Abstract
Several studies have explored the mechanisms of C-C motif chemokine ligand (CCL)2/CC receptor (R)2 function in tumorigenesis and inflammation. However, little is known about the role of CCL2/CCR2 in tumor recurrence, especially after radiotherapy. The present study aimed to determine the association between CCL2/CCR2 and glioma relapse. Moreover, the difference in the expression of CCL2/CCR2 between post-radiation and non-radiation recurrent glioma tissues was compared. A retrospective analysis of 80 patients with glioma who underwent tumor resection twice was performed. Primary group refers to glioma patients who received glioma resection surgery for the first time. Recurrent group refers to glioma patients who received glioma resection surgery after first relapse. In total, 10 patients with brain trauma who underwent partial resection of the normal brain as decompression treatment were used as controls. Protein expression levels of CCL2 and CCR2 were evaluated using immunohistochemistry. Prognostic analyses of patient survival using Kaplan-Meier curves and Cox regression models were performed. The expression levels of CCL2 and CCR2 were higher in recurrent glioma compared with the primary group. There was a positive correlation between tumor grade and protein expression of CCL2/CCR2. Furthermore, irradiation had a significant effect on CCR2 protein expression (P=0.014), but not on CCL2 protein expression (P=0.626). However, the expression of CCL2 and CCR2 showed no significant difference between primary and secondary glioblastoma. After adjusting for sex, radiotherapy and location of tumors in these gliomas, CCL2 was a prognostic factor for disease-free and overall survival (OS) times, as well as age and tumor grade. In the multivariate Cox modeling for glioma, CCR2 was significantly associated with OS rather than DFI. The significant correlations between CCL2/CCR2 expression and glioma tumor grade suggested that CCL2/CCR2 has a role in glioma progression. Combined with previous in vitro experiments, it was proposed that irradiation (radiotherapy)-induced expression of CCL2 is transient, while irradiation-induced expression of CCR2 is lasting. Therefore, CCL2/CCR2 is a potential therapeutic target for patients with glioma.
Keywords: glioma, C-C motif chemokine ligand 2, CCR2, relapse, irradiation, prognosis
Introduction
Glioma is the most common tumor type in the central nervous system, and glioblastoma multiforme (GBM; grade IV according to the the 2016 WHO Classification of Glioma) is the most lethal, with a median survival of 14.6 months (1-4). The median recurrence period of GBM is 6-12 months, and that of anaplastic glioma (grade III) is 18-36 months (5). Studies have shown that the malignancy and invasiveness of glioma is increased after recurrence (4,6). Considering that the recurrence and prognosis of malignant gliomas largely depends on the extent of invasion of tumors into normal brain parenchyma, brain radiotherapy with enlarged field is the recommended treatment to target the maximum number of tumor cells possible. However, clinical studies show that, even if patients with glioma received extended resection and field radiotherapy, ~80% of malignant glioma recurrence is located within 2-3 cm of the resected margin (7,8).
In addition to residual tumor cells and radioresistance, the tumor microenvironment also plays an important role in tumor recurrence (9). There are numerous different stromal cells aside from tumor cells in the tumor lesions, including include endothelial cells as well as inflammatory cells, that constitute tumor microenvironment (10). Recently, more and more studies have revealed that the tumor microenvironment plays an important role in the process of tumorigenesis, tumor development, chemo- and radio-resistance and tumor recurrence (11-15). However, until now, the underlying mechanisms for these processes in glioma were unclear.
The C-C motif chemokine ligand (CCL)2, one of the most important members of the chemokine family of proteins, was the first C family chemokine to be cloned and identified (16). CCL2 is highly expressed in several human central nervous system tumors, such as glioma, meningioma and Schwannoma (17-19). Production of CCL2 in tumor microenvironments can be stimulated by IL-1β and TNF-α (17). In normal brain tissues, CCL2 is primarily secreted by endothelial cells, fibroblasts, microglia, astrocytes and monocytes and its expression is relatively low (17). CCL2 functions by binding to the CC receptor (R)2, leading to accelerated Ca2+ influx, cAMP inhibition, and phospholipase C and phosphoinositol-3 kinase activation (20). The activation of CCL2/CCR2 signaling recruits monocytes from the bloodstream through the vascular endothelium and regulates the routine immunological surveillance of brain tissues (21).
Several studies have revealed that CCL2/CCR2 signaling is a promising target in patients with tumors and inflammation (16,22-24). However, little is known on the role of CCL2/CCR2 in tumor recurrence, especially after radiotherapy. Our previous study proposed that after radiotherapy, irradiated brain tissues promote the ability of tumor cells to invade and metastasize by secreting cytokines and proteases, which may partly result in the recurrence and progression of glioma (25). The present study investigated the expression of CCL2 and CCR2 in primary and recurrent glioma and analyzed the association with glioma relapse. The difference in the expression of CCL2/CCR2 between post-radiation and non-radiation recurrent glioma was also compared.
Materials and methods
Patients and tissue specimens
The records of 80 patients with glioma who underwent two glioma resections in Qilu Hospital of Shandong University (Jinan, China) between January 1995 and December 2015 were analyzed. The tissue specimens were evaluated by two pathologists experienced in glioma pathology. All specimens were collected retrospectively from primary tumors and their corresponding recurrent tumors. All patients were treated according to the guidelines used in our institutions. The present study complied with national regulations, and was approved by The Institutional Ethics and Investigation Committee of Qilu Hospital, Shandong University [approval no. KYLL-2015(KS)-068].
The inclusion criteria were as follows: i) All the cases were solitary lesions and pathologically diagnosed as glioma (2016 WHO classification of tumors of the central nervous system (3)); ii) both the first and second operations were performed in Qilu Hospital; iii) cranial MRI was performed after the first operation with detection of enhanced new lesions, or >25% larger than before, iv) the expected survival time was >8 weeks and v) the Karnofsky score (KPS) was >60(26). Patients with irradiation necrosis were excluded. According to whether the patients received radiotherapy after the first operation, they were divided into two groups: Post-radiation group (first operation, radiotherapy, recurrence, second operation) and non-radiation group (first operation, recurrence, second operation). 33 cases of primary (de novo) and 24 cases of secondary GBM (progressed from low-grade or anaplastic glioma) were included in this study. In addition, 10 non-tumor brain specimens were collected from patients with brain trauma who underwent partial resection of normal brain as decompression treatment for severe head injuries (Qilu Hospital of Shandong University) between January 2013 and December 2015. The clinicopathological characteristics of patients are listed in Table SI. Informed consent was provided by all individual participants included in the study (consent of patients <18 years old was provided by their guardians). Follow-up was performed over the phone and the deadline was April 2019. The time between surgery and disease recurrence was defined as the disease-free interval (DFI). The overall survival (OS) time of the primary tumors was calculated from the date of the operation following primary diagnosis to the date of death or last follow-up.
Immunohistochemistry
Paraffin sections (4 µm) were deparaffinized with xylene and ethanol gradient (100, 95 and 80%) methods at 65˚C for 30 h and treated with 3% hydrogen peroxide for 10 min to block the endogenous peroxidase, and then microwaved (98˚C) in 10 mM sodium citrate buffer (pH 6.0) to unmask the epitopes. After being blocked with goat serum (Beyotime Institute of Biotechnology) for 30 min at 37˚C, the sections were incubated with the rabbit anti-CCL2 antibody [1:100 in buffer (1% BSA, 99% PBS, pH 7.4); cat. no. ab73680] and the rabbit anti-CCR2 antibody [1:250 in buffer (0.75% glycine, 1.21% Tris, 10% glycerol) cat. no. ab155321] (both Abcam,) overnight at 4˚C. At the same time, the controls were treated similarly, except the primary antibody was replaced by PBS. After being washed three times with PBS, the sections were developed with DAB for ~1 min and counterstained with hematoxylin for 30 sec at room temperature. At last, the sections were examined and images were captured using a light microscope equipped with a digital camera (DM2000; Leica Microsystems GmbH) and stained cells were manually counted in five randomly selected fields of vision (original magnification, x400). Immunostaining intensity higher compared with the average background could be observed in the cytoplasmic staining. The mean number of cells expressing CCL2 and CCR2 was recorded. Immunohistochemically staining results were interpreted independently by two pathologists who were blinded to the clinical parameters of the individual cases. The scoring method used was described by Li et al (27) to determine positive CCL2 and CCR2 staining, The score of staining intensity of absent, low, medium and high was quantified as 0, 1, 2 and 3, respectively. The score of extent of staining (0, ≤25, ≤50 and ≤100%) was also classified as 0, 1, 2 and 3, respectively. The product of staining intensity and extent of staining was multiplied as the immunoreactive score (IRS), which ranged from 0 to 9. Then, the scores were divided into four groups: 0, 1-2, 3-5 and 6-9, corresponding to the absent, low, medium and high scores, respectively. Based on IRS, slides with scores of ≥3 were classified as positive expression, while slides with scores <3 were classified as absent expression.
Statistical analysis
The independent Student's t-test was used to analyze the continuous variables. The inconsistency rate for CCL2 is (21+4)/80=31.25%, and inconsistency rate for CCR2 is (26+6)/80=40.00%. Qualitative data were analyzed using χ2 tests, including grade and location, and Fisher's exact test was used when n<5 as appropriate. McNemar's test was used to compare changes in grade and protein expression between primary diagnosis and recurrence. Spearman's rank correlation method was used for correlation analysis. The total survival curve was drawn by Kaplan-Meier survival function, and log rank tests were used for statistical analysis. When there was cross-over of survival curves, a weighted test, such as ABS permutation, was used instead. In addition, Cox multivariate regression analysis was performed to determine independent prognostic factors. P<0.05 was considered to indicate a statistically significant difference. All calculations were performed using SPSS version 17.0 (SPSS Inc.).
Results
Patient distribution, survival and recurrent status
To explore the expression pattern of CCL2/CCR2 in glioma, protein expression was analyzed and clinical data were obtained. In total, 80 patients with glioma were included in the present study. Overall, two patients were lost during follow-up and no patients were still alive on the last day of follow-up. The status of these patients with respect to age, sex, adjuvant radiotherapy, histological grade, location of tumors, DFI and OS is listed in Table SI. The average age was 42.90 years (range, 9-77 years). Mean DFI and OS time were 30.16 months (range, 2.10-105.80) and 60.19 months (range, 6.00-169.20), respectively. There was a significant difference in tumor grade (P=1.097x10-5) when between primary and recurrent gliomas (Table I), which showed increased malignancy after tumor relapse. Among the 49 patients with high tumor grade in the first operation phase, only one patient (2.04%) developed low grade recurrent tumors. Non-brain tumor specimens from 10 patients with brain trauma were used as control, the characteristics of whom are presented in Table SII.
Table I.
Inconsistency rate of tumor grade and protein expressions of CCL2/CCR2 between primary and recurrent astrocytoma.
| Recurrent tumor | ||||||
|---|---|---|---|---|---|---|
| Grade | CCL2 | CCR2 | ||||
| Primary tumor | Low | High | Negative | Positive | Negative | Positive |
| Low/Negative, n (%) | 10 (12.50) | 21 (26.25) | 8 (10.00) | 21 (26.25) | 15 (18.75) | 26 (32.50) |
| High/Positive, n (%) | 1 (1.25) | 48 (60.00) | 4 (5.00) | 47 (58.75) | 6 (7.50) | 33 (41.25) |
| Total change, n (%) | 22 (27.50) | 25 (31.25) | 32 (40.00) | |||
| P-value | 1.097x10-5 | 0.001 | 0.001 | |||
All data were analyzed using McNemar's test.
Inconsistency rate of protein expression of CCL2/CCR2 between primary and recurrent gliomas
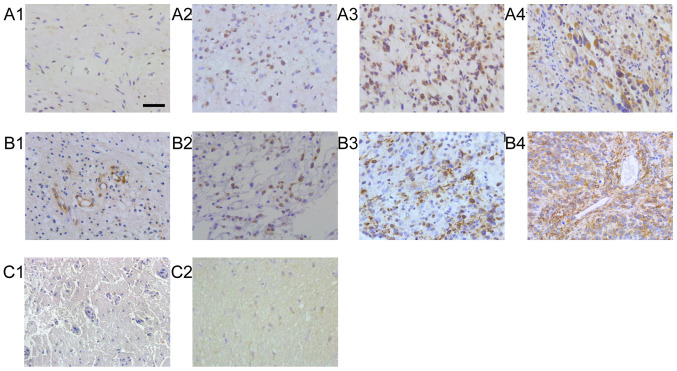
Protein expression of CCL2 and CCR2 in patients with glioma and brain trauma are presented in Fig. 1. The immunoreactivity of all proteins studied was mainly localized in tumor cells, but a considerable proportion of cases were localized in stromal cells. The inconsistency rate of CCL2/CCR2 between primary and recurrent tumors was 31.25 and 40.00%, respectively (Table I). The frequency of CCL2 and CCR2 expression was higher in the recurrent gliomas (85.00 vs. 63.75% for CCL2, P=0.001; 73.7% vs. 48.75% for CCR, P=0.001) compared with in the primary group (Table I).
Figure 1.
Representative results for the immunohistochemistry of CCL2 and CCR2 in primary glioma. CCL2 in glioma of WHO grade (A-1) I, (A-2) II, (A-3) III and (A-4) IV. CCR2 in glioma of WHO grade (B-1) I, (B-2) CCR2 II, (B-3) III and (B-4) C IV. CCL2 and CCR2 in normal tissues as normal control (C1 and C2). Scale bar, 200 μm. CCL, C-C motif chemokine ligand; CCR, C-C motif receptor; WHO, World Health Organisation.
Correlation between tumor grade and protein expression of CCL2/CCR2
CCL2 and CCR2 showed significant positive correlations with tumor grade in the recurrent tumors (P=4.014x10-5 and P=9.763x10-5, respectively), as well as in the primary tumors (P=0.006 and P=0.019, respectively; Table SIII).
Effect of radiotherapy on CCL2/CCR2 protein expression
There was no significant difference in the baseline of clinical characteristics between glioma with and without radiotherapy after the first operation (Table II). No difference was observed between irradiation and histological grade, location of tumor or CCL2 protein expression during the second operation (P=0.433, P=0.302 and P=0.108, respectively; Table III). However, irradiation was significantly associated with CCR2 expression in recurrent glioma (P=0.020; Table III). It was suggested that irradiation-induced expression of CCL2 is transient, while irradiation-induced expression of CCR2 is lasting.
Table II.
Baseline of clinical characteristics between patients with glioma with and without radiotherapy during the first operation.
| Radiotherapy | |||
|---|---|---|---|
| Characteristics | No | Yes | P-value |
| Mean age ± SD, years | 42.76±13.80 | 42.98±13.22 | 0.944 |
| Sex, n (%) | 0.227 | ||
| Male | 13 (16.25) | 30 (37.50) | |
| Female | 16 (20.00) | 21 (26.25) | |
| Location of tumor [n (%)] | 0.216a | ||
| Frontal lobe | 15 (18.75) | 29 (36.25) | |
| Temporal lobe | 4 (5.00) | 13 (16.25) | |
| Parietal lobe | 4 (5.00) | 4 (5.00) | |
| Occipital lobe | 1 (1.25) | 3 (3.75) | |
| Others | 5 (6.25) | 2 (2.50) | |
| Grade [n (%)] | 0.148a | ||
| I | 3 (3.75) | 4 (5.00) | |
| II | 13 (16.25) | 11 (13.75) | |
| III | 4 (5.00) | 12 (15.00) | |
| IV | 9 (11.25) | 24 (30.00) | |
| Expression of CCL2, n (%) | 0.472 | ||
| Negative | 12 (15.00) | 17 (21.25) | |
| Positive | 17 (21.25) | 34 (42.50) | |
| Expression of CCR2, n (%) | |||
| Negative | 13 (16.25) | 28 (35.00) | 0.386 |
| Positive | 16 (20.00) | 23 (28.75) | |
aR x C contingency table χ2 test for grade and location.
Table III.
Effect of radiotherapy on markers of recurrent astrocytoma.
| Radiotherapy | ||||
|---|---|---|---|---|
| Characteristics | No | Yes | χ2 | P-value |
| Histological grade, n (%)a | 2.750 | 0.433 | ||
| I | 1 (1.25) | 1 (1.25) | ||
| II | 5 (6.25) | 4 (5.00) | ||
| III | 4 (5.00) | 12 (15.00) | ||
| IV | 19 (23.75) | 34 (42.50) | ||
| Location of tumor, n (%)a | 4.747 | 0.302 | ||
| Frontal lobe | 17 (21.25) | 29 (36.25) | ||
| Temporal lobe | 4 (5.00) | 13 (16.25) | ||
| Parietal lobe | 3 (3.75) | 1 (1.25) | ||
| Occipital lobe | 1 (1.25) | 4 (5.00) | ||
| Others | 4 (5.00) | 4 (5.00) | ||
| Expression of CCL2, n (%)a | - | 0.108 | ||
| Negative | 7 (8.75) | 5 (6.25) | ||
| Positive | 22 (27.50) | 46 (57.50) | ||
| Expression of CCR2, n (%) | 5.379 | 0.020F | ||
| Negative | 12 (15.00) | 9 (11.25) | ||
| Positive | 17 (21.25) | 42 (52.50) | ||
aAnalyzed using Fisher's exact test. All other variables were analyzed using χ2 tests.
Difference of CCL2/CCR2 protein expression between primary and secondary GBM
GBM can be divided into de novo (primary GBM) or progression from low-grade or anaplastic glioma (secondary GBM) (3). Hence, the present study investigated the difference of CCL2/CCR2 protein expression between 33 cases of primary and 24 cases of secondary GBM. There was no significant difference in the baseline of clinical characteristics between primary GBM during the first operation and secondary GBM during the second operation (Table IV). In addition, no significant difference was observed for the protein expression of CCL2 and CCR2 between primary and secondary GBM (P=0.214 and P=0.346, respectively).
Table IV.
Difference of CCL2/CCR2 expression between primary and secondary glioblastoma.
| Characteristics | Primary glioblastoma | Secondary glioblastoma | P-value |
|---|---|---|---|
| Mean age ± SD, yearsa | 46.21±15.03 | 45.13±8.54 | 0.731 |
| Sex, n (%) | 0.157 | ||
| Male | 20 (60.61) | 10 (41.67) | |
| Female | 13 (39.39) | 14 (58.33) | |
| Location of tumor, n (%)b | 0.196 | ||
| Frontal lobe | 16 (48.49) | 15 (62.50) | |
| Temporal lobe | 6 (18.18) | 6 (25.00) | |
| Parietal lobe | 5 (15.15) | 2 (8.33) | |
| Occipital lobe | 3 (9.09) | 0 (0.00) | |
| Others | 3 (9.09) | 1 (4.17) | |
| Expression of CCL2, nb | 0.214 | ||
| Negative | 4 (12.12) | 0 (0.00) | |
| Positive | 29 (87.88) | 24 (100.00) | |
| Expression of CCR2, nb | |||
| Negative | 9 (27.27) | 4 (16.67) | 0.346 |
| Positive | 24 (72.73) | 20 (83.33) |
aAnalyzed using t-tests.
bAnalyzed using Fisher's exact test. Sex was analyzed using a χ2 test.
Impact of CCL2/CCR2 protein expression on patient survival
Kaplan-Meier analysis and results of analyses for DFI are shown in Table V. It was revealed that age, tumor grade and the protein expression of CCL2/CCR2 were significant prognostic factors in gliomas using Kaplan-Meier analysis (Fig. S1). To exclude possible confounding factors, multivariate Cox analysis was used to predict DFI considering multiple variables simultaneously. After adjusting for sex, radiotherapy and location of tumors, the significant prognostic factors for DFI were age [hazard ratio (HR) =1.846; 95% confidence interval (CI), 1.046-3.257; P=0.034), tumor grade (HR =2.247; 95% CI, 1.301-3.882; P=0.004) and expression of CCL2 (HR=1.663; 95% CI, 1.012-2.731; P=0.045) (Table V).
Table V.
Prognostic values of the clinicopathological parameters and markers.
| DFI | ||||||
|---|---|---|---|---|---|---|
| Mann-Whitney U | Cox | |||||
| Characteristics | Survival analysis P-value | Z | P-value | HR | 95% CI | P-value |
| Age, years | 4.186x10-4a | -2.739 | 0.006 | 1.846 | 1.046-3.257 | 0.034 |
| <50 | ||||||
| ≥50 | ||||||
| Sex | 0.594a | |||||
| Male | ||||||
| Female | ||||||
| Radiotherapy | 0.526a | |||||
| Yes | ||||||
| No | ||||||
| Grade | 7.263x10-6a | -4.079 | 4.500x10-5 | 2.247 | 1.301-3.882 | 0.004 |
| I-II | ||||||
| III-IV | ||||||
| Location | 0.359a | |||||
| Frontal | ||||||
| Temporal | ||||||
| Parietal | ||||||
| Occipital | ||||||
| Others | ||||||
| CCL2 | 0.001a | -3.898 | 9.700x10-5 | 1.663 | 1.012-2.731 | 0.045 |
| Negative | ||||||
| Positive | ||||||
| CCR2 | 0.026b | -2.224 | 0.026 | 1.133 | 0.694-1.849 | 0.618 |
| Negative | ||||||
| Positive | ||||||
aK-M, Kaplan-Meier;
bTwo-stage test; Cox, Cox regression; HR, hazard ratio; CI, confidence interval.
Using the Kaplan-Meier survival analysis model, it was demonstrated that age, tumor grade and protein expression of CCL2/CCR2 were significant prognostic factors for OS in gliomas (Fig. S2 and Table SIV). In Cox multivariate modeling, after adjusting for sex, radiotherapy and location of tumors, expression of CCL2 and CCR2 remained significant prognostic factors for OS (HR=1.879; 95% CI, 1.092-3.236; P=0.023 and HR =1.744; 95% CI, 1.033-2.945, P=0.037, respectively; Table SIV), as well as age and tumor grade (Table SIV).
Discussion
As one of the most important methods in the treatment of glioma, radiotherapy has been used in the clinic for over a century (28). But recurrence still occurs, even after whole brain radiotherapy. An increasing number of studies have shown that irradiation promotes malignant behaviors, including increased proliferation, invasion and migration (4,29).
Recently, the tumor microenvironment has attracted more and more attention regarding research into tumorigenesis, tumor development, antitumor resistance and tumor relapse (11-13). Morganti et al reported that single high-dose (10 Gy) γ-ray irradiation altered the microenvironment of brain tissue and induced increased infiltration of peripheral CCR2+ macrophages (30). A further study showed that CCL2 secreted by glioma cells induced microglia to infiltrate into tumor tissues, while microglia also secreted CCL2, which resulted in an amplifying effect for the recruitment of microglia (31). CCL2 is upregulated at both the mRNA and protein levels in the serum and tumor tissues of patients with glioma (21).
CCR2, the main receptor of CCL2, is overexpressed in most malignant glioma cells (20). The present study showed that there was significant positive correlation between CCL2/CCR2 and tumor grade in primary and recurrent glioma. CCL2 attracted microglia/macrophages, T lymphocytes, basophils, NK cells and hematopoietic progenitor cells to migrate and infiltrate into tumor tissues, all of which participate in a variety of tumor pathological processes, such as stimulating tumor proliferation and migration (32). CCL2 induces microglia/macrophages migration into glioma and indirectly induces the invasion and migration by secreting cytokines (33). An in vitro co-culture study showed that high expression of CCR2 in tumor cells directly promotes perineural invasion, a phenomenon in which cancer cells, especially prostate cancer, invaded the nerves and then reached other sites via the nervous system (34). Zhu et al reported that intraperitoneal injection of monoclonal antibodies against CCL2 significantly prolonged the survival of glioma xenograft mice (35). Therefore, CCL2/CCR2 signaling is closely associated with the progression of glioma.
GBM, the most lethal type of glioma, is classified into primary and secondary GBM (3). The two subgroups are histologically indistinguishable; however, more and more studies have distinguished between these using genetic, epigenetic, and molecular profiles (36,37). Telomerase reverse transcriptase promoter mutation, PTEN tumor suppressor gene mutation and high-level gene amplification of EGFR are hallmarks in primary GBMs, while mutations of isocitrate dehydrogenase, mitochondrial 1/2, TP53 and transcriptional regulator ATRX are more common in secondary GBMs (38). However, until now, little was known about the difference of chemokines in primary and secondary GBM. The present study analyzed the expression of CCL2/CCR2 in primary and secondary GBM. However, the results revealed that there was no significant difference.
Targeting CCL2/CCR2 has been shown to be effective in tumor chemosensitization [18], but whether it has a radiosensitizing effect is largely unknown. Recently, more and more studies reported that CCL2/CCR2 is involved in irradiation-induced damage of brain tissues and irradiation-induced malignant behaviors, such as increased migration and invasion (39-41). Therefore, the combination of radiotherapy and inhibition of CCL2/CCR2 may be promising to improve the prognosis of patients with glioma.
Overall, the present study revealed a significant correlation between CCL2 and CCR2 expression and glioma tumor grade. Furthermore, irradiation affected the expression of CCR2, but not CCL2. Hence, CCL2/CCR2 has potential as therapeutic target for patients with glioma.
Supplementary Material
Acknowledgements
The authors would like to thank Mr. Jian Wang, Mr. Bin Huang and Mrs. Anjing Chen (Brain Science Research Institute, Key Laboratory of Brain Functional Remodeling, Shandong University, Jinan, Shandong, P.R. China) for their excellent technical assistance.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WZ and ZJ conceived the study and designed most of the experiments. WZ and XZ interpreted the data and wrote the manuscript. QY performed most of the statistical analyses and generated all graphs and tables. JZ had primary responsibility for patient characterization and management. JZ, LM and WZ performed the immunohistological analysis. All authors discussed the results and approved the final submitted version. All authors read and approved the final manuscript. WZ and QY confirmed the authenticity of all the raw data.
Ethics approval and consent to participate
All procedures performed were in accordance with the Declaration of Helsinki and the study was approved by The Institutional Ethics and Investigation Committee of Qilu Hospital, Shandong University [approval no. KYLL-2015(KS) -068]. All patients provided informed written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann Oncol. 2017;28:1457–1472. doi: 10.1093/annonc/mdx106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao M, Lin Y, Liu X, Li Y, Zhang C, Wang Z, Wang Z, Wang Y, Guo Z. ISG20 promotes local tumor immunity and contributes to poor survival in human glioma. Oncoimmunology. 2019;8(e1534038) doi: 10.1080/2162402X.2018.1534038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Wang X, Xu R, Ji J, Xu Y, Han M, Wei Y, Huang B, Chen A, Zhang Q, et al. YM155 decreases radiation-induced invasion and reverses epithelial-mesenchymal transition by targeting STAT3 in glioblastoma. J Transl Med. 2018;16(79) doi: 10.1186/s12967-018-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19 (Suppl 5):v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann DF, Jenn J, Corradini S, Ruf V, Herms J, Forbrig R, Unterrainer M, Thon N, Kreth FW, Belka C, Niyazi M. Bevacizumab reduces toxicity of reirradiation in recurrent high-grade glioma. Radiother Oncol. 2019;138:99–105. doi: 10.1016/j.radonc.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Lara-Velazquez M, Al-Kharboosh R, Jeanneret S, Vazquez-Ramos C, Mahato D, Tavanaiepour D, Rahmathulla G, Quinones-Hinojosa A. Advances in brain tumor surgery for glioblastoma in adults. Brain Sci. 2017;7(166) doi: 10.3390/brainsci7120166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaspar LE, Fisher BJ, Macdonald DR, LeBer DV, Halperin EC, Schold SC Jr, Cairncross JG. Supratentorial malignant glioma: Patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24:55–57. doi: 10.1016/0360-3016(92)91021-e. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Ding K, Wang J, Li X, Zhao P. Chemoresistance caused by the microenvironment of glioblastoma and the corresponding solutions. Biomed Pharmacother. 2019;109:39–46. doi: 10.1016/j.biopha.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Wang X, Chen X, Zhang Q, Hong J. Novel immune-related gene signature for risk stratification and prognosis of survival in lower-grade glioma. Front Genet. 2020;11(363) doi: 10.3389/fgene.2020.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Au-Yeung CL, Yeung TL, Achreja A, Zhao H, Yip KP, Kwan SY, Onstad M, Sheng J, Zhu Y, Baluya DL, et al. ITLN1 modulates invasive potential and metabolic reprogramming of ovarian cancer cells in omental microenvironment. Nat Commun. 2020;11(3546) doi: 10.1038/s41467-020-17383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erin N, Grahovac J, Brozovic A, Efferth T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist Updat. 2020;53(100715) doi: 10.1016/j.drup.2020.100715. [DOI] [PubMed] [Google Scholar]
- 13.Uttam S, Stern AM, Sevinsky CJ, Furman S, Pullara F, Spagnolo D, Nguyen L, Gough A, Ginty F, Lansing Taylor D, Chakra Chennubhotla S. Spatial domain analysis predicts risk of colorectal cancer recurrence and infers associated tumor microenvironment networks. Nat Commun. 2020;11(3515) doi: 10.1038/s41467-020-17083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W, Yang S, Zhang F, Cheng F, Wang X, Rao J. Influence of the Hippo-YAP signalling pathway on tumor associated macrophages (TAMs) and its implications on cancer immunosuppressive microenvironment. Ann Transl Med. 2020;8(399) doi: 10.21037/atm.2020.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiffer D, Annovazzi L, Casalone C, Corona C, Mellai M. Glioblastoma: Microenvironment and Niche Concept. Cancers (Basel) 2018;11(5) doi: 10.3390/cancers11010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao Q, Vadgama JV, Wang P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun Signal. 2020;18(82) doi: 10.1186/s12964-020-00589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desbaillets I, Tada M, de Tribolet N, Diserens AC, Hamou MF, Van Meir EG. Human astrocytomas and glioblastomas express monocyte chemoattractant protein-1 (MCP-1) in vivo and in vitro. Int J Cancer. 1994;58:240–247. doi: 10.1002/ijc.2910580216. [DOI] [PubMed] [Google Scholar]
- 18.Nalla AK, Gogineni VR, Gupta R, Dinh DH, Rao JS. Suppression of uPA and uPAR blocks radiation-induced MCP-1 mediated recruitment of endothelial cells in meningioma. Cell Signal. 2011;23:1299–1310. doi: 10.1016/j.cellsig.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer S, Weishaupt A, Troppmair J, Martini R. Increase of MCP-1 (CCL2) in myelin mutant Schwann cells is mediated by MEK-ERK signaling pathway. Glia. 2008;56:836–843. doi: 10.1002/glia.20657. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura T. The production of monocyte chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments. Cytokine. 2017;98:71–78. doi: 10.1016/j.cyto.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Vakilian A, Khorramdelazad H, Heidari P, Sheikh Rezaei Z, Hassanshahi G. CCL2/CCR2 signaling pathway in glioblastoma multiforme. Neurochem Int. 2017;103:1–7. doi: 10.1016/j.neuint.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 22.He M, Yu W, Chang C, Miyamoto H, Liu X, Jiang K, Yeh S. Estrogen receptor α promotes lung cancer cell invasion via increase of and cross-talk with infiltrated macrophages through the CCL2/CCR2/MMP9 and CXCL12/CXCR4 signaling pathways. Mol Oncol. 2020;14:1779–1799. doi: 10.1002/1878-0261.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubos N, van der Gaag S, Gercek M, Kant S, Leube RE, Krusche CA. Inflammation shapes pathogenesis of murine arrhythmogenic cardiomyopathy. Basic Res Cardiol. 2020;115(42) doi: 10.1007/s00395-020-0803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong X, Duan Y, Zheng J, Ye Z, Hei TK. Tetramethylpyrazine prevents contrast-induced nephropathy via modulating tubular cell mitophagy and suppressing mitochondrial fragmentation, CCL2/CCR2-mediated inflammation, and intestinal injury. Oxid Med Cell Longev. 2019;2019(7096912) doi: 10.1155/2019/7096912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou W, Jiang Z, Xu YY, Li XG. Attention to normal brain volumes in glioblastoma radiotherapy: Potential role in tumor invasion and vasculogenesis. Med Hypotheses. 2013;80:501–504. doi: 10.1016/j.mehy.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Mistry AM, Mummareddy N, Salwi S, Davis LT, Ihrie RA. Glioblastoma distance from the subventricular neural stem cell niche does not correlate with survival. Front Oncol. 2020;10(564889) doi: 10.3389/fonc.2020.564889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Li Y, Li Z, Huang C, Yang Y, Lang M, Cao J, Jiang W, Xu Y, Dong J, Ren H. Hypoxia inducible factor 1 (HIF-1) recruits macrophage to activate pancreatic stellate cells in pancreatic ductal adenocarcinoma. Int J Mol Sci. 2016;17(799) doi: 10.3390/ijms17060799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Wang J, Li X, Wang D. Lysosomes contribute to radioresistance in cancer. Cancer Lett. 2018;439:39–46. doi: 10.1016/j.canlet.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Cui YH, Suh Y, Lee HJ, Yoo KC, Uddin N, Jeong YJ, Lee JS, Hwang SG, Nam SY, Kim MJ, Lee SJ. Radiation promotes invasiveness of non-small-cell lung cancer cells through granulocyte-colony-stimulating factor. Oncogene. 2015;34:5372–5382. doi: 10.1038/onc.2014.466. [DOI] [PubMed] [Google Scholar]
- 30.Morganti JM, Jopson TD, Liu S, Gupta N, Rosi S. Cranial irradiation alters the brain's microenvironment and permits CCR2+ macrophage infiltration. PLoS One. 2014;9(e93650) doi: 10.1371/journal.pone.0093650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherry JD, Meng G, Daley S, Xia W, Svirsky S, Alvarez VE, Nicks R, Pothast M, Kelley H, Huber B, et al. CCL2 is associated with microglia and macrophage recruitment in chronic traumatic encephalopathy. J Neuroinflammation. 2020;17(370) doi: 10.1186/s12974-020-02036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimura T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell Mol Immunol. 2018;15:335–345. doi: 10.1038/cmi.2017.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z, Feng X, Herting CJ, Garcia VA, Nie K, Pong WW, Rasmussen R, Dwivedi B, Seby S, Wolf SA, et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017;77:2266–2278. doi: 10.1158/0008-5472.CAN-16-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He S, He S, Chen CH, Deborde S, Bakst RL, Chernichenko N, McNamara WF, Lee SY, Barajas F, Yu Z, et al. The chemokine (CCL2-CCR2) signaling axis mediates perineural invasion. Mol Cancer Res. 2015;13:380–390. doi: 10.1158/1541-7786.MCR-14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu X, Fujita M, Snyder LA, Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neurooncol. 2011;104:83–92. doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Alessio A, Proietti G, Sica G, Scicchitano BM. Pathological and molecular features of glioblastoma and its peritumoral tissue. Cancers (Basel) 2019;11(469) doi: 10.3390/cancers11040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seifert M, Schackert G, Temme A, Schröck E, Deutsch A, Klink B. Molecular characterization of astrocytoma progression towards secondary glioblastomas utilizing patient-matched tumor pairs. Cancers (Basel) 2020;12(1696) doi: 10.3390/cancers12061696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129:829–848. doi: 10.1007/s00401-015-1432-1. [DOI] [PubMed] [Google Scholar]
- 39.Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, Ben-Josef E, Beatty GL. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23:137–148. doi: 10.1158/1078-0432.CCR-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas JG, Parker Kerrigan BC, Hossain A, Gumin J, Shinojima N, Nwajei F, Ezhilarasan R, Love P, Sulman EP, Lang FF. Ionizing radiation augments glioma tropism of mesenchymal stem cells. J Neurosurg. 2018;128:287–295. doi: 10.3171/2016.9.JNS16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, Singh R, Schall TJ, Datta M, Jain RK, et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci USA. 2020;117:1129–1138. doi: 10.1073/pnas.1910856117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.