Abstract
In cancer, myeloid cells have tumor-supporting roles. We reported that the protein GPNMB (glycoprotein nonmetastatic B) was profoundly upregulated in macrophages interacting with tumor cells. Here, using mouse tumor models, we show that macrophage-derived soluble GPNMB increases tumor growth and metastasis in Gpnmb-mutant mice (DBA/2J). GPNMB triggers in the cancer cells the formation of self-renewing spheroids, which are characterized by the expression of cancer stem cell markers, prolonged cell survival and increased tumor-forming ability. Through the CD44 receptor, GPNMB mechanistically activates tumor cells to express the cytokine IL-33 and its receptor IL-1R1L. We also determined that recombinant IL-33 binding to IL-1R1L is sufficient to induce tumor spheroid formation with features of cancer stem cells. Overall, our results reveal a new paracrine axis, GPNMB and IL-33, which is activated during the cross talk of macrophages with tumor cells and eventually promotes cancer cell survival, the expansion of cancer stem cells and the acquisition of a metastatic phenotype.
Keywords: cancer stem cells, GPNMB, IL-33, metastasis, tumor associated macrophages
Subject terms: Immunosurveillance, Cancer stem cells
Introduction
In the tumor microenvironment, tumor-associated macrophages (TAMs) represent a numerically abundant immune population that actively interacts with cancer cells. The hallmark of macrophages is their functional plasticity, which is regulated upon exposure to specific local cues. TAMs are strongly influenced by the presence of cancer cells and actively contribute to an inflammatory and immune-suppressive environment.1,2 The general consensus suggests that macrophages play crucial roles in the processes of cancer-promoting inflammation leading to disease progression. For example, TAMs efficiently sustain neo-angiogenesis switches, support tumor cell proliferation/survival and their invasion, have high protease activity and suppress antitumor immune responses. Accordingly, several studies have reported that high macrophage infiltration into human tumors is usually associated with resistance to therapy and poor patient prognosis.2–6
To gain further knowledge on the factors produced by macrophages that can directly support tumor cells, we investigated the transcriptome profile of human monocytes exposed to M-CSF-producing tumor cells, as previously reported;7 these monocytes differentiate into tumor-conditioned macrophages with an M2-like gene expression profile and, most notably, a global profile remarkably similar to that of freshly isolated human TAMs.7,8 Among the most upregulated genes, we identified Gpnmb, which encodes glycoprotein nonmetastatic B (GPNMB).
GPNMB is a highly glycosylated type I transmembrane protein localized on the cell surface or stored in endosomes/lysosomes. Membrane-bound GPNMB can be cleaved by metalloproteinases, such as ADAM10, to generate the soluble isoform.9 This protein was originally discovered in melanoma cell lines,10 osteoblasts11, and dendritic cells.12 In the literature, it is also known as osteoactivin or dendritic cell-associated heparan sulfate proteoglycan-integrin ligand (DC-HIL). GPNMB is expressed in many other cell types, such as osteoclasts, skin melanocytes and retinal pigment epithelial cells, hepatocytes, and leukocytes.10–14
GPNMB has a remarkably vast array of functions that are important in both physiological and pathological cellular processes. During homeostasis, a major role of GPNMB appears to be in cell adhesion mechanisms and cell differentiation, especially in the regulation of osteoblast maturation and cross talk with osteoclasts.15 By increasing the expression of MMP-3 and MMP-9 and the scavenging of debris, GPNMB is involved in tissue remodeling and repair processes after injury; some studies have also reported GPNMB effects limiting inflammation and favoring tissue protection at various sites (bone, kidney, and brain).16–19 Transgenic overexpression of GPNMB protected against fibrosis in the liver and muscles of mouse models.20,21 Some effects have also been described in the immune system. For example, Arizumi et al. reported that, when expressed by dendritic cells or myeloid-derived suppressor cells, GPNMB/DC-HIL mediated the suppression of T-cell activation by binding to syndecan-4 at the membrane.22,23
GPNMB is highly expressed in some tumors, such as melanoma, glioma, breast cancer (including triple-negative neoplasia), and cholangiocarcinoma, and it has been implicated in tumor progression and experimental metastasis.24–28 In vitro studies demonstrated that tumor cells with GPNMB silenced showed reduced in vitro migration and attenuated activation of MMPs.29 Furthermore, cross talk between cancer-associated fibroblasts and tumor cells induced greater cell motility, which was ascribed to the upregulation of GPNMB and the activation of MMPs in cancer cells.30 However, the roles of soluble GPNMB produced by macrophages, including paracrine effects on cancer cells remain unclear.
In a previous study, we found that a cleaved soluble form of GPNMB was produced in macrophages cocultured with tumor cells;7 therefore, here, we further investigate its biological role in cancer cells.
We show in this study that macrophage-released GPNMB binds to CD44 on tumor cells and activates the production of several mediators, including IL-33. The GPNMB/CD44/IL-33 axis leads to the expansion of cancer stem cells, prolongs cell survival and promotes the acquisition of a metastatic phenotype in mouse tumor models.
Materials and methods
Macrophage differentiation and polarization
Human monocytes were obtained from normal donor buffy coats by two-step gradient centrifugation. PBMCs were isolated by centrifugation with a Histopaque-1077 gradient (Sigma), and monocytes and T cells were separated from the PBMCs by centrifugation with a Percoll density gradient (GE Healthcare). Macrophages and tumor-conditioned macrophages (TC-macro) were obtained by culturing 106 human monocytes/ml in RPMI 1640 and 5% FBS for 6 days with either 25 ng/ml human recombinant M-CSF or 30% tumor cell line supernatant, as previously described.7 To induce M1/M2 polarization, M-CSF-differentiated macrophages were stimulated overnight with LPS (100 ng/ml) and IFNγ (50 ng/ml) or human IL-4 (20 ng/ml) (all reagents from PeproTech). Murine macrophages were obtained from bone marrow progenitors isolated from the femurs of 8-week-old mice and cultured in RPMI 1640 and 5% FBS with mouse M-CSF (25 ng/ml) for 6 days. The macrophages were polarized with murine cytokines as explained above; TC-macros were obtained in culture with 30% murine tumor cell line supernatant.
ELISA
To quantify the production of human/murine GPNMB and murine IL-6, CCL5, and IL-33 in the cell supernatants, commercial ELISA kits were used following the manufacturer’s instructions (R&D Systems). The data were analyzed with SoftMax Pro 5.3 software.
Intracellular signaling array
The phosphoproteome profile was evaluated with a human phospho-kinase array (ARY003B, R&D System) in MCA-1 mock cells starved in culture without serum for 24 h and then treated with 1% FBS with or without recombinant murine GPNMB (50 ng/ml).
A total of 3 × 106 cells were solubilized in 0.3 mL of lysis buffer 6. The protein lysate was quantified with a Pierce BCA protein assay kit (Thermo Fisher Scientific). The membranes were incubated overnight with 600 µg of protein lysates, and chemiluminescence was detected with a ChemiDoc imaging system (Bio-Rad Laboratories).
Immunofluorescence and immunohistochemistry
Paraffin embedded murine tissues were cut into 3 µm and placed on Superfrost slides. After dewaxing and rehydration, antigen unmasking was performed in a decloaking chamber with DIVA buffer (Biocare Medical) (for F4/80 staining) or in a microwave oven in which the following step was repeated once: 5 min at 800 MW in 0.25 mM EDTA buffer, pH 8.00. Endogenous peroxidases were blocked with 2% H2O2 for 20 min, and then, rodent block M (for F4/80 staining) or PBS/BSA 2% (for GPNMB staining) was used to block nonspecific binding sites. The sections were incubated with rat anti-mouse F4/80 1:400 (AbD Serotec) or 1:200 goat anti-mouse GPNMB (R&D). The sections were incubated in both primary antibodies for 1 h in a humid chamber. As a secondary antibody, we used a rat-on-mouse HRP polymer kit (Biocare Medical) for detecting F4/80-bound antibody and a goat-on-rodent kit (Biocare Medical) for detecting GPNMB. The reactions were developed with 3,3′-diaminobenzidine (Biocare Medical), counterstained with hematoxylin and mounted with Eukitt. In each experiment, at least 4 tumors/group were analyzed; the results are the mean of 4–10 immune-reactive areas for tumors. The analyses were performed with Image Pro-Analyzer 7.0 (Media Cybernetics) on pictures at the same magnifications.
Tumor cell lines and tumor spheroids
Human PT45, PANC1 and murine P815, MN-MCA-1, MCA-2, PANC02, MCA-1-GPNMB, and MCA-1-mock tumor cell lines were cultured at 37 °C and 5% CO2 in RPMI 1640 (Lonza) supplemented with 10% FBS (Sigma), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technologies, Inc.). The primary fibrosarcoma MCA-1 was induced in DBA/2J mice by subcutaneous inoculation of 50 µg of methylcholanthrene in 0.2 ml of peanut oil (Sigma-Aldrich), as previously described.31 MCA-1-mock and MCA-1-GPNMB spheroids were cultured in Iscove’s medium (Lonza) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin and specific factors, namely, mEGF (PeproTech, 20 ng/ml), mFGF (PeproTech, 20 ng/ml), B27 (Gibco, cat. no. 12587-010), and N2 (Gibco, cat. no. 17502-048), and referred to hereafter as spheroid medium. Murine recombinant GPNMB (R&D cat no. 2330-AC) and murine IL-33 (R&D cat no. 3626-ML-010) and macrophage supernatants from the TC-macros (with or without GPNMB exposure) were added to MCA-1-mock cells cultured in spheroid medium at the indicated concentrations. Anti-CD44 (Bio X Cell, clone IM7, cat no. BE0039), anti-IL-1R1L (R&D cat no. AF1004), and anti-human Trail R1 (Enzo Life Sciences, clone HS101, cat no. ALX-804-297-C100), which was used as an irrelevant antibody, were added to MCA-1 mock cells treated with GPNMB or IL-33 to inhibit sphere formation. All the cell lines were routinely checked for mycoplasma contamination.
GPNMB transduction
MCA-1 cells were transduced with a lentiviral plasmid that constitutively expressing endogenous GPNMB. The murine coding sequence of Gpnmb was cloned in frame with the fluorescent reporter mCherry under the CMV promoter in a lentiviral vector (GPNMB-mCherry pRRL-Sin), and an empty vector containing only mCherry (mCherry pRRL-Sin) was used as a control. Transduction of the MCA-1 cell line was performed by generating viral particles in HEK293T cells transfected with Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. The generated cell lines were expanded 24 h after the last transduction, and GPNMB expression was then assessed by flow cytometry and ELISA.
Tumor-conditioned medium (CM) preparation
Tumor-CM was prepared as described.7 Briefly, tumor cells growing to 90% confluence were incubated with fresh RPMI medium without FBS for 24 h; the CM was collected, passed through 0.2 mm filters and stored at −20 °C. To obtain supernatants from tumor-conditioned macrophages (TC-macros) of DBA/2J and DBA/2J/Gpnmb+ mice, BM-derived monocytes isolated from the femurs of 8-week-old healthy male mice were cultured for 6 days in RPMI 1640 with 10% FBS and 30% tumor cell line supernatant. TC-macro supernatants were collected, passed through 0.2 mm filters, and stored at −20 °C.
Mice
Mice were used in compliance with national (D.L.N. 26, G.U. March 4, 2014) and international law and policies (EEC Council Directive 2010/63/EU, OJL 276/33, 22-09-2010; NIH Guide for the Care and Use of Laboratory Animals, US National Research Council, 2011). Authorization from the Italian Ministry of Health number (489/2017-PR prot 6B2B3.32). DBA/2J/Gpnmb+ were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained and bred at Charles River (Calco, Milano); 8-week-old DBA/2J mice were also purchased from Charles River. Tumor cells were injected intramuscularly at the indicated concentrations. Tumor volume was measured with a caliper. At the end of the experiments, the tumors were explanted and then immunohistochemically assessed. Lungs and livers were placed in Bouin’s solution staining (picric acid, acetic acid and formaldehyde in aqueous solution), and the number of metastases was visually counted.
In the experiments of lung tumor colonization, DBA/2J mice were preconditioned before tumor injection by nasal inhalation of 25 µl of concentrated supernatants from DBA/2J and DBA/2J/Gpnmb+ TC-macro or supernatants from MCA-1-mock and MCA-1-GPNMB tumor cells. The cell supernatants were concentrated (>100×) by centrifugation over 50 MW cut-off filters (Sartorius). The Control mice received saline solution. After 10 min, the MCA-1-mock cells (105) were injected i.v. Two weeks later, the mice were sacrificed, and the lungs were explanted for the enumeration of lung colonies.
Real-time RT-PCR
Total RNA extraction was performed with pureZOL RNA isolation reagent (Bio-Rad). cDNA was synthesized by random priming with 2 μg of total RNA and a GeneAmp RNA PCR kit (Applied Biosystems) according to the manufacturer’s instructions. Real-time PCR was performed using SYBR Green dye and a 7900HT Fast Real-Time PCR system (Applied Biosystems). The sequences of primer pairs specific for each gene (Sigma) were designed with Primer Express Software (Applied Biosystems). The experiments for each sample were performed in triplicate. mRNA was normalized to GAPDH mRNA by subtracting the cycle threshold (Ct) value of GAPDH mRNA from the Ct value of the gene (ΔCt). The fold difference (2−ΔΔCt) was calculated by comparing ΔCt values. Arbitrary units are 2−ΔΔCt *100,000.
Primers
| mIL6 For | 5′ TAGTCCTTCCTACCCCAATTTCC 3′ |
| mIL6 Rev | 5′ TTGGTCCTTAGCCACTCCTTC 3′ |
| mCXCL10 For | 5′ CCAAGTGCTGCCGTCATTTTC 3′ |
| mCXCL10 Rev | 5′ GGCTCGCAGGGATGATTTCAA 3′ |
| mCCL5 For | 5′ GCTGCTTTGCCTACCTCTCC 3′ |
| mCCL5 Rev | 5′ TCGAGTGACAAACACGACTGC 3′ |
| mIL33 For | 5′ TCC TTG CTT GGC AGT ATC 3′ |
| mIL33 Rev | 5′ TGC TCA ATG TGT CAA CAG ACG 3′ |
| mGAPDH For | 5′ CCTTGACTGTGCCGTTGAATTT 3′ |
| mGAPDH Rev | 5′ GCAAAGTGGAGATTGTTGCCAT 3′ |
| mDNMT3 For | 5′ AGCGGGTATGAGGAGT GCAT 3′ |
| mDNMT3 Rev | 5′ GGGAGCATCCTTCGTGTCTG 3′ |
| mNanog For | 5′ TCTTCCTGGTCCCCACAGTTT 3′ |
| mNanog Rev | 5′ GCAAGAATAGTTCTCGGGATGAA 3′ |
| mOct3/4 For | 5′ AGAGGATCACCTTGGGGTACA 3′ |
| mOct3/4 Rev | 5′ CGAAGCGACAGATGGTGGTC 3′ |
| mSox2 For | 5′ GCGGAGTGGAAACTTTTGTCC 3′ |
| mSox2 Rev | 5′ CGGGAAGCGTGTACTTATCCTT 3′ |
| mBrachyury For | 5′ GCTTCAAGGAGCTAACTAACGAG 3′ |
| mBrachyury Rev | 5′ CCAGCAAGAAAGAGTACATGGC 3′ |
Flow cytometry
To measure the expression of cell surface molecules, the cells were pretreated with PBS+1% mouse serum for 30 min and then resuspended in FACS buffer (PBS without Ca++ or Mg++) and 1% FBS. The following mAbs were used: anti-CD117-PECy7, anti-Sca1-PECy7, anti-CD44-FITC (BD Bioscience), and anti-CD-PE (Invitrogen). The labeled cells were fixed in PBS+1% formalin. The data were acquired with a FACS Fortessa instrument (BD Biosciences) and analyzed by FACS Diva and FlowJo software version 6.1.1 (BD Biosciences).
To measure the phosphorylation status of p38, STAT3, and STAT5, cells were cultured for 24 h without serum. Then, 1% FBS was added with or without murine recombinant GPNMB (R&D cat no. 2330-AC) and with anti-CD44 and anti-IgG (Bio X Cell, clone IM7, cat no. BE0039 and clone LTF2, cat no. BE0090, respectively). We collected the cells 15 min after incubation to the antibodies and used a human T-cell activation kit (BD Bioscience cat no. 567050) following the manufacturer’s instructions. The data were acquired with a FACS Canto II (BD Biosciences) flow cytometer and analyzed by FACS Diva and FlowJo software version 6.1.1 (BD Biosciences).
Immunoprecipitation
The cells were lysed with NP40 lysis buffer (3 ml of NaCl 5 M, 10 mL of 10% NP40, 5 ml of 1 M Tris at pH 8, and 82 ml of H2O) supplemented with protease inhibitor. The proteins were quantified using a BCA protein assay kit (Thermo Fisher Scientific). Two hundred micrograms of protein was immunoprecipitated with 5 µg of anti-CD44 antibody (Abcam AB24504) previously bound to Dynabeads according to the manufacturer’s instructions (Dynabeads protein G, Thermo Fisher Scientific). The beads were washed three times in washing buffer (PBS with Tween 0,1%.) All the lysates were resuspended and denatured in Laemmli buffer at 95 °C for 5 min and separated by SDS-PAGE. The gels were transferred to nitrocellulose membranes (Bio-Rad) and blocked in 5% skim milk in TBS for 1 h prior to antibody incubation. The membranes were incubated overnight with primary antibodies (anti-GPNMB, AF2330 R&D) at 4 °C in 5% skim milk in TBS. An appropriate HRP‐conjugated secondary antibody (donkey anti-goat IgG conjugated with HRP, Invitrogen) was added for 1 h at room temperature. The proteins were detected with Immobilion ECL ultra substrate (Millipore).
Microarray analysis
A total of 150 ng of RNA was labeled with Cy3 using a Low Input Quick Amp Labeling Kit (Agilent Technologies, Palo Alto, CA, USA) and hybridized according to the manufacturer’s instructions. After 20 h of incubation at 65 °C while rotating at 20 rpm, arrays were washed and scanned with a laser confocal scanner (G2565B, Agilent Technologies, Santa Clara, USA). The data quality was assessed with arrayQualityMetrics software.32 the Data were processed with the limma package33 without background removal or quantile normalization. The appropriate corrections for technical replicates were applied. Differentially expressed genes (DEGs) were defined with a p value less than 0.01 and a q value less than 0.05. The DEGs were annotated through the database mmAgilentDesign028005.db made with AnnotationForge (Carlson M, Pagès H (2019). AnnotationForge: Tools for building SQLite-based annotation data packages. R package version 1.26.0). A pathway analysis was performed with the Bioconductor package clusterProfiler34 by querying the KEGG database.35 The pathways were deemed significant with a q value less than 0.05. In accordance with the MIAME guidelines, array data files were submitted to Array Express, ID E-MTAB-8082.
Statistical analysis
Statistical analysis was performed using the paired or unpaired Student’s t test with or without correction or one-way ANOVA; for multiple comparisons, we used a multiple t-test. P values less than 0.05 were considered significant.
Results
Soluble GPNMB is preferentially produced by M2-polarized macrophages and tumor-conditioned macrophages
In a previous analysis with human monocytes differentiated into macrophages in the presence of tumor-condition media (tumor-CM), we identified Gpnmb as among the most upregulated genes.7 From our Affydata gene array database, we evaluated the transcriptional profiles of monocytes, macrophages, and tumor-conditioned macrophages (TC-macro) obtained by coculturing with M-CSF-containing tumor supernatants. Gpnmb was expressed at higher levels in the rhM-CSF-differentiated M2-polarized macrophages and TC-macros (Fig. S1a). The protein GPNMB is located in the cell membrane and can be cleaved by proteases. The levels of soluble protein produced by human and murine macrophages were quantified by ELISA. Human monocytes exposed to the CM from pancreatic tumor cell lines (PT45 and PANC1) produced substantial levels of soluble GPNMB (Fig. S1b). In addition, M2-polarized macrophages produced GPNMB at higher levels than M1 macrophages (Fig. S1b). Similar findings were observed with bone marrow-derived murine macrophages (Fig. S1c) cultured in the presence of CM from mouse tumor cells (PANC02 and the fibrosarcoma MCA-2 or MN/MCA-1).
GPNMB expressed by TAMs is associated with aggressive tumor behavior
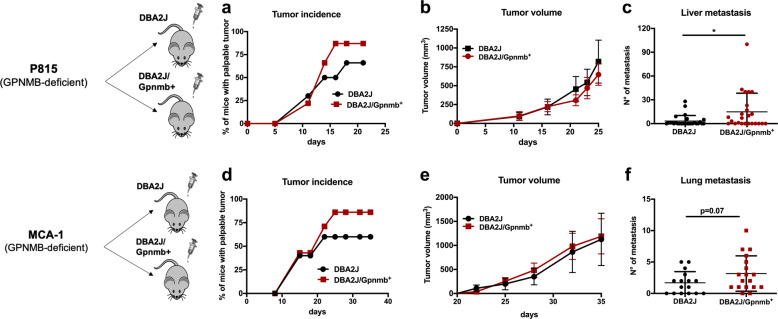
To investigate the functional role of GPNMB produced by macrophages in a tumor context in vivo, we used DBA/2J mice, a strain defective in Gpnmb due to a spontaneous mutation,36 and reconstituted functional GPNMB in the DBA/2J/Gpnmb+ mice. The syngeneic mastocytoma P815 (GPNMB defective) was inoculated i.m. (5 × 104 cells) in both mouse strains. Figure 1a shows that tumor incidence was increased in the DBA/2J/Gpnmb+ mice, in which stromal cells produced the protein, and that tumor growth, measured by volume, did not differ in the two mouse strains (Fig. 1b), while the number of spontaneous liver metastases was significantly higher in the DBA/2J/Gpnmb+ mice, with some mice having 40 or more spontaneous metastases in their liver (Figs. 1c and S2a).
Fig. 1.
Tumor incidence and metastases are increased in the DBA/2J/Gpnmb+ mice. In vivo growth of mouse tumors derived from cells with defective GPNMB production (P815 and MCA-1 primary methylcholanthrene-induced fibrosarcoma) in the DBA/2J mice (GPNMB defective) and in the DBA/2J/Gpnmb+ mice with GPNMB reconstituted. Tumor cells were inoculated intramuscularly at 5 × 104 cells. a P815 cells, tumor incidence; b tumor growth (10 mice/group mean ± SEM); c number of spontaneous liver metastases (three experiments, mean ± SD, total of 24 and 28 mice); d MCA-1 cells, tumor incidence; e tumor growth (10 mice/group mean ± SEM); f number of spontaneous lung metastases (three experiments, mean ± SD, total of 16 and 18 mice). Statistical analysis: t-test with Welch’s correction, ∗p < 0.05
To confirm these results, we generated 3-methylcholanthrene-induced fibrosarcoma in Gpnmb-deficient DBA/2J mice. One fibrosarcoma cell line (MCA-1) was established with a few transplants in vivo and was inoculated i.m. (5 × 104 cells) into both mouse strains. As observed in the P815 experiments, the tumor incidence and spontaneous lung metastases were increased in the competent DBA/2J/Gpnmb+ mice (Fig. 1d–f). The histological analysis of the tumor sections revealed immunoreactivity for GPNMB in the tumor stroma of the DBA/2J/Gpnmb+ mice (in the P815 tumors and especially the MCA-1 tumors); as expected, GPNMB was not detectable in the DBA/2J mice (Fig. S2c, d). F4/80-positive TAMs were indeed able to produce GPNMB (Fig. S2b). Notably, the global infiltration of F4/80+ TAMs was similar in the two strains, although the MCA-1 tumors implanted in the DBA/2J/Gpnmb+ mice showed a slightly higher number of TAMs (Fig. S2c, d). Taken together, these data indicate that the expression of GPNMB in TAMs was associated with increased tumor incidence and with the acquisition of aggressive tumor behavior and increased metastasis formation.
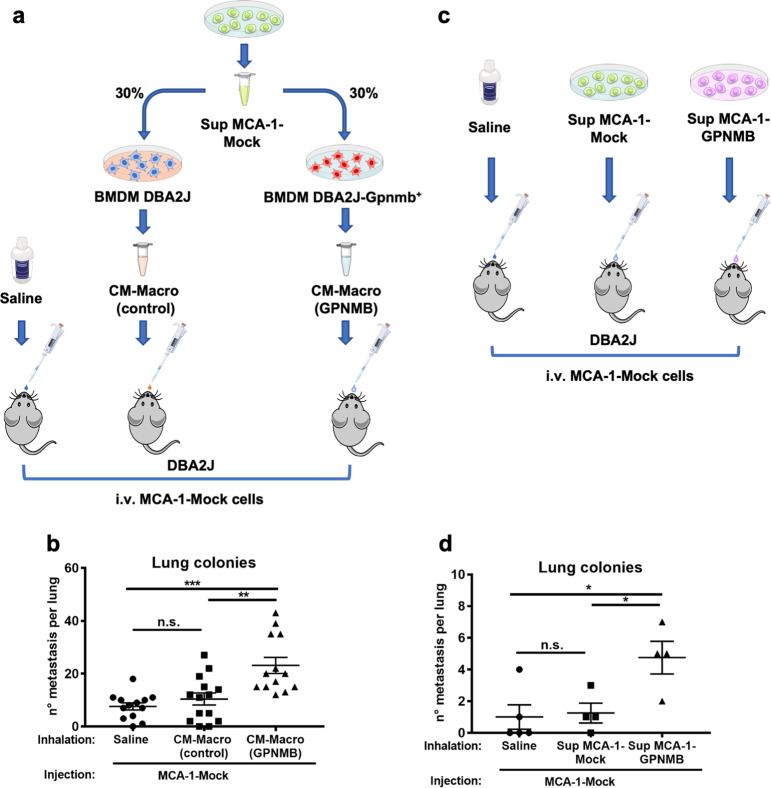
To further investigate in vivo the tumor-promoting effects of GPNMB produced by macrophages, we used CM from mouse macrophages that were induced to produce GPNMB in coculture with MCA-1 tumor cells. We hypothesized that concentrated GPNMB-containing CM administered by nasal inhalation could act in mouse lungs as a niche factor promoting the seeding and survival of intravenously injected tumor cells. BM-derived macrophages from DBA/2J/Gpnmb+ mice were cultured with tumor supernatant from the MCA-1 cells to induce the production of GPNMB; macrophages from the DBA/2J mice (deficient for GPNMB) were used as controls (Fig. 2a). Macrophage-conditioned medium (CM-macro) was concentrated in Vivaspin tubes with a 50 kDa MW cut-off threshold. ELISA quantification showed, as expected, no production of GPNMB in the concentrated medium from the control DBA/2J-macrophages and as much as 1.7 μg/ml produced in the CM from the DBA/2J/Gpnmb+ macrophages.
Fig. 2.
Macrophage-derived GPNMB increases the lung colonization of GPNMB-deficient MCA-1 tumor cells. a Conditioned medium (CM) from DBA/2J GPNMB-deficient macrophages (CM-macro control) or from DBA/2J/Gpnmb+ macrophages containing GPNMB (CM-macro GPNMB) was concentrated >100× in 25 µl. DBA/2J GPNMB-deficient mice inhaled concentrated macrophage-derived CM prior to injection i.v. with 105 MCA-1 sarcoma cells (GPNMB-deficient). Untreated mice inhaled saline solution. b Increased lung colonization of tumor cells in mice that inhaled CM-macro GPNMB. Results are shown for two independent experiments (total of 13–14 mice per group), with each dot representing an individual mouse. c The same type of experiment was performed with the tumor cell supernatant from GPNMB-transduced cells (Sup MCA-1-GPNMB) or mock-transduced cells (Sup MCA-1-Mock cells). d Mice that inhaled GPNMB-containing tumor cell supernatant had more lung colonies. Statistical analysis: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (b, d One-Way ANOVA). Data are calculated as the mean ± SEM
MCA-1 cells (GPNMB-deficient) were injected i.v. (105) in DBA/2J mice 10 min after nasal inhalation (25 μl) of GPNMB-containing CM-macro (~0.2 µg/kg) from the DBA/2J/Gpnmb+ mice or control CM from the DBA/2J-deficient mice. After 2 weeks, the results indicated that the mice that received GPNMB-containing CM-macro developed a significantly higher number of tumor colonies in their lungs (Fig. 2b). CM from the DBA/2J mice had the same effect as saline solution.
The results demonstrate that macrophage-produced GPNMB in the lung microenvironment acts as a paracrine factor on tumor cells to facilitate their survival, seeding and growth.
The same experiment was repeated using CM from MCA-1 cells transduced or not transduced with the Gpnmb gene (see below for characterization). In this case, a greater number of tumor colonies was observed in the lungs of mice that inhaled the CM from the MCA-1-GPNMB cells compared to those that inhaled CM from the MCA-1-mock cells or saline (Fig. 2c, d).
GPNMB activates several survival pathways in cancer cells through the CD44 receptor
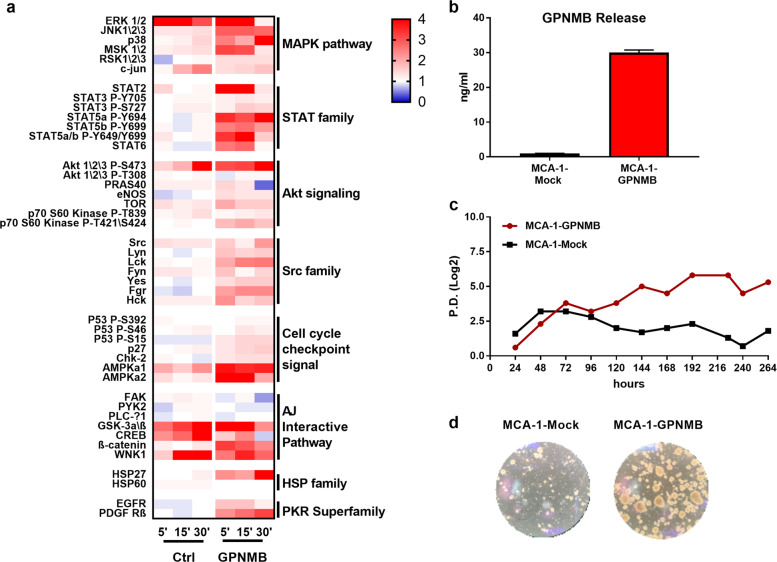
To understand which pathways are activated by GPNMB in tumor cells, we generated a phosphoproteomic profile of the MCA-1-sarcoma cells starved for 24 h and then exposed to recombinant murine GPNMB (50 ng/ml). The cells treated with GPNMB stimulated several pathways compared to the untreated cells (Fig. 3a). In particular, the most activated pathways belonged to the MAPK, STAT, Akt, and Src families.
Fig. 3.
GPNMB activates several pro-survival biochemical pathways in cancer cells. a Heatmap showing the phosphorylation profile of 45 proteins in MCA-1 sarcoma cells. Cells were starved without serum for 24 h and then treated or not with recombinant murine GPNMB (50 ng/ml) for the indicated times (5, 10, and 30 min). b ELISA quantification of the soluble isoform of GPNMB released by MCA-1-GPNMB and MCA-1-mock cells. c In vitro survival of MCA-1-GPNMB cells under conditions of serum deprivation over time. Data are expressed as population doubling (PD). d Representative images of spheroids from the MCA-1-GPNMB cells cultured in IMDM medium without serum and supplemented with N2, B27, bEGF, and FGF
The phosphorylation pattern of MAPKs and STATs, as well as HSP27 and β-catenin, suggested that GPNMB is a proliferation- and survival-inducing stimulus. The phosphorylation of AMPK (a1 and a2)—linked to survival under metabolic stress37—was also increased. The phosphorylation of Src family members has been associated with tumor metastases,38 a finding in line with the metastasis-promoting effect of GPNMB observed in our in vivo experiments. Together, these findings indicate that several pathways supporting cell survival, proliferation, and mobility are activated by GPNMB in tumor cells.
The results of the phosphoproteomic profiling also offered insights into the receptor used by GPNMB in our MCA-1 tumor model. GPNMB has different domains for binding to heparin, integrins, syndecan-4, and CD44.19,39–42 Binding of GPNMB to integrins leads to the activation of the FAK pathway.39,42,43 Because FAK phosphorylation was downregulated in the treated cells, we further explored the CD44/GPNMB axis. Using flow cytometry, we detected that MCA-1-sarcoma cells expressed high levels of CD44 on their surface (Fig. S3a). The activation of signaling downstream of CD44 was investigated in MCA-1 cells treated with GPNMB for 5–15 min and stained with mAb for detecting phospho-proteins. An increase in phospho-p38, phospho-STAT3 and phospho-STAT5 was detected in the treated cells (Fig. S3b), but only p38 was decreased upon pretreatment with the anti-CD44 mAb (Fig. S3c). To corroborate this finding, which is in line with data in the literature,44,45 we immunoprecipitated GPNMB-treated MCA-1 cells with anti-CD44 and subjected them to immunoblotting with anti-GPNMB. WB demonstrated the binding of soluble GPNMB to CD44 (Fig. S3d, e). The results demonstrate that, in our cellular system, GPNMB binds to the CD44 receptor to initiate its downstream effects.
To investigate the direct role of GPNMB in tumor cells, we overexpressed it in GPNMB-deficient MCA-1 fibrosarcoma cells. The murine Gpnmb gene was cloned in a lentiviral pRRL-Sin plasmid in which eGFP had been replaced with mCherry (Fig. S4a); after transduction, the mCherry-positive cells were selected by FACS sorting, propagated in vitro and characterized. MCA-1-GPNMB cells had high protein expression (Fig. S4b) and released as much as 30 ng/ml protein in soluble form (Fig. 3b). In the GPNMB-transduced cells, mCherry fluorescence was mainly localized at the cell membrane, while in the mock-transduced cells, mCherry was indicated by intracytoplasmic fluorescence (Fig. S4c).
To characterize the in vitro survival-promoting ability of GPNMB, MCA-1-mock and MCA-1-GPNMB cells were cultured under conditions of prolonged starvation. After 4–5 days, we noted that MCA-1-GPNMB cells quickly detached from the plastic and continued to proliferate in an anchorage-independent manner, forming spontaneous spheroids, while MCA-1-mock cells progressively died (Fig. 3c, d). By day 11, almost all the MCA-1-mock cells were dead, while spheres from the transduced cells were continuously proliferating. When transferred to low adherence plates with IMDM-supplemented medium, cells in the spheres formed secondary and tertiary spheroids. Sphere formation is characteristic of cancer stem cells; we hypothesized that GPNMB induced the expansion of an intrinsic population of cancer stem cells among the MCA-1-transduced cells.
Soluble GPNMB produced by macrophages activates a stemness program in the MCA-1-mock cells
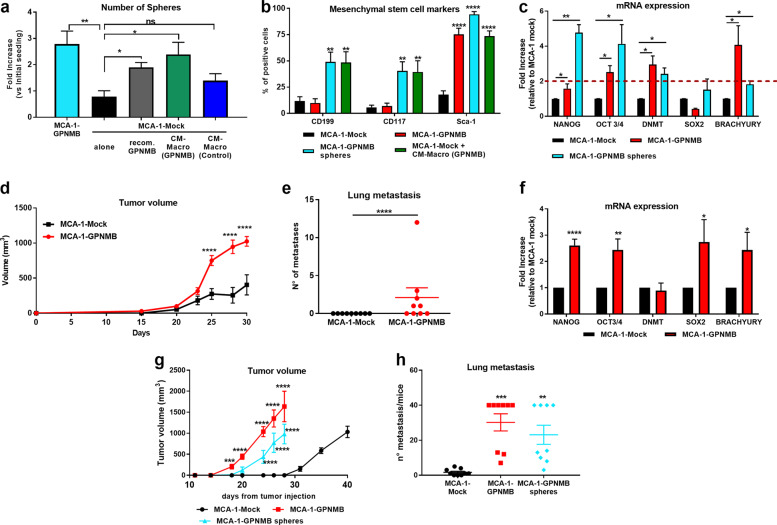
We next evaluated whether the soluble GPNMB produced by macrophages had the same sphere-forming effect as that produced by the GPNMB-transduced tumor cells. The CM of tumor-conditioned macrophages from the DBA/2J/Gpnmb+ mice or DBA/2J mice was prepared and concentrated as described above. MCA-1-mock cells were treated daily with CM-macro from the DBA/2J/Gpnmb+ macrophages (containing ~5 ng/ml GPNMB) or with an equal volume of CM-macro from the DBA/2J-deficient macrophages and observed for 1 week for self-renewal and sphere formation. As depicted in Fig. 4a, the MCA-1-mock cells generated spheres only when the CM-macro contained GPNMB (green bar) and not with the CM-macro from the DBA/2J-deficient mice (blue bar). In addition, the recombinant protein successfully induced the formation of spheres (gray bar), indicating that GPNMB alone was sufficient to promote the expansion of spheroids. The picture also shows for comparison the amount of spontaneous spheres formed by the MCA-1 GPNMB-transduced cells (Fig. 4a, turquoise bar).
Fig. 4.
GPNMB released by macrophages activates a stemness program in the MCA-1-mock cells. a Number of formed spheres in MCA-1-mock cells alone (black) or exposed to the CM from macrophages of DBA/2J/Gpnmb+ mice (CM-macro GPNMB, green) or DBA/2J mice (CM-macro control, blue) is shown. In addition, the number of spheres formed by MCA-1-mock cells exposed to murine recombinant GPNMB (gray) and by transduced MCA-1-GPNMB cells (turquoise) is shown. Results are expressed as the fold increase versus the number of initial cells seeded. Mean ± SD of three different experiments. b Flow cytometry analysis of stemness-related markers (CD199, CD117 and Sca1) in the MCA-1-Mock or MCA-1-GPNMB cells (black, red), in spheres derived from the MCA-1-GPNMB cells (turquoise) and in the MCA-1-mock cells treated with the GPNMB-containing CM from macrophages (green). (Mean ± SD six different experiments). c mRNA levels of stemness-related transcription factors in MCA-1-mock cells, MCA-1-GPNMB cells and their derived spheres. d Tumor growth of the MCA-1-GPNMB or MCA-1-mock cells injected i.m. (105) into DBA/2J mice. e Number of spontaneous lung metastases; each dot represents an individual mouse. Results are from one representative experiment of two perfomed with similar results. f mRNA quantification of the indicated transcription factors in the tumors formed by the MCA-1-mock and MCA-1-GPNMB cells grown in DBA/2J mice (5 mice/group). Data are expressed as fold increase relative to the MCA-1-mock tumors. All samples were tested in triplicate. g Tumor growth of MCA-1-mock cells, MCA-1-GPNMB cells and their derived spheres injected i.m. (102) into NSG mice. h Number of spontaneous lung metastasis. Each dot represents an individual mouse (pooled from two experiments). Statistical analysis: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 (one-way ANOVA, b–d and f–g multiple t-test, e and h unpaired t-test with the Welch’s correction). Data are presented as mean ± SEM
Cancer stem cells have specific features, including the expression of pluripotency genes and tissue-specific stemness markers, high tumorigenic ability and resistance to cytotoxic drugs. We therefore evaluated the expression of specific stem cell markers by flow cytometry. CD199 and CD117, two typical markers of undifferentiated mesenchymal cells, were upregulated in the spheres formed by MCA-1-GPNMB cells (Fig. 4b turquoise bars) and by MCA-1-mock cells cultured with GPNMB-containing CM-macro (Fig. 4b, green bars). Stem cell antigen 1 (Sca1) was endogenously expressed at high levels in the adherent MCA-1-GPNMB cells (red bars) but was expressed at a low level in the MCA-1-mock cells (black bars); treatment with macrophage-CM containing GPNMB induced high Sca-1 expression in MCA-1-mock cells (Fig. 4b, green).
Next, we determined the gene expression of other stemness-related markers. The mRNA levels of several pluripotency genes indicated that Nanog, Oct3/4 and DNA methyltransferase (DNMT) were significantly upregulated in the spheres (turquoise bars in Fig. 4c) compared to the MCA-1-mock cells (black bars). The MCA-1-GPNMB adherent cells (red bars) endogenously expressed the selected genes (e.g., Oct3/4, DNMT and Brachyury) prior to sphere formation (Fig. 4c).
To confirm that GPNMB induced the upregulation of a stemness program in different cancer cell types, we transduced two other murine cell lines: AB1 sarcomatoid and AB22 epithelioid mesothelioma cells.46 Transduced AB1 and AB22 mesothelioma cells released soluble GPNMB and were able to form spheres and express higher levels of stem cell markers, in contrast to their mock counterparts (Fig. S5a, b). These results demonstrate that the induction of a cancer stem cell program triggered by GPNMB is reproducible in different tumor cell lines.
We next investigated the in vivo growth of MCA-1 sarcoma cells (mock and GPNMB-transduced) when inoculated into NSG mice and DBA/2J mice. The results demonstrated, as expected, that the GPNMB-producing cells grew remarkably faster and formed lung metastasis, while the mock cells grew slowly and did not metastasize (Fig. S6a, b and Fig. 5d, e). The mRNA levels of the pluripotent genes in the explanted tumors were higher, particularly the Nanog, Oct3/4, Sox2, and Brachyury genes, in the MCA-1-GPNMB-derived tumors than in the MCA-1-mock-derived tumors (Fig. 4f and Fig. S6c).
Fig. 5.
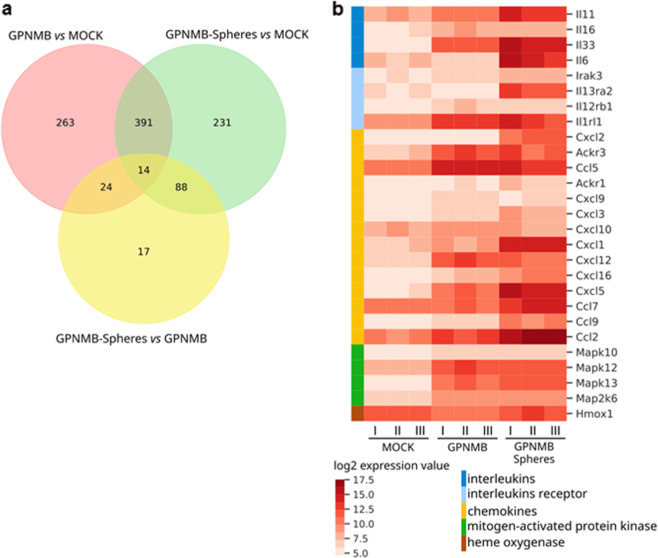
GPNMB activates the expression of several genes and survival pathways. a, b Gene expression analysis of the MCA-1-mock cells, MCA-1-GPNMB cells and their derived spheres. a Venn diagram of differentially expressed genes in three comparisons: MCA-1-GPNMB cells versus MCA-1-mock cells in pink; GPNMB-spheres versus MCA-1-mock cells in green; GPNMB-spheres versus MCA-1-GPNMB cells in yellow. Intersections indicate genes in common in the compared groups. b Heatmap of log2 expression values related to the set of genes indicated in the color legend. Genes are indicated in the rows, and samples are indicated in the columns, with three replicates per group (I, II, III)
Notably, the spheroids formed by MCA-1-GPNMB cells and MCA-1-mock cells treated with macrophage-produced GPNMB showed higher resistance to the cytotoxic effect of doxorubicin compared to MCA-1-mock cells (Fig. S6d), as expected from cells with stem cell features.
Finally, to demonstrate that GPNMB-induced spheroids have high tumorigenic potential, we injected i.m. as few as 100 cells/mouse in NSG mice. The MCA-1-GPNMB cells (red) and the cells derived from spheroids (turquoise) started growing ~17–20 days after inoculation, while the mock cells grew after 30 days (Fig. 4g). Furthermore, as observed in previous experiments, only the GPNMB-expressing tumors generated a high number of lung metastasis (Fig. 4h).
Taken together, the data indicate that GPNMB produced by either tumor cells or macrophages induced the activation of a survival and proliferation program in cancer cells with features of stem cells: upregulation of specific CSC markers, resistance to cytotoxic drugs, high tumorigenicity in vivo and distant spreading.
Mechanistic insights into the stemness-promoting ability of GPNMB
To further understand the signaling pathway driven by GPNMB, we used a microarray analysis to find genes that were differentially expressed in the MCA-1-GPNMB cells (adherent or spheres). Relative to the MCA-1-mock cells, 24 genes were differentially expressed in the adherent MCA-1-GPNMB cells, while 17 genes were expressed in the MCA-1-GPNMB spheres relative to the adherent MCA-1-GPNMB cells (Fig. 5a). Among the most upregulated genes, several were members of interleukin and chemokine families, most notably Il6, Il11, Il33, and its receptor Il1r1, Cxcl1/2, Cxcl5, Ccl2, Ccl5 and Ccl7 (Fig. 5b). In addition, genes involved in the MAPK pathway, a well-known survival pathway, were upregulated (Fig. 5b).
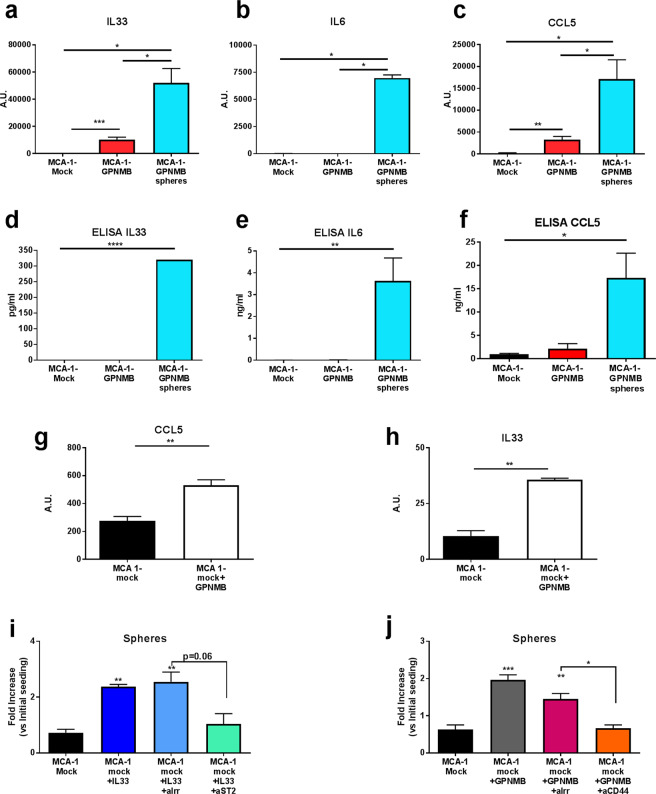
Validation experiments confirmed the high expression of IL-33, IL-6, and CCL5 mRNA, especially in the MCA-1-GPNMB spheroids (Fig. 6a–c). IL-33, IL-6, and CCL5 proteins were released only by sphere-forming cells (Fig. 6d–f). Similarly, the spheroids generated by the two mesothelioma cell lines (AB1 and AB22) transduced with GPNMB produced IL-33 and IL-6 (Fig. S7a, b).
Fig. 6.
GPNMB, via the CD44 receptor, stimulates the expression and release of IL-33, which promotes stemness in the MCA-1-mock cells. a–c Real-time PCR for IL-33, IL-6, and CCL5 (AU: arbitrary units) and d–f ELISA quantification of IL-33, IL-6, and CCL5 in MCA-1-mock cells, MCA-1-GPNMB cells and their derived spheres. g, h Real-time PCR for IL-33 and CCL5 in MCA-1-mock cells treated or not treated with the supernatant from MCA-1-GPNMB cells. i Number of the MCA-1-mock cells forming spheres induced by treatment with recombinant murine IL-33 (10 ng/ml) and the inhibitory effect of blocking by the anti-mST2 mAb (4 µg/ml) or the irrelevant mAb. j Number of MCA-1-mock cells forming spheres induced by recombinant murine GPNMB (10 ng/ml) and inhibitory effect of blocking by the anti-CD44 mAb (10 µg/ml) or irrelevant mAb. MCA-1-mock cells were cultured for 7 days in IMDM medium without serum and supplemented with factors bEGF, FGF, N2, and B27. Statistical analysis: ∗p < 0.05, ∗∗p < 0.01. Unpaired t-test (a–f and i, j) and unpaired t-test with the Welch’s correction (g, h). Data are presented as the mean ± SEM
To investigate the involvement of IL-33 in the GPNMB-induced formation of spheres and acquisition of a stemness phenotype, we first examined whether spheroids from MCA-1 mock cells (upon exposure to tumor CM containing GPNMB) also produced IL-33, as shown in Fig. 6g, h. We next treated MCA-1-mock cells with recombinant murine IL-33, which resulted in a twofold increase in the number of spheroids, and this increase was substantially inhibited upon blocking with anti-IL-1R1L mAb (Fig. 6i), supporting the supposition that IL-33 is involved in the GPNMB-induced activation of a stem cell program in cancer cells. Finally, in the MCA-1 mock cells treated with recombinant murine GPNMB, anti-CD44 completely inhibited sphere formation, confirming that, in our experimental model, soluble GPNMB binds to CD44 to initiate the expansion of spheres (Fig. 6l).
Overall, our results depict a scenario in which the tumor microenvironment macrophages, upon conditioning by tumor cells, produce the protein GPNMB, which triggers crucial pathways of survival and proliferation in cancer cells via its CD44 receptor, increasing the expression and release of IL-33 and ultimately promoting the expansion of cancer stem cells.
Discussion
Substantial evidence indicates that oncology therapy failure and disease recurrence are mainly due to the emergence of surviving cancer stem cells (CSCs).47,48 While several studies have been published on the tumor-promoting roles of macrophages, relatively few have addressed the supporting action of TAMs on CSCs. It is known that TAMs promote CSC formation by creating a protective niche where these cells comfortably reside.49,50 Macrophages also produce several cytokines (e.g., IL-6) or specific growth factors (PDGF, VEGF, and EGF) that enhance CSC survival and facilitate their differentiation into proliferating cells. Other mediators, such as MFG-E8 (milk-fat globule-epidermal growth factor VIII), hCAP-18/LL-37, and Ephrin family members, have also been reported.51,52
In this study, we show that a macrophage soluble product, the protein GPNMB, plays an important role in triggering the expansion of tumor cells with CSC properties. GPNMB-deficient cancer cells (MCA-induced primary sarcoma) exposed to the CM from macrophages containing GPNMB acquired the capacity to survive even in conditions of prolonged nutrient deprivation and spontaneously formed spheroids expressing typical mesenchymal stem cell markers. These sphere-forming cells actively expanded and showed drug resistance in vitro, a typical feature of CSCs.
Our in vivo studies confirmed that TAMs in the tumor microenvironment produce the protein GPNMB. Using two different transplantable mouse models, with GPNMB-negative tumor cells inoculated in mice with reconstituted GPNMB (DBA/2J/Gpnmb+ mice), in which this protein is expressed by stromal cells, we observed a higher number of tumors and spontaneous metastasis. More interestingly, when a preparation of concentrated macrophage-derived GPNMB was inhaled by the Gpnmb-deficient mice, the tumor cells subsequently inoculated i.v. resulted in a significantly higher number of lung tumor colonies. These results were confirmed using the soluble protein produced by GPNMB-transduced cancer cells. GPNMB-tumor cells in vivo showed a dramatic growth advantage and produced a high number of spontaneous lung metastasis. Notably, spheroids from GPNMB-transduced cells also expressed CSC markers, showed tumor-forming ability and extensive metastasis in vivo when as few of 100 cells were transplanted.
The results of our phosphoproteomic analysis with negative tumor cells treated with recombinant GPNMB revealed that several pathways associated with cell survival, proliferation and motility were activated. In addition to MAPKs and STATs, AMPK (a1 and a2) was strongly stimulated. AMPKs are sensors of energy status and are activated by metabolic stress to increase cell survival.37,53 The phosphorylation of Akt and Src family members is known to be linked to the acquisition of metastatic features, an outcome in line with the metastasis-promoting effect of GPNMB, as observed in our in vivo experiments.38,54 EGFR and PDGFR signaling is transduced through STAT5 and Src, and these pathways were also phosphorylated in the treated cells. Another relevant molecule from our analysis was HSP27, a stress-induced molecule with anti-apoptotic functions.55 Overall, these findings support the results that GPNMB-treated or GPNMB-expressing cells have a survival and metastatic advantage.
Our results are in accordance with those of previous studies. Activation of Akt and β-catenin in mouse mammary tumors transduced with GPNMB was also reported by Maric et al.24 In response to GPNMB, ERK is phosphorylated downstream of the CD44 receptor, as reported by Yu et al.42 CD44 is not the sole receptor for GPNMB.19,39–42 In our system, sarcoma cells were highly positive for CD44, and we further demonstrated that GPNMB directly binds CD44 to induce stemness, as blocking anti-CD44 mAbs significantly inhibited GPNMB-induced spheroid formation. It is known that CD44 has a prominent role in tumor progression and in cancer stem cell formation, and GPNMB is confirmed as an important activating ligand.56
Previous in vitro studies indicated that GPNMB/osteoactivin has important biological effects on normal progenitor cells; for instance, it acts as an osteoinductive agent, stimulating osteoprogenitor cells to differentiate into osteoblasts.57 Yu et al. reported that GPNMB also supports the survival, proliferation and migration of mesenchymal stem cells.42
In the tumor context, a promoting effect on tumor growth has been reported by the Siegel’s group. In breast cancer patients, including those with basal and triple-negative tumors, GPNMB expression correlated with reduced overall survival.25 In mouse models, the protein was preferentially expressed in tumor cells metastasizing to bones, and the authors identified upregulated MMP-3 and integrin alpha5beta1 as important molecules.43,58 In a recent paper, the same group, using a MMTV/Wnt-1 mouse model, demonstrated that when GPNMB was transduced into the mammary epithelium, tumor onset was dramatically increased, as characterized by elevated PI3K/AKT/mTOR signaling and increased β-catenin activity.24 Another recent study used a 3D sphere culture method with breast cancer cells. Chen et al., reported that tumor cells with high expression of surface GPNMB presented stem cell-like properties and upregulated EMT-inducing transcription factors. This phenomenon was induced through the hemITAM domain in the intracytoplasmic region of GPNMB.59 Thus, in contrast to our findings with the soluble form of GPNMB, they found that the intracellular portion of GPNMB was involved in the stimulation of cancer cells and acquisition of CSC-like properties.
To gain insights into the mechanism by which GPNMB exerts this CSC-promoting effect, we performed a transcriptomic analysis. Cells transduced with GPNMB, and especially their spheres, expressed IL-33 at much higher levels than mock cells. The involvement of IL-33 in the induction of stemness was confirmed by blocking its specific receptor IL-1R1L. IL-33, a member of the IL-1 superfamily, is a cytokine mainly involved in type 2 innate and adaptive immunity.60–62 IL-33 acts as an “alarmin” when released by necrotic cells after tissue damage, but can also be actively secreted. In addition to immune regulation, this cytokine has known anabolic roles in angiogenesis, osteogenesis, and hematopoiesis.63,64 IL-33 can be expressed during the progression of distinct cancer types (e.g., breast, lung, melanoma and colon cancer). Reports indicate that its role is controversial, context dependent, and may either promote or inhibit tumor growth, with direct effects on cancer and immune cells of the microenvironment.61,65,66 Recent studies have shown that IL-33 is involved in the support of CSCs, for instance, in colon and breast cancer, by stimulating in vitro sphere formation and activating stem cell genes.67–69 High IL-33 expression in cancer tissues, as well as systemic levels as detected by ELISA, correlate with poor patient prognosis.61,68 In normal hematopoietic stem cells, IL-33 supports myeloid progenitors, and its levels are increased in myeloproliferative neoplasms.70 Notably, in acute myeloid leukemia, a fusion oncogene generated by the inversion of chromosome 16 (CBFB-MYH11) induces the gene of the IL-33 receptor (IL-1RL1/ST2). The authors found that IL-1RL1-positive cells were enriched in stem cell activity, and that treatment with IL-33 increased the proliferation and survival of these cells.71 IL-33 signaling via IL-1RL1 activates NF-κB; accordingly, GPNMB-transduced cells producing high levels of IL-33 also secrete IL-6, another cytokine involved in cancer cell stemness, as well as several other inflammatory chemokines.48
Taken together, our results reveal a previously undiscovered molecular axis that is initiated by macrophages exposed to tumor cells and that plays a pivotal role in the survival and growth of cancer stem cells. The mechanism involves tumor-conditioned macrophages producing and releasing soluble GPNMB, which binds to CD44 on the tumor cell surface, and inducing the production of IL-33, ultimately promoting stem cell expansion and the acquisition of a metastatic phenotype in vivo.
Supplementary information
Acknowledgements
This work was supported by IG grants from the Italian Association for Cancer Research (AIRC) to P.A. and grants from the Italian Ministry of Health (GR-2013-02356521) to E.M.B. In addition, E.D. is a recipient of a fellowship from AIRC. We thank Fabio Pasqualini for his help in immunohistochemistry experiments and Marina Sironi and Roberta Migliore for their contributions to the in vivo experiments.
Author contributions
Supervising, researching and designing the study and writing the manuscript: C.B., P.A.; performing in vitro experiments and acquiring data: C.B., M.L., E.D., A.V., F.S.C., V.R., N.P., E.E., M.T., F.M.F.; performing phosphoproteomic assays and protein profiling: E.M.B., S.M., A.C.; performing in vivo experiment and acquiring data: C.B., R.A., E.D., M.L.; performing immunohistochemistry experiments: E.D., M.L.; performing gene expression analysis: L.M., I.C., S.M.; analyzing and interpreting data (e.g., statistical analysis, figures): E.D., C.B.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: M. Liguori, E. Digifico
These authors jointly supervised this work: P. Allavena, C. Belgiovine
Contributor Information
P. Allavena, Email: paola.allavena@humanitasresearch.it
C. Belgiovine, Email: Cristina.belgiovine@humanitasresearch.it
Supplementary information
The online version of this article (10.1038/s41423-020-0501-0) contains supplementary material.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belgiovine C, D’Incalci M, Allavena P, Frapolli R. Tumor-associated macrophages and anti-tumor therapies: complex links. Cell. Mol. life Sci. 2016;73:2411–2424. doi: 10.1007/s00018-016-2166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin. Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 5.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 6.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solinas G, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J. Immunol. 2010;185:642–652. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 8.Mayi TH, et al. Human adipose tissue macrophages display activation of cancer-related pathways. J. Biol. Chem. 2012;287:21904–21913. doi: 10.1074/jbc.M111.315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose AA, et al. ADAM10 releases a soluble form of the GPNMB/Osteoactivin extracellular domain with angiogenic properties. PLoS ONE. 2010;5:e12093. doi: 10.1371/journal.pone.0012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weterman MA, et al. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int. J. Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- 11.Safadi FF, et al. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J. Cell Biochem. 2001;84:12–26. doi: 10.1002/jcb.1259. [DOI] [PubMed] [Google Scholar]
- 12.Shikano S, Bonkobara M, Zukas PK, Ariizumi K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J. Biol. Chem. 2001;276:8125–8134. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- 13.Haralanova-Ilieva B, Ramadori G, Armbrust T. Expression of osteoactivin in rat and human liver and isolated rat liver cells. J. Hepatol. 2005;42:565–572. doi: 10.1016/j.jhep.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J. Immunol. 2007;178:6557–6566. doi: 10.4049/jimmunol.178.10.6557. [DOI] [PubMed] [Google Scholar]
- 15.Abdelmagid SM, et al. Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function. Exp. Cell Res. 2008;314:2334–2351. doi: 10.1016/j.yexcr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Frara N, et al. Transgenic expression of Osteoactivin/gpnmb enhances bone formation in vivo and osteoprogenitor differentiation ex vivo. J. Cell. Physiol. 2016;231:72–83. doi: 10.1002/jcp.25020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, et al. The melanoma-associated transmembrane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair. FASEB J. 2010;24:4767–4781. doi: 10.1096/fj.10-154757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano Y, et al. Glycoprotein nonmetastatic melanoma protein B (GPNMB) as a novel neuroprotective factor in cerebral ischemia-reperfusion injury. Neuroscience. 2014;277:123–131. doi: 10.1016/j.neuroscience.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 19.Neal ML, Boyle AM, Budge KM, Safadi FF, Richardson JR. The glycoprotein GPNMB attenuates astrocyte inflammatory responses through the CD44 receptor. J. Neuroinflamm. 2018;15:73. doi: 10.1186/s12974-018-1100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe H, et al. Transgenic expression of osteoactivin in the liver attenuates hepatic fibrosis in rats. Biochem. Biophys. Res. Commun. 2007;356:610–615. doi: 10.1016/j.bbrc.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa T, et al. Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am. J. Physiol. Cell Physiol. 2005;289:C697–C707. doi: 10.1152/ajpcell.00565.2004. [DOI] [PubMed] [Google Scholar]
- 22.Chung JS, Sato K, Dougherty II, Cruz PD, Jr., Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007;109:4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi M, et al. Blocking monocytic myeloid-derived suppressor cell function via Anti-DC-HIL/GPNMB antibody restores the in vitro integrity of t cells from cancer patients. Clin. Cancer Res. 2019;25:828–838. doi: 10.1158/1078-0432.CCR-18-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maric G, et al. GPNMB augments Wnt-1 mediated breast tumor initiation and growth by enhancing PI3K/AKT/mTOR pathway signaling and beta-catenin activity. Oncogene. 2019;38:5294–5307. doi: 10.1038/s41388-019-0793-7. [DOI] [PubMed] [Google Scholar]
- 25.Maric G, Rose AA, Annis MG, Siegel PM. Glycoprotein non-metastatic b (GPNMB): a metastatic mediator and emerging therapeutic target in cancer. OncoTargets Ther. 2013;6:839–852. doi: 10.2147/OTT.S44906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott PA, et al. A phase 2 study of glembatumumab vedotin, an antibody-drug conjugate targeting glycoprotein NMB, in patients with advanced melanoma. Cancer. 2019;125:1113–1123. doi: 10.1002/cncr.31892. [DOI] [PubMed] [Google Scholar]
- 27.Raggi C, et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J. Hepatol. 2017;66:102–115. doi: 10.1016/j.jhep.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose AA, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin. Cancer Res. 2010;16:2147–2156. doi: 10.1158/1078-0432.CCR-09-1611. [DOI] [PubMed] [Google Scholar]
- 29.Fiorentini C, et al. GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity. Exp. Cell Res. 2014;323:100–111. doi: 10.1016/j.yexcr.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Truong DD, et al. A human organotypic microfluidic tumor model permits investigation of the interplay between patient-derived fibroblasts and breast cancer cells. Cancer Res. 2019;79:3139–3151. doi: 10.1158/0008-5472.CAN-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Germano G, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Kauffmann A, Gentleman R, Huber W. arrayQualityMetrics—a bioconductor package for quality assessment of microarray data. Bioinformatics. 2009;25:415–416. doi: 10.1093/bioinformatics/btn647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson MG, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat. Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- 37.Zadra G, Batista JL, Loda M. Dissecting the dual role of AMPK in cancer: from experimental to human studies. Mol. Cancer Res. 2015;13:1059–1072. doi: 10.1158/1541-7786.MCR-15-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel A, Sabbineni H, Clarke A, Somanath PR. Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis. Life Sci. 2016;157:52–61. doi: 10.1016/j.lfs.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu H, et al. Osteoactivin inhibits dexamethasone-induced osteoporosis through up-regulating integrin β1 and activate ERK pathway. Biomed. Pharmacother. 2018;105:66–72. doi: 10.1016/j.biopha.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 40.Moussa FM, et al. Osteoactivin promotes osteoblast adhesion through HSPG and αvβ1 integrin. J. Cell. Biochem. 2014;115:1243–1253. doi: 10.1002/jcb.24760. [DOI] [PubMed] [Google Scholar]
- 41.Sondag GR, et al. Osteoactivin inhibition of osteoclastogenesis is mediated through CD44-ERK signaling. Exp. Mol. Med. 2016;48:e257. doi: 10.1038/emm.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu B, Sondag GR, Malcuit C, Kim MH, Safadi FF. Macrophage-associated Osteoactivin/GPNMB mediates mesenchymal stem cell survival, proliferation, and migration via a CD44-dependent mechanism. J. Cell Biochem. 2016;117:1511–1521. doi: 10.1002/jcb.25394. [DOI] [PubMed] [Google Scholar]
- 43.Maric G, et al. GPNMB cooperates with neuropilin-1 to promote mammary tumor growth and engages integrin alpha5beta1 for efficient breast cancer metastasis. Oncogene. 2015;34:5494–5504. doi: 10.1038/onc.2015.8. [DOI] [PubMed] [Google Scholar]
- 44.Furochi H, et al. Osteoactivin fragments produced by ectodomain shedding induce MMP-3 expression via ERK pathway in mouse NIH-3T3 fibroblasts. FEBS Lett. 2007;581:5743–5750. doi: 10.1016/j.febslet.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharyya S, Feferman L, Tobacman JK. Inhibition of phosphatase activity follows decline in sulfatase activity and leads to transcriptional effects through sustained phosphorylation of transcription factor MITF. PLoS ONE. 2016;11:e0153463. doi: 10.1371/journal.pone.0153463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mezzapelle R, et al. Human malignant mesothelioma is recapitulated in immunocompetent BALB/c mice injected with murine AB cells. Sci. Rep. 2016;6:22850. doi: 10.1038/srep22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge Y, Fuchs E. Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat. Rev. Genet. 2018;19:311–325. doi: 10.1038/nrg.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolli E, Movahedi K, Laoui D, Van Ginderachter JA. Novel insights in the regulation and function of macrophages in the tumor microenvironment. Curr. Opin. Oncol. 2017;29:55–61. doi: 10.1097/CCO.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 50.Sica A, Porta C, Amadori A, Pasto A. Tumor-associated myeloid cells as guiding forces of cancer cell stemness. Cancer Immunol. Immunother. 2017;66:1025–1036. doi: 10.1007/s00262-017-1997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jinushi M, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl Acad. Sci. USA. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu H, et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 2014;16:1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardie DG. AMPK—Sensing energy while talking to other signaling pathways. Cell Metab. 2014;20:939–952. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiao M, Sheng S, Pardee AB. Metastasis and AKT activation. Cell Cycle. 2014;7:2991–2996. doi: 10.4161/cc.7.19.6784. [DOI] [PubMed] [Google Scholar]
- 55.Choi K, Kim Park L. Targeting heat shock protein 27 in cancer: a druggable target for cancer treatment? Cancers. 2019;11:1195. doi: 10.3390/cancers11081195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Zuo X, Xie K, Wei D. The role of CD44 and cancer. Stem Cells. 2018;1692:31–42. doi: 10.1007/978-1-4939-7401-6_3. [DOI] [PubMed] [Google Scholar]
- 57.Abdelmagid SM, et al. Mutation in osteoactivin decreases bone formation in vivo and osteoblast differentiation in vitro. Am. J. Pathol. 2014;184:697–713. doi: 10.1016/j.ajpath.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rose AA, et al. Osteoactivin promotes breast cancer metastasis to bone. Mol. Cancer Res. 2007;5:1001–1014. doi: 10.1158/1541-7786.MCR-07-0119. [DOI] [PubMed] [Google Scholar]
- 59.Chen C, et al. Glycoprotein nmb Is exposed on the surface of dormant breast cancer cells and induces stem cell-like properties. Cancer Res. 2018;78:6424–6435. doi: 10.1158/0008-5472.CAN-18-0599. [DOI] [PubMed] [Google Scholar]
- 60.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 62.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50:778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi YS, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114:3117–3126. doi: 10.1182/blood-2009-02-203372. [DOI] [PubMed] [Google Scholar]
- 64.Kenswil KJG, et al. Characterization of endothelial cells associated with hematopoietic niche formation in humans identifies IL-33 As an anabolic factor. Cell Rep. 2018;22:666–678. doi: 10.1016/j.celrep.2017.12.070. [DOI] [PubMed] [Google Scholar]
- 65.Afferni C, et al. The pleiotropic immunomodulatory functions of IL-33 and its implications in tumor immunity. Front. Immunol. 2018;9:2601. doi: 10.3389/fimmu.2018.02601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen JX, Liu J, Zhang GJ. Interleukin-33 in malignancies: friends or foes? Front. Immunol. 2018;9:3051. doi: 10.3389/fimmu.2018.03051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang M, et al. IL33 promotes colon cancer cell stemness via JNK activation and macrophage recruitment. Cancer Res. 2017;77:2735–2745. doi: 10.1158/0008-5472.CAN-16-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu H, et al. IL-33 facilitates endocrine resistance of breast cancer by inducing cancer stem cell properties. Biochem. Biophys. Res. Commun. 2017;485:643–650. doi: 10.1016/j.bbrc.2017.02.080. [DOI] [PubMed] [Google Scholar]
- 69.Xie C, et al. Tobacco smoke induced hepatic cancer stem cell-like properties through IL-33/p38 pathway. J. Exp. Clin. Cancer Res. 2019;38:39. doi: 10.1186/s13046-019-1052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mager LF, et al. IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J. Clin. Investig. 2015;125:2579–2591. doi: 10.1172/JCI77347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Richter L, Becker M, Amador C, Hyde RK. IL1RL1 is dynamically expressed on Cbfb-MYH11(+) leukemia stem cells and promotes cell survival. Sci. Rep. 2019;9:1729. doi: 10.1038/s41598-018-38408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.