Chimeric antigen receptor T cell (CAR-T) therapy is one of the most promising approaches in cancer treatment.1 However, the limited availability of patient-derived T cells narrows its universal applicability. Thus, it is necessary to invent new methods to obtain alternative T-cell sources. Pluripotent stem cells (PSCs), which have unlimited culture potential and are amenable to gene editing, are ideal for generating induced T (iT) cells. Conventional PSC-derived iT cells in culture dishes exhibit extremely low activity in vivo, and their properties are only partially defined.2,3 Our group has recently developed a de novo two-step approach to generate functional iT cells from PSCs driven by the synergistic expression of Runx1 and Hoxa9.4 Here, we further tested the translational potential of these de novo iT cells in CAR-T cell therapy research.
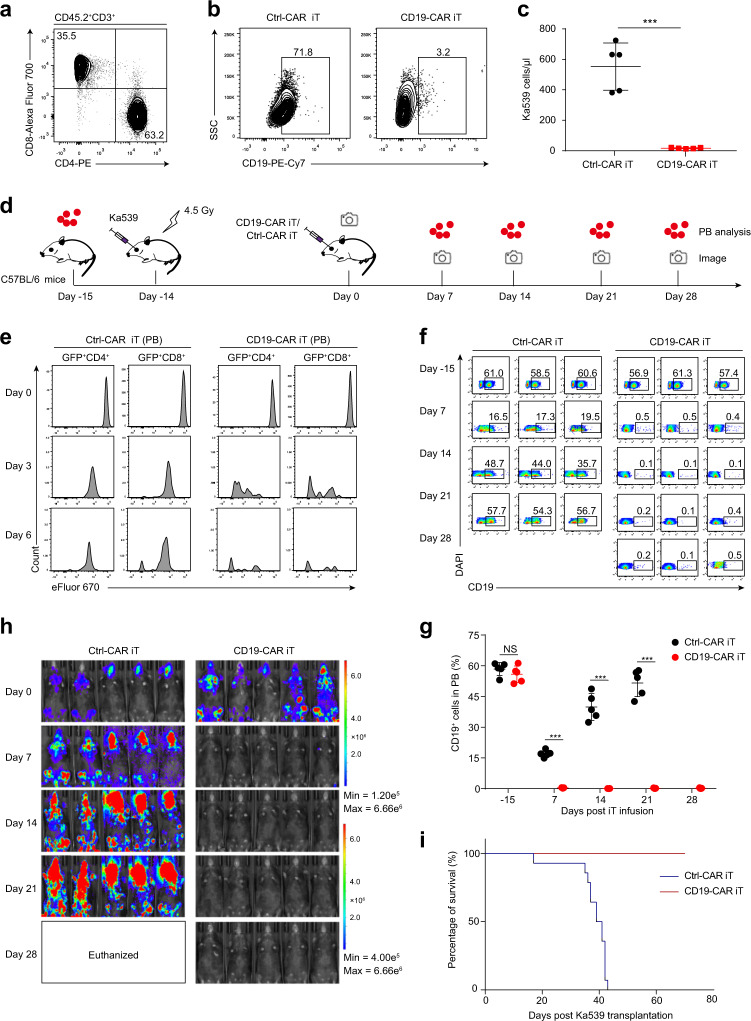
We developed a stepwise strategy to differentiate PSCs into CAR iT cells.4 We successfully generated induced hematopoietic endothelial cells (iHECs) from iRunx1-p2a-Hoxa9-PSCs. Next, the iHECs were sorted and cocultured with notch-ligand-expressing OP9 stromal cells (OP9-DL1)5 for hematopoietic progenitor cell (iHPC) maturation. Finally, the iHPCs were transplanted into irradiated B-NDG recipient mice (a commercial strain similar to NSG mice) for T lymphocyte reconstitution in vivo. Five weeks post transplantation, donor-derived CD45.2+CD3+ mature CD4SP (~63%) and CD8SP (~35%) iT cells were successfully detected in the spleens of B-NDG mice transplanted with iHPCs (Fig. 1a). We subsequently engineered iT cells with CD19-CAR to further assess the function of PSC-derived iT cells. We first constructed CD19-CAR and Ctrl-CAR retroviral vectors as described previously (Fig. S1a).6 CD19-CAR has the complete function of inducing immune reactions, but Ctrl-CAR is incompetent due to the lack of a CD3 zeta chain, which facilitates intracellular signal transduction. PSC-derived splenic iT cells were infected with the CD19-CAR or Ctrl-CAR retrovirus. The transduction efficiency of each retrovirus reached ~40%, as demonstrated by GFP expression (Fig. S1b). To test whether CD19-CAR iT cells can eliminate tumor cells in vitro, we cocultured CD19-CAR iT cells (1.0 × 104 cells) with B lymphoma cells (Ka539 cells, 1.0 × 104 cells) in 96-well plates for 36 h. As expected, the proportion and number of CD19+ Ka539 cells sharply decreased when the cells were cocultured with CD19-CAR iT cells for 36 h compared with Ctrl-CAR iT cells (n = 5, p < 0.001) (Fig. 1b, c). Thus, the results indicate that CD19-CAR iT cells display extremely powerful antitumor activity in vitro.
Fig. 1.
PSC-derived CD19-CAR iT cells effectively eradicate B-cell lymphoma. a Flow cytometry analysis of mature iT cells in the spleen of B-NDG mice transplanted with PSC-derived iHPCs. Each B-NDG mouse was transplanted with 1.0 × 106 bulk cells containing abundant iHPCs at day 21. Representative mice were sacrificed and analyzed at 5 weeks after transplantation. CD19-CAR/Ctrl-CAR iT cells were cocultured with CD19+ B lymphoma cells (Ka539 cells) for 36 h in vitro, and the percentage (b) and number (c) of CD19+ cells in each well were measured respectively by flow cytometry after 36 h coculture. Data are representative of two independent experiments. n = 5, ***p < 0.001, unpaired Studentʼs t-test (two-sided). d Schematic diagram of CD19-CAR/Ctrl-CAR iT cell therapy. The Ka539-luciferase tumor cell line (C57BL/6 background) was transplanted into irradiated (4.5 Gy) recipient mice (1.5 × 106 cells/mouse, C57BL/6) to construct a BCL mouse model. After 14 days, the activated CD19-CAR/Ctrl-CAR iT cells (6.0 × 106 cells/mouse) were adoptively transferred into tumor-bearing mice by retro-orbital injection. CD19+ cells were analyzed by flow cytometry in the peripheral blood (PB) of tumor-bearing mice, and tumor burden was measured weekly by bioluminescent imaging. e Proliferation of CD19-CAR/Ctrl-CAR iT cells. CD19-CAR/Ctrl-CAR iT cells (6.0 × 106) were labeled with the cell proliferation dye eFluor670 in vitro before transplantation. eFluor670 signals on CD19-CAR/Ctrl-CAR iT cells in PB of BCL mice were analyzed by flow cytometry at day 0, day 3 and day 6 after transplantation. Representative data are shown. f The kinetics of CD19+ cells in the PB of CD19-CAR/Ctr-CAR iT cell-treated recipients were analyzed at day -15, day 7, day 14, day 21, and day 28 by flow cytometry. The day when the mice were infused with CD19-CAR/Ctrl-CAR iT cells was defined as day 0. Representative data are shown (each group, n = 3). g Statistical analysis of CD19+ cells in the PB of CD19-CAR/Ctr-CAR iT cell-treated recipients (each group, n = 5). NS, no significance (p > 0.05), ***p < 0.001, unpaired Student’s t-test (two-sided). h B-cell lymphoma tumor burden was determined by weekly bioluminescent imaging (BLI, IVIS Spectrum PerkinElmer). For the BLI experiment, D-Luciferin (150 mg/kg, YEASEA) was administered by intraperitoneal injection 10–20 minutes before imaging. BLI was performed at day 0, day 7, day 14, day 21, and day 28 after CD19-CAR/Ctrl-CAR iT cell treatment (each group, n = 5 mice). The mice treated with Ctrl-CAR iT cells were euthanized when they became moribund due to heavy tumor burden between 21–28 days after treatment. i Kaplan–Meier survival curves of CD19-CAR/Ctr-CAR iT cell-treated mice with B-cell lymphoma (each group, n = 14 mice). p < 0.0001, log-rank test
The antitumor effect of regenerated CD19-CAR iT cells in vivo was further examined using a B-cell lymphoma (BCL) model established as previously reported.7 Briefly, to construct the BCL model, 1.5 × 106 Ka539-luciferase tumor cells were inoculated into irradiated C57BL/6 mice. After 14 days, the BCL mice were adoptively transferred retro-orbitally with 6.0 × 106 CD19-CAR iT cells, while the tumor-bearing mice treated with Ctrl-CAR iT cells were used as control. The percentages of BCL tumor cells (CD19+) were analyzed by flow cytometry in the peripheral blood (PB) of tumor-bearing mice, and the size of the tumor burden was measured weekly by bioluminescent imaging (BLI). (Fig. 1d). To evaluate the proliferating ability of the CD19-CAR iT cells in response to tumor stimulation in vivo, we prestained CD19-CAR iT cells with eFluor670 in vitro and chased the eFluor670 signals in PB at day 3 and day 6 after CD19-CAR iT cell infusion. Our results showed that CD19-CAR iT cells proliferated more vigorously than Ctrl-CAR iT cells in BCL mice (Fig. 1e). Then, we measured the percentage of CD19+ tumor cells in the PB of CD19-CAR iT cell-treated or Ctrl-CAR iT cell-treated mice. As early as 7 days after treatment with CD19-CAR iT cells, there was a decrease of PB tumor cells (lower than 0.5%), which was sustained for at least one month. In contrast, the percentage of CD19+ tumor cells in the PB of control mice was more than 32% at day 14 and day 21 (p < 0.001) (Fig. 1f, g). We also used BLI to monitor tumor development. As shown by BLI, CD19-CAR iT cells effectively eliminated tumor cells, while Ctrl-CAR iT cell-treated BCL mice developed tumors more rapidly, which resulted in euthanasia of these mice at a moribund phase (days 22–28 after iT cell infusion) (Fig. 1h). Compared with the observations in Ctrl-CAR iT-treated mice, complete tumor remission and no tumor recurrence were observed for over 70 days in CD19-CAR iT-treated mice. (Fig. 1i). These results further illustrate that PSC-derived CAR iT cells could effectively eliminate tumor cells in vivo.
In this study, we assessed the antitumor effect of PSC-derived iT cells based on the CAR-T cell therapy method. Our unique approach of initial PSC differentiation in vitro to generate T-cell precursors and subsequent complete T lymphoid development in vivo resulted in a large population of T lymphocytes that functionally resemble natural T cells. These CD19-CAR iT cells demonstrate promising therapeutic potential for treating blood cancers. CD19-CAR iT cells can significantly proliferate and effectively eradicate B lymphoma cells in tumor-bearing mice. The combination of PSCs and CAR technology here offers a potential new source of CAR iT cells that exhibit the capability of infinite proliferation and powerful potential of killing tumor cells. In the presence of an ideal animal incubator for generating iT cells using our approach, this system might pave the way for universal and personalized immunotherapy.
Supplementary information
Acknowledgements
This work was supported by the CAS Key Research Program of Frontier Sciences (QYZDB-SSW-SMC057), the Strategic Priority Research Program of Chinese Academy of Sciences (XDA16010601), the National Key R&D Program of China (2019YFA0110203), the Major Research and Development Project of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110104006), the Health and Medical Care Collaborative Innovation Program of Guangzhou Scientific and Technology (201803040017), the Science and Technology Planning Project of Guangdong Province (2017B030314056), and the National Natural Science Foundation of China (81925002, 81970099, 31900814).
Author contributions
C.L. and S.C. performed the experiments and analyzed the data. F.H. and D.H. participated in multiple experiments. J.W. and H.W. conceptualized and supervised this study and wrote and edited the manuscript. T.W. and J.D. reviewed the manuscript and provided feedback, and all authors approved the manuscript in its final form.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Cui Lv, Shoubing Chen
Contributor Information
Jinyong Wang, Email: wang_jinyong@gibh.ac.cn.
Hongling Wu, Email: wu_hongling@gibh.ac.cn.
Supplementary information
The online version of this article (10.1038/s41423-020-0429-4) contains supplementary material.
References
- 1.Davila ML, Sadelain M. Biology and clinical application of CAR T cells for B cell malignancies. Int. J. Hematol. 2016;104:6–17. doi: 10.1007/s12185-016-2039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmermans F, et al. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J. Immunol. 2009;182:6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy M, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Guo R, et al. Guiding T lymphopoiesis from pluripotent stem cells by defined transcription factors. Cell Res. 2020;30:21–33. doi: 10.1038/s41422-019-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt TM, et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat. Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 6.Davila ML, Kloss CC, Gunset G, Sadelain M. CD19 CAR-targeted T cells induce long-term remission and B Cell Aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS ONE. 2013;8:e61338. doi: 10.1371/journal.pone.0061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, et al. Deletions linked to TP53 loss drive cancer through p53-independent mechanisms. Nature. 2016;531:471–475. doi: 10.1038/nature17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.