Highlights
-
•
Ultrasound (US) and slightly acidic electrolyzed water (SAEW) were combined.
-
•
US + SAEW treatment inhibited mycelial growth and spore germination of R. stolonifer.
-
•
US + SAEW treatment disrupted the cell membranes and caused the leakage of cytoplasm.
-
•
US500 + SAEW50 at 40 and 55 °C increased cell membrane permeability and decreased MMP.
-
•
US500 + SAEW50 at 40 °C and US300 + SAEW50 at 55 °C controlled soft rot decay in TRs.
Keywords: Sweet potato, Rhizopus stolonifer, Ultrasound, Slightly acidic electrolyzed water, Combination
Abstract
Effects of ultrasound (US, 300, 400, and 500 W) and slightly acidic electrolyzed water (SAEW, 10, 30, and 50 mg/L) combination on inactivating Rhizopus stolonifer in sweet potato tuberous roots (TRs) were investigated. US at 300, 400, and 500 W simultaneous SAEW with available chlorine concentration of 50 mg/L at 40 and 55 °C for 10 min significantly inhibited colony diameters (from 90.00 to 6.00–71.62 mm) and spores germination (p < 0.05). US + SAEW treatment could destroy cell membrane integrity and lead to the leakage of nucleic acids and proteins (p < 0.05). Scanning and transmission electron microscopy results showed that US + SAEW treatment could damage ultrastructure of R. stolonifer, resulted in severe cell-wall pitting, completely disrupted into debris, apparent separation of plasma wall, massive vacuoles space, and indistinct intracellular organelles. US500 + SAEW50 treatment at 40 and 55 °C increased cell membrane permeability, and decreased mitochondrial membrane potential of R. stolonifer. In addition, US500 + SAEW50 at 40 °C and US300 + SAEW50 at 55 °C controlled R. stolonifer growth in sweet potato TRs during 20 days of storage, suggesting effective inhibition on the infection of R. stolonifer. Therefore, US + SAEW treatment could be a new efficient alternative method for storing and preserving sweet potato TRs.
1. Introduction
Sweet potato (Ipomoea batatas [L.] Lam.) belongs to morning glory family, Convolvulaceae, being rich in carbohydrates, proteins, dietary fiber, minerals, vitamins, and polyphenols [1]. As a food crop, sweet potato plays a significant role in the traditional diet of many countries and regions worldwide due to its higher dry matter content per unit land area than cereals [2]. However, sweet potato tuberous roots (TRs) are easily damaged by cuts and abrasion during postharvest due to their thin and delicate skin [3], thus they are easily infected by different pathogenic fungi [4]. On average, approximately 15–65% of sweet potato TRs is lost during the whole supply chain [5]. Rhizopus soft rot, one of the most severe postharvest diseases caused by R. stolonifer, is responsible for the significant decay losses of sweet potato TRs during storage [6]. Currently, to control the postharvest diseases including Rhizopus soft rot, synthetic fungicides are usually applied before storage, such as Botran, Carbendazim, etc. [7]. The application of synthetic fungicides results in various problems, such as increased resistance of pathogens to fungicides, environmental hazards, and residual chemical fungicides, which are harmful to human health [8]. Therefore, it is vital to look for alternative 'safer' methods to control postharvest diseases of sweet potato TRs.
Ultrasound (US), a form of energy generated by sound waves, can inactivate some microorganisms, and is considered to be an environmentally-friendly antimicrobial treatment in food industry [9]. The mechanical effect of US is generally regarded as the main inactivation mechanism resulting in complete rupture of microbial cell membranes and the death of microbe [10], [11]. US treatment was reported to reduce effectively mold growth in strawberries [12]. US also reduced the total number of bacteria colonies, mold, and yeast of green asparagus after 16 days of storage [13]. However, in some cases, US treatment alone was found to be no benefit for bactericidal efficiency [14]. Thus, US combined with other sanitizers had been used to enhance the inactivation effect on pathogenic in fruits and vegetables [15], [16], [17].
Slightly acidic electrolyzed water (SAEW), usually with a pH value of 5.0–6.5, is considered to be an effective antimicrobial agent in recent years [18]. SAEW exhibits strong antimicrobial activity against many different kinds of microorganisms such as Escherichia coli [19], Staphylococcus aureus [19], [20], Salmonella spp [21], Vibrio parahaemolyticus [22], and Bacillus cereus spores, etc. [23], [24]. SAEW has been used to disinfect fresh fruits [25], vegetables [26], [27], fish products [28], [29], and meat products [30]. In addition, SAEW shows potential on controlling some phytopathogenic fungi. SAEW resulted in 100% inactivation of Botrytis cinereaor in pure culture [31]. Spores of phytopathogenic fungi B. cinerea, Colletotrichum acutatum, and Phytophthora capsici were not cultivable after SAEW treatment [32]. Thus, SAEW has a powerful wide spectrum of antifungal activity, and is a potential alternative to fungicidal agents on the control of plant diseases. However, to our knowledge, there is no report about the application of US and SAEW combination on the inactivation of R. stolonifer in sweet potato.
Therefore, the study aimed to evaluate the effects of US combined with SAEW at the different temperatures on mycelial growth and spore germination of R. stolonifer, as well as its cell membrane structure and function. Besides, R. stolonifer was artificially inoculated to the fresh harvested sweet potato TRs, of which the disease severity of the TRs caused by R. stolonifer was observed. This new hurdle approach is thus expected to improve microbial safety of sweet potato during storage.
2. Materials and methods
2.1. Materials
Sweet potato TRs (cv. 'Xiguahong') were purchased from the local market (Beijing, China). Potato dextrose agar (PDA) medium and potato dextrose broth (PDB) medium were purchased from Beijing Aoboxing Bio-Technology Co., Ltd. (Beijing, China). Rhodamine 123 was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). The fluorescent dye SYTOX green was purchased from Nanjing Keygen Biotechnology Co., Ltd. (Nanjing, China).
2.2. Fungal cultivation and culture conditions
Rhizopus stolonifer (CICC40327) was obtained from China Center of Industrial Culture Collection (Beijing, China). R. stolonifer was maintained on PDA slants at 4 °C. Before each microbiological assay, it was activated on the PDA medium for 5 days at 28 °C.
2.3. Slightly acidic electrolyzed water (SAEW) preparation
SAEW was prepared by using a SAEW generation system (SWS050, Yantai Boxin Water Treatment Technology Co., Ltd., Yantai, China) as described previously [19] with modifications. SAEW was produced by electrolyzing 3% HCl aqueous solution at a setting of 8 A and 5 V. When the stable amps were reached after 30 min, SAEW with pH value 6.5–6.7, oxidation–reduction potential (ORP) value 800–900 mV, and an available chlorine concentration (ACC) of 80 mg/L was collected. The obtained SAEW was diluted in deionized water to produce SAEW with ACC 10 to 50 mg/L, and stored in polypropylene containers until use. The pH and ORP values were determined by a dual scale pH/ORP meter (PHB-4, Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China) with a pH electrode and an ORP electrode. The ACC was determined using a digital chlorine test kit (RC-3F, Kasahara Chemical Instruments Corp., Saitama, Japan).
2.4. Preparation of spores suspension of R. Stolonifer
An aliquot of 5 mL sterile water was added to the petri dish. The fungal suspensions were obtained by scraping the colony with a spreader, and then filtered twice to get a spore suspension. The spore suspension with a concentration of 1 × 108 spores/mL was prepared using a blood cell counting plate, thoroughly shaken on the vortex apparatus, and stored at 4 °C for further use on the same day.
2.5. US and SAEW combination (US + SAEW) treatment of spores suspension of R. Stolonifer
US + SAEW treatment of spores suspension of R. stolonifer was performed following the method by [31] with modification. Each aliquot of 0.5 mL of 1 × 108 spores/mL spores suspension of R. stolonifer was mixed 4.5 mL of SAEW with ACC of 10, 30, and 50 mg/L, respectively. Immediately, the mixture were treated with US by using a rectangular tank-type ultrasonic cleaner (KQ-500DE, Kunshan Ultrasonic Instrument Co., Ltd., Suzhou, China) filled with 5 L of sterile water with the conditions as following: US power 0, 300, 400, and 500 W, temperature 25, 40 and 55 °C, and treatment time 1, 5 and 10 min respectively. Following the experiments were stopped by diluting the mixture with sterile water to get a final concentration of R. stolonifer 1 × 106 spores/mL.
2.6. Mycelial growth of R. Stolonifer
The mycelial development of R. stolonifer was performed according to the method of Bevilacqua, et al. [33] with modification. Each aliquot of 5 μL treated R. stolonifer spore suspension was placed on the PDA plate. After the suspension was fully absorbed, the plates were sealed and placed in a constant temperature incubator at 28 °C for inverted culture. The colony diameter was measured every 24 h with the cross-over method.
2.7. Release of cellular content
The release of cellular content was conducted using the spectrophotometric method described by Chang, et al. [34] with modification. The mycelia were harvested from PDB incubated at 28 °C for 5 days with constant shaking at 120 rpm. The mycelia were washed twice with 0.1 M phosphate buffer (pH 7.0), resuspended in sterile deionized water, and treated by US + SAEW at different conditions mentioned above for 10 min. The treated mycelia were incubated at 28 °C for 12 h with shaking, and centrifuged at 8,000 × g at 4 °C for 10 min to get the supernatant. The absorbance at 260 and 280 nm of the supernatant was measured using a spectrophotometer (UV 2800, Shanghai Sunny Hengping Scientific Instrument Co., Ltd) to examine the nucleic acid and protein leakage, respectively.
2.8. Spore germination of R. Stolonifer
The spore germination of R. stolonifer was evaluated as described by Wang, et al. [35] with modification. Each treated R. stolonifer spore suspension (20 μL) was put on the concave slide, placed on a petri dish with sterile wet filter paper, and incubated for 24 h at 28 °C. The germination of spores was observed by an optical microscope (400 × ).
2.9. Scanning electron microscopy (SEM) analysis
Fungal morphology was assayed with SEM following the method of Oliveira, et al. [36]. The mycelia were harvested, washed, resuspended, and then treat by US + SAEW at different conditions mentioned above for 10 min as described in section 2.8. Then, the treated mycelia were fixed with 2.5% glutaraldehyde, washed three times (10 min each time) with 0.1 M phosphate buffer, post-fixed in 1% osmium tetroxide at 4 °C for 2 h, rinsed again with distilled water for three times with 3 min each, and then dehydrated with graded ethanol (30, 50, 60, 70, 80, 90, 95 and 100%) series using Critical point dryer (Leica CPD 030, Germany). The dehydrated mycelia were coated with gold–palladium, and observed with a Hitachi Model SU8010 SEM (Japan).
2.10. Transmission electron microscopy (TEM) analysis
Fungal ultrastructure was assayed by TEM following the method of Kong, et al. [37]. A portion of the dehydrated mycelia used for SEM were taken and washed with acetone at least 4 times (15 min each time), embedded in Spurr's resin, incubated overnight at room temperature, polymerized in an oven at 65 °C for one week, and sliced with a Leica EM UC7 (Germany) ultramicrotome, following by staining with uranyl acetate and alkaline lead citrate for 5–10 min. The ultrastructure of final samples was then examined using an H–7500 TEM (HITACH, Japan) at an accelerating voltage of 80 kV and a working current of 67 uA.
2.11. Laser scanning confocal microscope (LSCM) analysis
The cell membrane permeability and mitochondrial membrane potential (MMP) of R. stolonifer were detected by using the fluorescent dyes SYTOX green and Rhodamine 123 as described by [35]. The treated R. stolonifer mycelia were incubated with 5 μmol/L SYTOX green or 4 μg/mL Rhodamine 123 for 30 min in the dark, and then observed under a laser scanning confocal microscope (LSM 880, Carl Zeiss, Germany). The excitation and emission wavelengths observed by SYTOX green staining were 488 and 525 nm, respectively, while those observed by Rhodamine 123 staining were 561 and 595 nm, respectively.
2.12. Pathogenicity test
Sweet potato TRs with the weight of approximately 250 g/root and without visible external injuries were selected, washed with tap water, surface-disinfected with 75% alcohol for 2 min, and air-dried at room temperature. Three micro-wounds (5 mm deep) were made on one side of sweet potato TRs with a sterilized inoculation needle, and 20 μL of 1 × 106 spores/mL R. stolonifer conidial suspension were injected into the wounds. After 24 h incubation, sweet potato TRs were treated with US + SAEW under different conditions for 10 min, then put into a plastic turnover box (with gauze on the bottom and covered with gauze for moisturizing), and stored at 28 °C for 20 days. Sweet potato TRs treated with sterile water were marked as control. The incidence was checked during the storage.
2.13. Statistical analysis
The experiments were conducted in triplicates. The data were expressed as mean value ± standard deviation and tested by one-factor analysis of variance (ANOVA) using SPSS Statistics 17 software (IBM Co., USA). Duncan's multiple range tests were used to analyze the differences between the means. The confidence level for statistical significance was set at a probability value of p < 0.05.
3. Results and discussion
3.1. Effect of US + SAEW treatment on mycelial growth of R. Stolonifer
The mycelial growths of R. stolonifer as affected by US + SAEW treatment are presented in Table 1. At 25 °C, US + SAEW treatment with ACC 10, 30 and 50 mg/L at 0, 300 and 400 W for 1, 5 and 10 min, showed no effect on colony diameters of R. stolonifer (p > 0.05). The inhibitions of colony diameters were only observed in the R. stolonifer treated by US500 with ACC 30 and 50 mg/L for 5 min, as well as US500 with ACC 10–50 mg/L for 10 min (p < 0.05). At 40 °C, significant inhibitions of mycelial growth were shown in the R. stolonifer treated by US300, US400 and US500 with ACC 50 mg/L for different treatment time, or by US300, US400 and US500 with different ACC for 5 and 10 min (p < 0.05). And the colony diameter was the lowest for R. stolonifer treated by US500 at ACC 50 mg/L for 10 min, which was 6 mm, followed by US300 at ACC 50 mg/L for 10 min (22.45 mm, p < 0.05). At 55 °C, all US + SAEW treatments significantly inhibited the mycelial growth of R. stolonifer, suggesting the stronger inhibition effects on R. stolonifer compared to these at 25 and 40 °C. Among them, the colony diameters were the lowest for R. stolonifer treated by US300 and US400 at different ACC, and US500 at ACC 50 mg/L for 10 min, which were in the range of 6–13.66 mm (p < 0.05). The results above suggested that stronger US power (300, 400 and 500 W), higher ACC (50 mg/L), higher temperature (40 and 55 °C) and longer treatment time (10 min) presented better inhibition on mycelial growth of R. stolonifer.
Table 1.
Effect of ultrasound and slightly acidic electrolyzed water on mycelial growth (mm) of R. stolonifer at different temperature.
| Power | Time | ACC (mg/L) at 25 °C | ACC (mg/L) at 40 °C | ACC (mg/L) at 55 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (W) | (min) | 10 | 30 | 50 | 10 | 30 | 50 | 10 | 30 | 50 |
| 0 | 1 | 90.00 ± 0.00Bb | 90.00 ± 0.00Bb | 90.00 ± 0.00Bc | 90.00 ± 0.00Be | 90.00 ± 0.00Bf | 90.00 ± 0.00Bg | 52.81 ± 0.04Ae | 54.10 ± 3.41Ad | 53.52 ± 3.87Ag |
| 5 | 90.00 ± 0.00Cb | 90.00 ± 0.00Cb | 90.00 ± 0.00Cc | 90.00 ± 0.00Ce | 90.00 ± 0.00Cf | 90.00 ± 0.00Cg | 56.88 ± 1.67Be | 53.79 ± 0.92Ad | 53.01 ± 0.63Ag | |
| 10 | 90.00 ± 0.00Eb | 90.00 ± 0.00Eb | 90.00 ± 0.00Ec | 90.00 ± 0.00Ee | 90.00 ± 0.00Ef | 85.66 ± 0.35Dg | 54.32 ± 2.74Ce | 51.93 ± 0.49Bd | 49.32 ± 0.23Afgef | |
| US300 | 1 | 90.00 ± 0.00Eb | 90.00 ± 0.00Eb | 90.00 ± 0.00Ec | 90.00 ± 0.00Ee | 81.12 ± 0.30De | 75.36 ± 5.11Cf | 28.39 ± 2.49Bb | 22.37 ± 5.49Ab | 30.05 ± 5.90Bdc |
| 5 | 90.00 ± 0.00Eb | 90.00 ± 0.00Eb | 90.00 ± 0.00Ec | 71.52 ± 5.00Dc | 62.22 ± 4.53Cb | 62.53 ± 5.67Cd | 25.72 ± 9.86Ab | 36.28 ± 0.42Bc | 23.89 ± 1.10Ac | |
| 10 | 90.00 ± 0.00Eb | 90.00 ± 0.00Eb | 90.00 ± 0.00Ec | 64.80 ± 0.57Dc | 55.34 ± 1.41Ca | 22.45 ± 1.09Bb | 6.00 ± 0.00Aa | 11.45 ± 9.44Aa | 6.00 ± 0.00Aa | |
| US400 | 1 | 90.00 ± 0.00Db | 90.00 ± 0.00Db | 90.00 ± 0.00Dc | 90.00 ± 0.00De | 89.26 ± 1.05Df | 55.27 ± 11.67Cc | 45.17 ± 4.02ABd | 41.18 ± 0.20Abc | 46.93 ± 4.2Bdef |
| 5 | 90.00 ± 0.00Eb | 90.00 ± 0.00Eb | 90.00 ± 0.00Ec | 69.23 ± 0.93Db | 62.58 ± 9.43Cb | 69.26 ± 2.40Dde | 38.67 ± 0.15Ac | 42.86 ± 1.75ABc | 44.30 ± 3.04Ce | |
| 10 | 90.00 ± 0.00Eb | 90.00 ± 0.00Eb | 90.00 ± 0.00Ec | 71.50 ± 2.92Dc | 71.62 ± 0.95Dd | 64.14 ± 1.92Cde | 6.00 ± 0.00Aa | 13.66 ± 10.38Ba | 11.37 ± 7.59ABb | |
| US500 | 1 | 90.00 ± 0.00Eb | 90.00 ± 0.00Eb | 90.00 ± 0.00Ec | 90.00 ± 0.00Ee | 83.37 ± 0.9De | 68.81 ± 2.25Ce | 26.19 ± 2.82Ab | 35.44 ± 2.15Bc | 34.30 ± 3.62Bd |
| 5 | 90.00 ± 0.00 Gb | 69.10 ± 6.48DEa | 70.14 ± 5.90EFb | 76.33 ± 1.80Fd | 67.43 ± 0.74DEc | 63.02 ± 0.89Dd | 29.96 ± 5.64Bb | 36.29 ± 7.90Cc | 6.06 ± 0.00Aa | |
| 10 | 71.27 ± 1.31Ea | 67.70 ± 0.21Ea | 67.39 ± 6.87Ea | 68.37 ± 0.53Eb | 58.02 ± 1.87 Da | 6.00 ± 0.00Aa | 26.40 ± 0.98Bb | 36.04 ± 7.31Cc | 6.00 ± 0.00Aa | |
US, ultrasound; US300, US400 and US500, ultrasound treatment at 300, 400, and 500 W respectively; ACC, the concentration of available chlorine in slightly acidic electrolyzed water.
Values followed by different capital letters in the same row mean statistically significant differences (p < 0.05). Values followed by different lowercase letters in the same column mean statistically significant differences (p < 0.05).
The synergistic effect of US, SAEW and mild heat was also reported to inhibit the growth of some other pathogens. Luo and Oh [38] reported that US + SAEW treatment at 60 °C for 1 min resulted in an additional 2.72 log CFU/g reduction of L. monocytogenes compared to SAEW treatment alone. Luo, et al. [39] indicated that US (400 W/L) combined with SAEW (ACC 28–30 mg/L) at 60 °C achieved approximately 3.0 log CFU/g reduction in the Bacillus cereus. Ding, et al. [25] studied that US enhanced the bactericidal activity of SAEW (ACC = 34.33 mg/L) which resulted in 1.50 and 1.29 log reductions on yeasts and molds for cherry tomatoes and strawberries respectively. The inactivation effect of US + SAEW treatment might be due to thermosensation cavitation activity, which caused high pressure and temperature areas, destroying the bacterial cell wall, thus resulting in better penetration of SAEW [17].
3.2. Effect of US + SAEW treatment on cell membrane integrity
Effects of US + SAEW treatment on the leakage of nucleic acids at 260 nm and proteins at 280 nm from R. stolonifer cells are shown in Table 2. The absorbance at 260 nm of the untreated R. stolonifer US0 + SAEW0 was 0.33. At 25 °C, the absorbance at 260 nm of the mycelia treated by US500 + SAEW50 was significantly increased to 0.70 (p < 0.05). At 40 °C, with the increase of US power, the absorbance at 260 nm of the mycelia treated by US300 + SAEW50, US400 + SAEW50, US500 + SAEW50 were significantly increased to 0.67, 0.8 and 1.17 respectively (p < 0.05). At 55 °C, the absorbance at 260 nm was increased with the increase of US power and the ACC of SAEW. Obviously, the absorbance at 260 nm of the mycelia treated by US500 + SAEW50 was the highest (1.68), followed by US500 + SAEW50 (1.17, p < 0.05).
Table 2.
Effect of ultrasound and slightly acidic electrolyzed water on the leakage of 260 nm and 280 nm absorbing materials from R. stolonifer.
| Temperature (°C) | Treatment | Absorbance |
|
|---|---|---|---|
| 260 nm | 280 nm | ||
| 25 | US0 + SAEW0 | 0.33 ± 0.01a | 0.24 ± 0.00a |
| US500 + SAEW50 | 0.70 ± 0.20bcd | 0.54 ± 0.10abcde | |
| 40 | US300 + SAEW50 | 0.67 ± 0.07bcd | 0.47 ± 0.06abcde |
| US400 + SAEW50 | 0.80 ± 0.24 cd | 0.44 ± 0.13abcd | |
| US500 + SAEW50 | 1.17 ± 0.01ef | 0.73 ± 0.03def | |
| 55 | US300 + SAEW10 | 0.47 ± 0.15ab | 0.33 ± 0.04ab |
| US300 + SAEW30 | 0.61 ± 0.01abcd | 0.40 ± 0.02abc | |
| US300 + SAEW50 | 0.92 ± 0.06de | 0.53 ± 0.04abcde | |
| US400 + SAEW10 | 1.32 ± 0.03 fg | 0.78 ± 0.01ef | |
| US400 + SAEW30 | 1.50 ± 0.28gh | 0.61 ± 0.38bcde | |
| US400 + SAEW50 | 0.51 ± 0.07abc | 0.55 ± 0.11abcde | |
| US500 + SAEW10 | 0.73 ± 0.14bcd | 0.67 ± 0.19cdef | |
| US500 + SAEW30 | 0.65 ± 0.03bcd | 0.73 ± 0.17def | |
| US500 + SAEW50 | 1.68 ± 0.12 h | 0.97 ± 0.01f | |
US, ultrasound; SAEW, slightly acidic electrolyzed water, US + SAEW, ultrasound with the power of 300, 400, and 500 W combined SAEW with the concentration of available chlorine of 10, 30, and 50 mg/L respectively.
Values followed by different lowercase letters in the same column mean statistically significant differences (p < 0.05).
Similarly, the absorbance at 280 nm of the untreated R. stolonifer was 0.24. At 25 °C, the absorbance at 280 nm of the mycelia treated by US500 + SAEW50 was increased to 0.54, but with no significant difference compared to that of the untreated one (p > 0.05). At 40 °C, the absorbance at 280 nm of the mycelia treated by US500 + SAEW50 was significantly increased to 0.73 (p < 0.05). At 55 °C, US500 + SAEW50 treatment also resulted in the highest absorbance at 280 nm of the mycelia, which was 0.97 (p < 0.05). For both absorbances at 260 and 280 nm, US500 + SAEW50 treatment at 55 °C for 10 min showed the highest values, suggesting the most severe leakage of intracellular materials. The leak of nucleic acid and protein in R. stolonifer treated with US + SAEW indicated that the cell membrane was disrupted, resulting in the release of internal components of the cell [37]. Lin, et al. [40] revealed that the integrity and permeability of the E. coli cell membrane was disrupted by US treatment. SAEW (ACC = 25.27 mg/L) was reported to damage the cell membrane of E. coli, S. aureus, and B. subtilis [41]. US + SAEW treatment caused the release of cell contents and the disintegration of the cell wall and plasma membrane on S. aureus bacteria [42]. It might be due to that the high pressure and temperature in the local regions created by US damaged the bacterial cell structure and caused higher uptake of SAEW, thus resulting in maximum cellular damage [43].
3.3. Effect of US + SAEW treatment on spore germination
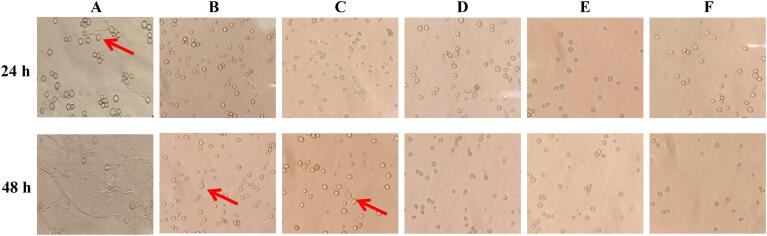
The spore germination of R. stolonifer as affected by US + SAEW treatment is shown in Fig. 1. After 24 h incubation, the germ tubes and spore germination were observed in the untreated R. stolonifera (Fig. 1A), while only swollen spores were observed in US + SAEW treated samples (Fig. 1B-F). After 48 h incubation, the spores were completely germinated, and typical hyphae were shown in the untreated R. stolonifer (Fig. 1A). The spores of R. stolonifer treated by US500 + SAEW50 (25 °C, 10 min) and US500 + SAEW50 (40 °C, 10 min) were only enlarged (Fig. 1B and C). There are no spores germinated after US300 + SAEW50, US400 + SAEW50, and US500 + SAEW50 treatment at 55 °C for 10 min (Fig. 1D-F), suggesting US + SAEW treatment inhibited the spore germination ultimately and affected the development of hyphae of R. stolonifer. Evelyn, et al. [44] stated that US at 75 °C could inactivate the vegetative cells and spores of the bacterial and fungal. Guerra Sierra, et al. [45] found that SAEW with ACC 10–50 ppm for 7 min could inhibit the conidia germination of R. stolonifer and B. cinerea..
Fig. 1.
Effects of ultrasound and slightly acid electrolytic water combination on spore germination. US, ultrasound; SAEW, slightly acid electrolytic water. A, untreated; B, US500 + SAEW50 (25 °C, 10 min); C, US500 + SAEW50 (40 °C, 10 min); D, US300 + SAEW50 (55 °C, 10 min); E, US400 + SAEW50 (55 °C, 10 min); F, US500 + SAEW50 (55 °C, 10 min).
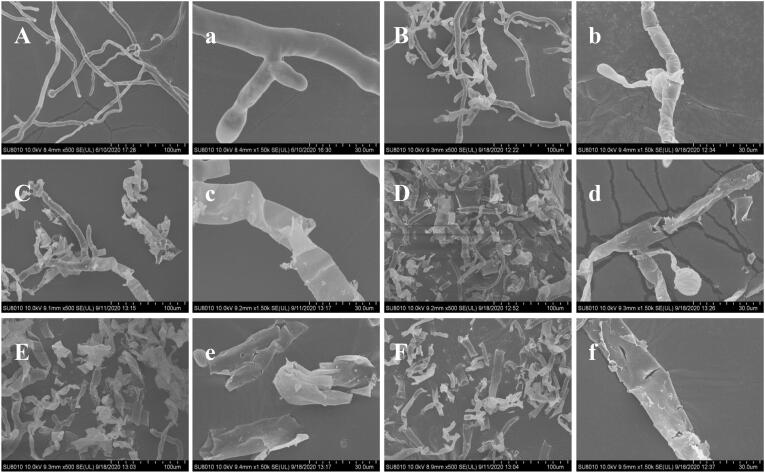
3.4. Scanning electron microscopy (SEM)
SEM micrographs of R. stolonifer as affected by US + SAEW treatment are shown in Fig. 2. The untreated R. stolonifer presented homogeneous, regular and long tubular hyphae with a smooth external surface (Fig. 2 A-a). US500 + SAEW50 (25 °C, 10 min) treatment induced superficial wrinkles and partially collapsed hyphae (Fig. 2 B-b). US500 + SAEW50 (40 °C, 10 min) treated hyphae was distorted and destructed, resulting in flowing out of part of cellular contents (Fig. 2 C-c). Severe cell-wall pitting was observed, and part of hyphae were completely disrupted into debris after US300 + SAEW50, US400 + SAEW50, and US500 + SAEW50 treatment at 55 °C for 10 min respectively (Fig. 2, D-d, E-e, and F-f). It was reported that US + SAEW had a significant impact on the biological structure of S. aureus [42], which might be due to the cavitation efficacy of US, which could produce micro-cracks in the bacterial cell membranes that allowed SAEW to enter inside the bacteria cells [46].
Fig. 2.
Effect of ultrasound and slightly acid electrolytic water combination on the fungal morphology of R. stolonifer by SEM. US, ultrasound; SAEW, slightly acid electrolytic water. A-a, untreated; B-b, US500 + SAEW50 (25 °C, 10 min); C-c, US500 + SAEW50 (40 °C, 10 min); D-d, US300 + SAEW50 (55 °C, 10 min); E-e, US400 + SAEW50 (55 °C, 10 min); F-f, US500 + SAEW50 (55 °C, 10 min).
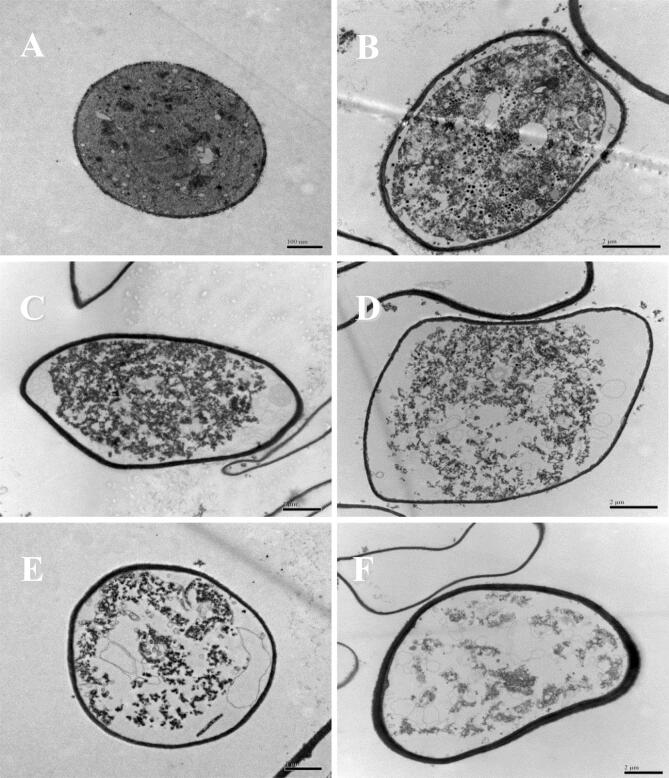
3.5. Transmission electron microscopy (TEM)
Ultrastructure changes of R. stolonifer observed by TEM as affected by US + SAEW treatment are shown in Fig. 3. The untreated R. stolonifer showed typical fungal ultrastructure with normal cell wall thickness, the plasma membrane was regular and intact, the cytoplasmic matrix was abundant, and the prominent organelles such as mitochondria, vacuoles and nucleus were normal and uniform (Fig. 3A). Compared with the untreated one, the ultrastructure of R. stolonifer was changed after different US + SAEW treatment. An apparent separation of plasma wall and decreased cytoplasm were observed in R. stolonifer treated by US500 + SAEW50 (25 °C, 10 min) (Fig. 3B). This result might be because the free radicals and hydrogen peroxide were generated during US treatment, which changes cell membrane permeability through the sonochemical reaction and oxidative damage, thus resulting in the leakage of intracellular potassium and the loss of osmotic pressure balance. More serious separation of plasma wall and intracellular components damages were observed in R. stolonifer treated by US500 + SAEW50 (40 °C, 10 min) (Fig. 3C). Much more severe damaged intracellular components, increased vacuoles space, indistinct intracellular organelles, and cytoplasm loss were shown in stolonifer after US300 + SAEW50, US400 + SAEW50, and US500 + SAEW50 treatment at 55 °C for 10 min respectively (Fig. 3D, E, and F). Among them, the most severe cell damage was observed after US500 + SAEW50 (55 °C, 10 min) treatment (Fig. 3F). Markedly damaged, destroyed, or distinct separation of the cytoplasmic cellular material was also observed in the cell membrane of E.coli O157:H7 cells treated by SAEW with ACC10 or 30 mg/L for 3 min [47].
Fig. 3.
Effect of ultrasound and slightly acid electrolytic water combination on the fungal ultrastructure of R. stolonifer by TEM. US, ultrasound; SAEW, slightly acid electrolytic water. A, untreated; B, US500 + SAEW50 (25 °C, 10 min); C, US500 + SAEW50 (40 °C, 10 min); D, US300 + SAEW50 (55 °C, 10 min); E, US400 + SAEW50 (55 °C, 10 min); F, US500 + SAEW50 (55 °C, 10 min).
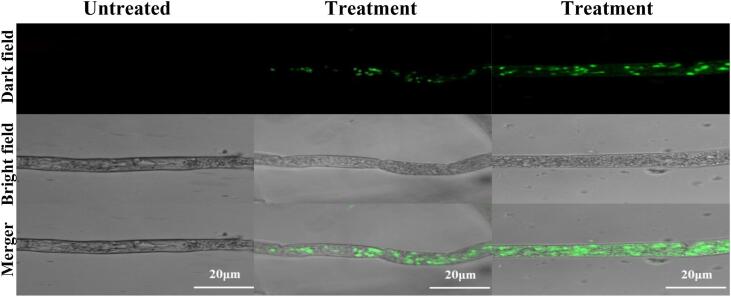
3.6. Cell membrane permeability and mitochondrial membrane potential (MMP)
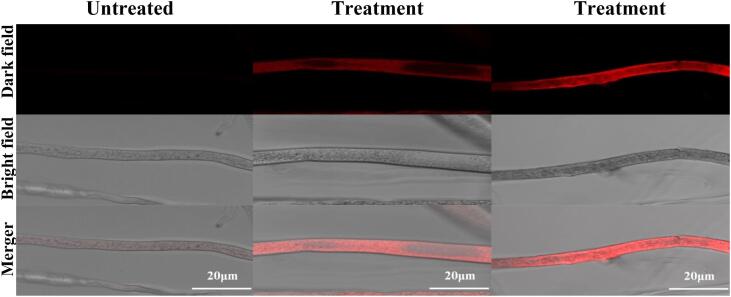
The cell membrane plays an essential role in maintaining the normal physiological functions of microorganisms, including the transportation of necessary materials and adenine triphosphate generation. Once the membrane is damaged irreversibly, microbial cells will be dead immediately. To assess the effect of US + SAEW treatment on the cell membrane permeability of R. stolonifer, a fluorescent dye based on a high-affinity nucleic acid stain SYTOX-Green was used. The dye can easily penetrate the cells with compromised plasma membranes, but can't cross the membranes of non-compromised living cells, being used to assess the integrity of biological membranes [48]. As shown in Fig. 4, no fluorescent signal was observed in the untreated hyphal cell. However, after US + SAEW treatment, the fluorescent signal was distinct and clear. The hyphal cell treated by US500 + SAEW50 (55 °C, 10 min) showed stronger fluorescence intensity than that treated by US500 + SAEW50 (40 °C, 10 min). US treatment was found to significantly increase the membrane permeability of E. coli O157:H7 [40]. The cell membrane permeability of Escherichia coli was also enhanced treated by SAEW with ACC 60 mg/L for 5 min [49].
Fig. 4.
Ultrasound combined with slightly acid electrolytic water elicited an increase in cell membrane permeability of R. stolonifer observed by SYTOX green staining. US, ultrasound; SAEW, slightly acid electrolytic water. Treatment 1, US500 + SAEW50 (40 °C, 10 min); Treatment 2, US500 + SAEW50 (55 °C, 10 min). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To evaluate MMP changes of R. stolonifer as affected by US + SAEW treatment, Rhodamine 123 staining was used. Rhodamine 123 is a cationic fluorescent dye that penetrates cell membranes, which is an indicator of MMP. In normal cells, the transmembrane potential of mitochondria could be relied on to enter the mitochondrial matrix, and the fluorescence intensity would be reduced or disappeared [50]. As shown in Fig. 5, the fluorescent signal was very weak in the untreated hyphal cell. After US + SAEW treatment, the fluorescent signal was obviously enhanced. The hyphal cell treated by US500 + SAEW50 (55 °C, 10 min) showed stronger fluorescence intensity than that treated by US500 + SAEW50 (40 °C, 10 min), indicating that the MMP of the cells was substantially decreased. As a sensitive indicator reflecting the energy status of mitochondria and cells, the decrease in MMP suggested that the normal physiological activities of cells could be affected [51]. These results indicated that the function of R. stolonifer cell membrane were damage after US + SAEW treatment.
Fig. 5.
Ultrasound combined with slightly acid electrolytic water elicited a decrease in mitochondrial membrane potential of R. stolonifer observed by Rhodamine 123 staining. US, ultrasound; SAEW, slightly acid electrolytic water. Treatment 1, US500 + SAEW50 (40 °C, 10 min); Treatment 2, US500 + SAEW50 (55 °C, 10 min).
3.7. R. Stolonifer infections in sweet potato TRs
The effect of US + SAEW treatment on R. stolonifer development in sweet potato TRs is shown in Fig. 6. For the untreated samples, the TRs were rapidly infected after inoculation with R. stolonifer for only 5 days, with some mycelia appeared and started to diffuse at the wound (Fig. 6A). With the increase of storage time (10, 15, and 20 days), the infection was significantly increased and diffused. The TRs were completely infected and turned soft at 15 days of storage (Fig. 6A). For the TRs treated by US500 + SAEW50 (25 °C, 10 min), after 10 days storage, the infection was diffused; after 15 days storage, nearly a half part of rotting inside TRs was observed; but after 20 days storage, a very large part of rotting inside TRs was observed (Fig. 6B). For the TRs treated by US500 + SAEW50 (25 °C, 10 min), after 10 days storage, the infection was diffused; after 15 days storage, part of rotting inside TRs was observed; but after 20 days storage, a very large part of rotting inside TRs was observed (Fig. 6B). Similar phenomenon was observed in the TRs treated by US400 + SAEW50 (55 °C, 10 min) and US500 + SAEW50 (55 °C, 10 min) (Fig. 6 E and F). Whereas for the TRs treated by US500 + SAEW50 (40 °C, 10 min) and US300 + SAEW50 (55 °C, 10 min), small-localized scabs appeared in the puncture wound locations after 5 and 10 days storage; then only partial enlarged infection was observed after 15 and 20 days storage (Fig. 6C and D), suggesting US + SAEW treatment at appropriate conditions could effectively prevent the spreading of soft rot pathogens. It had been reported that the application of US at 400 W/L and 40 °C for 3 min could significantly enhance the efficacy of 5 mg/L SAEW on the reduction of pathogens and spoilage microorganisms in kale [52]. However, it was noteworthy that the TRs in the US400 + SAEW50 (55 °C, 10 min) and US500 + SAEW50 (55 °C, 10 min) groups was rotting faster than the other treatment groups at the end of the storage, although US400 + SAEW50 and US500 + SAEW50 (55 °C, 10 min) were good at inhibiting the mycelial growths of R. stolonifer (Table 1). These results might be due to the explosion of the bubbles generated by US at relative higher power (400 and 500 W) and temperature (55 °C), which might induce damages to the internal tissue of TRs. The damages might promptly accelerate the growth of surface microorganisms, leading to the decay of the plant tissue [14].
Fig. 6.
Effect of ultrasound and slightly acid electrolytic water combination on transverse section of sweet potato TRs artificially inoculated with R. stolonifer. US, ultrasound; SAEW, slightly acid electrolytic water. A, untreated; B, US500 + SAEW50 (25 °C, 10 min); C, US500 + SAEW50 (40 °C, 10 min); D, US300 + SAEW50 (55 °C, 10 min); E, US400 + SAEW50 (55 °C, 10 min); F, US500 + SAEW50 (55 °C, 10 min).
4. Conclusions
In this study, the effect of US combined with SAEW at different temperature on R. stolonifer of sweet potato TRs was evaluated. US + SAEW treatment at proper power, temperature and time could synergistically inhibit the mycelial growth and spore germination of R. stolonifer. The cavitation bubbles caused by US might lead to the penetration of SAEW into R. stolonifer cells, so as to enhance the effectiveness of SAEW on the permeability increase of cell membranes and the leakage of intracellular nucleic acids and proteins. The damaged R. stolonifer cells were further ruptured by US, which resulted in the cell wall and membrane fragmentation of lysed cells. In addition, US + SAEW treatment could control the growth of R. stolonifer in sweet potato TRs to some extent, indicating that US + SAEW treatment could be a new way to control R. stolonifer in sweet potato.
CRediT authorship contribution statement
Lulu Li: Investigation, Formal analysis, Writing - original draft. Tai-Hua Mu: Conceptualization, Methodology, Writing - review & editing, Resources, Supervision, Funding acquisition. : . Miao Zhang: Writing - review & editing, Project administration, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the Beijing Municipal Science and Technology Project (Z191100004019012). We also thank the earmarked fund for the China Agriculture Research System (CARS-10-B21) and the National Key R & D Program of China (2016YFE0133600).
Contributor Information
Tai-Hua Mu, Email: mutaihua@126.com.
Miao Zhang, Email: zhangmiao@caas.cn.
References
- 1.Wang S., Nie S., Zhu F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016;89:90–116. doi: 10.1016/j.foodres.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 2.Wamalwa L.N., Cheseto X., Ouna E., Kaplan F., Maniania N.K., Machuka J., Torto B., Ghislain M. Toxic Ipomeamarone accumulation in healthy parts of Sweetpotato (Ipomoea batatas L. Lam) storage roots upon infection by Rhizopus stolonifer. J. Agric. Food. Chem. 2015;63(1):335–342. doi: 10.1021/jf504702z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty C., Roychowdhury R., Chakraborty S., Chakravorty P., Ghosh D. A Review on Post-Harvest Profile of Sweet Potato. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(5):1894–1903. [Google Scholar]
- 4.Yuan B., Xue L.-W., Zhang Q.-Y., Kong W.-W., Peng J., Kou M., Jiang J.-H. Essential Oil from Sweet Potato Vines, a Potential New Natural Preservative, and an Antioxidant on Sweet Potato Tubers: Assessment of the Activity and the Constitution. J. Agric. Food. Chem. 2016;64(40):7481–7491. doi: 10.1021/acs.jafc.6b03175. [DOI] [PubMed] [Google Scholar]
- 5.P V., Dash S.K., Rayaguru K. Post-Harvest Processing and Utilization of Sweet Potato: A Review. Food Rev. Int. 2019;35(8):726–762. [Google Scholar]
- 6.Nafady N.A., Alamri S.A.M., Hassan E.A., Hashem M., Mostafa Y.S., Abo-Elyousr K.A.M. Application of ZnO-nanoparticles to manage Rhizopus soft rot of sweet potato and prolong shelf-life. Folia Horticulturae. 2019;31:319–329. [Google Scholar]
- 7.Edmunds B.A., Holmes G.J. Evaluation of Alternative Decay Control Products for Control of Postharvest Rhizopus Soft Rot of Sweetpotatoes. Plant Health Progress. 2009;10:26. [Google Scholar]
- 8.Xing K., Li T.J., Liu Y.F., Zhang J., Zhang Y., Shen X.Q., Li X.Y., Miao X.M., Feng Z.Z., Peng X., Li Z.Y., Qin S. Antifungal and eliciting properties of chitosan against Ceratocystis fimbriata in sweet potato. Food Chem. 2018;268:188–195. doi: 10.1016/j.foodchem.2018.06.088. [DOI] [PubMed] [Google Scholar]
- 9.Luo K., Oh D.H. Inactivation kinetics of Listeria monocytogenes and Salmonella enterica serovar Typhimurium on fresh-cut bell pepper treated with slightly acidic electrolyzed water combined with ultrasound and mild heat. Food Microbiol. 2016;53:165–171. doi: 10.1016/j.fm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Pinheiro J., Alegria C., Abreu M., Goncalves E.M., Silva C.L.M. Influence of postharvest ultrasounds treatments on tomato (Solanum lycopersicum, cv. Zinac) quality and microbial load during storage. Ultrasonics - Sonochemistry. 2015;27:552–559. doi: 10.1016/j.ultsonch.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Huang G., Chen S., Dai C., Sun L., Sun W., Tang Y., Xiong F., He R., Ma H. Effects of ultrasound on microbial growth and enzyme activity. Ultrasonics - Sonochemistry. 2017;37:144–149. doi: 10.1016/j.ultsonch.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Aday M.S., Temizkan R., Büyükcan M.B., Caner C. An innovative technique for extending shelf life of strawberry: Ultrasound. LWT - Food Sci. Technol. 2013;52(2):93–101. [Google Scholar]
- 13.Wang J., Fan L. Effect of ultrasound treatment on microbial inhibition and quality maintenance of green asparagus during cold storage. Ultrasonics - Sonochemistry. 2019;58 doi: 10.1016/j.ultsonch.2019.104631. [DOI] [PubMed] [Google Scholar]
- 14.Wang W., Ma X., Zou M., Jiang P., Hu W., Li J., Zhi Z., Chen J., Li S., Ding T., Ye X., Liu D. Effects of Ultrasound on Spoilage Microorganisms, Quality, and Antioxidant Capacity of Postharvest Cherry Tomatoes. J. Food Sci. 2015;80(10):C2117–C2126. doi: 10.1111/1750-3841.12955. [DOI] [PubMed] [Google Scholar]
- 15.Mu Y., Feng Y., Wei L., Li C., Cai G., Zhu T. Combined effects of ultrasound and aqueous chlorine dioxide treatments on nitrate content during storage and postharvest storage quality of spinach (Spinacia oleracea L.) Food Chem. 2020;333 doi: 10.1016/j.foodchem.2020.127500. [DOI] [PubMed] [Google Scholar]
- 16.Guo M., Zhang L., He Q., Arabi S.A., Zhao H., Chen W., Ye X., Liu D. Synergistic antibacterial effects of ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157:H7. Ultrasonics - Sonochemistry. 2020;66:104988. doi: 10.1016/j.ultsonch.2020.104988. [DOI] [PubMed] [Google Scholar]
- 17.Mansur A.R., Oh D.H. Combined effects of thermosonication and slightly acidic electrolyzed water on the microbial quality and shelf life extension of fresh-cut kale during refrigeration storage. Food Microbiol. 2015;51:154–162. doi: 10.1016/j.fm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Rahman SME, Khan I., Oh D.-H. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Comprechensive Reviews in Food Science and Food Safety. 2016;15(3):471–490. doi: 10.1111/1541-4337.12200. [DOI] [PubMed] [Google Scholar]
- 19.Issa-Zacharia A., Kamitani Y., Morita K., Iwasaki K. Sanitization potency of slightly acidic electrolyzed water against pure cultures of Escherichia coli and Staphylococcus aureus, in comparison with that of other food sanitizers. Food Control. 2010;21:740–745. [Google Scholar]
- 20.Liao X., Xuan X., Li J., Suo Y., Liu D., Ye X., Chen S., Ding T. Bactericidal action of slightly acidic electrolyzed water against Escherichia coli and Staphylococcus aureus via multiple cell targets. Food Control. 2017;79:380–385. [Google Scholar]
- 21.Issa-Zacharia A., Kamitani Y., Tiisekwa A., Morita K., Iwasaki K. In vitro inactivation of Escherichia coli, Staphylococcus aureus and Salmonella spp. using slightly acidic electrolyzed water. J. Biosci. Bioeng. 2010;110:308–313. doi: 10.1016/j.jbiosc.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Chen T.Y., Kuo S.H., Chen S.T., Hwang D.F. Differential proteomics to explore the inhibitory effects of acidic, slightly acidic electrolysed water and sodium hypochlorite solution on Vibrio parahaemolyticus. Food Chem. 2016;194:529–537. doi: 10.1016/j.foodchem.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Kim H.J., Tango C.N., Chelliah R., Oh D.H. Sanitization Efficacy of Slightly Acidic Electrolyzed Water against pure cultures of Escherichia coli Salmonella enterica, Typhimurium, Staphylococcus aureus and Bacillus cereus spores, in Comparison with Different Water Hardness. Sci. Rep. 2019;9:4348. doi: 10.1038/s41598-019-40846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain M.S., Tango C.N., Oh D.H. Inactivation kinetics of slightly acidic electrolyzed water combined with benzalkonium chloride and mild heat treatment on vegetative cells, spores, and biofilms of Bacillus cereus. Food Res. Int. 2019;116:157–167. doi: 10.1016/j.foodres.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Ding T., Ge Z., Shi J., Xu Y.-T., Jones C.L., Liu D.-H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT - Food Sci. Technol. 2015;60:1195–1199. [Google Scholar]
- 26.Koide S., Shitanda D., Note M., Cao W. Effects of mildly heated, slightly acidic electrolyzed water on the disinfection and physicochemical properties of sliced carrot. Food Control. 2011;22(3-4):452–456. [Google Scholar]
- 27.Li L., Song S., Nirasawa S., Hung Y.-C., Jiang Z., Liu H. Slightly Acidic Electrolyzed Water Treatment Enhances the Main Bioactive Phytochemicals Content in Broccoli Sprouts via Changing Metabolism. J. Agric. Food. Chem. 2019;67(2):606–614. doi: 10.1021/acs.jafc.8b04958. [DOI] [PubMed] [Google Scholar]
- 28.Park S.Y., Ha S.-D. Reduction of Escherichia coli and Vibrio parahaemolyticus Counts on Freshly Sliced Shad (Konosirus punctatus) by Combined Treatment of Slightly Acidic Electrolyzed Water and Ultrasound Using Response Surface Methodology. Food Bioprocess Technol. 2015;8(8):1762–1770. [Google Scholar]
- 29.Lin T., Wang J.J., Li J.B., Liao C., Pan Y.J., Zhao Y. Use of acidic electrolyzed water ice for preserving the quality of shrimp. J. Agric. Food. Chem. 2013;61(36):8695–8702. doi: 10.1021/jf4019933. [DOI] [PubMed] [Google Scholar]
- 30.Cichoski A.J., Flores D.R.M., De Menezes C.R., Jacob-Lopes E., Zepka L.Q., Wagner R., Barin J.S., de Moraes Flores É.M., da Cruz Fernandes M., Campagnol P.C.B. Ultrasound and slightly acid electrolyzed water application: An efficient combination to reduce the bacterial counts of chicken breast during pre-chilling. Int. J. Food Microbiol. 2019;301:27–33. doi: 10.1016/j.ijfoodmicro.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Guentzel J.L., Callan M.A., Liang Lam K., Emmons S.A., Dunham V.L. Evaluation of electrolyzed oxidizing water for phytotoxic effects and pre-harvest management of gray mold disease on strawberry plants. Crop Prot. 2011;30:1274–1279. [Google Scholar]
- 32.Song J.Y., Kim N., Nam M.H., Park B., Whang E.-I., Choi J.M., Kim H.G. Fungicidal Effect of Slightly Acidic Hypochlorous Water against Phytopathogenic Fungi. The Korean Journal of Mycology. 2013;41:274–279. [Google Scholar]
- 33.Bevilacqua A., Campaniello D., Sinigaglia M., Corbo M.R. Combination of ultrasound and antimicrobial compounds towards Pichia spp. and Wickerhamomyces anomalus in pineapple juice. LWT - Food Science and Technology. 2015;64:616–622. [Google Scholar]
- 34.Chang C.H., Chiang M.L., Chou C.C. The effect of temperature and length of heat shock treatment on the thermal tolerance and cell leakage of Cronobacter sakazakii BCRC 13988. Int. J. Food Microbiol. 2009;134:184–189. doi: 10.1016/j.ijfoodmicro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Wang C., Yuan S., Zhang W., Ng T., Ye X. Buckwheat Antifungal Protein with Biocontrol Potential To Inhibit Fungal (Botrytis cinerea) Infection of Cherry Tomato. J. Agric. Food. Chem. 2019;67:6748–6756. doi: 10.1021/acs.jafc.9b01144. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira J., Parisi M.C.M., Baggio J.S., Silva P.P.M., Paviani B., Spoto M.H.F., Gloria E.M. Control of Rhizopus stolonifer in strawberries by the combination of essential oil with carboxymethylcellulose. Int. J. Food Microbiol. 2019;292:150–158. doi: 10.1016/j.ijfoodmicro.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Kong J., Zhang Y., Ju J., Xie Y., Guo Y., Cheng Y., Qian H., Quek S.Y., Yao W. Antifungal effects of thymol and salicylic acid on cell membrane and mitochondria of Rhizopus stolonifer and their application in postharvest preservation of tomatoes. Food Chem. 2019;285:380–388. doi: 10.1016/j.foodchem.2019.01.099. [DOI] [PubMed] [Google Scholar]
- 38.Luo K., Oh D.H. Synergistic Effect of Slightly Acidic Electrolyzed Water and Ultrasound at Mild Heat Temperature in Microbial Reduction and Shelf-Life Extension of Fresh-Cut Bell Pepper. J. Microbiol. Biotechnol. 2015;25:1502–1509. doi: 10.4014/jmb.1505.05021. [DOI] [PubMed] [Google Scholar]
- 39.Luo K., Kim S.Y., Wang J., Oh D.-H. A combined hurdle approach of slightly acidic electrolyzed water simultaneous with ultrasound to inactivate Bacillus cereus on potato. LWT - Food Science and Technology. 2016;73:615–621. [Google Scholar]
- 40.Lin L., Wang X., Li C., Cui H. Inactivation mechanism of E. coli O157:H7 under ultrasonic sterilization. Ultrasonics - Sonochemistry. 2019;59:104751. doi: 10.1016/j.ultsonch.2019.104751. [DOI] [PubMed] [Google Scholar]
- 41.Hao J., Wu T., Li H., Liu H. Differences of Bactericidal Efficacy on Escherichia coli, Staphylococcus aureus, and Bacillus subtilis of Slightly and Strongly Acidic Electrolyzed Water. Food Bioprocess Technol. 2016;10:155–164. [Google Scholar]
- 42.Li J., Ding T., Liao X., Chen S., Ye X., Liu D. Synergetic effects of ultrasound and slightly acidic electrolyzed water against Staphylococcus aureus evaluated by flow cytometry and electron microscopy. Ultrasonics - Sonochemistry. 2017;38:711–719. doi: 10.1016/j.ultsonch.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 43.Ding T., Xuan X.T., Li J., Chen S.G., Liu D.H., Ye X.Q., Shi J., Xue S.J. Disinfection efficacy and mechanism of slightly acidic electrolyzed water on Staphylococcus aureus in pure culture. Food Control. 2016;60:505–510. [Google Scholar]
- 44.Evelyn H.J., Kim F.V.M. Silva, Modeling the inactivation of Neosartorya fischeri ascospores in apple juice by high pressure, power ultrasound and thermal processing. Food Control. 2016;59:530–537. [Google Scholar]
- 45.Guerra Sierra B.E., Sandoval A., Torcoroma L. Antifungal activity of acidic electrolyzed water against strawberry postharvest molds (Fragaria x ananassa Duch cv. Camarosa) Acta Agronómica. 2019;68:126–133. [Google Scholar]
- 46.Wu S., Nie Y., Zhao J., Fan B., Huang X., Li X., Sheng J., Meng D., Ding Y., Tang X. The Synergistic Effects of Low-Concentration Acidic Electrolyzed Water and Ultrasound on the Storage Quality of Fresh-Sliced Button Mushrooms. Food Bioprocess Technol. 2017;11:314–323. [Google Scholar]
- 47.Nan S., Li Y., Li B., Wang C., Cui X., Cao W. Effect of Slightly Acidic Electrolyzed Water for Inactivating Escherichia coli O157:H7 and Staphylococcus aureus Analyzed by Transmission Electron Microscopy. J. Food Prot. 2010;73:2211–2216. doi: 10.4315/0362-028x-73.12.2211. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B., Dong C., Shang Q., Han Y., Li P. New insights into membrane-active action in plasma membrane of fungal hyphae by the lipopeptide antibiotic bacillomycin L. BBA. 1828;2013:2230–2237. doi: 10.1016/j.bbamem.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 49.Ye Z., Wang S., Chen T., Gao W., Zhu S., He J., Han Z. Inactivation Mechanism of Escherichia coli Induced by Slightly Acidic Electrolyzed Water. Sci. Rep. 2017;7:6279. doi: 10.1038/s41598-017-06716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh P., Roy A., Hess D., Ghosh A., Das S. Deciphering the mode of action of a mutant Allium sativum Leaf Agglutinin (mASAL), a potent antifungal protein on Rhizoctonia solani. BMC Microbiol. 2015;15:237. doi: 10.1186/s12866-015-0549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brand M.D., Chien L.-F., Ainscow E.K., Rolfe D.F.S., Porter R.K. The causes and functions of mitochondrial proton leak. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 52.Mansur A.R., Oh D.-H. Combined Effect of Thermosonication and Slightly Acidic Electrolyzed Water to Reduce Foodborne Pathogens and Spoilage Microorganisms on Fresh-cut Kale. J. Food Sci. 2015;80(6):M1277–M1284. doi: 10.1111/1750-3841.12888. [DOI] [PubMed] [Google Scholar]