Invasive fungal diseases (IFD) impact morbidity, mortality, hospital stay and healthcare costs in critically ill patients, constituting an unmet medical need.1 Pathogenic fungi rarely cause IFD in immunocompetent individuals but become life-threatening in cases of immunodeficiency, young infants, disrupted mucosal barriers or polyantibiotic therapy, highlighting the role of immune surveillance in IFD control.2
Host fungal recognition relies on pattern recognition receptors (PRRs) mainly expressed by innate immune cells to induce protective responses. PRRs from different structural families, such as Toll-like receptors (TLRs), C-type lectin receptors (CLR), scavenger receptors (SRs), nucleotide-binding and oligomerization domain-like receptors (NLR), and retinoid acid-inducible gene-like receptors (RLR) target conserved structural components of fungal cells (e.g., mannans, β-glucans, or chitins)—collectively designated microbial-associated molecular patterns (MAMPs).2 The identification of genetic variants in patients and the development of knockout mouse models have paved road to the discovery of PRR mechanisms in antifungal immunity and novel immunomodulatory strategies against IFD.2
MAMPs indirectly promote T cell-mediated responses through innate immune cells by upregulating antigen presentation and both co-stimulatory molecule and cytokine production. Incipient evidence indicates that different T cell subsets directly modulate ongoing immune responses by responding to MAMPs.3,4 We previously showed that human CD5, a lymphoid-specific class I SR, binds to and senses the presence of fungal cells through β-glucan recognition.5 CD5 is a signal transducing receptor physically associated with the antigen-specific receptor complex of T and B1a cells that downmodulates activating intracellular signals upon specific antigen recognition.6 In this way, CD5 binding to MAMPs is thought to prevent autoimmunity by dampening the activation of low-affinity self-reactive immune cells and favoring the expansion of high-affinity non-self-reactive immune cells to optimize anti-infectious responses.7
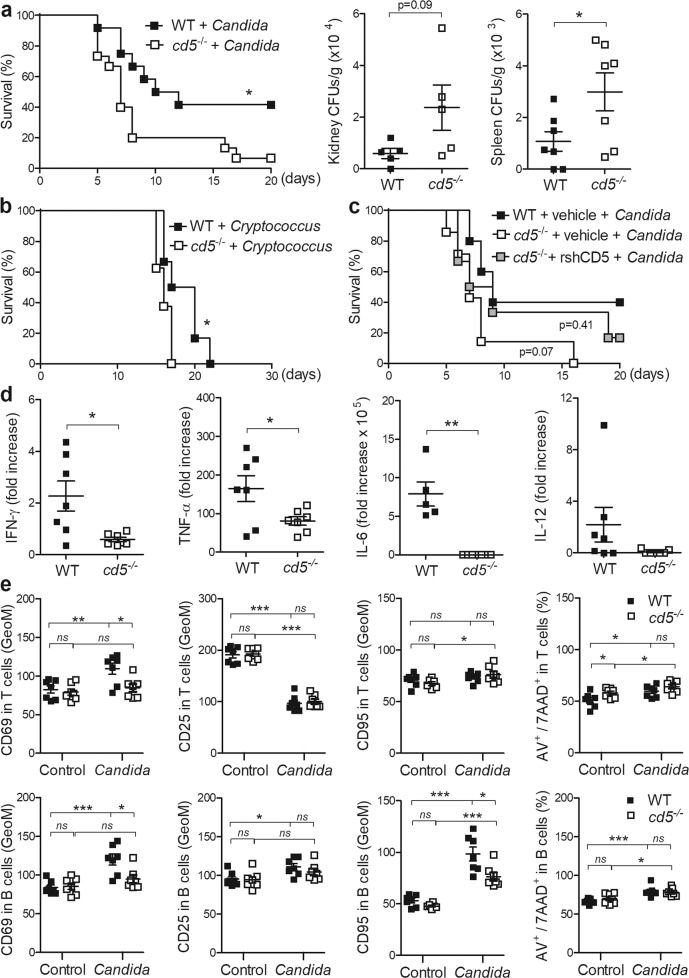
Fungal β-glucan recognition occupies a substantial number of PRRs (e.g., dectin-1, CD23, SCARF1, CD36, and TLR2) on innate immune cells.2 This raises the question of the redundancy of CD5 on adaptive immune cells during antifungal defense. We investigated CD5 deficiency (cd5−/−) in mice susceptibility to fungal infection. cd5−/− mice showed lower survival and increased fungal burden compared with wild-type (WT) mice following sublethal systemic Candida albicans infection (Fig. 1a) or lethal challenge with Cryptococcus neoformans (Fig. 1b), supporting widespread fungal susceptibility. Interestingly, no survival differences between C. albicans-infected WT and cd5−/− mice were observed following therapeutic infusion of recombinant soluble human CD5 protein (rshCD5) (Fig. 1c), in line with previous findings using rshCD5 in a zymosan-induced fungal sepsis-like mouse model.5
Fig. 1.
Effect of CD5 deficiency on in vivo and in vitro fungal challenge. a Survival of WT (n = 12) and cd5−/− (n = 15) C57BL/6 mice infected with the C. albicans SC5314 strain (3 × 103 CFU/g, tail vein) (left). Log-rank (Mantel–Cox) test. Fungal burden from kidney (n = 5) and spleen (n = 7) at 72 h postinfection (right). Mann–Whitney test. b Survival of WT (n = 6) and cd5−/− (n = 8) mice infected with the C. neoformans H99 strain (3 × 104 CFU/g, intranasal). c Survival of C. albicans-infected (as in a) WT mice treated with vehicle (n = 5) and cd5−/− mice treated with vehicle (n = 7) or rshCD5 (1.25 mg/kg; n = 6) at 18 h postinfection. d IFN-γ, TNF-α, IL-6, and IL-12 ELISA levels in culture supernatants from total splenocytes (2 × 105 cells/well) from cd5−/− (n = 7) and WT (n = 7) mice exposed to heat-killed C. albicans SC5314 (105 CFUs/well) for 24 h. The results are plotted as fold induction from unstimulated cells. Unpaired t-test. e Flow cytometry of unfractionated splenocytes from cd5−/− (n = 7) and WT (n = 7) mice challenged with heat-killed C. albicans (as in d) and analyzed for CD69, CD25, CD95/Fas, and annexin V/7-AAD positivity on gated T (CD3+B220−) and B (CD3−B220+) cells. Represented is the geometric mean of fluorescence intensity (GeoM). Unpaired t-test. *p < 0.05; **p < 0.01; ***p < 0.001
T cells are the main immune cell subset expressing CD5, which downmodulates T cell activation.6 Thus, mechanistic in vitro studies were performed using unfractionated splenocytes from cd5−/− and WT mice exposed for 24 h to heat-killed C. albicans. As shown in Fig. 1d, lower production of IFN-γ, TNF-α, IL-6, and IL-12 was detected in cd5−/− splenocytes than in WT splenocytes. Fungal exposure differentially influenced activation and/or apoptosis in WT versus cd5−/− splenocytes, as illustrated in Fig. 1e. The expression of the early activation CD69 marker was upregulated in WT but not in cd5−/− spleen T and B cells following a 24-h challenge with heat-killed C. albicans, whereas no differences in CD25 expression were detected (Fig. 1e). Under these conditions, activation-induced and apoptosis-inducing cell surface marker CD95/Fas revealed no differences between cd5−/− and WT spleen T cells (Fig. 1e) but showed upregulation in B cells, albeit with lower levels in cd5−/− cells (Fig. 1e). The lack of differences in annexin V/7-ADD positivity (Fig. 1e) indicates that CD5 deficiency desensitizes T and B cells to activation during C. albicans exposure.
Our results support a nonredundant role for CD5 in antifungal immune responses. The higher susceptibility to fungal infection in CD5-deficient mice resembles that of other immune cell fungal receptors, such as dectin-1 and dectin-2, whose deficiency increases C. albicans infection susceptibility, enhances fungal dissemination, lowers cell recruitment and decreases pro-inflammatory cytokine production as a consequence of impaired fungal recognition.8,9 Our in vitro conditions mirroring innate immunity show lower cytokine production and lower T and B cell activation upon fungal exposure under CD5 deficiency. Thus, we propose that in the absence of specific antigen recognition, fungal sensing by CD5 provides co-stimulatory signals, potentiating T and B cell functions. The latter agrees with the activating properties of the casein kinase 2-binding domain of CD5 (via AKT activation), which is necessary for Th17 cell differentiation through the IFN-γ response and RORγt localization.10
We postulate that CD5 protects against IFD by sensitizing T cells to fungal constituents and consequently contributing to Th17 and/or Th1 responses. Research on other CD5+ non-T (B1a, Breg, iNKT, and ILCs) and nonlymphoid (macrophages and DCs) cell subsets awaits full understanding of the IFD-protective mechanism of CD5.6
Acknowledgements
We thank Marcos Isamat for critically reviewing the paper and Joaquin García-Luna for excellent technical assistance. This work was supported by the Spanish Ministerio de Economía y Competitividad (SAF-2016-80535-R), co-financed by the European Development Regional Fund “A way to achieve Europe” to F.L. M.V.-d-A., C.C., S.C.-L., I.S., and E.C. are recipients of fellowships from the Ministerio de Economía y Competitividad (BES-2014-069237; BES-2017-082107), the Ministerio de Educación Cultura y Deporte (FPU15/02897), the Fundação para a Ciência e a Tecnologia (SFRH/BD/75738/2011), and the European Community Seventh Framework Program (BIOTRACK, FP7/2007/2013; 229673).
Author contributions
M.V.-d-A., C.C., I.S., S.C.-L., and E.C. performed the experiments. M.V.-d-A. and F.L. wrote the paper. E.C., O.Z., and F.L. designed and supervised the project. All authors discussed the results and participated in revising the paper.
Competing interests
The authors declare no competing interests.
References
- 1.Klingspor L, et al. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (2006–2008) Clin. Microbiol. Infect. 2015;21:87.e1–87.e10. doi: 10.1016/j.cmi.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Salazar F, Brown GD. Antifungal innate immunity: a perspective from the last 10 years. J. Innate Immun. 2018;10:373–397. doi: 10.1159/000488539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu G, Zhang L, Zhao Y. Modulation of immune responses through direct activation of Toll-like receptors to T cells. Clin. Exp. Immunol. 2010;160:168–175. doi: 10.1111/j.1365-2249.2010.04091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imanishi T, Saito T. T cell co-stimulation and functional modulation by innate signals. Trends Immunol. 2020;41:200–212. doi: 10.1016/j.it.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Vera J, et al. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc. Natl Acad. Sci. USA. 2009;106:1506–1511. doi: 10.1073/pnas.0805846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgueño‐Bucio E, Mier‐Aguilar CA, Soldevila G. The multiple faces of CD5. J. Leukoc. Biol. 2019;105:891–904. doi: 10.1002/JLB.MR0618-226R. [DOI] [PubMed] [Google Scholar]
- 7.Lenz LL. CD5 sweetens lymphocyte responses. Proc. Natl Acad. Sci. USA. 2009;106:1303–1304. doi: 10.1073/pnas.0812579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor PR, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ifrim DC, et al. The role of Dectin-2 for host defense against disseminated candidiasis. J. Interferon Cytokine Res. 2016;36:267–276. doi: 10.1089/jir.2015.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGuire DJ, et al. CD5 enhances Th17-cell differentiation by regulating IFN-γ response and RORγt localization. Eur. J. Immunol. 2014;44:1137–1142. doi: 10.1002/eji.201343998. [DOI] [PMC free article] [PubMed] [Google Scholar]