Abstract
Breast cancer (BC) is one of the most common types of cancer with the highest morbidity rate amongst all cancers in women worldwide. Arctigenin is isolated from the seeds of Asteraceae lappa and exhibits anti-inflammatory and anti-viral effects. The present study aimed to investigate the effect of arctigenin on BC cells and to explore the regulation of arctigenin on eukaryotic translation initiation factor 4E binding protein 1 (4EBP1) expression. To do so, MDA-MB-231 and BT549 cells were treated with arctigenin at various concentrations (0, 5, 10, 20 and 40 µM). Cells treated with 40 µM arctigenin were transfected with pcDNA3.1-4EBP1 or NC control. Cell Counting Kit-8 assay was used to determine cell proliferation, reverse transcription quantitative PCR was used to evaluate the transfection efficiency, western blotting was used to detect relative protein expression and Transwell assays were performed to evaluate the migratory and invasive abilities of BC cells. The results demonstrated that arctigenin could inhibit the proliferation, migratory and invasive abilities, and epithelial to mesenchymal transition (EMT) of MDA-MB-231 and BT549 cells. Furthermore, arctigenin downregulated the expression of 4EBP1 in MDA-MB-231 and BT549 cells, whereas 4EBP1 overexpression could reverse the inhibiting effect of arctigenin on proliferation, migratory and invasive abilities, and EMT in MDA-MB-231 and BT549 cells. The findings suggested that arctigenin may inhibit human BC cell proliferation, migratory and invasive abilities, and EMT by targeting 4EBP1.
Keywords: arctigenin, eukaryotic translation initiation factor 4E binding protein 1
Introduction
Breast cancer (BC) is one of the most common and deadly cancers in women worldwide (1-3), and >20 distinct subtypes of breast cancer have been identified (4). Next-generation sequencing studies have drawn comprehensive molecular BC portraits, and >1600 driver mutations have been identified in 93 BC genes (5). Among all cases, hormone-receptor-positive BCs account for half of the disease subtypes (6). According to the presence or absence of molecular markers for estrogen or progesterone receptors and human epidermal growth factor 2 (ERBB2; formerly HER2), BC is divided into three major subtypes (luminal, basal-like and Her-2+) (7). The subtype would determine the type of systemic therapy given to patient, including endocrine therapy, chemotherapy, and ERBB2-targeted antibody or small-molecule inhibitor therapy combined with chemotherapy (8). In addition, surgical resection is also considered for patients with non-metastatic BC (9). At present, palliative care can improve the quality of life and prolong the life in patients with metastatic BC treated according to subtypes (10). It is therefore necessary to identify new therapeutic targets and to determine the underlying mechanisms of BC.
Arctigenin, a bioactive lignin, can be isolated from the seeds of Asteraceae lappa and has exhibited some anti-inflammatory and anti-viral effects (11). Furthermore, arctigenin has been reported to increase the chemosensitivity of several cancer cells, including HepG2, HeLa and K562(12). Arctigenin has also been applied to the treatment of various types of cancer, and the anti-tumor function has been illustrated in various cancers, including gallbladder cancer (13), human retinoblastoma cells (14), lung cancer (15) and prostate tumor (16). Wang et al (17) reported that arctigenin could trigger autophagy, induce apoptosis and enhance the sensitivity of colorectal cancer cell to chemotherapy. In addition, arctigenin can inhibit the migratory and invasive abilities of breast cancer cells by downregulating heparanase and matrix metalloproteinases (MMPs) 2 and 9 in MDA-MB-231 cells (18). Huang et al (12) also demonstrated that arctigenin could promote the anti-metastasis effect and inhibit triple-negative breast cancer by downregulating the protein cancerous inhibitor of protein phosphatase 2A. In addition, it was demonstrated that arctigenin can target the transcription factor signal transducer and activator of transcription 3, which is involved in epithelial to mesenchymal transition (EMT) (19). However, limited studies have focused on the underlying mechanism of arctigenin on metastasis, migration and EMT in BC.
Eukaryotic translation initiation factor 4E binding protein 1 (4EBP1) is a type of translation-repressor protein and represents one of the main downstream effector of mammalian target of rapamycin (mTOR) (20). As a tumor suppressor, 4EBP1 serves crucial roles in various types of cancer. For example, 4EBP1 can be reactivated by mTOR inhibition and act as a tumor suppressor in head and neck squamous cell carcinomas (21). Furthermore, overexpressed 4EBP1 is an independent predictor of outcome for patients with ovarian cancer (22). There is also some evidence that 4EBP1 is overexpressed in BC cells where it might serve as an oncogene (23,24). However, the underlying mechanism of 4EBP1 in BC remains unknown.
Therefore, the present study aimed to investigate the effect and underlying mechanisms of arctigenin on BC cells and to explore the regulation relationship between arctigenin and 4EBP1.
Materials and methods
Chemicals and reagents
Arctigenin (purity, up to 98%) was obtained from Shanghai Yuanye Bio-Technology Co., Ltd. Arctigenin was dissolved in DMSO at a stock solution of 50 mM and stored at -20˚C. The solution was then diluted in culture medium to the appropriate final concentrations prior to use (5, 10, 20 and 40 µM).
Cell treatment and transfection
The human breast cancer cell lines MDA-MB-231 and BT549 were purchased from the American Type Culture Collection. Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.), containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), and 100 µg/ml streptomycin and 100 U/ml penicillin (Gibco; Thermo Fisher Scientific, Inc.) and placed at 37˚C in a humidified incubator containing 5% CO2. MDA-MB-231 and BT549 cells were treated with arctigenin at various concentrations (5, 10, 20 and 40 µM) or vehicle as the control for 24, 48 and 72 h.
For 4EBP1 overexpression, 50 nM pcDNA3.1-4EBP1 or pcDNA3.1-NC (Invitrogen; Thermo Fisher Scientific, Inc.) were diluted by 250 µl of serum-free Opti-MEM and incubated at room temperature for 5 min and mixed with 5-µl aliquot of Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) for another 20 min at room temperature and added to culture well of MDA-MB-231 or BT549 cell (5x104/well). Subsequently, the cells were cultured for 6-8 h at 37˚C with 5% CO2, the complete medium was refreshed and the cells were cultured for a further 48 h prior to the following experiments.
Cell proliferation assay
MDA-MB-231 and BT549 cells were seeded at the density of 5x103 cells per well in 96-well plates and cultured in DMEM medium containing 10% FBS overnight. Subsequently, cells were treated with different concentrations of arctigenin and cultured for various times (24, 48 and 72 h). Cell Counting Kit-8 (CCK8; 10 µl; Dojindo Molecular Technologies, Inc.) reagent, which was used to assess cell proliferation, was added to the wells and cells were incubated for 3 h at 37˚C. Absorbance was measured at a wavelength of 450 nm on a microplate reader.
Transwell migration and invasion assay
To evaluate the migratory and invasive abilities of cells, 24-well Transwell chambers (8-µm pore size; Corning Inc.) were used. MDA-MB-231 and BT549 cells were treated with different concentrations of arctigenin for 48 h. For the migration assay, 1x105 treated cells were resuspended in serum-free medium containing 1% bovine serum albumin (Sigma-Aldrich; Merck KGaA) and seeded in the upper chamber of the Transwell, while DMEM with 10% FBS was added to the lower chamber. After incubation at 37˚C for 24 h, cells in the lower chamber were fixed with 4% paraformaldehyde and stained with 1% crystal violet for 15 min at 37˚C (JRDUN Biotechnology Co., Ltd.), whereas cells in the upper wells were removed. For the invasion assay, the method was similar to the cell migration assay, but the Transwell membrane was pre-treated with Matrigel (BD Biosciences) at a concentration of 2 mg/ml. In addition, the results were assessed 36 h after incubation. For qualification, five random fields per filter were counted under a light microscope at a magnification of x100 (Leica Microsystems GmbH).
RNA extraction and reverse transcription quantitative (RT-q) PCR
MDA-MB-231 and BT549 cells were collected and total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). PrimeScript RT reagent Kit (Takara Biotechnology Co., Ltd.) was used for reverse transcription. RT-qPCR was conducted with SYBR green master reagent (Toyobo Life Science). The PCR reactions were conducted as follows: Initial denaturation at 95˚C for 2 min followed by 28 cycles at 95˚C for 30 sec, 58˚C for 30 sec, and 72˚C for 30 sec. The sequences of the primers used were as follows: 4EBP1, forward 5'-GATACCTCCTTGTGCCTCCA-3', reverse 5'-TCGTTCTTGTCCACTTCCTG-3'; and GAPDH, forward 5'-ATCCCATCACCATCTTCCAG-3' and reverse 5'-TTCTAGACGGCAGGTCAGGT-3'. The relative expression levels of 4EBP1were normalized to endogenous control GAPDH and were expressed as 2-ΔΔCq (25).
Western blotting
Western blotting was performed to detect the protein expression of E-cadherin, N-cadherin, vimentin and 4EBP1. Cells were harvested, washed with PBS and lysed in RIPA buffer (Sigma-Aldrich; Merck KGaA) and 1% protease inhibitors cocktail (Merck KGaA). Protein concentration was determined using BCA protein reagent (Pierce; Thermo Fisher Scientific, Inc.). Proteins (20 µg) were separated by 10% SDS-PAGE gel and transferred onto PVDF membranes (Merck KGaA). Membranes were blocked with 5% skimmed milk for 1 h at 37˚C and were incubated overnight at 4˚C with primary antibodies against E-cadherin (cat. no. ab1416; 1:100), N-cadherin (cat. no. ab76057; 1:1,000), vimentin (cat. no. ab92547; 1:1,000), 4EBP1 (cat. no. ab32024; 1:5,000) and GAPDH (cat. no. ab181602; 1:10,000; all from Abcam). Membranes were then incubated with a diluted horseradish peroxidase-labeled goat anti-rabbit secondary antibody (cat. no. SE134; 1:2,000, Beijing Solarbio Science and Technology Co., Ltd.) at room temperature for 1 h. SuperSignal® West Pico Trial kit (Pierce; Thermo Fisher Scientific, Inc.) was used to detect the signal on the membrane and optical densities of the bands were measured using ImageJ software (version 1.38; National Institutes of Health).
Statistical analysis
Statistical analyses were performed using SPSS 22.0 software (IBM Corp.). The data were presented as the means ± standard deviation. Comparison among three or more groups was conducted using one-way ANOVA followed by Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
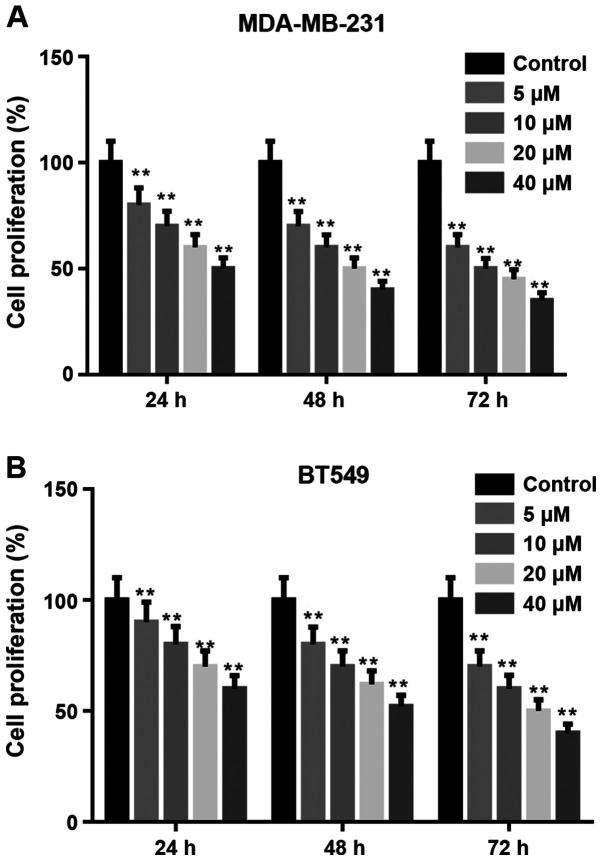
Arctigenin inhibits the proliferation of MDA-MB-231 and BT549 cells
CCK-8 assay was used to measure the proliferation of MDA-MB-231 and BT549 cells treated with arctigenin (0, 5, 10, 20, and 40 µM) for 24, 48 and 72 h. As presented in Fig. 1A and B, arctigenin significantly decreased the proliferation of MDA-MB-231 and BT549 cells in a concentration-dependent manner compared with the control. Subsequently, the concentrations of 20 and 40 µM were selected to further evaluate the effects of arctigenin on the migratory and invasive abilities and EMT of cells.
Figure 1.
Arctigenin inhibited the proliferation of MDA-MB-231 and BT549 cells. (A and B) Proliferation of MDA-MB-231 and BT549 cells treated with arctigenin (0, 5, 10, 20, and 40 µM) for 24, 48 or 72 h detected by Cell Counting-Kit 8 assay. **P<0.01 vs. Control group.
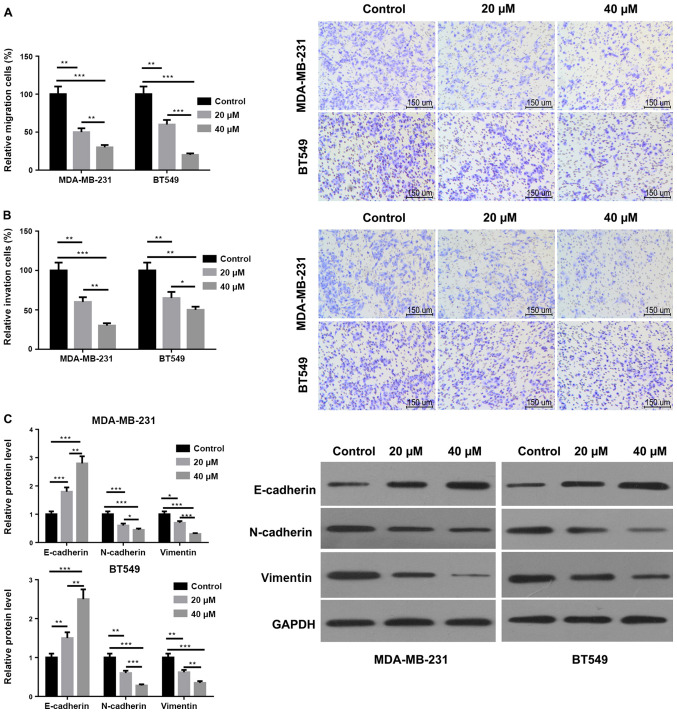
Arctigenin inhibits the migratory and invasive abilities and EMT of MDA-MB-231 and BT549 cells
As presented in Fig. 2A, the migration ability of MDA-MB-231 and BT549 cells following treatment with 20 and 40 µM arctigenin was significantly decreased compared with the control. Furthermore, the migration ability of MDA-MB-231 and BT549 cells treated with 40 µM arctigenin was significantly decreased compared with cells treated with 20 µM arctigenin. The results from Fig. 2B demonstrated that arctigenin could also inhibit the invasive ability of MDA-MB-231 and BT549 cells in a concentration-dependent manner. In addition, as presented in Fig. 2C, the protein expression of N-cadherin and vimentin was significantly decreased in MDA-MB-231 and BT549 cells following treatment with 20 and 40 µM arctigenin compared with the control. The expression of E-cadherin was significantly increased in MDA-MB-231 and BT549 cells. These results demonstrated also that arctigenin may inhibit EMT in a dose-dependent manner. Taken together, these findings indicated that arctigenin may serve a crucial role in the processes of migration, invasion and EMT of MDA-MB-231 and BT549 cells.
Figure 2.
Arctigenin inhibited the migratory and invasive abilities and EMT of MDA-MB-231 and BT549 cells. (A) Migratory ability of MDA-MB-231 and BT549 cells treated with arctigenin assessed by Transwell assay (scale bar, 150 µm). (B) Invasive ability of MDA-MB-231 and BT549 cells treated with arctigenin assessed by Transwell assay (scale bar, 150 µm). (C) Protein expression of N-cadherin and vimentin in MDA-MB-231 and BT549 cells treated with arctigenin evaluated by western blotting. *P<0.05 **P<0.01 and ***P<0.001 vs. Control group or 20 µM group.
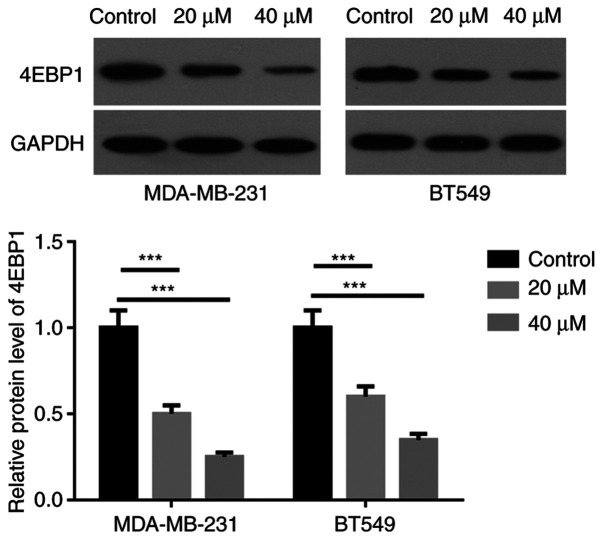
Arctigenin downregulates the expression of 4EBP1 in MDA-MB-231 and BT549 cells
The effect of arctigenin on 4EBP1 expression in BC cells was evaluated. As seen in Fig. 3, the expression of 4EBP1 was significantly decreased in MDA-MB-231 and BT549 cells following treatment with 20 and 40 µM arctigenin compared with the control. In addition, 4EBP1 expression in cells treated with 40 µM arctigenin was significantly decreased compared with cells treated with 20 µM arctigenin.
Figure 3.
Arctigenin downregulated the expression of 4EBP1 in MDA-MB-231 and BT549 cells. Protein expression of 4EBP1 in MDA-MB-231 and BT549 cells treated with arctigenin was assessed by western blotting and quantified by densitometric analysis. ***P<0.001 vs. Control group.
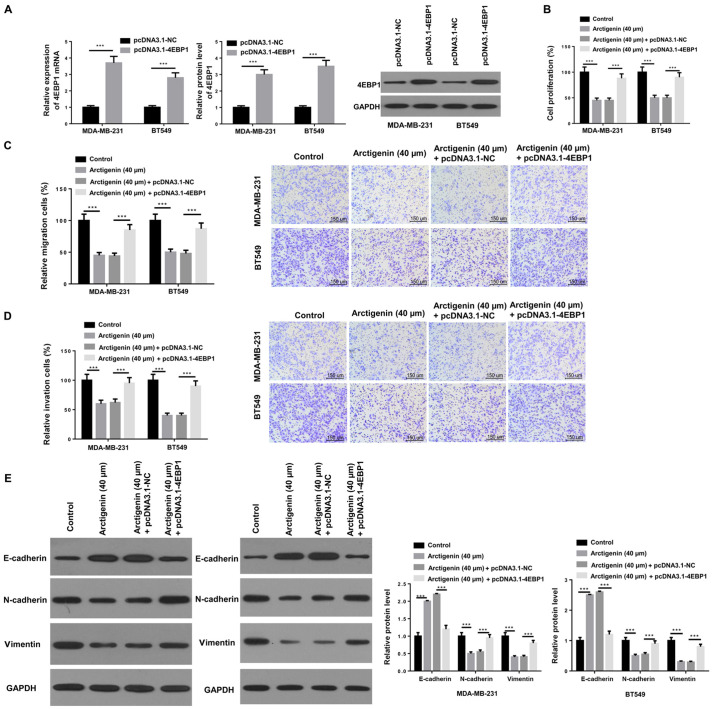
4EBP1 overexpression can reverse the inhibitory effect of arctigenin on the proliferation, migratory and invasive abilities and EMT in MDA-MB-231 and BT549 cells
The effect of 4EBP1 on cell migratory and invasive abilities and EMT in MDA-MB-231 and BT549 cells was subsequently further investigated. 4EBP1 was overexpressed by transfection with pcDNA-4EBP1, and the transfection efficiency was examined by RT-qPCR and western blotting. As presented in Fig. 4A, the mRNA and protein expression of 4EBP1 was significantly increased in transfected MDA-MB-231 and BT549 cells compared with NC. Furthermore, MDA-MB-231 and BT549 cell proliferation was significantly decreased following treatment with arctigenin, which was reversed following 4EBP1 overexpression (Fig. 4B). The results from Transwell assays demonstrated that 4EBP1 overexpression could reverse the inhibitory effect of arctigenin on the cell migratory and invasive abilities (Fig. 4C and D). In addition, 4EBP1 overexpression significantly increased the expression of E-cadherin but decreased the expression of N-cadherin and vimentin in MDA-MB-231 and BT549 cells treated with arctigenin (Fig. 4E). These findings suggested that overexpression of 4EBP1 may reverse the inhibitory effect of arctigenin on the proliferation, migratory and invasive abilities and EMT in MDA-MB-231 and BT549 cells.
Figure 4.
4EBP1 overexpression could reverse the inhibitory effect of arctigenin on the proliferation, migratory and invasive abilities and EMT in MDA-MB-231 and BT549 cells. (A) Transfection efficiency was examined by reverse transcription quantitative PCR and western blotting. (B) Cell proliferation of transfected MDA-MB-231 and BT549 cells treated with arctigenin was detected using Cell Counting-Kit 8 assay. Transwell assay was used to evaluate the (C) migratory ability or (D) invasive ability of MDA-MB-231 and BT549 cells treated with arctigenin and following transfection with pcDNA3.1-NC or pcDNA3.1-4EBP1 (scale bar, 150 µm). (E) Western blotting was used to evaluate the protein expression of E-cadherin, N-cadherin and vimentin in MDA-MB-231 and BT549 cells treated with arctigenin and following transfection with pcDNA3.1-NC or pcDNA3.1-4EBP1. ***P<0.001 vs. pcDNA3.1-NC group, Control group or Arctigenin (40 µM) + pcDNA3.1-NC group.
Discussion
BC is one of the most prevalent carcinomas in women worldwide, and the development of BC metastasis leads to a high mortality rate. However, there is no Food and Drug Administration-approved targeted therapy for BC (26). It is therefore urgent to identify new targeting therapy and drugs against BC. In the last decades, arctigenin and 4EBP1 have been reported in several studies. As previously reported, arctigenin inhibits the degradation of topoisomerase IIα and reduces the expression of GRP78 in solid tumors, which can attenuate anticancer drug resistance (27). Maheshwari et al (28) also found that arctigenin shows anti-tumor activity against a set of human solid tumor cell lines, including pancreatic-PANC-1, colon-H116, lung-H125, liver-HepG2, OVC-5 and brain-U251N. However, the role and underlying mechanism of arctigenin and 4EBP1 in the proliferation, migratory and invasive abilities and EMT of BC cells remain unclear. The present study demonstrated that arctigenin could inhibit the proliferation, migratory and invasive abilities and EMT of BC cells, which was reversed following 4EBP1 overexpression.
Arctigenin is a member of the Asteraceae family that could inhibit the growth of several cancer cells (29). Previous studies revealed that arctigenin has anti-viral, anti-inflammatory and anti-tumor activities (30,31). Furthermore, arctigenin was reported to be a therapeutic agent against cancer and to inhibit some oncogenic signaling pathways (32). As demonstrated by Maxwell et al (33), arctigenin has some anti-metastatic effects on human BC cells by inhibiting MMP-9 and urokinase plasminogen activator via the Akt, NF-κB, and MAPK signaling pathways. Lee et al (34) also demonstrated that arctigenin can decrease the proliferation of MCF-7 and MDA-MB-231 human BC cells and induce apoptosis in MCF-7 cells. In the present study, arctigenin inhibited the proliferation and migratory and invasive abilities of MDA-MB-231 and BT549 cells. These results were consistent with the study of Lou et al (18), in which the effect of arctigenin on the inhibition of BC cell migration and invasion is confirmed. Another study reported that arctigenin can inhibit the proliferation of MDA-MB-231 cells in a dose-dependent manner and from a concentration as low as 0.4 µΜ (19). In the present study, arctigenin was found to inhibit BC cell proliferation at the low concentration of 5 µΜ and to inhibit BC cell migratory and invasive abilities at the low concentration at 20 µΜ. The difference may be due to detection methods and cell culture conditions. As reported by Xu et al (15), arctigenin can inhibit TGF-β-induced EMT and suppress the progression and metastasis of lung cancer cells. Lu et al (35) also demonstrated that arctigenin can attenuate tumor metastasis by inhibiting EMT in hepatocellular carcinoma. However, only a few studies have investigated the effect of arctigenin on EMT in BC cells. To the best of our knowledge, the present study was the first to demonstrate that arctigenin could inhibit EMT in MDA-MB-231 and BT549 cells.
As a major substrate of mTORC1, 4EBP1 plays an essential role in the regulation of cancer cell proliferation (36). In addition, 4EBP1 can slow tumor progression in phosphatase and tensin homolog (PTEN)-driven prostate cancer (37). Significant upregulation and dephosphorylation of 4EBP1 serve an important role in the promotion of pancreatic cancer cell death (38). The results from these studies suggest that 4EBP1 might serve as a tumor suppressor factor and inhibit the migratory and invasive abilities of various cancer cells. However, only limited studies have investigated the role and underlying mechanism of 4EBP1 in BC cells in the last decades. Conversely, it was reported that integrated analysis of PTEN and p4EBP1 protein levels could be considered as predictors for pathological complete response in patients with HER2-positive BC receiving neoadjuvant therapy (39). Besides, 4EBP1 is considered as an oncogene and was found to be upregulated in BC cells (23,24). The present study demonstrated for the first time that overexpressing 4EBP1 could reverse the inhibitory effect of arctigenin on the proliferation, migratory and invasive abilities and EMT of BC cells. These result suggested that 4EBP1 may promote tumor progression and act as an oncogene in BC. All these results indicated that 4EBP1 might serve different roles in cell proliferation, migration, invasion and EMT in various types of cancer cell. Further investigation is therefore essential.
In summary, the present study demonstrated that arctigenin could inhibit human BC cell proliferation, migratory and invasive abilities and EMT by targeting 4EBP1. These findings may bring a new direction for the development of targeting therapy against BC.
Acknowledgements
Not applicable.
Funding Statement
Funding: Not applicable.
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WL conducted the majority of the experiments and wrote the manuscript; FW and HLuo conducted experiments, analyzed the data and confirm the authenticity of all the raw data. HLiu designed the study and revised the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mavaddat N, Michailidou K, Dennis J, Lush M, Fachal L, Lee A, Tyrer JP, Chen TH, Wang Q, Bolla MK, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104:21–34. doi: 10.1016/j.ajhg.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Wu L, Zheng W, Wen W, Wang S, Shu X, Long J, Shen CY, Wu PE, Saloustros E, et al. Genetic evidence for the association between schizophrenia and breast cancer. J Psychiatry Brain Sci. 2018;3(7) doi: 10.20900/jpbs.20180007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Bossche JV. Lipid-laden macrophages cross the border to cancer. Immunometabolism. 2020;2(e200006) [Google Scholar]
- 4.Sudharshan PJ, Petitjean C, Spanhol F, Oliveira LE, Heutte L, Honeine P. Multiple instance learning for histopathological breast cancer image classification. Exp Syst Appl. 2019;117:103–111. [Google Scholar]
- 5.Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373–386.e10. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Qi Y, Kong X, Zhai J, Li Y, Song Y, Wang J, Feng X, Fang Y. Immunological therapy: A novel thriving area for triple-negative breast cancer treatment. Cancer Lett. 2019;442:409–428. doi: 10.1016/j.canlet.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Song W, Zhou Y, Mao F, Lin Y, Guan J, Sun Q. Expression and function of MutT homolog 1 in distinct subtypes of breast cancer. Oncol Lett. 2017;13:2161–2168. doi: 10.3892/ol.2017.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toi M, Tanaka S, Bando M, Hayashi K, Tominaga T. Outcome of surgical resection for chest wall recurrence in breast cancer patients. J Surg Oncol. 1997;64:23–26. doi: 10.1002/(sici)1096-9098(199701)64:1<23::aid-jso5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Waks AG, Winer EP. Breast cancer treatment: A review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell T, Lee KS, Kim S, Nam KS. Arctigenin inhibits the activation of the mTOR pathway, resulting in autophagic cell death and decreased ER expression in ER-positive human breast cancer cells. Int J Oncol. 2018;52:1339–1349. doi: 10.3892/ijo.2018.4271. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q, Qin S, Yuan X, Zhang L, Ji J, Liu X, Ma W, Zhang Y, Liu P, Sun Z, et al. Arctigenin inhibits triple-negative breast cancers by targeting CIP2A to reactivate protein phosphatase 2A. Oncol Rep. 2017;38:598–606. doi: 10.3892/or.2017.5667. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Cai S, Zuo B, Gong W, Tang Z, Zhou D, Weng M, Qin Y, Wang S, Liu J, et al. Arctigenin induced gallbladder cancer senescence through modulating epidermal growth factor receptor pathway. Tumour Biol. 2017;39(1010428317698359) doi: 10.1177/1010428317698359. [DOI] [PubMed] [Google Scholar]
- 14.Ke N, Liu Q, Pi L, Fang J, Chen L, Chen X. The antitumor function of arctigenin in human retinoblastoma cells is mediated by jagged-1. Mol Med Rep. 2019;19:3642–3648. doi: 10.3892/mmr.2019.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Lou Z, Lee SH. Arctigenin represses TGF-β-induced epithelial mesenchymal transition in human lung cancer cells. Biochem Biophys Res Commun. 2017;493:934–939. doi: 10.1016/j.bbrc.2017.09.117. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Diaz T, Henning S, Vadgama J. Abstract 5253: Arctigenin inhibits prostate tumor growth in vitro and in vivo in obese state. Cancer Res. 2017;77 (Suppl 13)(S5253) doi: 10.1016/j.yclnex.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Lina L, Xu L, Yang Z, Qian Z, Zhou J, Suoni L. Arctigenin enhances the sensitivity of cisplatin resistant colorectal cancer cell by activating autophagy. Biochem Biophys Res Commun. 2019;520:20–26. doi: 10.1016/j.bbrc.2019.09.086. [DOI] [PubMed] [Google Scholar]
- 18.Lou C, Zhu Z, Zhao Y, Zhu R, Zhao H. Arctigenin, a lignan from Arctium lappa L., inhibits metastasis of human breast cancer cells through the downregulation of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol Rep. 2017;37:179–184. doi: 10.3892/or.2016.5269. [DOI] [PubMed] [Google Scholar]
- 19.Feng T, Cao W, Shen W, Zhang L, Gu X, Guo Y, Tsai HI, Liu X, Li J, Zhang J, et al. Arctigenin inhibits STAT3 and exhibits anticancer potential in human triple-negative breast cancer therapy. Oncotarget. 2017;8:329–344. doi: 10.18632/oncotarget.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, Guo J, Li H, Wang J. Meta-analysis of the prognostic value of p-4EBP1 in human malignancies. Oncotarget. 2017;9:2761–2769. doi: 10.18632/oncotarget.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Feng X, Molinolo AA, Martin D, Vitale-Cross L, Nohata N, Ando M, Wahba A, Amornphimoltham P, Wu X, et al. 4E-BP1 Is a tumor suppressor protein reactivated by mTOR inhibition in head and neck cancer. Cancer Res. 2019;79:1438–1450. doi: 10.1158/0008-5472.CAN-18-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alabdullah ML, Ahmad DA, Moseley P, Madhusudan S, Chan S, Rakha E. The mTOR downstream regulator (p-4EBP1) is a novel independent prognostic marker in ovarian cancer. J Obstet Gynaecol. 2019;39:522–528. doi: 10.1080/01443615.2018.1534091. [DOI] [PubMed] [Google Scholar]
- 23.Rutkovsky AC, Yeh ES, Guest ST, Findlay VJ, Muise-Helmericks RC, Armeson K, Ethier SP. Eukaryotic initiation factor 4E-binding protein as an oncogene in breast cancer. BMC Cancer. 2019;19(491) doi: 10.1186/s12885-019-5667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristina A, Bartolacci C, Wijnant K, Crinelli R, Bianchi M, Magnani M, Hysi A, Iezzi M, Amici A, Marchini C. Resveratrol fuels HER2 and ERα-positive breast cancer behaving as proteasome inhibitor. Aging (Albany NY) 2017;9:508–520. doi: 10.18632/aging.101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 27.Yoon SB, Park HR. Arctigenin inhibits etoposide resistance in HT-29 colon cancer cells during microenvironmental stress. J Microbiol Biotechnol. 2019;29:571–576. doi: 10.4014/jmb.1901.01061. [DOI] [PubMed] [Google Scholar]
- 28.Maheshwari M, Jia Q, Valeriote FA. Arctin and arctigenin as a potential treatment for solid tumors. Cancer Res. 2019;79 (Suppl 13)(S366) [Google Scholar]
- 29.Naoe A, Tsuchiya T, Kondo Y, Uga N, Watanabe S, Yasui T, Hara F, Suzuki T. Arctigenin induces apoptosis in human hepatoblastoma cells. Pediatr Surg Int. 2019;35:723–728. doi: 10.1007/s00383-019-04473-6. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Tan YJ, Lu ZZ, Li BB, Sun CH, Li T, Zhao LL, Liu Z, Zhang GM, Yao JC, Li J. Arctigenin inhibits liver cancer tumorigenesis by inhibiting gankyrin expression via C/EBPα and PPARα. Front Pharmacol. 2018;9(268) doi: 10.3389/fphar.2018.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CY, Hsieh PL, Liao YW, Peng CY, Yu CC, Lu MY. Arctigenin reduces myofibroblast activities in oral submucous fibrosis by LINC00974 inhibition. Int J Mol Sci. 2019;20(1328) doi: 10.3390/ijms20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y, Fan Q, Cai T, Huang W, Xie X, Wen Y, Shi Z. Molecular mechanisms of the action of arctigenin in cancer. Biomed Pharmacother. 2018;108:403–407. doi: 10.1016/j.biopha.2018.08.158. [DOI] [PubMed] [Google Scholar]
- 33.Maxwell T, Chun SY, Lee KS, Kim S, Nam KS. The anti-metastatic effects of the phytoestrogen arctigenin on human breast cancer cell lines regardless of the status of ER expression. Int J Oncol. 2017;50:727–735. doi: 10.3892/ijo.2016.3825. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Imm JY, Lee SH. β-catenin mediates anti-adipogenic and anticancer effects of arctigenin in preadipocytes and breast cancer cells. J Agric Food Chem. 2017;65:2513–2520. doi: 10.1021/acs.jafc.7b00112. [DOI] [PubMed] [Google Scholar]
- 35.Lu Z, Chang L, Zhou H, Liu X, Li Y, Mi T, Tong D. Arctigenin attenuates tumor metastasis through inhibiting epithelial-mesenchymal transition in hepatocellular carcinoma via suppressing GSK3β-dependent Wnt/β-catenin signaling pathway in vivo and in vitro. Front Pharmacol. 2019;10(937) doi: 10.3389/fphar.2019.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Huang F, Zhang Z, Wang P, Luo Y, Li H, Li N, Wang J, Zhou J, Wang Y, Li S. Feedback activation of SGK3 and AKT contributes to rapamycin resistance by reactivating mTORC1/4EBP1 axis via TSC2 in breast cancer. Int J Biol Sci. 2019;15:929–941. doi: 10.7150/ijbs.32489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding M, Van der Kwast TH, Vellanki RN, Foltz WD, McKee TD, Sonenberg N, Pandolfi PP, Koritzinsky M, Wouters BG. The mTOR targets 4E-BP1/2 restrain tumor growth and promote hypoxia tolerance in PTEN-driven prostate cancer. Mol Cancer Res. 2018;16:682–695. doi: 10.1158/1541-7786.MCR-17-0696. [DOI] [PubMed] [Google Scholar]
- 38.Elia A, Henry-Grant R, Adiseshiah C, Marboeuf C, Buckley RJ, Clemens MJ, Mudan S, Pyronnet S. Implication of 4E-BP1 protein dephosphorylation and accumulation in pancreatic cancer cell death induced by combined gemcitabine and TRAIL. Cell Death Dis. 2017;8(3204) doi: 10.1038/s41419-017-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loibl S, Darb-Esfahani S, Huober J, Klimowicz A, Furlanetto J, Lederer B, Hartmann A, Eidtmann H, Pfitzner B, Fasching PA, et al. Integrated analysis of PTEN and p4EBP1 protein expression as predictors for pCR in HER2-positive breast cancer. Clin Cancer Res. 2016;22:2675–2683. doi: 10.1158/1078-0432.CCR-15-0965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.