Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common inherited trait and affects ~7% of the global population. The G6PD A− variant (class III), which has mild-to-moderate residual enzymatic activity (10–60%), is present in 11–14% of African American (AA) individuals. Meta-analysis and epidemiological studies from different countries have shown that individuals with G6PD deficiency have an increased risk of developing diabetes and cardiovascular disease.1,2 G6PD deficiency increases the risk of elevated systolic blood pressure and the incidence of CVD among populations in USA, the Mediterranean region, and China.1,3,4 G6PD deficiency is also associated with fibrosis, autoimmune diseases, metabolic disorders, and sporadic Alzheimer’s disease, indicating that the clinical risks associated with G6PD deficiency are likely to be underestimated.
Differentiation of monocytes to resident macrophages occurs when monocytes enter the tissue and are under the control of local environmental cues. Monocytes and macrophages play vital roles in the innate immune defense against invading pathogens and support adaptive immunity, tumor surveillance, and cardiovascular homeostasis.5 Two extreme states of activated macrophages are M1-like macrophages (in response to inflammatory stimuli such as LPS and IFNγ) and M2-like macrophages (in response to cytokines such as IL4 and IL-13).5 However, the contribution of G6PD deficiency to monocyte/macrophage polarization is currently unknown.
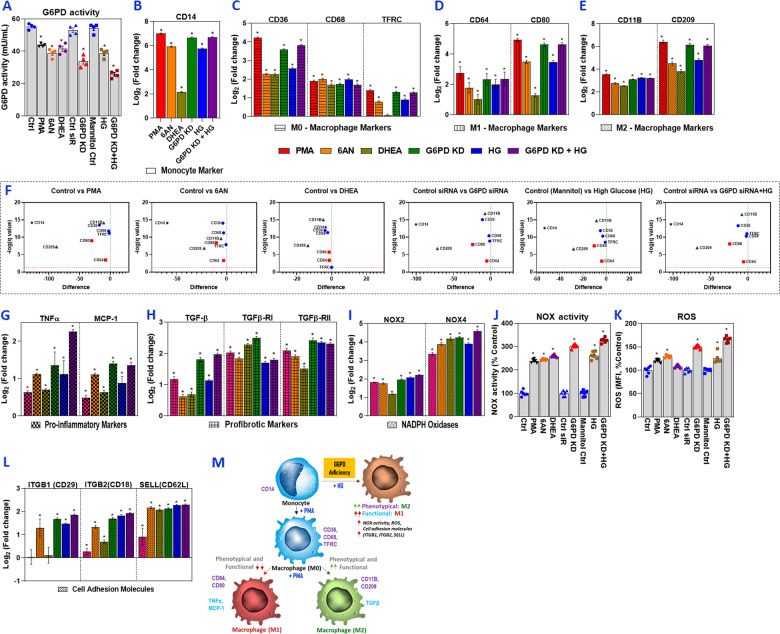
This study investigated whether G6PD deficiency conditions (G6PD A− variant; mild–moderate) influence the polarization of human monocytes and macrophages. G6PD activity was experimentally decreased to mimic that of the G6PD A− variant (Fig. 1a). Monocytes were treated with PMA, which induced the resting macrophage (Mφ) state (M0 phenotype) (Fig. 1a–f). G6PD-deficient (G6PD knockdown) monocytes were also polarized to the Mφ macrophage state; these cells showed increased expression of cell surface markers for the M1 (CD64 and CD80) and M2 (CD11B and CD209) phenotypes compared with the levels in the respective untreated controls (Fig. 1a–f). The fold change in intensity was much more significant for CD80 and CD209 than the other markers and was comparable with that of the PMA group (Fig. 1a–f). Similarly, treatment with pharmacological inhibitors of the G6PD (6AN and DHEA) and high glucose (HG) significantly increased CD80 and CD209, but the fold change was lower than that of the PMA and G6PD KD groups. Polarization marker expression results indicated that G6PD deficiency activates monocytes and induces polarization that closely resembles the alternatively activated M2 phenotype. It appears that both the in vivo tissue milieu and homeostatic signals influence the different polarization states of activated macrophages and influence the change in phenotype.5 The phenotypes of macrophages can switch between different functional states, and the stability of M1, M2, and other phenotypes is unclear.
Fig. 1.
G6PD deficiency activates monocytes and their effect on functional and phenotypical M1/M2 macrophage polarization. Human monocytes/macrophages (SC cells) were treated with PMA (150 nM for 48 h), G6PD inhibitors (6AN/DHEA; 100 µM for 24 h), or G6PD siRNA (100 nM for 24 h). G6PD-normal (control) and G6PD-deficient cells were then exposed to high glucose (HG; 25 mM) for an additional 24 h. For PMA and G6PD inhibitors, normal cells were used as a control, and control siRNA served as a control for G6PD-siRNA-treated groups. For cells treated with HG, mannitol was used as an osmolarity control. a G6PD activity. RT-qPCR was performed to assess the level of target genes as indicated; the data are presented as log base twofold change over the respective controls (b–e). b Monocyte marker (CD14). c M0 macrophage markers (CD36, CD68, and TFRC/CD71). d M1 macrophage markers (CD64 and CD80). e M2 macrophage markers (CD11B/ITGAM and CD209). f Multiple Student’s t tests were used to analyze control vs. treated conditions; volcano plots with false discovery rate (FDR) were created using the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli with Q = 1%. g mRNA levels of proinflammatory markers (TNFα and MCP-1). h mRNA levels of profibrotic markers (TGF-β1, TGF-βR1, and TGF-βR2). i mRNA levels of NADPH oxidase (NOX2 and NOX4). j NOX activity. k ROS levels. l mRNA levels of cell adhesion molecules (ITGB1/CD29, ITGB2/CD18, and SELL(CD62L)). m Schematic illustration of the proposed mechanism of glucose-6-phosphate dehydrogenase (G6PD) deficiency-induced human monocyte/macrophage activation/polarization. The results are shown as the mean ± SEM (n = 3–6). An asterisk indicates significance. The p values were calculated with ANOVA, followed by Dunnett’s post hoc test (*p < 0.05)
Diabetes/hyperglycemia tends to shift monocytes toward a functionally proinflammatory phenotype, which induces chronic inflammation and may impair wound healing due to insufficient M2 functional profibrotic TGFβ signaling. Thus, we examined whether G6PD deficiency influences functional cytokine gene expression. The results showed increased expression of proinflammatory markers, such as TNF and MCP-1 (Fig. 1g), but reduced levels of the profibrotic or M2 functional cytokine TGFβ (Fig. 1h). Exposure to HG induced the expression of inflammatory cytokines to levels higher than those of TGFβ and its receptors (Fig. 1g, h). This suggests that metabolic status may polarize resting M0 macrophages to either the M1 or M2 phenotype.
Glutathione (GSH) recycling is impaired in G6PD deficiency due to insufficient levels of the reduced form of NADPH, which induces a redox imbalance. G6PD deficiency causes excess oxidative stress, which in turn activates the TGF-β/p-Smad signaling pathway and increases NOX enzymes. G6PD deficiency also suppresses antioxidant pathways, including the synthesis of GSH, and accelerates inflammation. Epithelial and endothelial cells are capable of cellular transdifferentiation into mesenchymal cells (EMT and EndMT). Transforming growth factor-1, which is considered the primary transdifferentiation inducer, plays a role in the pathogenesis of various diseases, including malignant, vascular, inflammatory, and fibrotic disorders, as well as pulmonary arterial hypertension and atherosclerosis. An increased risk for fibrotic diseases has been reported in G6PD-deficient subjects.6,7 The expression of TGF-β and its receptors in G6PD-deficient monocytes/macrophages was higher than that in the HG-treated groups (Fig. 1g, h). The expression of proinflammatory cytokines showed the opposite result. These results indicate that the metabolic milieu influences the functional heterogeneity of macrophages.
This study also demonstrated that TGF-β1 signaling upregulated NADPH oxidases and ROS under G6PD-deficient conditions (Fig. 1i, k). Previously, we reported that G6PD deficiency promotes an oxidative shift via several mechanisms, including reduced recycling of GSH, reduced NADPH levels, and derangements in NO metabolism.8–10 In addition, compared with healthy subjects, G6PD-deficient subjects have significantly increased TGF-β levels in cultured peripheral blood mononuclear cells.11 This suggests that redox imbalance contributes to TGF-β-mediated pathophysiological effects under G6PD-deficient conditions.
The expression of leukocyte cell adhesion molecules (CAM) were evaluated. G6PD deficiency and HG treatment caused upregulation of CAM compared with expression in the respective controls (Fig. 1l). Thus, G6PD deficiency, along with hyperglycemia, may favor monocytic dysfunction by increasing levels of inflammatory cytokines, upregulating CAM, and facilitating the invasion and adhesion of circulating monocytes.1,8,9 This suggests that the presence of an inherited G6PD deficiency in addition to diabetes is a double burden that dysregulates redox homeostasis, increases oxidative stress, assaults the endothelium, and increases the risk of CVD.
AA individuals are prone to G6PD deficiency and an increased incidence of CVD.1,2,10 This study is the first to show that G6PD deficiency activates monocytes and alters macrophage polarization. The schematic presented in Fig. 1m shows that while G6PD-deficient cells functionally behave similar to M1 macrophages (proinflammatory), their phenotypes more closely resemble those of M2 (profibrotic) cells. G6PD deficiency-induced ROS may shift in cytokine TGF-β signaling, which upregulates NADPH oxidases. Activation of these factors generates excess oxidative stress and induces cytokines (TNFα and MCP-1) and the expression of CAMs. These observations may be relevant with respect to the initiation and progression of CVD. Early diagnostic and therapeutic interventions may reduce the risk of cardiovascular disease in the susceptible G6PD-deficient population. In addition to the critical role of G6PD deficiency in the polarization of monocytes/macrophages, it may also be associated with the clinical risks inherent to other immune-metabolic disorders.
Supplementary information
Acknowledgements
The Malcolm W. Feist Cardiovascular Research Fellowship provided support for this study to RP and the Endowed Chair in Diabetes to SKJ from the Center for Cardiovascular Diseases and Sciences, LSUHSC-Shreveport. SKJ also received support from grants from the National Institutes of Health/National Center for Complementary and Integrative Health (RO1 AT007442, 2013-16). We thank Ms Paula Polk, Manager, and Dr Wiola Luszczek, Research Specialist, at the Research Core Facility at LSUHSC-Shreveport, for their expert technical assistance. We also thank Mr William E. McLean and Mr Christopher M. Stevens for lab assistance. The authors thank Ms Georgia Morgan for excellent editing.
Author contributions
RP and SKJ conceived and designed the experiment and analyzed the data; RP performed the experiments and wrote the original draft; RP and SKJ reviewed and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-020-0428-5) contains supplementary material.
References
- 1.Parsanathan, R. & Jain S. K. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is linked with cardiovascular disease. Hypertens. Res. 10.1038/s41440-020-0402-8 (2020). [DOI] [PubMed]
- 2.Parsanathan R, Jain SK. Novel invasive and noninvasive cardiac-specific biomarkers in obesity and cardiovascular diseases. Metab. Syndr. Relat. Disord. 2020;18:10–30. doi: 10.1089/met.2019.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J, et al. The association between glucose-6-phosphate dehydrogenase deficiency and abnormal blood pressure among prepregnant reproductive-age Chinese females. Hypertens. Res. 2019;42:75–84. doi: 10.1038/s41440-018-0118-1. [DOI] [PubMed] [Google Scholar]
- 4.Pes GM, Parodi G, Dore MP. Glucose-6-phosphate dehydrogenase deficiency and risk of cardiovascular disease: a propensity score-matched study. Atherosclerosis. 2019;282:148–153. doi: 10.1016/j.atherosclerosis.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Yang J, Wei Y, Wei X. Epigenetic regulation of macrophages: from homeostasis maintenance to host defense. Cell Mol. Immunol. 2020;17:36–49. doi: 10.1038/s41423-019-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Congdon PJ, Littlewood JM, Aggarwal RK, Shapiro H. Glucose 6-phosphate dehydrogenase deficiency and cystic fibrosis. Postgrad. Med. J. 1981;57:453–454. doi: 10.1136/pgmj.57.669.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elko EA, et al. Age-dependent dysregulation of redox genes may contribute to fibrotic pulmonary disease susceptibility. Free Radic. Biol. Med. 2019;141:438–446. doi: 10.1016/j.freeradbiomed.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsanathan R, Jain SK. L-cysteine in vitro can restore cellular glutathione and inhibits the expression of cell adhesion molecules in G6PD-deficient monocytes. Amino Acids. 2018;50:909–921. doi: 10.1007/s00726-018-2559-x. [DOI] [PubMed] [Google Scholar]
- 9.Parsanathan R, Jain SK. Glucose-6-phosphate dehydrogenase deficiency increases cell adhesion molecules and activates human monocyte-endothelial cell adhesion: protective role of l-cysteine. Arch. Biochem. Biophys. 2019;663:11–21. doi: 10.1016/j.abb.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Jain SK, Palmer M. Effect of glucose-6-phosphate dehydrogenase deficiency on reduced and oxidized glutathione and lipid peroxide levels in the blood of African-Americans. Clin. Chim. Acta. 1996;253:181–183. doi: 10.1016/0009-8981(96)06371-1. [DOI] [PubMed] [Google Scholar]
- 11.Sanna F, et al. Production of inflammatory molecules in peripheral blood mononuclear cells from severely glucose-6-phosphate dehydrogenase-deficient subjects. J. Vasc. Res. 2007;44:253–263. doi: 10.1159/000100903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.