Abstract
Abnormal activation of the cyclin-dependent kinases (CDKs), which result in aberrant cell proliferation, is one of the inherent characteristics of tumor. Thus targeting the activity of CDKs represents a promising tumor therapeutic strategy. Currently, the specific inhibitors that target CDK4 and CDK6 have been approved for the treatment of estrogen receptor positive, human epidermal growth factor receptor 2 negative (ER+ HER2−) breast cancer in combination with endocrine therapy; other combination strategies are being tested in a number of clinical trials. However, the acquired resistance to CDK4/6 inhibitors has emerged. As the cell cycle is orchestrated by a series of biological events, the alterations of other molecular events that regulate the cell cycle progression may be involved in intrinsic resistance to CDK4/6 inhibitors. In this review we mainly discuss the mechanisms underlying intrinsic resistance and acquired resistance to CDK4/6 inhibitors as well as combination strategies with other signal pathway inhibitors being tested in clinical and pre-clinical studies, to extend the use of CDK4/6 inhibitors in tumor treatment.
Keywords: cancer, cell cycle, Rb, CDK4/6 inhibitors, intrinsic resistance, acquired resistance
Introduction
Aberrant cell cycle activity is one of central hallmarks of cancer [1]. CDK4 and CDK6 combine with D-type cyclins to control cell cycle progression in the early G1 phases. Early in the G1 phase, cyclins D1, D2, and D3 combine with CDK4 and CDK6 activated by promitotic signaling, and this complex subsequently phosphorylates retinoblastoma (Rb) protein, which in turn releases E2F transcription factors to allow for transcription of E-type cyclins and other proteins, for example, CDK2. Subsequently, the cyclin E-CDK2 complex further phosphorylates Rb, promoting S-phase entry [2]. In this process, the activity of the cyclin D-CDK4/6 complex is negatively regulated by cyclin-dependent kinase inhibitors (CKIs). Some CKIs are members of the inhibitor of CDK4 (INK4) family, which consists of four proteins: p16INK4A, p15INK4B, p18INK4C and p19INK4D, and these proteins can specifically bind to CDK4 and CDK6 to prevent their association with D-type cyclins, thereby inhibiting the activity of CDK4 and CDK6 [3–5].
The cyclin D-CDK4/6-Rb pathway is commonly mutated in human cancers [6]. In ER+ breast cancer, multiple oncogenic signals promote cyclin D1 expression and activate CDK4/6 to drive breast cancer progression [7]. Endocrine therapy is the main treatment for ER+ breast cancer. Previous studies suggested that cyclin D1 and CDK4 remain active against endocrine therapy [8]. Preclinical studies found that ER+ breast cancers harboring elevated expression of cyclin D1 and Rb protein were sensitive to palbociclib [9]. Palbociclib was the first CDK4/6 inhibitor to demonstrate clinical efficacy in ER+ breast cancer, and ~60%–70% of all breast cancers respond to palbociclib [10]. Subsequently, two other CDK4/6 inhibitors followed. Ribociclib is very similar in structure to palbociclib and is also used in combination with hormone therapy. While abemaciclib is structurally less similar to both ribociclib and palbociclib, and it can be adopted as a monotherapy [11, 12]. Compared to endocrine therapy, this combination therapy indeed improved the overall survival time, but ~25%–35% of patients did not respond, and almost all patients eventually acquired resistance [13]. Therefore, it is urgent to find the mechanism(s) of drug resistance and choose appropriate strategies against acquired resistance.
This review mainly discusses the mechanisms of resistance to CDK4/6 inhibitors from two aspects: (1) intrinsic resistance mechanisms including amplification or mutation of certain genes in the cyclin D-CDK4/6-Rb pathway; and (2) acquired resistance mechanisms such as activation of other compensatory signaling pathways, tumor metabolism and the tumor immune microenvironment. In addition, this review will summarize the strategies for combating CDK4/6 inhibitors resistance and that are under clinical development in various tumor types.
The intrinsic resistance mechanisms
Currently, the CDK4/6 inhibitors: palbociclib, ribociclib, and abemaciclib have been approved in combination with endocrine therapy as standard treatment for ER+ breast cancer. In Table 1, we summarized the specific CDK4/6 inhibitors used in combination with endocrine therapy in advanced HR+HER2− breast cancer in various clinical settings.
Table 1.
CDK4/6 inhibitors in combination with endocrine therapy in advanced HR+ HER2− breast cancer in various clinical settings.
| Drug | Combination | Line | Menopausal status | Clinical settings |
|---|---|---|---|---|
| Palbociclib | Letrozole | 1st | Post | PALOMA-2 |
| Fulvestrant | 2nd | Pre and Post | PALOMA-3 | |
| Ribociclib | Letrozole | 1st | Pre | MONALEESA-7 |
| Letrozole | 1st | Post | MONALEESA-2 | |
| Fulvestrant | 1st and 2nd | Post | MONALEESA-3 | |
| Abemaciclib | Letrozole | 1st | Post | MONARCH 3 |
| Fulvestrant | 2nd | Pre and Post | MONARCH 2 | |
| – | 2+ | Pre | MONARCH 1 |
As CDK4/6 inhibitors perform their anti-tumor activities by inhibiting the Rb pathway, Rb loss or CDK4 amplification is involved in intrinsic resistance. Additionally, other elements that participate in regulating cell cycle or bypassing the cyclin D-CDK6-Rb pathway such as CDK2 overexpression, and other signaling pathways that directly regulate the cell cycle have the potential to contribute to CDK4/6 inhibitors resistance.
The Rb-E2F pathway
Rb loss
Rb loss has been shown to facilitate cell cycle progression, compromising G1/S arrest [14]. Finn et al. detected that breast tumor cell lines with high expression of Rb and cyclin D1 and relatively low expression of p16 were sensitive to PD0332991 (palbociclib) and showed that Rb loss yielded therapeutic failure [9]. Similarly, Konecny et al. demonstrated that cancer cell lines that were sensitive to PD0332991 (palbociclib) exhibited functional Rb and decreased p16 and cyclin E1 expression by testing many ovarian cancer cell lines [15].
In human papilloma virus (HPV)-positive cervical cancer and head and neck cancers, the viral oncoprotein E7 promotes RB protein degradation and disrupt its function, leading to accumulation of p16INK4A, which in turn results in resistance to CDK4/6 inhibitors [16, 17]. However, in advanced bladder cancer, regardless of Rb status, palbociclib as a monotherapy or in combination with cisplatin has demonstrated significant efficacy and antitumor effects. Mechanically, palbociclib exerted these antitumor effects by inhibiting FOXM1 phosphorylation [18].
By analyzing circulating tumor DNA (ctDNA) from the breast cancer patients who had been treated with a CDK4/6 inhibitor for several months, Condorelli et al. detected acquired RB mutations, and these alterations could lead to Rb functional loss, conferring CDK4/6 inhibitor resistance [19].
E2F
The overexpression of E2F activating transcription factors is capable of bypassing CDK4/6 inhibition, directly driving DNA replication and mitosis, which is involved in the inherent resistance to CDK4/6 inhibitors [20]. In letrozole-resistant ER+ breast cancers, the activity of E2F4 was increased, and most of the E2F4 target genes were upregulated; in addition, treatment with palbociclib in letrozole-resistance patients before surgery significantly decreased the expression of E2F4 target genes [21]. In BRAF-mutant and NRAS-mutant melanomas, E2F reactivation has been identified as the mechanism by which tumors acquire resistance to combined MEKi and CDK4/6i [22].
CDKs
CDK4
Amplification of CDK4 exists in a number of tumors, for example, approximately 50% of glioblastomas (GBMs) bear CDK4 amplification, and in melanomas, one specific point mutation, R24C in CDK4, leads to constitutively activated CDK4, which in turn results in CDK4 being insensitive to inhibition by INK4 family members [23, 24]. In rhabdomyosarcoma (RMS), CDK4 amplification and overexpression could reduce the sensitivity of ribociclib [25]. However, in CDK4-amplified liposarcoma and neuroblastoma, palbociclib and ribociclib have shown significant anti-proliferative activity [26, 27]. Additionally, the T172 phosphorylation of CDK4 was shown to control the cell cycle process, and breast tumors harboring high T172 phosphorylation of CDK4 were more sensitive to palbociclib than those without CDK4 T172 phosphorylation [28].
CDK2
Many tumors show noncanonical cyclin D1-CDK2-mediated S-phase entry. In addition, the noncanonical cyclin D1-CDK2-mediated S-phase transition, in part, can confer resistance to CDK4/6 inhibitors in ER-positive breast cancer [29, 30]. The luminal androgen receptor (LAR) subtype, a subtype of triple-negative breast cancer (TNBC), was sensitive to CDK4/6 inhibitors initially but subsequently developed drug resistance. Compared to that in the sensitive-cells, higher levels of CDK2 activity have been demonstrated in resistant cells. In addition, PI3K inhibition antagonized CDK2 activity and re-sensitized tumors to CDK4/6 inhibitors [31].
Cyclins
Cyclin D1
The CCND1 3’UTR mutation in endometrial cancer has been shown to increase CCND1 expression and can activate D-type cyclins, which enhances the sensitivity to the CDK4/6 inhibitor abemaciclib [32]. In the phase II Paloma 1 study, all enrolled patients harbored cyclin D1 amplification because cyclin D1 overexpression presents the potential for increased CDK4/6 inhibitor sensitivity. The treatment results were not as expected, and these selected patients did not show better therapeutic effects than unselected patients [10].
The SWI/SNF chromatin remodeling protein SMARCA4 (BRG1), is frequently lost in the cells of small-cell carcinoma of the ovary, hypercalcemic type (SCCOHT), a subtype of ovarian cancer. SMARCA4 loss was shown to cause cyclin D1 deficiency in SCCOHT cells, in turn leading to high sensitivity to CDK4/6 inhibitors [33]. In addition, researchers have found that losses of SMARCA4 and SMARCA2 also exist in a subgroup of non-small-cell lung cancer (NSCLC), and these losses restricted CCND1 mRNA expression, suppressed the activity of c-Jun to reduce cyclin D1 expression and increased the sensitivity to CDK4/6 inhibitors [34].
Cyclin E
Cyclin E overexpression in endometrial cancer and CCNE1 amplification in TNBC were shown to be associated with aggressive tumor grade and poor patient survival [35, 36]. In gastric cancer, cyclin E overexpression conferred resistance to palbociclib [37].
One large-scale clinical trial (NCT01942135) randomly assigned patients to different treatment arms to identify biomarkers associated with the effectiveness of adding palbociclib to fulvestrant in HR+HER2− metastatic breast cancer. This study demonstrated that high CCNE1 mRNA expression was associated with poor anti-proliferative activity of palbociclib, and there were no significant interactions between treatment and the expression levels of CDK4, CDK6, cyclin D1, and Rb. Thus, the level of CCNE1 mRNA was identified as a potential biomarker to predict intrinsic resistance to the combination of palbociclib with fulvestrant [38].
CDK4 inhibitors
p16INK4A
CDKN2A loss is a frequent event in many tumors [39]. In 143 patients with primary invasive melanoma, 56% of patients possessed hemizygous or homozygous loss of CDKN2A. Further research found that in patients with either CDKN2A methylation or CDKN2A mutation, loss of p16INK4A expression was potentially good for palbociclib sensitivity [40]. In sporadic pancreatic ductal adenocarcinoma (PDAC), loss of function mutations in CDKN2A activated CDK4 or CDK6 to promote cell division, but these p16INK4A-deficient PDAC cells showed intrinsic resistance to CDK4/6 inhibitors. Insulin-like growth factor-1 (IGF-1) inhibitors could enhance CDK4/6 inhibitor activity to synergistically inhibit the growth of p16INK4A-deficient PDAC cells [41]. In GBM xenograft models, by analyzing mutation or amplification of the components in the p16INK4A-CDK4-Rb axis, the study indicated that GBMs harboring p16 deficiency, non-amplified CDK4 and wild-type Rb status could be more susceptible to palbociclib than GBMs without these factors [42].
p53
p53 activation can result in cell cycle arrest and negative regulation of cell cycle genes at the transcription level [43]. The CDK4 inhibitor p21/CDKN1A (WAF1, CIP1) was the first transcriptional target identified for p53. p53 loss is associated with lower levels of the p53 target gene p21Cip1, which increases CDK2 activity to promote the cell cycle [44]. In a recent clinical study, p53 mutation was linked with poor clinical response to abemaciclib in breast cancer patients [45]. However, Fernández-Aroca et al. found that CDK4/6 inhibition was effective in many tumor models that had Rb or p53 mutation. Additionally, palbociclib has been reported to be a novel radio-sensitizing agent, and wild-type p53 was strictly required for palbociclib to execute its radio-sensitizing effects [46].
miRNAs
Deregulation of microRNAs (miRNAs) plays an important role in tumor progression [47]. Novel miRNAs that target CDK4/6 and exhibit potential for therapeutic development in multiple tumors have been identified. The microRNA miR-302 suppressed both the cyclin D-CDK4/6 and cyclin E-CDK2 pathways to enhance G1 phase arrest to inhibit the tumorigenicity of human pluripotent stem cells [48]. miRNA-138 can induce cell cycle G1/S arrest to inhibit the growth of GBM cells by directly targeting cell cycle genes such as CDK6, E2F2 and E2F3 [49]. In addition, overexpression of miR-506 in ovarian carcinoma also inhibits cell proliferation by directly targeting CDK4 and CDK6, thereby preventing CDK4/6-FOXM1 signaling [50]. Lulla et al. have demonstrated that a family of miRNAs including miR-6883–5p, miR-149*, miR-6785-5p, and miR-4728-5p directly target the UTRs of CDK4/6 mRNAs, and ectopic expression of miR-6883-5p or miR-149*, two novel miRNAs, downregulated CDK4 and CDK6 levels in human colorectal cancer cells. Restoring expression of miR-6883-5p and miR-149* could block cell growth, leading to colorectal cancer cell apoptosis [51]. CDK6 overexpression has been identified as a key determinant to confer acquired resistance to CDK4/6i, and Cornell et al. have reported that miR-432-5p suppresses the TGF-β pathway to promote CDK6 overexpression in ER+ breast cancer cells [52].
Other signaling pathways
In nonmalignant cells, D-type cyclins can be activated by extracellular mitogens signals through estrogen receptor and human epidermal growth factor receptors [53]. As D-type cyclins accumulate, they combine with CDK4 and CDK6 to phosphorylate Rb, which promotes cell cycle progression [2]. Mitogenic signaling pathways such as the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling pathway were shown to be aberrantly activated by specific molecular events, which may play a role in resistance to CDK4/6 inhibitors. The detailed underlying mechanisms will be discussed in the acquired resistance section.
To better understand the mechanisms responsible for intrinsic resistance to CDK4/6 inhibitors, we summarized the dysregulated expression of the cyclin D-CDK4/6-Rb pathway in different types of tumors and its contribution to the mechanisms of intrinsic resistance to CDK4/6 inhibitors in Table 2a.
Table 2.
a Overview of the dysregulated expression and functions of the major cell cycle proteins contribute to the intrinsic resistance to CDK4/6 inhibitors. b Overview of other mitogenic signaling activation to CDK4/6 inhibition and combination strategies.
| Gene | Expression | Cancer type | Functions | References |
|---|---|---|---|---|
| RB1 | Rb1 loss | Breast cancer | Resistance to palbociclib | [9] |
| RB1 | Rb1 loss | Ovarian cancer | Resistance to palbociclib | [15] |
| RB1 | Rb1degradation | HPV-positive cervical cancer and head and neck cancer | Resistance to CDK4/6 inhibitors | [16, 17] |
| CDK4 | CDK4-amplification | Liposarcoma and Neuroblastoma | Sensitive to CDK4/6 inhibitors | [26, 27] |
| CDK4 | Activation T172 phosphorylation | Breast cancer | Enhance the sensitivity to palbociclib | [28] |
| CDK4 | CDK4-amplification | Rhabdomyosarcoma | Reduce the sensitivity to ribociclib | [25] |
| CCND1 | Cyclin D1-overexpression | Endometrial cancer | Enhance the sensitivity to abemaciclib | [32] |
| SMARCA4 | SMARCA4 loss | Small-cell carcinoma of the ovary, hypercalcemic type (SCCOHT) cells | Cause cyclin D1 deficiency and enhance the sensitivity to CDK4/6 inhibitors | [33] |
| ]SMARCA2/4 | SMARCA2/4 loss | Non-small cancer cell | Cause cyclin D1 deficiency and increase the sensitivity to CDK4/6 inhibitors | [34] |
| CDKN2A | p16INK4A loss | Melanoma | Good for palbociclib sensitivity | [40] |
| CDKN2A | p16INK4A loss | Pancreatic ductal adenocarcinoma | Resistance to CDK4/6 inhibitors | [41] |
| CDKN2A | p16INK4A deficiency | Glioblastoma | More susceptible to palbociclib | [42] |
| CCNE1 | Cyclin E1 overexpression | Gastric cancer | Resistance to palbociclib | [37] |
| CCNE1 | High cyclin E1 expression | HR+ HER2- breast cancer | Resistance to palbociclib | [38] |
| E2F4 | E2F4 activation | ER+ breast cancer | Resistance to palbociclib | [21] |
| E2F | E2F activation | Mutant BRAF and NRAS melanoma | Resistance to palbociclib | [22] |
| TP53 | p53 loss | Breast cancer | Resistance to abemaciclib | [45] |
| CDK2 | High levels of CDK2 activity | Luminal androgen receptor breast cancer | Resistance to CDK4/6 inhibitors | [31] |
| Other mitogenic signaling | Cancer type | Functions | Combination with other inhibitors | References |
|---|---|---|---|---|
| FGFR1 amplification | ER+ breast cancer | Resistance to CDK4/6 inhibitors | Lucitanib (FGFR inhibitors) | [54] |
| FGFR1 signaling pathway activation | KRAS-mutant non-small lung cancer | Resistance to CDK4/6 inhibitors | MEK inhibitors | [55] |
| MAPK signaling pathway activation | prostate cancer | Resistance to CDK4/6 inhibitors | MEK inhibitors | [56] |
| PIK3CA E545K mutation | NRAS-mutant melanoma | Resistance to CDK4/6 inhibitors | mTOR inhibitors and S6K1 inhibitors | [57] |
| mTOR activation | Pancreatic cancer | Resistance to CDK4/6 inhibitors | mTOR inhibitors | [60] |
| PDK1 activation | ER+ breast cancer | Resistance to ribociclib | PDK1 inhibitors or CDK2 inhibitors | [61] |
| NF-κB-HGF pathway | Glioblastoma | Resistance to ribociclib | Altiratinib (c-Met/Trk inhibitor) | [62] |
| Androgen Receptor activation | Breast cancer | Resistance to palbociclib | Androgen receptor inhibitors | [63] |
| FAT1 loss (Hippo pathway suppression) | ER+ breast cancer | CDK6 overexpression | [64, 65] | |
| MYC-driven (mTOR activation) | Colorectal carcinoma | Resistance to CDK4/6 inhibitors | [66] | |
| Fbxo4 loss (Gln-addition) | Esophageal squamous cell carcinoma | Resistance to CDK4/6 inhibitors | Glutaminase1plus metformin or phenformin | [67] |
| IL6/STAT3 activation | ER+ breast cancer | Resistance to CDK4/6 inhibitors | STAT3 inhibitor plus PARP inhibitor | [68] |
Mechanisms of adaptation and acquired resistance
To date, the CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) have achieved great success in the treatment of ER+HER2− breast cancer. With these successes, acquired resistance to CDK4/6 inhibitors has emerged. Many preclinical studies have suggested that activation of other mitogenic signaling pathways, amplification of specific genes, alteration of the tumor environment and modification of tumor immunity result in the emergence of drug resistance.
Activation of other mitogenic signaling pathways and combination therapies to target them
FGFR1 signaling pathway
In the MONALEESA-2 trial, abnormal fibroblast growth factor receptor (FGFR) signaling was revealed to participate in resistance to fulvestrant ± ribociclib. FGFR1 amplification has been identified to play an important role in acquired resistance in patients with shorter progression-free survival but not in patients with wild-type FGFR1. In addition, combination treatment with the FGFR tyrosine kinase inhibitor (TKI) lucitanib could abrogate this resistance [54].
Similarly, KRAS-mutant NSCLCs are initially sensitive to palbociclib but readily acquire resistance. In the resistant cells, the expression of CDK6, D-type cyclins, and cyclin E increased, and upstream FGFR1 signaling activated by the extracellular secretion of FGF ligands was observed, which in turn drove extracellular signal-regulated kinase (ERK) to activate mTOR; in this setting, combination treatment with a mitogen-activated protein kinase kinase (MEK) inhibitor was able to re-sensitize the resistant cells [55].
MAPK signaling pathway
The mitogen-activated protein kinase (MAPK) signaling pathway was found to be activated in CDK4/6 inhibitor-resistant prostate cancer cells by using integration of RNA sequencing analysis and phosphoproteomics, and studies further found that CDK4/6 inhibitor-resistant models were sensitized by MEK inhibitors. Combination treatments that use MEK inhibition could be adopted as promising therapies to treat or prevent CDK4/6 inhibitor resistance in cancer [56]. In NRAS-mutant melanoma, the strategy of combining MEK and CDK4/6 inhibitors (MEKi + CDK4i) at first demonstrated efficacy, but the patients eventually developed resistance. By whole-exome sequencing, the PIK3CA E545K mutation was detected and identified as the main mechanism of resistance. S6K1, a critical downstream protein of the MAPK pathway, was activated and played a role in PIK3CA E545K-induced drug resistance [57].
PI3K/AKT/mTOR signaling pathway
The AKT or mTOR signaling pathways remain active and drive acquired resistance to CDK4/6 inhibitors in several tumors [58, 59]. In breast cancer, aberrant mTORC1 activation increased cyclin D1 overexpression, participating in CDK4/6 inhibitors resistance. In addition, the upregulation of both cyclin D1 and cyclin E was also observed in pancreatic cancer models that were sensitive to mTOR inhibitors [60]. In ribociclib-resistant ER-positive breast cancer, pyruvate dehydrogenase kinase 1 (PDK1) was activated and served as a key modifier of CDK4/6 inhibitors, and CDK2 directly phosphorylated AKT at S477/T479 to activate the AKT pathway. Treatment with a PDK1 inhibitor or the CDK2 inhibitor dinaciclib was able to re-sensitize ribociclib-resistant cells to CDK4/6 inhibitors [61].
NF-κB-HGF pathway
Inhibition of CDK4/6 drives NF-κB-mediated upregulation of secreted factors such as hepatocyte growth factor and nerve growth factor, which in turn activate both the c-Met and TrkA-B pathways, eventually leading to development of resistance to abemaciclib in GBM treatment. Combination treatment with a c-Met/Trk inhibitor and altiratinib abrogated this resistance, leading to a significant synergic effect against GBM [62].
Androgen receptor (AR)
In a palbociclib-resistant breast cancer cell line (MCF-7pR), ER signaling loss and AR signaling activation have been demonstrated. The AR signaling pathway regulates cell cycle processes, and thereby reduces sensitivity to CDK4/6 inhibitors. Blocking AR could overcome the resistance to palbociclib [63].
Tumor metabolism pathways contribute to acquired resistance to CDK4/6 inhibitors
Hippo pathway
In a genomic analysis of 348 ER+ breast cancers treated with CDK4/6i, a loss-of-function mutation in FAT atypical cadherin 1 (FAT1) was detected in CDK4/6i-resistant patients. FAT1 loss caused Hippo pathway suppression, inducing Yes-associated protein (YAP)/tafazzin (TAZ) nuclear localization to induce CDK6 expression. Neurofibromin 2 (NF2), a Hippo pathway component, was inactivated, which also increased CDK6 expression to reduce sensitivity to CDK4/6i [64].
In radiation-resistant esophageal cancer cells, overexpression of YAP1 increased CDK6 transcription to promote CDK6 expression, and using the YAP1 inhibitor CA3 and the CDK6 inhibitor LEE001 (ribociclib) significantly suppressed esophageal cancer cell growth [65].
MYC-driven combinations
In HCT116 human colorectal carcinoma cells, CDK4/6 inhibition leads to metabolic reprogramming mainly through the MYC transcription factor. Upon inhibition of CDK4/6, MYC overexpression drives glucose, glutamine, and amino acid metabolism, leading to metabolic reprogramming and activation of the mTOR pathway. Hence, MYC-driven adaptions to CDK4/6 inhibition can make cancer cells highly sensitive to inhibitors of MYC, glutaminase or mTOR. The metabolic adaptations caused by anti-proliferative drugs may unveil novel targets to be exploited by treatment combinations to overcome acquired drug resistance [66].
Gln addiction
Aberrations in the Fbxo4-cyclin D1 axis are frequently observed in a number of tumors, and Fbxo4 loss results in hyperactivation of cyclin D1-CDK4/6 to promote esophageal squamous cell carcinoma (ESCC) progression, which drives glutamine addiction (Gln addiction) in ESCC cells. Palbociclib-resistant (PDR) TE7-PDR and TE10-PDR ESCC cells exhibited characteristics of Gln-addiction phenotypically, as well as upregulation of glutaminase 1 (GLS1). In addition, in accordance with these characteristics, with Gln-depletion or GLS1 knockdown, the PDR cells became sensitive to CDK4/6i. Combination treatment with B-839 (glutaminase 1 inhibitor) and metformin/phenformin can increase sensitivity to CDK4/6 inhibitors [67].
Alterations of the tumor immune microenvironment involved in CDK4/6 inhibitor resistance
Whole-exome sequencing and genome-wide expression analysis of palbociclib-resistant MCF-7 and T47D breast cancer cells generated in a stepwise dose-escalating fashion and matched tumor samples showed that the interleukin 6 (IL-6)/signal transducer and activator of transcription 3 (STAT3) pathway was activated. Administration of a specific STAT3 inhibitor combined with a poly ADP-ribose polymerase (PARP) inhibitor effectively treated this acquired resistance to palbociclib [68].
Targeting immune checkpoints, for example, blocking programmed cell death protein 1 (PD-1) and its ligand PD-L1, has been shown to have significant efficacy in several tumors [69]. Zhang et al. found that CDK4/6 inhibitors enhanced PD-L1 stability. Cyclin D-CDK4 could directly phosphorylate speckle-type BTB/POZ protein (SPOP) at Ser6, thereby promoting the physical interaction between 14-3-3γ and SPOP. Inhibition of the interaction of SPOP with 14-3-3γ increased SPOP binding to cadherin 1 (Cdh1), resulting in SPOP poly-ubiquitination, which promoted PD-L1 degradation. Depletion/inhibition of cyclin D-CDK4 directly reduced the interaction of SPOP with 14-3-3γ. Hence, the combination of a CDK4/6 inhibitor with anti-PD-1 immunotherapy could be a promising strategy for tumor treatment [70].
In prostate cancer, the tumor suppressor Rb directly interacts with the NF-κB protein p65 in a p-S249/T252-dependent manner. Rb knockdown or CDK4/6 inhibition decreased Rb phosphorylation at S249/T252 and upregulated the activity of the NF-κB pathway to increase PD-L1 transcription. Administration of an Rb-derived S249/T252 phosphorylation-mimetic peptide suppressed the radiotherapy-induced upregulation of PD-L1 to enhance the efficacy of radiation [71].
Additionally, CDK4/6 inhibitors can remodel the tumor environment by increasing intracellular levels of double-stranded RNA, stimulating type III interferon production and markedly inhibiting the growth of regulatory T cells (Tregs). CDK4/6 inhibitors can also reduce the activity of DNA methyltransferase 1. All these molecular alterations can help cytotoxic T cells clear tumor cells, and combination treatment with immune checkpoint blockade inhibitors further enhanced this effect [72].
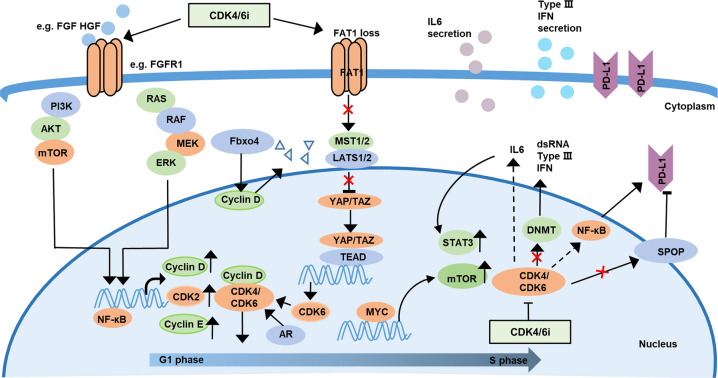
In Fig. 1, we summarize the known mechanisms of acquired resistance to CDK4/6 inhibitors, such as activation of other mitogenic signaling pathways and CDK6 gene amplification, and provide rational combination strategies to combat the acquired resistance to CDK4/6 inhibitors in multiple tumor types in Table 2b. Other combination strategies in different types of tumors are being tested in multiple clinical trials, and these are summarized in Table 3.
Fig. 1. Mechanisms of resistance to CDK4/6 inhibitors.
Upon CDK4/6 inhibition, FGFR1 amplification can drive ERK signaling pathway activation, increasing CDK6, D-type cyclins and cyclin E expression. In addition, the activity of the MAPK signaling pathway or the PI3K/AKT/mTOR pathway is activated or enhanced. The NF-κB pathway can upregulate HGF, which in turn activates the c-Met and TrkA-B pathways. Simultaneously, androgen receptor expression is increased to promote cell cycle progression in palbociclib-resistant breast cancer cells. In CDK4/6 inhibitor-resistant ER+ breast cancer, loss of FAT1 can suppress the Hippo pathway to induce YAP/TAZ nuclear localization, increasing CDK6 expression. Upon inhibition of CDK4/6, MYC overexpression can reprogram tumor metabolism and activate the mTOR pathway. Loss of Foxb4 can increase the accumulation of cyclin D1 in the nucleus, which induces glutamine addiction and promotes tumor progression. In palbociclib-resistant breast cancer cells, the IL-6/STAT3 pathway is activated and promotes SPOP degradation, thereby increasing PD-L1 levels and enhancing the levels of double-stranded RNA, stimulating the production of type III interferon.
Table 3.
Clinical trials studying on CDK4/6 inhibitors in combination with other therapies in other cancer treatment.
| Disease | Combination | Phase | Trial |
|---|---|---|---|
| Palbociclib | |||
| KRAS mutant non-small-cell lung cancer, solid tumors | PD-0325901(MEK inhibitor) | I/II | NCT02022982 |
| Squamous cell carcinoma of the head and neck (SCCHN) | Cetuximab | II | NCT02499120 |
| Squamous cell carcinoma of the head and neck | Carboplatin | II | NCT03194373 |
| Recurrent mantle cell lymphoma | Ibrutinib (BTK Inhibitor) | I | NCT02159755 |
| Advanced solid tumors, breast cancer | Taselisib or pictilisib (PI3K inhibitor) | I | NCT02389842 |
| Endometrial cancer | Letrozole | II | NCT02730429 |
| Ovarian epithelial carcinoma | II | NCT01536743 | |
| Advanced solid tumor malignancies | 5-FU and oxaliplatin | I | NCT01522989 |
| Palbociclib | |||
| Advanced hepatocellular carcinoma, | II | NCT01356628 | |
| HCC, liver cancer | |||
| Non-small-cell lung cancer | PF-06747775 and avelumab | II | NCT02349633 |
| Ribociclib | |||
| Glioblastoma glioma | I | NCT02345824 | |
| Acute lymphoblastic leukemia ALL | Dexamethasone and everolimus | I | NCT03740334 |
| High grade glioma, | I/II | NCT02607124 | |
| Diffuse intrinsic pontine glioma, | |||
| Bithalamic high grade glioma | |||
| Gastrointestinal cancer | II | NCT02420691 | |
| Metastatic pancreatic | Everolimus | II | NCT02985125 |
| Ribociclib | |||
| Adenocarcinoma | |||
| Liposarcoma | HDM201 | I/II | NCT02343172 |
| Solid tumors harboring | LGX818 and MEK162 | I/II | NCT01543698 |
| Abemaciclib | |||
| Glioblastoma | II | NCT02981940 | |
| Non-small-cell lung cancer stage IV | Docetaxel | II | NCT02450539 |
| Non-small-cell lung cancer | Erlotinib | III | NCT02152631 |
| Non-small-cell lung cancer | Pembrolizumab and anastrozole | I | NCT02779751 |
| Breast cancer, mantle cell lymphoma | II | NCT01739309 | |
Conclusions and perspectives
Targeting the cell-cycle machinery is a potentially promising strategy for cancer therapy, and CDK4/CDK6 inhibitors, palbociclib, ribociclib and abemaciclib have achieved great therapeutic effects in ER+ breast cancer. However, patients eventually become insensitive to treatment or acquire drug resistance. The potential mechanisms of acquired resistance to CDK4/6 inhibitors include the following: (1) alteration of the cyclin D-CDK4/6-Rb pathway, for instance, CDK6 or CDK4 amplification or cyclin D, cyclin E or E2F overexpression; (2) activation of alternate pathways, including the FGFR1 signaling pathway and the PI3K/AKT/mTOR pathway; and (3) modulation of the immune microenvironment by the CDK4/6 inhibitors, such as changes in PD-L1 expression. Investigation of the mechanisms of intrinsic resistance and acquired resistance is necessary for choosing the tumors that will respond best and provides potential combinatory strategies with inhibitors of other compensatory signaling pathways to overcome drug resistance.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81773754 to Ling Ding).
Author contributions
XQX drafted the manuscript. XHP, TTW, JW, and BY critically revised the manuscript. LD and QJH designed the article. All authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xia-qing Xu, Xiao-hui Pan
Contributor Information
Qiao-jun He, Email: qiaojunhe@zju.edu.cn.
Ling Ding, Email: ld362@zju.edu.cn.
References
- 1.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 3.Hannon GJ, Beach D. p15INK4b is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–61. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 4.Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 2015;15:2672–81. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan FK, Zhang J, Cheng L, Shapiro DN, Winoto A. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol Cell Biol. 2015;15:2682–8. doi: 10.1128/mcb.15.5.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann J, Bohlmann R, Heinrich N, Hofmeister H, Kroll J, Künzer H, et al. Characterization of new estrogen receptor destabilizing compounds: effects on estrogen-sensitive and tamoxifen-resistant breast cancer. J Natl Cancer Inst. 2004;96:210–8. doi: 10.1093/jnci/djh022. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18:1–11. doi: 10.1186/s13058-015-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:1–13. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 11.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 12.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–46. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 13.Rinnerthaler G, Gampenrieder SP, Greil R. ASCO 2018 highlights: metastatic breast cancer. Memo - Mag Eur Med Oncol. 2018;11:276–9. doi: 10.1007/s12254-018-0450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J, Thijssen B, McDermott U, Garnett M, Wessels LF, Bernards R. Targeting the RB-E2F pathway in breast cancer. Oncogene. 2016;35:4829–35. doi: 10.1038/onc.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konecny GE, Winterhoff B, Kolarova T, Qi J, Manivong K, Dering J, et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res. 2011;17:1591–602. doi: 10.1158/1078-0432.CCR-10-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witkiewicz AK, Knudsen KE, Dicker AP, Knudsen ES. The meaning of p16 ink4a expression in tumors: functional significance, clinical associations and future developments. Cell Cycle. 2011;10:2497–503. doi: 10.4161/cc.10.15.16776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aagaard L, Lukas J, Bartkova J, Kjerulff AA, Strauss M, Bartek J. Aberrations of p16Ink4 and retinoblastoma tumor-suppressor genes occur in distinct sub-sets of human cancer cell lines. Int J Cancer. 1995;61:115–20. doi: 10.1002/ijc.2910610120. [DOI] [PubMed] [Google Scholar]
- 18.Rubio C, Martínez-Fernández M, Segovia C, Lodewijk I, Suarez-Cabrera C, Segrelles C, et al. CDK4/6 inhibitor as a novel therapeutic approach for advanced bladder cancer independently of RB1 status. Clin Cancer Res. 2019;25:390–402. doi: 10.1158/1078-0432.CCR-18-0685. [DOI] [PubMed] [Google Scholar]
- 19.Condorelli R, Spring L, O’Shaughnessy J, Lacroix L, Bailleux C, Scott V, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2018;29:640–5. doi: 10.1093/annonc/mdx784. [DOI] [PubMed] [Google Scholar]
- 20.Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer. 2019;19:326–38. doi: 10.1038/s41568-019-0143-7. [DOI] [PubMed] [Google Scholar]
- 21.Guerrero-Zotano AL, Stricker TP, Formisano L, Hutchinson KE, Stover DG, Lee KM, et al. ER+ Breast cancers resistant to prolonged neoadjuvant letrozole exhibit an e2f4 transcriptional program sensitive to cdk4/6 inhibitors. Clin Cancer Res. 2018;24:2517–29. doi: 10.1158/1078-0432.CCR-17-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SH, Gong X, Zhang Y, Van Horn RD, Yin T, Huber L, et al. RAF inhibitor LY3009120 sensitizes RAS or BRAF mutant cancer to CDK4/6 inhibition by abemaciclib via superior inhibition of phospho-RB and suppression of cyclin D1. Oncogene. 2018;37:821–32. doi: 10.1038/onc.2017.384. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt EE, Ichimura K, Reifenberger G, Collins VP. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 1994;54:6321–4. [PubMed] [Google Scholar]
- 24.Chawla R, Procknow JA, Tantravahi RV, Khurana JS, Litvin J, Reddy EP. Cooperativity of Cdk4R24C and Ras in melanoma development. Cell Cycle. 2010;9:3305–14. doi: 10.4161/cc.9.16.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olanich ME, Sun W, Hewitt SM, Abdullaev Z, Pack SD, Barr FG. CDK4 amplification reduces sensitivity to CDK4/6 inhibition in fusion-positive rhabdomyosarcoma. Clin Cancer Res. 2015;21:4947–59. doi: 10.1158/1078-0432.CCR-14-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickson MA, Tap WD, Keohan ML, D’Angelo SP, Gounder MM, Antonescu CR, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31:2024–8. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geoerger B, Bourdeaut F, DuBois SG, Fischer M, Geller JI, Gottardo NG, et al. A phase I study of the CDK4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumors. Clin Cancer Res. 2017;23:2433–41. doi: 10.1158/1078-0432.CCR-16-2898. [DOI] [PubMed] [Google Scholar]
- 28.Raspé E, Coulonval K, Pita JM, Paternot S, Rothé F, Twyffels L, et al. CDK4 phosphorylation status and a linked gene expression profile predict sensitivity to palbociclib. EMBO Mol Med. 2017;9:1052–66. doi: 10.15252/emmm.201607084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malumbres M, Sotillo R, Santamaría D, Galán J, Cerezo A, Ortega S, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Spencer SL, Cappell SD, Tsai FC, Overton KW, Wang CL, Meyer T. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell. 2013;155:369–83. doi: 10.1016/j.cell.2013.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asghar US, Barr AR, Cutts R, Beaney M, Babina I, Sampath D, et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple negative breast cancer. Clin Cancer Res. 2017;23:5561–72. doi: 10.1158/1078-0432.CCR-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong X, Litchfield LM, Webster Y, Chio LC, Wong SS, Stewart TR, et al. Genomic aberrations that activate D-type cyclins are associated with enhanced sensitivity to the CDK4 and CDK6 inhibitor Abemaciclib. Cancer Cell. 2017;32:761–76. doi: 10.1016/j.ccell.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Xue Y, Meehan B, Macdonald E, Venneti S, Wang XQD, Witkowski L, et al. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat Commun. 2019;10:558. doi: 10.1038/s41467-018-06958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue Y, Meehan B, Fu Z, Wang XQD, Fiset PO, Rieker R, et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat Commun. 2019;10:557. doi: 10.1038/s41467-019-08380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santala S, Talvensaari-Mattila A, Soini Y, Santala M. Cyclin E expression correlates with cancer-specific survival in endometrial endometrioid adenocarcinoma. Anticancer Res. 2015;35:3393–7. [PubMed] [Google Scholar]
- 36.Zhao ZM, Yost SE, Hutchinson KE, Li SM, Yuan YC, Noorbakhsh J, et al. CCNE1 amplification is associated with poor prognosis in patients with triple negative breast cancer. BMC Cancer. 2019;19:1–11. doi: 10.1186/s12885-019-5290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min A, Kim JE, Kim YJ, Lim JM, Kim S, Kim JW, et al. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 2018;430:123–32. doi: 10.1016/j.canlet.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 38.Chandarlapaty S, Razavi P. Cyclin E mRNA: assessing cyclin-dependent kinase (CDK) activation state to elucidate breast cancer resistance to CDK4/6 inhibitors. J Clin Oncol. 2019;37:1148–50. doi: 10.1200/JCO.19.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001;264:42–55. doi: 10.1006/excr.2000.5149. [DOI] [PubMed] [Google Scholar]
- 40.Young RJ, Waldeck K, Martin C, Foo JH, Cameron DP, Kirby L, et al. Loss of CDKN2A expression is a frequent event in primary invasive melanoma and correlates with sensitivity to the CDK4/6 inhibitor PD0332991 in melanoma cell lines. Pigment Cell Melanoma Res. 2014;27:590–600. doi: 10.1111/pcmr.12228. [DOI] [PubMed] [Google Scholar]
- 41.Heilmann AM, Perera RM, Ecker V, Nicolay BN, Bardeesy N, Benes CH, et al. CDK4/6 and IGF1 receptor inhibitors synergize to suppress the growth of p16INK4A-deficient pancreatic cancers. Cancer Res. 2014;74:3947–58. doi: 10.1158/0008-5472.CAN-13-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cen L, Carlson BL, Schroeder MA, Ostrem JL, Kitange GJ, Mladek AC, et al. p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol. 2012;14:870–81. doi: 10.1093/neuonc/nos114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engeland K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018;25:114–32. doi: 10.1038/cdd.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox LS. Multiple pathways control cell growth and transformation: overlapping and independent activities of p53 and p21cip/WAF1/Sdi1. J Pathol. 1997;183:134–40. doi: 10.1002/(SICI)1096-9896(199710)183:2<134::AID-PATH960>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 45.Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and safety of Abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non–small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6:740–53. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 46.Fernández-Aroca DM, Roche O, Sabater S, Pascual-Serra R, Ortega-Muelas M, Sánchez Pérez I, et al. P53 pathway is a major determinant in the radiosensitizing effect of Palbociclib: implication in cancer therapy. Cancer Lett. 2019;451:23–33. doi: 10.1016/j.canlet.2019.02.049. [DOI] [PubMed] [Google Scholar]
- 47.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 48.Lin SL, Chang DC, Ying SY, Leu D, Wu DT. MicroRNA miR-302 inhibits the tumorigenecity of human pluripotent stem cells by coordinate suppression of the CDK2 and CDK4/6 cell cycle pathways. Cancer Res. 2010;70:9473–82. doi: 10.1158/0008-5472.CAN-10-2746. [DOI] [PubMed] [Google Scholar]
- 49.Qiu S, Huang D, Yin D, Li F, Li X, Kung HF, et al. Suppression of tumorigenicity by MicroRNA-138 through inhibition of EZH2-CDK4/6-pRb-E2F1 signal loop in glioblastoma multiforme. Biochim Biophys Acta - Mol Basis Dis. 2013;1832:1697–707. doi: 10.1016/j.bbadis.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Liu G, Sun Y, Ji P, Li X, Cogdell D, Yang D, et al. MiR-506 suppresses proliferation and induces senescence by directly targeting the CDK4/6-FOXM1 axis in ovarian cancer. J Pathol. 2014;233:308–18. doi: 10.1002/path.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lulla AR, Slifker MJ, Zhou Y, Lev A, Einarson MB, Dicker DT, et al. miR-6883 family miRNAs target CDK4/6 to induce G1 phase cell-cycle arrest in colon cancer cells. Cancer Res. 2017;77:6902–13. doi: 10.1158/0008-5472.CAN-17-1767. [DOI] [PubMed] [Google Scholar]
- 52.Cornell L, Wander SA, Visal T, Wagle N, Shapiro GI. MicroRNA-mediated suppression of the TGF-β pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell Rep. 2019;26:2667–80. doi: 10.1016/j.celrep.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torii S, Yamamoto T, Tsuchiya Y, Nishida E. ERK MAP kinase in G1 cell cycle progression and cancer. Cancer Sci. 2006;97:697–702. doi: 10.1111/j.1349-7006.2006.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Formisano L, Lu Y, Servetto A, Hanker AB, Jansen VM, Bauer JA, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10:1–14. doi: 10.1038/s41467-019-09068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haines E, Chen T, Kommajosyula N, Chen Z, Herter-Sprie GS, Cornell L, et al. Palbociclib resistance confers dependence on an FGFR-MAP kinase-mTOR-driven pathway in KRAS-mutant non-small cell lung cancer. Oncotarget. 2018;9:31572–89. doi: 10.18632/oncotarget.25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Leeuw R, McNair C, Schiewer MJ, Neupane NP, Brand LJ, Augello MA, et al. MAPK reliance via acquired CDK4/6 inhibitor resistance in cancer. Clin Cancer Res. 2018;24:4201–14. doi: 10.1158/1078-0432.CCR-18-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romano G, Chen PL, Song P, McQuade JL, Liang RJ, Liu M, et al. A preexisting rare PIK3CA e545k subpopulation confers clinical resistance to MEK plus CDK4/6 inhibition in NRAS melanoma and is dependent on S6K1 signaling. Cancer Disco. 2018;8:556–67. doi: 10.1158/2159-8290.CD-17-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor–positive breast cancer. Cancer Res. 2016;76:2301–13. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J, Xu K, Liu PD, Geng Y, Wang B, Gan WJ, et al. Inhibition of Rb phosphorylation leads to mTORC2-mediated activation of Akt. Mol Cell. 2017;62:929–42. doi: 10.1016/j.molcel.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knudsen ES, Kumarasamy V, Ruiz A, Sivinski J, Chung SJ, Grant A, et al. Cell cycle plasticity driven by MTOR signaling: integral resistance to CDK4/6 inhibition in patient-derived models of pancreatic cancer. Oncogene. 2019;38:3355–70. doi: 10.1038/s41388-018-0650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Michaloglou C, Crafter C, Siersbaek R, Delpuech O, Curwen JO, Carnevalli LS, et al. Combined inhibition of mTOR and CDK4/6 is required for optimal blockade of E2F function and long-term growth inhibition in estrogen receptor–positive breast cancer. Mol Cancer Ther. 2018;17:908–20. doi: 10.1158/1535-7163.MCT-17-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olmez I, Zhang Y, Manigat L, Benarmar M, Brenneman B, Nakano I, et al. Combined c-Met/Trk inhibition overcomes resistance to CDK4/6 inhibitors in glioblastoma. Cancer Res. 2018;78:4360–9. doi: 10.1158/0008-5472.CAN-17-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ji WF, Shi YQ, Wang X, He WW, Tang L, Tian SW, et al. Combined androgen receptor blockade overcomes the resistance of breast cancer cells to palbociclib. Int J Biol Sci. 2019;15:522–32. doi: 10.7150/ijbs.30572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li ZQ, Razavi P, Li Q, Toy WY, Liu B, Ping C, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell. 2018;34:893–905. doi: 10.1016/j.ccell.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li F, Xu Y, Liu B, Singh PK, Zhao W, Jin JK, et al. YAP1-mediated CDK6 activation confers radiation resistance in esophageal cancer – Rationale for the combination of YAP1 and CDK4/6 inhibitors in esophageal cancer. Clin Cancer Res. 2019;25:2264–77. doi: 10.1158/1078-0432.CCR-18-1029. [DOI] [PubMed] [Google Scholar]
- 66.Castellarnau MT, Atauri PD, Celada JT, Perarnau J, Yuneva M, Thomson TM, et al. De novo MYC addiction as an adaptive response of cancer cells to CDK4/6 inhibition. Mol Syst Biol. 2017;13:940–55. doi: 10.15252/msb.20167321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qie S, Yoshida A, Parnharm S, Oleinik N, Beeson GC, Beeson CC, et al. Targeting glutamine-addiction and overcoming CDK4/6 inhibitor resistance in human esophageal squamous cell carcinoma. Nat Commun. 2019;10:1–15. doi: 10.1038/s41467-019-09179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kettner NM, Vijayarahavan S, Durak MG, Bui T, Kohansal M, Ha MJ, et al. Combined inhibition of STAT3 and DNA repair in palbociclib-resistant ER-positive breast cancer. Clin Cancer Res. 2019;25:3996–4013. doi: 10.1158/1078-0432.CCR-18-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang JF, Bu X, Wang HZ, Zhu YS, Geng Y, Tan YY, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via Cul3 SPOP to control cancer immune surveillance. Nature. 2018;553:91–5. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin X, Ding DL, Yan YQ, Li H, Wang B, Ma LL, et al. Phosphorylated RB promotes cancer immunity by inhibiting NF-κB activation and PD-L1 expression. Mol Cell. 2019;73:22–35. doi: 10.1016/j.molcel.2018.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumor immunity. Nature. 2017;548:471–5. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]