Abstract
Three-dimensional trans-thoracic echo (3DE)-derived heart models have not previously been utilized to guide catheter ablation. In this case report, we describe creation of a 3DE model from TTE, import of the model into CARTO3, and successful use of the model as a guide during mapping and ablation of a right lateral accessory pathway. We believe this technique represents a valuable alternative to integration of CT or MRI-derived anatomic data, and that it has potential to improve definition of the AV valve annuli during catheter ablation of accessory pathways.
Keywords: Catheter ablation, accessory pathway, Wolff-Parkinson-White, three-dimensional echocardiography, three-dimensional electroanatomic mapping
Introduction:
Anatomic data from CT and MRI can be imported into electroanatomic mapping (EAM) systems to guide catheter ablation. In the pediatric population, however, CT and MRI data are not practical to guide routine catheter ablation due to the radiation exposure of CT and the potential need for sedation in young patients undergoing MRI. In contrast, transthoracic echocardiography (TTE) is often a standard component of pre-procedural evaluation and has negligible risk; three-dimensional imaging can be acquired as part of routine TTE with minimal added time or cost. Three-dimensional TTE (3DE)-derived heart models are emerging as a powerful imaging tool for the planning of both surgical and transcatheter interventions.1–2 We therefore sought to evaluate whether 3DE models could feasibly be imported using the CARTO Merge module, in a manner analogous to importing CT or MRI anatomical data.
To our knowledge, there are presently no published data in the pediatric or adult literature on the feasibility of integration of 3DE models with EAM systems. In this case report, we describe successful integration of a 3DE-derived heart model into the CARTO3 environment, and demonstrate preliminary utility for guiding mapping and ablation in a young person with Wolff-Parkinson-White syndrome (WPW).
Case:
A 21 year old female with WPW and SVT presented for EP study and catheter ablation. Pre-procedural TTE demonstrated normal intracardiac anatomy. TTE data were imported into custom modeling software within 3D Slicer (www.slicer.org), which has been previously described.3–5 Using an end-diastolic frame, atrial and ventricular structures were segmented, resulting in a heart model focused on the atrioventricular (AV) valve annuli and adjacent structures (Figure 1). Segmentation was accomplished using assisted and semi-automatic tools available within 3D Slicer. The segmentation surface was smoothed and exported as a .vtk format surface mesh. At the start of the procedure, this 3DE model was imported into CARTO3. A shell of the right atrium was constructed via the fast anatomic mapping (FAM) technique, and the Inferior Vena Cava-Right Atrial junction, Superior Vena Cava-Right Atrial junction, Coronary Sinus os, His bundle, and a few tricuspid annular points were tagged. Using these anatomic points, the 3DE model was registered (aligned) to the EAM, using the CARTO Merge module.
Figure 1: Generation of 3DE Model in 3D Slicer from TTE Data.
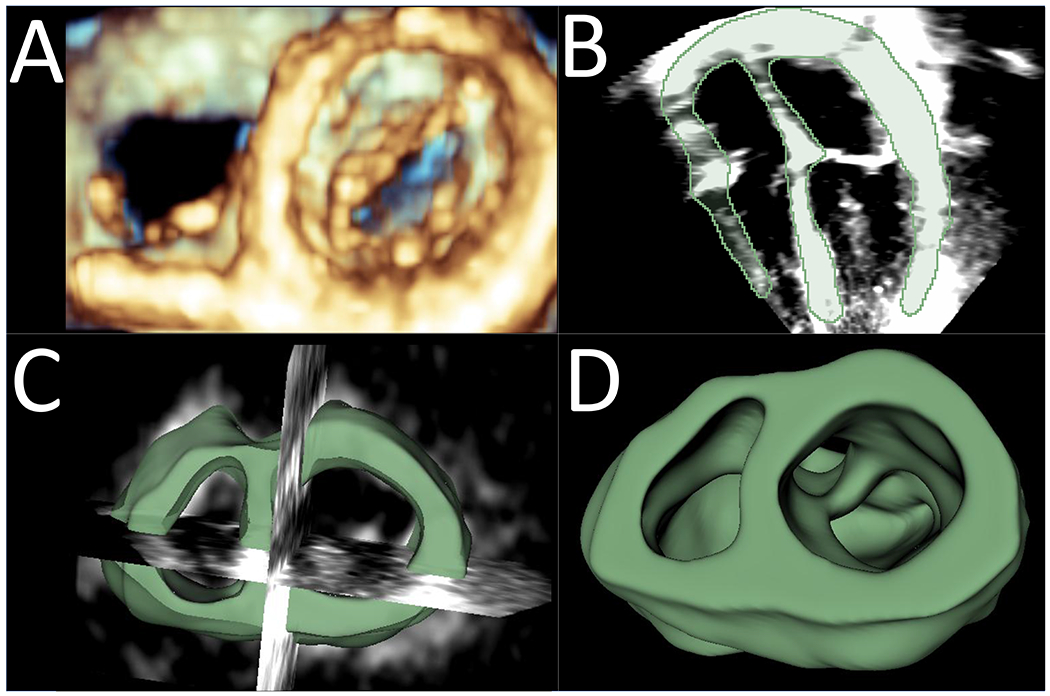
A. Three-dimensional echo-derived volume rendering of AV valves, viewed from the ventricle; B. Two-dimensional 4-chamber view with the segmentation of the atrial and ventricular structures shown in green; C. Visualization of the 3DE heart model focused on the AV valve annuli and adjacent structures with intersection of 2D cutting planes; D. Final 3DE heart model created in 3D Slicer, viewed from the ventricle.
EP testing demonstrated two accessory pathways (AP), one with a left-sided and one with a right-sided activation pattern. The left-sided AP was addressed first, via trans-septal approach. During mapping of the mitral annulus, the 3DE model was subtracted from view, but points acquired during mapping were subsequently used to further refine registration of the 3DE model with the EAM. A left lateral AP was successfully eliminated with radiofrequency energy (RF). The tricuspid annulus was then mapped, and activation localized to a right lateral position. At this point, the 3DE model was brought into view to facilitate identification of annular positions along the free wall. An initial RF lesion eliminated AP conduction in 3.5 seconds, but AP conduction recurred immediately following the lesion. Guided by the 3DE model, as well as electrogram signals, a more ventricular site was targeted in the same plane as this first lesion; RF application at this site eliminated AP conduction in 1.4 seconds (Figure 2). At 1 year follow-up, there has been no recurrence of pre-excitation or SVT.
Figure 2: 3DE Model within CARTO3.
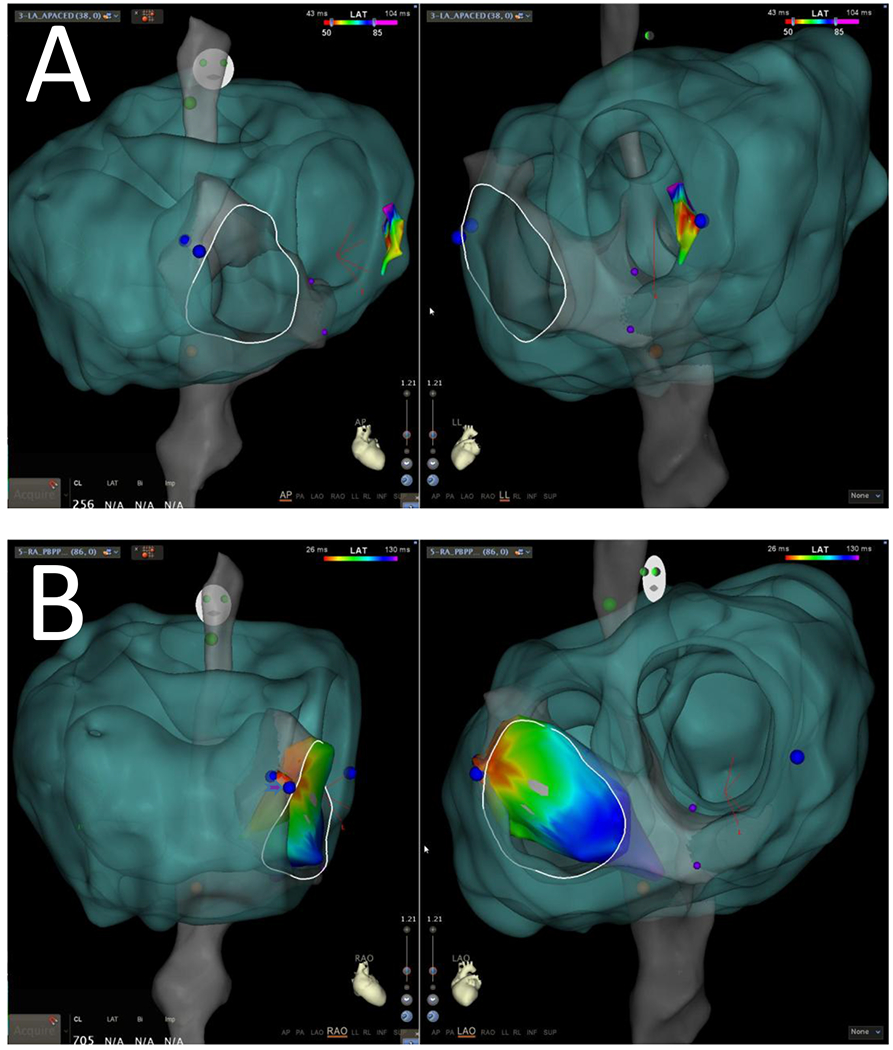
A. The 3DE model (green) has been aligned with the FAM-derived shell (grey). The tricuspid valve annulus has been manually drawn in white, based on registered annular electrograms. In panel A, AP and lateral views are shown, with overlying left-sided activation map (color scale). B. In panel B, RAO and LAO views are shown, with overlying right-sided activation map. Ablation sites are marked in blue. The more ventricular lesion on the lateral tricuspid valve (arrowhead) was the site of definitive pathway elimination. This site can be seen to align well with the 3DE defined valve border.
Discussion:
In this report, we describe a novel technique for the import of 3DE-derived heart models into an EAM system to guide catheter ablation. We believe this technique may be of significant benefit, particularly within the pediatric population and potentially within congenital heart disease (CHD) patients.
While EAM systems have improved the efficacy and safety of catheter ablation,6 limitations in the fidelity of these maps exist, in part owing to the anatomic complexities of the surfaces being reconstructed. Surface complexity is most pronounced at the junction between chambers, namely the AV valves.7 Additional complexity stems from individual variations in anatomy, which are magnified by the heterogeneity of anatomy seen in the CHD population. Catheter ablation of APs in children relies on precise and stable catheter contact with the AV valve annuli. Improved anatomic definition of the valve borders using 3DE data may therefore enhance procedural efficacy.
3DE-derived valve geometry may be especially beneficial in ablation of APs on the lateral border of the tricuspid annulus, as demonstrated by the present case. It is notoriously challenging to define the lateral border of the tricuspid annulus, due to its larger circumference, and to absence of a right-sided annular venous structure (in contrast to the smaller mitral annulus, which is approximated by the coronary sinus).8 These difficulties contribute in part to the observed lower acute success and higher recurrence rates in ablation of right lateral APs, as compared to left-sided APs.9 One reported strategy for delineation of the right lateral annulus involves placement of a microelectrode catheter in the right coronary artery; however, this technique carries the risks attendant to arterial access and coronary instrumentation.8,10 In the presented case, we employed a non-invasive technique to create a roadmap of the tricuspid annulus. The 3DE-defined border of the tricuspid valve guided placement of a more ventricular lesion, facilitating ablation success. 3DE defined valve anatomy may also have particular relevance to specific CHD substrates, such as Ebstein’s anomaly.
Modules such as CARTO Merge were developed to address limitations in the anatomic accuracy of EAM, and incorporation of CT and MRI data during catheter ablation has been well described.11–14 In young patients, use of an alternative 3D imaging modality such as TTE is desirable, in order to reduce unnecessary radiation exposure, avoid sedation, and minimize cost. TTE is often obtained during the pre-procedural evaluation of young patients undergoing catheter ablation, and 3DE can be incorporated into these studies with negligible risk and minimal added time or cost.
Although this technique worked well in the present case, there are some limitations, which may affect broad application of the technology to other cases. Quality of the 3DE model relies on good trans-thoracic echocardiographic windows, which may be suboptimal in larger patients or post-operative patients. This patient had normal anatomy, so the relevance of 3DE models in CHD ablation remains unclear, although prior reports demonstrate that this technique accurately reconstructs congenitally abnormal valves.15 Finally, we acknowledge that the present case was unique in that there was a left-sided pathway, in addition to the right-sided pathway. The mitral annular points allowed for additional refinement of model registration; this fine-tuning would not be possible in the typical case, though may not be necessary for accurate registration.
Integration of TTE-derived 3DE models to guide ablation has not been previously described in the literature. Intracardiac echo (ICE)-derived 3D data has been used,16 though requires use of an 8.5 Fr vascular sheath, which may not be appropriate depending on the size of the patient. This case report is the first to describe the feasibility and methodology of this novel strategy. Further study is needed to determine the accuracy of anatomic registration with 3DE models, the impact of these models on ablation outcomes, and the relevance to CHD. We believe this technique represents a low cost, easily accessible tool with potential to significantly improve safety and efficacy of catheter ablation, particularly in children.
Acknowledgements:
The authors would like to acknowledge Frank Gavigan and Thomas Young (Biosense Webster) for their technical support during this study.
Funding: This work was supported by a Children’s Hospital of Philadelphia (CHOP) Cardiac Center Grant, a CHOP Innovation Grant, Big Hearts to Little Hearts, a Canarie Research Software Foundation grant, and NIH R01HL153166.
Footnotes
Disclosures: The authors have no relevant conflicts of interest.
References:
- 1.Jolley MA, Lasso A, Nam HH, Dinh PV, Scanlan AB, Nguyen AV, Ilina A, Morray B, Glatz AC, McGowan FX, Whitehead K, Dori Y, Gorman JH 3rd, Gorman RC, Fichtinger G, Gillespie MJ. Toward predictive modeling of catheter-based pulmonary valve replacement into native right ventricular outflow tracts. Catheter Cardiovasc Interv 2019. February 15;93(3):E143–E152. doi: 10.1002/ccd.27962. Epub 2018 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nam HH, Herz C, Lasso A, Drouin S, Posada A, Morray B, O’Byrne ML, Paniagua B, Joffe D, Mackensen B, Rogers L, Fichtinger G, Jolley MA. Simulation of Transcatheter Atrial and Ventricular Septal Defect Device Closure Within Three-Dimensional Echocardiography-Derived Heart Models on Screen and in Virtual Reality. J Am Soc Echocardiogr 2020. March 2. doi: 10.1016/j.echo.2020.01.011. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lasso A, Nam HH, Dinh PV, Pinter C, Fillion-Robin JC, Pieper S, Jhaveri S, Vimort JB, Martin K, Asselin M, McGowan FX, Kikinis R, Fichtinger G and Jolley MA. Interaction with Volume-Rendered Three-Dimensional Echocardiographic Images in Virtual Reality. J Am Soc Echocardiogr 2018; 31(10): 1158–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanlan AB, Nguyen AV, Ilina A, Lasso A, Cripe L, Jegatheeswaran A, Silvestro E, McGowan FX, Mascio CE, Fuller S, Spray TL, Cohen MS, Fichtinger G and Jolley MA. Comparison of 3D Echocardiogram-Derived 3D Printed Valve Models to Molded Models for Simulated Repair of Pediatric Atrioventricular Valves. Pediatr Cardiol 2018; 39(3): 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi PS, Nam HH, Lasso A, Herz C, Drouin S, Harrild DM, Quartermain M, Fichtinger G, Mascio CE, Emani S, Jolley MA. 3D Modeling of Surgically Implanted Stent-Based Valves in the Mitral Position in Children. Ann Thorac Surg 2020. March 18;.doi: 10.1016/j.athoracsur.2020.02.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceresnak SR, Dubin AM, Kim JJ, Valdes SO, Fishberger SB, Shetty I, Zimmerman F, Tanel RE, Epstein MR, Motonaga KS, Capone CA, Nappo L, Gates GJ and Pass RH. Success rates in pediatric WPW ablation are improved with 3-dimensional mapping systems compared with fluoroscopy alone: a multicenter study. J Cardiovasc Electrophysiol 2015; 26(4): 412–416. [DOI] [PubMed] [Google Scholar]
- 7.Packer DL Three-dimensional mapping in interventional electrophysiology: techniques and technology. J Cardiovasc Electrophysiol 2005; 16(10): 1110–1116. [DOI] [PubMed] [Google Scholar]
- 8.Fishberger SB1, Hernandez A, Zahn EM. Electroanatomic mapping of the right coronary artery: a novel approach to ablation of right free-wall accessory pathways. J Cardiovasc Electrophysiol 2009. May;20(5):526–9. doi: 10.1111/j.1540-8167.2008.01370. [DOI] [PubMed] [Google Scholar]
- 9.Van Hare GF, Javitz H, Carmelli D, et al. Prospective assessment after pediatric cardiac ablation: Demographics, medical profiles, and initial outcomes. J Cardiovasc Electrophysiol 2004. doi: 10.1046/j.1540-8167.2004.03645. [DOI] [PubMed] [Google Scholar]
- 10.Shah MJ, Jones TK, Cecchin F. Improved localization of right-sided accessory pathways with microcatheter-assisted right coronary artery mapping in children. J Cardiovasc Electrophysiol 2004. November;15(11):1238–43. [DOI] [PubMed] [Google Scholar]
- 11.Dong J, Dickfeld T, Dalal D, Cheema A, Vasamreddy CR, Henrikson CA, Marine JE, Halperin HR, Berger RD, Lima JA, Bluemke DA and Calkins H. Initial experience in the use of integrated electroanatomic mapping with three-dimensional MR/CT images to guide catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2006; 17(5): 459–466. [DOI] [PubMed] [Google Scholar]
- 12.Bertaglia E, Bella PD, Tondo C, Proclemer A, Bottoni N, De Ponti R, Landolina M, Bongiorni MG, Coro L, Stabile G, Dello Russo A, Verlato R, Mantica M and Zoppo F. Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMerge Italian Registry. Europace 2009; 11(8): 1004–1010. [DOI] [PubMed] [Google Scholar]
- 13.Pflaumer A, Deisenhofer I, Hausleiter J and Zrenner B. Mapping and ablation of atypical flutter in congenital heart disease with a novel three-dimensional mapping system (Carto Merge). Europace 2006; 8(2): 138–139. [DOI] [PubMed] [Google Scholar]
- 14.Tovia-Brodie O, Belhassen B, Glick A, Shmilovich H, Aviram G, Rosso R and Michowitz Y. Use of New Imaging CARTO(R) Segmentation Module Software to Facilitate Ablation of Ventricular Arrhythmias. J Cardiovasc Electrophysiol 2017; 28(2): 240–248. [DOI] [PubMed] [Google Scholar]
- 15.Pouch AM, Aly AH, Lasso A, Nguyen AV, Scanlan AB, McGowan FX, Fichtinger G, Gorman RC, Gorman JH 3rd, Yushkevich PA, and Jolley MA. Image Segmentation and Modeling of the Pediatric Tricuspid Valve in Hypoplastic Left Heart Syndrome. Funct Imaging Model Heart 2017; 10263:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okumura Y, Henz BD, Johnson SB, Bunch TJ, O’Brien CJ, Hodge DO, Altman A, Govari A and Packer DL. Three-dimensional ultrasound for image-guided mapping and intervention: methods, quantitative validation, and clinical feasibility of a novel multimodality image mapping system. Circ Arrhythm Electrophysiol 2008; 1(2): 110–119. [DOI] [PubMed] [Google Scholar]


