Abstract
Gold nanoparticles (AuNPs) are biocompatible nanomaterials with potential application in the food industry. The safety of AuNPs oral consumption remains inconclusive, and information on possible long-term toxicity is limited. The current study aimed to evaluate the subchronic oral toxicity of AuNPs in male and female Institute of Cancer Research (ICR) mice. Citrate-coated spherical AuNPs with 53 nm diameters were prepared and orally administered to the mice. No mortality or clinical abnormalities were observed following daily administration of AuNPs at the dosages of 0.2, 2, and 20 mg/kg for 90 days. There was no significant difference in body weight or the relative organs' weights between the control and AuNPs-treated mice. No gross abnormalities or histopathological changes were observed except that the male mice treated with high dose (20 mg/kg AuNPs) showed minor infiltration in the kidneys, and female mice showed a reduced A/G ratio and elevated platelet indices. Overall, the 90-day long-term oral consumption of AuNPs did not cause significant toxicity in mice.
Keywords: Gold, Nanoparticle, Oral, Chronic toxicity, Tissue histopathology
Gold; Nanoparticle; Oral; Chronic toxicity; Tissue histopathology
1. Introduction
Gold compounds have been used in medicine for decades. Gold nanoparticles (AuNPs) are more biologically active than bulk gold owing to their small size and large surface area. AuNPs are advantageous because of their facile synthesis, chemical stability, easy surface modification, and distinct optical properties. Capping agents, such as citric acid, are often added during the synthetic process to help stabilize colloidal AuNPs solutions [1]. AuNPs are considered a biocompatible reagent for biomedical applications, such as drug delivery, cancer treatment, biosensors, photothermal therapy, and imaging technologies [2, 3]. However, contact of AuNPs with biological fluids may induce irreversible aggregation [4]. Long-term colloidal stability also needs to be considered in the application of AuNPs.
The application of AuNPs in the food industry is also increasing, particularly regarding storage and food safety. It has been documented that AuNPs are used in advanced food analysis to detect food contaminants [5]. AuNPs-conjugated DNA, enzymes, or antibodies are used in biosensors for the detection of foodborne pathogens, serving as potential tools for food safety evaluation [6](Kumar et al., 2020). Moreover, AuNPs have attracted attention owing to their application in the development of antimicrobial agents for protection and preservation in food packaging [7].
Currently, regulations on the use of AuNPs in the medical and food industry are limited. As the information of toxicological studies including biodistribution and metabolism of nanosized particles are insufficient, the safe dose of gold nanoparticles for humans are not yet established by European Food Safety Authority (EFSA) or US Environmental Protection Agency (EPA). Although AuNPs are considered to be relatively safe, toxicity is still a concern because of their wide applications. A number of in vivo studies have been conducted to evaluate the potential toxic effects of AuNPs; however, the results remain inconclusive [8, 9]. The size, shape, surface chemistry, stabilization coatings, as well as the administration (dosage, duration, route of administration, etc.) may contribute to the different in vivo toxic effects of AuNPs [9]. Among them, only one long-term chronic study reported a toxic effect of AuNPs. Although i.p. injection of 1 and 2 mg/kg AuNPs (50 nm) for 90 days showed high toxicity and organ damage [10], information on the 90-day oral toxicity of AuNPs in both male and female mice is not available. Even though the current application of AuNPs in the food industry is relatively less than that in biology and medicine, their long-term oral safety is an important concern for consumers when utilized as food ingredients or food-packaging materials. Thus, the present study aimed to evaluate the possible toxicity in male and female ICR mice after oral administration of AuNPs at different dosages for 90 days. This chronic oral safety evaluation of AuNPs is essential for future application in the medical and food industries.
2. Materials and methods
2.1. Preparation and characterization of colloidal AuNPs
The AuNPs colloidal solution, supplied by the Tripod Nano Technology Corporation (Taoyuan City, Taiwan), was synthesized according to a patented method [11]. In brief, HAuCl4 aqueous solution (0.25 mL of 0.2 M aqueous solution, 0.05 mmol) and citric acid (40.3 mg, 0.21 mmol) were placed in a 100 mL reaction flask. The solution was heated at 150 °C for 12 min to complete the reduction of HAuCl4. An appropriate medium (e.g. 50 mL pure water) was added to disperse the resulting AuNPs, and the solution was heated at 70 °C for 10 min to obtain colloidal AuNPs.
The physicochemical properties of the synthesized AuNPs were fully characterized by ultraviolet-visible (UV-vis) spectroscopy, infrared spectroscopy, inductively coupled plasma atomic emission spectroscopy, transmission electron microscopy (TEM), dynamic light scattering (DLS) analysis, and potentiometric titration. The transformation in the surface plasmonic resonance of AuNPs was measured by UV-vis spectroscopy on an Agilent Technologies Cary60 UV-vis spectrophotometer operating at a resolution of 2 nm. The UV-vis spectrum (200–800 nm) of AuNPs colloidal solution (0.2 mM) in a quartz cuvette with 1.0 cm path length was recorded at room temperature. Infrared spectra were recorded on an Agilent Technologies Cary630 FT-IR spectrometer. TEM studies of AuNPs were performed on an FEI Tecnai™ G2 F-20 S-TWIN operating at an accelerating voltage of 200 kV. The sample (20 μL) was dropped onto a 200-mesh copper-coated grid for 10 min, and the excess sample was wiped off using a filter paper. Samples were given 30 min to dry completely prior to visualization with TEM. The particle size and zeta potential of the produced AuNPs were determined using a Zetasizer 300 HAS (Malvern Instruments, Malvern, U.K.) according to the method of photon correlation spectroscopy. All the analyses were carried out with triplicate measurements for a single sample during the 60 s time duration. For zeta potential, all studies were measured without dilution of the nanoparticulate dispersion. The same samples prepared for the DLS analysis were injected into a prerinsed folded capillary cell for the zeta potential measurements at an applied voltage of 100 V.
2.2. Subchronic toxicity study
Totally 40 male and 40 female ICR mice of five weeks old (25–28 g) were obtained from BioLASCO Experimental Animal Center (Taipei, Taiwan). All animals were held in plastic cages with stainless steel wire-bar lid and kept under standard environmental conditions (23 ± 1 °C, 50–60% relative humidity, and 12 h light/12 h dark cycle) with free access to water and commercial mouse feed (MF-18). All animals were acclimatized to this environment for at least one week before the subchronic toxicity test. The experimental protocols for this study were approved by the National Kaohsiung University of Science and Technology Laboratory Animal Care and Use Committee (No. 0107-AAAP-008).
The subchronic toxicity study was performed in accordance with the Safety Assessment Guidelines, approved by the Ministry of Health and Welfare (Taiwan, 1999) and the OECD Guidelines 408 (OECD, 2018) for the Testing of Chemicals. Mice were randomly divided into four groups of ten males and ten females each. Different dosages of AuNPs were prepared in ddH2O. Mice in the low dose (LD), medium dose (MD), and high dose (HD) group received daily oral administration of 0.2 mg/kg, 2 mg/kg, and 20 mg/kg colloidal AuNPs, respectively, while the mice in the control group received ddH2O. The clinical signs of toxicity and mortality including convulsion, tremor, vocalization, diarrhea, piloerection, salivation, lacrimation, changes in skin and fur, dyspnea, lethargy and death were monitored at least once per day. Body weight and food consumption were measured and recorded weekly throughout the experiment. The livers, spleens, kidneys, lungs, brains and hearts were collected, weighed, and subjected to detailed gross necropsy.
2.3. Hematological analysis
Whole blood samples (10 μL) were subjected to measure the hematological parameters by an automatic blood cell analyzer (Microsemi LC-662 G, HORIBA), including white blood cells (WBC), red blood cells (RBC), hemoglobin (Hgb), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet count (PLT), mean platelet volume (MPV), platelet volume ratio, plateletcrit value (PCT), and platelet distribution width (PDW).
2.4. Biochemical analysis
Blood samples were collected centrifuged at 705× g for 10 min at 4 °C to separate the serum. The serum biochemical parameters including glutamic oxaloacetic transaminase (GOT), glutamyl pyruvic transaminase (GPT), alkaline phosphatase (ALP), total protein (TP), albumin, globulin, albumin/globulin ratio (A/G Ratio), total bilirubin (T-BIL), glucose, calcium (Ca), blood urea nitrogen (BUN), and creatinine (CREA) were analyzed by an automatic biochemical analyzer (FUJI DRI-CHEM 4000i).
2.5. Histopathological examination
The liver and kidney were fixed in 10% neutral formalin for further histopathological examination. The formalin-fixed tissue was dehydrated in a graded series of ethanol (70%, 80%, 90%, 95% and 100%), embedded in paraffin, and sectioned into 5 μm-thick sections. The sections were stained with hematoxylin and eosin (H&E) and examined under a light microscope (Olympus, Tokyo, Japan).
2.6. Sirius Red staining
The paraffin-embedded liver and kidney sections were subjected to deparaffinize and rehydrate with a graded series ethanol (100%, 95% and 80%), and then the sections were incubated with Sirius Red solution for 1 h. After washed, the sections were counterstained with Fast Green solution for 5 min. Sections were rinsed with water and rehydrated by grade series of ethanol (80%, 90% and 100%), mounted with a resinous medium and examined under a light microscope (Olympus, Tokyo, Japan).
2.7. Statistical analysis
Data are presented as mean ± SEM. Statistically significant differences (p < 0.05) among the various groups were evaluated using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. All statistical analyses were performed using SAS v 9.1 software.
3. Results
3.1. Physical characterization of colloidal AuNPs
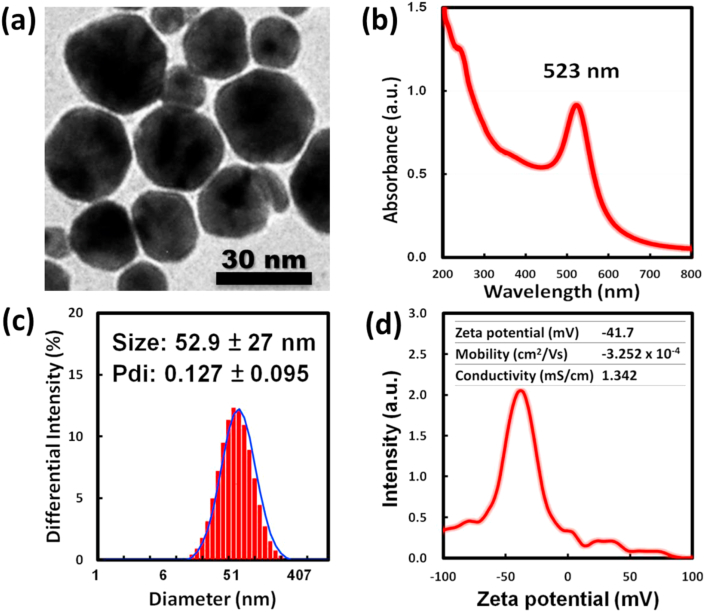
TEM analysis was used to investigate the morphology of the synthesized colloidal AuNPs (Figure 1a). The UV-vis absorption spectrum of the colloidal AuNPs solution exhibited a high intense absorption band at λmax = 523 nm (Figure 1b), which was consistent with the surface plasmonic resonance of AuNPs of ~20 nm diameter. The TEM images revealed that the AuNPs were highly dispersed spheres with a core size of 25.4 ± 6.1 nm. The DLS analysis indicated that the citrate-encapsulated AuNPs had a mean diameter of 52.9 ± 27 nm with a narrow size distribution (Figure 1c). The colloidal AuNPs exhibited distinct negative surface charges with a zeta potential of −41.7 mV at pH 2.5 (Figure 1d) and electrical conductivity of 1.342 mS/cm. The high zeta potential renders the high stability of the colloidal AuNPs to avoid severe aggregation.
Figure 1.
Characterization of colloidal gold nanoparticles. (a) TEM image shows the AuNPs of spheric shape with a core size of 25.4 ± 6.1 nm, (b) The colloidal AuNPs solution has an absorption band at λmax = 523 nm in the UV-vis spectrum, (c) The citrate-encapsulated AuNPs have a mean diameter of 52.9 ± 27 nm in DLS analysis, and (d) The colloidal AuNPs solution shows zeta potential of −41.7 mV at pH 2.5 and electrical conductivity of 1.342 mS/cm.
3.2. Mortality and clinical observations
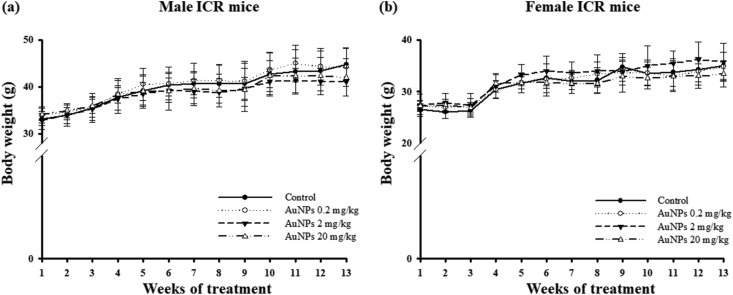
There were no cases of mortality or abnormal clinical signs or behavioral changes, such as skin and fur conditions, changes in the eyes, diarrhea, abdominal breathing, lethargy, and tremor, observed in both sexes of mice throughout the experimental period of AuNPs treatment. No significant differences in mean body weight and weight gain were found between the male and female mice among the groups (Figure 2 and Table 1). Mean food consumption was not significantly different between the AuNPs-treated and control mice during the experimental period (data not shown).
Figure 2.
Mean body weight of (a) male and (b) female ICR mice orally treatment with AuNPs for 90 days. No significant change of weights on the AuNPs treatment.
Table 1.
Body weight gain and relative organ weight of male and female ICR mice after 90-day oral administration of AuNPs.
| Gender | Control | AuNPs |
F value | P value | Degree of freedom | |||
|---|---|---|---|---|---|---|---|---|
| 0.2 mg/kg | 2 mg/kg | 20 mg/kg | ||||||
| Body weight | ||||||||
| Initial body weight (g) | Male | 33.1 ± 1.3a | 34.0 ± 1.7a | 32.8 ± 1.9a | 34.1 ± 1.3a | 1.42 | 0.25 | 37 |
| Female | 26.5 ± 1.0a | 27.3 ± 1.0a | 27.3 ± 2.2a | 27.2 ± 1.0a | 0.69 | 0.56 | 39 | |
| Final body weight (g) | Male | 44.8 ± 3.4a | 44.2 ± 4.1a | 41.1 ± 3.0a | 42.0 ± 4.0a | 2.15 | 0.11 | 37 |
| Female | 35.0 ± 2.6a | 34.8 ± 2.8a | 35.7 ± 3.6a | 33.4 ± 2.5a | 1.08 | 0.37 | 39 | |
| Weight gain (g) |
Male | 11.2 ± 0.6a | 11.1 ± 1.6a | 10.1 ± 1.7a | 10.5 ± 1.9a | 3.10 | 0.04 | 37 |
| Female |
8.5 ± 2.7a |
7.5 ± 3.0a |
8.4 ± 3.1a |
6.2 ± 2.2a |
1.40 |
0.26 |
39 |
|
| Relative organ weight | ||||||||
| Liver (%) | Male | 5.05 ± 0.35a | 4.96 ± 0.37a | 5.09 ± 0.24a | 5.10 ± 1.04a | 0.08 | 0.97 | 36 |
| Female | 4.54 ± 0.54ab | 4.98 ± 0.57a | 4.46 ± 0.45b | 4.79 ± 0.24ab | 2.56 | 0.07 | 39 | |
| Kidney (%) | Male | 1.67 ± 0.11a | 1.81 ± 0.34a | 1.79 ± 0.18a | 1.67 ± 0.14a | 1.18 | 0.33 | 36 |
| Female | 1.36 ± 0.09b | 1.52 ± 0.20a | 1.44 ± 0.18ab | 1.48 ± 0.14ab | 1.98 | 0.13 | 39 | |
| Spleen (%) | Male | 0.22 ± 0.02a | 0.26 ± 0.04a | 0.28 ± 0.06a | 0.36 ± 0.38a | 0.91 | 0.45 | 36 |
| Female | 0.36 ± 0.08ab | 0.41 ± 0.09a | 0.34 ± 0.06b | 0.34 ± 0.06ab | 2.09 | 0.12 | 39 | |
Data were presented as the mean ± SEM (n = 10 per group). Mean values within each column with different labels are significantly different (p < 0.05) according to one-way ANOVA followed by Tukey's post hoc test. The relative organ weight was expressed as a percentage of body weight.
3.3. Hematological and biochemical parameters
The results of the hematological parameters of AuNPs-treated and control mice are summarized in Table 2. All hematological parameters for male and female mice in the AuNPs -treated groups were in the normal range compared to that of the control group, except for the platelet indices of female mice at the highest dose of AuNPs. The PLT, MPV, PCT, and PDW were significantly higher in female mice administered 20 mg/kg AuNPs than mice in the control group. The significant platelet indices in female mice were considered to be due to differences in sex. Although some of those increased hematological parameters were in reference values [12], oral administration of a high dosage of AuNPs may affect platelet and coagulation function in female mice.
Table 2.
Effect of 90 days oral administration of AuNPs on haematological parameters in mice.
| Parameter | Male |
F value | P value | Degree of freedom | |||
|---|---|---|---|---|---|---|---|
| Control | AuNPs 0.2 mg/kg | AuNPs 2 mg/kg | AuNPs 20 mg/kg | ||||
| WBC (103/μL) | 13.02 ± 5.10a | 12.89 ± 8.20a | 13.13 ± 6.03a | 15.77 ± 10.88a | 0.31 | 0.82 | 38 |
| RBC (106/μL) | 8.33 ± 0.39a | 8.03 ± 0.45b | 8.50 ± 0.22a | 8.09 ± 0.54a | 2.65 | 0.07 | 35 |
| Hgb (g/dL) | 13.66 ± 0.79a | 12.97 ± 0.72a | 13.50 ± 0.58a | 12.91 ± 1.27a | 1.78 | 0.17 | 38 |
| Hct (%) | 42.22 ± 3.20a | 39.90 ± 2.70a | 41.57 ± 1.58a | 40.11 ± 1.63a | 2.11 | 0.12 | 37 |
| MCV (μm3) | 48.76 ± 2.23a | 49.67 ± 1.77a | 48.91 ± 2.60a | 47.33 ± 3.89a | 1.20 | 0.32 | 38 |
| MCH (pg) | 15.82 ± 0.99a | 16.17 ± 0.61a | 15.90 ± 0.87a | 15.80 ± 1.05a | 0.32 | 0.81 | 38 |
| MCHC (g/dL) | 32.93 ± 0.51a | 32.91 ± 0.21a | 32.73 ± 0.47a | 33.17 ± 0.51a | 1.25 | 0.31 | 28 |
| RDW (%) | 12.69 ± 0.70a | 12.73 ± 0.69a | 12.77 ± 0.72a | 12.91 ± 0.68a | 0.19 | 0.90 | 38 |
| PLT (103/μL) | 1225.60 ± 135.54a | 1158.60 ± 138.22a | 1245.80 ± 71.89aa | 1186.50 ± 69.11a | 0.68 | 0.58 | 20 |
| MPV (μm3) | 7.08 ± 1.58a | 6.50 ± 0.85a | 6.34 ± 0.64a | 6.54 ± 1.77a | 0.60 | 0.62 | 38 |
| PCT (%) | 0.52 ± 0.24a | 0.55 ± 0.16a | 0.67 ± 0.20a | 0.67 ± 0.18a | 1.47 | 0.24 | 37 |
| PDW (%) |
9.65 ± 1.04a |
10.19 ± 0.96a |
9.70 ± 0.72a |
10.01 ± 0.94a |
0.42 |
0.74 |
34 |
| Parameter |
Female | F value |
P value |
Degree of freedom |
|||
| Control |
AuNPs 0.2 mg/kg |
AuNPs 2 mg/kg |
AuNPs 20 mg/kg |
||||
| WBC (103/μL) | 11.48 ± 2.46a | 9.74 ± 3.25a | 11.00 ± 2.62a | 9.97 ± 2.31a | 0.95 | 0.43 | 39 |
| RBC (106/μL) | 8.38 ± 0.49a | 8.20 ± 0.47ab | 8.33 ± 0.43a | 8.24 ± 0.69ab | 0.20 | 0.90 | 33 |
| Hgb (g/dL) | 13.79 ± 0.92a | 13.16 ± 0.65a | 13.47 ± 0.57a | 13.30 ± 0.68a | 1.30 | 0.29 | 37 |
| Hct (%) | 41.13 ± 2.84a | 40.08 ± 1.81a | 40.69 ± 2.11a | 41.19 ± 2.20a | 0.41 | 0.75 | 32 |
| MCV (μm3) | 49.86 ± 2.50a | 49.08 ± 2.78a | 48.89 ± 1.94a | 47.48 ± 2.38a | 1.68 | 0.19 | 39 |
| MCH (pg) | 16.46 ± 0.77a | 16.54 ± 0.81a | 16.17 ± 0.50a | 16.34 ± 0.39a | 0.59 | 0.63 | 35 |
| MCHC (g/dL) | 33.04 ± 0.54b | 33.35 ± 0.52b | 33.15 ± 0.73b | 33.24 ± 0.92b | 0.36 | 0.78 | 39 |
| RDW (%) | 12.98 ± 0.75a | 13.14 ± 0.49a | 12.78 ± 0.84a | 13.20 ± 0.74a | 0.69 | 0.56 | 39 |
| PLT (103/μL) | 1089.25 ± 102.89b | 1061.43 ± 99.66b | 1014.14 ± 116.83b | 1240.00 ± 78.15a | 6.61 | 0.002 | 28 |
| MPV (μm3) | 6.03 ± 0.25b | 6.47 ± 0.53ab | 6.29 ± 0.49b | 6.83 ± 0.53a | 5.19 | 0.004 | 39 |
| PCT (%) | 0.70 ± 0.09b | 0.63 ± 0.11b | 0.67 ± 0.10b | 0.79 ± 0.12a | 2.92 | 0.05 | 31 |
| PDW (%) | 9.78 ± 0.42ab | 9.26 ± 0.85b | 9.56 ± 0.76b | 10.41 ± 0.90a | 3.44 | 0.03 | 33 |
Data were presented as the mean ± SEM (n = 10 per group). Mean values within each column with different labels are significantly different (p < 0.05) according to one-way ANOVA followed by Tukey's post hoc test.
Table 3 shows the results of biochemical parameters in AuNPs-treated male and female mice. A slight and significant decrease in serum calcium was found in female mice administered AuNPs at 2 and 20 mg/kg, respectively, while the values were in the normal range [12]. Serum levels of TP and albumin were increased in male mice after oral treatment with AuNPs, but without a dose-dependent effect; therefore, they were not considered toxic responses. The A/G ratio was not different among the groups in male mice. A significant decrease in TP and albumin followed by increased globulin was found in female mice orally treated with AuNPs at 20 mg/kg; however, the decreased values of TP and albumin were in physiological range as referenced animals [12]. The A/G ratio also significantly decreased in the same group. The decrease in A/G ratio may due to an effect that responding to elevated globulin. GOT value seems to be higher in male and female mice treatment with three dosages of AuNPs but no statistically significant difference was found when compared to control group. Significantly elevated serum GPT and ALP levels were found in male mice administered AuNPs at dosages of 0.2 and/or 2 mg/kg but not 20 mg/kg. The increased serum GPT and ALP levels indicated damage to liver function, while no histopathological change occurred in all AuNPs-administered male mice (see Figure 3). The levels of serum GTP, ALP, and T-BIL were not different in all AuNPs-treated female mice. A slight and significantly increased and decreased BUN level was found in male and female mice orally with 2 mg/kg of AuNPs, respectively. However, a dose-dependent effect was not observed. No significant difference in serum CREA was found in either male or female mice among the groups.
Table 3.
Effect of 90 days oral administration of AuNPs on biochemical parameters in mice.
| Parameter | Male |
F value | P value | Degree of freedom | |||
|---|---|---|---|---|---|---|---|
| Control | AuNPs 0.2 mg/kg | AuNPs 2 mg/kg | AuNPs 20 mg/kg | ||||
| Ca (mg/dL) | 13.60 ± 4.20a | 15.11 ± 1.76a | 15.70 ± 2.16a | 13.20 ± 1.40a | 2.03 | 0.13 | 38 |
| Glucose (mg/dL) | 270.00 ± 88.96a | 265.71 ± 49.95a | 337.78 ± 58.48a | 326.25 ± 80.52a | 2.86 | 0.05 | 35 |
| TP (g/dL) | 5.40 ± 0.70b | 7.00 ± 1.12a | 7.10 ± 0.99a | 6.30 ± 0.67a | 7.75 | 0.0004 | 28 |
| Albumin (g/dL) | 2.90 ± 0.74b | 3.78 ± 0.97a | 4.00 ± 0.82a | 3.59 ± 0.86ab | 3.12 | 0.04 | 38 |
| Globulin (g/dL) | 2.50 ± 1.18a | 3.22 ± 0.97a | 3.10 ± 0.57a | 2.71 ± 0.68a | 1.41 | 0.26 | 38 |
| A/G Ratio | 1.51 ± 1.02a | 1.30 ± 0.56a | 1.34 ± 0.37a | 1.45 ± 0.63a | 0.19 | 0.90 | 38 |
| GOT (U/L) | 90.00 ± 37.80a | 141.76 ± 76.27a | 142.50 ± 32.84a | 111.43 ± 68.42a | 1.63 | 0.21 | 28 |
| GPT (U/L) | 38.00 ± 7.89b | 56.67 ± 10.00a | 48.00 ± 14.76ab | 44.00 ± 18.97ab | 2.70 | 0.06 | 38 |
| ALP (U/L) | 41.00 ± 19.69b | 62.22 ± 14.81a | 66.00 ± 17.13a | 53.00 ± 17.67ab | 4.01 | 0.01 | 38 |
| T-BIL (mg/dL) | 0.16 ± 0.10a | 0.21 ± 0.12a | 0.20 ± 0.08a | 0.20 ± 0.10a | 0.46 | 0.71 | 38 |
| BUN (mg/dL) | 24.00 ± 3.59b | 26.33 ± 4.50ab | 27.70 ± 3.65a | 24.30 ± 3.02ab | 2.22 | 0.10 | 38 |
| CREA (mg/dL) |
0.23 ± 0.01a |
0.23 ± 0.02a |
0.23 ± 0.02a |
0.23 ± 0.01a |
0.42 |
0.74 |
38 |
| Parameter |
Female | F value |
P value |
Degree of freedom |
|||
| Control |
AuNPs 0.2 mg/kg |
AuNPs 2 mg/kg |
AuNPs 20 mg/kg |
||||
| Ca (mg/dL) | 12.07 ± 1.38ab | 12.80 ± 1.55a | 11.10 ± 1.85b | 11.00 ± 1.56b | 2.87 | 0.05 | 39 |
| Glucose (mg/dL) | 270.89 ± 60.39a | 294.44 ± 38.77a | 301.11 ± 47.55a | 264.00 ± 79.17a | 0.19 | 0.90 | 39 |
| TP (g/dL) | 5.52 ± 0.51a | 6.00 ± 0.67a | 5.70 ± 0.95a | 4.80 ± 0.92b | 4.25 | 0.01 | 39 |
| Albumin (g/dL) | 3.84 ± 0.59a | 3.90 ± 0.74a | 3.60 ± 0.70a | 2.30 ± 0.78b | 11.32 | 0.00002 | 39 |
| Globulin (g/dL) | 1.68 ± 0.47b | 2.10 ± 0.32ab | 2.10 ± 0.88ab | 2.50 ± 0.78a | 2.63 | 0.07 | 39 |
| A/G Ratio | 2.59 ± 1.25a | 1.90 ± 0.46a | 2.13 ± 1.18a | 1.02 ± 0.49b | 5.11 | 0.004 | 39 |
| GOT (U/L) | 115.50 ± 45.01a | 181.43 ± 92.09a | 121.25 ± 47.64a | 152.50 ± 79.42a | 1.33 | 0.29 | 28 |
| GPT (U/L) | 41.40 ± 11.63a | 45.00 ± 11.79a | 44.00 ± 8.43a | 48.00 ± 9.19a | 0.69 | 0.56 | 39 |
| ALP (U/L) | 90.20 ± 24.41a | 84.44 ± 33.58a | 68.89 ± 29.34a | 66.67 ± 25.00a | 1.58 | 0.21 | 36 |
| T-BIL (mg/dL) | 0.20 ± 0.17a | 0.19 ± 0.09a | 0.14 ± 0.05a | 0.19 ± 0.08a | 0.79 | 0.51 | 39 |
| BUN (mg/dL) | 21.97 ± 4.27ab | 23.80 ± 3.79a | 19.50 ± 3.34b | 24.10 ± 2.73a | 3.5 | 0.03 | 39 |
| CREA (mg/dL) | 0.25 ± 0.04a | 0.24 ± 0.01a | 0.24 ± 0.02a | 0.26 ± 0.01a | 1.12 | 0.35 | 39 |
Data were presented as the mean ± SD (n = 10 per group). Mean values within each column with different labels are significantly different (p < 0.05) according to one-way ANOVA and followed by Tukey's post hoc test.
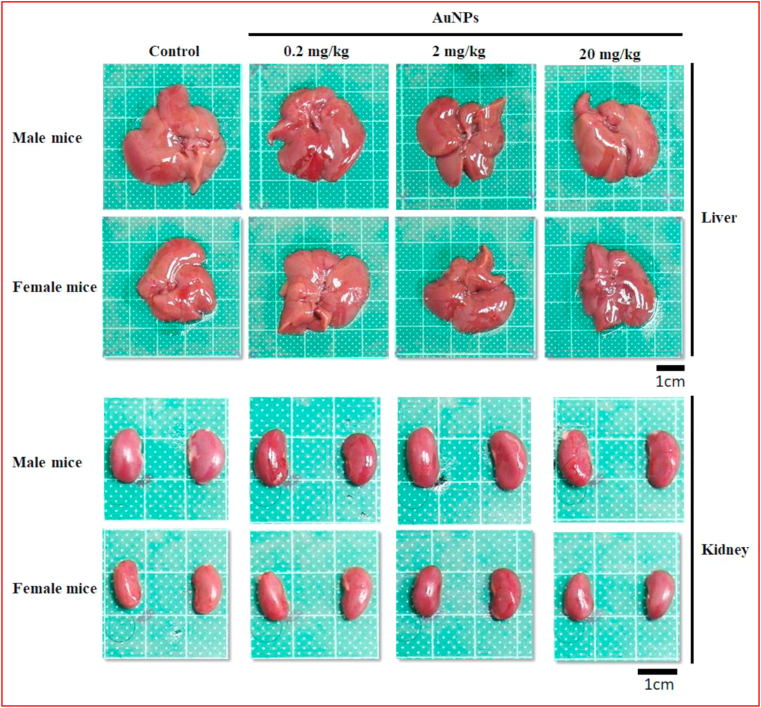
Figure 3.
Representative photographs of liver and kidney from male and female ICR mice after orally treatment with AuNPs for 90 days. No apparent morphological alteration on AuNPs treatment at the dosages of 0.2, 2 and 20 mg/kg body weight. Scale bar = 1 cm.
3.4. Gross pathology and histopathological examination
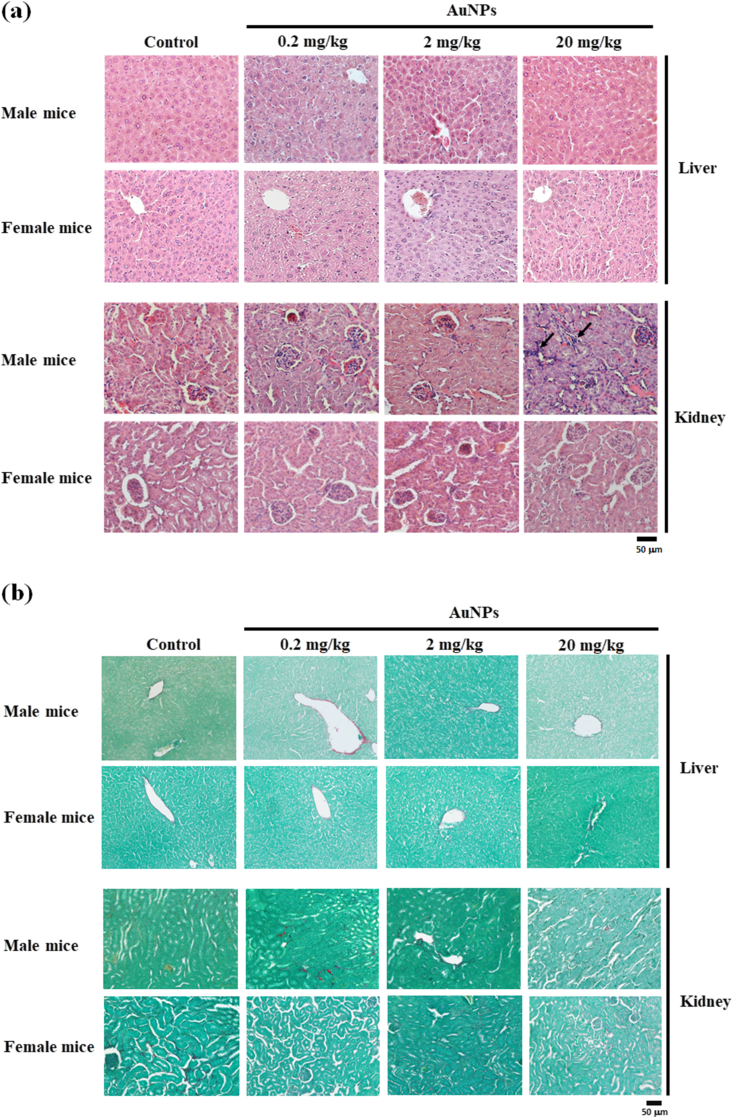
The relative weights of liver, kidney, and spleen of male and female mice are presented in Table 1. No significant differences were observed in the relative organ weights of both male and female mice in all AuNPs-treated groups compared with the control mice, except for the increased relative kidney weight in female mice by oral AuNPs at 0.2 mg/kg. Figure 3 shows the liver and kidney appearance of male and female mice. No morphological alterations occurred in the liver and kidney of both male and female mice by AuNPs treatment at all dosages when compared with mice in the control group. No grossly obvious lesions of other vital organs were identified in either male or female mice in any of the AuNPs-treated groups compared with the control group during necropsy (data not shown), indicating that AuNPs did not cause adverse effects in either male or female mice after 90 days of treatment. Both male and female mice treated with different dosages of AuNPs for 90 days showed no histopathological lesions in the liver (Figure 4a, upper). Similar results were observed in the kidney, while a slight infiltration of inflammatory cells occurred in male mice orally administered 20 mg/kg AuNPs (Figure 4a, lower). No other histopathological lesions were observed. In addition, the results of Sirius red staining showed AuNPs treatment at different dosages presented no obvious collagen fiber deposition in the hepatic and renal tissue in both male and female mice (Figure 4b).
Figure 4.
Effects of oral administration of AuNPs for 90 days on liver and renal histology in male and female mice. (a) H&E staining shows no histopathological lesion in liver and kidney, except for a slight inflammatory infiltration of the male mice kidney cells (indicated by black arrows) on treatment with high dosage of AuNPs. (b) Sirius red staining of hepatic and renal tissues shows no obvious interstitial fibrosis in male and female mice treatment with AuNPs. Scale bar = 50 μm.
4. Discussion
AuNPs are used in a broad range of applications including biological, pharmaceutical, and diagnostic techniques in recent years because of their unique physical and chemical properties. For example, AuNPs with surface modified by bioactive materials and small molecules are used as synthetic vaccines or for disease diagnosis by acting as sensing probes for detecting metal ions, biomolecules, bacterias, and cells [13]. In addition, AuNPs are used as drug carriers for the delivery of anti-cancer drugs and antibiotics [2]. The application of AuNPs is increasing in the food industry in recent years. AuNPs are acting as nanoprobes to detect toxins, food pathogens, and pesticides in foods as well as ensuring food quality and safety [14]. They are also used as an antimicrobial agent that applied in packaging materials for food protection and preservation. It is showed bioactive film using cereal starch incorporating AuNPs not only improves its mechanical property but also exhibits strong antibacterial activity against food-borne pathogens [15]. Another study demonstrated chlorhexidine-conjugated AuNPs displayed biofilm inhibitory effect against Klebsiella pneumonia [16]. Because of their unique properties and ease of synthesis, AuNPs nowadays are attractive for medicine, food industry, and even for healthcare applications. There are some AuNPs products that have been developed as commercial health and dietary supplements [17]; however, toxicological effect of AuNPs was observed in fibroblasts [18]. The potential toxicity of AuNPs remains an important concern to human health, even though Au is considered inert for biological systems. It is necessary to identify potential long-term toxic effects via oral administration for further application in food and medicine. Selection of AuNPs in appropriate shape, size and capping agent may influence the body uptake and tissue distribution. AuNPs in the range of 25–50 nm diameter have been found to exhibit the highest uptake, whereas particles >50 nm showed lower levels of uptake [19]. In this study, the AuNPs are prepared in spherical form. In general, spherical metal nanoparticles are non-toxic or less toxic than that in other shapes [20, 21]. We thus chose the 53 nm sized AuNPs of spherical shape for the current study.
The toxicity of NPs not only depends on their physiochemical properties but also the difference in experimental conditions in vivo [22, 23]. Most acute and subacute toxicity studies revealed a low or nontoxic effect of colloidal AuNPs, while others showed conflicting results [10, 24, 25]. It appears that AuNPs toxicity is not determined by single determinants but rather by multiple factors [8, 9]. For example, the size and surface area of NPs are believed to play important roles in the toxicity of living systems. The smaller size of AuNPs (<10 nm) more easily enters the cell nucleus and interacts with DNA, exhibiting a higher cytotoxicity than larger ones [26, 27]. Studies have shown widespread organ distribution in rodent administration of smaller AuNPs (5–15 nm) than that of larger AuNPs (50–100 nm), which indicates the potential in vivo toxicity for smaller AuNPs [24, 28, 29, 30]. Two in vivo studies have suggested that AuNPs toxicity is size-dependent. Smaller AuNPs (5 and 10 nm) were found to cause significant histopathological changes in the livers of mice, whereas larger particles (20 and 50 nm) showed minor effects [31, 32].
However, another study reported that i.p. injection of AuNPs (8–35 nm) showed severe sickness and high mortality, which was not observed with smaller (3–5 nm) and larger (50–100 nm) particles [24]. A similar result was found in the comparison of AuNPs toxicity studies by oral administration (Table 4). This information implies that the in vivo toxicity of AuNPs is not only size-dependent but also related to other factors such as surface coating, dosage, exposure route, duration, and species.
Table 4.
Comparison of animal studies of AuNPs toxicity.a
| Size (nm)b | Surface coating | Rodent | Administration | Dosage (mg/kg/day) | Duration (days) | Toxicityc | Reference | |
|---|---|---|---|---|---|---|---|---|
| 53 | Citrate | Male ICR mice Female ICR mice |
Oral | 0.2, 2 and 20 | 90 | – | Current study | |
| 5 (8.6) 5 (8.7) 5-60 (11.6–89.8) |
Citrate PVP Tannic acid |
Male C57BL/6 mice | Oral | 0.025 0.025 0.015–0.025 |
8 | – | Zhu et al. [33] | |
| 13.5 | Citrate | Male ICR mice | Oral | 0.1375–2.2 1.1 |
14 28 |
± ± |
Zhang et al. [8] | |
| 10–25 | Citrate | Male Wistar rats | Oral | 0.02 | 21 | + | Rathore et al. [25] | |
| 5–15 | Citrate | Female SD rats | Oral | 0.325–1.3 | 14 | – | Jo et al. [34] | |
| 1–25 | Polyphenold | Male Wistar rats | Oral | 5 and 10 | 28 | - | Mata et al. [35] | |
AuNPs are prepared in spherical shape by reduction of AuClO4 in citrate solution unless otherwise specified.
The size of citrate coated AuNPs determined by TEM. The number in parenthesis indicates the size determined by DLS.
Significant toxicity, +; no or insignificant toxicity, –; some or ambiguous toxicity, ±.
AuNPs (spherical shape) were prepared in plant extract of Abutilon Indicum.
Although the physiochemical properties are most critical for the toxicity of AuNPs, studies have shown that the route of administration also influences the toxicity, which is summarized in a review paper [9]. Zhang et al. conducted a subchronic toxicity study of 13.5-nm sized AuNPs in mice by three different administration methods (oral, intraperitoneal, and intravenous) [8]. They found that intraperitoneal and oral administration of 13.5-nm AuNPs at 1.1 mg/kg for 28 days showed higher toxicity than tail vein injection. Oral administration seems to cause the highest toxicity compared to intraperitoneal and intravenous injections, which was evidenced by significantly reduced body weight, increased spleen weight, and a decrease in red blood cells. The high toxic effect of oral AuNPs may be attributed to intestinal damage [8]. Because the oral route is the most common route for intake of NPs from diet and food products, potential toxicity may occur for long-term accumulation of NPs in organisms, which cannot be neglected. In the current study, we evaluated the chronic toxicity of AuNPs in male and female mice by oral administration for 90 days. Table 4 summarizes the current results along with other toxicity studies on oral AuNPs in vivo.
Two studies reported the oral toxicity of AuNPs in rodents. Oral administration of 13.5 nm AuNPs at dosages of 0.55, 1.1, and 2.2 mg/kg for 14 days significantly reduced body weight, but not at a lower dosage (0.1375 and 0.275 mg/kg) [8]. In the same study, mice orally administered 13.5 nm AuNPs at 1.1 mg/kg for 28 days showed decreased body weight and red blood cells but increased spleen weight [8]. Rathore et al. reported that oral administration of 10–25 nm AuNPs for 21 days revealed alveolar inflammation and hepatic and renal toxicity [25]. The size, surface coating (citrate) of AuNPs, exposure route, and duration of the animal study were similar in the above two studies, but different dosages of AuNPs and rodents were used. The use of citrate as a capping agent may not contribute to the toxicity of AuNPs, which were not found in the other two in vivo studies [33, 34] and our current results (Table 4). The oral toxicity of AuNPs may also not be attributed to the different surface coatings [33, 35]. In addition, dosage and duration were not correlated with the oral toxicity of AuNPs by Rathore et al. and Mata et al., which had the same and/or similar size of AuNPs, animal strain, and gender in their studies [25, 35].
When comparing our current study to Zhang et al., we presumed that size could be a major cause of the oral toxicity of AuNPs. Zhang et al. reported male ICR mice orally administered citrate-coated AuNPs of 13.5 nm at 2.2 mg/kg for 14 days showed a significant reduction in body weight and organ indices (heart, liver, spleen, lung, kidneys, and brain), whereas the thymus and spleen index increased [8]. In the current study, male ICR mice orally administered citrate-coated AuNPs of 53 nm at 2 mg/kg for 90 days showed no abnormal effects. Previous studies have suggested that AuNPs with a small particle size (<50 nm) showed a wider tissue distribution and higher accumulation compared to larger particles (50–250 nm) by intraperitoneal or intravenous injection [24, 28, 29, 30]. A size-dependent pattern was also found in the in vivo studies by oral administration. Two studies suggested that a higher level of Au accumulated in tissue when smaller sized AuNPs (<50 nm) were administered to animals, and a lower or undetected content was found in the case of larger AuNPs (>50 nm) [33, 36]. Therefore, we suggest the low-toxic effect of oral administration of AuNPs in the current study compared to that of Zhang et al. may partially be due to the low tissue distribution caused by the larger size of the AuNPs (53 nm). However, further investigation is required.
In the current study, it appears that GOT value was increased in both mice and female mice administered AuNPs at three dosages; however, there was no statistically significant difference was found between each AuNPs-treated and control group. This may attribute to the diversity in genetic variation in individual outbred ICR mice [37] leading to a higher standard deviation observed in the GOT value. Further histopathological examination of the liver confirmed no evidence of pathological change and fibrosis in all AuNPs treated mice compared with control animals. Based on these examinations, we suggested the slightly elevated GOP values were not considered toxic responses.
The significant influence of oral AuNPs at the highest dosage (20 mg/kg) on platelet indices and serum protein was found in female mice in our study, indicating a sex-dependent difference. Chen et al. reported C57 mice administered intraperitoneal injections of various sizes of PEG-coated AuNPs (<50 nm) at 4 mg/kg for 28 days showed sex differences in the toxicological response. They found that male mice presented significant liver toxicity and an obvious increase in WBC and RBC counts, while high kidney toxicity was observed in female mice [38]. The different sex-related toxicity of oral AuNPs may be attributed to particle size, surface coating, exposure route, and most importantly, distinct metabolism and hormones between male and female individuals. Evidence has revealed sex-based differences in xenobiotic toxicity and efficiency, including drugs and environmental pollutants [39]. Female are indicated more likely to show adverse effect than male in response to drug and toxicant [40, 41]. This gender-based variation is proposed to contribute to the difference in hormonal-dependent regulation of proteins and enzyme activity involved in drug metabolism via a direct and indirect mechanism. For example, males and female rodents display a different pattern of growth hormone secretion from the pituitary that leads to differential regulation of male- and female-specific drug metabolizing enzymes [39]. Platelet biology may also be related to sex hormones. Male and female platelets have a differential response to ligand-induced activation, and female platelets show an increased activation potential. Male platelets pretreatment estradiol displayed approximate female platelet activation induced by platelet-activating factor [42]. A case-control study demonstrated women oral contraceptives increased the risk of venous thrombosis, and showed a positive association with oestrogen dose [43]. This information reflecting the possibility of hormonal effect involved in the sex-dependent differences in platelet indices and serum protein observed in 20 mg/kg AuNPs treated male and female mice in our study. Although we found that oral administration of AuNPs caused a decrease in serum protein and A/G ratio in female, other abnormal effects were not observed for liver and renal function (serum biochemistry and histopathology). More in vivo evidence regarding the sex-related differences in AuNPs toxicology is necessary in the future.
5. Conclusion
The results of the current subchronic toxicity study demonstrated no mortality and obvious adverse effects in male mice orally administered 53 nm AuNPs for 90 days. Female mice daily administrated 53 nm AuNPs at 20 mg/kg for 90 days showed statistical differences of hematological (PLT, MPV, PCT, and PDW) and biochemical parameters (Ca and serum proteins), whereas the differences were slight and none of these differences were determined to be of toxicological significance. The repeated oral exposure of spheric citrate-coated 53 nm AuNPs up to 20 mg/kg was low toxic for mice following 90 days of treatment. These data provide important safety information for the application of AuNPs in food products and their management in the food chain. Gold compounds have been used in medicine for decades and most studies on animal models support that AuNPs do not cause appreciable toxicity.
Declarations
Author contribution statement
Pei-Pei Sun: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Ching-Shu Lai: Conceived and designed the experiments; Performed the experiments.
Chung-Jung Hung: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Periyathambi Dhaiveegan: Analyzed and interpreted the data; Wrote the paper.
Mei-Ling Tsai: Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data.
Chun-Lun Chiu: Contributed reagents, materials, analysis tools or data.
Jim-Min Fang: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Tripod Nano Technology Company.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Fernandez-Ponce C., Munoz-Miranda J.P., de Los Santos D.M., Aguado E., Garcia-Cozar F., Litran R. Influence of size and surface capping on photoluminescence and cytotoxicity of gold nanoparticles. J. Nanopart. Res. 2018;20:305. doi: 10.1007/s11051-018-4406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dykman L.A., Khlebtsov N.G. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3:34–55. [PMC free article] [PubMed] [Google Scholar]
- 3.Balfourier A., Kolosnjaj-Tabi J., Luciani N., Carn F., Gazeau F. Gold-based therapy: from past to present. Proc. Natl. Acad. Sci. U.S.A. 2020;117:22639–22648. doi: 10.1073/pnas.2007285117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A., Moyano D.F., Parnsubsakul A., Papadopoulos A., Wang L.S., Landis R.F., Das R., Rotello V.M. Ultrastable and biofunctionalizable gold nanoparticles. ACS Appl. Mater. Interfaces. 2016;8:14096–14101. doi: 10.1021/acsami.6b02548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H., Zhou K., Zhao G. Gold nanoparticles: from synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018;78:83–94. [Google Scholar]
- 6.Kumar H., Kuca K., Bhatia S.K., Saini K., Kaushal A., Verma R., Bhalla T.C., Kumar D. Applications of nanotechnology in sensor-based detection of foodborne pathogens. Sensors.(Basel) 2020;20 doi: 10.3390/s20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pissuwan D., Gazzana C., Mongkolsuk S., Cortie M.B. Single and multiple detections of foodborne pathogens by gold nanoparticle assays. Wiley. Interdiscip. Rev .Nanomed. Nanobiotechnol. 2020;12:e1584. doi: 10.1002/wnan.1584. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X.D., Wu H.Y., Wu D., Wang Y.Y., Chang J.H., Zhai Z.B., Meng A.M., Liu P.X., Zhang L.A., Fan F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010;5:771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adewale O.B., Davids H., Cairncross L., Roux S. Toxicological behavior of gold nanoparticles on various models: influence of physicochemical properties and other factors. Int. J. Toxicol. 2019;38:357–384. doi: 10.1177/1091581819863130. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta J., Datta P., Patra H.K., Dasgupta A.K., Gomes A. In vivo interaction of gold nanoparticles after acute and chronic exposures in experimental animal models. J. Nanosci. Nanotechnol. 2013;13:1660–1670. doi: 10.1166/jnn.2013.7113. [DOI] [PubMed] [Google Scholar]
- 11.Lu L., an K.S., Wang C.H., Chiu C.L., Chang T.W., Wang C.D., Fang J.M. Method of making colloidal metal nanoparticles. US Patent US10099191B1. 2018 [Google Scholar]
- 12.River Charles. 2011. CD-1 IGS Mouse Model Information Sheet. [Google Scholar]
- 13.Zhang J., Mou L., Jiang X. Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 2021;11:923–936. doi: 10.1039/c9sc06497d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafiq M., Anjum S., Hano C., Anjum I., Abbasi B.H. An overview of the applications of nanomaterials and nanodevices in the food industry. Foods. 2020;9 doi: 10.3390/foods9020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagno C.H., Costa T.M., de Menezes E.W., Benvenutti E.V., Hertz P.F., Matte C.R., Tosati J.V., Monteiro A.R., Rios A.O., Flores S.H. Development of active biofilms of quinoa (Chenopodium quinoa W.) starch containing gold nanoparticles and evaluation of antimicrobial activity. Food Chem. 2015;173:755–762. doi: 10.1016/j.foodchem.2014.10.068. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A., Khan A.K., Anwar A., Ali S.A., Shah M.R. Biofilm inhibitory effect of chlorhexidine conjugated gold nanoparticles against Klebsiella pneumoniae. Microb. Pathog. 2016;98:50–56. doi: 10.1016/j.micpath.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Frohlich E., Roblegg E. Models for oral uptake of nanoparticles in consumer products. Toxicology. 2012;291:10–17. doi: 10.1016/j.tox.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaicherd S., Killingsworth M.C., Pissuwan D. Toxicity of gold nanoparticles in a commercial dietary supplement drink on connective tissue fibroblast cells. SN Appl. Sci. 2019;1:336. [Google Scholar]
- 19.Chithrani B.D., Chan W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 20.Murphy C.J., Gole A.M., Stone J.W., Sisco P.N., Alkilany A.M., Goldsmith E.C., Baxter S.C. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc. Chem. Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 21.Schaeublin N.M., Braydich-Stolle L.K., Maurer E.I., Park K., MacCuspie R.I., Afrooz A.R., Vaia R.A., Saleh N.B., Hussain S.M. Does shape matter? Bioeffects of gold nanomaterials in a human skin cell model. Langmuir. 2012;28:3248–3258. doi: 10.1021/la204081m. [DOI] [PubMed] [Google Scholar]
- 22.Sukhanova A., Bozrova S., Sokolov P., Berestovoy M., Karaulov A., Nabiev I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale. Res. Lett. 2018;13:44. doi: 10.1186/s11671-018-2457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yah C.S., Simate G.S., Iyuke S.E. Nanoparticles toxicity and their routes of exposures. Pak. J Pharm Sci. 2012;25:477–491. [PubMed] [Google Scholar]
- 24.Chen Y.S., Hung Y.C., Liau I., Huang G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale. Res. Lett. 2009;4:858–864. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathore M., Mohanty I.R., Maheswari U., Dayal N., Suman R., Joshi D.S. Comparative in vivo assessment of the subacute toxicity of gold and silver nanoparticles. J. Nano Res. 2014;16:2338. [Google Scholar]
- 26.Huo S., Jin S., Ma X., Xue X., Yang K., Kumar A., Wang P.C., Zhang J., Hu Z., Liang X.J. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano. 2014;8:5852–5862. doi: 10.1021/nn5008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Y., Neuss S., Leifert A., Fischler M., Wen F., Simon U., Schmid G., Brandau W., Jahnen-Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 28.De Jong W.H., Hagens W.I., Krystek P., Burger M.C., Sips A.J., Geertsma R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 29.Sonavane G., Tomoda K., Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids Surf. B Biointerfaces. 2008;66:274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.H., Kim J.H., Kim K.W., Kim M.H., Yu Y.S. Intravenously administered gold nanoparticles pass through the blood-retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20:505101. doi: 10.1088/0957-4484/20/50/505101. [DOI] [PubMed] [Google Scholar]
- 31.Abdelhalim M.A., Jarrar B.M. Renal tissue alterations were size-dependent with smaller ones induced more effects and related with time exposure of gold nanoparticles. Lipids Health Dis. 2011;10:163. doi: 10.1186/1476-511X-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim K.E., Al-Mutary M.G., Bakhiet A.O., Khan H.A. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules. 2018;23 doi: 10.3390/molecules23081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu S., Jiang X., Boudreau M.D., Feng G., Miao Y., Dong S., Wu H., Zeng M., Yin J.J. Orally administered gold nanoparticles protect against colitis by attenuating Toll-like receptor 4- and reactive oxygen/nitrogen species-mediated inflammatory responses but could induce gut dysbiosis in mice. J. Nanobiotechnol. 2018;16:86. doi: 10.1186/s12951-018-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jo M.R., Bae S.H., Go M.R., Kim H.J., Hwang Y.G., Choi S.J. Toxicity and biokinetics of colloidal gold nanoparticles. Nanomaterials.(Basel) 2015;5:835–850. doi: 10.3390/nano5020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mata R., Nakkala J.R., Chandra V.K., Raja K., Sadras S.R. In vivo bio-distribution, clearance and toxicity assessment of biogenic silver and gold nanoparticles synthesized from Abutilon indicum in Wistar rats. J. Trace Elem. Med. Biol. 2018;48:157–165. doi: 10.1016/j.jtemb.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Hillyer J.F., Albrecht R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2001;90:1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- 37.Chia R., Achilli F., Festing M.F., Fisher E.M. The origins and uses of mouse outbred stocks. Nat. Genet. 2005;37:1181–1186. doi: 10.1038/ng1665. [DOI] [PubMed] [Google Scholar]
- 38.Chen J., Wang H., Long W., Shen X., Wu D., Song S.S., Sun Y.M., Liu P.X., Fan S., Fan F., Zhang X.D. Sex differences in the toxicity of polyethylene glycol-coated gold nanoparticles in mice. Int. J. Nanomed. 2013;8:2409–2419. doi: 10.2147/IJN.S46376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolson T.J., Mellor H.R., Roberts R.R. Gender differences in drug toxicity. Trends Pharmacol. Sci. 2010;31:108–114. doi: 10.1016/j.tips.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Miller M.A. Gender-based differences in the toxicity of pharmaceuticals--the Food and Drug Administration's perspective. Int. J. Toxicol. 2001;20:149–152. doi: 10.1080/109158101317097728. [DOI] [PubMed] [Google Scholar]
- 41.Mennecozzi M., Landesmann B., Palosaari T., Harris G., Whelan M. Sex differences in liver toxicity-do female and male human primary hepatocytes react differently to toxicants in vitro? PloS One. 2015;10 doi: 10.1371/journal.pone.0122786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coleman J.R., Moore E.E., Kelher M.R., Samuels J.M., Cohen M.J., Sauaia A., Banerjee A., Silliman C.C., Peltz E.D. Female platelets have distinct functional activity compared with male platelets: implications in transfusion practice and treatment of trauma-induced coagulopathy. J. Trauma Acute. Care Surg. 2019;87:1052–1060. doi: 10.1097/TA.0000000000002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Hylckama Vlieg A., Helmerhorst F.M., Vandenbroucke J.P., Doggen C.J., Rosendaal F.R. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ. 2009;339:b2921. doi: 10.1136/bmj.b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.