a retrospective study of patients with GPP published in 1971 on 155 patients with GPP, of the 106 patients followed over time, 34 had died, and a major proportion of these deaths (26) were attributable to the disease or its treatment.25 In a more recent study of a Malaysian cohort of 95 patients with acute GPP, 7% mortality was reported, although this was thought to be underestimated given the sporadic follow-up.13 In a study of 63 patients with pustular psoriasis published in 1991, 3% mortality was observed during the acute presentation, but the long-term prognosis was not assessed.26 A limitation of these studies is the inclusion of several milder, more localized subtypes of pustular psoriasis, thereby likely underestimating the severity and complications of classic GPP.
Interestingly, and in stark contrast to plaque psoriasis, which is equally common in men and women,27 pustular psoriasis is more common in women, with approximately twice as many women affected as men, as shown in both Asian28 and European cohort studies.7 The average age of onset of GPP is ~31 years of age, which is lower than that of PPP (44 years of age) or ACH (52 years of age).7 In contrast, the onset of plaque psoriasis occurs most frequently between the ages of 15–22 years, with a second, but smaller, peak of onset between the ages of 55–70 years.29
Prevalence and comorbidities
Pustular psoriasis is more prevalent among Asian populations. This might be partly explained by the presence of population-specific rare genetic variants associated with pustular psoriasis.30 Thus, GPP prevalence is higher among East Asians, affecting ~7.5 per million in Japan in contrast to 1.76 per million in the French population.31–33 Although no reports directly related to the economic burden of pustular psoriasis exist, two groups have reported that patients experience a significant impact on quality of life along with emotional problems, including anger, concern, shame, and difficulties in daily/social activities,33,34 and concomitant depression is commonly observed.34 Similar to plaque psoriasis,35–37 pustular psoriasis is associated with metabolic syndrome, including hypertension, hyperlipidemia, diabetes, and obesity.13,34 In addition, the presence of seronegative arthritis (psoriatic arthritis) has been reported in GPP13 and is a common comorbidity of PPP, where it is found in up to 16% of patients.34 PPP is also known to coexist with a rare syndrome called synovitis–acne–pustulosis–hyperostosis–osteitis, a rare disease with an arthritic and cutaneous presentation.38,39
Predisposing factors
While the genetic basis of pustular psoriasis has become clearer in recent years, the cause of pustular psoriasis is still mostly unknown and unexplored. Most of the evidence gathered to date is based on individual case reports but hints toward interactions between established (or still unknown) genetic predisposing factors and environmental factors such as microbiota dysbiosis or exposure to triggering drugs. With regard to the latter, pustular psoriasis has been reported to be triggered by different medications, including terbinafine, penicillin, lithium, iodine, progestins, and hydroxychloroquine.40–42 Steroid withdrawal is also a known predisposing factor, and pustular psoriasis can arise as a paradoxical reaction to ongoing treatment with anti-TNF agents.43,44 Bacterial and viral species, including Staphylococcus aureus, Epstein–Barr virus, and cytomegalovirus, have been associated with GPP flares.45–47 Smoking is one of the best-known risk factors for pustular psoriasis, particularly PPP, where this association is best established.48,49 However, once pustular psoriasis is triggered, smoking cessation may improve palmoplantar disease but typically does not lead to full resolution.50
Pathogenesis
In recent years, substantial progress has been made in shedding light on some of the central genetic and immunological processes involved in pustular psoriasis. A common thread is the convergence of IL-36 cytokines to the pro-inflammatory, epithelial-derived IL-1 cytokine family.51 IL-1 family members differ from classic IL-1 cytokines in that they are activated extracellularly by proteolytic cleavage.52 In contrast, classic IL-1 family members produced by many cell types are usually activated intracellularly via inflammasome-mediated cleavage.53 Available evidence indicates that IL-36 cytokines are likely the critical driver of the autoinflammatory responses that characterize pustular psoriasis, as recently evidenced by the rapid and marked clinical response seen with IL-36 receptor inhibition in GPP.54 In contrast to autoimmunity, an adaptive immune-mediated process, autoinflammation is a process by which innate immune cells inflict host tissue damage in the absence of an infectious stimulus. Autoinflammation can be initiated by aberrant inflammatory cytokine production and/or alterations in cytokine signaling.
The term “autoinflammation” emerged in 1999, describing the role of mutations in tumor necrosis factor receptor (TNFR1) resulting in periodic fever syndrome.55 Since then, many similar diseases have been described.53,56 In contrast to autoimmune diseases, which are driven by cells of the adaptive immune system (T and B cells) reacting against self-tissues through T-cell responses or autoantibodies, autoinflammatory diseases are caused by genetic mutations in molecules and pathways that are involved in innate immune responses.55,56 Here, we will discuss the current knowledge and understanding of the genetic basis of pustular psoriasis and the immunological processes involved.
Genetic architecture
Genetic variants in six different genes have been implicated in pustular psoriasis, including clinical syndromes with limited features of pustular psoriasis. These include genes encoding the interleukin-36 receptor antagonist (IL36RN), the caspase recruitment domain-containing protein 14 (CARD14), adapter protein complex 1 subunit sigma 3 (AP1S3), TNFAIP3-interacting protein 1 (TNIP1), the serine protease inhibitor gene serpin family A member 3 (SERPINA3), and the IL-1 receptor antagonist (IL-1RA) gene (IL1RN), which is mutated in a severe autoinflammatory syndrome of the skin and bones that occasionally presents with widespread pustules and bone inflammation.53
The first evidence for genetic defects involving pustular skin disease was the identification of homozygous mutations in IL1RN in six families with a deficiency of IL-1 receptor antagonist (DIRA).53 The resulting IL-1RA deficiency leads to unopposed activity of the pro-inflammatory cytokines IL-1α and IL-1β. Of the nine children in the original report, eight presented with cutaneous pustulosis, ranging from discrete crops of pustules to generalized severe pustulosis, with patients either presenting at birth or by 2.5 weeks of age.53 A similar presentation has been reported by other groups.57,58
DIRA was the first genetic disorder with mutations leading to cutaneous pustules. Two years after the finding of IL1RN mutations as a cause for DIRA, mutations in the IL-36 receptor antagonist gene (IL36RN) were identified in nine Tunisian multiplex families with autosomal recessive GPP.59 Nearly simultaneously, another research group published IL36RN mutations in three out of five unrelated individuals with GPP.51 The term “deficiency of interleukin thirty-six-receptor antagonist”, or DITRA is sometimes used for this disease.59 IL-36 is a member of the IL-1 family of cytokines,52 and similar to the function of the IL-1RA on IL-1α and IL-1β function, the IL-36 receptor antagonist opposes binding of the pro-inflammatory IL-36 cytokine members IL-36α, IL-36β, and IL-36γ to the IL-36 receptor.59 Thus, under normal circumstances, the IL-36 receptor antagonist prevents IL-36-induced NF-κB activation, but with GPP-associated mutations in the IL36RN gene, the absence or malfunction of the IL-36 receptor antagonist leads to unopposed signaling of IL-36 cytokines.60 Patients with IL36RN mutations have a typical GPP clinical course defined by repeated flares of sudden onset, with diffuse erythematous skin eruption characterized by rapid coverage of pustules along with high-grade fever, leukocytosis, and elevated C-reactive protein.59 Of the original cohort, the disease presented in 75% of patients during childhood and in the remaining quarter during early adult life, including pregnancy (impetigo herpetiformis).59
The mutations identified in the IL36RN gene in the initial Tunisian families with GPP were homozygous missense mutations with the substitution of proline for leucine at position 27 (p.Leu27Pro).59 This mutation affected the stability of the IL-36 receptor antagonist protein, resulting in unopposed activity of the three pro-inflammatory IL-36 cytokines.59 In the initial European study of five individuals with GPP, three were found to have mutations in the IL36RN gene, including a new homozygous missense mutation (p.Ser113Leu) and one compound heterozygote carrier (p.Ser113Leu and p.Arg48Trp).51 To date, multiple types of mutations have been reported in the IL36RN gene and associated with GPP, including substitution, frameshift, and splicing defects.61 The range of mutations associated with pustular psoriasis subtypes is outlined in Table 2. The mutation allele frequency of IL36RN in GPP is close to 24%, with a frequency that is slightly lower in ACH (~17%) and lower still in PPP (~5%).7 A similar low frequency of IL36RN mutations in PPP has been reported by other groups.62 Notably, single nucleotide variants in the IL36RN gene were not of pathogenic importance among patients from various ethnic groups, including European,61 Japanese,63 and Chinese populations,64 further bringing into question the link between IL36RN and PPP.64 IL36RN mutations do not appear to contribute to the risk of plaque psoriasis, and the great majority of IL36RN mutations are seen in patients with GPP that do not have concomitant plaque psoriasis.65 Finally, in light of the role of the IL-36 axis in pustular forms of psoriasis, it is notable that a single nucleotide polymorphism in the IL36G gene region has been associated with plaque psoriasis.66 However, no studies have yet examined this polymorphism in pustular psoriasis subtypes.
Table 2.
Genetic risk variants associated with the pustular psoriasis subtypes
| Genetic variant | Ethnicity | Pustular psoriasis subtype |
|---|---|---|
| IL36RN (encoding IL-36Ra) | ||
| p.Leu27Pro | Tunisian families | GPP59 |
| p.Ser113Leu | European | GPP,51 PPP61 |
| p.Ser113Leu /p.Arg48Trp | GPP,51 ACH61 | |
| p.Ser113Leu/p.Ser113Leu | GPP,61 ACH,61 PPP61 | |
| p.Lys35Arg/p.Ser113Leu | GPP61 | |
| p.Arg48Trp/p.Ser113Leu | GPP7 | |
| p.Val44Met/p.Ser113Leu | GPP7 | |
| p.Arg102Trp/p.Ser113Leu | ACH7, 61 | |
| p.Glu94X/p.Ser113Leu | GPP127 | |
| p.Pro76Leu/p.Ser113Leu | GPP127 | |
| c.115+6T > C | Asian | GPP,61 ACH + GPP61 |
| c.115+6T > C/c.115+6T > C | GPP,61 ACH + GPP61 | |
| c.115+6T > C/p.Ser113Leu | GPP61 | |
| c.115+6T > C;p.Pro76Leu | GPP7,54 | |
| p.Arg102Trp | GPP61 | |
| c.115+5G > A/p.Ser113Leu | GPP61 | |
| p.Arg10Arg/p.Ser113Leu | GPP61 | |
| p.Arg10X | GPP128 | |
| p.Arg10Arg/p.Thr123Arg | GPP129 | |
| p.Arg10Arg/p.Arg10X | GPP129 | |
| p.Pro76Leu | GPP130 | |
| p.Pro76Leu/p.Pro76Leu | Turkish | GPP61 |
| p.Leu27Pro/p.Leu27Pro | Algerian | GPP131 |
| p.Gly141Met/p.Gly141Met | Spanish | GPP131 |
| CARD14 | ||
| p.Glu138Ala | European | GPP67 |
| p.Ser602Leu | GPP, PPP132 | |
| p.Ser200Asn | PPP62 | |
| p.Glu197Lys | PPP62 | |
| p.Thr591Met | PPP7 | |
| p.Lys78Asn | PPP7 | |
| p.Arg69Gln | GPP133 | |
| p.Arg179His | GPP133 | |
| p.Gly117Ser | GPP133 | |
| p.Asp176His | GPP133 | |
| p.Gly117Ser | Tunisian | GPP132 |
| p.Arg69Trp | GPP132 | |
| p.Glu197Lys | GPP132 | |
| p.Gly117Ser | Asian | Familial GPP67 |
| p.Asp176His | GPP with PV,134 PPP135 | |
| p.Arg275His | GPP54 | |
| p.Cys50Tyr | Chinese Han | PPP73 |
| p.Pro479Arg | PPP73 | |
| p.Gly648Ser | PPP73 | |
| p.Met119Val | GPP with PV136 | |
| p.Arg166His | GPP with PV136 | |
| p.Arg682Trp | PPP, GPP with PV7,136 | |
| AP1S3 | ||
| p.Phe4Cys | European | GPP,75 PPP,75 PV75 |
| p.Arg33Trp | GPP,75 PPP,75 ACH7,75 | |
| p.Gln17Lys | GPP, PPP, ACH75 | |
| p.Thr22Ala | GPP, PPP, ACH75 | |
| p.Thr32IIe | GPP, PPP, ACH75 | |
| p.Leu79Val | GPP, PPP, ACH75 | |
| p.Ile83Thr | GPP, PPP, ACH75 | |
| TNIP1 | Chinese | |
| rs3805435, rs3792798, rs3792797, rs869976, rs17728338, and rs999011 | GPP82,84 | |
| SERPINA3 | European | GPP79 |
| c.966delT/p.Tyr322Ter | ||
| IL1RN | Puerto Rican | GPP58 |
GPP generalized pustular psoriasis, PPP palmoplantar pustular psoriasis, ACH acrodermatitis continua of Hallopeau, PV psoriasis vulgaris
Gain-of-function mutations in CARD14 were identified in 2012 in several extended families with multiple cases of patients with plaque psoriasis, psoriatic arthritis, and pustular psoriasis,67 capping the search of the gene behind the psoriasis susceptibility 2 (PSORS2) locus reported 18 years prior.68 CARD14 belongs to the CARD-containing, membrane-associated guanylate kinase-like domain-containing protein (CARMA) family of scaffolding proteins that play a critical role in the recruitment and activation of IKK proteins and activation of the NF-κB signaling pathway.69 Psoriasis-associated variants have been shown to have a gain of function effect by disrupting CARD14 autoinhibition, leading to BCL10- and MALT1-dependent NF-κB activation in keratinocytes.70 Notably, keratinocytes transfected with psoriasis-associated CARD14 genetic variants had increased NF-κB activity and increased mRNA expression of IL36G, the potent neutrophil chemoattractant CXCL8 (IL-8), and CCL20.71 CARD14 gain-of-function mutant mice (Card14E138A/+) develop spontaneous skin inflammation that resembles psoriasis and has increased Il17a expression in the skin,72 and conversely, CARD14-deficient mice are resistant to the development of psoriasis-like inflammation in an inducible model of psoriasis.72 Mutations in the CARD14 gene are associated with both plaque psoriasis and GPP, with different mutations predisposing to different types of psoriasis. Thus, the p.Gly117Ser variant is associated with plaque psoriasis, whereas GPP is strongly associated with the p.Glu138Ala variant.71–74 In a cohort of 251 patients of European American and German ethnicity, PPP was also shown to be associated with CARD14 mutations.62 Similar findings have been reported in Han Chinese, where three novel variants were shown to be associated with PPP.73
In 2014, mutations in AP1S3 were identified to predispose patients to pustular psoriasis.75 AP1S3 is a component of the adapter protein 1 complex, which is important for vesicle trafficking between the Golgi network and endosomes.76 Pustular psoriasis-associated AP1S3 mutations were shown to disrupt the endosomal translocation of TLR3, an innate pattern-recognition receptor for double-stranded RNA,75 which recognizes double-stranded RNA.77 Further work by this same group78 demonstrated that AP1S3 interferes with keratinocyte autophagy and in turn results in abnormal activation of NF-κB signaling through accumulation of p62. AP1S3-deficient cells have increased mRNA expression of both IL1B and IL36A and increased expression and secretion of CXCL8 (IL-8).78 AP1S3 mutations have been reported in all pustular psoriasis subtypes and appear to be most frequent in the ACH subtype.75
Recently, a loss-of-function mutation in SERPINA3 was identified in 2 of 25 GPP patients by whole exome sequencing.79 SERPINA3 encodes a keratinocyte-derived serine protease that interacts with different proteases to inhibit their activity. Its strongest interaction is with the neutrophil protease cathepsin G.15,80 Cathepsin G has been shown to process full-length secreted IL-36 cytokines to their more active truncated forms, thereby increasing their pro-inflammatory activity by a factor of >500.16,81
Other genetic variants implicated in the predisposition to GPP include variants in the TNIP1 gene locus.82 TNFAIP3-interacting protein 1, encoded by TNIP1, interacts with the deubiquitinating enzyme A20 to inhibit NF-κB signaling.83 In a study of 73 patients with GPP, 67 patients with PPP, and 476 healthy controls in the Han Chinese population, 6 polymorphisms were identified in the TNIP1 gene locus and shown to be weakly associated with GPP but not PPP.84
In summary, most of the genetic variants identified to date that predispose to pustular psoriasis involve signaling pathways or biological processes involving IL-1 or IL-36 activity and thereby emphasize the prominent autoinflammatory nature of the pustular psoriasis subtypes.
Immunology and cytokine signaling network in pustular psoriasis
The most prominent inflammatory response in pustular forms of psoriasis involves activation of IL-1 and IL-36 cytokine signaling.15 While both IL-1 and IL-36 are increased in plaque forms of psoriasis, they are expressed approximately tenfold higher in pustular forms of psoriasis,16 whereas IL-17 responses appear to play a lesser role in pustular psoriasis (Gudjonsson, unpublished observation). Thus, psoriasis subtypes can be envisioned to exist on a spectrum, where on one end, plaque psoriasis is characterized by adaptive immune processes involving CD4 and CD8 T cells and the dominance of the IL-17/IL-23 immune axis,85 and on the opposite end, innate immune responses dominate involving neutrophil infiltration and IL-36 activation, features more characteristic of autoinflammatory responses.16 While targeting the IL-1 axis in GPP has shown some success,86 the response appears to be significantly more modest than what is seen with inhibition of the IL-36 axis.54
IL-36 cytokines are part of the IL-1 family, which consists of 11 members: IL-1 (IL-1α, IL-1β, IL-1RA), IL-18, IL-33, IL-36 (IL-36α, IL-36β, IL-36γ, IL-36RA), IL-37, and IL-38.87 The IL-36 cytokines act in a very similar manner to IL-1, with IL-36α, IL-36β, and IL-36γ having pro-inflammatory activity and IL-36Ra inhibiting their effect at the receptor level.88 Several notable differences exist between the IL-1 and IL-36 cytokines, with the expression of IL-36 cytokines being mostly limited to epithelial tissues such as those of the skin, lung, and gastrointestinal tract.88 Furthermore, whereas IL-1β is dependent upon inflammasome activation and processing by intracellular caspases prior to secretion,89 IL-36 cytokines are secreted, usually after some type of danger signal,90 as full-length proteins and require processing by extracellular proteases for their full activity.81,91
The main proteases that have been implicated in the processing of IL-36 cytokines include the neutrophil-derived proteases cathepsin G, elastase, and proteinase-3,81 with neutrophil extracellular traps, which are abundant in pustular psoriasis skin lesions,15 serving as platforms for their processing and activation.92 This processing increases the biological activity of the IL-36 cytokines by ~500-fold.81 In addition, inducible keratinocyte-derived proteases, such as Cathepsin S, can effectively process IL-36γ.91
IL-36 signals to keratinocytes in an autocrine manner, inducing the expression and production of more IL-36 cytokines as well as other pro-inflammatory cytokines, antimicrobial peptides, and neutrophil chemokines, such as CXCL1, CXCL2, and CXCL8 (IL-8) (Fig. 3), acting through the metalloreductases STEAP1 and STEAP4,93 thus creating a self-sustaining cycle of inflammation in the epidermis.90 IL-36 also acts on both T cells and dendritic cells. In dendritic cells, IL-36 activation promotes maturation and increases the expression of MHC class II along with the costimulatory molecules CD80 and CD86,94 in addition to promoting the secretion of pro-inflammatory cytokines, including IL-1, IL-23, TNF, and IL-6.94,95 IL-36 leads to the induction of IFN-γ, IL-4, and IL-17 by T cells94 and has also been shown to promote clonal CD4 T-cell expansion and IL-17A production in GPP.96 This activation and contribution of both T cells and dendritic cells in IL-36 responses may explain the therapeutic efficacy seen in many patients with GPP treated with anti-TNF or anti-IL-17A agents.97,98
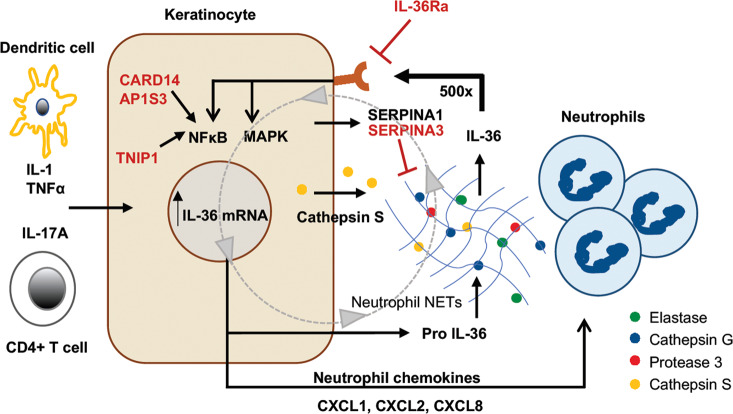
Fig. 3.
IL-36 autocrine and autoinflammatory circuits. Keratinocytes are the major source of IL-36 in the skin. IL-36 expression can be induced by other pro-inflammatory cytokines, including IL-1, TNF, and IL-17A. IL-36 is secreted as full-length “pro-IL-36.” When exposed to neutrophil-derived proteases, including elastase, cathepsin G, or protease 3, IL-36 cytokines are enzymatically cleaved into a truncated form that has >500-fold greater biological activity. This truncated IL-36 can act back on keratinocytes via the IL-36 receptor (IL-36R) to induce even more IL-36 expression, amplifying the circuit, as well as expression of neutrophil chemokines such as CXCL1, CXCL2, CXCL6, and CXCL8 (IL-8) that attract progressively greater numbers of neutrophils into the skin. The serine protease inhibitors SERPINA1 and SERPINA3 can inhibit neutrophil proteases. Genes shown in red have been shown to genetically predispose to pustular forms of psoriasis
Importantly, to prevent unchecked amplification of this IL-36-mediated inflammatory cascade, keratinocytes have the ability to regulate this pathway through the secretion of protease inhibitors, many of which are induced by the same inflammatory mediators that turn on IL-36.15 While this overall picture is still incompletely understood, it has been shown that IL-36α processing by neutrophil elastase can be inhibited by keratinocyte-derived SERPINA1.15 Similarly, IL-36γ processing by Cathepsin G can be inhibited by SERPINA3.15 This would explain how loss-of-function mutations in the SERPINA3 gene, as recently described79 and outlined earlier in this article, contribute to GPP.
It should be noted that IL-36 activation is not limited to pustular psoriasis, as IL-36 is also active and likely to contribute to the pathophysiology of chronic plaque psoriasis as part of the feed-forward amplification in the epidermis.99,100 Therapies currently in clinical trials, such as the IL-36R antagonist, are likely to help to shed light on this mechanism. However, the main difference between plaque and pustular psoriasis is that pustular psoriasis appears to involve “hyperactivation” of the IL-36 axis (Fig. 4), leading to a marked and robust shift toward neutrophil chemotaxis and neutrophil-driven inflammatory responses.
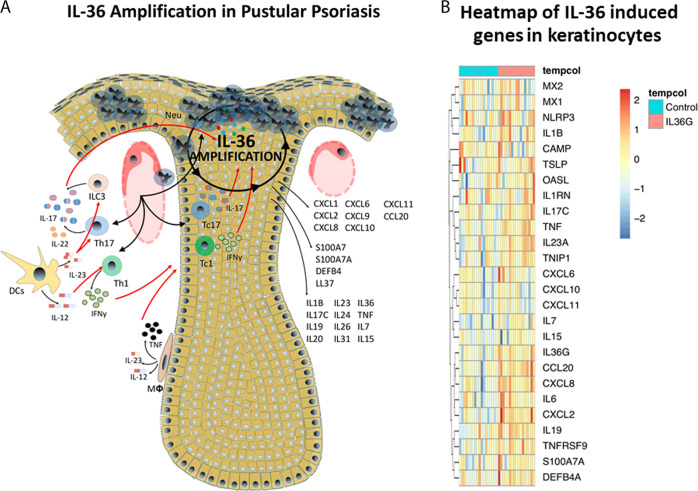
Fig. 4.
IL-36 amplification circuit in pustular psoriasis. a Shows the role of the IL-36 autocrine circuit in amplifying inflammation in pustular psoriasis. IL-36 leads to the induction of a number of cytokines and chemokines that drive various aspects of psoriasis, such as neutrophil chemotaxis (CXCL1, CXCL2, CXCL6, CXCL8), Th17 chemotaxis (CCL20), antimicrobial responses (S100A7, S100A7A, DEFB4, LL-37), and pro-inflammatory cytokines (IL-7, IL-15, IL-17C, IL-19, IL-36). b demonstrates the induction of gene expression via RNA sequencing in human primary keratinocytes upon IL-36 stimulation for 24 h, which again includes genes important for neutrophil infiltration, antimicrobial responses, pro-inflammatory cytokines, and type I interferon responses (MX1, MX2, OASL)
Treatment perspectives
Pustular psoriasis is difficult to treat, and no drugs are yet approved specifically for therapy against this disease. Historically, therapeutic options have been limited to cyclosporine, methotrexate, and acitretin as first-line therapies,101 often with variable treatment responses. In the last two decades, many of the existing biologics approved for plaque psoriasis have been used to treat patients with pustular psoriasis. The anti-TNF agent infliximab has been shown to be effective in case reports and small case series of patients with acute GPP.97,102 Notably, however, paradoxical psoriasis can arise after treatment with anti-TNF agents, and these are often pustular in nature,103 presenting most frequently as PPP.104 Ustekinumab, which targets the common p40 subunit of IL-12 and IL-23, was found to exhibit efficacy in four patients with GPP;105 however, three of them had preexisting plaque psoriasis, making interpretation of the results difficult. Ustekinumab has further shown limited therapeutic efficacy in PPP.106,107 The therapeutic response to the anti-IL-17A agent secukinumab was shown in an open-label single-arm study in 12 Japanese GPPs over a period of 16 weeks.98 Another trial looking at the clinical response to secukinumab in PPP showed modest improvement at week 16, with ~27% of patients achieving 75% improvement from baseline.108 Treatments targeting IL-1 have been reported by several groups. Thus, anakinra, a recombinant IL-1RA, was shown to be effective in a single patient with a history of plaque psoriasis and GPP.86 Similarly, gevokizumab, a novel IL-1β inhibitor, showed beneficial effects in two patients with GPP without a prior history of plaque psoriasis.109 Another IL-1β inhibitor, canakinumab, showed successful outcomes in a patient with a severe form of GPP110 and partial response in two patients with severe PPP.111 More recently, a monoclonal antibody targeting the IL-36 receptor (spesolimab) was shown to be effective in five out of seven patients with GPP, with all patients exhibiting rapid skin improvement within 4 weeks after the administration of a single dose of spesolimab.54 Given the prominence of IL-36 activation in pustular psoriasis, two anti-IL-36R agents, spesolimab and ANB019, are currently in phase II clinical trials for GPP and PPP. Table 3 lists treatments that are under preclinical and clinical trials targeting IL-1/IL-36 for pustular psoriasis subtypes.
Table 3.
IL-1/IL-36 targets and treatments for pustular psoriasis that are under preclinical and clinical trials
| Treatment/target | Pustular psoriasis subtype: results | Trial |
|---|---|---|
| Gevokizumab/IL-1β | GPP: beneficial109 | Case report |
| Canakinumab/IL-1β | GPP: beneficial110 | Case report |
| PPP: unresponsive111 | Case report | |
| Anakinra/IL-1RA | GPP: beneficial86 | Case report |
| PPP: partial response137 | Case report | |
| Spesolimab/IL-36R | GPP: beneficial54, 138 | Phase III clinical trial |
| PPP: beneficial139 | Phase II clinical trial | |
| ANB019/IL-36R | GPP: beneficial140 | Phase II clinical trial |
| PPP: beneficial141 | Phase II clinical trial |
GPP generalized pustular psoriasis, PPP palmoplantar pustular psoriasis
Animal models for pustular psoriasis
Psoriasis is a disease unique to humans.112 However, certain aspects of the disease, such as activation of specific inflammatory pathways or infiltration of specific leukocyte populations such as neutrophils, can be modeled in mice. These models can be transgenic, xenografts, or induced via intradermal cytokine injection or via topical application of the TLR7 agonist imiquimod (IMQ). We will address some of the mouse models that may have applicability for the study of GPP. No models currently exist to model PPP or ACH.
As discussed above, IL-36 activation is central to pustular psoriasis pathogenesis. Transgenic mice directing overexpression of the Il36a gene to the epidermis with the keratin 14 promoter were found to be small and showed flaky skin. Histology revealed a thickened epidermis, with leukocyte infiltration and increased expression of neutrophil chemokines.99 Interestingly, this phenotype peaked at postnatal day 5 and resolved by postnatal day 21.99 However, when these mice were backcrossed to Il36rn−/− mice, they developed a dramatically more severe skin phenotype characterized histologically by intracorneal and intraepithelial pustules, parakeratosis and dilated superficial dermal blood vessels.99 Intradermal injection of Il-36α into mouse skin113,114 has been used to induce an inflammatory phenotype, but this phenotype is less robust than the transgenic Il36rn−/− model.
The topical IMQ mouse model of psoriasis is commonly used to study various inflammatory mechanisms in skin.115 IMQ-treated mice have increased expression of both IL-1 and IL-36 cytokines and increased neutrophil infiltration into the skin, with, interestingly, female mice developing more severe disease than male mice.116 IMQ treatment in Il1r1 KO, Il36a KO, and Il1r1/Il36a double KO mice resulted in a decreased inflammatory response compared to wild-type mice.116 Furthermore, the IMQ model has been used to demonstrate the contribution of infiltrating neutrophils to the overall inflammatory burden through TLR4/IL-36R cross-talk.117
Finally, a mouse model based on the p.Glu138Ala gain-of-function mutation in CARD14 has been developed. These mice develop spontaneous psoriasis-like skin inflammation characterized by epidermal hyperplasia72 and have increased expression of IL-36 cytokines and neutrophil chemokines such as CXCL1.118 To date, no mouse models have been generated to model the effect of psoriasis-associated mutations in the AP1S3 or SERPINA3 genes.
None of these models fully capture the complexity of human pustular psoriasis, and differences in mouse vs. human biology and immunology, along with the limited genetic background of these mice due to inbreeding, may limit their usability. However, while transgenic Il36a mice spontaneously resolved the skin phenotype by 3 weeks of age, Il36rn deficiency in Il36a transgenic mice had a strong resemblance to GPP,99 and as the disease is based on abnormal activation of the IL-36 cytokine axis, such mice might be the most suitable model for the study of GPP. However, to our knowledge, this transgenic model is not commercially available.
Outstanding questions and conclusions
Enormous progress has been made in recent years to expand our understanding of the pathogenesis of pustular psoriasis. This includes the identification of predisposing genetic variants and the development of novel therapeutic agents targeting central inflammatory circuits in pustular psoriasis, such as therapies targeting IL-36, which are currently undergoing clinical trials.54 However, much work still remains to be done.
First, known genetic risk variants that predispose to pustular psoriasis explain only approximately one-third of the total number of cases.7 Therefore, there is a need for additional studies to help identify other predisposing and possibly ethnic-specific genetic variants given the high frequency of pustular psoriasis in certain populations31 and their associated biological pathways.
Second, the three IL-36 cytokines have very similar biologic activity119 but are not processed81 or expressed at equal levels in pustular psoriasis. IL36G is most highly expressed, followed by IL36A, and IL36B has the lowest expression in skin.15 Therefore, these differences hint at a deeper biological role(s), and the contribution of each of the three pro-inflammatory IL-36 members remains unknown, but elucidation of this mechanism could allow more targeted therapeutic approaches for pustular psoriasis.
Third, our understanding of the complex interactions between various proteases and protease inhibitors in psoriatic and pustular psoriasis skin is incomplete. Deeper exploration of this system will provide novel insights not only into IL-36 biology but also into a broad range of inflammatory responses in the skin.
Fourth, our understanding of the differences and relationships between different subtypes of pustular psoriasis remains limited. This includes PPP and ACH. In addition, the nature of the link to smoking remains unanswered, as well as the strong female bias, which is not seen in chronic plaque psoriasis.4
Finally, much remains to be done to elucidate the natural history of psoriasis. Currently, we have a very limited understanding of how this disease behaves over time in different patients and its associated comorbidities. Increasing our understanding of the disease course will influence not only how we study psoriasis but also how patients will be managed and treated.
Acknowledgements
This work was supported by the Babcock Endowment Fund (L.C.T. and J.E.G.), the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01-AR060802 (J.E.G.), P30-AR075043 (J.E.G.), and K01-AR072129 (L.C.T.), and the National Institute of Allergy and Infectious Diseases under award number R01-AR069071 (J.E.G.), the A. Alfred Taubman Medical Research Institute (J.E.G. and J.M.K.), the National Psoriasis Foundation (J.E.G, N.L.W., J.M.K., E.M., and L.C.T.), and the Parfait Emerging Scholar Award (J.M.K.). L.C.T. is supported by the Dermatology Foundation, the Arthritis National Research Foundation, and the National Psoriasis Foundation.
Competing interests
The authors declare no competing interests.
References
- 1.Sarac G, Koca TT, Baglan T. A brief summary of clinical types of psoriasis. North Clin. Istanb. 2016;3:79–82. doi: 10.14744/nci.2016.16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019;20:1475. doi: 10.3390/ijms20061475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestle FO, Kaplan DH, Barker J. Psoriasis. N. Engl. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 4.Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin. Dermatol. 2007;25:535–546. doi: 10.1016/j.clindermatol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev. Clin. Immunol. 2019;15:907–919. doi: 10.1080/1744666X.2019.1648209. [DOI] [PubMed] [Google Scholar]
- 6.Bissonnette R, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J. Dermatol. Sci. 2017;85:20–26. doi: 10.1016/j.jdermsci.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Twelves S, et al. Clinical and genetic differences between pustular psoriasis subtypes. J. Allergy Clin. Immunol. 2019;143:1021–1026. doi: 10.1016/j.jaci.2018.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangale-Daflapurkar S, Danve A. Pustular psoriasis of pregnancy successfully treated with cyclosporine. Am. J. Ther. 2016;23:e1250–e1252. doi: 10.1097/MJT.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 9.Owczarczyk-Saczonek A, Znajewska-Pander A, Owczarek W, Maciejewska-Radomska A, Placek W. Clinicopathologic retrospective analysis of annular pustular psoriasis. Acta Dermatovenerol. Alp. Pannonica Adriat. 2018;27:215–219. [PubMed] [Google Scholar]
- 10.Huang YW, Tsai TF. Juvenile-onset pustular psoriasis: case series and literature review. Br. J. Dermatol. 2020;182:816–817. doi: 10.1111/bjd.18473. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez NP, Perry HO, Muller SA, Winkelmann RK. Subcorneal pustular dermatosis and pustular psoriasis. A clinicopathologic correlation. Arch. Dermatol. 1983;119:715–721. [PubMed] [Google Scholar]
- 12.Zhu T, Jin H, Shu D, Li F, Wu C. Association of IL36RN mutations with clinical features, therapeutic response to acitretin, and frequency of recurrence in patients with generalized pustular psoriasis. Eur. J. Dermatol. 2018;28:217–224. doi: 10.1684/ejd.2018.3245. [DOI] [PubMed] [Google Scholar]
- 13.Choon SE, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J. Dermatol. 2014;53:676–684. doi: 10.1111/ijd.12070. [DOI] [PubMed] [Google Scholar]
- 14.Navarini AA, et al. European consensus statement on phenotypes of pustular psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017;31:1792–1799. doi: 10.1111/jdv.14386. [DOI] [PubMed] [Google Scholar]
- 15.Johnston A, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J. Allergy Clin. Immunol. 2017;140:109–120. doi: 10.1016/j.jaci.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 2017;49:1–8. doi: 10.1016/j.coi.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 18.Li M, et al. Prevalent and rare mutations in IL-36RN gene in Chinese patients with generalized pustular psoriasis and psoriasis vulgaris. J. Invest. Dermatol. 2013;133:2637–2639. doi: 10.1038/jid.2013.267. [DOI] [PubMed] [Google Scholar]
- 19.Lohr S, et al. Association analysis of psoriasis vulgaris and psoriatic arthritis with loss-of-function mutations in IL36RN in German patients. Br. J. Dermatol. 2016;175:639–641. doi: 10.1111/bjd.14624. [DOI] [PubMed] [Google Scholar]
- 20.Asumalahti K, et al. Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. J. Invest. Dermatol. 2003;120:627–632. doi: 10.1046/j.1523-1747.2003.12094.x. [DOI] [PubMed] [Google Scholar]
- 21.Borges-Costa J, et al. Clinical and laboratory features in acute generalized pustular psoriasis: a retrospective study of 34 patients. Am. J. Clin. Dermatol. 2011;12:271–276. doi: 10.2165/11586900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths C, Barker J, Chalmers R, Bleiker T, Creamer D. Rook’s Textbook of Dermatology. Hoboken: John Wiley & Sons, Incorporated; 2016. [Google Scholar]
- 23.Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int. J. Mol. Sci. 2016;17:1214. doi: 10.3390/ijms17081214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br. J. Dermatol. 1968;80:771–793. doi: 10.1111/j.1365-2133.1968.tb11947.x. [DOI] [PubMed] [Google Scholar]
- 25.Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br. J. Dermatol. 1971;85:407–411. doi: 10.1111/j.1365-2133.1971.tb14044.x. [DOI] [PubMed] [Google Scholar]
- 26.Zelickson BD, Muller SA. Generalized pustular psoriasis. A review of 63 cases. Arch. Dermatol. 1991;127:1339–1345. [PubMed] [Google Scholar]
- 27.Armstrong AW. Psoriasis. JAMA Dermatol. 2017;153:956. doi: 10.1001/jamadermatol.2017.2103. [DOI] [PubMed] [Google Scholar]
- 28.Jin H, et al. Clinical features and course of generalized pustular psoriasis in Korea. J. Dermatol. 2015;42:674–678. doi: 10.1111/1346-8138.12863. [DOI] [PubMed] [Google Scholar]
- 29.Larsabal M, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br. J. Dermatol. 2019;180:647–656. doi: 10.1111/bjd.17147. [DOI] [PubMed] [Google Scholar]
- 30.Yan D, Afifi L, Jeon C, Cordoro KM, Liao WA. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol. Online J. 2018;24:4. [PubMed] [Google Scholar]
- 31.Ohkawara A, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm. Venereol. 1996;76:68–71. doi: 10.2340/00015555766871. [DOI] [PubMed] [Google Scholar]
- 32.Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur. J. Dermatol. 2006;16:669–673. [PubMed] [Google Scholar]
- 33.Kharawala S, Golembesky AK, Bohn RL, Esser D. The clinical, humanistic, and economic burden of generalized pustular psoriasis: a structured review. Expert Rev. Clin. Immunol. 2020;16:239–252. doi: 10.1080/1744666X.2019.1708193. [DOI] [PubMed] [Google Scholar]
- 34.Trattner H, et al. Quality of life and comorbidities in palmoplantar pustulosis—a cross-sectional study on 102 patients. J. Eur. Acad. Dermatol. Venereol. 2017;31:1681–1685. doi: 10.1111/jdv.14187. [DOI] [PubMed] [Google Scholar]
- 35.Kozlowska D, et al. Serum sphingolipid level in psoriatic patients with obesity. Postepy Dermatol. Alergol. 2019;36:714–721. doi: 10.5114/ada.2019.91422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goolam Mahyoodeen N, Crowther NJ, Snyman T, Pillay L, Tikly M. High burden of the metabolic syndrome and its component disorders in South Africans with psoriasis. Int J. Dermatol. 2019;58:557–562. doi: 10.1111/ijd.14348. [DOI] [PubMed] [Google Scholar]
- 37.Namiki K, et al. Thyroid dysfunction in patients with psoriasis: higher prevalence of thyroid dysfunction in patients with generalized pustular psoriasis. J. Dermatol. 2020;47:133–139. doi: 10.1111/1346-8138.15178. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Li C, Zhang W. The coexistence of SAPHO syndrome and rheumatoid arthritis: a case report. Medicine. 2017;96:e5724. doi: 10.1097/MD.0000000000005724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rozin AP, Nahir AM. Is SAPHO syndrome a target for antibiotic therapy? Clin. Rheumatol. 2007;26:817–820. doi: 10.1007/s10067-006-0274-6. [DOI] [PubMed] [Google Scholar]
- 40.Ozturk G, et al. Generalized pustular eruptions due to terbinafine. Cutan. Ocul. Toxicol. 2012;31:81–84. doi: 10.3109/15569527.2011.607202. [DOI] [PubMed] [Google Scholar]
- 41.Gammoudi R, et al. Acute generalized exanthematous pustulosis induced by oxacillin confirmed by patch testing. Contact Dermat. 2018;79:108–110. doi: 10.1111/cod.13005. [DOI] [PubMed] [Google Scholar]
- 42.Webster GF. Pustular drug reactions. Clin. Dermatol. 1993;11:541–543. doi: 10.1016/0738-081x(93)90163-7. [DOI] [PubMed] [Google Scholar]
- 43.Saeki H, et al. Juvenile pustular psoriasis associated with steroid withdrawal syndrome due to topical corticosteroid. J. Dermatol. 2008;35:601–603. doi: 10.1111/j.1346-8138.2008.00531.x. [DOI] [PubMed] [Google Scholar]
- 44.Vasconcellos JB, et al. Paradoxical psoriasis after the use of anti-TNF in a patient with rheumatoid arthritis. Bras. Dermatol. 2016;91:137–139. doi: 10.1590/abd1806-4841.20164456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiyad Z, Moriarty B, Creamer D, Higgins E. Generalized pustular psoriasis associated with Epstein-Barr virus. Clin. Exp. Dermatol. 2015;40:146–148. doi: 10.1111/ced.12493. [DOI] [PubMed] [Google Scholar]
- 46.Yoneda K, Matsuoka-Shirahige Y, Demitsu T, Kubota Y. Pustular psoriasis precipitated by cytomegalovirus infection. Br. J. Dermatol. 2012;167:1186–1189. doi: 10.1111/j.1365-2133.2012.11044.x. [DOI] [PubMed] [Google Scholar]
- 47.Pouessel G, et al. Childhood pustular psoriasis associated with Panton-Valentine leukocidin-producing Staphylococcus aureus. Pediatr. Dermatol. 2007;24:401–404. doi: 10.1111/j.1525-1470.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 48.Miot HA, Miot LD, Lopes PS, Haddad GR, Marques SA. Association between palmoplantar pustulosis and cigarette smoking in Brazil: a case-control study. J. Eur. Acad. Dermatol. Venereol. 2009;23:1173–1177. doi: 10.1111/j.1468-3083.2009.03282.x. [DOI] [PubMed] [Google Scholar]
- 49.Wilsmann-Theis, D. et al. Palmoplantar pustulosis—a cross-sectional analysis in Germany. Dermatol. Online J.23 (2017). [PubMed]
- 50.Michaelsson G, Gustafsson K, Hagforsen E. The psoriasis variant palmoplantar pustulosis can be improved after cessation of smoking. J. Am. Acad. Dermatol. 2006;54:737–738. doi: 10.1016/j.jaad.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 51.Onoufriadis A, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am. J. Hum. Genet. 2011;89:432–437. doi: 10.1016/j.ajhg.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boutet MA, et al. Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin. Exp. Immunol. 2016;184:159–173. doi: 10.1111/cei.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aksentijevich I, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N. Engl. J. Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bachelez H, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N. Engl. J. Med. 2019;380:981–983. doi: 10.1056/NEJMc1811317. [DOI] [PubMed] [Google Scholar]
- 55.McDermott MF, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 56.Brydges S, Kastner DL. The systemic autoinflammatory diseases: inborn errors of the innate immune system. Curr. Top. Microbiol. Immunol. 2006;305:127–160. doi: 10.1007/3-540-29714-6_7. [DOI] [PubMed] [Google Scholar]
- 57.Jesus AA, et al. A novel mutation of IL1RN in the deficiency of interleukin-1 receptor antagonist syndrome: description of two unrelated cases from Brazil. Arthritis Rheumatol. 2011;63:4007–4017. doi: 10.1002/art.30588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minkis K, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch. Dermatol. 2012;148:747–752. doi: 10.1001/archdermatol.2011.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marrakchi S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N. Engl. J. Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 60.Tauber M, et al. IL36RN mutations affect protein expression and function: a basis for genotype-phenotype correlation in pustular diseases. J. Invest. Dermatol. 2016;136:1811–1819. doi: 10.1016/j.jid.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 61.Setta-Kaffetzi N, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J. Invest. Dermatol. 2013;133:1366–1369. doi: 10.1038/jid.2012.490. [DOI] [PubMed] [Google Scholar]
- 62.Mossner R, et al. Palmoplantar pustular psoriasis is associated with missense variants in CARD14, but not with loss-of-function mutations in IL36RN in European patients. J. Invest. Dermatol. 2015;135:2538–2541. doi: 10.1038/jid.2015.186. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi T, Fujimoto N, Kabuto M, Nakanishi T, Tanaka T. Mutation analysis of IL36RN gene in Japanese patients with palmoplantar pustulosis. J. Dermatol. 2017;44:80–83. doi: 10.1111/1346-8138.13551. [DOI] [PubMed] [Google Scholar]
- 64.Xiaoling Y, Dan S, Hongzhong J. Lack of association between mutation in IL36RN and palmoplantar pustular psoriasis in Chinese patients. Bras. Dermatol. 2019;94:658–663. doi: 10.1016/j.abd.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capon F. IL36RN mutations in generalized pustular psoriasis: just the tip of the iceberg? J. Invest. Dermatol. 2013;133:2503–2504. doi: 10.1038/jid.2013.361. [DOI] [PubMed] [Google Scholar]
- 66.Traks T, et al. Polymorphisms in IL36G gene are associated with plaque psoriasis. BMC Med. Genet. 2019;20:10. doi: 10.1186/s12881-018-0742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jordan CT, et al. PSORS2 is due to mutations in CARD14. Am. J. Hum. Genet. 2012;90:784–795. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomfohrde J, et al. Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science. 1994;264:1141–1145. doi: 10.1126/science.8178173. [DOI] [PubMed] [Google Scholar]
- 69.Blonska M, Lin X. CARMA1-mediated NF-kappaB and JNK activation in lymphocytes. Immunol. Rev. 2009;228:199–211. doi: 10.1111/j.1600-065X.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howes A, et al. Psoriasis mutations disrupt CARD14 autoinhibition promoting BCL10-MALT1-dependent NF-kappaB activation. Biochem J. 2016;473:1759–1768. doi: 10.1042/BCJ20160270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jordan CT, et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am. J. Hum. Genet. 2012;90:796–808. doi: 10.1016/j.ajhg.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang M, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66–79. doi: 10.1016/j.immuni.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 73.Fu F, et al. Rare CARD14 missense variants associated with palmoplantar pustulosis (PPP) in the Chinese Han population. Eur. J. Dermatol. 2019;29:99–100. doi: 10.1684/ejd.2018.3457. [DOI] [PubMed] [Google Scholar]
- 74.Tsoi LC, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 2012;44:1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Setta-Kaffetzi N, et al. AP1S3 mutations are associated with pustular psoriasis and impaired toll-like receptor 3 trafficking. Am. J. Hum. Genet. 2014;94:790–797. doi: 10.1016/j.ajhg.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 78.Mahil SK, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and up-regulating IL-36 production. J. Invest. Dermatol. 2016;136:2251–2259. doi: 10.1016/j.jid.2016.06.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frey S, et al. Rare loss-of-function mutation in SERPINA3 in generalized pustular psoriasis. J. Invest. Dermatol. 2020;140:1451–1455. doi: 10.1016/j.jid.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 80.Beatty K, Bieth J, Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J. Biol. Chem. 1980;255:3931–3934. [PubMed] [Google Scholar]
- 81.Henry CM, et al. Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines. Cell Rep. 2016;14:708–722. doi: 10.1016/j.celrep.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Z, Xu JH. Investigation of psoriasis susceptibility loci in psoriatic arthritis and a generalized pustular psoriasis cohort. J. Investig. Dermatol. Symp. Proc. 2018;19:S83–S85. doi: 10.1016/j.jisp.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 83.Heyninck K, Kreike MM, Beyaert R. Structure-function analysis of the A20-binding inhibitor of NF-kappa B activation, ABIN-1. FEBS Lett. 2003;536:135–140. doi: 10.1016/s0014-5793(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 84.Han JW, et al. Tumor necrosis factor-alpha induced protein 3 interacting protein 1 gene polymorphisms and pustular psoriasis in Chinese Han population. Chin. Med. J. 2016;129:1519–1524. doi: 10.4103/0366-6999.184470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nograles KE, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huffmeier U, Watzold M, Mohr J, Schon MP, Mossner R. Successful therapy with anakinra in a patient with generalized pustular psoriasis carrying IL36RN mutations. Br. J. Dermatol. 2014;170:202–204. doi: 10.1111/bjd.12548. [DOI] [PubMed] [Google Scholar]
- 87.Towne JE, et al. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity. J. Biol. Chem. 2011;286:42594–42602. doi: 10.1074/jbc.M111.267922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Debets R, et al. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J. Immunol. 2001;167:1440–1446. doi: 10.4049/jimmunol.167.3.1440. [DOI] [PubMed] [Google Scholar]
- 89.He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnston A, et al. IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J. Immunol. 2011;186:2613–2622. doi: 10.4049/jimmunol.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ainscough JS, et al. Cathepsin S is the major activator of the psoriasis-associated proinflammatory cytokine IL-36gamma. Proc. Natl Acad. Sci. USA. 2017;114:E2748–E2757. doi: 10.1073/pnas.1620954114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clancy DM, Henry CM, Sullivan GP, Martin SJ. Neutrophil extracellular traps can serve as platforms for processing and activation of IL-1 family cytokines. FEBS J. 2017;284:1712–1725. doi: 10.1111/febs.14075. [DOI] [PubMed] [Google Scholar]
- 93.Liang Y, et al. Six-transmembrane epithelial antigens of the prostate comprise a novel inflammatory nexus in patients with pustular skin disorders. J. Allergy Clin. Immunol. 2017;139:1217–1227. doi: 10.1016/j.jaci.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vigne S, et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood. 2011;118:5813–5823. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- 95.Mutamba S, Allison A, Mahida Y, Barrow P, Foster N. Expression of IL-1Rrp2 by human myelomonocytic cells is unique to DCs and facilitates DC maturation by IL-1F8 and IL-1F9. Eur. J. Immunol. 2012;42:607–617. doi: 10.1002/eji.201142035. [DOI] [PubMed] [Google Scholar]
- 96.Arakawa A, et al. Unopposed IL-36 activity promotes clonal CD4(+) T-cell responses with IL-17A production in generalized pustular psoriasis. J. Invest. Dermatol. 2018;138:1338–1347. doi: 10.1016/j.jid.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 97.Benoit S, Toksoy A, Brocker EB, Gillitzer R, Goebeler M. Treatment of recalcitrant pustular psoriasis with infliximab: effective reduction of chemokine expression. Br. J. Dermatol. 2004;150:1009–1012. doi: 10.1111/j.1365-2133.2004.05960.x. [DOI] [PubMed] [Google Scholar]
- 98.Imafuku S, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J. Dermatol. 2016;43:1011–1017. doi: 10.1111/1346-8138.13306. [DOI] [PubMed] [Google Scholar]
- 99.Blumberg H, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carrier Y, et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J. Invest. Dermatol. 2011;131:2428–2437. doi: 10.1038/jid.2011.234. [DOI] [PubMed] [Google Scholar]
- 101.Robinson A, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J. Am. Acad. Dermatol. 2012;67:279–288. doi: 10.1016/j.jaad.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 102.Torii H, Nakagawa H, Japanese Infliximab Study Investigators Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J. Dermatol. 2011;38:321–334. doi: 10.1111/j.1346-8138.2010.00971.x. [DOI] [PubMed] [Google Scholar]
- 103.Kimura U, et al. Generalized pustular psoriasis-like eruptions induced after the first use of adalimumab in the treatment of psoriatic arthritis. J. Dermatol. 2012;39:286–287. doi: 10.1111/j.1346-8138.2011.01344.x. [DOI] [PubMed] [Google Scholar]
- 104.Wendling D, et al. Onset or exacerbation of cutaneous psoriasis during TNFalpha antagonist therapy. Jt. Bone Spine. 2008;75:315–318. doi: 10.1016/j.jbspin.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 105.Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825–828. doi: 10.1001/jamadermatol.2016.0751. [DOI] [PubMed] [Google Scholar]
- 106.Bissonnette R, et al. Increased expression of IL-17A and limited involvement of IL-23 in patients with palmo-plantar (PP) pustular psoriasis or PP pustulosis; results from a randomised controlled trial. J. Eur. Acad. Dermatol. Venereol. 2014;28:1298–1305. doi: 10.1111/jdv.12272. [DOI] [PubMed] [Google Scholar]
- 107.Husson, B. et al. Efficacy and safety of TNF blockers and of ustekinumab in palmoplantar pustulosis and in acrodermatitis continua of Hallopeau. J. Eur. Acad. Dermatol. Venereol.10.1111/jdv.16265 (2020). [DOI] [PubMed]
- 108.Mrowietz U, et al. Secukinumab for moderate-to-severe palmoplantar pustular psoriasis: results of the 2PRECISE study. J. Am. Acad. Dermatol. 2019;80:1344–1352. doi: 10.1016/j.jaad.2019.01.066. [DOI] [PubMed] [Google Scholar]
- 109.Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br. J. Dermatol. 2015;173:239–241. doi: 10.1111/bjd.13614. [DOI] [PubMed] [Google Scholar]
- 110.Skendros P, et al. Successful response in a case of severe pustular psoriasis after interleukin-1beta inhibition. Br. J. Dermatol. 2017;176:212–215. doi: 10.1111/bjd.14685. [DOI] [PubMed] [Google Scholar]
- 111.Mansouri B, Kivelevitch D, Campa M, Menter A. Palmoplantar pustular psoriasis unresponsive to the interleukin-1beta antagonist canakinumab. Clin. Exp. Dermatol. 2016;41:324–326. doi: 10.1111/ced.12759. [DOI] [PubMed] [Google Scholar]
- 112.Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J. Invest. Dermatol. 2007;127:1292–1308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- 113.Campbell JJ, et al. Efficacy of chemokine receptor inhibition in treating IL-36alpha-induced psoriasiform inflammation. J. Immunol. 2019;202:1687–1692. doi: 10.4049/jimmunol.1801519. [DOI] [PubMed] [Google Scholar]
- 114.Foster AM, et al. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J. Immunol. 2014;192:6053–6061. doi: 10.4049/jimmunol.1301481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hawkes JE, Gudjonsson JE, Ward NL. The snowballing literature on imiquimod-induced skin inflammation in mice: a critical appraisal. J. Invest. Dermatol. 2017;137:546–549. doi: 10.1016/j.jid.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alvarez P, Jensen LE. Imiquimod treatment causes systemic disease in mice resembling generalized pustular psoriasis in an IL-1 and IL-36 dependent manner. Mediators Inflamm. 2016;2016:6756138. doi: 10.1155/2016/6756138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shao S, et al. Neutrophil extracellular traps promote inflammatory responses in psoriasis via activating epidermal TLR4/IL-36R crosstalk. Front. Immunol. 2019;10:746. doi: 10.3389/fimmu.2019.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sundberg JP, et al. Gain of function p.E138A alteration in Card14 leads to psoriasiform skin inflammation and implicates genetic modifiers in disease severity. Exp. Mol. Pathol. 2019;110:104286. doi: 10.1016/j.yexmp.2019.104286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swindell WR, et al. RNA-Seq Analysis of IL-1B and IL-36 responses in epidermal keratinocytes identifies a shared MyD88-dependent gene signature. Front. Immunol. 2018;9:80. doi: 10.3389/fimmu.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J. Women’s Health. 2018;10:109–115. doi: 10.2147/IJWH.S125784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ross MG, Tucker DC, Hayashi RH. Impetigo herpetiformis as a cause of postpartum fever. Obstet. Gynecol. 1984;64:49S–51S. doi: 10.1097/00006250-198409001-00013. [DOI] [PubMed] [Google Scholar]
- 122.Yamashita T, et al. An effective and promising treatment with adalimumab for impetigo herpetiformis with postpartum flare-up. Int J. Dermatol. 2019;58:350–353. doi: 10.1111/ijd.14141. [DOI] [PubMed] [Google Scholar]
- 123.Tay YK, Tham SN. The profile and outcome of pustular psoriasis in Singapore: a report of 28 cases. Int J. Dermatol. 1997;36:266–271. doi: 10.1046/j.1365-4362.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 124.Xiao T, Li B, He CD, Chen HD. Juvenile generalized pustular psoriasis. J. Dermatol. 2007;34:573–576. doi: 10.1111/j.1346-8138.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 125.Wang Q, Liu W, Zhang L. Clinical features of von Zumbusch type of generalized pustular psoriasis in children: a retrospective study of 26 patients in southwestern China. Bras. Dermatol. 2017;92:319–322. doi: 10.1590/abd1806-4841.20175536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Oliveira ST, Maragno L, Arnone M, Fonseca Takahashi MD, Romiti R. Generalized pustular psoriasis in childhood. Pediatr. Dermatol. 2010;27:349–354. doi: 10.1111/j.1525-1470.2010.01084.x. [DOI] [PubMed] [Google Scholar]
- 127.Korber A, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J. Invest. Dermatol. 2013;133:2634–2637. doi: 10.1038/jid.2013.214. [DOI] [PubMed] [Google Scholar]
- 128.Sugiura K, et al. A novel IL36RN/IL1F5 homozygous nonsense mutation, p.Arg10X, in a Japanese patient with adult-onset generalized pustular psoriasis. Br. J. Dermatol. 2012;167:699–701. doi: 10.1111/j.1365-2133.2012.10953.x. [DOI] [PubMed] [Google Scholar]
- 129.Farooq M, et al. Mutation analysis of the IL36RN gene in 14 Japanese patients with generalized pustular psoriasis. Hum. Mutat. 2013;34:176–183. doi: 10.1002/humu.22203. [DOI] [PubMed] [Google Scholar]
- 130.Li M, et al. IL36RN gene mutations are not associated with sporadic generalized pustular psoriasis in Chinese patients. Br. J. Dermatol. 2013;168:452–455. doi: 10.1111/j.1365-2133.2012.11195.x. [DOI] [PubMed] [Google Scholar]
- 131.Hussain S, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J. Allergy Clin. Immunol. 2015;135:1067–1070. e1069. doi: 10.1016/j.jaci.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 132.Ammar M, et al. CARD14 alterations in Tunisian patients with psoriasis and further characterization in European cohorts. Br. J. Dermatol. 2016;174:330–337. doi: 10.1111/bjd.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mossner R, et al. The genetic basis for most patients with pustular skin disease remains elusive. Br. J. Dermatol. 2018;178:740–748. doi: 10.1111/bjd.15867. [DOI] [PubMed] [Google Scholar]
- 134.Sugiura K, Muto M, Akiyama M. CARD14 c.526G>C (p.Asp176His) is a significant risk factor for generalized pustular psoriasis with psoriasis vulgaris in the Japanese cohort. J. Invest. Dermatol. 2014;134:1755–1757. doi: 10.1038/jid.2014.46. [DOI] [PubMed] [Google Scholar]
- 135.Tobita R, et al. A novel CARD14 variant, homozygous c.526G>C (p.Asp176His), in an adolescent Japanese patient with palmoplantar pustulosis. Clin. Exp. Dermatol. 2019;44:694–696. doi: 10.1111/ced.13926. [DOI] [PubMed] [Google Scholar]
- 136.Qin P, et al. Variant analysis of CARD14 in a Chinese Han population with psoriasis vulgaris and generalized pustular psoriasis. J. Invest. Dermatol. 2014;134:2994–2996. doi: 10.1038/jid.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tauber M, et al. Partial clinical response to anakinra in severe palmoplantar pustular psoriasis. Br. J. Dermatol. 2014;171:646–649. doi: 10.1111/bjd.13012. [DOI] [PubMed] [Google Scholar]
- 138.ClinicalTrialNCT03886246. A 5-year study to test BI 655130 in patients with generalized pustular psoriasis who took part in previous studies with BI 655130. Clinical Trial. https://clinicaltrials.gov/ct2/show/NCT03886246 (2019).
- 139.ClinicalTrialNCT03135548. Initial dosing of BI 655130 in palmoplantar pustulosis patients. Clinical Trial. https://clinicaltrials.gov/ct2/show/NCT03135548 (2019).
- 140.ClinicalTrialNCT03619902. A study to evaluate the efficacy and safety of ANB019 in subjects with generalized pustular psoriasis (GPP). Clinical Trial. https://clinicaltrials.gov/ct2/show/NCT03619902 (2019).
- 141.ClinicalTrialNCT03633396. A study to evaluate the efficacy and safety of ANB019 in subjects with palmoplantar pustulosis (PPP). Clinical Trial. https://clinicaltrials.gov/ct2/show/NCT03633396 (2019).