Abstract
Background/objectives
To evaluate the management of conjunctival melanoma with local excision and adjuvant brachytherapy.
Subjects/methods
Data of all patients who received local excision and adjuvant brachytherapy for conjunctival melanoma between 1999 and 2016 in a Dutch national referral centre were reviewed. A protocol with Sr-90 was used until 2012, a protocol with Ru-106 was used hereafter. Local recurrence, metastasis, survival, visual acuity and treatment complications were assessed.
Results
A total of 58 patients was identified: 32 patients were treated with Sr-90 and 26 with Ru-106. Mean follow-up time was 97.3 months (143.1 months after Sr-90, and 40.2 months after Ru-106). All lesions were epibulbar, the median tumour thickness was 0.9 mm. Local recurrence occurred in 13/58 cases (22%), with a 5-year recurrence rate of 21%. Local recurrence occurred equally often in both protocols, with 5-year recurrence rates of 19% (Sr-90) versus 23% (Ru-106) (p = 0.68). Metastasis developed in 3/58 cases (5%), with 2 cases after Sr-90, and 1 after Ru-106 (p = 1.00). The most reported complications were pain (29%), dry eyes (21%), symblepharon (9%), ptosis (12%) and cataract (9%). No severe corneal or scleral complications were observed. Median visual acuity was 1.00 pre-surgery, at the end of follow-up this was 1.00 (Sr-90) and 0.95 (Ru-106).
Conclusion
Local excision with adjuvant brachytherapy provides good tumour control with excellent visual outcome and mild side effects in patients with limited conjunctival melanoma. Results after Sr-90 or Ru-106 were comparable; a choice for either treatment may be based on experience of the clinician and availability of materials.
Subject terms: Eye cancer, Outcomes research
Introduction
Conjunctival melanoma (CoM) is a rare disease with a yearly incidence of 0.4–0.8/million in Caucasians [1–3]. Originating in melanocytes of the conjunctiva, it shares many features with the more commonly occurring cutaneous melanoma. Both the epibular and the non-epibulbar conjunctiva can be affected in CoM, and the clinical presentation may therefore vary between patients. Treatment of CoM may range from local excision with adjuvant therapy in smaller lesions, up to more extensive procedures such as orbital exenteration in larger or advanced CoM [4]. Commonly used modalities of adjuvant therapy include cryotherapy, topical chemotherapy (mitomycin-C, interferon-alfa) and/or radiotherapy (brachytherapy or external beam). While an inferior outcome was demonstrated for excision alone compared with excision with adjuvant therapy in various retrospective analyses [5, 6], the preferred method of adjuvant therapy can be debated [7].
Brachytherapy can be delivered to the ocular surface using various sources, such as Iodine-125 plaques, Ruthenium-106 plaques or the Strontium-90 handheld applicator. The aim of applying localised high-dose radiation to the ocular surface is similar for all techniques, but the devices vary in characteristics such as the properties of the specific radio-isotope, size and method of use. As CoM is considered to be relatively radio insensitive [8], radiotherapy is not advised as sole therapy, and brachytherapy will therefore usually follow excision. Adverse events of brachytherapy are considered mild, with (transient) dry eye complaints or corneal erosions being frequently reported; but more severe events as corneal ulcers and scleral necrosis may also occur [4]. With surface radiation doses ranging from 50 to 300 Gy [9], different profiles of adverse events may be expected between modalities, and the upper limits may be weighed against gains in treatment outcome. This debate regarding dosages and different radiation modalities is similarly seen in brachytherapy of the more common uveal melanoma [10]. Few reports have been published on the various radiation modalities for CoM, due to the rarity of the disease. As a result, brachytherapy modalities are often pooled in analyses, limiting the ability to obtain knowledge on the individual treatment characteristics and outcomes. Importantly, in the absence of prospective studies on this topic in CoM, recommendations on the usage of brachytherapy are often based on personal preferences, which may vary between authors [11, 12].
In this study, we present the treatment outcome of a large series of Dutch CoM patients who were treated with local excision and adjuvant brachytherapy. In our institution, a treatment protocol with adjuvant Sr-90 brachytherapy was applied for CoM patients until 2012, followed by a protocol with Ru-106 brachytherapy hereafter [13]. The aim of this study was to evaluate the clinical outcome of both protocols, and to give recommendations for clinical use of brachytherapy in CoM.
Materials and methods
Patients
A retrospective analysis was performed on the clinical data of all patients who were treated with adjuvant brachytherapy for CoM between January 1999 and December 2016 at the Leiden University Medical Center (LUMC, Leiden, The Netherlands), a national referral centre for ocular melanoma. Patients with a histologically proven primary invasive tumour without prior irradiation were included; this could be a new lesion or a recurrence. Patients with recurrent disease who received multiple treatments with brachytherapy were categorised by their first episode. The institutional medical ethics committee of the Leiden University Medical Center agreed with this retrospective study. The study adhered to the tenets of the Declaration of Helsinki.
Treatments
During the study time, local excision down to bare sclera with adjuvant brachytherapy was the preferred treatment strategy for limited CoM at our institution. A margin of 2 mm was applied; for lesions near the cornea, this included an epitheliectomy with alcohol application and cell removal with a surgical blade. Cryotherapy to the conjunctival edges was not included in the protocol. Up to 2012, brachytherapy was administered using Sr-90. From 2012 onwards, brachytherapy was applied post surgery with Ru-106. In both protocols, several weeks (ranging from 2 to 4 weeks) were usually in between excision and radiation treatment; allowing for histologic confirmation of the diagnosis, assessment of surgical margins and wound healing [13].
Strontium-90 protocol
While diagnosis, excision and follow-up of patients were performed in the LUMC, Sr-90 treatment was administered in the Academic Medical Center (Amsterdam, The Netherlands) or Catharina Hospital (Eindhoven, The Netherlands) as in those centres a Sr-90 applicator was available. Sr-90 treatment was administered under lidocaine drops with a handheld Sr-90 applicator (Type SIQ3, diameter 12 mm, Amersham Int., Buckinghamshire, UK) at the outpatient clinic (Fig. 1). A dose of 60 Gy was administered in six sessions of 10 Gy each, prescribed at the conjunctival surface. Treatment time was 60–90 s per application. In case of large lesions (>10 mm) more than one area was consecutively treated at each session; possible overlap of treated areas was accepted.
Fig. 1. Brachytherapy devices.
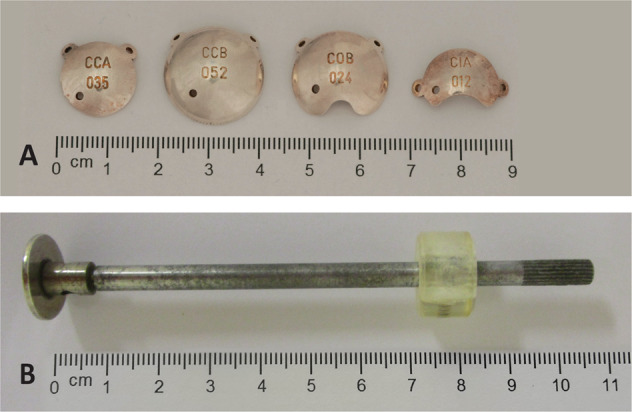
a Ruthenium-106 plaques in various sizes and shapes. From left to right: a CCA and CCB plaque (round), followed by a COB and CIA plaque (crescent shaped; originally designed for use near the optic nerve and limbal region, respectively). The radiation source is located on the concave—hollow—side of the plaque. The two eyelets on the margin are used to suture the plaque to the ocular surface. The depicted plaques are dummies for educational use, with a small perforation near the centre of the plaque that is absent in plaques used for treatment. b Strontium-90 handheld applicator. The radiation source is located at the end of the tip (left), and is applied against the ocular surface to deliver radiation treatment.
Ruthenium-106 protocol
Ru-106 brachytherapy was administered solely in the LUMC. Brachytherapy was applied to deliver 100 Gy in a single dose, specified to a default depth of 2 mm as all lesions had been excised. Application time was calculated taking into account the dose rate of the applicator on the specific date of treatment [14, 15]. Patients were admitted to the hospital for the duration of treatment, lasting several hours to days. Six types of Ru-106 plaques (Bebig co, Berlin, Germany) were used: four round applicators (CCX, CCA, CCD and CCB), and two crescent shaped (CIA and COB) with diameters of 11.6 to 20.2 mm (Fig. 1). Ru-106 plaques were sutured to the sclera via its two eyelets, commonly with an additional matrass suture overlying the plaque to secure its location [13].
From 2012 onwards, as an addition to the treatment protocol with Ru-106, topical treatment with mitomycin-C (drops of 0.04%, four times daily, in two consecutive series of 14 days with 1 week in between) was considered in patients with a component of primary acquired melanosis (PAM, based on histology) besides the CoM [13]. Actual application was reserved for the more severe cases, and following patient preferences for this additional treatment.
Clinical data and outcomes
At diagnosis, regular greyscale ultrasound of the cervical/neck lymph nodes was performed to detect possible regional spread. During follow-up, ultrasound of the lymph nodes was repeated every 6 months. There was no common surveillance protocol for systemic metastases; imaging or biopsies followed individual indications.
Patient clinical charts, pathology reports and clinical photographs were reviewed for patient and tumour characteristics. Tumours were staged according to the 8th edition of the AJCC TNM classification [16].
Data on recurrences, metastasis, survival and side effects of treatment were extracted from the patient charts, in addition to data provided by the Dutch National Cancer Registry.
Local recurrence was defined as any histologically proven recurrence of invasive CoM. Metastasis was defined as systemic spread, based on findings of histology or imaging.
Complications of treatment were evaluated at each follow-up visit. Visual acuity (VA) was measured with Snellen charts. A visual loss of more than two lines on a Snellen chart was considered functional loss. Useful vision was defined as >0.33 as proposed by the World Health Organisation.
Statistics
Differences between nominal data were analysed with Chi-square or Fisher’s exact test. Differences between continuous data were analysed with the independent samples t-test or the Mann–Whitney U test. When applicable, statistical tests were two-sided. The median and mean follow-up time were estimated by using reverse Kaplan–Meier’s methodology [17]. Time to local recurrence, metastasis, exenteration and death were calculated from the first day of brachytherapy to the date of the event with censoring at the date of last follow-up alive and event-free using Kaplan–Meier’s methodology. Differences in survival between groups were tested using the log rank test. Data were analysed with SPSS software (v.23). For all analyses, p values < 0.05 were considered statistically significant.
Results
Patient characteristics
A total of 58 patients who were treated with local excision and adjuvant brachytherapy was included; 32 were treated with adjuvant Sr-90, and 26 with adjuvant Ru-106.
Mean age at diagnosis of all patients was 58.9 years. All tumours were epibulbar, with a median thickness of 0.9 mm (IQR 0.5–1.5). Of the patients who were treated with Sr-90, 31 cases (97%) had stage cT1, and 1 case (3%) had stage cT2; all patients who were treated with Ru-106 had stage cT1 (p = 1.00). None of the patients had lymph node involvement or metastasis at diagnosis. There were no differences in baseline tumour characteristics between those cases who were treated with Sr-90 and those who were treated with Ru-106 (Table 1).
Table 1.
Baseline characteristics of the study population (n = 58). Sr-90 was administered between 1999 and 2012, Ru-106 was administered from 2012 onwards.
| Item | Total cases (%) |
Sr-90 cases (%) |
Ru-106 cases (%) |
P value |
|---|---|---|---|---|
| Total | 58 (100) | 32 (100) | 26 (100) | |
| Sex | ||||
| Female | 28 (48) | 17 (53) | 11 (42) | 0.41 |
| Male | 30 (52) | 15 (47) | 15 (58) | |
| Age at diagnosis (years) | ||||
| Mean (SD) | 58.9 (18) | 52.7 (20) | 66.5 (14) | 0.003 |
| Age at diagnosis | ||||
| <60 years | 30 (52) | 19 (59) | 11 (42) | 0.20 |
| ≥60 years | 28 (48) | 13 (41) | 15 (58) | |
| Eye involved | ||||
| Right | 30 (52) | 18 (56) | 12 (46) | 0.44 |
| Left | 28 (48) | 14 (44) | 14 (54) | |
| Location | ||||
| Epibulbar | 58 (100) | 32 (100) | 26 (100) | N.A. |
| Non-epibulbar | 0 (0) | 0 (0) | 0 (0) | |
| cTNM | ||||
| T1 | 57 (98) | 31 (97) | 26 (100) | 1.00 |
| T2 | 1 (2) | 1 (3) | 0 (0) | |
| Thickness (mm) | ||||
| Median (IQR) | 0.9 (0.5–1.5) | 1.0 (0.6–1.7) | 0.8 (0.2–1.3) | 0.14 |
| Largest Basal Diameter (mm) | ||||
| Median (IQR) | 6.0 (5.0–8.0) | 6.0 (4.5–9.0) | 6.0 (5.0–8.0) | 0.93 |
| Tumour pigmentationa | ||||
| Pigmented | 32 (64) | 19 (70) | 13 (57) | 0.31 |
| Non-pigmented/Mixed | 18 (36) | 8 (30) | 10 (43) | |
| PAMb | ||||
| Present | 52 (90) | 30 (94) | 22 (85) | N.A. |
| Absent | 3 (5) | 0 (0) | 3 (12) | |
| Unknown | 3 (5) | 2 (6) | 1 (4) | |
| Timing of treatment | ||||
| Primary CoM | 49 (85) | 25 (78) | 24 (92) | 0.17 |
| Recurrence | 9 (15) | 7 (22) | 2 (8) | |
| Location initial treatmentc | ||||
| LUMC | 20 (34) | 11 (34) | 9 (35) | 0.99 |
| Elsewhere | 38 (66) | 21 (66) | 17 (65) | |
SD standard deviation, PAM primary acquired melanosis, IQR inter-quartile range, cTNM clinical AJCC TNM classification, LUMC Leiden University Medical Center, Sr-90 strontium-90 brachytherapy, Ru-106 ruthenium-106 brachytherapy, N.A. not applicable.
aTumour pigmentation describes the clinical appearance of the conjunctival melanoma (melanin pigment) and was assessed visually and via colour photography [34].
bPAM status was determined histologically.
cInitial treatment for all patients was excision. This procedure was followed by adjuvant brachytherapy as described.
In the Sr-90 cohort, 25 cases (78%) were treated for a primary CoM, and 7 (22%) for a recurrence. Previous treatments of those seven patients included excision only, or excision with cryotherapy or mitomycin-C. In the Ru-106 cohort, 24 cases (93%) were treated for a primary CoM, and 2 (8%) for a recurrence. Previous treatments of these two patients were excision only and excision with cryotherapy.
Treatment characteristics
All included patients received adjuvant brachytherapy following local tumour excision. All Sr-90 patients received the prescribed surface dose of 60 Gy in six sessions of 10 Gy each (Table 2). The total dose at 2 mm depth from the conjunctival surface was 12.9 Gy [18]. The Ru-106 patients received a median dose of 100.5 Gy at 2 mm depth, with a median application time of 25.1 h. The median surface dose was 171.8 Gy. Two patients in the Sr-90 group (6%), and 13 patients in the Ru-106 group (50%) received mitomycin-C drops for concomitant PAM as a further treatment during follow up.
Table 2.
Treatment characteristics of Sr-90 and Ru-106 brachytherapy.
| Sr-90 | Ru-106 | |
|---|---|---|
| Characteristics | ||
| Device | Hand-held applicator | Plaque |
| Radio-isotope | Strontium-90 | Ruthenium-106 |
| Emitter type | Bèta ray emitter | Bèta ray emitter |
| Half-life | 28.8 yearsa | 374 daysa |
| Half-value layer in tissue | 0.9 mmb | 2.4 mma |
| Protocol | ||
| Total surface dose | 60 Gy | N.A. |
| Total 2-mm-depth dosec | N.A. | 100 Gyd |
| Fractions | 6 | 1 |
| Received | ||
| Total surface dose (median) | 60.0 Gy | 171.8 Gy (range 146.8–265.2) |
| Total 2-mm-depth dosec (median) | 12.9 Gy | 100.5 Gy (range 95.2–140.5) |
| Total duration (median) | 0.13 he | 25.1 h (range 12.7–95.0) |
| Dose rate at 2-mm depthc (median) | 103.2 Gy/h | 4.0 Gy/h |
Sr-90 Strontium-90 brachytherapy, Ru-106 Ruthenium-106 brachytherapy, N.A. not applicable.
aReference: [35].
bReference: [18].
cThe aim is set by default at 2-mm depth as the treatment is post surgery.
dOne patient (early in the protocol) was aimed at 130 Gy.
eThe duration per each of the six fractions of treatment was estimated at 60–90 s.
Clinical outcome
The mean follow-up time was 97.3 months (range: 9.3–229). This was 143.1 months (range: 16.0–229) in patients treated with Sr-90, and 40.2 months (range: 9.3–76.1) in patients treated with Ru-106 (p < 0.001) (Table 3).
Table 3.
Clinical outcome of the study population after adjuvant treatment with Sr-90 or Ru-106 for conjunctival melanoma (n = 58).
| Item | Total cases (%) |
Sr-90 cases (%) |
Ru-106 cases (%) |
P value |
|---|---|---|---|---|
| Length of follow-up (months) | ||||
| Mean (SD) | 97.3 (9.2) | 143.1 (10.6) | 40.2 (3.47) | <0.001 |
| Recurrence | ||||
| Overall recurrence | ||||
| No | 45 (78) | 24 (75) | 21 (81) | 0.60 |
| Yes | 13 (22) | 8 (25) | 5 (19) | |
| Recurrence-free survival (%) | ||||
| 1-year | 91 | 91 | 92 | 0.68 |
| 3-year | 81 | 84 | 77 | |
| 5-year | 79 | 81 | 77 | |
| 10-year | 71 | 73 | N.A. | |
| Recurrence location | ||||
| Centre | 2 (15) | 1 (13) | 1 (20) | N.A. |
| Margin | 7 (55) | 4 (50) | 3 (60) | |
| New location | 2 (15) | 2 (25) | 0 (0) | |
| Uncertaina | 2 (15) | 1 (13) | 1 (20) | |
| Metastasis | ||||
| Overall metastasis | ||||
| No | 55 (95) | 30 (94) | 25 (96) | 1.00 |
| Yes | 3 (5) | 2 (6) | 1 (4) | |
| Metastasis-free survival (%) | ||||
| 3-year | 96 | 97 | 96 | 0.96 |
| 5-year | 94 | 93 | 96 | |
| 10-year | 94 | 93 | N.A. | |
| Melanoma-related survival | ||||
| Overall melanoma-related status | ||||
| Alive | 55 (95) | 29 (91) | 26 (100) | 0.25 |
| Death | 3 (5) | 3 (9) | 0 (0) | |
| Melanoma-related survival (%) | ||||
| 3-year | 100 | 100 | 100 | 0.65 |
| 5-year | 97 | 97 | 100 | |
| 10-year | 89 | 88 | N.A. | |
| Exenteration | ||||
| Overall exenteration | ||||
| No | 54 (93) | 30 (94) | 24 (92) | 1.00 |
| Yes | 4 (7) | 2 (6) | 2 (8) | |
| Exenteration-free survival (%) | ||||
| 3-year | 97 | 100 | 92 | 0.13 |
| 5-year | 97 | 100 | 92 | |
| 10-year | 89 | 91 | N.A. | |
SD standard deviation, Sr-90 strontium-90 brachytherapy, Ru-106 ruthenium-106 brachytherapy, N.A. not applicable.
aThe location of the recurrence was uncertain in two patients of whom not enough data on the location of the primary lesion could be retrieved.
Local recurrence occurred in 13/58 cases (22%), with a 3- and 5-year recurrence rate of 19 and 21%. Local recurrence occurred equally often in both treatment groups, with 3- and 5-year recurrence rates of 16% and 19% for Sr-90, versus 23% and 23% for Ru-106 (overall log rank p = 0.68) (Fig. 2). Overall, 3 patients (9% of the total 32) developed a second recurrence after initial treatment with Sr-90, and 1 patient (4% of the total 26) did so after initial treatment with Ru-106. Of the nine patients who were treated with brachytherapy for a recurrence (i.e., not for their first episode of CoM), three (33%) developed another recurrence during follow-up. This is not significantly different from the recurrence rate observed in others (10/49 (20%), p = 0.73).
Fig. 2. Recurrence-free survival per treatment protocol.
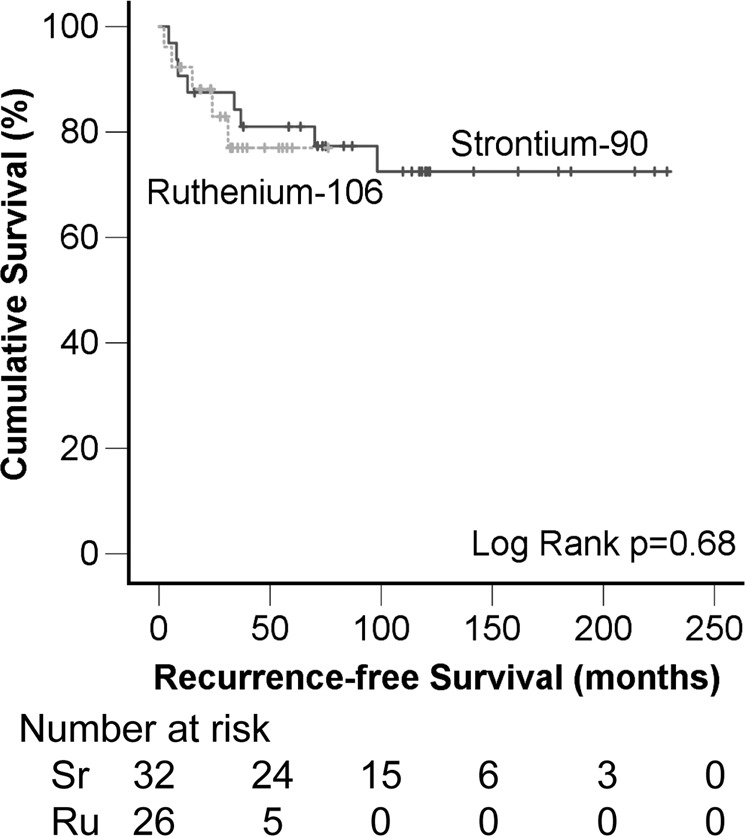
No difference in recurrence-free survival was observed between the Sr-90 group and Ru-106 group (log rank p = 0.68), calculated over the first 76 months of follow-up time with data on both groups.
Metastasis was detected in 3/58 patients (5%), with a 5-year metastasis rate of 6%. In the Sr-90 group, two patients (6%) developed metastasis (one case of lung metastasis, one case of liver metastasis), and one patient (4%) did so after Ru-106 treatment (metastases in both lungs and liver) (p = 1.00). At the end of follow-up, three patients (9%) had died due to melanoma-related causes in the Sr-90 cohort, and none in the Ru-106 cohort (p = 0.25).
The most frequently reported adverse events after excision and brachytherapy were pain (17 cases, 29%), dry eyes (12 cases, 21%), symblepharon (5 cases, 9%), ptosis (7 cases, 12%) and cataract (4 cases, 9%).
In 20 patients treated with Sr-90 (63%) and in 21 patients treated with Ru-106 (81%), any adverse event was noted (p = 0.37). All adverse events, stratified to early (i.e., within 6 months of treatment) and late onset, are presented in Supplementary Table 1.
The median VA of all patients was 1.00 pre-surgery as well as at the end of follow-up. For the Sr-90 cohort, this was 1.00 at the end of follow-up, and for the Ru-106 cohort this was 0.95 (Supplementary Table 2).
Discussion
This study evaluates the treatment outcome of CoM with local excision and adjuvant brachytherapy in a Dutch national referral centre. An overall 5-year recurrence rate of 21%, and an overall 5-year metastasis rate of 6% were observed. Side effects of therapy were mostly transient and mild. During study time, adjuvant treatment with either Sr-90 or Ru-106 was administered: no statistically significant differences were detected in the development of recurrences, metastases or melanoma-related deaths.
To our knowledge, few studies report on the clinical outcome of adjuvant Sr-90 or Ru-106 for CoM. Our 5-year recurrence rate after Sr-90 (19%) is comparable to the rate of 18% reported by Cohen in a study of 20 patients [19]. The results are favourable compared with another study, which reported a recurrence rate of 9 in 15 cases (60%) with a mean follow-up time of 35 months [20]. Missotten reported a recurrence rate of 6 in 46 cases (13%) but the exact follow-up time is unclear from the available data [21]. Our total recurrence rate after Ru-106 (19%) is comparable to the rate of 6 in 36 patients (17%) treated with adjuvant Ru-106 by Damato, who applied a similar dose of 100 Gy at 2 mm depth but later used 100 Gy at 1 mm [22].
For iodine plaque treatment, recurrence rates of 1 in 14 cases (7%) after 39 months follow-up [23], and 3 in 19 cases (16%) in 43.1 months follow-up have been reported [24], which seems comparable to our results of both Sr-90 and Ru-106 as the confidence intervals are wide. Other reports on brachytherapy for CoM were considered too small for a comparison of results [12, 25, 26].
To overcome the problem of small series, Wong presented a composite series of Sr-90 and I-125, with a recurrence rate of 15 in 74 patients (20%) with a mean follow-up time of 63.9 months [4]. This is comparable to the overall 5-year recurrence rate (21%) for both therapies in our study of 58 patients. As expected, since only smaller melanoma qualify for brachytherapy, these rates are far below the general reported recurrence rates of CoM, with up to 61% recurrences in 5 years [6].
The reported complications after treatment with excision and Sr-90 or Ru-106 were mostly mild (Supplementary Table 1). The complaints of pain or discomfort were noticed shortly after the surgery and radiation and resolved within days. One patient treated with Sr-90, and four patients treated with Ru-106, developed cataract which was likely caused or accelerated by radiation effects. Radiation retinopathy was not observed.
In the Ru-106 group, a symblepharon occurred frequently together with double vision, probably related to the excision and adherence of conjunctiva. Ptosis was mentioned remarkably more often after treatment with Ru-106 (seven cases, 27%) compared with Sr-90 (zero cases), which might be related to the manipulation of the eyelids to deliver treatment, the additional use of mitomycin-C drops or the formation of symblepharon. In two patients a small area of bare sclera was seen after excision with Sr-90, requiring no further treatment, and two patients developed a limbal dellen after excision with Ru-106, resolving spontaneously in one case and requiring treatment with surgery in the other case.
The more severe complications occurred after additional treatments, often for recurrences (Supplementary Table 2). Following further excision, another Ru-106 plaque and mitomycin-C drops for recurrences of CoM and PAM, one patient developed severe limbal stem cell failure requiring autologous stem cell transplantation. This was the only patient in this study with an expected long-lasting decrease of sight.
Because of the limited availability of data, we could not compare the complication rate statistically with other isotopes as iodine, but a similar pattern of mild adverse events has been reported [23, 24].
There appears to be a remarkable difference between the radiation doses of the two used isotopes in this study. The median dose at 2 mm depth of Sr-90 was 12.9 versus 100.5 Gy for Ru-106. These two dosages can, however, not be directly compared. The biological effect of radiation is not only determined by the total dose, but also for example by the dose rate and overall treatment time. As Sr-90 is applied in short sessions, the dose rate is much higher compared with that of Ru-106 (103.2 Gy/h for Sr-90 versus 4.0 Gy/h for Ru-106) (Table 2), and this can partially explain why Sr-90 treatment with a lower total dose suffices compared with the higher dose with Ru-106. This would be in line with findings from uveal melanoma, indicating that higher dose rates are more effective [27, 28]. However, the dose rate effect is challenged by others as they could not find such an association [29, 30]. Future studies may investigate whether lower doses of brachytherapy can be applied for CoM while retaining good clinical outcome.
The choice for treatment with Sr-90 or Ru-106 is usually based on the experience of the clinician and availability of the materials. While Sr-90 is applied in an outpatient setting, with multiple short fractions of therapy, Ru-106 is applied in one continuous setting, often requiring patients to stay in the hospital overnight. By the design of the devices, it could be argued that Sr-90 applicators are somewhat more suitable for treatment of melanomas on the non-epibulbar conjunctiva compared with the (concave shaped) Ru-106 plaques (Fig. 1). But, if the melanoma is located deep into the inner lining of the eyelids, other adjuvant therapies will be required as reverse mounted iodine plaques [12], external beam irradiation or topical chemotherapy. As Sr-90 applicators are currently out of production [19], it may be difficult for centres to obtain these. There is a lobby for renewed production, however. Once obtained, Sr-90 applicators have a much longer life span by the considerably longer half-life value of the radio-isotope compared with Ru-106 (Table 2).
Of patients with a conclusion on margin status, in 8 (38%) of Sr-90 and 17 (65%) of Ru-106 patients, all margins were free (p = 0.06). It is likely that the true percentage of margin-free excisions in the Sr-90 cohort is higher, however, as in 11 (34%) patients of the Sr-90 group (but in none of the Ru-106 group) there was no final verdict on margin status due to tissue processing techniques as tangential cutting. Overall, recurrences occurred in 4/25 (16%) of patients with tumour-free margins, and in 6/22 (27%) of patients with incomplete resections, which was not significantly different (p = 0.65). It should be mentioned, however, that margin status of the scleral and (if applicable) corneal side is often difficult to assess histopathologically. Therefore, regarding limbal lesions we advise to include the adjacent corneal margin in the field of radiation.
Our study demonstrated good clinical outcome for adjuvant brachytherapy in CoM, but one may wonder how this relates to other adjuvant therapies as the topical application of mitomycin-C. Literature on the adjuvant use of mitomycin-C in CoM is scarce, however, with small series reporting recurrence rates of 33–50% [31, 32], and denominating long-term risks as the development of limbal stem cell insufficiency [33]. A potential benefit of mitomycin-C can be that it reaches the conjunctiva as a whole, compared with the localised effect of brachytherapy. As it does not cross the basement membrane, however, it has been proposed that mitomycin-C is most effective in intraepithelial and superficial melanoma [32], and it should not be used as a primary treatment for CoM [4]. Although the current evidence for mitomycin-C as a single adjuvant therapy in localised CoM is limited, it may be a reasonable alternative in specific patients (e.g., with contra-indications to radiotherapy, or more widespread lesions). We do advise to consider adding mitomycin-C drops to excision and brachytherapy in patients with limited CoM and concomitant PAM, with the intention to prevent or delay recurrences [13]. As there is no final proof for using this addition, we feel that other factors may guide this decision as well. These include the severity of the clinical image, and patient preferences.
This study presents a large cohort of CoM patients who were treated with local excision and adjuvant brachytherapy. As two isotopes were used in our institution, the clinical outcome of both protocols could be reported. The criteria for receiving Sr-90 or Ru-106 were similar, being routine applications after CoM excision, and the cohorts had no significant differences in tumour characteristics at baseline. Even so, initial treatment was performed equally often in a tertiary centre, which we identified before as a relevant prognostic parameter [13]. By the national cancer registry, recurrences, survival time and cause of death could be retrieved for all patients; this contributed to a long and informative follow-up time of the study. A strict comparison between the Sr-90 and Ru-106 is limited by various factors, however. Due to the relatively short follow-up time for Ru-106 compared with Sr-90, a statistical analysis of long-term outcome is hampered. An important matter is that mitomycin-C drops were administered more commonly to patients with CoM and co-occurring PAM from 2012 onwards [13]. As PAM should be considered a pre-malignant disorder, this treatment might be relevant, affecting mainly patients in the more recent Ru-106 cohort. In an additional analysis, we analysed all patients without additional mitomycin-C treatment (comparing 30 patients in the Sr-90 group with 13 patients in the Ru-106 group), which yielded similar results to the overall analysis for tumour characteristics and outcome (Supplementary Tables 3 and 4). Due to small numbers, we were not able to compare patients with and without mitomycin-C. Further, Sr-90 treatment could not be administered in our own institution, requiring patients to be referred for treatment to another centre in The Netherlands. Referral contains the risk of information loss and is logistically challenging. This was the main reason for changing the protocol to treatment with Ru-106 plaques that could be administered at the LUMC itself. As during and shortly after Ru-106 treatment, patients could be monitored more closely compared with the patients who received Sr-90, this might have—partially—led to the higher detection of early adverse events. Importantly, as no prospective studies on this topic exist in CoM, retrospective series (as the current analysis) provide helpful clues for clinicians. To overcome the aforementioned limitations, we call for prospective multi-centre studies to optimise CoM treatment.
Concluding, local excision with adjuvant brachytherapy showed good tumour control with excellent visual outcome and mild side effects in a retrospective analysis of CoM patients. Results after treatment with either Sr-90 or Ru-106 were comparable; a choice between either treatment may be based on the experience of the clinician and the availability of required materials. Prospective, multi-centre studies should be encouraged to optimise treatments as CoM is rare.
Summary
What was known before
Local excision with adjuvant therapy is the preferred treatment for smaller CoM.
Brachytherapy is commonly used as adjuvant therapy, and can be administered with various radiation sources.
What this study adds
Results after treatment with Ruthenium-106 and Strontium-90 are comparable, with good tumour control and mild side effects.
A choice for either treatment may be based on experience of the clinician and availability of materials.
Supplementary information
Acknowledgements
The authors like to thank Leo Blank, MD (Academic Medical Center/University of Amsterdam, The Netherlands) for his contributions to the Strontium-90 treatment of the study population and critical revision of the manuscript.
Funding
NJB is the recipient of a MD/PhD-programme grant of the Leiden University Medical Center. The sponsor or funding organisation had no role in the design or conduct of this research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Niels J. Brouwer, Marina Marinkovic
Supplementary information
The online version of this article (10.1038/s41433-020-0879-z) contains supplementary material, which is available to authorised users.
References
- 1.Tuomaala S, Eskelin S, Tarkkanen A, Kivela T. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399–408. [PubMed] [Google Scholar]
- 2.Yu GP, Hu DN, McCormick S, Finger PT. Conjunctival melanoma: is it increasing in the United States? Am J Ophthalmol. 2003;135:800–6. doi: 10.1016/S0002-9394(02)02288-2. [DOI] [PubMed] [Google Scholar]
- 3.Triay E, Bergman L, Nilsson B, All-Ericsson C, Seregard S. Time trends in the incidence of conjunctival melanoma in Sweden. Br J Ophthalmol. 2009;93:1524–8. doi: 10.1136/bjo.2009.157933. [DOI] [PubMed] [Google Scholar]
- 4.Wong JR, Nanji AA, Galor A, Karp CL. Management of conjunctival malignant melanoma: a review and update. Expert Rev Ophthalmol. 2014;9:185–204. doi: 10.1586/17469899.2014.921119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Potter P, Shields CL, Shields JA, Menduke H. Clinical predictive factors for development of recurrence and metastasis in conjunctival melanoma: a review of 68 cases. Br J Ophthalmol. 1993;77:624–30. doi: 10.1136/bjo.77.10.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Missotten GS, Keijser S, De Keizer RJ, De Wolff-Rouendaal D. Conjunctival melanoma in the Netherlands: a nationwide study. Invest Ophthalmol Vis Sci. 2005;46:75–82. doi: 10.1167/iovs.04-0344. [DOI] [PubMed] [Google Scholar]
- 7.Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, Vlajkovic S, Cekic S, Stefanovic V. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol. 2013;6:1230–44. [PMC free article] [PubMed] [Google Scholar]
- 8.Lommatzsch PK, Lommatzsch RE, Kirsch I, Fuhrmann P. Therapeutic outcome of patients suffering from malignant melanomas of the conjunctiva. Br J Ophthalmol. 1990;74:615–9. doi: 10.1136/bjo.74.10.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause L, Mladenova A, Bechrakis NE, Kreusel KM, Plath T, Moser L, et al. Treatment modalities for conjunctival melanoma. Klin Monbl Augenheilkd. 2009;226:1012–6. doi: 10.1055/s-0028-1109651. [DOI] [PubMed] [Google Scholar]
- 10.Reichstein D, Karan K. Plaque brachytherapy for posterior uveal melanoma in 2018: improved techniques and expanded indications. Curr Opin Ophthalmol. 2018;29:191–8. doi: 10.1097/ICU.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 11.Westekemper H, Schallenberg M, Tomaszewski A, Nuckel H, Sauerwein W, Meller D, et al. Malignant epibulbar tumours: new strategies in diagnostics and therapy. Klin Monbl Augenheilkd. 2011;228:780–92. doi: 10.1055/s-0029-1246068. [DOI] [PubMed] [Google Scholar]
- 12.Shields JA, Shields CL, Freire JE, Brady LW, Komarnicky L. Plaque radiotherapy for selected orbital malignancies: preliminary observations: the 2002 Montgomery Lecture, part 2. Ophthal Plast Reconstr Surg. 2003;19:91–5. doi: 10.1097/01.IOP.0000056020.66654.33. [DOI] [PubMed] [Google Scholar]
- 13.Brouwer NJ, Marinkovic M, van Duinen SG, Bleeker JC, Jager MJ, Luyten GPM. Treatment of conjunctival melanoma in a Dutch referral centre. Br J Ophthalmol. 2018;102:1277–82. doi: 10.1136/bjophthalmol-2017-311082. [DOI] [PubMed] [Google Scholar]
- 14.Marinkovic M, Horeweg N, Fiocco M, Peters FP, Sommers LW, Laman MS, et al. Ruthenium-106 brachytherapy for choroidal melanoma without transpupillary thermotherapy: Similar efficacy with improved visual outcome. Eur J Cancer. 2016;68:106–13. doi: 10.1016/j.ejca.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Ellis F, Sorensen A. A method of estimating biological effect of combined intracavitary low dose rate radiation with external radiation in carcinoma of the cervix uteri. Radiology. 1974;110:681–6. doi: 10.1148/110.3.681. [DOI] [PubMed] [Google Scholar]
- 16.Coupland SE, Barnhill RL, Conway M, Damato B, Esmaeli B, Albert DM. Conjunctival melanoma. In: Amin MB ES, Green F, editors. AJCC canger staging manual, 8th ed. New York: Springer; 2017. p. 795–803.
- 17.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
- 18.Supe SJ, Cunningham JR. A physical study of a strontium 90 beta-ray applicator. Am J Roentgenol Radium Ther Nucl Med. 1963;89:570–4. [PubMed] [Google Scholar]
- 19.Cohen VM, Papastefanou VP, Liu S, Stoker I, Hungerford JL. The use of strontium-90 Beta radiotherapy as adjuvant treatment for conjunctival melanoma. J Oncol. 2013;2013:349162. doi: 10.1155/2013/349162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause L, Ritter C, Wachtlin J, Kreusel KM, Hocht S, Foerster MH, et al. Recurrence rate following adjuvant strontium-90 brachytherapy after excision of conjunctival melanoma. Klin Monbl Augenheilkd. 2008;225:649–52. doi: 10.1055/s-2008-1027432. [DOI] [PubMed] [Google Scholar]
- 21.Missotten GS, De Keizer RJ, Spileers W, Blank L. Strontium brachytherapy in conjunctival melanoma (abstract). Acta Ophthalmol. 2011;89:s248. 10.1111/j.1755-3768.2011.4262.x.
- 22.Damato B, Coupland SE. An audit of conjunctival melanoma treatment in Liverpool. Eye. 2009;23:801–9. doi: 10.1038/eye.2008.154. [DOI] [PubMed] [Google Scholar]
- 23.Stannard CE, Sealy GR, Hering ER, Pereira SB, Knowles R, Hill JC. Malignant melanoma of the eyelid and palpebral conjunctiva treated with iodine-125 brachytherapy. Ophthalmology. 2000;107:951–8. doi: 10.1016/S0161-6420(00)00061-0. [DOI] [PubMed] [Google Scholar]
- 24.Karim R, Conway RM. Conservative resection and adjuvant plaque brachytherapy for early-stage conjunctival melanoma. Clin Exp Ophthalmol. 2011;39:293–8. doi: 10.1111/j.1442-9071.2010.02469.x. [DOI] [PubMed] [Google Scholar]
- 25.Zehetmayer M, Menapace R, Kulnig W. Combined local excision and brachytherapy with ruthenium-106 in the treatment of epibulbar malignancies. Ophthalmologica. 1993;207:133–9. doi: 10.1159/000310419. [DOI] [PubMed] [Google Scholar]
- 26.Langmann G, Faschinger C, Kleinert R, Poier E, Langmann A. Zur bulbuserhaltenden therapie von Bindehautmelanomen. Spektrum Augenheilkd. 1991;5:266–9. doi: 10.1007/BF03163971. [DOI] [Google Scholar]
- 27.Mossbock G, Rauscher T, Winkler P, Kapp KS, Langmann G. Impact of dose rate on clinical course in uveal melanoma after brachytherapy with ruthenium-106. Strahlenther Onkol. 2007;183:571–5. doi: 10.1007/s00066-007-1734-x. [DOI] [PubMed] [Google Scholar]
- 28.van Ginderdeuren R, van Limbergen E, Spileers W. 18 years’ experience with high dose rate strontium-90 brachytherapy of small to medium sized posterior uveal melanoma. Br J Ophthalmol. 2005;89:1306–10. doi: 10.1136/bjo.2005.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fili M, Lundell G, Lundell M, Seregard S. High dose rate and low dose rate ruthenium brachytherapy for uveal melanoma. No association with ocular outcome. Br J Ophthalmol. 2014;98:1349–54. doi: 10.1136/bjophthalmol-2014-305055. [DOI] [PubMed] [Google Scholar]
- 30.Damato B, Patel I, Campbell IR, Mayles HM, Errington RD. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol Phys. 2005;63:385–91. doi: 10.1016/j.ijrobp.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Ditta LC, Shildkrot Y, Wilson MW. Outcomes in 15 patients with conjunctival melanoma treated with adjuvant topical mitomycin C: complications and recurrences. Ophthalmology. 2011;118:1754–9. doi: 10.1016/j.ophtha.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 32.Kurli M, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: 12 years’ experience. Graefes Arch Clin Exp Ophthalmol. 2005;243:1108–14. doi: 10.1007/s00417-004-1080-y. [DOI] [PubMed] [Google Scholar]
- 33.Russell HC, Chadha V, Lockington D, Kemp EG. Topical mitomycin C chemotherapy in the management of ocular surface neoplasia: a 10-year review of treatment outcomes and complications. Br J Ophthalmol. 2010;94:1316–21. doi: 10.1136/bjo.2009.176099. [DOI] [PubMed] [Google Scholar]
- 34.Brouwer NJ, Marinkovic M, Luyten GPM, Shields CL, Jager MJ. Lack of tumour pigmentation in conjunctival melanoma is associated with light iris colour and worse prognosis. Br J Ophthalmol. 2019;103:332–7. doi: 10.1136/bjophthalmol-2018-312018. [DOI] [PubMed] [Google Scholar]
- 35.Stannard C, Sauerwein W, Maree G, Lecuona K. Radiotherapy for ocular tumours. Eye. 2013;27:119–27. doi: 10.1038/eye.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


