Abstract
Hepatic ischemic reperfusion injury (IRI) is a common complication of liver surgery. Although an imbalance between mitochondrial fission and fusion has been identified as the cause of IRI, the detailed mechanism remains unclear. Augmenter of liver regeneration (ALR) was reported to prevent mitochondrial fission by inhibiting dynamin-related protein 1 (Drp1) phosphorylation, contributing partially to its liver protection. Apart from phosphorylation, Drp1 activity is also regulated by small ubiquitin-like modification (SUMOylation), which accelerates mitochondrial fission. This study aimed to investigate whether ALR-mediated protection from hepatic IRI might be associated with an effect on Drp1 SUMOylation. Liver tissues were harvested from both humans and from heterozygous ALR knockout mice, which underwent IRI. The SUMOylation and phosphorylation of Drp1 and their modulation by ALR were investigated. Hepatic Drp1 SUMOylation was significantly increased in human transplanted livers and IRI-livers of mice. ALR-transfection significantly decreased Drp1 SUMOylation, attenuated the IRI-induced mitochondrial fission and preserved mitochondrial stability and function. This study showed that the binding of transcription factor Yin Yang-1 (YY1) to its downstream target gene UBA2, a subunit of SUMO-E1 enzyme heterodimer, was critical to control Drp1 SUMOylation. By interacting with YY1, ALR inhibits its nuclear import and dramatically decreases the transcriptional level of UBA2. Consequently, mitochondrial fission was significantly reduced, and mitochondrial function was maintained. This study showed that the regulation of Drp1 SUMOylation by ALR protects mitochondria from fission, rescuing hepatocytes from IRI-induced apoptosis. These new findings provide a potential target for clinical intervention to reduce the effects of IRI during hepatic surgery.
Subject terms: Sumoylation, Gastroenteritis
Introduction
A variety of hepatic surgery procedures, particularly during partial hepatectomy and liver transplantation, require temporarily blocking the blood perfusion into the liver [1, 2]. Ischemia-reperfusion injury (IRI) results from a prolonged ischemic insult followed by restoration of blood perfusion and it is an important cause of liver damage due to the surgical operation. Currently, because of the shortage of organ supplies, more transplant programs have begun to use marginal grafts in liver transplantation, increasing the incidence of IRI and the concomitant risk of liver graft dysfunction [3]. Although the pathophysiology of IRI is not completely understood, several crucial events such as oxidative stress, inflammation and apoptosis have been proposed to contribute to the hepatic injury [4]. Recently, mitochondrial dysfunction has been considered as another critical contributor towards hepatic IRI [5, 6].
Mitochondria are highly dynamic organelles that constantly undergo a coordinated process of fission and fusion to maintain their shape, distribution, and size [7]. An increasing number of studies confirm that effective inhibition of mitochondrial fission might protect various tissues from stress-induced injury, such as IRI [8, 9]. In line with regulation of mitochondrial fission dynamin-related protein 1 (Drp1) plays an essential role [10–12]. Drp1 activity is regulated by several post-translational modifications, which integrate to control mitochondrial fission. Drp1 activity is tightly controlled by phosphorylation at Ser637 and Ser616 [13, 14]. SUMOylation also modulates Drp1 recruitment and cell apoptosis [15]. Regulation of substrates involved in cerebral and myocardial IRI by SUMOylation has been reported [16, 17].
Augmenter of liver regeneration (ALR, genetic name, Gfer), formerly called hepatic stimulator substance (HSS), was originally identified in the liver of weanling rats in 1975 [18]. This ALR is involved in liver protection against a variety of injuries, including carbon tetrachloride, d-galactosamine and hydrogen peroxide (H2O2) [19–21]. Changes of ALR expression have been linked to the pathogenesis of several liver diseases, for example, its depletion accelerates development of steatohepatitis and hepatocellular carcinoma [22]. We previously demonstrated that ALR protects the liver from IRI-induced apoptosis, probably by inhibiting Drp1 phosphorylation at Ser616 to prevent its translocation [23], preserving mitochondrial dynamics and functionality against reactive oxygen species (ROS) attack [24].
We recently found that Drp1 mutated only at Ser616 (S616A) could still be partly translocated to mitochondria, while SUMOylation of Drp1 was also increased (data not show). As SUMOylation of Drp1 is also a determinant factor for its mitochondrial localization, it was hypothesized that following inhibition of Drp1 phosphorylation, compensatory Drp1 SUMOylation might be enhanced, facilitating Drp1 translocation and subsequent mitochondrial fission, reducing hepatic IRI. To address this question, ALR+/– mouse model (heterozygous deletion of ALR gene) was used to investigate the contribution of Drp1 SUMOylation, in combination with its dephosphorylation, to mitochondrial fission. These experiments showed that ALR can significantly inhibit Drp1 SUMOylation, preventing mitochondrial fragmentation during hepatic IRI. A potential mechanism might be the interaction of ALR with YY1, inhibiting its nuclear import, decreasing transcription of ubiquitin-like modifier-activating enzyme 2 (UBA2), suppressing Drp1 SUMOylation and preserving mitochondrial function and rescuing hepatocytes from IRI.
Materials and methods
Human liver samples
Human liver samples were obtained from the Surgical Department of Chaoyang Hospital, Capital Medical University. Transplanted livers had suffered from 10-h ischemia and 2 h of reperfusion. The collection of human samples complied with the guidelines of the Ethics Committee of Capital Medical University and was only performed with informed consent of the patients.
Animal model of hepatic IRI
The C57BL/6J mice were bred in specific pathogen-free conditions, following the guidelines of Animal Ethics Association of Capital Medical University, Beijing China. Mouse heterozygous knockout of the ALR gene (ALR+/–) was prepared as previously published [23]. Six-week-old male ALR+/− and wild-type (WT) mice were used for the hepatic IRI model. Atraumatic clips were used to clamp the arterial and portal venous flow to temporally block 70% of blood supply in liver lobes. After 90-min ischemia, the clips were removed and the reperfusion to ischemic liver sustained for 3 h, before the trial mice were euthanized. The sham-operation in ALR+/+ and ALR+/− mice were also performed, with the peritoneum opened and arteries and veins of the liver separated only, without occlusion and restoration of hepatic blood flow.
Histological examination of liver tissues
Liver tissues were embedded in paraffin wax, sectioned to 5 μm-thick histological slices, stained with hematoxylin and eosin (H & E) and examined under a microscope (DM5000 B; Leica Microsystems, Wetzlar, Germany). IRI-induced hepatic damage was categorized based upon Suzuki’s criteria [25]. Hepatic expression levels of ALR, Drp1 and SUMO-related proteins were detected by immunohistochemistry and western blot.
Cell culture and transfection
The cell lines HepG2 and HEK-293T were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). All cells were incubated with Dulbecco’s modified Eagle medium (DMEM) (Thermo, Waltham, WA, USA) supplemented with 10% fetal bovine serum (FBS) at 37 °C and 5% CO2. To simulate IRI in vitro (designated as H/R), the cells were subjected to hypoxic treatment with 1% oxygen, 5% carbon dioxide and 94% nitrogen for 5 h in serum- and glucose-free DMEM, followed by reoxygenation in conventional DMEM medium in normoxic environment for 2 h. Cell transfection was performed using Lipofectamine 3000 Transfection Reagent (Cat.# L3000008, Invitrogen, Waltham, WA, USA) or Dharma FECT 4 Reagent (Cat.# T-2004, Thermo, Waltham, WA, USA), following the manufacturers’ instructions.
Antibodies and reagents
The antibodies applied in our experiments were as follows: Drp1 (Cat.# ab219596, diluted 1:1000, Abcam, Cambridge, UK); phospho-Drp1 Ser616 (Cat.# 3455, diluted 1:300, Cell Signaling Technology [CST], Danvers, MA, USA); phosphor-Drp1 Ser637 (Cat.# 4867, diluted 1:300, CST); SUMO1 (Cat.# ab32058, diluted 1:1000, Abcam); UBA2 (Cat.# 15347-1-AP, diluted 1:1000, Proteintech, Chicago, IL, USA); Ubc9 (Cat.# ab75854, diluted 1:5000, Abcam); Mul1 (Cat.# ab84067, diluted 1:300, Abcam); Myc (Cat.# ab32, diluted 1:1000, Abcam); YY1 (Cat.# 63227, diluted 1:1000, CST); YY1AP1 (Cat.# 10425-1-AP, diluted 1:1000, Proteintech); Caspase-3 (Cat.# 14220, diluted 1:1000, CST); Alexa Fluor 488 goat anti-rabbit IgG (H+L) (Cat.# A11001, diluted 1:200, Invitrogen Waltham, WA, USA); Goat anti-Rabbit IgG-HRP (Cat.# SA00001-2, diluted 1:5000, Proteintech); and Goat anti-Mouse IgG-HRP (Cat.# SA00001-1, diluted 1:5000, Proteintech). Reagents in our study were purchased from the following suppliers: 2-D08 (Cat.# S8696, Selleck, Houston, TX, USA); Ro-3306 (Cat.# S7747, Selleck); MitoTracker DeepRed (Cat.# M22426, Invitrogen); DAPI (Cat.# C1005, Beyotime, Beijing, China).
Plasmids and small-interfering RNA (siRNAs)
The plasmids pcDNA3.0-Flag-ALR, Myc-Drp1 S616A, S616D and 4KR (K594R, K597R, K606R, K608R) were constructed and maintained in our laboratory. Myc-tagged Drp1 (variant 3) was purchased from Vigenebio (Rockville, MD, USA). A set of siRNAs was designed and synthesized by GenePharma (Shanghai, China). The sequences of siRNAs are shown in Suppl. Table S1. YY1 siRNA (Cat.# sc-44330) and YY1AP1 siRNA (Cat.# sc-78797) were purchased from Santa Cruz Biotechnology (CA, USA).
Reverse-transcription quantitative PCR
TRIzol Reagent (Cat.# 15596018, Thermo) was used to extract total RNA from liver tissues and cells, and total RNA was reverse transcribed into complementary DNA for quantitative PCR (qPCR) preparation (Cat.# A3800, Promega, Madison, WI, USA), conducted using AceQ Universal SYBR qPCR Master Mix (Cat.# Q511-02, Vazyme, Nanjing, China) and analyzed as described previously [23]. The 18S ribosomal RNA or β-actin was used to normalize gene expression. Primer information is shown in Suppl. Table S2.
In vivo SUMOylation assay
Cells and tissues were lysed in RIPA lysis buffer made up of 50 mM Tris-HCl at a pH of 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS, which was supplemented with phenylmethylsulfonyl fluoride (PMSF), Na3VO4, DL-Dithiothreitol (DTT) and 20 mM N-ethylmaleimide (NEM). Cell extracts were incubated with pretreated Anti-Myc magnetic beads (Cat.# B26301, Bimake, Houston, TX, USA) overnight on a roller at 4 °C. Beads were washed in lysis buffer and resuspended once with SDS-loading buffer. The protein samples were detected by western blotting as described previously [23].
GST protein pull-down assay
Bacterium-expressed GST or GST-YY1 proteins were immobilized on glutathione-sepharose 4B beads (GE Healthcare Australia) and washed, then beads were incubated with His-ALR. Beads were washed with GST-binding buffer made up of 100 mM NaCl, 50 mM NaF, two mM EDTA, 1% Nonidet P40 and protease inhibitor cocktail and the proteins were eluted, followed by western blot analysis.
Isolation of mitochondria and detection by the membrane potential assay
Mitochondria were isolated from HepG2 cells by using a Minute™ Mitochondria Isolation Kit for Mammalian Cells and Tissues (Cat.# MP-007, Invent Biotechnologies, Plymouth, MN, USA). Mitochondrial membrane potential was detected by JC-1 (Cat.# HY-15534, MedChemExpress, Monmouth, NJ, USA). Collected cells were incubated with JC-1 staining buffer for 30 min at 37 °C and 5% CO2. Fluorescence of samples was measured by NovoCyte® Flow Cytometer (ACEA, San Diego, CA, USA).
Apoptosis assay
After H/R treatment, the cells were collected and stained by an Annexin V-EGFP Apoptosis Detection Kit (Beyotime, Shanghai, China). Apoptosis induced by H/R was measured by a NovoCyte® Flow Cytometer (ACEA).
Immunostaining and confocal microscopy
Cells plated at glass bottom dishes were fixed with 4% paraformaldehyde for 10 min. For blocking the nonspecific-binding sites, cells were incubated in phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA) (Cat.# 0332-100G, VWR Life Science, Radnor, PA, USA) for 30 min. Then, the cells were incubated with proper primary and secondary antibodies in 5% BSA. For mitochondrial staining, 60 nM MitoTracker DeepRed (Cat.# M22426, Thermo) was used to incubate cells for 20 min at 37 °C. Fluorescent images were captured by Laser Confocal Microscopy (Leica TCS-NT SP8).
Immunoblots and immunoprecipitation
Proteins for immunoblot analysis were quantified by using a BCA Protein Assay Kit (Cat.# 23229, Thermo) and denatured at 99 °C. The proteins were detected by western blotting. For immunoprecipitation analysis, cells were lysed in NP-40 lysis buffer (50 mM Tris, pH7.4; 150 mM NaCl; 1% NP-40) supplemented with appropriate inhibitors. Cell lysates were incubated with primary antibodies for 8 h at 4 °C, then Protein A/G beads (Cat.# sc-2003, Santa Cruz) were added for overnight incubation at 4 °C. The beads were washed three times with lysis buffer, resuspended in SDS-loading buffer. The proteins were detected by western blot analysis.
Quantitative chromatin immunoprecipitation (qChIP) assay
The qChIP assay was performed using a SimpleChIP Enzymatic Chromatin IP Kit (Cat.# 9003, CST). YY1 antibody was used for the immunoprecipitation assay. Primer information is shown in Suppl. Table S2.
Electrophoretic mobility shift assay (EMSA)
Double-stranded oligonucleotides of UBA2 used for EMSA were end-labeled with biotin. The labeled probes were incubated with 100 ng YY1 for 30 min in binding buffer of 10 mM Tris-HCl at a pH of 7.5, 5 mM KCl, 5 mM MgCl2, 10 mM ZnSO4, 50 mg/ml of poly(dI-dC), 5 mg/ml bovine serum albumin, 0.67 mM dithiothreitol, 0.67 mM phenylmethyl sulphonyl fluoride and 2.5% glycerol, in the presence or absence of unlabeled probes. The antibody and protein were preincubated for 20 min to detect both the shift- and the super-shifted bands. The EMSA assays were performed as described previously [26]. The probe sequences are listed as in the following: UBA2-probe: 5ʹ-TTATTCCCATGATGGCCATCCCTGTA-3′
UBA2-MP: 5′-TTATTCCCTTCTACCGCATCCCTGTA-3′
Statistical analysis
Samples and animals were allocated randomly, and the sample size of each experiment is indicated in corresponding figure legend. Investigators were not blinded to the group allocation during the experiments including animal studies. The results are presented as means ± standard deviation (SD) of at least three independent experiments. The MA-plot-based method was performed to identify differentially expressed genes in RNA sequencing using R-STUDIO (version 3.5.2) and a DEGseq package. ANOVA was performed for multiple comparisons and Student’s t-test was performed to compare the differences between two groups using GraphPad Prism 6 software, P < 0.05 was considered significant. Data that exceeding more than three standard deviations from mean were excluded. The variation was estimated, and the variance between the groups that are compared is similar.
Results
SUMOylation of Drp1 is increased in patient transplant livers
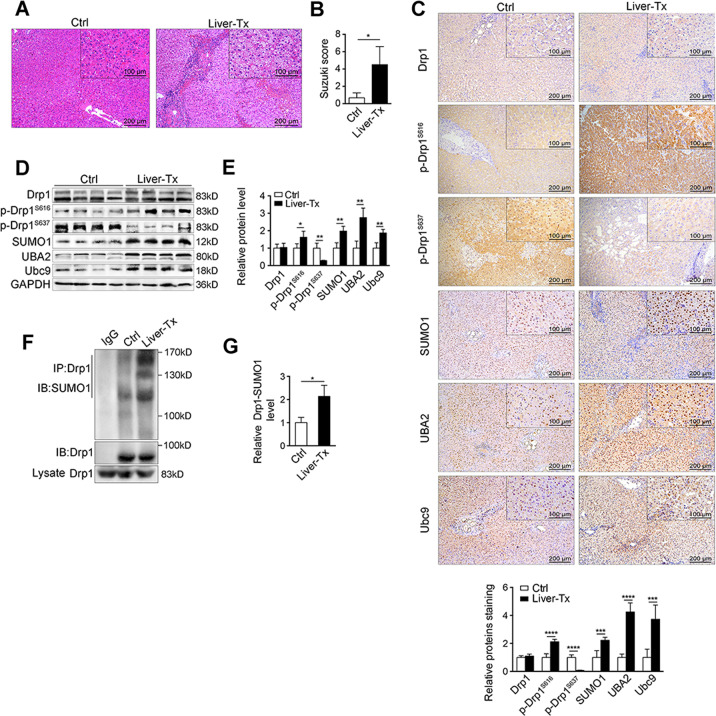
To observe the effects of IRI, transplant liver tissues were collected from five patients, who had undergone 10-h ischemia and 2-h reperfusion (Tx). As a control (Ctrl) liver tissues from other five non-IRI liver surgery cases were obtained. The IRI livers exhibited severe histological damage compared to non-IRI livers (Fig. 1A, B). The Drp1 protein levels showed no difference between IRI and non-IRI liver tissues (Fig. 1C–E), but Drp1 phosphorylated at Ser616 was clearly increased in IRI liver tissues, while Ser637 was decreased (Fig. 1C–E). The levels of SUMO-related proteins (SUMO1, UBA2, Ubc9) were also increased in IRI-liver tissues (Fig. 1D, E and Suppl. Fig. S1A). SUMOylation of Drp1 in IRI-liver tissues was obviously enhanced compared with non-IRI ones (Fig. 1F, G), suggesting that Drp1 protein modifications such as phosphorylationS616 and SUMOylation were significantly increased after IRI.
Fig. 1. SUMOylation of Drp1 is increased in transplant livers from patients.
A H&E staining in liver tissues of patients w/i liver transplant (Liver-Tx, n = 5, IRI, 10 h/2 h) or w/o Tx (as control, n = 5). Scale bar, 200 μm, 100 μm. B Liver damage induced by IRI was evaluated by Suzuki’s histological criterion. C Immunohistochemical staining and D western blotting of indicated proteins in human liver tissues w/i Liver-Tx or w/o Tx. GAPDH was used as loading control. E The quantifications of indicated protein contents in human liver tissues (n = 3). F SUMOylation of Drp1 was analyzed by immunoprecipitation (IP) in liver of patients w/i Liver-Tx or w/o Tx. Anti-Drp1 immunoprecipitants were analyzed by western blotting with anti-SUMO1 antibody. G The quantifications of Drp1 SUMOylation in human liver tissues (n = 3). P-values were calculated by Student’s t-test. Data represent mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Abbreviations: Liver-Tx liver transplant patients, Ctrl control patients, w/o without, w/i with, p-Drp1 phosphorylated-Drp1.
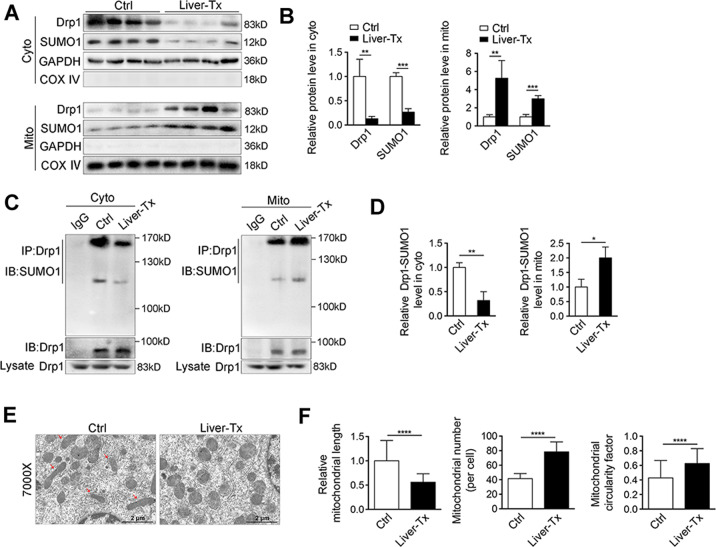
Based on these alterations, it was speculated that these modifications might be associated with Drp1 mitochondrial recruitment. To investigate this hypothesis, cytosol and mitochondria were fractionated and the subcellular distribution of Drp1 was analyzed. As shown in Fig. 2A, B, the majority of Drp1 was recruited into mitochondria in IRI-livers, with a significantly decreased in the cytosolic fraction and SUMO1 showed a similar distribution to Drp1. Quantification of SUMOylated Drp1 revealed a marked increase of mitochondrial-located protein in Tx IRI-liver tissues compared to non-IRI Ctrl livers, and it was conversely in cytosol (Fig. 2C, D and Suppl. Fig. S1B). Accordingly, excessive mitochondrial fragmentation was seen in the IRI-liver tissues (Fig. 2E, F). Overall, recruitment of SUMOylated Drp1 to mitochondria was significantly increased in IRI-liver tissues during liver transplantation.
Fig. 2. SUMOylated Drp1 in mitochondria is increased in human transplant livers.
A Western blotting of Drp1 and SUMO1 in cytosolic fraction and mitochondrial compartment from liver tissues of patients w/i Liver-Tx or w/o Tx. GAPDH and COX IV were used as loading controls, respectively. B The quantifications of Drp1 and SUMO1 in cytosolic fraction and mitochondrial compartment from human liver tissues. The proteins in cytosolic fraction and mitochondrial compartment were normalized by GAPDH and COX IV respectively (n = 3). C SUMOylation of Drp1 was analyzed by IP in cytosolic fraction and mitochondrial compartment from liver tissues of patients w/i Liver-Tx or w/o Tx. Anti-Drp1 immunoprecipitants were analyzed by western blotting with anti-SUMO1 antibody. D The quantifications of Drp1 SUMOylation in cytosolic fraction and mitochondrial compartment from human liver tissues (n = 3). E Electron microscopy analysis of liver tissues in patients w/i Liver-Tx or w/o Tx. The arrows in red indicated the elongated mitochondria. Scale bar, 2 μm. F The length of mitochondria was measured in 20 cells of each group, respectively (left). Mitochondrial number (per cell) was counted in 20 cells (middle). Mitochondrial circularity factor in 20 cells (right). The values were recorded from 0 to 1 (0 represents perfect linear, and 1 represents perfect circular). P-values were calculated by Student’s t-test. Data represent mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Abbreviations: Liver-Tx liver transplant patients, Ctrl control patients, w/o without, w/i with, Cyto cytosolic fraction, Mito mitochondrial compartment.
ALR knockdown increases the expression of SUMO-related proteins after IRI
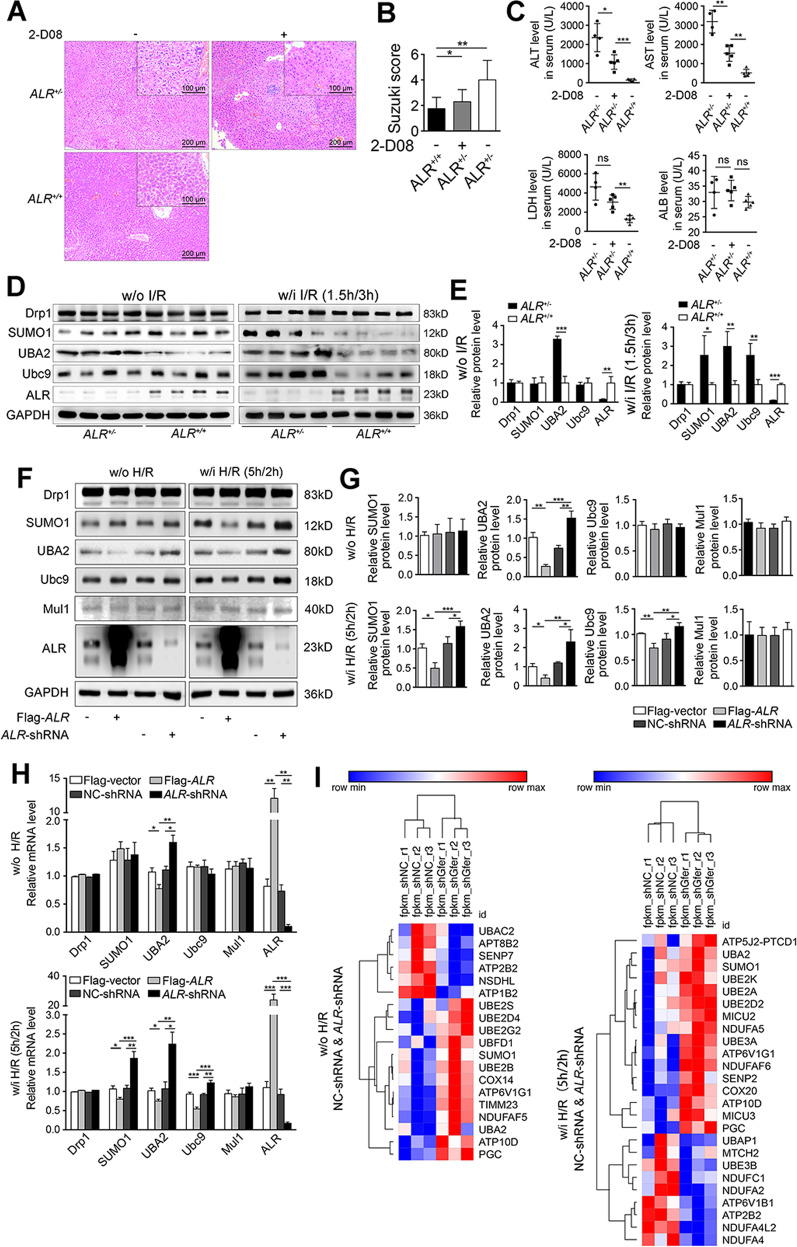
The Drp1 phosphorylationSer616 and SUMOylation cooperatively promoted its translocation to mitochondria and enhanced its activity to cause mitochondrial fission. While it was reported that ALR inhibits Drp1 phosphorylationSer616 and protects hepatocytes from IRI by reducing mitochondrial fission [23], the question remains whether ALR might also modulate Drp1 SUMOylation. As a result, four ALR heterozygous knockout mice (ALR+/–) were employed to test this hypothesis in a hepatic IRI-animal model. The results showed that the hepatic damage in ALR+/− mice appeared to be more serious than in five wild-type mice (ALR+/+) (Fig. 3A, left, B). Inhibition of SUMOylation by 2-D08 intraperitoneal injection, which prevents the transfer of SUMO from the Ubc9-SUMO thioester to the substrate [27], alleviated hepatic IRI in ALR+/− mice (Fig. 3A, right, B). Serum ALT, AST and LDH were also significantly elevated in ALR+/− mice relative to wild-type animals (Fig. 3C). This increase in liver damage could also be prevented by 2-D08 treatment (Fig. 3C), suggesting that haploin sufficiency of ALR exacerbates hepatic IRI and this effect could be deminished in part by the inhibition of Drp1 SUMOylation.
Fig. 3. ALR knockdown increases the expression of SUMO-related proteins after IRI.
A H&E staining in liver tissues of C57BL/6J wild-type mice (ALR+/+, n = 5) and heterozygote knockout of ALR mice w/i (ALR+/−, n = 4) or w/o 2-D08 (24 h, 10 mg/kg) intraperitoneal injection (n = 5) after IRI. Scale bar, 200 μm, 100 μm. B Liver damage induced by IRI was evaluated by Suzuki’s histological criterion. C Liver function parameters (ALT, AST, ALB and LDH) after IRI in serum. D Western blotting of indicated proteins in ALR+/− and ALR+/+ mice w/o or w/i IRI (ALR+/+, n = 4; ALR+/−, n = 4). GAPDH was used as loading control. E The quantifications of indicated proteins in mouse liver (n = 3). F Western blotting of indicated proteins in HepG2 cells transfected with Flag-ALR or ALR-shRNA. Cells were treated either w/i H/R (5 h/2 h) or w/o H/R. GAPDH was used as loading control. G The quantifications of indicated proteins (n = 3). H RT-qPCR of indicated genes in Flag-ALR or ALR-shRNA transfected HepG2 cells. Cells were treated either w/i H/R or w/o H/R (n = 3). I Heatmap of RNA-seq was created in ALR-shRNA and NC-shRNA-transfected HepG2 cells. P-values were calculated by Student’s t-test. Data represent mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations: I/R ischemia-reperfusion, H/R hypoxia/reoxygenation, w/o without, w/i with.
Regarding the observed alleviation of liver injury by a SUMO inhibitor 2-D08, it was hypothesized that if the enhanced mitochondrial fission caused by Drp1 SUMOylation was regulated by ALR, the IRI-induced liver injury could be prevented by ALR overexpression. To confirm this, SUMO-related proteins were analyzed and as indicated in Fig. 3D, E, hepatic expression of SUMO1, UBA2 and Ubc9 were significantly increased in liver tissues of ALR+/− mice after hepatic IRI (Right). The levels of mRNA displayed similar patterns (Suppl. Fig. S1C). While without IRI treatment, there were no differences in the levels of SUMO-related proteins except UBA2 (Fig. 3D, E, left).
To further verify the regulation of SUMO-related proteins by ALR, ALR-overexpressing or knockdown HepG2 cells were used. As shown in Fig. 3F, G, expression of SUMO1, UBA2 and Ubc9 in ALR-knockdown cells significantly increased after H/R and conversely, expression levels of these three proteins in ALR-overexpressing cells were decreased. Mul1 is an E3 ubiquitin-protein ligase that plays a role in SUMOlyation [15, 28]. ALR did not alter the mRNA level of Mul1 (Fig. 3F, G). Importantly, the mRNA of UBA2 was profoundly upregulated in ALR-knockdown cells, while markedly downregulated in ALR-overexpressing cells (Fig. 3H).
Furthermore, transcriptome profiles of shNC (scrambled control) and shALR cells with or without H/R treatment were analyzed. The results of RNA-seq indicated that, at non-H/R condition, 133 genes (Suppl. Fig. S2A) were differently expressed in ALR-shRNA cells, while 131 genes were altered at H/R condition (Suppl. Fig. S2B). Interestingly, in ALR-shRNA cells, pathways related to mitochondrial oxidative phosphorylation, non-alcoholic fatty liver disease (NAFLD), ubiquitin and ubiquitin-like modification were found significantly changed (Suppl. Fig. S2C, D). Further analysis revealed that the levels of UBA2 and SUMO1 were substantially elevated in ALR-shRNA cells (Fig. 3I). Identically to previous reports, several key enzymes responsible for electronic respiratory chain were transcriptionally downregulated in ALR-shRNA cells after H/R (Fig. 3I). Quantification confirmed the increase in SUMO1 and UBA2 in ALR-shRNA cells (Suppl. Fig. S2E, F). Hence, knockdown of ALR gene expression increased the abundance of SUMO-related proteins after hepatic IRI.
Insufficiency of ALR gene amplifies Drp1 SUMOylation and recruitment to mitochondria
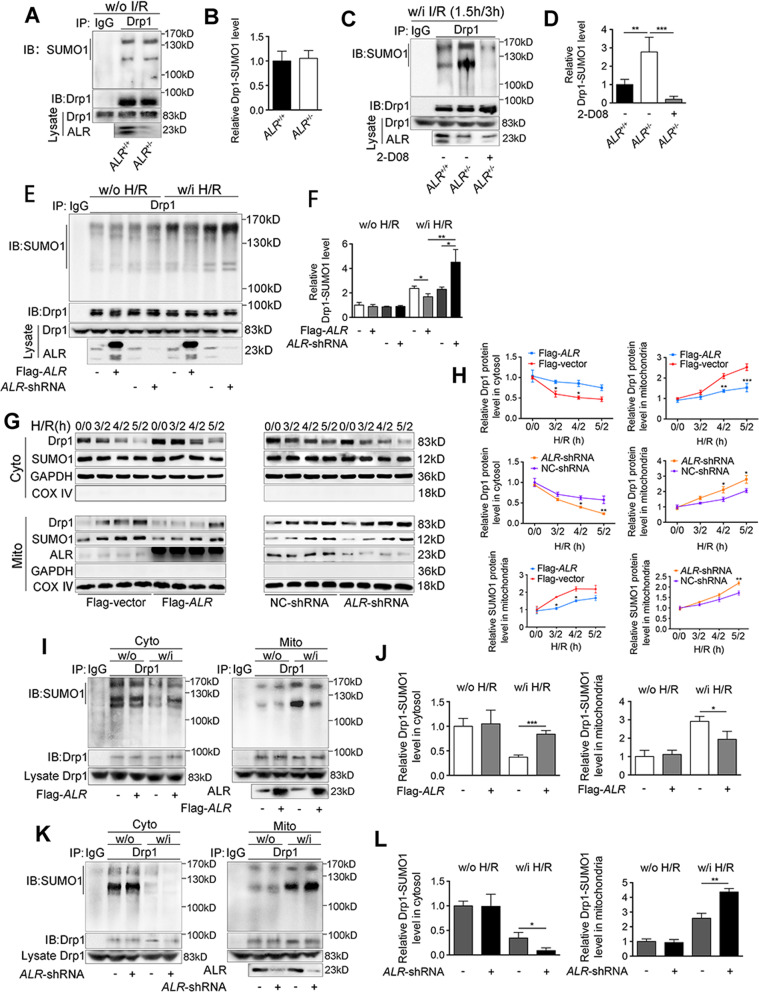
Since ALR inhibited the expressions of SUMO-related proteins, the question was whether SUMOylation of Drp1 could be suppressed as well. The results showed that ALR exerted no effect on Drp1 SUMOylation under non-IRI condition (Fig. 4A, B), however, Drp1 SUMOylation was markedly increased in ALR+/− mice after IRI (Fig. 4C, D). Under H/R condition, Drp1 SUMOylation in ALR-shRNA cells increased (Fig. 4E, lane 9, 4F), while ALR overexpression reverted this modification (Fig. 4E, lane 7, 4F).
Fig. 4. Insufficiency of ALR gene amplifies Drp1 SUMOylation and recruitment to mitochondria.
A Drp1 SUMOylation was analyzed by IP in liver tissues from ALR+/+ and ALR+/− mice w/o IRI, anti-Drp1 for IP and anti-SUMO1 for western blotting. B The quantifications of Drp1 SUMOylation in mouse livers w/o IRI (n = 3). C Drp1 SUMOylation was analyzed by IP in livers from ALR+/+ and ALR+/− mice after IRI, anti-Drp1 for IP and anti-SUMO1 for western blotting. ALR+/− mice with 2-D08 treatment (24 h, 10 mg/kg) were used as control. D The quantifications of Drp1 SUMOylation in mouse livers w/i IRI (n = 3). E IP of Drp1 SUMOylation in HepG2 cells transfected with Flag-ALR or ALR-shRNA. Cells were either treated w/i H/R or w/o H/R. Anti-Drp1 immunoprecipitants were analyzed by western blotting with anti-SUMO1 antibody. F The quantifications of Drp1 SUMOylation in HepG2 cells (n = 3). G Western blotting of Drp1 and SUMO1 in cytosolic fraction and mitochondrial compartment of HepG2 cells transfected with Flag-ALR (left) or ALR-shRNA (right). Cells were treated under the condition of H/R with prolonged hypoxia time (0–5 h/2 h). GAPDH and COX IV were used as loading controls, respectively. H The quantifications of Drp1 and SUMO1 in cytosolic fraction and mitochondrial compartment of HepG2 cells. The proteins in cytosol and mitochondria were normalized by GAPDH and COX IV, respectively (n = 3). SUMOylation of Drp1 was analyzed by IP in cytosolic fraction and mitochondrial compartment from HepG2 cells transfected either with Flag-ALR (I) or ALR-shRNA (K). Anti-Drp1 immunoprecipitants were analyzed by western blotting with anti-SUMO1 antibody. The quantifications of Drp1 SUMOylation in cytosolic fraction and mitochondrial compartment from Flag-ALR (J) or ALR-shRNA (L) transfected cells (n = 3). P-values were calculated by both Student’s t-test (B, D, F, J, L) and two-way ANOVA test (H). Data represent mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations: I/R ischemia-reperfusion, H/R hypoxia/reoxygenation, w/o without, w/i with, IP immunoprecipitation, IB immunoblotting, Cyto cytosolic fraction, Mito mitochondrial compartment.
These data suggested ALR could suppress Drp1 SUMOylation, but it was unclear whether this effect could influence Drp1 recruitment to mitochondria. As shown in Fig. 4G, H, Drp1 recruitment to mitochondria appeared to be in a time-dependent manner following hypoxia, but this phenomenon appeared to be stopped by ALR-transfection. The SUMO1 protein expression in mitochondria exhibited a similar pattern to Drp1, but conversely, ALR-shRNA accelerated Drp1 accumulation in mitochondria, to SUMO1 (Fig. 4G, H). In addition to this in vitro data, the in vivo results in ALR+/− mice also demonstrated that abundant Drp1 was recruited to mitochondria after hepatic IRI (Suppl. Fig. S3A, B).
The SUMOylated Drp1 in mitochondria and cytosol was then separately measured (Suppl. Fig. S3C, D). Figure 4I, J reveals that SUMOylated Drp1 in mitochondria was decreased in ALR-transfected cells after H/R, while it was increased in the cytosol. This alternation was reversed in ALR-shRNA cells (Fig. 4K, L). ALR deletion altered the distribution of SUMOylated Drp1 between mitochondria and cytosol in vivo, which was identical to in vitro results. (Suppl. Fig. S3E–G). Collectively, ALR inhibited Drp1 SUMOylation and blocked its translocation from cytosol to mitochondria.
ALR reciprocally modulates phosphorylation and SUMOylation to restrict Drp1 mitochondrial translocation during hepatic IRI
ALR restricts Drp1 phosphorylationSer616 and SUMOylation. However, it is still unclear how the phosphorylation and SUMOylation mutually cooperate to promote Drp1 mitochondrial recruitment. The full-length Drp1 contains four domains, including GTPase domain, Middle domain, B domain, and GTPase-effector (GED) domain. Several splicing forms of Drp1 gene exist, such as variant 1 (Var 1, a full-length 736-aa), variant 2 (Var 2, partial deletion of 27-aa in B domain) and variant 3 (Var 3, NM_005690.4, partial deletion of 37-aa in B domain). Drp1 could be SUMO-modified on K532, K535, K558, K568, K594, K597, K606, K608 sites in B domain [29]. To completely delete Drp1 SUMO1-modification, Drp1 plasmid (variant 3) lacking K532, K535, K558, K568 underwent further mutation at K594R, K597R, K606R and K608R, abbreviated as Drp1-4KR (Suppl. Fig. S4A, left). The Drp1-plasmid with substitutions of S616A and S616D was also constructed. The results showed that Drp1 SUMOylation after transfecting Drp1-S616A mutant was increased compared to Drp1-WT, but the SUMOylation after transfecting Drp1-S616D mutant was significantly decreased (Fig. 5A, B and Suppl. Fig. S4A, B). Additionally, the transfection of cells with non-SUMOylated Drp1-4KR mutant notably increased Drp1 phosphorylation at site Ser616, but not Ser637 (Fig. 5A, B and Suppl. Fig. S4A, B). Inhibition of SUMOylation by 2-D08 also enhanced Drp1 phosphorylationSer616 in a dose-depended manner (Fig. 5C, D), but phosphorylation at Ser637 only decreased in H/R condition (Fig. 5C and Suppl. Fig. S4C). These results indicated that there might be an interplay between Drp1 phosphorylation and SUMOylation.
Fig. 5. ALR reciprocally modulates phosphorylation and SUMOylation to restrict Drp1 mitochondrial translocation during hepatic IRI.
A SUMOylation and phosphorylation of Drp1 were analyzed by IP in HepG2 cells transfected with Myc-Drp1 or mutants of S616A, S616D, and 4KR, respectively. Cells were treated w/i or w/o H/R. Anti-Drp1 immunoprecipitants were analyzed by western blotting with anti-SUMO1, anti-p-Drp1 at Ser616 and Ser637 site antibodies. B The quantifications of Drp1 SUMOylation, p-Drp1S616 and p-Drp1S637 in HepG2 cells transfected either with Myc-Drp1 or indicated mutants. The results were normalized to the values of Drp1-WT group (n = 3). C SUMOylation and phosphorylation of Drp1 were analyzed by IP in HepG2 cells transfected with ALR-shRNA. Cells were treated with 2-D08 (18 h, 0 μM, 50 μM, 100 μM). NC-shRNA was used as negative control. Anti-Drp1 immunoprecipitants were analyzed by western blotting with anti-SUMO1, anti-p-Drp1 at Ser616 and Ser637 site antibodies. D The quantifications of Drp1 SUMOylation and p-Drp1S616 in cells with 2-D08 treatment (24 h, 100 μM). The results were normalized to the values of NC-shRNA group without 2-D08 and H/R treatment (n = 3). E IP of Drp1 SUMOylation and western blotting of Drp1, SUMO1 and p-Drp1 in cytosolic fraction and mitochondrial compartment from HepG2 cells transfected with ALR-shRNA. UBA2 was knockdown by siRNA. The β-actin and COX IV were used as loading controls, respectively. F Flow cytometry plots for Annexin V-EGFP and PI staining in HepG2 cells transfected with Flag-ALR or ALR-shRNA under the condition of H/R. The ALR-shRNA cells were transfected with siUBA2 or treated with 2-D08 (24 h, 100 μM). G The percentage of EGFP+/PI+ and EGFP+/PI− cells. The values were normalized to negative control group transfected with Flag-tagged vector (n = 3). H Western blotting of indicated proteins in HepG2 cells transfected with ALR-shRNA. The cells were transfected either with Myc-Drp1 WT or 4KR mutant. GAPDH was used as loading control. I The quantifications of indicated proteins were analyzed (n = 3). J Western blotting of p-Drp1S616 and p-Drp1S637 in cytosolic fraction and mitochondrial compartment from ALR-shRNA HepG2 cells transfected either with Myc-Drp1 WT or 4KR mutant. The β-actin and COX IV were used as loading control, respectively. K The quantifications of indicated proteins in cytosolic fraction and mitochondrial compartment were analyzed in cells subject to w/o (upper) or w/i (lower) H/R (n = 3). L IP of Drp1 SUMOylation, western blotting of Drp1, p-Drp1, ATPS-β, NDUFB8, Caspase-3, and cleaved Caspase-3 in HepG2 cells transfected either with Myc-Drp1 WT or 4KR-S616A mutant. GAPDH was used as loading control. M Western blotting of Drp1 in cytosolic fraction and mitochondrial compartment from HepG2 cells transfected either with Myc-Drp1 WT or 4KR-S616A mutant. The β-actin and COX IV were used as loading control, respectively. N The quantifications of Drp1 in cytosolic fraction and mitochondrial compartment (n = 3). P-values were calculated by both Student’s t-test (B, G, I, K, N) and one-way ANOVA (D). Data represent mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations: p-Drp1 phosphorylated-Drp1, H/R hypoxia/reoxygenation, w/o without, w/i with, IP, immunoprecipitation, IB, immunoblotting, Cyto, cytosolic fraction, Mito mitochondrial compartment.
It was next investigated if enhanced Drp1 phosphorylation at Ser616 due to its reduced SUMOylation contributed to Drp1 mitochondrial translocation. As indicated in Fig. 5E, depletion of UBA2 inhibited Drp1 SUMOylation and the translocation of SUMO1 and Drp1 from cytoplasm to mitochondria was decreased after H/R (Suppl. Fig. S4F). Blockage of SUMOylation by UBA2 knockdown or 2-D08 treatment both strengthened Drp1 phosphorylation at Ser616 primarily in mitochondria rather than in cytosolic fraction (Fig. 5E and Suppl. Fig. S4D–F). The apoptosis was decreased in H/R-induced ALR-shRNA cells after UBA2 knockdown or 2-D08 treatment (Fig. 5F, G and Suppl. Fig. S4G). Next, ALR-shRNA cells were transfected with Drp1-WT or Drp1-4KR. In H/R condition, the protein levels of NDUFB8, a subunit of NADH ubiquinone oxidoreductase and ATPS-β, a β-polypeptide of ATP synthase were decreased more in Drp1-WT-transfected cells than in Drp1-4KR-transfected cells and the Caspase-3 activity was reversed (Fig. 5H, I). More importantly, 4KR-transfected cells showed a higher Drp1 phosphorylation at Ser616 in mitochondria than in WT-transfected cells (Fig. 5J, K).
The Drp1 4KR-S616A mutant was generated (Fig. 5L and Suppl. Fig. S4H) and compared with Drp1 wild-type; it completely blocked the translocation of Drp1 from cytosol to mitochondria (Fig. 5M, N). The protein levels of NDUFB8 and ATPS-β were increased in 4KR-S616A-transfected cells and the activity of Caspase-3 was correspondingly reduced (Fig. 5L and Suppl. Fig. S4H), which indicated that the mitochondrial function was preserved after inhibition of Drp1 phosphorylation and SUMOlyation. These results suggested that the Drp1 translocation to mitochondria induced by either SUMOylation or phosphorylationSer616 seemed to be coordinately compensated and also verified that ALR could simultaneously inhibit both these modification processes, which might be beneficial for the amelioration of hepatic IRI.
ALR blocks Drp1 mitochondrial translocation and maintains the mitochondrial homeostasis after IRI
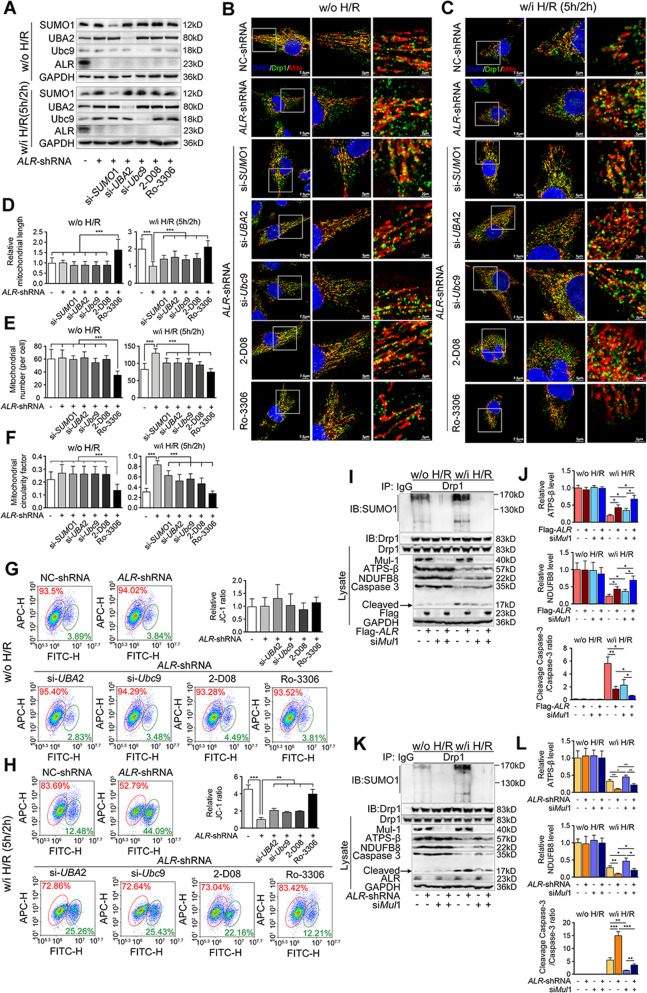
Considering that ALR inhibits Drp1 recruitment to mitochondria through controlling its phosphorylation and SUMOylation, it was investigated whether this inhibition could protect mitochondria from H/R injury. Mitochondrial morphology was examined and the results indicated that mitochondrial fission increased after H/R injury (Fig. 6B, C, row 1). Depletion of ALR clearly worsened mitochondrial fission (Fig. 6B, C, row 2), whereas, inhibition of SUMO-related protein expression (Fig. 6A and Suppl. Fig. S5A) or suppression of SUMOylation with 2-D08 partly abolished mitochondrial fission (Fig. 6B, C, row 3-6, Fig. 6D–F). CDK1 has been reported to phosphorylate Drp1 at Ser616 [14] and to further phosphorylate Ubc9, which could also enhance Drp1 SUMOylation [30]. Treatment of cells with Ro-3306, an inhibitor of CDK1 [31] to block the phosphorylation/SUMOylation of Drp1 simultaneously, could result in elongation of mitochondria after the H/R (Fig. 6B, C, row 7, Fig. 6D–F).
Fig. 6. ALR blocks Drp1 mitochondrial translocation and maintains the mitochondrial homeostasis after IRI.
A Western blotting of indicated proteins in ALR-shRNA HepG2 cells transfected with siSUMO1, siUBA2, siUbc9, or treated with 2-D08 (18 h, 100 μM) and Ro-3306 (20 h, 35 nM), respectively. GAPDH was used as loading control. B, C Representative images of immunofluorescence staining on Drp1 (green), mitochondria (red), and DAPI (blue) in HepG2 cells with ALR-shRNA-transfected w/o (B) or w/i H/R (C). Scale bar, 7.5, 5, 2 μm. D Quantifications of mitochondrial length in about 50 cells of each group. The results were normalized to the values of ALR-shRNA group. E Quantifications of mitochondrial number (per cell) in about 50 cells. F Quantifications of mitochondrial circularity factor in about 50 cells. The values were recorded as 0 to 1 (0 means perfect linear and 1 means perfect circular). G, H Flow cytometry plots for JC-1 aggregates (red) and monomer (green) in ALR-shRNA HepG2 cells w/o (G) or w/i H/R (H). The cells were treated with siRNA against UBA2 or Ubc9, or treated with 2-D08 (24 h, 100 μM) and Ro-3306 (20 h, 35 nM), respectively. JC-1 ratios for aggregates/monomer were calculated (right) (n = 3). IP of Drp1 SUMOylation, western blotting of Drp1, Mul1, ATPS-β, NDUFB8, Caspase-3 and cleaved Caspase-3 in HepG2 cells transfected either with Flag-ALR (I) or ALR-shRNA (K). The cells were transfected with siMul1. GAPDH was used as loading control. The graphs of indicated ratios in Flag-ALR (J) or ALR-shRNA (L) cells were quantified and normalized to the values of Flag-vector or NC-shRNA transfected with siNC w/o H/R, respectively (n = 3). P-values were calculated by Student’s t-test. Data represent mean ± S.D. *P < 0.05; **P < 0.01; ****P < 0.0001. Abbreviations: H/R hypoxia/reoxygenation, w/o without, w/i with, DAPI 4′,6‐diamidino‐2‐phenylindole.
Loss of mitochondrial membrane potential (MMP) severely disrupted mitochondrial function. This MMP was reduced by H/R (Fig. 6G, H) while inhibition of ALR clearly worsened the H/R-induced loss of MMP. Similarly, knockdown of UBA2/Ubc9 or blockage of SUMOylation by 2-D08 partially rescued the MMP collapse. The MMP was also rescued following Ro-3306 treatment. Measurement of NDUFB8 and ATPS-β verified that the mitochondrial function could be preserved by ALR-overexpressing (Suppl. Fig. S5B–E). The in vivo experiments provided similar data for NDUFB8 and ATPS-β as those in in vitro (Suppl. Fig. S5F–I).
Knockdown of Mul1 significantly inhibited Drp1 SUMOylation (Fig. 6I, K and Suppl. Fig. S5J). The reductions of NDUFB8 and ATPS-β induced by H/R were prevented by knockdown of Mul1 and Caspase-3 activity was suppressed as well (Fig. 6I–L). During Mul1 knockdown, ALR could still preserve mitochondrial function and inhibit cell apoptosis during H/R, suggesting that ALR protection may be partially due to its regulation on Drp1 phosphorylation.
Additional parameters related to IRI such as reactive oxygen species (ROS) and ATP content in ALR+/− mice were also determined. The results showed an increase in production of H2O2 (Suppl. Fig. S6A) and ROS levels (Suppl. Fig. S6E, F) in ALR+/− mice after IRI compared to ALR+/+ mice, but treatment with 2-D08 was able to reduce these (Suppl. Fig. S6A, E, F). Antioxidant molecules such as superoxide dismutase (SOD) level (Suppl. Fig. S6B), glutathione peroxidase (GPx) activity (Suppl. Fig. S6C), and reduced/oxidized glutathione (GSH/GSSG) ratio (Suppl. Fig. S6D) were decreased in ALR+/− mice. As a consequence, hepatic ATP levels (Suppl. Fig. S6G, H) declined as well. Treatment with 2-D08 preserved these antioxidant markers (Suppl. Fig. S6B–D and G, H). Taken together, these results showed that ALR might also protect liver against IRI-induced mitochondrial dysfunction by inhibiting SUMOylated Drp1 mitochondrial translocation.
ALR interacts with transcriptional factor YY1 and inhibits UBA2 transcription
To explore the mechanism by which ALR modulates Drp1 SUMOylation, immunoprecipitation and mass spectrometry (IP-MS) were employed to screen for potential proteins interacting with ALR (Suppl. Fig. S7A). It indicated that putative proteins interacting with ALR include Mia40, Tim8 and Cox17, which were previously reported [32, 33] (Suppl. Fig. S7B and Suppl. Table S3). Transcription factor YY1 and YY1-associated protein 1 (YY1AP1), which is a regulator of YY1 [34, 35], were also identified (Suppl. Fig. S7B). These results were further confirmed by Co-IP (Fig. 7A) and a GST pull-down assay (Fig. 7B). However, ALR interacted with YY1AP1 only in the condition of H/R (Fig. 7A, lane 8), without affecting the protein stability of YY1 and YY1AP1 (Fig. 7C–F), which appeared to translocate into the nucleus during H/R (Fig. 7G–K). The interaction between ALR and YY1/YY1AP1 mostly occurred in the cytoplasm (Fig. 7G). YY1 seemed to be increased in the cytoplasm when ALR was overexpressed, whereas depletion of ALR enhanced YY1 nuclear localization (Fig. 7G–K). Simultaneously, YY1AP1 exhibited the distribution like YY1 after H/R (Fig. 7G–K), implying that ALR interaction with YY1AP1 might be assisting YY1 to stay in the cytoplasm.
Fig. 7. ALR interacts with transcriptional factor YY1 and inhibits UBA2 transcription.
A Co-IP of interaction between ALR and YY1 or YY1AP1 in HepG2 cells transfected with Flag-ALR. Anti-Flag immunoprecipitants were analyzed by western blotting with anti-YY1, anti-YY1AP1 antibodies. Cells were treated w/i or w/o H/R. B Direct interaction between YY1 and ALR was revealed by GST pull-down assays. Input and pull-down samples were both subjected to western blotting with anti-GST and anti-His antibodies. Ten percent of bacterial lysate was used for input. C Western blotting of UBA2, YY1 and YY1AP1 in HepG2 cells transfected with Flag-ALR. Cells were either treated w/i or w/o H/R. GAPDH was used as loading control. D The quantifications of indicated proteins (n = 3). E Western blotting of UBA2, YY1 and YY1AP1 in HepG2 cells transfected with ALR-shRNA. The ALR-shRNA cells were transfected with Flag-ALR for rescue experiment. GAPDH was used as loading control. Cells were either treated w/i or w/o H/R. F The quantifications of indicated proteins (n = 3). G Co-IP of interaction between ALR and YY1 or YY1AP1, western blotting of YY1 and YY1AP1 in cytoplasm and nucleus from HepG2 cells transfected with Flag-ALR. The α-tubulin and lamin A/C were used as loading controls, respectively. H The quantifications of YY1 and YY1AP1 in cytoplasm and nucleus. The proteins were normalized by α-tubulin and lamin A/C, respectively (n = 3). I Representative images of immunofluorescence staining on YY1 (upper)/YY1AP1 (lower) (green), Flag (red) and DAPI (blue) in HepG2 cells transfected with Flag-ALR. Flag-vector was a negative control. Scale bar, 10 μm. J Western blotting of YY1 and YY1AP1 in cytoplasm and nucleus from HepG2 cells transfected with ALR-shRNA. The ALR-shRNA cells were transfected with Flag-ALR for rescue experiment. Cells were either treated w/o or w/i H/R. The α-tubulin and lamin A/C were used as loading controls, respectively. K The quantifications of YY1 and YY1AP1 in cytoplasm and nucleus (n = 3). L EMSA was performed with 5 μl aliquot containing 100 ng of GST-YY1 and 5 pmol of biotin-labeled UBA2 promoter oligonucleotides, YY1 antibody and an unlabeled competitor probe were added as indicated. The unlabeled competitor at a 10- or 50-fold excess was included in the incubation mixture prior to the EMSA. M, N IP of Drp1 SUMOylation, western blotting of UBA2, YY1 or YY1AP1 in HepG2 cells transfected with Flag-ALR. Cells were transfected either with siYY1 (M) or siYY1AP1 (N). Cells were either treated w/i or w/o H/R. GAPDH was used as loading control. O, P IP of Drp1 SUMOylation, western blotting of UBA2, YY1 or YY1AP1 in HepG2 cells transfected with ALR-shRNA. The ALR-shRNA cells were transfected with Flag-ALR for rescue experiment. Cells were transfected with siYY1 (O) or siYY1AP1 (P). Cells were treated w/i or w/o H/R. GAPDH was used as loading control. P-values were calculated by Student’s t-test. Data represent mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0. 001. Abbreviations: H/R hypoxia/reoxygenation, w/o without, w/i with IP immunoprecipitation, IB immunoblotting, DAPI 4′,6‐diamidino‐2‐phenylindole, Mut mutant, Cyto cytoplasm, Nu nucleus.
Leptomycin b (LMB) [36], a nuclear export inhibitor, can potently block CRM1/exportin and completely interrupt the exit of nuclear proteins such as APC [37] (Suppl. Fig. S7C). Similarly, the results in Suppl. Fig. S7D, E show that LMB did not affect the distribution of YY1 and YY1AP1 in ALR-transfected cells, suggesting that ALR might restrict YY1 import to the nucleus, which relies either on simultaneous application of a nuclear import inhibitor, or co-transfection of YY1-bearing plasmids.
Based upon the TRANSFAC database (ALGGEN, http://alggen.lsi.upc.es), a putative transcription factor of UBA2 gene was searched and YY1 was top-ranked among the candidate transcription factors. Subsequent an mRNA assay verified that siRNA to YY1/YY1AP1 substantially decreased both the protein and mRNA level of UBA2 (Suppl. Fig. S7F). The qChIP assay (Suppl. Fig. S7G) and EMSA assay (Fig. 7L) also revealed YY1 binding to the UBA2 promoter region.
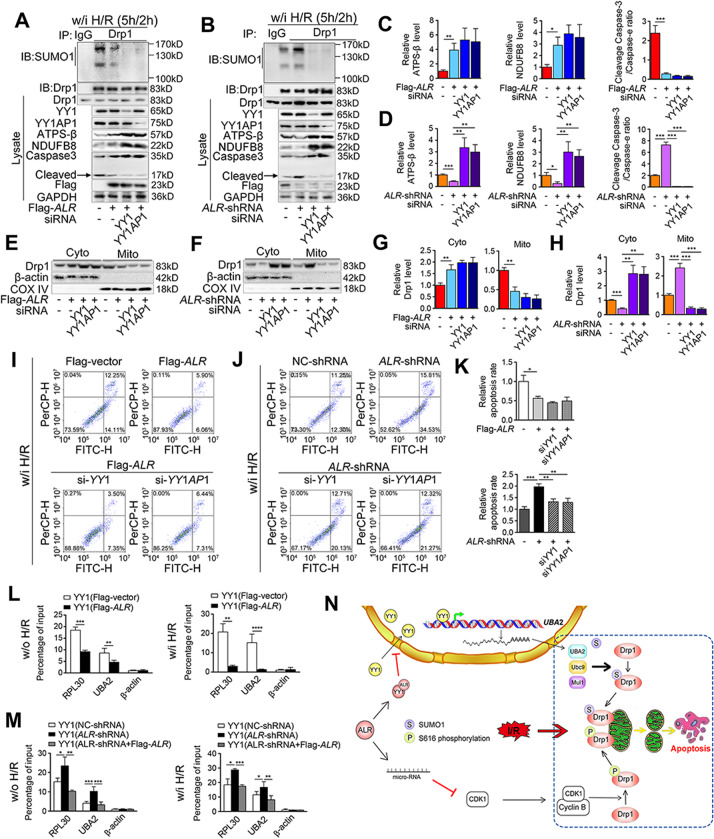
The study results showed that ALR was able to downregulate UBA2 (Fig. 7M–P and Suppl. Fig. S7H–K) and this regulation was abolished when YY1 was blocked with siRNA (Fig. 7M, O and Suppl. Fig. S7H, J). A similar result was observed when YY1AP1 was blocked during H/R (Fig. 7N, P and Suppl. Fig. S7I, K), further confirming that ALR downregulation of UBA2 was YY1-dependent. During H/R, Drp1 SUMOylation and subsequently mitochondrial translocation were inhibited after YY1 or YY1AP1 knockdown (Fig. 8A–H and Suppl. Fig. S7L, M), while the protein levels of NDUFB8 and ATPS-β were increased, indicating that the mitochondrial function was well preserved (Fig. 8A–D). YY1/YY1AP1 silencing also decreased the H/R-induced apoptosis rate in ALR-shRNA cells (Fig. 8I–K).
Fig. 8. Knockdown of YY1 alleviates mitochondrial dysfunction and apoptosis caused by H/R.
A, B IP of Drp1 SUMOylation, western blotting of YY1, YY1AP1, ATPS-β, NDUFB8, Caspase-3 and cleaved Caspase-3 in HepG2 cells transfected either with Flag-ALR (A) or ALR-shRNA (B) under the condition of H/R. The cells were transfected either with siYY1 or siYY1AP1. GAPDH was used as loading control. C, D The graphs of indicated ratios in Flag-ALR (C) or ALR-shRNA (D) cells were quantified, respectively (n = 3). E, F Western blotting of Drp1 in cytosolic fraction and mitochondrial compartment from Flag-ALR (E) or ALR-shRNA (F) HepG2 cells transfected with siYY1 or siYY1AP1 under the condition of H/R. The β-actin and COX IV were used as loading control, respectively. G, H The quantifications of Drp1 in cytosolic fraction and mitochondrial compartment from Flag-ALR (G) or ALR-shRNA (H) HepG2 (n = 3). I–J Flow cytometry plots for Annexin V-EGFP and PI staining in HepG2 cells transfected with Flag-ALR (I) and ALR-shRNA (J) under the condition of H/R. The cells were transfected with siYY1 or siYY1AP1. K The percentage of EGFP+/PI+ and EGFP+/PI− cells. The values were normalized to the negative control groups, respectively (n = 3). L, M The qChIP assays for UBA2 and RPL30 promoter binding using anti-YY1 in HepG2 cells with Flag-ALR (L) or ALR-shRNA (M) transfection. The ALR-shRNA cells were transfected with Flag-ALR for rescue. Cells were treated either w/i or w/o H/R. The results were represented as fold change over control (IgG) (n = 3). N Working model of ALR-maintaining mitochondrial dynamics and preventing apoptosis during IRI. P-values were calculated by Student’s t-test. Data represent mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations: H/R hypoxia/reoxygenation, w/o without, w/i with, IP immunoprecipitation, IB immunoblotting, Cyto cytosolic fraction, Mito mitochondrial compartment.
The qChIP assay showed that binding of YY1 to the UBA2 promoter region was significantly reduced in ALR-transfected cells and strengthened in ALR-shRNA cells inversely (Fig. 8L, M). Taken together, these data strongly suggested that the restriction of Drp1 SUMOylation by ALR and the subsequent inhibition of mitochondrial fission were associated with the downregulation of YY1-dependent UBA2 transcription under IRI condition.
Discussion
Mitochondrial homeostasis is maintained by fission and fusion [38], which allows for damaged mitochondria to be segregated for removal and facilitates the equilibration of mitochondrial components [39, 40]. Ischemia-reperfusion injury (IRI) is one of the major reasons for failed liver transplantations, where excessive mitochondrial fission has been described [41]. Mitochondrial fission and fusion are controlled by a family of dynamin-related proteins and the master mediator of fission is dynamin-related protein 1 (Drp1) [12, 42]. Deletion of this gene Drp1−/− in mice results in early postnatal death because of brain hypoplasia, with apoptosis induced by mitochondrial fission [43]. Drp1 has been well-known as a controller in mitochondrial dynamic during the IRI process in liver and heart [11, 44]. Drp1 translocation to the mitochondria is a key step to induce mitochondrial fission, which is mainly controlled by phosphorylation and SUMOylation [28, 45]. Drp1 SUMOylation of in myocardial IRI produced different effects at different stages of injury. Inhibiting SUMO1 modification of Drp1 and subsequently excessive mitochondrial fission by DJ-1 protects heart from myocardial IRI [46]. Drp1 SUMOylation also leads to mitophagy induced by zinc as well, which clears the damaged mitochondria and prevents ROS generation in cardiac IRI [47]. The upstream regulatory mechanism of Drp1 phosphorylation and SUMOylation that affects Drp1 recruitment to mitochondria remains unclear. Although we have previously shown that ALR inhibited CDK1/cyclin B-mediated Drp1 phosphorylation and mitochondrial fission, it was not clear if ALR was involved in the regulation of Drp1 SUMOylation. This hypothesis was confirmed by both in vitro and in vivo experiments (Fig. 4A–F). As indicated in Fig. 4I–L and Suppl. Fig. S3C–G, SUMOylated Drp1 accumulated around mitochondria in the liver of ALR-lacking mice or in ALR-shRNA hepatocytes and this accumulation was effectively abolished in ALR-transfected cells. The ALR-induced inhibition of Drp1 SUMOylation coincided with a significant decrease in mitochondrial fragmentation, and with a significant restoration of mitochondrial function (Fig. 6 and Suppl. Fig. S6). Mechanistically, the downregulation of Drp1 SUMOylation by ALR was apparently associated with its negative transcriptional control of UBA2, at the promoter level.
Identification of the transcriptional regulation of UBA2 by ALR encouraged further investigation of transcription factors that regulated UBA2, which have not been characterized to date. The transcription factor YY1 was identified by bioinformatics tools in silico, and subsequently verified in vivo (Fig. 7L and Suppl. Fig. S7G). It is known as a multifunctional transcription factor responsible for activating or inhibiting transcription of target genes [48]. Despite YY1 residing in the nucleus as a transcriptional regulator, there are reports showing that YY1 could also stay in the cytoplasm [49, 50]. In this study, it was found that ALR was one of the potential proteins that interacted with YY1 in cytoplasm. It is known that the YY1-associated protein 1 (YY1AP1) is a novel identified co-activator of YY1 [34], so by interacting with YY1 and its regulator YY1AP1, ALR blocked their nuclear import and sustainably diminished the transcriptional level of UBA2 (Fig. 7). Although Leptomycin B, a potent nuclear export inhibitor, effectively interrupted the export of YY1 from the nucleus, an additional experiment using an inhibitor to block nuclear import along with Leptomycin B should be simultaneously applied to compare YY1 net-flow between cytosol and nucleus. The lack of a validated nuclear import inhibitor prevented the performance of this supplemental experiment at the present time. Although the mechanism of how ALR interacts with YY1 to block its nuclear import remains unclear, the IP-MS analysis identified that ALR was co-purified with more one importin proteins, including importin 5, 11, and α5 (Suppl. Fig. S7B and Suppl. Table. S3) [51–55]. Nuclear protein import requires the nuclear pore-targeting complex, which contains the NLS-cargo and NLS receptor complex, also called as importin proteins [56, 57], so in this case, ALR may interact with a nuclear pore-targeting protein, providing the possible explanation of how ALR inhibits YY1 nuclear import. Further studies will be required to confirm these hypotheses.
Phosphorylation and SUMOylation are often interacting to regulate protein function, an example of which is the antagonism of SUMOylation of CESTA protein to regulate its subnuclear localization [58]. It has been also reported that SUMOylation occurs in a phosphorylation-dependent manner during the heat shock response [59]. This study showed that via either phosphorylation or SUMOylation, ALR plays an important role in regulating Drp1 activity, and mitochondrial dynamics, during hepatic IRI. This ALR-governed complementary regulation of Drp1 by phosphorylation and SUMOylation represents a coordinated yet independent manner to regulate Drp1 activity in either direction (Fig. 5) by two different molecular mechanisms. Further study could focus on the causal effect relationship between ALR and Drp1 in hepatic IRI such as whether Drp1 knockout or 4KR mutant can rescue the phenotype of ALR+/− during the hepatic IRI.
In conclusion, these results demonstrated that ALR provided an elegant route towards exploiting Drp1 and its impact on mitochondrial fission via inhibiting SUMOylation and phosphorylation as a potential tool to prohibit mitochondrial fission and reducing IRI during liver transplantation surgery or other ischemic conditions. Considering the paucity of donor organs for liver transplantation, optimal conservation of these precious resources is of prime importance.
Supplementary information
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC), Grant/Award Number: 31771279. Surgery samples were generously provided by Prof. Dr. Lang in the Surgical Department of Chaoyang Hospital, Capital Medical University.
Author contributions
Study concept and design: PX, WA; acquisition of patient specimens: JH, YD; acquisition of data: JH, YD; analysis and interpretation of data: PX, JH; drafting of the manuscript: JH; critical revision of the manuscript: PX, WA; funding for PI: WA.
Data availability
The transcriptome data sets are available at NCBI (SAR accession: PRJNA598942).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Capital Medical University Ethics Committee.
Footnotes
Edited by L. Scorrano
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jing Huang, Ping Xie
Supplementary information
The online version of this article (10.1038/s41418-020-00641-7) contains supplementary material, which is available to authorized users.
References
- 1.Huguet C, Addario-Chieco P, Gavelli A, Arrigo E, Harb J, Clement RR. Technique of hepatic vascular exclusion for extensive liver resection. Am J Surg. 1992;163:602–5. doi: 10.1016/0002-9610(92)90567-b. [DOI] [PubMed] [Google Scholar]
- 2.Abdalla EK, Roger N, Jacques B. Hepatic vascular occlusion: which technique? Surg Clin North Am. 2004;84:563–85. doi: 10.1016/S0039-6109(03)00231-7. [DOI] [PubMed] [Google Scholar]
- 3.Selzner M, Rüdiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–8. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 4.Odeh M. The role of reperfusion-induced injury in the pathogenesis of the crush syndrome. N. Engl J Med. 1991;324:1417–22. doi: 10.1056/NEJM199105163242007. [DOI] [PubMed] [Google Scholar]
- 5.Elias-Miró M, Jiménez-Castro MB, Rodés J, Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res. 2013;47:555–68. doi: 10.3109/10715762.2013.811721. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwenhuijs VB, De Bruijn MT, Padbury RT, Barritt GJ. Hepatic ischemia-reperfusion injury: roles of Ca2+ and other intracellular mediators of impaired bile flow and hepatocyte damage. Dig Dis Sci. 2006;51:1087–102. doi: 10.1007/s10620-006-8014-y. [DOI] [PubMed] [Google Scholar]
- 7.Zemirli N, Morel E, Molino D. Mitochondrial dynamics in basal and stressful conditions. Int J Mol Sci. 2018;19:564. doi: 10.3390/ijms19020564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, et al. Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc. 2017;6:e005328. doi: 10.1161/JAHA.116.005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Liu X. Novel insights into the role of mitochondrial fusion and fission in cardiomyocyte apoptosis induced by ischemia/reperfusion. J Cell Physiol. 2018;233:5589–97. doi: 10.1002/jcp.26522. [DOI] [PubMed] [Google Scholar]
- 10.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–25. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 11.Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis, and decreasing oxidative stress. Redox Biol. 2019;20:296–306. doi: 10.1016/j.redox.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca TB, Sánchez-Guerrero Á, Milosevic I, Raimundo N. Mitochondrial fission requires DRP1 but not dynamins. Nature. 2019;570:E34–42. doi: 10.1038/s41586-019-1296-y. [DOI] [PubMed] [Google Scholar]
- 13.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–7. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 14.Zaja I, Bai X, Liu Y, Kikuchi C, Dosenovic S, Yan Y, et al. Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem Biophys Res Commun. 2014;453:710–21. doi: 10.1016/j.bbrc.2014.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–50. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu J, Yu HM, Chiu SY, Mirando AJ, Maruyama EO, Cheng JG, et al. Disruption of SUMO-specific protease 2 induces mitochondria mediated neurodegeneration. PLoS Genet. 2014;10:e1004579. doi: 10.1371/journal.pgen.1004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A, Jeong D, Mitsuyama S, Oh JG, Liang L, Ikeda Y, et al. The role of SUMO-1 in cardiac oxidative stress and hypertrophy. Antioxid Redox Signal. 2014;21:1986–2001. doi: 10.1089/ars.2014.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;248:273–84. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei MH, An W, Zhang BH, Shao Q, Gong DZ. Hepatic stimulator substance protects against acute liver failure induced by carbon tetrachloride poisoning in mice. Hepatology. 1993;17:638–44. doi: 10.1002/hep.1840170418. [DOI] [PubMed] [Google Scholar]
- 20.Ho DW, Fan ST, To J, Woo YH, Zhang Z, Lau C, et al. Selective plasma filtration for treatment of fulminant hepatic failure induced by D-galactosamine in a pig model. Gut. 2002;50:869–76. doi: 10.1136/gut.50.6.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Chen L, Yu H, Liu H, An W. Transfection of hepatic stimulator substance gene desensitizes hepatoma cells to H2O2-induced cell apoptosis via preservation of mitochondria. Arch Biochem Biophys. 2007;464:48–56. doi: 10.1016/j.abb.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Rani R, Karns R, Gandhi CR. Augmenter of liver regeneration protein deficiency promotes hepatic steatosis by inducing oxidative stress and microRNA-540 expression. FASEB J. 2019;33:3825–40. doi: 10.1096/fj.201802015R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Huang J, An W. Hepatic stimulator substance resists hepatic ischemia-reperfusion injury by regulating Drp1 translocation and activation. Hepatology. 2017;66:1989–2001. doi: 10.1002/hep.29326. [DOI] [PubMed] [Google Scholar]
- 24.Jiang SJ, Li W, An W. Adenoviral gene transfer of hepatic stimulator substance confers resistance against hepatic ischemia-reperfusion injury by improving mitochondrial function. Hum Gene Ther. 2013;24:443–56. doi: 10.1089/hum.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–72. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Tan Y, Chen Y, Du M, Peng Z, Xie P. USF2 inhibits the transcriptional activity of Smurf1 and Smurf2 to promote breast cancer tumorigenesis. Cell Signal. 2019;53:49–58. doi: 10.1016/j.cellsig.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Kim YS, Keyser SG, Schneekloth JS., Jr Synthesis of 2′,3′,4′-trihydroxyflavone (2-D08), an inhibitor of protein sumoylation. Bioorg Med Chem Lett. 2014;24:1094–7. doi: 10.1016/j.bmcl.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prudent J, Zunino R, Sugiura A, Mattie S, Shore GC, McBride HM. MAPL SUMOylation of Drp1 stabilizes an ER/mitochondrial platform required for cell death. Mol Cell. 2015;17:941–55. doi: 10.1016/j.molcel.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Figueroa-Romero C, Iñiguez-Lluhí JA, Stadler J, Chang CR, Arnoult D, Keller PJ, et al. SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. FASEB J. 2009;23:3917–27. doi: 10.1096/fj.09-136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su YF, Yang T, Huang H, Liu LF, Hwang J. Phosphorylation of Ubc9 by Cdk1 enhances SUMOylation activity. PLoS ONE. 2012;7:e34250. doi: 10.1371/journal.pone.0034250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci USA. 2006;103:10660–5. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daithankar VN, Farrell SR, Colin T. Augmenter of liver regeneration: substrate specificity of a flavin-dependent oxidoreductase from the mitochondrial intermembrane space. Biochemistry. 2009;48:4828–37. doi: 10.1021/bi900347v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banci L, Bertini I, Calderone V, Cefaro C, Ciofi-Baffoni S, Gallo A, et al. Molecular recognition, and substrate mimicry drive the electron-transfer process between MIA40 and ALR. Proc Natl Acad Sci USA. 2011;108:4811–6. doi: 10.1073/pnas.1014542108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang CY, Liang YJ, Lin YS, Shih HM, Jou YS, Yu WC. YY1AP, a novel co-activator of YY1. J Biol Chem. 2004;279:17750–5. doi: 10.1074/jbc.M310532200. [DOI] [PubMed] [Google Scholar]
- 35.Ohtomo T, Horii T, Nomizu M, Suga T, Yamada J. Molecular cloning of a structural homolog of YY1AP, a coactivator of the multifunctional transcription factor YY1. Amino Acids. 2007;33:645–52. doi: 10.1007/s00726-006-0482-z. [DOI] [PubMed] [Google Scholar]
- 36.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–7. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brocardo M, Näthke IS, Henderson BR. Redefining the subcellular location and transport of APC: new insights using a panel of antibodies. EMBO Rep. 2005;6:184–90. doi: 10.1038/sj.embor.7400329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development, and disease. Nat Rev Mol Cell Biol. 2014;15:634–46. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 40.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–9. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 41.Maneechote C, Palee S, Chattipakorn SC, Chattipakorn N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med. 2017;21:2643–53. doi: 10.1111/jcmm.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–56. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishihara N1, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–66. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 44.Wu D, Dasgupta A, Chen KH, Neuber-Hess M, Patel J, Hurst TE, et al. Identification of novel dynamin-related protein 1 (Drp1) GTPase inhibitors: Therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia-reperfusion injury. FASEB J. 2020;34:1447–64. doi: 10.1096/fj.201901467R. [DOI] [PubMed] [Google Scholar]
- 45.Jahani-Asl A, Slack RS. The phosphorylation state of Drp1 determines cell fate. EMBO Rep. 2007;8:912–3. doi: 10.1038/sj.embor.7401077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu Y, Lambert JP, Nicholson CK, Kim JJ, Wolfson DW, Cho HC, et al. DJ-1 protects the heart against ischemia-reperfusion injury by regulating mitochondrial fission. J Mol Cell Cardiol. 2016;97:56–66. doi: 10.1016/j.yjmcc.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bian X, Xu J, Zhao H, Zheng Q, Xiao X, Ma X, et al. Zinc-induced SUMOylation of dynamin-related protein 1 protects the heart against ischemia-reperfusion injury. Oxid Med Cell Longev. 2019;2019:1232146. doi: 10.1155/2019/1232146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang M, Zhang Y, Yang S, Zhou J, Gao W, Yang X, et al. Multifunctional YY1 in liver diseases. Semin Liver Dis. 2017;37:363–76. doi: 10.1055/s-0037-1607451. [DOI] [PubMed] [Google Scholar]
- 49.Ficzycz A, Ovsenek N. The Yin Yang 1 Transcription factor associates with ribonucleoprotein (mRNP) complexes in the cytoplasm of Xenopus oocytes. J Biol Chem. 2002;277:8382–7. doi: 10.1074/jbc.M110304200. [DOI] [PubMed] [Google Scholar]
- 50.Palko L, Bass HW, Beyrouthy MJ, Hurt MM. The Yin Yang-1 (YY1) protein undergoes a DNA-replication-associated switch in localization from the cytoplasm to the nucleus at the onset of S phase. J Cell Sci. 2004;117:465–76. doi: 10.1242/jcs.00870. [DOI] [PubMed] [Google Scholar]
- 51.Baas R, Sijm A, van Teeffelen HA, van Es R, Vos HR, Marc Timmers HT. Quantitative proteomics of the SMAD (suppressor of mothers against decapentaplegic) transcription factor family identifies Importin 5 as a bone morphogenic protein receptor SMAD-specific Importin. J Biol Chem. 2016;291:24121–32. doi: 10.1074/jbc.M116.748582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mis M, O’Brien S, Steinhart Z, Lin S, Hart T, Moffat J, et al. IPO11 mediates βcatenin nuclear import in a subset of colorectal cancers. J Cell Biol. 2020;219:e201903017. doi: 10.1083/jcb.201903017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen M, Nowak DG, Narula N, Robinson B, Watrud K, Ambrico A, et al. The nuclear transport receptor Importin-11 is a tumor suppressor that maintains PTEN protein. J Cell Biol. 2017;216:641–56. doi: 10.1083/jcb.201604025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Yu W, Qian X, Xia Y, Zheng Y, Lee J-H, et al. Nucleus-translocated ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Mol Cell. 2017;66:684–97. doi: 10.1016/j.molcel.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sang Y, Li Y, Zhang Y, Alvarez AA, Yu B, Zhang W, et al. CDK5-dependent phosphorylation and nuclear translocation of TRIM59 promotes macroH2A1 ubiquitination and tumorigenicity. Nat Commun. 2019;10:4013. doi: 10.1038/s41467-019-12001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoneda Y. Nuclear pore-targeting complex and its role on nuclear protein transport. Arch Histol Cytol. 1996;59:97–107. doi: 10.1679/aohc.59.97. [DOI] [PubMed] [Google Scholar]
- 57.Beck M, Hurt E. The nuclear pore complex: understanding its function through structural insight. Nat Rev Mol Cell Biol. 2017;18:73–89. doi: 10.1038/nrm.2016.147. [DOI] [PubMed] [Google Scholar]
- 58.Khan M, Rozhon W, Unterholzner SJ, Chen T, Eremina M, Wurzinger B, et al. Interplay between phosphorylation and SUMOylation events determines CESTA protein fate in brassinosteroid signaling. Nat Commun. 2014;5:4687. doi: 10.1038/ncomms5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohideen F, Capili AD, Bilimoria PM, Yamada T, Bonni A, Lima CD. A molecular basis for phosphorylation-dependent SUMO conjugation by the E2 Ubc9. Nat Struct Mol Biol. 2009;16:945–52. doi: 10.1038/nsmb.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The transcriptome data sets are available at NCBI (SAR accession: PRJNA598942).