Abstract
Pigment epithelium–derived factor (PEDF) is a widely expressed 50-kDa glycoprotein belonging to the serine protease inhibitor family, with well-established anti-inflammatory functions. Recently, we demonstrated the immunoregulatory role played by PEDF in dry eye disease (DED) by suppressing the maturation of antigen-presenting cells at the ocular surface following exposure to the desiccating stress. In this study, we evaluated the effect of PEDF on the immunosuppressive characteristics of regulatory T cells (Tregs), which are functionally impaired in DED. In the presence of PEDF, the in vitro cultures prevented proinflammatory cytokine (associated with type 17 helper T cells)-induced loss of frequency and suppressive phenotype of Tregs derived from normal mice. Similarly, PEDF maintained the in vitro frequency and enhanced the suppressive phenotype of Tregs derived from DED mice. On systemically treating DED mice with PEDF, moderately higher frequencies and significantly enhanced suppressive function of Tregs were observed in the draining lymphoid tissues, leading to the efficacious amelioration of the disease. Our results demonstrate that PEDF promotes the suppressive capability of Tregs and attenuates their type 17 helper T-cell–mediated dysfunction in DED, thereby playing a role in the suppression of DED.
Dry eye disease (DED) is a highly prevalent multifactorial disease characterized by ocular surface inflammation, loss of tear film homeostasis, and neurosensory dysfunction.1 DED may present as either a stand-alone disease or a common ocular complication of systemic disorders, such as diabetes and rheumatoid arthritis.2,3 Epidemiologic studies have estimated the prevalence of symptomatic DED in the United States to be 14.5%.4 The annual cost for management of DED in the country has been estimated to be $3.84 billion USD.5 DED complicates virtually every other ocular surface condition, such as wound healing, infection, allergy, and drug toxicity; and yet, the therapeutic options are minimal despite ample evidence implicating immunologic processes in the pathogenesis of DED.6
The laboratory and clinical research conducted in the past two decades has facilitated development of a deeper understanding of the underlying immunologic mechanisms in the pathogenesis of DED that lead to the induction and exacerbation of ocular surface epitheliopathy. Previous studies from our laboratory have determined the dominant role played by IL-17–secreting CD4+ T helper 17 (Th17) cells in mediating epithelial damage in DED.7, 8, 9, 10 Moreover, we have also characterized the critical role of CD4+CD25+ regulatory T cells (Tregs) in attenuating DED pathogenesis and highlighted the inability of Tregs derived from DED mice (in comparison to Tregs from normal mice) to suppress Th17 effector cells.9
Regulatory T cells play a critical role as master regulators of the immune system, and the current evidence suggests their influence on nearly every aspect of T-cell function by either direct interaction with T cells or by suppressing the activation of antigen-presenting cells.11 Tregs express a wide variety of membrane-associated regulatory factors, including cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and glucocorticoid-induced TNFR-related protein (GITR) that mediate their cell-cell contact suppressive function.12, 13, 14, 15, 16 In addition, Tregs secrete immunomodulatory cytokines, such as transforming growth factor-β1 (TGF-β1), and interleukin (IL-10) that suppress T-cell proliferation, differentiation, and antigen-presenting cell maturation in a paracrine manner.11,17 It is well known that Treg function is dependent mostly on forkhead box P3 (FOXP3) expression, and even modest alterations in this transcription factor expression can significantly affect their functionality.18, 19, 20, 21, 22 An association between decreased FOXP3 expression in Tregs results in worsening of DED.23 Moreover, Th17 effectors are resistant to Treg suppression in DED, and in vivo blockade of IL-17 restores Treg function, linking Th17-dominant immunity with Treg dysfunction.9 Despite the current evidence of Treg dysfunction due to DED and other Th17-mediated disorders, there is an unmet need to identify the factors that can preserve Treg function to ameliorate the disease severity, and can be used translationally for the development of novel therapeutics.
Pigment epithelium–derived factor (PEDF) is a ubiquitously expressed 50-kDa glycoprotein belonging to the serine protease inhibitor family.24 PEDF is expressed by multiple ocular tissues in the cornea, choroid, retina, and ciliary muscles.25 Recent studies have shown the critical role played by endogenous PEDF in tumor suppression, and inhibiting inflammation and neovascularization.26, 27, 28, 29, 30, 31, 32 The immunoregulatory effects of PEDF depend on the target tissue and the underlying etiology of inflammation. Recently, we demonstrated the immunosuppressive effect of PEDF on the maturation of ocular surface dendritic cells (DCs) in dry eye disease.33 In mice with herpes simplex virus keratitis, subconjunctival injection of PEDF has been shown to be efficacious in the suppression of ocular surface inflammation by inhibiting neutrophilic infiltration and reducing expression of proinflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), 7 days after viral inoculation.34 More importantly, intravitreal injections of PEDF significantly reduced expression levels of inflammatory factors, including vascular endothelial growth factor, vascular endothelial growth factor receptor-2, monocyte chemoattractant protein-1, TNF-α, and intercellular adhesion molecule 1, in diabetic retinopathy murine models.32
Previous studies have demonstrated the conversion of naïve CD4+ T cells into Tregs in vitro and in vivo, after exposure to supernatants of human retinal pigment epithelium cells.35 Amongst the wide array of immunoregulatory factors expressed by the retinal pigment epithelium cells, PEDF has been speculated to play a critical role in Treg induction and maintenance.36 Herein, we investigated the immunoregulatory function of PEDF on Tregs using a validated murine model of dry eye disease (Figure 1). In vitro data demonstrate that PEDF treatment promotes the suppressive properties of Tregs when exposed to the inflammatory environment mimicking DED. In vivo administration of recombinant PEDF (rPEDF) augments Treg frequencies as well as enhances their immunosuppressive properties, thereby ameliorating corneal epitheliopathy in DED.
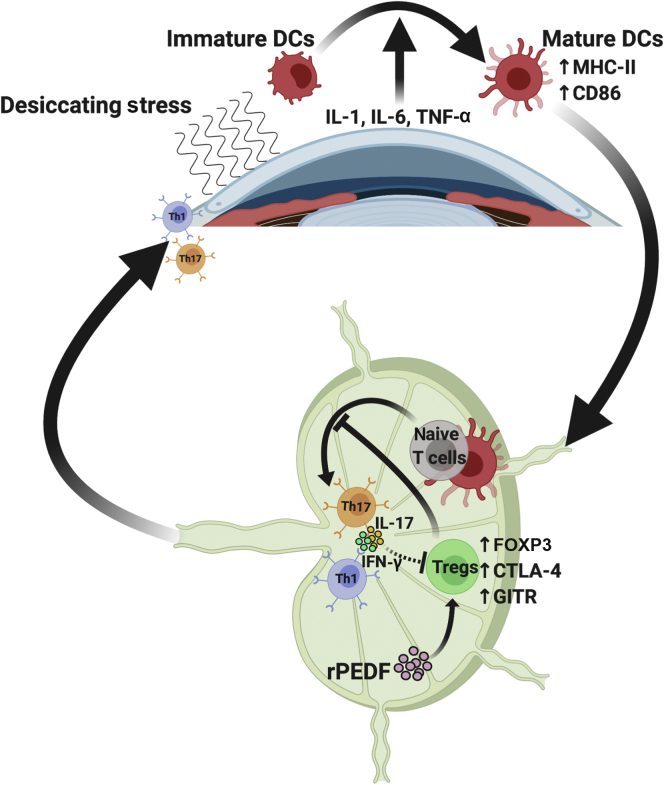
Figure 1.
Pigment epithelium–derived factor (PEDF) enhances the suppressive phenotype of regulatory T cells in a murine model of dry eye disease (DED). Dendritic cells (DCs) are activated in response to the proinflammatory cytokines, released by corneal epithelium on exposure to desiccating stress, and migrate from the cornea to the draining lymph nodes (DLNs) to form the afferent arm of immunopathogenesis in DED. In the DLNs, DCs prime naïve T cells to generate proinflammatory cytokines secreting type 17 helper T (Th17) cells. The regulatory T cells (Tregs) are known to limit the interaction between DCs and naïve T cells, thereby preventing the generation of Th17 cells. However, in DED, Tregs are rendered dysfunctional, leading to loss of their immunosuppressive functions. This study shows a protective effect of recombinant PEDF (rPEDF) on Tregs in DED and subsequent amelioration of DED. CD86, cluster of differentiation 86; CTLA-4, cytotoxic T-lymphocyte–associated protein 4; FOXP3, forkhead box P3; GITR, glucocorticoid-induced TNFR-related protein; IFN-γ, interferon-γ; MHC, major histocompatibility complex; Th1, T helper cells type 1; TNF-α, tumor necrosis factor-α.
Materials and Methods
Animals
Six- to 8-week–old female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). All the animals used in the experiments were housed in the secure, pathogen-free animal vivarium located in the Schepens Eye Research Institute and treated as per the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research guidelines.37 The Schepens Eye Research Institute Animal Care and Use Committee approved all the experiments performed on the animals. Three to four mice were used per group for each experiment, which were conducted at least four times, independently.
Dry Eye Disease Induction
DED was induced in mice, as described in previous studies.38,39 In brief, mice were placed in a controlled environment chamber with relative humidity between 15% and 20%, ambient temperature between 21°C and 23°C, and airflow of 15 L/minute for 14 days. Age- and sex-matched mice housed in the standard vivarium were used as controls for all experiments.
CD4+ CD25+ T-Cell Isolation and Culture
Submandibular and cervical draining lymph nodes (DLNs) were harvested from naïve and DED mice. Single-cell suspensions were prepared using a 70-μm cell strainer (BD Biosciences, Franklin Lakes, NJ), as described previously.9 CD4+CD25+ Tregs were isolated by magnetic separation using the Treg isolation kit (Miltenyi Biotec Inc., Somerville, MA). The purity of sorted cells (>95% FOXP3+ Tregs) was confirmed by flow cytometric analysis. Tregs were resuspended in RPMI 1640 (Corning Life Sciences Inc., Tewksbury, MA)-based complete medium supplemented with IL-2 (100 ng/mL; PeproTech Inc., Rocky Hill, NJ) at a concentration of 1 × 106 cells/mL. Subsequently, 100 μL of cell suspension was plated in each well of a 96-well plate (Corning Life Sciences Inc.).
Real-Time PCR
The sorted cells were frozen in TRIzol (Invitrogen Inc., Carlsbad, CA) at −80°C. The total RNA was extracted and purified with RNeasy Micro Kit (Qiagen Inc., Germantown, MD), as per the instructions from the manufacturer. The first strand of cDNA was synthesized with random hexamers using reverse transcriptase (Super Script III; Invitrogen Inc.). Real-time PCR was performed using TaqMan PCR Mastermix and 6-carboxyfluorescein (FAM) dye-labeled predesigned primers (Applied Biosystems Inc., Foster City, CA) for IL-10 (Mm00439615_g1), TGF-β (Mm00441727_g1), and GAPDH (Mm99999915_g1). GAPDH was used as an endogenous reference for each reaction. The quantitative PCR results were analyzed using the comparative CT method using the Light Cycler 480 Software version 1.5 (Roche Diagnostics Corp., Indianapolis, IN).
I.P. rPEDF Treatment Regimen
After induction of DED, mice were randomly divided into two groups; the treatment group received an i.p. injection of 1 μg/mL recombinant murine PEDF (LifeSpan BioSciences Inc., Seattle, WA), and the control group received an i.p. injection of murine serum albumin (Sigma-Aldrich Corp., St. Louis, MO) once daily for 14 days. All the dosages used in this study were evaluated and optimized in our preliminary experiments.
Treg Suppression Assay
Treg suppression assay was performed as described previously.40 Tregs and responder CD4+ T cells were isolated from the DLNs of DED-treated (rPEDF), untreated, or normal mice. Purified total CD4+ responder T cells (1 × 105) were co-cultured with CD4+CD25+ Tregs (5 × 104), T cell–depleted syngeneic splenocytes (1 × 105), in previously anti-CD3 (Biolegend Inc., San Diego, CA) coated flat-bottom microplates (96-well) for 3 days in the presence of additional 1 μg/mL of soluble anti-CD3 antibody (Biolegend Inc.). Proliferation of CD3-stimulated responder CD4+ T cells without adding Tregs was considered control proliferation with 0% suppression. Proliferation was measured using the bromodeoxyuridine proliferation kit (Millipore Sigma Inc., Burlington, MA), and percentage of suppression was calculated using the following formula:
| (1) |
Corneal Fluorescein Staining Scoring
Corneal epitheliopathy after continuous exposure to desiccating stress was evaluated by administering 1 μL of 2.5% fluorescein (Sigma-Aldrich Corp.) to the lateral conjunctival sac with a micropipette. Eye examination was performed 3 minutes later, using a slit-lamp biomicroscope under cobalt blue light to record the corneal fluorescein staining scores. The evaluation of the punctate corneal staining was performed in a masked manner, and the observer graded the disease as per the National Eye Institute Scoring System, assigning a score between 0 and 3 for each of the five areas of the cornea.41
Flow Cytometry
The magnetically sorted cells were treated with an anti-mouse CD16/32 antibody (anti-FcR antibody; Biolegend Inc.) in 0.5% bovine serum albumin for 30 minutes at 4°C, to block non-specific staining. For surface markers, cells were incubated with fluorescein isothiocyanate–conjugated anti-CD4, phosphatidylethanolamine-Cy7 conjugated CTLA4, and phosphatidylethanolamine-conjugated GITR (Biolegend Inc.) for 30 minutes on ice, in the dark. Then, the cells were washed twice and permeabilized for intracellular staining with fixation/permeabilization solution (Thermo Fischer Inc., Waltham, MA) at 4°C for 1 hour. Cells were subsequently washed using Permeabilization/Wash Buffer (Thermo Fischer Inc.) and stained with antigen-presenting cell–conjugated anti-FOXP3 (eBioscience/Thermofisher Inc., San Diego, CA) antibody for 20 minutes on ice, in the dark, and then washed twice. The cells were analyzed using the LSR II flow cytometer (BD Biosciences Inc., San Jose, CA), and the acquired data were analyzed using the Summit version 4.3 software package (Dako Colorado, Inc., Fort Collins, CO).
Statistical Analysis
Data distribution from multiple groups was compared with normal data distribution using one-way analysis of variance, followed by Bonferroni multiple comparisons tests. Unpaired, two-tailed t-test was used for the comparison of the two groups with the normal distribution of data. The differences were considered statistically significant at P < 0.05. Data from all experiments are presented as means ± SEM of at least four independent experiments. Statistical analysis of the acquired data was performed using Prism for Windows software package version 8.0 (GraphPad Software Inc., San Diego, CA).
Results
PEDF Prevents Proinflammatory Cytokine-Induced Loss of Treg Frequency and Suppressive Phenotype
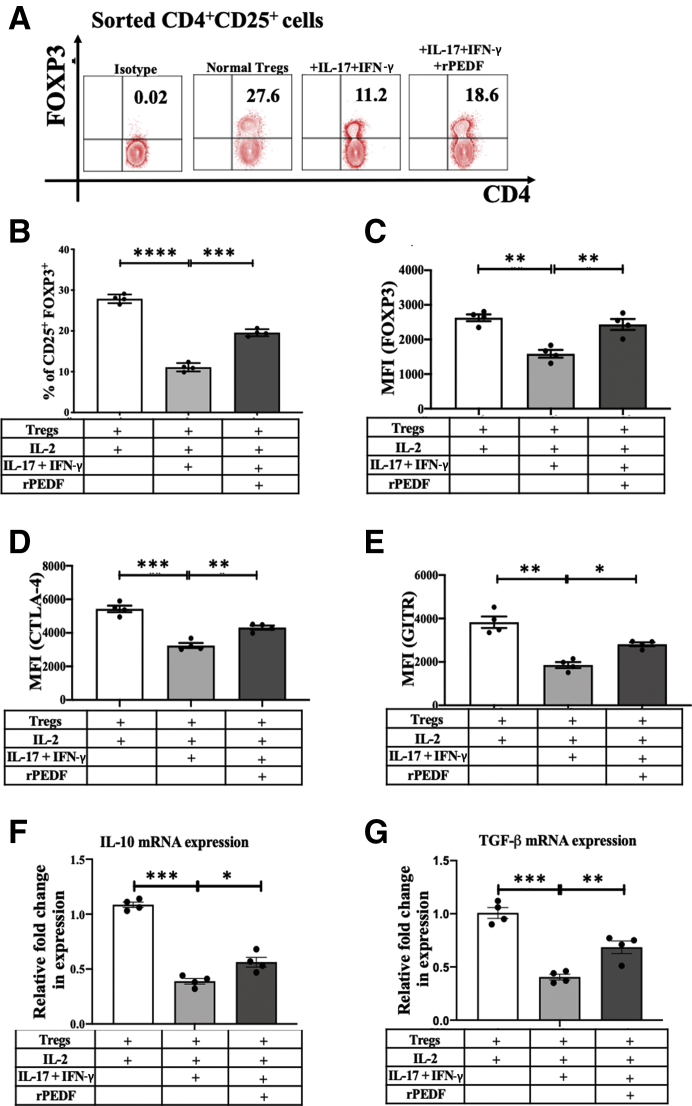
The direct effect of PEDF on Tregs in the Th17-associated inflammatory cytokine milieu was investigated in vitro. To determine this, CD4+CD25+ Tregs (approximately 90% FOXP3+) were magnetic-activated cell-sorted from normal mice and were cultured in the presence of dysfunction, inducing proinflammatory cytokines IL-17A (100 ng/mL) and interferon-γ (IFN-γ) (10 ng/mL), with or without rPEDF (100 ng/mL), for 72 hours. Treg dysfunction has been previously evaluated as a transient loss of FOXP3 expression on changes in the microenvironment, specifically on exposure to proinflammatory cytokines.9,42 Our data show that supplementing the media with rPEDF resulted in maintenance of significantly higher frequency of Tregs (19.58 ± 0.51 versus 11.10 ± 0.43; P < 0.0001), with a significantly higher expression (mean fluorescence intensity) of FOXP3 (2434 ± 159 versus 1587 ± 110; P = 0.0012), CTLA-4 (4315 ± 122 versus 3245 ± 150; P = 0.001), and GITR (2812 ± 91 versus 1855 ± 136; P = 0.0046) compared with that in untreated controls (Figure 2, A–E). Tregs were further evaluated for their expression of anti-inflammatory cytokines IL-10 and TGF-β1, which have been reported to suppress T-cell immune responses. The real-time PCR analysis revealed that both TGF-β1 and IL-10 expression levels were significantly increased (P = 0.0008 and P = 0.0011, respectively) on supplementation of the media with PEDF compared with those in untreated controls (Figure 2, F and G).
Figure 2.
Pigment epithelium-derived factor (PEDF) prevents proinflammatory cytokine–induced loss of regulatory T-cell (Treg) frequency and suppressive phenotype. Cervical and submandibular draining lymph nodes were harvested from normal mice, and CD4+CD25+ cells were isolated by magnetic sorting. Sorted regulatory T cells (Tregs) were cultured with dysfunction, inducing proinflammatory cytokines IL-17A (100 ng/mL) and interferon (IFN)-γ (10 ng/mL) for 72 hours. The culture medium was supplemented with IL-2 (100 ng/mL) for Treg maintenance in vitro. Representative flow cytometry plots (A) and bar charts (B), showing frequencies of CD4+CD25+ FOXP3+ Tregs cultured with (100 ng/mL) or without recombinant pigment epithelium–derived factor (rPEDF). Bar charts showing forkhead box P3 (FOXP3) (C), cytotoxic T-lymphocyte–associated protein 4 (CTLA-4; D), and glucocorticoid-induced TNFR-related protein (GITR) (E) expression levels [mean fluorescence intensity (MFI)] in Tregs cultured with or without rPEDF. Real-time analysis showing expression levels of IL-10 (F) and transforming growth factor (TGF)-β (G) by Tregs cultured with (100 ng/mL) or without rPEDF. Data are presented as a representative experiment of the four performed, each consisting of four mice per group. Data are presented as means ± SEM (B–G). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
PEDF Maintains the Frequency and Enhances the Function of Tregs Derived from DED Mice
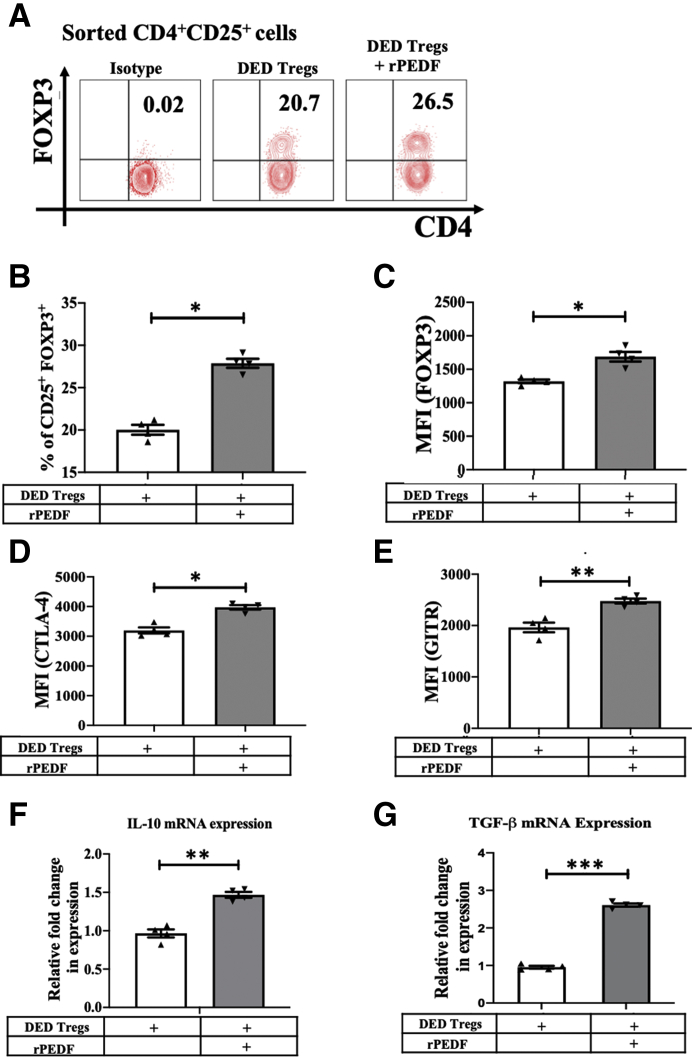
Next, the direct effect of rPEDF on dysfunctional Tregs derived from mice with DED was evaluated. Tregs were harvested from DLNs after 14 days of disease induction and cultured the cells with or without rPEDF (100 ng/mL) for 72 hours. Our data show that supplementing the media with rPEDF resulted in a significantly higher frequency of DED Tregs (27.95 ± 0.54 versus 20.15 ± 0.58; P = 0.028), as well as significantly enhanced expression (mean fluorescence intensity) of FOXP3 (1693 ± 72 versus 1324 ± 27; P = 0.03), CTLA-4 (4014 ± 174 versus 3142 ± 150; P = 0.05), and GITR (2477 ± 93 versus 1963 ± 48; P = 0.0027) compared with Tregs without rPEDF treatment (Figure 3, A–E). The supplementation of media with rPEDF also resulted in a 1.5-fold (P = 0.008) and a 2.6-fold (P < 0.0001) increase in DED Treg expression levels of IL-10 and TGF-β, respectively (Figure 3, F and G).
Figure 3.
Pigment epithelium–derived factor (PEDF) maintains the frequency and enhances the suppressive phenotype of regulatory T cells (Tregs) derived from dry eye disease (DED) mice. Mice were placed in the controlled environment chamber for 14 days to induce DED. The cervical and submandibular draining lymph nodes were harvested from mice, and CD4+CD25+ cells were isolated by magnetic sorting. Representative flow cytometry plots (A) and bar charts (B), showing frequencies of CD4+CD25+ FOXP3+ Tregs cultured with or without recombinant PEDF (rPEDF). Bar charts showing forkhead box P3 (FOXP3) (C), cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) (D), and glucocorticoid-induced TNFR-related protein (GITR) (E) expression levels [mean fluorescence intensity (MFI)] in Tregs cultured with or without rPEDF. Real-time analysis, showing the expression levels of IL-10 (F) and transforming growth factor-β (TGF-β) (G) by Tregs cultured with or without rPEDF. Data are presented as a representative experiment of the four performed, each consisting of four mice per group. Data are presented as means ± SEM (B–G). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Systemic Treatment with rPEDF Maintains Suppressive Capacity of Tregs in Dry Eye Disease
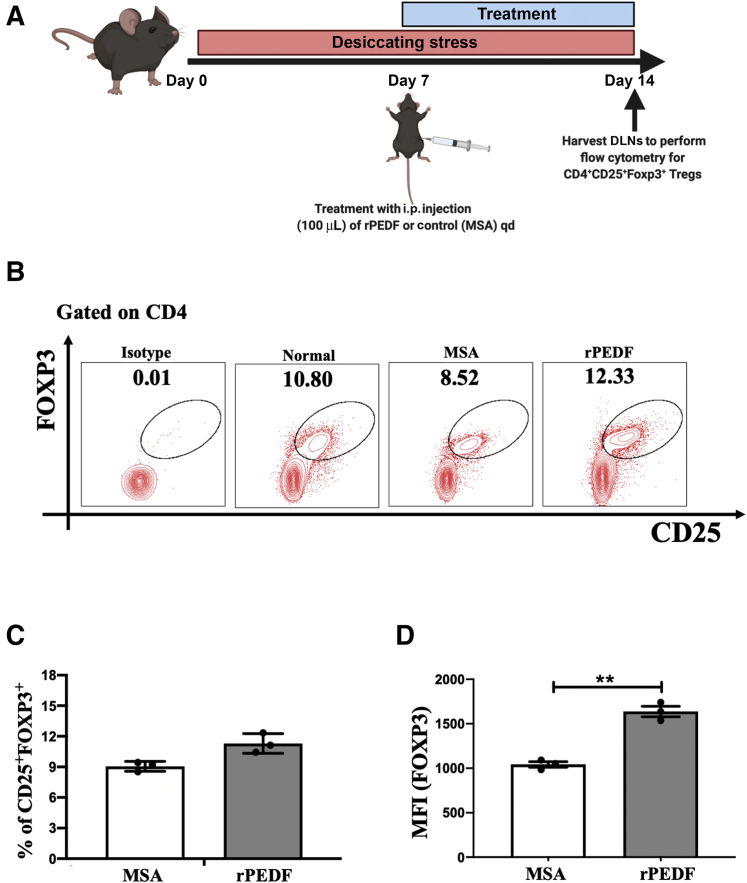
Having established the effect of rPEDF on Treg frequency and function in vitro, we assessed the efficacy of systemic injection of PEDF on Tregs and consequently on disease severity in mice with DED. The mice in the control group were injected with an equal volume of murine serum albumin. The analysis of the flow cytometric data showed moderately higher frequencies of FOXP3+ Tregs in cervical and submandibular DLNs in mice treated with rPEDF (9.11 ± 1.11 versus 12.01 ± 0.95; P = 0.413) compared with murine serum albumin–treated controls (Figure 4, A and B). However, a significantly higher expression of FOXP3 was observed in Tregs derived from the DLNs of the rPEDF-treated group (1637 ± 59 versus 1025 ± 21; P = 0.0052) compared with the controls (Figure 4C).
Figure 4.
Systemic treatment with recombinant pigment epithelium–derived factor (rPEDF) maintains regulatory T-cell (Treg) phenotype in dry eye disease (DED). To delineate the effect of PEDF on dysfunctional Tregs in DED, mice were treated with i.p. injection of rPEDF (1 μg/mL) or murine serum albumin (MSA; control) once daily for 7 days. A: Schematic diagram showing the time points of DED induction, i.p. rPEDF or MSA injections, and tissue harvesting. B: Representative flow cytometry plots showing the frequency of CD4+CD25+FOXP3+ Tregs. C: Bar charts showing the frequency of forkhead box P3 (FOXP3), in mice treated with rPEDF or MSA. D: Bar charts showing the expression levels of FOXP3 in mice treated with rPEDF or MSA. Data are presented as a representative experiment of the four performed, each consisting of three mice per group. Data are presented as means ± SEM (C and D). ∗∗P < 0.01. DLN, draining lymph node; MFI, mean fluorescence intensity; qd, once a day.
Systemic Treatment with rPEDF Maintains Treg Function in Dry Eye Disease
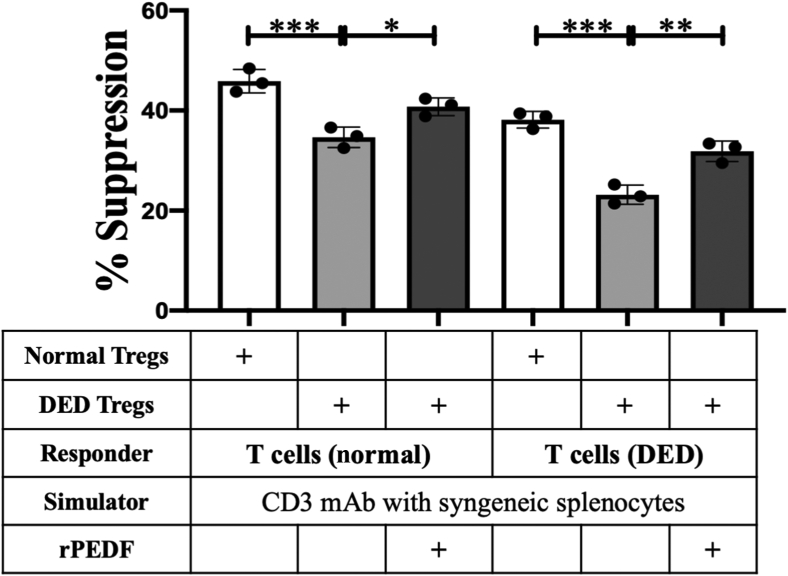
Tregs were isolated from the submandibular and cervical DLNs of DED (rPEDF-treated and untreated) and normal mice. An in vitro co-culture suppression assay system was used to compare the regulatory potential of purified Tregs from the three groups, to suppress the proliferation of CD3-antibody stimulated naïve T cells (isolated from the DLNs of normal mice) and primed T cells (isolated from the DLNs of DED mice) (Figure 5). Tregs isolated from DED mice treated with systemic rPEDF showed significantly higher potential in suppressing proliferation of both naïve T cells (P = 0.0022) and primed T cells (P = 0.0002) compared with those from untreated DED mice. In addition, Tregs isolated from rPEDF-treated DED mice had a moderately lower capacity compared with that of normal Tregs in suppressing the proliferation of naïve and primed T cells. These results demonstrate the effect of systemically administered rPEDF on maintenance of Treg function in mice with dry eye disease.
Figure 5.
In vitro regulatory T-cell (Treg) suppression assay. Naïve or primed T cells isolated from the draining lymph nodes (DLNs) of normal and dry eye disease (DED) mice, respectively, were stimulated with CD3 antibody for 3 days in the presence of Tregs isolated from the DLNs of normal or DED [pigment epithelium–derived factor (PEDF)–treated and untreated] mice. The activity of Tregs is measured at Treg/Teff (effector T cell) ratio of 1:2, as standardized previously. Proliferation was measured using the bromodeoxyuridine incorporation assay, compared with the proliferative responses of respective CD3-stimulated T cells in the absence of Tregs, and the percentage suppression calculated. Data are presented as a representative experiment of the four performed, each consisting of three mice per group. Data are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. mAb, monoclonal antibody; rPEDF, recombinant PEDF.
PEDF Treatment Limits Dry Eye Disease Severity
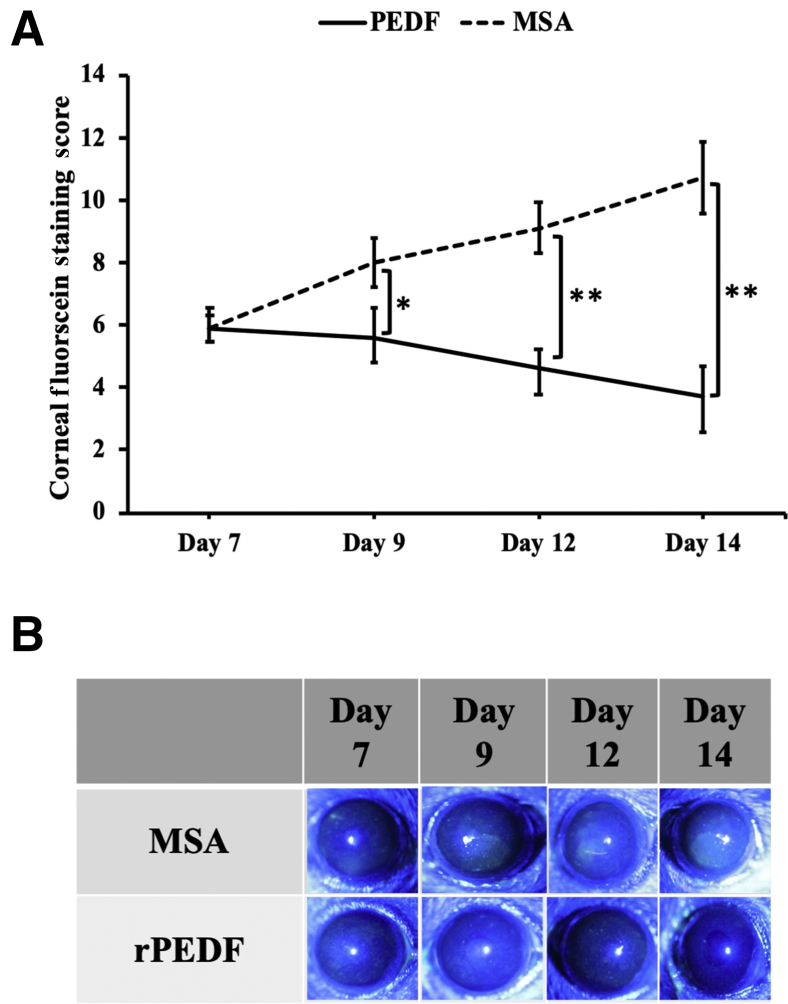
Finally, the therapeutic effect of rPEDF on clinical disease severity was evaluated. DED was induced in mice for 14 days, and the mice were i.p. injected with 1 μg/mL rPEDF daily for 7 days from day 7 (Figure 4A). There was a significant reduction in corneal fluorescein staining scores at all time points (days 9, 12, and 14) in corneas of mice receiving i.p. rPEDF in comparison to the murine serum albumin–treated control group (Figure 6).
Figure 6.
Treatment with recombinant pigment epithelium–derived factor (rPEDF) reduces dry eye disease (DED) severity. Efficacy of systemic rPEDF treatment initiated after DED induction for 7 days was assessed. A: Clinical severity of DED on treatment with murine serum albumin (MSA; control) or rPEDF was evaluated using corneal fluorescein staining on days 7, 9, 12, and 14. B: Representative corneal fluorescein staining images captured on days 7, 9, 12, and 14. Data are presented as a representative experiment of the four performed, each consisting of four mice per group. Data are presented as means ± SEM (A). ∗P < 0.05, ∗∗P < 0.01.
Discussion
The discovery of endogenous immunoregulatory factors has enabled us to develop novel therapeutic modalities that directly act on the inflammatory cells involved in the pathophysiological mechanisms of immune-mediated diseases. In this study, we investigated the direct effect of one such immunomodulatory factor—pigment epithelium–derived factor—on the immunosuppressive phenotype of regulatory T cells. PEDF is an endogenous anti-inflammatory protein first isolated from the conditioned medium of cultured human fetal retinal pigment epithelium cells.43 Imai et al44 performed a series of experiments to generate regulatory T cells on incubating peripheral blood mononuclear cells with supernatants of human retinal pigment epithelium cells, attributing this phenomenon to the intrinsic factors expressed by the cells, including PEDF. Although multiple groups have highlighted the suppressive effect of PEDF on macrophages and DCs, this study is the first attempt to investigate the direct effect of PEDF on regulatory T cells.33,45,46
Antigen-presenting cells, primarily composed of DCs, are activated in response to the proinflammatory cytokines (released by corneal epithelium after exposure to desiccating stress) and migrate from the cornea to the DLNs, forming the critical afferent arm in the immunopathogenesis of DED.47 In the DLNs, the DCs interact with naïve T cells and generate inflammatory type 1 helper T-cells (Th1) and Th17 cells, the primary effectors in the pathogenesis of DED, which subsequently migrate to the ocular surface.48 These inflammatory changes in the lymphoid compartment promote migration of Tregs residing in the thymus to the secondary lymphoid tissue, guided by the homing receptors CD62L and CCR7, where they generate stable interactions with activated and mature DCs.49 Tregs prevent generation of T-effector cells by obstructing the interface between naïve T cells and mature DCs by suppressing the expression of costimulatory molecules CD80 and CD86 and instead induce DCs to produce pro-apoptotic molecules.50,51
IL-17 and IFN-γ were introduced to the culture system to replicate the proinflammatory milieu between the ocular surface and secondary lymphoid tissue axis, primarily attributed to a marked increase in Th1 and Th17 cell frequencies in DED.52 In addition, Chen et al10 have also identified Th17-derived IFN-γ–secreting Th17/Th1 as a primary source of increased IFN-γ in DED. These IL-17A+IFN-γ+ double-positive Th17/Th1 cells, emerging from Th17 cells on disease induction, are a significant source of IFN-γ during DED progression, and increased IFN-γ expression plays a role in the pathogenic Th17 response in severe DED. Our data suggest that addition of rPEDF to the co-culture system effectively preserved the frequency as well as suppressive phenotype of Tregs, as evident from significantly higher expression of FOXP3, CTLA-4, and GITR.
Niederkorn et al53 have outlined the critical immunosuppressive function of Tregs in limiting DED pathogenesis by showing exacerbation of disease in mice exposed to desiccating stress, after depletion of CD4+CD25hiFOXP3+ Tregs. A previous study from our laboratory has demonstrated that, despite similar Treg frequencies in naïve and DED mice, the CD4+CD25hiFOXP3+ Tregs in DED mice are less effective in suppressing the generation of pathogenic T effector cells compared with Tregs in naïve mice.9 To further validate our hypothesis regarding the effect of rPEDF on dysfunctional Tregs, we cultured Tregs derived from DED mice and supplemented the media with rPEDF. Flow cytometric data showed a significantly higher frequency of CD4+CD25+FOXP3+ Tregs and expression of FOXP3, GITR, and CTLA-4 in DED Tregs treated with rPEDF, after 72 hours of culture.
After establishing the effect of PEDF on Tregs in vitro, its translational potential was evaluated in a validated murine model of DED. DED mice were systemically treated with rPEDF for a period of 7 days and observed a moderately higher frequency of Tregs in treated mice compared with the controls. However, the Tregs derived from the mice treated with rPEDF exhibited a significantly higher expression of FOXP3 compared with the controls. Our hypothesis was further corroborated by the maintenance of Treg-suppressive function in DED mice treated with rPEDF compared with that in untreated mice. Furthermore, the effect of rPEDF on Treg function resulted in significant reduction of DED severity in treated mice compared with that in controls.
In summary, the experimental data from our study establish a novel immunoregulatory function of PEDF through functional enhancement of regulatory T cells. We demonstrate that PEDF preserves the frequency and phenotype of Tregs exposed to proinflammatory cytokines capable of inducing dysfunction. Our data also show a similar effect of PEDF on dysfunctional Tregs derived from DED. Lastly, we confirm the protective effect of PEDF on Tregs in vivo, thus demonstrating its therapeutic potential in reducing disease severity by limiting Treg dysfunction in DED.
Acknowledgments
We thank Santanu Mukherjee, Ph.D., for valuable feedback regarding the experimental design and data analysis. The visual abstract (Figure 1) was designed by R.B.S. using Biorender.com.
Footnotes
Supported by the National Eye Institute/NIH grants R01 EY020889 (R.D.), T32-EY007145 (H.A.), and core grant P30EY003790.
Disclosures: None.
Author Contributions
R.B.S., T.B., S.K.C., and R.D. designed the study; R.B.S., T.B., and S.K.M. acquired data; R.B.S., T.B., H.A., and Y.C. analyzed the data; R.B.S. prepared the manuscript; R.B.S., T.B., and H.A. revised the manuscript; S.K.C. and R.D. supervised the study; all authors read and approved the final manuscript.
References
- 1.Craig J.P., Nichols K.K., Akpek E.K., Caffery B., Dua H.S., Joo C.K., Liu Z., Nelson J.D., Nichols J.J., Tsubota K., Stapleton F. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Dana R., Meunier J., Markowitz J.T., Joseph C., Siffel C. Patient-reported burden of dry eye disease in the United States: results of an online cross-sectional survey. Am J Ophthalmol. 2020;216:7–17. doi: 10.1016/j.ajo.2020.03.044. [DOI] [PubMed] [Google Scholar]
- 3.Tsubota K., Pflugfelder S.C., Liu Z., Baudouin C., Kim H.M., Messmer E.M., Kruse F., Liang L., Carreno-Galeano J.T., Rolando M., Yokoi N., Kinoshita S., Dana R. Defining dry eye from a clinical perspective. Int J Mol Sci. 2020;21:9271. doi: 10.3390/ijms21239271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulsen A.J., Cruickshanks K.J., Fischer M.E., Huang G.-H.H., Klein B.E.K.K., Klein R., Dalton D.S. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J., Asche C.V., Fairchild C.J. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30:379–387. doi: 10.1097/ICO.0b013e3181f7f363. [DOI] [PubMed] [Google Scholar]
- 6.McMonnies C.W. Dry eye disease immune responses and topical therapy. Eye Vis (Lond) 2019;6:12. doi: 10.1186/s40662-019-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan S.K., Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol. 2009;2:375–376. doi: 10.1038/mi.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Chauhan S.K., Soo Lee H., Saban D.R., Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014;7:38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan S.K., El Annan J., Ecoiffier T., Goyal S., Zhang Q., Saban D.R., Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Chauhan S.K., Shao C., Omoto M., Inomata T., Dana R. IFN-γ–expressing Th17 cells are required for development of severe ocular surface autoimmunity. J Immunol. 2017;199:1163–1169. doi: 10.4049/jimmunol.1602144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Paterson A.M., Sharpe A.H. Taming tissue-specific T cells: CTLA-4 reins in self-reactive T cells. Nat Immunol. 2010;11:109–111. doi: 10.1038/ni0210-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomon B., Lenschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., Bluestone J.A. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 14.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens G.L., McHugh R.S., Whitters M.J., Young D.A., Luxenberg D., Carreno B.M., Collins M., Shevach E.M. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu J., Yamazaki S., Takahashi T., Ishida Y., Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 17.Shevach E.M. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Rubtsov Y.P., Niec R.E., Josefowicz S., Li L., Darce J., Mathis D., Benoist C., Rudensky A.Y. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 20.Deng G., Song X., Fujimoto S., Piccirillo C.A., Nagai Y., Greene M.I. Foxp3 post-translational modifications and treg suppressive activity. Front Immunol. 2019;10:2486. doi: 10.3389/fimmu.2019.02486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 22.Fontenot J.D., Gavin M.A., Rudensky A.Y., Pillars Article Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003. 4: 330-336. J Immunol. 2017;198:986–992. [PubMed] [Google Scholar]
- 23.Hua J., Inomata T., Chen Y., Foulsham W., Stevenson W., Shiang T., Bluestone J.A., Dana R. Pathological conversion of regulatory T cells is associated with loss of allotolerance. Sci Rep. 2018;8:7059. doi: 10.1038/s41598-018-25384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson D.W., Volpert O.V., Gillis P., Crawford S.E., Xu H., Benedict W., Bouck N.P. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 25.Karakousis P.C., John S.K., Behling K.C., Surace E.M., Smith J.E., Hendrickson A., Tang W.X., Bennett J., Milam A.H. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol Vis. 2001;7:154–163. [PubMed] [Google Scholar]
- 26.Chandolu V., Dass C.R. Cell and molecular biology underpinning the effects of PEDF on cancers in general and osteosarcoma in particular. J Biomed Biotechnol. 2012;2012:740295. doi: 10.1155/2012/740295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elahy M., Baindur-Hudson S., Dass C.R. The emerging role of PEDF in stem cell biology. J Biomed Biotechnol. 2012;2012:239091. doi: 10.1155/2012/239091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J.T., Chen Y.L., Chen W.C., Chen H.Y., Lin Y.W., Wang S.H., Man K.M., Wan H.M., Yin W.H., Liu P.L., Chen Y.H. Role of pigment epithelium-derived factor in stem/progenitor cell-associated neovascularization. J Biomed Biotechnol. 2012;2012:871272. doi: 10.1155/2012/871272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda S., Yamagishi S.-I., Okuda S. Anti-vasopermeability effects of PEDF in retinal-renal disorders. Curr Mol Med. 2010;10:279–283. doi: 10.2174/156652410791065291. [DOI] [PubMed] [Google Scholar]
- 30.Yabe T., Sanagi T., Yamada H. The neuroprotective role of PEDF: implication for the therapy of neurological disorders. Curr Mol Med. 2010;10:259–266. doi: 10.2174/156652410791065354. [DOI] [PubMed] [Google Scholar]
- 31.Yamagishi S., Kikuchi S., Nakamura K., Matsui T., Takeuchi M., Inoue H. Pigment epithelium-derived factor (PEDF) blocks angiotensin II-induced T cell proliferation by suppressing autocrine production of interleukin-2. Med Chem. 2006;2:265–269. doi: 10.2174/157340606776930826. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S.X., Wang J.J., Gao G., Shao C., Mott R., Ma J. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006;20:323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 33.Singh R.B., Blanco T., Mittal S.K., Taketani Y., Chauhan S.K., Chen Y., Dana R. Pigment epithelium-derived factor secreted by corneal epithelial cells regulates dendritic cell maturation in dry eye disease. Ocul Surf. 2020;18:460–469. doi: 10.1016/j.jtos.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian X., Wang T., Zhang S., Wang Q., Hu X., Ge C., Xie L., Zhou Q. PEDF reduces the severity of herpetic simplex keratitis in mice. Investig Ophthalmol Vis Sci. 2018;59:2923–2931. doi: 10.1167/iovs.18-23942. [DOI] [PubMed] [Google Scholar]
- 35.Zamiri P., Sugita S., Streilein J.W. Immunosuppressive properties of the pigmented epithelial cells and the subretinal space. Chem Immunol Allergy. 2007;92:86–93. doi: 10.1159/000099259. [DOI] [PubMed] [Google Scholar]
- 36.Masli S., Vega J.L. Ocular immune privilege sites. Methods Mol Biol. 2011;677:449–458. doi: 10.1007/978-1-60761-869-0_28. [DOI] [PubMed] [Google Scholar]
- 37.The Association for Research in Vision and Opthamology . Baltimore, MD: Association for Research in Vision and Ophthalmology. 1995. ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. [Google Scholar]
- 38.Chen Y., Chauhan S.K., Tan X., Dana R. Interleukin-7 and -15 maintain pathogenic memory Th17 cells in autoimmunity. J Autoimmun. 2017;77:96–103. doi: 10.1016/j.jaut.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Chauhan S.K., Lee H.S., Stevenson W., Schaumburg C.S., Sadrai Z., Saban D.R., Kodati S., Stern M.E., Dana R. Effect of desiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunopathogenesis. Investig Ophthalmol Vis Sci. 2013;54:2457–2464. doi: 10.1167/iovs.12-11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taketani Y., Marmalidou A., Dohlman T.H., Singh R.B., Amouzegar A., Chauhan S.K., Chen Y., Dana R. Restoration of regulatory T-cell function in dry eye disease by antagonizing substance P/neurokinin-1 receptor. Am J Pathol. 2020;190:1859–1866. doi: 10.1016/j.ajpath.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amparo F., Wang H., Yin J., Marmalidou A., Dana R. Evaluating corneal fluorescein staining using a novel automated method. Invest Ophthalmol Vis Sci. 2017;58:BIO168–BIO173. doi: 10.1167/iovs.17-21831. [DOI] [PubMed] [Google Scholar]
- 42.Cunnusamy K., Niederkorn J.Y. IFN-γ blocks CD4+CD25+ Tregs and abolishes immune privilege of minor histocompatibility mismatched corneal allografts. Am J Transpl. 2013;13:3076–3084. doi: 10.1111/ajt.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steele F.R., Chader G.J., Johnson L.V., Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993;90:1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai A., Sugita S., Kawazoe Y., Horie S., Yamada Y., Keino H., Maruyama K., Mochizuki M. Immunosuppressive properties of regulatory T cells generated by incubation of peripheral blood mononuclear cells with supernatants of human RPE cells. Investig Ophthalmol Vis Sci. 2012;53:7299–7309. doi: 10.1167/iovs.12-10182. [DOI] [PubMed] [Google Scholar]
- 45.Ren K., Jiang T., Chen J., Zhao G.J. PEDF ameliorates macrophage inflammation via NF-κB suppression. Int J Cardiol. 2017;247:42. doi: 10.1016/j.ijcard.2017.07.069. [DOI] [PubMed] [Google Scholar]
- 46.Wen H., Liu M., Liu Z., Yang X., Liu X., Ni M., Dong M., Luan X., Yuan Y., Xu X., Lu H. PEDF improves atherosclerotic plaque stability by inhibiting macrophage inflammation response. Int J Cardiol. 2017;235:37–41. doi: 10.1016/j.ijcard.2017.02.102. [DOI] [PubMed] [Google Scholar]
- 47.Schaumburg C.S., Siemasko K.F., De Paiva C.S., Wheeler L.A., Niederkorn J.Y., Pflugfelder S.C., Stern M.E. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 48.El Annan J., Chauhan S.K., Ecoiffier T., Zhang Q., Saban D.R., Dana R. Characterization of effector T cells in dry eye disease. Investig Opthalmol Vis Sci. 2009;50:3802. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szanya V., Ermann J., Taylor C., Holness C., Fathman C.G. The subpopulation of CD4 + CD25 + splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 50.Campbell D.J. Control of regulatory T cell migration, function, and homeostasis. J Immunol. 2015;195:2507–2513. doi: 10.4049/jimmunol.1500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fallarino F., Grohmann U., Hwang K.W., Orabona C., Vacca C., Bianchi R., Belladonna M.L., Fioretti M.C., Alegre M.L., Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 52.Pflugfelder S.C., Corrales R.M., de Paiva C.S. T helper cytokines in dry eye disease. Exp Eye Res. 2013;117:118–125. doi: 10.1016/j.exer.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niederkorn J.Y., Stern M.E., Pflugfelder S.C., De Paiva C.S., Corrales R.M., Gao J., Siemasko K. Desiccating stress induces T cell-mediated Sjögren’s syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]