Key Points
Question
Can a brief blended digital therapy targeting reasoning (SlowMo) improve paranoia for adults with psychosis when added to usual care?
Findings
This randomized clinical trial of 361 individuals with clinical paranoia did not demonstrate that SlowMo therapy reduced the primary outcome of self-reported paranoia at 24 weeks compared with usual care only, although secondary beneficial effects were found on this measure at 12 weeks. Self-reported persecution and observer-rated paranoia were improved at both points.
Meaning
SlowMo, a digitally supported reasoning intervention, indicated a beneficial effect on paranoia; further work to optimize the effects of SlowMo is warranted.
This randomized clinical trial investigates the effects and mechanisms of action of SlowMo, a digitally supported reasoning intervention, plus usual care compared with usual care only among individuals with clinical paranoia.
Abstract
Importance
Persistent paranoia is common among patients with psychosis. Cognitive-behavioral therapy for psychosis can be effective. However, challenges in engagement and effectiveness remain.
Objective
To investigate the effects on paranoia and mechanisms of action of SlowMo, a digitally supported reasoning intervention, plus usual care compared with usual care only.
Design, Setting, and Participants
This parallel-arm, assessor-blinded, randomized clinical trial recruited participants at UK community health services from May 1, 2017, to May 14, 2019. Eligible participants consisted of a referral sample with schizophrenia-spectrum psychosis and distressing, persistent (≥3 months) paranoia.
Interventions
Individuals were randomized 1:1 to SlowMo, consisting of 8 digitally supported face-to-face sessions and a mobile app, plus usual care (n = 181) and usual care only (n = 181).
Main Outcomes and Measures
The primary outcome was paranoia, measured by the Green et al Paranoid Thoughts Scale (GPTS) total score at 24 weeks. Secondary outcomes included GPTS total score at 12 weeks and GPTS Part A (reference) and Part B (persecutory) scores, the Psychotic Symptom Rating Scales (PSYRATS Delusion subscale), reasoning (belief flexibility, possibility of being mistaken [Maudsley Assessment of Delusions, rated 0%-100%]), and jumping to conclusions (Beads Task).
Results
A total of 361 participants were included in intention-to-treat analysis, of whom 252 (69.8%) were male and 249 (69.0%) were White; the mean (SD) age was 42.6 (11.6) years. At 24 weeks, 332 participants (92.0%) provided primary outcome data. Of 181 participants in the SlowMo group, 145 (80.1%) completed therapy. SlowMo plus usual care was not associated with greater reductions than usual care in GPTS total score at 24 weeks (Cohen d, 0.20; 95% CI, −0.02 to 0.40; P = .06). There were significant effects on secondary paranoia outcomes at 12 weeks, including GPTS total score (Cohen d, 0.30; 95% CI, 0.09-0.51; P = .005), Part A score (Cohen d, 0.22; 95% CI, 0.06-0.39; P = .009), and Part B score (Cohen d, 0.32; 95% CI, 0.08-0.56; P = .009), and at 24 weeks, including Part B score (Cohen d, 0.25; 95% CI, 0.01-0.49; P = .04) but not Part A score (Cohen d, 0.12; 95% CI, −0.05 to 0.28; P = .18). Improvements were observed in an observer-rated measure of persecutory delusions (PSYRATS delusion) at 12 weeks (Cohen d, 0.47; 95% CI, 0.17-0.78; P = .002) and 24 weeks (Cohen d, 0.50; 95% CI, 0.20-0.80; P = .001) and belief flexibility at 12 weeks (Cohen d, 0.29; 95% CI, 0.09-0.49; P = .004) and 24 weeks (Cohen d, 0.28; 95% CI, 0.08-0.49; P = .005). There were no significant effects on jumping to conclusions. Improved belief flexibility and worry mediated paranoia change (range mediated, 36%-56%).
Conclusions and Relevance
SlowMo did not demonstrate significant improvements in the primary measure of paranoia at 24 weeks; however, a beneficial effect of SlowMo on paranoia was indicated by the results on the primary measure at an earlier point and on observer-rated paranoia and self-reported persecution at 12 and 24 weeks. Further work to optimize SlowMo’s effects is warranted.
Trial Registration
isrctn.org Identifier: ISRCTN 32448671
Introduction
Paranoia, or fear of deliberate harm from others, is among the most common symptoms of schizophrenia spectrum disorders and is associated with substantial distress and disruption.1 Developing effective interventions for paranoia is a clinical priority. Meta-analyses of first-generation cognitive-behavioral therapy for psychosis (CBTp) have indicated associations with delusions2 and broader positive symptoms.3 However, marked challenges to treatment engagement, adherence, and effectiveness remain.1,4
SlowMo therapy adopts an interventionist-causal approach5 to increasing CBTp effectiveness by targeting reasoning processes considered causal in paranoia.6 These biased processes include jumping to conclusions (JTC) (ie, forming rapid judgments using limited information) and belief inflexibility (reduced metacognitive capacity for reflecting on and reviewing one’s beliefs and considering alternatives).6,7,8 SlowMo aims to build awareness of a tendency to JTC and develop increased belief flexibility. SlowMo is the end point of a decade of development, during which preliminary evidence that the intervention reduced paranoia severity, mediated by increased belief flexibility, was found.9,10,11,12 Over time, the intervention has focused increasingly on belief flexibility, adopting the terms fast and slow thinking to communicate reasoning concepts.8,13,14 SlowMo also uses digital technology and inclusive, human-centered design to improve the user experience with the aim of enhancing engagement and adherence for the widest possible range of people.14,15,16 SlowMo builds on the encouraging findings for stand-alone and blended mobile phone apps for psychosis17,18,19,20 and, to our knowledge, is the first blended digital psychological intervention for paranoia (using digitally supported face-to-face therapy and a mobile app).
This randomized clinical trial aimed to test the efficacy of SlowMo in reducing paranoia and improving reasoning. We hypothesized that SlowMo would improve paranoia and reasoning together with outcomes prioritized by the trial’s service-user consultants: self-concept, quality of life, and well-being. We also hypothesized that the treatment effects on paranoia would be mediated through reasoning, specifically belief flexibility and JTC. We also examined worry as an outcome and mediator because worry mediates change in paranoia.21 However, because worry was not directly targeted by the treatment, we hypothesized that worry would not mediate the treatment effects of SlowMo on paranoia.
Methods
Research Design
This parallel-group randomized clinical trial (ISRCTN32448671) used 1:1 allocation and blinded assessors to test the efficacy of adding SlowMo therapy to treatment as usual (TAU) to reduce paranoia severity compared with TAU alone (trial protocol given in Supplement 1). The trial was performed from May 1, 2017, to October 31, 2019. Recruitment was from UK community mental health services across 3 main sites. The trial received ethical approval from the Camberwell St Giles research ethics committee, and all participants gave written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.22
Participants
Eligible participants met the following criteria: 18 years or older; persistent (≥3 months) distressing paranoia (assessed using the Schedules for Clinical Assessment in Neuropsychiatry23); score of greater than 29 on the Green et al Paranoid Thoughts Scale (GPTS) Part B, the Persecutory subscale24; a diagnosis of schizophrenia spectrum psychosis (codes F20-29 from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision)25; capacity to provide informed consent; and sufficient English to participate in trial processes. Participants were excluded if they had profound visual or hearing impairment, were unable to engage in assessments, were currently receiving psychological therapy for paranoia, and had a primary diagnosis of substance use disorder, personality disorder, organic syndrome, or learning disability.
Randomization and Masking
After baseline assessment, we randomly assigned (1:1) eligible patients using a secure, independent, web-based service hosted by King’s Clinical Trials Unit. Randomly varying sized blocks were used and stratified by site and baseline paranoia (median split of ≥62 on GPTS Part B score24). Research assessors were masked to allocation. The site coordinators (T.W., M.R.-C., A.M., C.S., and N.C.) conducted randomization and informed participants. If unmasking occurred, reallocation to another rater occurred when operationally feasible. Breaks in masking were recorded.
Interventions
SlowMo is a digitally supported CBTp consisting of 8 individual, face-to-face sessions (60-90 minutes) in accordance with a clinical manual that was delivered within 12 weeks. The intervention builds awareness of unhelpful fast thinking and supports individualized formulation. SlowMo then assists people with slowing down for a moment to find ways of feeling safer. Sessions are assisted by the SlowMo web app delivered using a touchscreen laptop, with interactive features including information, animated vignettes, games, and personalized thought bubbles. The web app synchronizes to a native android mobile app providing access in daily life to SlowMo strategies and individualized safer-thought bubbles.14,26 A device was provided to all participants. Behavioral work outside the clinic room was encouraged, with the aim of practicing strategies. Therapy was delivered in clinic settings or at home (eMethods 1 and eFigure 1 in Supplement 2 give further details on the intervention).
Therapists included 11 trained doctoral-level psychologists (M.R.-C., T.W., A.M., C.S., and N.C. and 6 others), with therapists supervised weekly using recorded sessions. Therapy uptake was assessed by number and duration of sessions attended, with fidelity to the clinical manual defined as no more than 1 web app component missed per session (mean calculated across all attended sessions). Mobile app adherence was operationalized as at least 1 home screen interaction after a minimum of 3 therapy sessions and was recorded by system analytics (eMethods 2 in Supplement 2).
Treatment as usual was delivered according to UK national and local service guidelines and typically involved antipsychotic therapy, contact with a mental health worker, and outpatient psychiatric appointments. Participation did not alter pharmacologic or psychosocial treatment decisions (recorded in both groups using the modified Client Service Receipt Inventory27).
Measurements
Assessments were performed at 0 (baseline), 12 weeks (postintervention), and 24 weeks (follow-up). Blinded assessors conducted enrollment and assessments at clinics or in participants’ homes. Participants were compensated £20 (approximately US $28) at each point.
Outcomes
The primary outcome was self-reported paranoia severity at 24 weeks, measured by the GPTS total score24 (range, 32-160, with higher scores indicating more severe paranoia). The GPTS consists of two 16-item subscales assessing ideas of social reference (Part A) and persecution (Part B) during the previous month, with reported scores being secondary outcomes. Detail on all measures is provided in Supplement 1 and eMethods 3 and 4 in Supplement 2. Secondary paranoia measures also consisted of 2 observer-rated scales: the Psychotic Symptom Rating Scales (PSYRATS) delusions subscale,28 scored as a total and as 2 factors (conviction and distress29), and individual persecutory delusions and ideas of reference items from the Scales for Assessment of Positive Symptoms (SAPS).30 We also assessed outcomes on the Revised GPTS (R-GPTS)31 (total and subscale scores); this revised measure was published during the trial and was added to the statistical analysis plan before statistical analysis commenced (Supplement 1). It consists of 2 scales assessing thinking relevant to paranoia based on the original items: ideas of social reference (8 items) and persecution (10 items).
Reasoning was assessed as an outcome and as a potential mediator by 2 established methods of assessing belief flexibility relating to delusions8: possibility of being mistaken (self-rated 0%-100% and observer-rated yes or no) from the Maudsley Assessment of Delusions Schedule32 and alternative explanations from the Explanations of Experiences interview,33 with increased flexibility being desirable; and by the JTC Beads Task,7 versions 85:15 and 60:40. The Fast and Slow Thinking Questionnaire34 (previously the TAPS26), consisting of 2 scales, one assessing fast (intuitive) thinking and one measuring slow (analytic) thinking, was included as a reasoning outcome but was not prespecified as a hypothesized mediator. Other secondary outcomes were well-being (the Warwick-Edinburgh Mental Well-being Scale35), quality of life (the Manchester Short Assessment of Quality of Life36), self and other schemas (the Brief Core Schema Scales37), and worry (the Penn State Worry Questionnaire38). Adverse events were actively monitored throughout the study until the 24-week follow-up.
Statistical Analysis
We powered the study to detect a 10-point reduction in the GPTS total score (effect size, 0.40) and accounted for the partial nested design owing to therapist clustering in the SlowMo arm.39 With 1:1 allocation and a statistical significance level of 2-tailed P < .05, a simple 2-tailed t test with 150 people per group had 90% power to detect an effect size of 0.40 and 80% power for an effect size of 0.35. To allow for 20% attrition, we aimed to recruit 360 patients at baseline split equally across 3 sites. All analyses were performed using the intention-to-treat population. Statisticians (R.E. and K.J.) were only unblinded after database lock, and the statistical analysis was performed unblinded owing to the need to account for therapist effects in the SlowMo arm. No interim analysis was performed. All analyses were conducted in Stata, version 16.0 (StataCorp LLC).40
To test the primary hypothesis that the intervention would reduce paranoia severity during the 24-week study, we fitted a linear mixed model allowing for clustering by participants and therapists to the repeated measures of the GPTS with fixed effects of randomization, time, time by randomization interaction, site, paranoia severity (stratifier), and baseline GPTS. The treatment effect (adjusted between-group mean difference) was estimated from the model for each point separately. All secondary outcome measures were analyzed using linear mixed models for continuous outcomes and logistic mixed models for binary outcomes. Cohen d effect sizes at 12 and 24 weeks were calculated as the adjusted mean difference divided by the sample SD of the outcome at baseline and are shown in a forest plot. Mediation analysis used parametric regression models, whereas moderation analyses were conducted by adding interaction terms between randomized groups and a set of prespecified moderators; further detail of the moderation and mediation analyses and the methods and results of a compliance-adjusted analysis are provided in eMethods 5, 6, and 8 in Supplement 2.
Missing data on measures were prorated if more than 90% of items were completed; otherwise the measure was considered missing. We checked for covariates associated with missing outcomes by comparing responders with nonresponders on key baseline variables. Maximum likelihood estimation in the mixed models accounted for missing outcome data under a missing-at-random assumption, conditional on the covariates included in the model.
Results
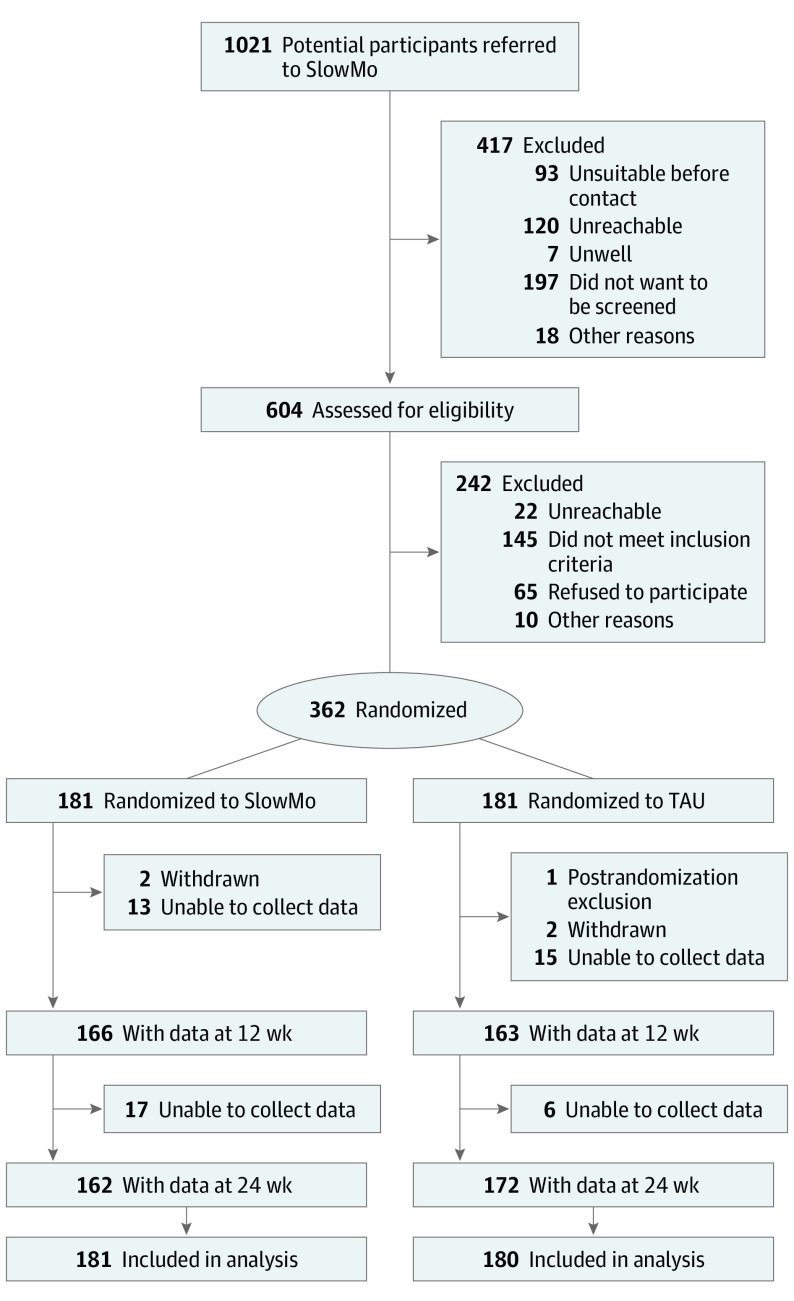
Of 604 people assessed for eligibility, 362 were recruited; 181 were randomized to the SlowMo group and 181 to the TAU group (Figure 1). One participant in the TAU group withdrew consent to use data after randomization. The final sample was therefore 361 participants.
Figure 1. Trial Profile.
TAU indicates treatment as usual.
Data on the primary outcome were available on 328 participants (90.6%) at 12 weeks and 332 (92.0%) at 24 weeks. Unmasking without replacement of an assessor occurred for 22 participants (6.7%) at 12 weeks and 19 participants (5.7%) at 24 weeks (eTable 1 in Supplement 2).
Participant baseline characteristics and stratification factors (paranoia severity and site) are shown in Table 1. Typical of samples with persisting psychosis, participants were predominantly male (252 [69.8%]) and White (249 [69.0%]), with a mean (SD) age of 42.6 (11.6) years. Other clinical characteristics (diagnosis, years in contact with services, and medication equivalent doses) were also unexceptional. There were no marked differences between the groups. Most participants had severe paranoia; 170 (94.4%) in the TAU arm and 169 (93.4%) in the SlowMo arm met criteria31 for likely presence of persecutory delusions on the GPTS (eTable 2 in Supplement 2). Baseline characteristics are also shown by site in eTable 3 in Supplement 2. eTable 4 in Supplement 2 shows baseline values of clinical and cognitive measures examined as putative moderators.
Table 1. Baseline Characteristics of the Intention-to-Treat Population.
| Characteristic | Study arma | ||
|---|---|---|---|
| SlowMo (n = 181) | TAU (n = 180) | Overall (N = 361) | |
| Age, mean (SD), y | 43.1 (11.7) | 42.2 (11.6) | 42.6 (11.6) |
| Sex | |||
| Male | 132 (72.9) | 120 (66.7) | 252 (69.8) |
| Female | 49 (27.1) | 60 (33.3) | 109 (30.2) |
| Marital status | |||
| Single | 145 (80.1) | 137 (76.1) | 282 (78.1) |
| Cohabiting | 6 (3.3) | 6 (3.3) | 12 (3.3) |
| Married or civil partnership | 22 (12.2) | 24 (13.3) | 46 (12.7) |
| Divorced | 7 (3.9) | 10 (5.6) | 17 (4.7) |
| Widowed | 1 (0.6) | 3 (1.7) | 4 (1.1) |
| Self-defined race/ethnicity | |||
| White | 120 (66.3) | 129 (71.7) | 249 (69.0) |
| Black Caribbean | 9 (5.0) | 9 (5.0) | 18 (5.0) |
| Black African | 12 (6.6) | 10 (5.6) | 22 (6.1) |
| Black other | 16 (8.8) | 12 (6.7) | 28 (7.8) |
| Indian | 0 | 3 (1.7) | 3 (0.8) |
| Pakistani | 4 (2.2) | 4 (2.2) | 8 (2.2) |
| Chinese | 1 (0.6) | 0 | 1 (0.3) |
| Other | 19 (10.5) | 12 (6.7) | 31 (8.6) |
| Missing | 0 | 1 (0.6) | 1 (0.3) |
| Highest level of schooling | |||
| Primary school | 4 (2.2) | 3 (1.7) | 7 (1.9) |
| Secondary, no examinations or qualifications | 30 (16.6) | 34 (18.9) | 64 (17.7) |
| Secondary O/CSE equivalent | 50 (27.6) | 51 (28.3) | 101 (28.0) |
| Secondary A-level equivalent | 23 (12.7) | 16 (8.9) | 39 (10.8) |
| Vocational education or college | 43 (23.8) | 44 (24.4) | 87 (24.1) |
| University degree or professional qualification | 31 (17.1) | 30 (16.7) | 61 (16.9) |
| Missing | 0 | 2 (1.1) | 2 (0.6) |
| Current working status | |||
| Unemployed | 141 (77.9) | 150 (83.3) | 291 (80.6) |
| Employed | |||
| Full-time | 8 (4.4) | 8 (4.4) | 16 (4.4) |
| Part-time | 15 (8.3) | 14 (7.8) | 29 (8.0) |
| Self-employed | 4 (2.2) | 2 (1.1) | 6 (1.7) |
| Retired | 10 (5.5) | 2 (1.1) | 12 (3.3) |
| Student | 1 (0.6) | 3 (1.7) | 4 (1.1) |
| Housewife or househusband | 2 (1.1) | 1 (0.6) | 3 (0.8) |
| Normal living situation | |||
| Alone | 108 (59.7) | 103 (57.2) | 211 (58.4) |
| With partner | 19 (10.5) | 28 (15.6) | 47 (13.0) |
| With parents | 25 (13.8) | 30 (16.7) | 55 (15.2) |
| With other relatives | 4 (2.2) | 4 (2.2) | 8 (2.2) |
| With others | 25 (13.8) | 15 (8.3) | 40 (11.1) |
| Site | |||
| London | 66 (36.5) | 64 (35.6) | 130 (36.0) |
| Oxford | 49 (27.1) | 50 (27.8) | 99 (27.4) |
| Sussex | 66 (36.5) | 66 (36.7) | 132 (36.6) |
| GPTS Part B score (stratification factor) | |||
| <62 | 110 (60.8) | 109 (60.6) | 219 (60.7) |
| ≥62 | 71 (39.2) | 71 (39.4) | 142 (39.3) |
| Diagnosis | |||
| Schizophrenia | 116 (64.1) | 109 (60.6) | 225 (62.3) |
| Schizoaffective disorder | 30 (16.6) | 34 (18.9) | 64 (17.7) |
| Delusional disorder | 3 (1.7) | 3 (1.7) | 6 (1.7) |
| Psychosis (other) | 32 (17.7) | 34 (18.9) | 66 (18.3) |
| Time in contact with services, y | |||
| <1 | 7 (3.9) | 6 (3.3) | 13 (3.6) |
| 1-5 | 22 (12.2) | 33 (18.3) | 55 (15.2) |
| 6-10 | 40 (22.1) | 44 (24.4) | 84 (23.3) |
| 11-20 | 70 (38.7) | 70 (38.9) | 140 (38.8) |
| >20 | 42 (23.2) | 27 (15.0) | 69 (19.1) |
| Chlorpromazine-equivalent dose of antipsychotic drug, mean (SD), mg/d | 452.96 (399.45) | 519.97 (419.80) | 486.37 (410.53) |
Abbreviations: GPTS, Green et al Paranoid Thoughts Scale; O/CSE, O-level/Certificate of Secondary Education; TAU, treatment as usual.
Unless otherwise indicated, data are expressed as number (percentage) of patients.
The mean (SD) number of SlowMo sessions attended was 6.8 (2.6), increasing to 7.3 (1.9) for those attending 1 or more sessions. Among the 181 participants in the SlowMo arm, 145 (80.1%) completed all 8 therapy sessions, 13 (7.2%) attended no sessions, and 23 (12.7%) discontinued therapy between sessions 1 and 7. Mean (SD) session duration, including behavioral work, was 75 (29) minutes. Therapy fidelity was high; of the 168 individuals who attended at least 1 session, 159 (94.6%) met a priori criteria for web app delivery, and 100 of 140 (71.4%) met adherence criteria for mobile app use.
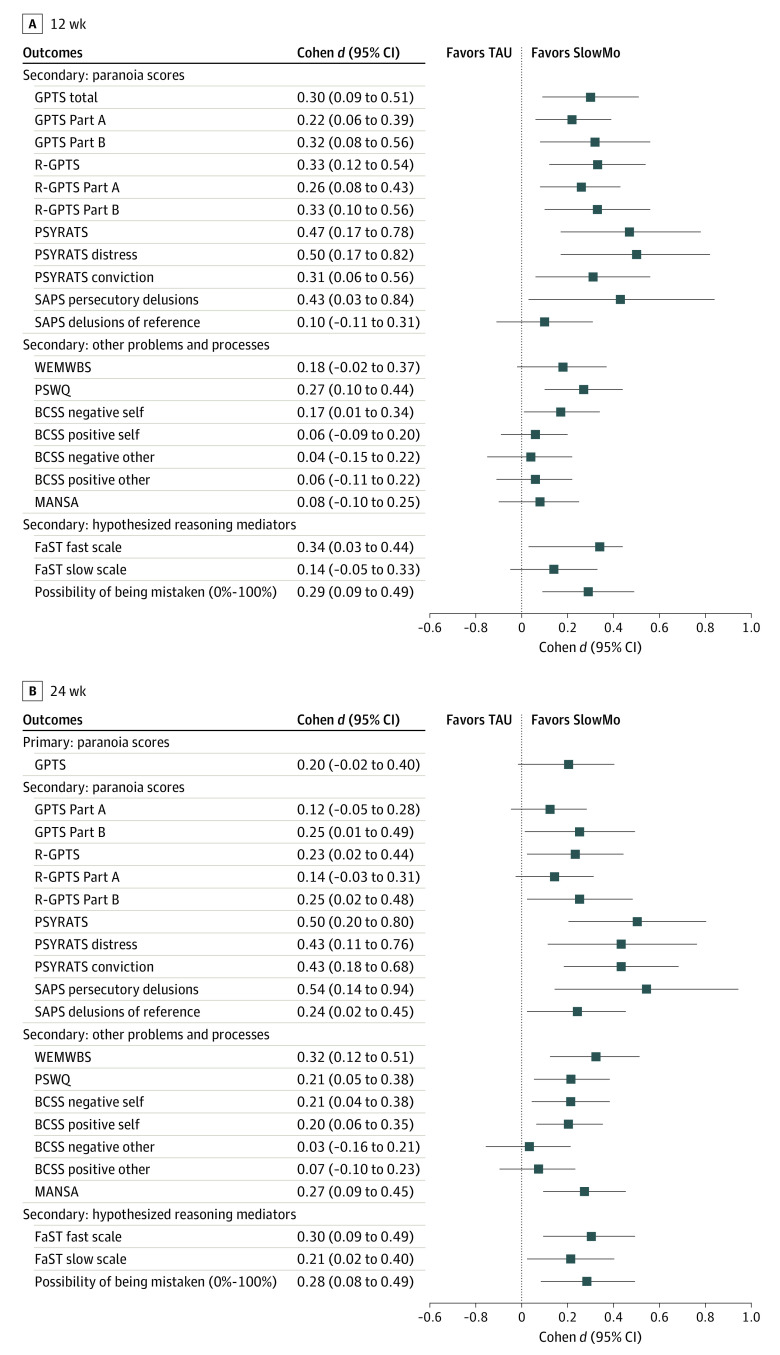
Descriptive statistics, between-group mean differences and their associated P values and 95% CIs, and standardized effect sizes for all primary and secondary paranoia outcomes are given in Table 2. Figure 2 shows standardized effect sizes for continuous variables as a forest plot (eFigure 2 in Supplement 2 gives a forest plot of binary secondary outcomes). SlowMo plus TAU was not associated with greater reductions than TAU alone in the primary outcome of GPTS total paranoia score at 24 weeks (Cohen d, 0.20; 95% CI, −0.02 to 0.40; P = .06). At 12 weeks, SlowMo plus TAU was associated with greater reductions than TAU alone in GPTS total score (Cohen d, 0.30; 95% CI, 0.09-0.51; P = .005), Part A score (Cohen d, 0.22; 95% CI, 0.06-0.39; P = .009), and Part B score (Cohen d, 0.32; 95% CI, 0.08-0.56; P = .009). At 24 weeks, SlowMo was significantly associated with lower Part B score (Cohen d, 0.25; 95% CI, 0.01-0.49; P = .04) but not Part A score (Cohen d, 0.12; 95% CI, −0.05 to 0.28; P = .18).
Table 2. Primary and Secondary Paranoia Outcomes.
| Outcome | SlowMo groupa | TAU groupa | Adjusted mean difference (SE) [95% CI] | P value | Cohen d effect size (95% CI)b | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | No. of participants | Mean (SD) | No. of participants | ||||
| Primary | |||||||
| GPTS total score | |||||||
| Baseline | 104.7 (27.6) | 180 | 105.9 (26.0) | 179 | NA | NA | NA |
| 24 wk | 81.7 (31.6) | 161 | 86.3 (33.2) | 171 | −5.27 (2.84) [−10.83 to 0.29] | .06 | 0.20 (−0.02 to 0.40) |
| Secondary | |||||||
| GPTS total score | |||||||
| Baseline | 104.7 (27.6) | 180 | 105.9 (26.0) | 179 | NA | NA | NA |
| 12 wk | 84.8 (30.8) | 165 | 92.5 (33.1) | 163 | −8.06 (2.85) [−13.64 to −2.48] | .005 | 0.30 (0.09 to 0.51) |
| GPTS Part A score | |||||||
| Baseline | 48.6 (15.9) | 181 | 50.3 (15.1) | 179 | NA | NA | NA |
| 12 wk | 40.2 (14.9) | 165 | 44.2 (15.8) | 163 | −3.49 (1.34) [−6.12 to −0.86] | .009 | 0.22 (0.06 to 0.39) |
| 24 wk | 39.2 (15.0) | 161 | 42.0 (15.8) | 172 | −1.79 (1.34) [−4.41 to −0.83] | .180 | 0.12 (−0.05 to 0.28) |
| GPTS Part B score | |||||||
| Baseline | 56.2 (14.4) | 180 | 55.9 (13.8) | 180 | NA | NA | NA |
| 12 wk | 44.6 (18.1) | 166 | 48.2 (18.7) | 163 | −4.51 (1.71) [−7.87 to −1.15] | .009 | 0.32 (0.08 to 0.56) |
| 24 wk | 42.2 (18.2) | 161 | 45.1 (18.9) | 171 | −3.53 (1.71) [−6.89 to −0.18] | .04 | 0.25 (0.01 to 0.49) |
| R-GPTS total score | |||||||
| Baseline | 40.7 (15.4) | 179 | 41.5 (14.8) | 180 | NA | NA | NA |
| 12 wk | 29.6 (17.2) | 166 | 34.3 (18.6) | 163 | −5.00 (1.61) [−8.16 to −1.86] | .002 | 0.33 (0.12 to 0.54) |
| 24 wk | 27.5 (17.6) | 160 | 31.1 (18.6) | 169 | −3.42 (1.61) [−6.57 to −0.27] | .03 | 0.23 (0.02 to 0.44) |
| R-GPTS social reference score | |||||||
| Baseline | 16.0 (7.8) | 180 | 17.1 (8.2) | 180 | NA | NA | NA |
| 12 wk | 11.9 (7.6) | 166 | 14.2 (8.1) | 163 | −2.05 (0.70) [−3.42 to −0.68] | .003 | 0.26 (0.08 to 0.43) |
| 24 wk | 11.3 (7.6) | 160 | 13.1 (8.0) | 172 | −1.15 (0.70) [−2.51 to 0.22] | .099 | 0.14 (−0.03 to 0.31) |
| R-GPTS persecution score | |||||||
| Baseline | 24.7 (9.3) | 180 | 24.4 (8.7) | 180 | NA | NA | NA |
| 12 wk | 17.7 (11.1) | 166 | 20.1 (11.7) | 163 | −2.97 (1.07) [−5.07 to −0.88] | .005 | 0.33 (0.10 to 0.56) |
| 24 wk | 16.3 (11.2) | 161 | 18.0 (11.6) | 169 | −2.25 (1.07) [−4.34 to −0.16] | .04 | 0.25 (0.02 to 0.48) |
| PSYRATS total score | |||||||
| Baseline | 16.5 (3.3) | 180 | 16.2 (3.1) | 180 | NA | NA | NA |
| 12 wk | 13.2 (4.9) | 166 | 14.5 (5.0) | 162 | −1.53 (0.50) [−2.50 to −0.56] | .002 | 0.47 (0.17 to 0.78) |
| 24 wk | 12.5 (5.2) | 161 | 14.0 (5.5) | 171 | −1.62 (0.49) [−2.59 to −0.65] | .001 | 0.50 (0.20 to 0.80) |
| PSYRATS distress score | |||||||
| Baseline | 8.1 (1.8) | 181 | 7.9 (1.7) | 180 | NA | NA | NA |
| 12 wk | 6.3 (2.8) | 166 | 7.0 (2.8) | 162 | −0.87 (0.29) [−1.44 to −0.30] | .003 | 0.50 (0.17 to 0.82) |
| 24 wk | 6.0 (3.0) | 161 | 6.8 (3.0) | 171 | −0.76 (0.29) [−1.32 to −0.19] | .009 | 0.43 (0.11 to 0.76) |
| PSYRATS conviction score | |||||||
| Baseline | 8.4 (2.0) | 180 | 8.3 (1.9) | 180 | NA | NA | NA |
| 12 wk | 6.9 (2.5) | 166 | 7.4 (2.6) | 163 | −0.62 (0.25) [−1.11 to −0.13] | .01 | 0.31 (0.06 to 0.56) |
| 24 wk | 6.4 (2.5) | 161 | 7.2 (2.8) | 172 | −0.84 (0.25) [−1.33 to −0.35] | .001 | 0.43 (0.18 to 0.68) |
| SAPS persecutory delusions score | |||||||
| Baseline | 3.5 (0.8) | 181 | 3.4 (0.9) | 180 | NA | NA | NA |
| 12 wk | 2.8 (1.3) | 164 | 3.0 (1.3) | 161 | −0.37 (0.18) [−0.71 to −0.03] | .04 | 0.43 (0.03 to 0.84) |
| 24 wk | 2.5 (1.5) | 161 | 2.8 (1.4) | 171 | −0.46 (0.18) [−0.80 to −0.12] | .009 | 0.54 (0.14 to 0.94) |
| SAPS ideas and delusions of reference score | |||||||
| Baseline | 2.5 (1.8) | 181 | 2.7 (1.7) | 180 | NA | NA | NA |
| 12 wk | 2.2 (1.9) | 165 | 2.4 (1.8) | 161 | −0.18 (0.19) [−0.55 to 0.19] | .35 | 0.10 (−0.11 to 0.31) |
| 24 wk | 1.9 (1.9) | 160 | 2.4 (1.9) | 171 | −0.41 (0.19) [−0.79 to −0.04] | .03 | 0.24 (0.02 to 0.45) |
Abbreviations: GPTS, Green et al Paranoid Thoughts Scale; NA, not applicable; PSYRATS, Psychotic Symptom Rating Scales; R-GPTS, Revised GPTS; SAPS, Scale for the Assessment of Positive Symptoms; TAU, treatment as usual.
Low score indicates better outcomes.
Negative effects indicate benefit of SlowMo compared with TAU.
Figure 2. Primary and Secondary Outcomes of SlowMo.
Markers represent point estimates, with horizontal lines representing 95% CIs. BCSS indicates Brief Core Schema Scales; FaST, Fast and Slow Thinking Questionnaire; GPTS, Green et al Paranoid Thoughts Scales; MANSA, Manchester Short Assessment of Quality of Life; PSWQ, Penn State Worry Questionnaire; PSYRATS, Psychotic Symptom Rating Scales; R-GPTS, Revised GPTS; SAPS, Scale for the Assessment of Positive Symptoms; TAU, treatment as usual; and WEMWBS, Warwick-Edinburgh Mental Well-being Scale.
SlowMo was also associated with improvements in observer-rated measures of persecutory delusions, including PSYRATS Delusions subscale score at 12 (Cohen d, 0.47; 95% CI, 0.17-0.78; P = .002) and 24 (Cohen d, 0.50; 95% CI, 0.20-0.80; P = .001) weeks and SAPS Persecutory Delusions subscale score at 12 (Cohen d, 0.43; 95% CI, 0.03-0.84; P = .04) and 24 (Cohen d, 0.54; 95% CI, 0.14-0.94; P = .009) weeks. The reduction in paranoia on the R-GPTS total score was significant at both points (Cohen d at 12 weeks, 0.33 [95% CI, 0.12-0.54; P = .002]; Cohen d at 24 weeks, 0.23 [95% CI, 0.02-0.44; P = .03]), as were the reductions on the PSYRATS distress (Cohen d at 12 weeks, 0.50 [95% CI, 0.17-0.82; P = .003]; Cohen d at 24 weeks, 0.43 [95% CI, 0.11-0.76; P = .009]) and conviction (Cohen d at 12 weeks, 0.31 [95% CI, 0.06-0.56; P = .01]; Cohen d at 24 weeks, 0.43 [95% CI, 0.18-0.68; P = .001]) subscale scores. Referential ideas assessed using GPTS Part A, R-GPTS Reference, and SAPS Ideas of Reference subscale scores showed less consistent effects, with significant effects either at 12 weeks or 24 weeks but not both.
Treatment effects were found for some but not all reasoning measures. Belief flexibility (possibility of being mistaken) improved at 12 (Cohen d, 0.29; 95% CI, 0.09-0.49; P = .004) and 24 (Cohen d, 0.28; 95% CI, 0.08-0.49; P = .005) weeks but alternative explanations did not. The binary measure of the possibility of being mistaken was significant at 12 but not 24 weeks. Jumping to conclusions did not improve (with 1 significant finding, beads drawn at 12 weeks). The fast-thinking scale of the Fast and Slow Thinking Questionnaire showed improvements at both time points, and the slow-thinking scale showed improvement at 24 weeks. Significant improvements were found for SlowMo in well-being (Cohen d at 24 weeks, 0.32; 95% CI, 0.12-0.51; P = .001), quality of life (Cohen d at 24 weeks, 0.27; 95% CI, 0.09-0.45; P = .003), worry (Cohen d at 12 weeks, 0.27 [95% CI, 0.10-0.44; P = .002]; Cohen d at 24 weeks, 0.21 [95% CI, 0.05-0.38; P = .01]), and self-concept (Cohen d for negative self at 12 weeks, 0.17 [95% CI, 0.01-0.34; P = .04]; Cohen d for negative self at 24 weeks, 0.21 [95% CI, 0.04-0.38; P = .01]; Cohen d for positive self at 24 weeks, 0.20 [95% CI, 0.06-0.35; P = .006]) (Table 3 and Figure 2).
Table 3. Secondary Outcomes and Hypothesized Mediators.
| Outcome | SlowMo groupa | TAU groupa | Adjusted mean difference (SE) [95% CI]b | P value | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Participants, No, | Mean (SD) | Participants, No. | |||
| WEMWBS scorec | ||||||
| Baseline | 39.3 (9.1) | 179 | 40.5 (8.7) | 175 | NA | NA |
| 12 wk | 42.2 (9.4) | 164 | 41.6 (9.1) | 157 | 1.56 (0.89) [−0.18 to 3.30] | .08 |
| 24 wk | 43.3 (11.0) | 157 | 41.2 (9.6) | 165 | 2.82 (0.89) [1.08 to 4.56] | .001 |
| PSWQ score | ||||||
| Baseline | 56.9 (10.8) | 179 | 56.6 (10.1) | 175 | NA | NA |
| 12 wk | 53.2 (11.6) | 158 | 55.4 (11.5) | 157 | −2.81 (0.90) [−4.57 to −1.04] | .002 |
| 24 wk | 52.2 (11.6) | 154 | 54.5 (11.5) | 163 | −2.24 (0.90) [−4.00 to −0.48] | .01 |
| BCSS negative self score | ||||||
| Baseline | 9.9 (5.8) | 181 | 10.3 (5.5) | 178 | NA | NA |
| 12 wk | 9.0 (6.0) | 162 | 10.0 (6.0) | 159 | −0.98 (0.48) [−1.92 to −0.04] | .04 |
| 24 wk | 8.4 (5.9) | 160 | 9.7 (5.8) | 167 | −1.19 (0.48) [−2.12 to −0.25] | .01 |
| BCSS positive self scorec | ||||||
| Baseline | 10.7 (5.6) | 181 | 10.8 (5.4) | 178 | NA | NA |
| 12 wk | 11.5 (5.6) | 164 | 11.5 (5.6) | 159 | 0.33 (0.41) [−0.48 to 1.13] | .43 |
| 24 wk | 12.5 (5.5) | 159 | 11.6 (5.8) | 168 | 1.11 (0.41) [0.31 to 1.92] | .006 |
| BCSS negative other score | ||||||
| Baseline | 13.3 (6.1) | 181 | 13.3 (5.8) | 178 | NA | NA |
| 12 wk | 12.9 (6.1) | 163 | 13.0 (6.0) | 159 | −0.21 (0.55) [−1.30 to 0.88] | .70 |
| 24 wk | 12.6 (6.2) | 159 | 12.7 (6.3) | 168 | −0.16 (0.55) [−1.25 to 0.92] | .77 |
| BCSS positive other scorec | ||||||
| Baseline | 11.6 (5.2) | 180 | 11.1 (4.9) | 177 | NA | NA |
| 12 wk | 12.2 (5.1) | 164 | 11.8 (4.8) | 159 | 0.28 (0.42) [−0.55 to 1.12] | .50 |
| 24 wk | 12.4 (4.8) | 158 | 12.1 (4.8) | 168 | 0.34 (0.42) [−0.49 to 1.17] | .42 |
| MANSA scorec | ||||||
| Baseline | 46.8 (9.9) | 161 | 48.1 (10.2) | 164 | NA | NA |
| 12 wk | 48.1 (10.7) | 145 | 48.9 (10.6) | 146 | 0.76 (0.91) [−1.02 to 2.55] | .40 |
| 24 wk | 50.5 (11.7) | 135 | 49.1 (9.5) | 148 | 2.75 (0.92) [0.94 to 4.55] | .003 |
| Possibility of being mistaken (0%-100%)c | ||||||
| Baseline | 34.6 (30.9) | 181 | 35.1 (31.0) | 180 | NA | NA |
| 12 wk | 48.9 (32.2) | 165 | 39.9 (33.2) | 161 | 9.02 (3.16) [2.83 to 15.21] | .004 |
| 24 wk | 45.3 (31.8) | 161 | 37.7 (31.1) | 172 | 8.88 (3.16) [2.70 to 15.07] | .005 |
| Possibility of being mistaken, No. yes/no (% yes/% no)c | ||||||
| Baseline | 105/76 (58/42) | NA | 106/74 (59/41) | NA | NA | NA |
| 12 wk | 124/41 (75/25) | NA | 100/61 (62/38) | NA | 3.83 (1.53 to 9.59)d | .004 |
| 24 wk | 105/56 (65/35) | NA | 100/72 (58/42) | NA | 2.01 (0.86 to 4.70)d | .11 |
| Alternative explanations, No. yes/no (% yes/% no)c | ||||||
| Baseline | 79/102 (44/56) | NA | 85/94 (48/52) | NA | NA | NA |
| 12 wk | 90/74 (55/45) | NA | 78/83 (48/52) | NA | 1.74 (0.90 to 3.36)d | .10 |
| 24 wk | 87/73 (54/46) | NA | 87/82 (52/48) | NA | 1.33 (0.70 to 2.55)d | .39 |
| JTC version 85:15, No. yes/no (% yes/% no) | ||||||
| Baseline | 103/77 (57/43) | NA | 83/96 (46/54) | NA | NA | NA |
| 12 wk | 70/95 (42/58) | NA | 68/91 (42/58) | NA | 0.71 (0.31 to 1.62)d | .42 |
| 24 wk | 55/105 (34/66) | NA | 62/107 (37/63) | NA | 0.58 (0.25 to 1.34)d | .20 |
| JTC version 85:15 (No. of beads) | ||||||
| Baseline | 3.8 (4.4) | NA | 3.9 (4.0) | NA | NA | NA |
| 12 wk | 4.3 (4.3) | NA | 4.1 (3.9) | NA | 0.39 (0.43) [−0.45 to 1.22] | .37 |
| 24 wk | 5.2 (4.8) | NA | 4.1 (3.3) | NA | 0.99 (0.42) [0.16 to 1.83] | .02 |
| JTC version 60:40 (yes or no) | ||||||
| Baseline | 72/108 (40/60) | NA | 59/120 (33/67) | NA | NA | NA |
| 12 wk | 47/118 (29/71) | NA | 42/117 (26/74) | NA | 0.82 (0.26 to 2.51)d | .72 |
| 24 wk | 43/117 (27/73) | NA | 44/125 (26/74) | NA | 0.69 (0.22 to 2.18)d | .53 |
| JTC version 60:40 (No. of beads) | ||||||
| Baseline | 5.7 (5.4) | NA | 5.7 (5.1) | NA | NA | NA |
| 12 wk | 7.0 (5.7) | NA | 6.8 (5.4) | NA | 0.28 (0.49) [−0.68 to 1.25] | .56 |
| 24 wk | 7.0 (5.2) | 6.5 (4.9) | 0.49 (0.49) [−0.47 to 1.45] | .32 | ||
| FaST fast scale score | ||||||
| Baseline | 16.9 (4.7) | 174 | 16.7 (4.3) | 169 | NA | NA |
| 12 wk | 15.3 (4.9) | 165 | 16.2 (5.0) | 160 | −1.07 (0.47) [−1.98 to −0.16] | .02 |
| 24 wk | 15.0 (4.4) | 160 | 16.2 (5.1) | 168 | −1.33 (0.46) [−2.23 to −0.42] | .004 |
| FaST slow scale scorec | ||||||
| Baseline | 19.9 (4.7) | 174 | 19.7 (4.8) | 169 | NA | NA |
| 12 wk | 20.3 (4.8) | 165 | 19.3 (4.8) | 160 | 0.66 (0.45) [−0.22 to 1.55] | .14 |
| 24 wk | 20.3 (4.4) | 160 | 19.3 (4.8) | 168 | 1.00 (0.45) [0.12 to 1.88] | .03 |
Abbreviations: BCSS, Brief Core Schema Scale; FaST, Fast and Slow Thinking Questionnaire; JTC, jumping to conclusions; MANSA, Manchester Short Assessment of Quality of Life; NA, not applicable; PSWQ, Penn State Worry Questionnaire; TAU, treatment as usual; WEMWBS, Warwick-Edinburgh Mental Well-being Scale.
Unless otherwise indicated, low score indicates better outcomes.
Unless otherwise indicated, negative effects indicate benefit of SlowMo compared with TAU.
High score indicates better outcomes; positive effects indicate benefit of SlowMo compared with TAU.
Odds ratio (95% CI).
The moderation analysis (eTable 5 in Supplement 2) revealed no differential effects on paranoia as measured by the GPTS or R-GPTS.31 There were 2 moderation effects (on PSYRATS), at P < .05. However, given the number of tests, this finding may have occurred by chance.
The mediation analysis results on the GPTS, R-GPTS, and PSYRATS at 12 and 24 weeks are shown in eTables 6 to 8 in Supplement 2. The possibility of being mistaken (0%-100%)32 and worry38 mediated the treatment effects on all paranoia outcomes at 12 and 24 weeks. Approximately 40% of the total effect was mediated through each mediator at 12 weeks and 56% at 24 weeks.
Fifty-four adverse events were reported, of which 51 were serious, occurring in 19 participants in the SlowMo group and 21 in the TAU group; no deaths were recorded (eMethods 7 and eTable 9 in Supplement 2). A compliance-adjusted analysis showed significant treatment effects of SlowMo therapy on the primary outcome compared with TAU in those adherent to treatment at all points, with treatment effects increasing as the number of sessions increased (eTable 10 in Supplement 2). Data on concomitant treatments and service use are shown in eMethods 9 and eTables 11 and 12, respectively, in Supplement 2.
Discussion
Treatment with SlowMo, a brief blended digital therapy, did not result in significant improvements in the primary outcome of total GPTS paranoia score at 24 weeks. However, the pattern of results indicates that SlowMo had a beneficial effect on paranoia in general. Effects on total self-rated GPTS paranoia after treatment and on self-rated GPTS persecution during the 24-week study and significant sustained moderate effects on all observer-rated measures of persecutory delusions were seen. SlowMo treatment was associated with improvements in reasoning, in belief flexibility (possibility of being mistaken), and Fast and Slow Thinking Questionnaire scores. Change in both belief flexibility and worry mediated improvements in paranoia. There were effects on outcomes prioritized at the design stage by service-user consultants41 in well-being, self-esteem, quality of life, and worry, with the most consistent change at 24 weeks. Therapy uptake and adherence were high. Treatment effects were not moderated by clinical or demographic variables, indicating benefits regardless of cognitive capacity, symptoms, or caregiver relationships. There was no evidence of the intervention being harmful, with similar numbers of serious adverse events in both groups.
Although GPTS effects were small, most met the threshold of P < .05, suggesting consistent effects on secondary self-reported paranoia outcomes. Of note, the effects for the observer-rated and widely used PSYRATS total score were in the moderate range. Although adjustment of type I error in the reporting of secondary outcomes in clinical trials is not mandated,42 this improvement (at P < .001) would remain significant at 24 weeks even if a conservative adjustment for multiple testing were to be applied. In addition to reduced PSYRATS conviction scores, the clinically important target of distress also showed a sustained reduction. Taken together, the secondary paranoia outcomes indicate small to moderate effects that were equal to or greater than rates reported in meta-analyses of longer-term CBTp for delusions.2,43 However, given this overall pattern of results, the absence of an effect on the primary outcome of GPTS total score at 24 weeks and the failure to reach the a priori threshold for clinical importance merit further consideration. Examination of the results and the GPTS subscales constituting the total score indicates that persecutory beliefs showed stronger effects across a range of measures, whereas milder referential ideas (self or observer rated) did not show consistent improvement. One potential explanation may be that as persecutory beliefs improved, they changed into milder ideas of reference (thus shifting down the hierarchy of paranoid beliefs44,45), but that the therapy prevented such ideas and their experiential components from being elaborated into paranoid fears of intentional harm. Given this finding, we believe that future iterations of SlowMo therapy should enhance work on referential ideas.
High uptake, fidelity, and adherence and the absence of moderation by baseline characteristics suggest that the inclusive, human-centered design facilitated engagement across a wide range of users and settings, which is crucial to real-world implementation.15,16 Usability and acceptability will be the subjects of future studies. Barriers to accessing psychological therapy for paranoia are widely reported,46 with effective, usable brief treatments such as SlowMo offering a potential solution.
A second goal was to evaluate reasoning as a mechanism. Improvements were observed in belief flexibility. Consistent with a proof-of-concept study,9 the possibility of being mistaken mediated the change in paranoia, explaining 36% to 56% of the variance after the intervention and at follow-up. In contrast, JTC showed little evidence of change. This finding, together with meta-analytic results,47 suggests that JTC may be associated with vulnerability to persecutory beliefs but be relatively unresponsive to change over time. This evolving understanding supports foregrounding the promotion of slow thinking and greater flexibility with the aim of generating compensatory strategies for real-world fast thinking.8
Worry also mediated paranoia reduction, with a similar proportion of the variance explained by the mediation by belief flexibility. This was not hypothesized because worry was not explicitly targeted in SlowMo. However, given that worry causes paranoia21 and that SlowMo altered worry, the finding suggests that worry reduction plausibly constitutes part of the treatment route for SlowMo. Of note, SlowMo shares features with worry interventions.21 Both involve noticing thoughts, decentering, and refocusing attention. Furthermore, the extent to which worry and belief flexibility are independent routes to change or whether other mechanisms for treatment effects, such as the parallel improvements in self-concept and well-being, might occur could not be determined in the present study. Our original hypotheses derived from a theory of change in which the primary process underpinning SlowMo was via reasoning. However, the evidence from this study suggests the potential for other processes also to be involved in treatment effects. Our cognitive model of paranoia proposes multifactorial causality, particularly highlighting both reasoning and emotional processes.48 We plan additional investigations of these and other potential mechanisms to inform further causal understanding of paranoia.
Limitations
This study has limitations. The trial design did not control for effects of time with a therapist, with TAU being selected as the comparator condition. There is a low penetration of evidence-based psychological treatment in clinical services,49 and thus a key efficacy question is whether SlowMo therapy confers benefits beyond those of TAU. Garety et al9 previously established the superiority of an earlier brief version of the intervention against an active control intervention. Examination of mechanisms of change also required a control condition as much as possible inert with respect to reasoning. A further limitation is that our primary outcome, the GPTS score, uses self-report and was revised during the trial.31 However, the more psychometrically robust revision31 yielded similar results but with slightly larger effects. In addition, the use of blinded observer-rated measures of delusions (yielding moderate effect sizes) was consistent with improvement in clinically severe paranoia. Furthermore, we did not assess functioning; however, we did measure quality of life36 and well-being,35 indicating improvements in satisfaction with a range of domains of everyday life and function.
Conclusions
This is the first randomized clinical trial, to our knowledge, to test a blended digital therapy for paranoia in people with psychosis. Although no effect was demonstrated on the primary paranoia outcome at 24 weeks, the pattern of results on secondary outcomes indicates SlowMo had a positive effect on paranoia, mostly sustained at follow-up, that matched or exceeded effects observed for standard CBTp albeit delivered in fewer sessions.50 Improvements in well-being, quality of life, and self-concept also occurred. The results indicate that the treatment was effective, in part, through helping people to slow down their thinking and to worry less. Further understanding of the mechanisms of action of SlowMo is warranted. The trial results also indicate the need for future work to enhance and translate the effects of SlowMo.
Trial Protocol
eMethods 1. SlowMo Therapy Description
eFigure 1. SlowMo Webapp Formulation
eMethods 2. SlowMo Mobile App Adherence Criterion
eMethods 3. Data Quality and Interrater Reliability
eMethods 4. Data Completeness and Timing of Assessments
eTable 1. Unblinding by Site
eTable 2. Number (Percentage) Above Threshold for a Potential Persecutory Delusion
eFigure 2. Forest Plot Binary Outcomes
eTable 3. Baseline Characteristics by Site
eMethods 5. Moderation Analysis: Further Detail
eTable 4. Baseline Clinical and Cognitive Characteristics as Potential Moderators of the Intention-to-Treat Population
eTable 5. Moderation Analysis Results
eMethods 6. Mediation Analysis: Further Detail
eTable 6. Mediation Effects of SlowMo on GPTS: Mediator Variables at 12 Weeks and GPTS at 12 and 24 Weeks
eTable 7. Mediation Effects of SlowMo: Mediator Variables at 12 Weeks and PSYRATS at 12 and 24 Weeks
eTable 8. Mediation Effects of SlowMo: Mediator Variables at 12 Weeks and R-GPTS at 12 and 24 Weeks
eMethods 7. Adverse Events
eTable 9. Adverse Events and Trial-Related Adverse Events
eMethods 8. Compliance Analysis
eTable 10. Analysis of Treatment Received
eMethods 9. Concomitant Therapy and Medication and Service Use Data
eTable 11. Concomitant Therapy and Medication
eTable 12. Service Use Over Preceding 6 Months at Baseline and Follow-up
References.
Data Sharing Statement
References
- 1.Schizophrenia Commission . The Abandoned Illness: A Report From the Schizophrenia Commission. Rethink Mental Illness; November 2012. [Google Scholar]
- 2.Turner DT, Burger S, Smit F, Valmaggia LR, van der Gaag M. What constitutes sufficient evidence for case formulation–driven CBT for psychosis? cumulative meta-analysis of the effect on hallucinations and delusions. Schizophr Bull. 2020;46(5):1072-1085. doi: 10.1093/schbul/sbaa045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bighelli I, Salanti G, Huhn M, et al. Psychological interventions to reduce positive symptoms in schizophrenia: systematic review and network meta-analysis. World Psychiatry. 2018;17(3):316-329. doi: 10.1002/wps.20577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman D, Dunn G, Garety P, et al. Patients’ beliefs about the causes, persistence and control of psychotic experiences predict take-up of effective cognitive behaviour therapy for psychosis. Psychol Med. 2013;43(2):269-277. doi: 10.1017/S0033291712001225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendler KS, Campbell J. Interventionist causal models in psychiatry: repositioning the mind-body problem. Psychol Med. 2009;39(6):881-887. doi: 10.1017/S0033291708004467 [DOI] [PubMed] [Google Scholar]
- 6.Garety PA, Freeman D. The past and future of delusions research: from the inexplicable to the treatable. Br J Psychiatry. 2013;203(5):327-333. doi: 10.1192/bjp.bp.113.126953 [DOI] [PubMed] [Google Scholar]
- 7.Garety PA, Freeman D, Jolley S, et al. Reasoning, emotions, and delusional conviction in psychosis. J Abnorm Psychol. 2005;114(3):373-384. doi: 10.1037/0021-843X.114.3.373 [DOI] [PubMed] [Google Scholar]
- 8.Ward T, Garety PA. Fast and slow thinking in distressing delusions: a review of the literature and implications for targeted therapy. Schizophr Res. 2019;203:80-87. doi: 10.1016/j.schres.2017.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garety P, Waller H, Emsley R, et al. Cognitive mechanisms of change in delusions: an experimental investigation targeting reasoning to effect change in paranoia. Schizophr Bull. 2015;41(2):400-410. doi: 10.1093/schbul/sbu103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross K, Freeman D, Dunn G, Garety P. A randomized experimental investigation of reasoning training for people with delusions. Schizophr Bull. 2011;37(2):324-333. doi: 10.1093/schbul/sbn165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waller H, Freeman D, Jolley S, Dunn G, Garety P. Targeting reasoning biases in delusions: a pilot study of the Maudsley Review Training Programme for individuals with persistent, high conviction delusions. J Behav Ther Exp Psychiatry. 2011;42(3):414-421. doi: 10.1016/j.jbtep.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waller H, Emsley R, Freeman D, et al. Thinking well: a randomised controlled feasibility study of a new CBT therapy targeting reasoning biases in people with distressing persecutory delusional beliefs. J Behav Ther Exp Psychiatry. 2015;48:82-89. doi: 10.1016/j.jbtep.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahneman D. Thinking, Fast and Slow. Farrar, Straus and Giroux; 2011. [Google Scholar]
- 14.Hardy A, Wojdecka A, West J, et al. How inclusive, user-centered design research can improve psychological therapies for psychosis: development of SlowMo. JMIR Ment Health. 2018;5(4):e11222. doi: 10.2196/11222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham AK, Lattie EG, Mohr DC. Experimental therapeutics for digital mental health. JAMA Psychiatry. 2019;76(12):1223-1224. doi: 10.1001/jamapsychiatry.2019.2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohr DC, Riper H, Schueller SM. A solution-focused research approach to achieve an implementable revolution in digital mental health. JAMA Psychiatry. 2018;75(2):113-114. doi: 10.1001/jamapsychiatry.2017.3838 [DOI] [PubMed] [Google Scholar]
- 17.Ben-Zeev D, Brian RM, Jonathan G, et al. Mobile Health (mHealth) versus clinic-based group intervention for people with serious mental illness: a randomized controlled trial. Psychiatr Serv. 2018;69(9):978-985. doi: 10.1176/appi.ps.201800063 [DOI] [PubMed] [Google Scholar]
- 18.Bucci S, Barrowclough C, Ainsworth J, et al. Actissist: proof-of-concept trial of a theory-driven digital intervention for psychosis. Schizophr Bull. 2018;44(5):1070-1080. doi: 10.1093/schbul/sby032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Depp CA, Perivoliotis D, Holden J, Dorr J, Granholm EL. Single-session mobile-augmented intervention in serious mental illness: a three-arm randomized controlled trial. Schizophr Bull. 2019;45(4):752-762. doi: 10.1093/schbul/sby135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlosser DA, Campellone TR, Truong B, et al. Efficacy of PRIME, a mobile app intervention designed to improve motivation in young people with schizophrenia. Schizophr Bull. 2018;44(5):1010-1020. doi: 10.1093/schbul/sby078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman D, Dunn G, Startup H, et al. Effects of cognitive behaviour therapy for worry on persecutory delusions in patients with psychosis (WIT): a parallel, single-blind, randomised controlled trial with a mediation analysis. Lancet Psychiatry. 2015;2(4):305-313. doi: 10.1016/S2215-0366(15)00039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montgomery P, Grant S, Mayo-Wilson E, et al. ; CONSORT-SPI Group . Reporting randomised trials of social and psychological interventions: the CONSORT-SPI 2018 Extension. Trials. 2018;19(1):407. doi: 10.1186/s13063-018-2733-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization . SCAN Schedules for Clinical Assessment in Neuropsychiatry, Version 1.0. World Health Organization; 1992. [Google Scholar]
- 24.Green CE, Freeman D, Kuipers E, et al. Measuring ideas of persecution and social reference: the Green et al Paranoid Thought Scales (GPTS). Psychol Med. 2008;38(1):101-111. doi: 10.1017/S0033291707001638 [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. World Health Organization; 2010. [Google Scholar]
- 26.Garety PA, Ward T, Freeman D, et al. SlowMo, a digital therapy targeting reasoning in paranoia, versus treatment as usual in the treatment of people who fear harm from others: study protocol for a randomised controlled trial. Trials. 2017;18(1):510. doi: 10.1186/s13063-017-2242-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beecham J. Collecting and estimating costs. In: Knapp M, ed. The Economic Evaluation of Mental Health Care. Arena; 1995. [Google Scholar]
- 28.Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the Psychotic Symptom Rating Scales (PSYRATS). Psychol Med. 1999;29(4):879-889. doi: 10.1017/S0033291799008661 [DOI] [PubMed] [Google Scholar]
- 29.Steel C, Garety PA, Freeman D, et al. The multidimensional measurement of the positive symptoms of psychosis. Int J Methods Psychiatr Res. 2007;16(2):88-96. doi: 10.1002/mpr.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreasen NC. The Scale of the Assessment of Positive Symptoms (SAPS). University of Iowa; 1984. [Google Scholar]
- 31.Freeman D, Loe BS, Kingdon D, et al. The revised Green et al Paranoid Thoughts Scale (R-GPTS): psychometric properties, severity ranges, and clinical cut-offs. Psychol Med. 2019;51(2):244-253. doi: 10.1017/S0033291719003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wessely S, Buchanan A, Reed A, et al. Acting on delusions, I: prevalence. Br J Psychiatry. 1993;163(1):69-76. doi: 10.1192/bjp.163.1.69 [DOI] [PubMed] [Google Scholar]
- 33.Freeman D, Garety PA, Fowler D, Kuipers E, Bebbington PE, Dunn G. Why do people with delusions fail to choose more realistic explanations for their experiences? an empirical investigation. J Consult Clin Psychol. 2004;72(4):671-680. doi: 10.1037/0022-006X.72.4.671 [DOI] [PubMed] [Google Scholar]
- 34.Hardy A, Tolmeijer E, Edwards V, et al. Measuring reasoning in paranoia: development of the Fast and Slow Thinking Questionnaire. Schizophr Bull Open. 2020;1(1):sgaa035. doi: 10.1093/schizbullopen/sgaa035 [DOI] [Google Scholar]
- 35.Tennant R, Hiller L, Fishwick R, et al. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes. 2007;5(1):63. doi: 10.1186/1477-7525-5-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (MANSA). Int J Soc Psychiatry. 1999;45(1):7-12. doi: 10.1177/002076409904500102 [DOI] [PubMed] [Google Scholar]
- 37.Fowler D, Freeman D, Smith B, et al. The Brief Core Schema Scales (BCSS): psychometric properties and associations with paranoia and grandiosity in non-clinical and psychosis samples. Psychol Med. 2006;36(6):749-759. doi: 10.1017/S0033291706007355 [DOI] [PubMed] [Google Scholar]
- 38.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28(6):487-495. doi: 10.1016/0005-7967(90)90135-6 [DOI] [PubMed] [Google Scholar]
- 39.Batistatou E, Roberts C, Roberts S. Sample size and power calculations for trials and quasi-experimental studies with clustering. Stata J. 2014;14(1):159-175. doi: 10.1177/1536867X1401400111 [DOI] [Google Scholar]
- 40.Stata Statistical Software, Release 16. [computer program]. StataCorp LLC; 2019.
- 41.Lancet Psychiatry . Measuring success: the problem with primary outcomes. Lancet Psychiatry. 2020;7(1):1. doi: 10.1016/S2215-0366(19)30483-3 [DOI] [PubMed] [Google Scholar]
- 42.Li G, Taljaard M, Van den Heuvel ER, et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol. 2017;46(2):746-755. [DOI] [PubMed] [Google Scholar]
- 43.Mehl S, Werner D, Lincoln TM. Corrigendum: does cognitive behavior therapy for psychosis (CBTp) show a sustainable effect on delusions? a meta-analysis. Frontiers in Psychology. 1868;2019:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman D, Garety PA, Bebbington PE, et al. Psychological investigation of the structure of paranoia in a non-clinical population. Br J Psychiatry. 2005;186(5):427-435. doi: 10.1192/bjp.186.5.427 [DOI] [PubMed] [Google Scholar]
- 45.Freeman D. Suspicious minds: the psychology of persecutory delusions. Clin Psychol Rev. 2007;27(4):425-457. doi: 10.1016/j.cpr.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 46.van der Gaag M. The efficacy of CBT for severe mental illness and the challenge of dissemination in routine care. World Psychiatry. 2014;13(3):257-258. doi: 10.1002/wps.20162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudley R, Taylor P, Wickham S, Hutton P. Psychosis, delusions and the “jumping to conclusions” reasoning bias: a systematic review and meta-analysis. Schizophr Bull. 2016;42(3):652-665. doi: 10.1093/schbul/sbv150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE. A cognitive model of persecutory delusions. Br J Clin Psychol. 2002;41(pt 4):331-347. doi: 10.1348/014466502760387461 [DOI] [PubMed] [Google Scholar]
- 49.Haddock G, Eisner E, Boone C, Davies G, Coogan C, Barrowclough C. An investigation of the implementation of NICE-recommended CBT interventions for people with schizophrenia. J Ment Health. 2014;23(4):162-165. doi: 10.3109/09638237.2013.869571 [DOI] [PubMed] [Google Scholar]
- 50.van der Gaag M, Valmaggia LR, Smit F. The effects of individually tailored formulation-based cognitive behavioural therapy in auditory hallucinations and delusions: a meta-analysis. Schizophr Res. 2014;156(1):30-37. doi: 10.1016/j.schres.2014.03.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. SlowMo Therapy Description
eFigure 1. SlowMo Webapp Formulation
eMethods 2. SlowMo Mobile App Adherence Criterion
eMethods 3. Data Quality and Interrater Reliability
eMethods 4. Data Completeness and Timing of Assessments
eTable 1. Unblinding by Site
eTable 2. Number (Percentage) Above Threshold for a Potential Persecutory Delusion
eFigure 2. Forest Plot Binary Outcomes
eTable 3. Baseline Characteristics by Site
eMethods 5. Moderation Analysis: Further Detail
eTable 4. Baseline Clinical and Cognitive Characteristics as Potential Moderators of the Intention-to-Treat Population
eTable 5. Moderation Analysis Results
eMethods 6. Mediation Analysis: Further Detail
eTable 6. Mediation Effects of SlowMo on GPTS: Mediator Variables at 12 Weeks and GPTS at 12 and 24 Weeks
eTable 7. Mediation Effects of SlowMo: Mediator Variables at 12 Weeks and PSYRATS at 12 and 24 Weeks
eTable 8. Mediation Effects of SlowMo: Mediator Variables at 12 Weeks and R-GPTS at 12 and 24 Weeks
eMethods 7. Adverse Events
eTable 9. Adverse Events and Trial-Related Adverse Events
eMethods 8. Compliance Analysis
eTable 10. Analysis of Treatment Received
eMethods 9. Concomitant Therapy and Medication and Service Use Data
eTable 11. Concomitant Therapy and Medication
eTable 12. Service Use Over Preceding 6 Months at Baseline and Follow-up
References.
Data Sharing Statement